Hepatitis B virus testing of adults seeking evaluation and treatment of sexually transmitted infection who are unvaccinated and do not know their hepatitis B virus infection status is cost saving compared with the current recommendation for vaccination alone.
Abstract
Background
The estimated number of people living with hepatitis B virus (HBV) infection acquired through sexual transmission was 103,000 in 2018, with an estimated incidence of 8300 new cases per year. Although hepatitis B (HepB) vaccination is recommended by the Advisory Committee for Immunization Practices for persons seeking evaluation and treatment for sexually transmitted infections (STIs), prevaccination testing is not yet recommended. Screening may link persons with chronic hepatitis B to care and reduce unnecessary vaccination.
Methods
We used a Markov model to calculate the health impact and cost-effectiveness of 1-time HBV testing combined with the first dose of the HepB vaccine for adults seeking care for STI. We ran a lifetime, societal perspective analysis for a hypothetical population of 100,000 aged 18 to 69 years. The disease progression estimates were taken from recent cohort studies and meta-analyses. In the United States, an intervention that costs less than $100,000 per quality-adjusted life-year (QALY) is generally considered cost-effective. The strategies that were compared were as follows: (1) vaccination without HBV screening, (2) vaccination and hepatitis B surface antigen (HBsAg) screening, (3) vaccination and screening with HBsAg and anti-HBs, and (4) vaccination and screening with HBsAg, anti-HBs, and anti-HBc. Data were obtained from Centers for Medicare & Medicaid services reimbursement, the Centers for Disease Control and Prevention vaccine price list, and additional cost-effectiveness literature.
Results
Compared with current recommendations, the addition of 1-time HBV testing is cost-saving and would prevent an additional 138 cases of cirrhosis, 47 cases of decompensated cirrhosis, 90 cases of hepatocellular carcinoma, 33 liver transplants, and 163 HBV-related deaths, and gain 2185 QALYs, per 100,000 adults screened. Screening with the 3-test panel would save $41.6 to $42.7 million per 100,000 adults tested compared with $41.5 to $42.5 million for the 2-test panel and $40.2 to $40.3 million for HBsAg alone.
Conclusions
One-time HBV prevaccination testing in addition to HepB vaccination for unvaccinated adults seeking care for STI would save lives and prevent new infections and unnecessary vaccination, and is cost-saving.
Hepatitis B virus (HBV) can be efficiently transmitted by sexually active persons, and sexual transmission is a common route of HBV infection in low-endemic countries.1 The risks of HBV transmission between persons with acute or chronic hepatitis B (CHB) and an unprotected or unvaccinated partner is as high as 40% through sexual contact.1 The Centers for Disease Control and Prevention (CDC) estimated that from 2013 to 2018, an estimated 38% of acute HBV infections, or 47,000, in the United States were attributable to sexual transmission.2 The estimated prevalence of sexually transmitted HBV infections in the United States in 2018 was 103,000, with an estimated incidence of 8300 new cases.2
To decrease the risk of sexually transmitted HBV infection, the CDC Advisory Committee for Immunization Practices (ACIP) recommends hepatitis B (HepB) vaccination for persons seeking evaluation or treatment for sexually transmitted infection (STI) who reported not completing a HepB vaccine series.3 Although there are many HepB cost-effectiveness analyses, very few examine vaccination in high-risk populations, and very few examine prevaccination screening. A study published in 2008 suggested that universal HepB vaccination of persons at STI clinics who reported no prior vaccination would be cost-saving to society.4 However, that was based on a cohort aged 25 years, with only 10% self-reported prior vaccination or infection, and based on the much lower prior federal contract price for the 3-dose HepB vaccine of $24 per dose. Hepatitis B virus prevaccination testing is not currently recommended in the absence of other risk factors for persons seeking evaluation and treatment for STI. One-time HBV testing would provide a diagnosis for those living with CHB, enabling linkage to care and treatment. Testing could also save vaccine costs by identifying persons with natural immunity or current infection who would not require second or third doses of the vaccine, and identifying people with vaccine-induced immunity who might not need further doses.
The purpose of this study was to assess the cost-effectiveness of 1-time prevaccination HBV testing of adults seeking evaluation and treatment for STI in any clinical setting who reported no prior HepB vaccination and do not know their HBV infection status. We compared screening strategies using the hepatitis B surface antigen (HBsAg) test for CHB or a 2-test panel (HBsAg and hepatitis surface antibody [anti-HBs]) for CHB and immunity or a 3-test panel (HBsAg, HepB core antibody [anti-HBc], and anti-HBs) for CHB, prior infection, and immunity, and CHB treatment and HepB vaccination compared with HepB vaccination alone.
METHODOLOGY
Because the impact of HepB vaccination and infection may take place over many years or decades, we used a Markov model (Appendix Fig. 1, http://links.lww.com/OLQ/A809) to simulate progression through a discrete series of health states in response to alternative screening, treatment, and vaccination policies. Markov models are appropriate for simulating people moving from health state to health state over time, such as how persons with inactive or latent chronic HepB infection can reactivate to cause active hepatitis with liver inflammation. The model starts with a cohort of the eligible population distributed across health states of susceptible, immune (from vaccination or prior infection), inactive CHB, active CHB, and cirrhosis. Events and outcomes measured in the model included CHB screening, monitoring and treatment costs, quality-adjusted life-years (QALYs), and clinical end points. This mathematical model has been shown to closely match HBV natural history, cirrhosis incidence, and survival.5 We use age proportions based on the age distribution seen at STI clinics,6 as shown in Table 1. The estimated HBsAg prevalence in the STI population in the United States was 4.2%.7 We assumed that, as with the general population, 67% of adults with CHB are not aware of their infection.9 Results were presented as weighted averages over age and sex where 61.9% of the risk group was male.8 The proportion with reported prior HepB vaccination ranged across age groups from 15.9% to 91.3%10,11 (Table 1). The age group–specific annual incidence of developing acute HBV infection ranged between 0.33% and 1.0%.4 We ran a lifetime analysis to compute results for a hypothetical population of 100,000 men and women aged 18 to 69 years eligible to be screened due to seeking care for an STI.
TABLE 1.
Key Input Variables and Ranges
| Variable | Base Case | Range | Distribution* | Reference |
|---|---|---|---|---|
| Age/birth cohort, y | ≥18 | 18–69 | ||
| Proportion of population (at an STI clinic), % | Hechter et al.6 | |||
| 18–24 y | 35 | |||
| 25–34 y | 39 | |||
| 35–44 y | 17 | |||
| 45–54 y | 7 | |||
| 55+ y | 3 | |||
| HBsAg prevalence in STI US population, % | 4.17 | Marseille et al.7 | ||
| Male-to-female ratio within the risk group, % | 61.9 | Pathela et al.8 | ||
| Ratio of prevalence in men vs. women | 1.38 | Patel et al.9 | ||
| Proportion with prior vaccination, % | ||||
| 18–29 y | 91.3 | 16–95 | Beta | Rosenthal et al.10† |
| 30–39 y | 40.1 | 37.0–91.3 | Beta | Lu et al.,11 CDC, 2021, unpublished |
| 40–49 y | 31.9 | 29.1–40.1 | Beta | Lu et al.,11 CDC, 2021, unpublished |
| 50–59 y | 24.9 | 22.6–31.9 | Beta | Lu et al.,11 CDC, 2021, unpublished |
| 60+ y | 15.6 | 14.3–24.9 | Beta | Lu et al.,11 CDC, 2021, unpublished |
| Proportion unvaccinated who have prior infection (anti-HBc positive regardless of anti-HBs), % | ||||
| 18–24 y | 8.8 | 4.2–13.3 | Beta | Trepka et al.48s |
| 25–34 y | 26.1 | 20.1–32.1 | Beta | Trepka et al.48s |
| 35+ y | 33.1 | 27.4–38.8 | Beta | Trepka et al.48s |
| Annual incidence of developing acute HBV infection, % | ||||
| 18–39 y | 1.0 | Beta | Miriti et al.4 | |
| 40+ y | 0.33 | Beta | Miriti et al.4 | |
| Percent not aware of their infection | 67 | 49–82 | Beta | Patel et al.9 |
| Percent aware of prior vaccination | ||||
| 19–49 y | 70 | 63.6–97.6 | Beta | Rolnick et al.12 |
| 50+ y | 80.8 | 75.5–86.2 | Beta | Rolnick et al.12 |
| Proportion adults diagnosed with CHB and linked to care and received antiviral treatment, % | 18 | 17–19 | Beta | Harris et al.41s |
| Screening costs, US$ | ||||
| Cost of hepatitis B serologic tests | CMS38s | |||
| HBsAg | 10.33 | 7.50–20.00 | Gamma | |
| Anti-HBc | 12.05 | 7.50–20.00 | Gamma | |
| Anti-HBs | 10.74 | 7.50–20.00 | Gamma | |
| All 3 hepatitis B tests | 28.27 | 25.00–40.00 | Gamma | |
| Vaccination costs, US$ | ||||
| Engerix-B/Recombivax HB private sector cost per dose | 61.54 | 25.43–73.85 | Gamma | CDC49s |
| Twinrix private sector cost per dose | 112.35 | 62.04–134.83 | Gamma | CDC49s |
| Heplisav-B private sector cost per dose | 121.25 | 73.05–145.50 | Gamma | CDC49s |
| Vaccine administration (physician) | 25.84 | 20.67–31.01 | Gamma | Tsai et al.50s |
| Vaccine administration (pharmacist) | 17.50 | 14–21 | Gamma | Tsai et al.50s |
| Patient time, vaccination-specific visit, physician's office, h | 2.00 | Ray et al.51s | ||
| Patient time, vaccination-specific visit, pharmacy, h | 0.20 | 0.17–0.29 | Gamma | Prosser et al.52s |
| Proportion of patients requiring vaccination-specific visit | ||||
| First dose | 0 | |||
| Second and third doses | 0.5 | 0–1 | Beta | CDC53s |
| Proportion of patients receiving vaccine in pharmacy setting | 0.3 | Singhal and Zhang15 | ||
| Mean hourly earnings, US$ | 29.96 | BLS54s | ||
| Linkage to care and treatment costs, US$ | ||||
| Antiviral drug costs per year | 502.00 | 326.00–1,464.00‡ | Gamma‡ | |
| Initial baseline tests (HBeAg, CBC, CMP, HBV DNA) | 71.40 | 35.70–107.10 | Gamma | CMS38s |
| Total annual monitoring costs‡ | 355.00 | 177.50–532.50 | Gamma | CMS38s |
| Clinic visit × 2 | 74.00 | § | CMS38s | |
| Ultrasound × 1 (50% none, 50% × 2) | 125.00 | § | CMS38s | |
| AFP × 1 (50% none, 50% × 2) | 17.00 | § | CMS38s | |
| CMP × 2 | 11.00 | § | CMS38s | |
| HBV DNA × 1 | 43.00 | § | CMS38s | |
| Annual disease management costs | ||||
| Medical costs, US$ | ||||
| CHB | 1694.00 | 183.00–5061.00 | Gamma | Liu et al.42s |
| Cirrhosis | 5057.00 | 183.00–5061.00 | Gamma | Liu et al.42s |
| Decompensated cirrhosis | 13,405.00 | 6709.00–20,115.00 | Gamma | Liu et al.42s |
| Hepatocellular carcinoma | 53,366.00 | 26,761.00–80,054.00 | Gamma | Liu et al.42s |
| Liver transplantation 1st year | 175,745.00 | 87,879.00–263,612.00 | Gamma | Liu et al.42s |
| Liver transplantation 2nd year | 30,687.00 | 15,343.00–46,043.00 | Gamma | Liu et al.42s |
| Acute symptomatic (not hospitalized) | 591.00 | 306.00–1029.00 | Gamma | Kim et al.61s |
| Acute symptomatic (hospitalized) | 17,340.00 | 3691.00–17,340.00 | Gamma | Kim et al.61s |
| Fulminant hepatitis | 27,707.00 | 27,791.00–74,414.00 | Gamma | Kim et al.61s |
| Patient and caregiver time costs, US$ | ||||
| Active HBV (w/o cirrhosis) | 5876.00 | 3289.00–8462.00 | Gamma | Federico et al.55s |
| Cirrhosis | 8639.00 | 6139.00–11,241.00 | Gamma | Federico et al.55s |
| HCC | 7816.00 | 5317.00–10,316.00 | Gamma | Federico et al.55s |
| Transplant | 16,440.00 | 13,940.00–18,940.00 | Gamma | Federico et al.55s |
| Sustained viral response | 960.00 | 0.00–7311.00 | Gamma | Assumption: 32 h/y |
| Health state utilities | ||||
| Immune state | 0.99 | 0.98–1.00 | Beta | Chahal et al.13 |
| Susceptible state | 0.99 | 0.98–1.00 | Beta | Chahal et al.13 |
| Active CHB | 0.91 | (0.80–0.92) | Beta | Woo et al.45s |
| Cirrhosis | 0.88 | (0.78–0.88) | Beta | Woo et al.45s |
| Inactive CHB | 1 | (0.90–1.00 | Woo et al.45s | |
| Decompensated cirrhosis | 0.73 | (0.49–0.82) | Beta | Woo et al.45s |
| Hepatocellular carcinoma | 0.81 | (0.77–0.85) | Beta | Woo et al.45s |
| Liver transplantation | 0.84 | (0.72–0.84) | Beta | Woo et al.45s |
| HBsAg seroclearance | 1 | (0.95–1.00) | Woo et al.45s | |
| Viral suppression | 1 | (0.95–1.00) | Woo et al.45s | |
| Vaccination, % | ||||
| Fraction receiving 1st dose | 74 | 66.6–81.4 | Beta | Miriti et al.4 |
| Fraction receiving 2nd dose (of those receiving 1st dose) | 61 | 47.7–62.7 | Beta | Bruxvoort et al.14 |
| Fraction receiving 3rd dose (of those receiving 1st dose) | 32 | 27.0–33.0 | Beta | Bruxvoort et al.14 |
| Seroprotection rate for Twinrix/Engerix-B/Recombivax HB (0-, 1-, and 6-mo schedule), % | ||||
| Vaccine effectiveness, 1st dose | ||||
| 18–29 y | 20.4 | 4–28.8 | Beta | Levie et al.56s |
| 30–39 y | 20.4 | 4–28.8 | Beta | Levie et al.56s |
| 40–49 y | 12 | 4–17.2 | Beta | Treadwell et al.57s |
| 50–59 y | 12 | 4–17.2 | Beta | Treadwell et al.57s |
| 60+ y | 7.5 | 4–8.6 | Beta | Joines et al.58s |
| Vaccine effectiveness, 2nd dose | ||||
| 18–29 y | 77.0 | 24–87.2 | Beta | Levie et al.56s |
| 30–39 y | 77.0 | 24–87.2 | Beta | Levie et al.56s |
| 40–49 y | 63 | 24–72.0 | Beta | Treadwell et al.57s |
| 50–59 y | 63 | 24–72.0 | Beta | Treadwell et al.57s |
| 60+ y | 50.4 | 24–60 | Beta | Joines et al.58s |
| Vaccine effectiveness, 3rd dose | ||||
| 18–29 y | 99.3 | 80–100 | Beta | Levie et al.56s |
| 30–39 y | 99.3 | 80–100 | Beta | Levie et al.56s |
| 40–49 y | 97 | 80–100 | Beta | Treadwell et al.57s |
| 50–59 y | 97 | 80–100 | Beta | Treadwell et al.57s |
| 60+ y | 92.2 | 80–100 | Beta | Joines et al.58s |
| Seroprotection rate for Heplisav-B (0- and 1-mo schedule), % | ||||
| Vaccine effectiveness, 1st dose | ||||
| 18–29 y | 22 | 12–32 | Beta | Hirst et al.59s |
| 30–39 y | 22 | 12–32 | Beta | Hirst et al.59s |
| 40–49 y | 22 | 12–32 | Beta | Hirst et al.59s |
| 50–59 y | 22 | 12–32 | Beta | Hirst et al.59s |
| 60+ y | 22 | 12–32 | Beta | Hirst et al.59s |
| Vaccine effectiveness, 2nd dose | ||||
| 18–29 y | 96 | 80–100 | Beta | Hirst et al.59s |
| 30–39 y | 96 | 80–100 | Beta | Hirst et al.59s |
| 40–49 y | 96 | 80–100 | Beta | Hirst et al.59s |
| 50–59 y | 96 | 80–100 | Beta | Hirst et al.59s |
| 60+ y | 96 | 80–100 | Beta | Hirst et al.59s |
| Discount rate, % | 3 | 0–5 |
*These are the distributions used for the probabilistic sensitivity analysis. The distributions are set such that the means are centered on the base-case value and the SDs of the distributions are set to match one-quarter of the ranges specified in the “Range” column of this table. Parameters with no distribution identified were not varied in probabilistic sensitivity analysis. More details are described in the Appendix (http://links.lww.com/OLQ/A809).
†The 2013 National Immunization Survey–Teen indicate that 91.3% of 17-year-olds received 3 doses of HepB vaccine in 2013.60s This coverage estimate of 17-year-olds from 2013 would be 25 years old in 2021, so we used this estimate for those aged 18 to 29 years of age for vaccination coverage.
‡The range of $326.00 to $16,464.00 is for 1-way sensitivity analysis, but for the probabilistic sensitivity analysis, an SD of 173 is used.
§Annual monitoring costs are implemented as a single parameter in the model. The subcomponents are shown to explain how the annual monitoring costs were calculated. However, for purposes of sensitivity analysis, only the total annual monitoring costs are varied.
All costs in 2021 US dollars, with inflation adjusting using the personal consumption expenditures index.
AFP indicates α-fetoprotein; HBc, hepatitis B core antibody; ALT, alanine aminotransferase; CBC, complete blood count; CHB, chronic hepatitis B; CMP, complete metabolic panel; HBeAg, hepatitis B e antigen; HBs, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; STI, sexually transmitted infections.
The following scenarios (Appendix Fig. 2, http://links.lww.com/OLQ/A809) were assessed:
-
1)
Vaccination without HBV screening (current practice): Offer HepB vaccination (2-dose or 3-dose vaccines) if the adult reported no prior vaccination (no HBV testing).
-
2)
HepB vaccination and HBsAg screening: Offer HepB vaccination (2-dose or 3-dose vaccines) if the adult reported no prior vaccination. If the HBsAg test result is positive, no further vaccine doses are given, and the patient is connected to CHB care and treatment.
-
3)
HepB vaccination and screening with HBsAg and anti-HBs: Offer HepB vaccination if the adult reported no prior vaccination. If the test results are positive for HBsAg or anti-HBs ≥10 mIU/mL*, no further vaccine doses are given. An adult testing positive for HBsAg is connected to CHB care and treatment.
-
4)
HepB vaccination and screening with HBsAg, anti-HBs, and anti-HBc: Offer HepB vaccination if the adult reported no prior vaccination. If the test results are positive for HBsAg or anti-HBc or anti-HBs >10 mIU/mL*, no further vaccine doses are given. An adult testing positive for HBsAg is connected to CHB care and treatment.
In sensitivity analyses, we explored comparisons with 2 additional strategies with no HepB vaccination for comparison:
A) No HepB vaccination or HBV screening
B) HBV screening, and CHB care and treatment. No HepB vaccination
Vaccination
HepB vaccination was modeled including both the dollar costs of vaccination and the productivity costs for patient time. We modeled a vaccine-specific visit at the physician's office or pharmacy for subsequent doses. The proportion of patients receiving subsequent doses of HepB vaccine in a pharmacy setting was 30%.15 Age group–specific seroprotection rates for the 3-dose HepB vaccines (Twinrix or Engerix-B or Recombinvax HB) and the 2-dose vaccine (Heplisav-B) by age are shown in Table 1. Based on the reported HepB vaccination coverage among individuals seeking care for STI who reported no prior vaccination, 74% received the first HepB vaccine dose.4 Among persons who received the first dose, 61% received a second dose and 32% received a third dose,14 as shown in Table 1. We assumed that 70% of the adults aged 19 to 49 years and 80.8% of adults 50 years and older were aware whether they have been vaccinated.12 See additional details on vaccination rates in the Appendix (Appendix Table 1, http://links.lww.com/OLQ/A809).
Disease Progression and Treatment Related Estimates
Disease transition estimates for acute infection are shown in Table 1 and were obtained from Chahal et al.13 and Hutton et al.16 The natural history of CHB and disease progression rates were derived from recent cohort studies and meta-analyses mainly from North America for HBV monoinfected patients (Appendix Tables 2 and 3, http://links.lww.com/OLQ/A809).17–27 A 50% reduction in disease progression estimates was applied for female patients, based on recent sex-specific studies.28–30 Treatment effectiveness estimates were expressed as reductions in disease progression risks for treatment-naive patients (Appendix Table 4, http://links.lww.com/OLQ/A809).31s–36s We assumed that effective antiviral suppression would reduce liver cancer risks in cirrhotic and noncirrhotic patients by 50% and 70%, respectively, compared with natural history.33s,34s,36s Disease progression between health states, conditional on treatment, age (where available), and sex was simulated in 1-year cycles. Causes of death that were not related to CHB were included in the model, based on age-specific mortality rates from life tables in the National Statistics Report.37s
Costs and Utilities
The costs of HBsAg ($10.33), anti-HBc ($10.74), anti-HBs ($12.05) tests, and a 3 HepB tests package ($28.27) were based on Medicare reimbursement.38s For our base-case analysis, we used the private sector costs for the 3-dose monovalent HepB vaccines (Engerix-B and Recombivax-HB) at $61.86 to $61.22 per dose, the 3-dose combination hepatitis A and HepB vaccine (Twinrix) at $112.35 per dose, and the 2-dose monovalent vaccine (Heplisav-B) at $121.25 per dose.39s The lower CDC federal contract prices for the vaccines were also used in the sensitivity analysis. The analysis included administration costs of $25.84 at the providers' office or clinic and $17.50 at the pharmacy. Although the lowest price for antiviral drugs is generic tenofovir disoproxil fumarate at $325 per year (RedBook 202140s), we used an annual antiviral drug cost of $502 with the assumption that 60% of the patients will be dispensed generic tenofovir disoproxil fumarate and 40% generic entecavir.41s We obtained other medical management costs for CHB, cirrhosis, decompensated cirrhosis, and liver cancer from Liu et al.42s Medical management costs were adjusted for inflation using the personal consumption expenditure index to reflect 2021 US dollars.43s For patients diagnosed with CHB and linked to care, we assumed that they will receive initial baseline tests (hepatitis B e antigen, complete blood count, complete metabolic panel, HBV DNA), and twice yearly clinic visits with alanine aminotransferase blood tests and yearly HBV DNA viral load tests, and that eligible patients (50%) would receive additional hepatocellular carcinoma surveillance consisting of liver ultrasound and α-fetoprotein every 6 months as recommended by the American Association for the Study of Liver Diseases44s (Table 1). Costs of testing and clinic visits were based on Medicare reimbursement rates38s (Table 1). We assumed patients who achieved HBsAg loss would continue to incur annual costs for long-term CHB monitoring. All costs and QALYs were discounted at a rate of 3% per year. The analysis was performed from the societal perspective. We used EuroQol 5 Dimensions utility assessments calculated by Woo et al.45s based on a Canadian CHB patient sample and included age adjustments, and for the immune and susceptible health states, we used Chahal et al.13 estimates.
In our analysis, we evaluated the costs and QALYs for each testing and vaccination strategy combination. We rank-ordered the strategies in terms of lowest to highest costs. In cases where an intervention had higher costs but better health outcomes, we calculated an incremental cost-effectiveness ratio (ICER) between the more expensive strategy and the less-expensive strategy. In some sensitivity analyses, we calculated a net monetary value, which converts dollars and QALYs into a single measure where QALYs are valued at $100,000 each. Our main goal was to evaluate the impact of the HBV testing strategies. In a secondary analysis, we evaluated the cost-effectiveness of HepB vaccination compared with no vaccination.
In addition to conducting a base-case analysis, we also conducted sensitivity analyses. We first conducted 1-way sensitivity analyses where we varied each parameter one at a time. We also examined results assuming 3-dose vaccination. We next evaluated several pairs of parameters in 2-way sensitivity analysis. Finally, we conducted a Monte-Carlo simulation where each parameter was varied according to probability distributions (described in the Appendix, http://links.lww.com/OLQ/A809) to get a distribution of outcomes with which we created cost-effectiveness acceptability curves.
The disease model was created using TreeAge Pro Healthcare 2011 and analyzed with Microsoft Excel 365.
RESULTS
Among adults seeking care for an STI, prevaccination HBV testing for HepB unvaccinated persons and CHB care and treatment improved health outcomes while lowering overall net costs when compared with vaccination alone of adults who reported no prior HepB vaccination (Fig. 1). The addition of 1-time adult prevaccination HBV testing and connection to CHB care would prevent an additional 138 cases of cirrhosis, 90 cases of hepatocellular carcinoma, and 163 HBV-related deaths and would gain 2185 QALYs for every 100,000 adults screened, irrespective of the HepB vaccine used (Table 2; Appendix Table 5, http://links.lww.com/OLQ/A809). Because CHB treatment is so inexpensive and these disease outcomes are so costly, a strategy that combined HepB vaccination with CHB screening with HBsAg and treatment produced an estimated $40.2 to $40.3 million in net savings for every 100,000 adults tested depending on the vaccine used (Table 2; Appendix Table 5, http://links.lww.com/OLQ/A809). HBV testing that includes anti-HBc to test for prior infection or anti-HBs to test for immunity would identify people who would not benefit from vaccination, saving the cost of a second or third dose of vaccine. Screening with the 2-test panel “HBsAg, anti-HBs” would lower costs by $41.5 to $42.5 million when compared with the current practice that only offers vaccination. Testing with the HepB 3-test panel “HBsAg, anti-HBs, anti-HBc” would have the highest cost savings compared with vaccination alone, but it only had an additional $0.44 to $1.84 in savings per person than testing with the 2-test panel.
Figure 1.
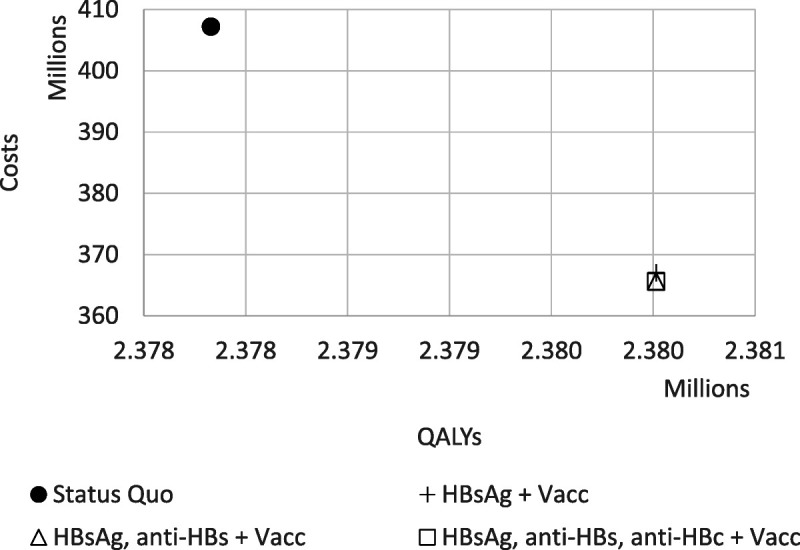
Comparison in costs and QALYs of status quo (HepB vaccination with Heplisav) with the various prevaccination HBV testing strategies combined with vaccination among a cohort of 100,000 persons. The HBsAg + Vacc; HBsAg, anti-HBS + Vacc; and HBsAg, anti-HBS, anti-HBC + Vacc all are very closely overlapping. All have the same QALYs. See Figure 3 for a closer examination of the cost differences. Results for Engerix/Recombivax and Twinrix are similar and can be found in Appendix Table 4 (http://links.lww.com/OLQ/A809).
TABLE 2.
Health and Economic Results of Screening Interventions for a Population of 100,000 Adults
| Strategy | Cost | QALYs | Cirrhosis | Decompensated Cirrhosis | HCC | Transplants | HBV Deaths |
|---|---|---|---|---|---|---|---|
| Heplisav-B | |||||||
| Status quo | 407,218,391 | 2,377,829 | 461 | 131 | 431 | 156 | 690 |
| HBsAg + Vacc | 367,022,663 | 2,380,014 | 323 | 84 | 341 | 123 | 526 |
| HBsAg, anti-HBs + Vacc | 365,641,803 | 2,380,014 | 323 | 84 | 341 | 123 | 526 |
| HBsAg, anti-HBs, anti-HBc + Vacc | 365,591,884 | 2,380,014 | 323 | 84 | 341 | 123 | 526 |
| Engerix-B/Recombivax HB | |||||||
| Status quo | 405,264,020 | 2,377,824 | 462 | 131 | 431 | 156 | 690 |
| HBsAg + Vacc | 365,073,912 | 2,380,009 | 324 | 84 | 341 | 123 | 527 |
| HBsAg, anti-HBs + Vacc | 363,727,272 | 2,380,009 | 324 | 84 | 341 | 123 | 527 |
| HBsAg, anti-HBs, anti-HBc + Vacc | 363,683,205 | 2,380,009 | 324 | 84 | 341 | 123 | 527 |
| Twinrix | |||||||
| Status quo | 408,974,805 | 2,377,824 | 462 | 131 | 431 | 156 | 690 |
| HBsAg + Vacc | 368,650,275 | 2,380,009 | 324 | 84 | 341 | 123 | 527 |
| HBsAg, anti-HBs + Vacc | 366,485,066 | 2,380,009 | 324 | 84 | 341 | 123 | 527 |
| HBsAg, anti-HBs, anti-HBc + Vacc | 366,300,996 | 2,380,009 | 324 | 84 | 341 | 123 | 527 |
Cost: in US dollars. Dominant: the intervention has lower costs and higher QALYs than the status quo.
ICER indicates incremental cost-effectiveness ratio; QALYs, quality-adjusted life-years.
The cost-effectiveness of HepB vaccination of adults seeking care for an STI, compared with no vaccination, varied depending on the vaccine used. Vaccination with the 2-dose Heplisav vaccine would cost $5.4 million, prevent 1490 acute infections, prevent 6 HBV-related deaths, and would have an ICER of $96,794 per QALY for every 100,000 adults seen at STI clinics (Appendix Table 5, http://links.lww.com/OLQ/A809). Using the Engerix-B or Recombivax HB vaccine had an ICER of $68,225 per QALY, and the Twinrix vaccine had an ICER of $141,297 per QALY.
Vaccination of the cohort with the 2-dose vaccine (Heplisav) was $1.94 million more expensive than vaccination with the 3-dose monovalent vaccines (Engerix-B/Recombivax HB), but added 5 more QALYs per 100,000 population. Heplisav was $1.76 million less expensive and had better protection against HBV infection than Twinrix, adding 5 more QALYs (Appendix Tables 5 and 6a–c, http://links.lww.com/OLQ/A809).
SENSITIVITY ANALYSIS
The main conclusion that HBV screening and CHB treatment was cost-effective did not change because parameter assumptions were varied (Fig. 2, Appendix Figs. 3a–c, http://links.lww.com/OLQ/A809). If CHB prevalence in the STI population were zero instead of 4.2%, the benefits of screening would be smaller, but screening would still be less costly than the status quo because it would identify people who had already been vaccinated and would not need additional vaccine doses (Appendix Figs. 4a–c, http://links.lww.com/OLQ/A809). Anti-HBc prevalence did not substantially affect the value of screening (Appendix Figs. 5a–c, http://links.lww.com/OLQ/A809).
Figure 2.
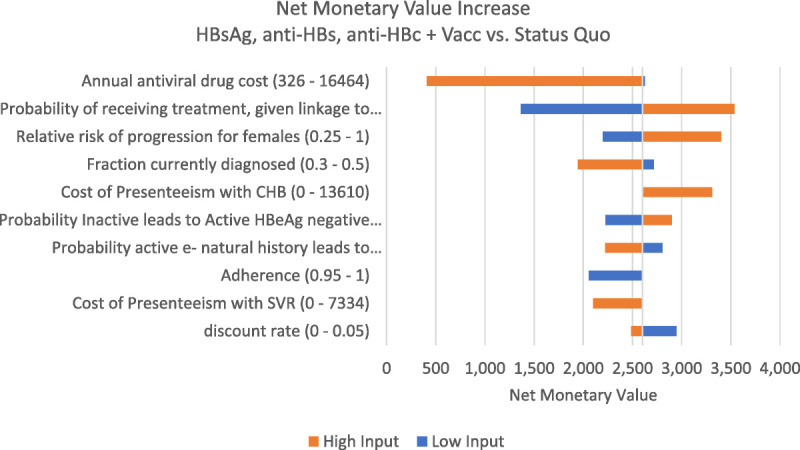
Net monetary value increase with HBsAg, anti-HBs, anti-HBc + Vacc versus status quo for a single person screened. Heplisav vaccine. Net monetary value calculates the incremental value of the HBsAg, anti-HBs, anti-HBc + Vacc strategy compared with the status quo strategy by valuing dollars at a rate of $1 = $1 and QALYs gained at a value of 1 QALY = $100,000. Positive values indicate the HBsAg, anti-HBs, anti-HBc + Vacc strategy is preferred when compared with the status quo if a policy maker is willing to pay $100,000 per QALY gained.
Because the 3 tests (HBsAg, anti-HBs, anti-HBc) strategy only saved a net $0.44 to $1.84 above the 2 test (HBsAg, anti-HBs) strategy, many small changes in test costs could lead to a switch between which of these testing strategies were the lowest cost. However, the net cost difference between these 2 strategies did not vary more than a few dollars per person because parameters were varied (Appendix Figs. 6a–c, http://links.lww.com/OLQ/A809).
The Appendix (http://links.lww.com/OLQ/A809) contains comparisons of policies of screening and vaccination to strategies with screening without HepB vaccination. Screening and vaccination helps avoid unnecessary vaccine doses, so under these conditions, vaccination is much more cost-effective when compared with no-vaccination strategies.
Sensitivity analysis results were similar with all 3 types of vaccines (Appendix Figs. 3–6, http://links.lww.com/OLQ/A809).
Using CDC federal contract vaccine pricing instead of private payer pricing would make immunization much more cost-effective. The ICER of current practice (vaccination alone) compared with no HepB vaccination would become much more favorable at $44,374 per QALY for Heplisav, $16,298 for Engerix-B/Recombivax HB, and $68,944 for Twinrix (Appendix Tables 7a–c, http://links.lww.com/OLQ/A809).
Probabilistic sensitivity analysis highlights overall uncertainty about which specific testing and vaccination strategy is the most cost-effective, given joint uncertainty around all model parameters (Fig. 3). When evaluating various screening policies with vaccination and using the Heplisav vaccine, screening with the 2 tests (HBsAg, anti-HBs) and HepB vaccination was optimal in 40% to 49% of simulations and screening with the 3 tests (HBsAg, anti-HBs, anti-HBc), and vaccination was optimal in 47% to 55% of simulations; HBsAg screening with vaccination was optimal in 4% to 8% of simulations. Current practice (HepB vaccination alone) was not optimal compared with the strategies involving screening. If using the other vaccines, it was slightly more likely that screening with the 3 tests (HBsAg, anti-HBs, anti-HBc) was cost-effective compared with the 2 tests (HBsAg, anti-HBs), but there still was substantial uncertainty and the status quo was highly unlikely to be cost-effective (Appendix Figs. 12a and b, http://links.lww.com/OLQ/A809). If considering policies without vaccination, there was additional uncertainty (Appendix Figs. 13a–c, http://links.lww.com/OLQ/A809).
Figure 3.
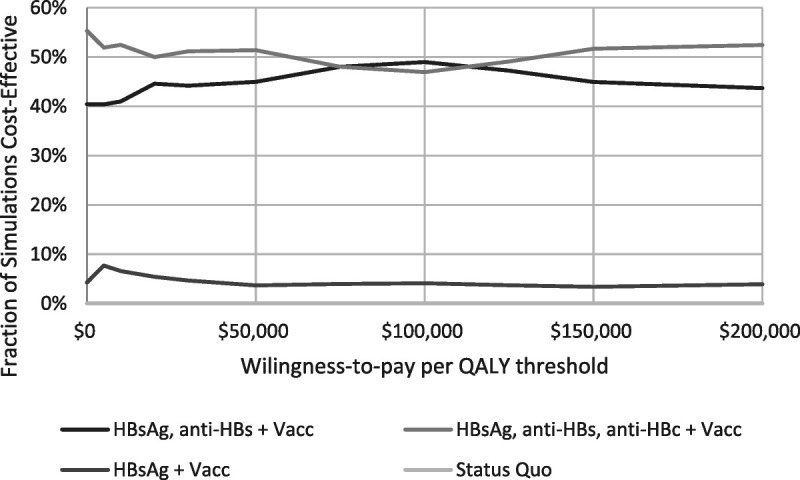
Probability strategy is preferred under various thresholds for willingness to pay for 1 QALY. Evaluated for Heplisav. The QALY differences do not vary substantially, so these results are relatively stable for other willingness-to-pay values between $0 and $100,000 per QALY.
DISCUSSION
Adding a 1-time HBV testing for adults at STI clinics who reported they have not been vaccinated and have not been previously tested to the current recommendation to offer HepB vaccination would be cost saving. Prevaccination testing with HBsAg and linking those diagnosed with CHB to care and antiviral treatment will prevent costly complications and deaths, and can reduce sexual transmission of HBV to sex partners. The current approach of vaccinating without prevaccination testing likely results in missed opportunities for CHB diagnosis. The optimal cost savings were seen screening using the 3-test panel, which also detected past infection or immunity to reduce unnecessary vaccine doses. However, the cost difference between testing with the 3-test panel and the 2-test panel was minor: only $0.44 to $1.84 per person.
The conclusion that HBV prevaccination testing of adults and referral for CHB treatment is valuable is consistent with other studies of the value of screening and treating high-risk groups.13,16,46s Hutton et al.16 found screening and treatment to be cost-effective for Asian and Pacific Islanders in the United States. They found that ring vaccination of partners of identified positive individuals may be cost-effective, but that broad vaccination of these adults would not be cost-effective. However, that analysis is from 2007 and the adult Asian and Pacific Islander population may have lower infection risk than individuals seeking care for STI. A 2018 study by Toy et al.46s reported that reaching World Health Organization screening and treating goals would be highly cost-effective or cost-saving. However, it did not focus on vaccination or people at higher risk of STI. A 2019 study by Chahal et al.13 found screening, treatment, and vaccination of men who have sex with men to be highly cost-effective.
Our analysis is the first to specifically evaluate HBV screening and vaccination policies for individuals at risk of STI. We show that the current ACIP recommendation to vaccinate persons seeking care for STI who reported no prior HepB vaccination would likely prevent 1338 to 1490 acute HepB infections and 6 HBV-related deaths for every 100,000 persons evaluated for STI. The current recommendation (HepB vaccination alone) based on the commercial vaccine prices has an ICER of $68,225 for the 3-dose Engerix-B or Recombivax HB vaccine, $141,297 for the 3-dose Twinrix combined hepatitis A and HepB vaccine, and $96,794 for the 2-dose Heplisav-B vaccine.
The value of HepB vaccination in this high-risk STI population was addressed by Miriti et al.4 in 2008. In a base-case analysis of people aged 25 years with only 10% reported having prior HepB vaccination, Miriti's study suggested that a national program for routine HepB vaccination would likely be cost saving for the society if loss productivity from illness was included in the analysis. Since that study was published, many adolescents and young adults have received HepB vaccination.11 The CDC estimated that among adults aged 18 to 29 years, about 91% have received the HepB vaccine.10 We calculated that only 24% of individuals at STI clinics would benefit from HepB vaccination because today's population seen at STI clinics is mostly young with high vaccination coverage. In Addition, of those unprotected, about 30% are 40 years and older and face a higher incidence of acute infection. Miriti's analysis used federal contract vaccine pricing for the 3-dose monovalent HepB vaccines at $24.25 per dose, whereas in this study, we used the commercial pricing for the HepB vaccines that ranged from $61.86 to $121.25 per dose. If CDC federal contract vaccine prices were used, our study found vaccination alone with the 3-dose monovalent vaccines (Engerix-B or Recombinvax HB) and the 2-dose monovalent vaccine (Heplisav-B) would be cost-effective at $27,778 per QALY and $55,969 per QALY, respectively. The ICER for vaccination alone with the combined hepatitis A and HepB vaccine (Twinrix) was $80,424 per QALY. Miriti's study did not consider the impact of CHB treatment but included the costs of productivity loss from disease complications that were not included in this study. Since 2005, CHB antiviral drug treatment costs have dramatically dropped, lowering the costs of chronic infection treatment. Thus, it has become less expensive to treat persons diagnosed with CHB.
Our study has several limitations. We did not evaluate all possible screening, vaccination, and treatment policies. We only included vaccination policies with the first dose given at the initial visit because of concerns about loss to follow-up and because that policy is in line with ACIP recommendations.3 In addition, we did not further stratify the population of individuals seeking care for an STI, based on either patient characteristics (e.g., men who have sex with men) or setting of care. Presumably, higher-risk populations would benefit more from vaccination. However, even moderate-risk groups are likely to benefit from screening that would link patients to highly cost-effective care.47s
Like all modeling studies, the quality of the results is predicated upon the quality of the input assumptions. Data are somewhat scarce on the prevalence and incidence of HepB in STI clinic populations. Because of this limitation, we conducted a sensitivity analysis on these parameters and found they did not have a meaningful impact on the overall conclusions. Many of our costs are based on Medicare fee schedules. Although Medicare payments are commonly used for cost-effectiveness analyses, the rates are designed for other purposes, and in addition, the average patient age is older than the population studied here. However, we assume that the economic costs of testing and clinic visits may not be substantially different even given the age differences. We did not include the benefits of prevention of secondary infections, which may be a concern in a population that engages in higher levels of sexual activity. However, the secondary benefits could be attenuated if younger people with higher levels of sexual activity assortatively mix with younger people with higher baseline levels of HBV vaccination. We also did not include productivity losses from disease, which may underestimate cost savings from screening and vaccination. We did not include the added benefit in protecting against hepatitis A if the combined hepatitis A and HepB vaccine (Twinrix) was used for vaccination. Our model did not include any impact of the clinical value from knowing anti-HBc status beyond avoiding unnecessary vaccine doses. However, it may be possible that knowing anti-HBc status may be useful knowledge for certain patients like patients who would be at risk for HepB reactivation when receiving immunosuppressive therapy.
A 1-time HBV prevaccination testing of adults seeking evaluation and treatment of STI in addition to the current recommendation to vaccinate persons who reported no prior HepB vaccination is cost saving. Compared with HepB vaccination alone, a combined strategy that includes immunization, screening with the HBV 3-test panel, and treatment of CHB would save more than $40 million and prevent 163 HBV-related deaths/100,000 adults screened.
Supplementary Material
Appendix
For further references, please see “Supplemental References,” http://links.lww.com/OLQ/A810.
Assumes that anti-HBs ≥10 mIU/mL alone indicates a complete HepB vaccine series if records are not available, and that persons without knowledge or records of prior vaccination with anti-HBs <10 mIU/mL might have been fully vaccinated in the past but have waning antibody and not necessarily waning immunity.
D.W.H. and M.T. are co-first authors.
Conflict of Interest and Sources of Funding: The authors have no conflict of interest to declare. This work was supported by The US Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (NU38PS004651).
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention or the authors' affiliated institutions.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
Contributor Information
Mehlika Toy, Email: mtoy@stanford.edu.
Joshua A. Salomon, Email: salomon1@stanford.edu.
Erin E. Conners, Email: ola3@cdc.gov.
Noele P. Nelson, Email: xdg9@cdc.gov.
Aaron M. Harris, Email: ieo9@cdc.gov.
Samuel So, Email: samso@stanford.edu.
REFERENCES
- 1.Brook M. Sexually acquired hepatitis. Sex Transm Infect 2002; 78:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts H Jiles R Harris AM, et al. Incidence and prevalence of sexually transmitted hepatitis B, United States, 2013–2018. Sex Transm Dis 2021; 48:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schillie S Vellozzi C Reingold A, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 2018; 67:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miriti MK Billah K Weinbaum C, et al. Economic benefits of hepatitis B vaccination at sexually transmitted disease clinics in the U.S. Public Health Rep 2008; 123:504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toy M Hutton D Harris AM, et al. Cost-effectiveness of one-time universal screening for chronic hepatitis B infection in adults in the United States. Clin Infect Dis 2022; 74:210–217. [DOI] [PubMed] [Google Scholar]
- 6.Hechter RC Jacobsen SJ Luo Y, et al. Hepatitis B testing and vaccination among adults with sexually transmitted infections in a large managed care organization. Clin Infect Dis 2014; 58:1739–1745. [DOI] [PubMed] [Google Scholar]
- 7.Marseille E Harris AM Horvath H, et al. Hepatitis B prevalence association with sexually transmitted infections: A systematic review and meta-analysis. Sex Health 2021; 18:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pathela P Klingler EJ Guerry SL, et al. Sexually transmitted infection clinics as safety net providers: Exploring the role of categorical sexually transmitted infection clinics in an era of health care reform. Sex Transm Dis 2015; 42:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel EU Thio CL Boon D, et al. Prevalence of hepatitis B and hepatitis D virus infections in the United States, 2011–2016. Clin Infect Dis 2019; 69:709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal EM Hall EW Rosenberg ES, et al. Assessing the cost-utility of preferentially administering Heplisav-B vaccine to certain populations. Vaccine 2020; 38:8206–8215. [DOI] [PubMed] [Google Scholar]
- 11.Lu PJ Hung MC Srivastav A, et al. Surveillance of vaccination coverage among adult populations—United States, 2018. MMWR Surveill Summ 2021; 70:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolnick S Parker E Nordin J, et al. Self-report compared to electronic medical record across eight adult vaccines: Do results vary by demographic factors? Vaccine 2013; 31:3928–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chahal HS Peters MG Harris AM, et al. Cost-effectiveness of hepatitis B virus infection screening and treatment or vaccination in 6 high-risk populations in the United States. Open Forum Infect Dis 2019; 6:ofy353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruxvoort K Slezak J Huang R, et al. Association of Number of doses with hepatitis B vaccine series completion in US adults. JAMA Netw Open 2020; 3:e2027577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singhal PK, Zhang D. Costs of adult vaccination in medical settings and pharmacies: An observational study. J Manag Care Spec Pharm 2014; 20:930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton DW Tan D So SK, et al. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med 2007; 147:460–469. [DOI] [PubMed] [Google Scholar]
- 17.Yang HI Hung HL Lee MH, et al. Incidence and determinants of spontaneous seroclearance of hepatitis B e antigen and DNA in patients with chronic hepatitis B. Clin Gastroenterol Hepatol 2012; 10:527–534.e1–2. [DOI] [PubMed] [Google Scholar]
- 18.Thiele M Gluud LL Fialla AD, et al. Large variations in risk of hepatocellular carcinoma and mortality in treatment naive hepatitis B patients: Systematic review with meta-analyses. PLoS One 2014; 9:e107177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: A systematic review and meta-analysis. Liver Int 2016; 36:1239–1251. [DOI] [PubMed] [Google Scholar]
- 20.Lin X Robinson NJ Thursz M, et al. Chronic hepatitis B virus infection in the Asia-Pacific region and Africa: Review of disease progression. J Gastroenterol Hepatol 2005; 20:833–843. [DOI] [PubMed] [Google Scholar]
- 21.Kanwal F Gralnek IM Martin P, et al. Treatment alternatives for chronic hepatitis B virus infection: A cost-effectiveness analysis. Ann Intern Med 2005; 142:821–831. [DOI] [PubMed] [Google Scholar]
- 22.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J Hepatol 2008; 48:335–352. [DOI] [PubMed] [Google Scholar]
- 23.Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol 2009; 104:1693–1699. [DOI] [PubMed] [Google Scholar]
- 24.Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: Appreciably high rates during a long-term follow-up. Hepatology 2007; 45:1187–1192. [DOI] [PubMed] [Google Scholar]
- 25.Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology 2010; 51:435–444. [DOI] [PubMed] [Google Scholar]
- 26.Burra P Germani G Adam R, et al. Liver transplantation for HBV-related cirrhosis in Europe: An ELTR study on evolution and outcomes. J Hepatol 2013; 58:287–296. [DOI] [PubMed] [Google Scholar]
- 27.Ahn SH Park YN Park JY, et al. Long-term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J Hepatol 2005; 42:188–194. [DOI] [PubMed] [Google Scholar]
- 28.Le AK Toy M Yang HI, et al. Age and Gender-Specific Disease Progression Rates to Cirrhosis and Hepatocellular Carcinoma (HCC) in Treated and Untreated Patients With Chronic Hepatitis B. Washington DC: The American Association for the Study of Liver Diseases, 2017. [Google Scholar]
- 29.Guy J, Peters MG. Liver disease in women: The influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol (N Y) 2013; 9:633–639. [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen E, Tran TT. Hepatitis B in the female population. Gastroenterol Clin North Am 2016; 45:359–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


