Abstract
Human parturition at term and preterm is an inflammatory process synchronously executed by both fetomaternal tissues to transition them from a quiescent state t an active state of labor to ensure delivery. The initiators of the inflammatory signaling mechanism can be both maternal and fetal. The placental (fetal)-maternal immune and endocrine mediated homeostatic imbalances and inflammation are well reported. However, the fetal inflammatory response (FIR) theories initiated by the fetal membranes (amniochorion) at the choriodecidual interface are not well established. Although immune cell migration, activation, and production of proparturition cytokines to the fetal membranes are reported, cellular level events that can generate a unique set of inflammation are not well discussed. This review discusses derangements to fetal membrane cells (physiologically and pathologically at term and preterm, respectively) in response to both endogenous and exogenous factors to generate inflammatory signals. In addition, the mechanisms of inflammatory signal propagation (fetal signaling of parturition) and how these signals cause immune imbalances at the choriodecidual interface are discussed. In addition to maternal inflammation, this review projects FIR as an additional mediator of inflammatory overload required to promote parturition.
Keywords: aging, amniochorion, choriodecidua, EMT, exosomes, inflammation, premature rupture of the membranes, preterm birth, senescence, signaling
1 |. INTRODUCTION
Human pregnancy and parturition are fascinating phenomena but perplexing to comprehend. Two independent biological and physiological systems, the fetal and the maternal, must be considered simultaneously to maintain pregnancy and aid in fetal growth and development.1 Parturition is a unique physiological process that reverses all homeostatic states of pregnant uterine tissues to ensure timely delivery at term.2–6 Preterm labor and delivery (preterm birth, <37 weeks), a major complication impacting ~12% of all pregnancies, contribute to 1 million neonatal deaths globally.7–10 Preterm prelabor rupture of the fetal membranes or amniochorion membranes (pPROM), a disease of the fetal membranes, is the antecedent to 40% of all preterm births in the USA.11–13 Preterm birth is a syndrome initiated by failures in many fetomaternal uterine tissues that work together to maintain pregnancy, resulting in early initiation of labor and delivery.1,14–18 Neonates born preterm are predisposed to mortalities and morbidities and often have significant health issues throughout their lives.19–23 Mothers who deliver preterm are also at risk of postpartum complications.15,20,21 Therefore, reducing the risk of preterm birth is a global healthcare priority.9,24 The mechanisms of delivery in normal and preterm birth parturition are likely similar but with different initiators of labor at term and preterm; nevertheless, the facts remain unclear.25,26 Advances in reproductive biology research have improved our knowledge of various fetomaternal organs and their contribution to pregnancy and parturition, both term and preterm.27–30
A well-orchestrated interplay between the various uterine tissues and fetomaternal immune mediators maintains a harmonious state for 40 weeks. Parturition is a physiologic alteration of homeostatic balance in multiple systems, including the immune system. However, several challenges persist in our understanding of the mechanisms leading to a homeostatic imbalance in response to various risk factors, which prevent us from significantly reducing the incidence of preterm birth.19 Reducing preterm birth risk remains a significant challenge, as this condition may arise with fetomaternal medical indications or maybe spontaneous with an unknown etiology.1,6 It is unclear what initiates the events that disturb this balance to promote the parturition phenotype in the uterine compartments. The critical question still debated in the field is whether the initiator of parturition is (1) fetal signaling,6,31,32 (2) maternal signaling,30,33 or (3) synchronized fetomaternal signaling.4,34,35 Regarding # 1, fetal organ maturation can release biochemicals into the amniotic fluid that include but are not limited to surfactants from the lung,6,36–38 endothelins from the kidneys,39,40 brain-derived neurotrophic factor from brain cells,41 and platelet activation factor from the liver.42 These biochemicals are proinflammatory and cause a further increase in proinflammatory cytokines in the amniotic fluid that can induce parturition-associated changes in uterine tissues.43 This is due to their ability to increase pro-parturition-associated signaling in both fetomaternal compartments and increase oxidative stress (OS) and reactive oxygen species in the fetal membranes and placenta.44–46 These inflammatory molecules in amniotic fluid and reactive oxygen radicals induce fetal membrane and placental tissue damage,47–49 setting off a cascade of inflammation in other uterine compartments.50 More precisely, at the placental/decidual and membrane/decidual interfaces, decidua and resident immune cells function as amplifiers of fetal-derived biochemical, inflammatory, and reactive oxygen species signals, increasing the inflammatory load and creating immune intolerance on the maternal side.5,33,36,45,51 In # 2, maternal biologic mechanisms responding to endocrine and inflammatory changes can initiate inflammatory changes in the decidua and propagate them toward the fetal compartments, increasing fetal inflammation. Finally, in # 3, both fetomaternal signals originate simultaneously and contribute to parturition. The maternal decidua is the key organ that forms the major junction (interface) between both fetal units, that is, the placenta and fetal membranes.52–56 Importantly, regardless of their origin, all signals are propagated toward the decidua, the junction where inflammation amplification primarily involves immune cells and inflammatory mediators.57–63 Decidual activation causes immune cells and other proinflammatory mediators to propagate in both directions, tilting the threshold of immune balance in both fetal and maternal tissues.5,33,64–66
2 |. CURRENT STRATEGIES FOR MITIGATING PRETERM BIRTH AND LESSONS LEARNED
As shown in Figure 1, the pathophysiologic pathways of preterm birth, regardless of their etiologies, involve inflammation and oxidative stress.67,68 These two mechanisms are inseparable and can be initiated by various endogenous and exogenous factors. The signaling mechanism activated by these pathways causes terminal events leading to labor and delivery, specifically the transition of quiescent myometrium to an active state and ripening of the cervix.5,65,69–72 Although these are events physiologically seen to facilitate normal parturition at term, premature activation of overwhelming inflammatory and oxidative stress signaling can contribute to preterm birth or pPROM. Current intervention strategies have primarily focused on delaying preterm delivery by reducing activation of maternal uterine tissues in response to various signals, including inflammation. A steady rate of preterm births around the globe suggests that current strategies are insufficient to reduce the risk of preterm birth.65 Multiple factors can contribute to the clinical dilemma of how to intervene during preterm labor to reduce the risk of preterm birth, but primarily heterogeneities in etiologies, pathways, and biomarkers create ambiguity, and approaches to stop contractions using tocolytics73–76 or anti-inflammatory endocrine supplementation (eg, progesterone) are unlikely to be universally successful, as the pathogenesis and pathophysiologic pathways that promote oxidative stress and inflammation, which in turn lead to the terminal events culminating in labor and delivery, are variable.77 These reasons highlight the need to better understand the fetomaternal uterine homeostatic mechanisms that operate during pregnancy and the initiator and effector signals that can create changes to facilitate the parturition process at term and preterm. Mechanistically, parturition collapses all homeostatic balance in various reproductive tissues that maintain pregnancy. Facilitation of this process by immune-cell-mediated imbalances at the fetomaternal interface is well documented. This review will focus on cellular and extracellular immunobiological mechanisms that can disrupt the fetomaternal interface systems that may eventually lead to parturition at term and preterm.
FIGURE 1.
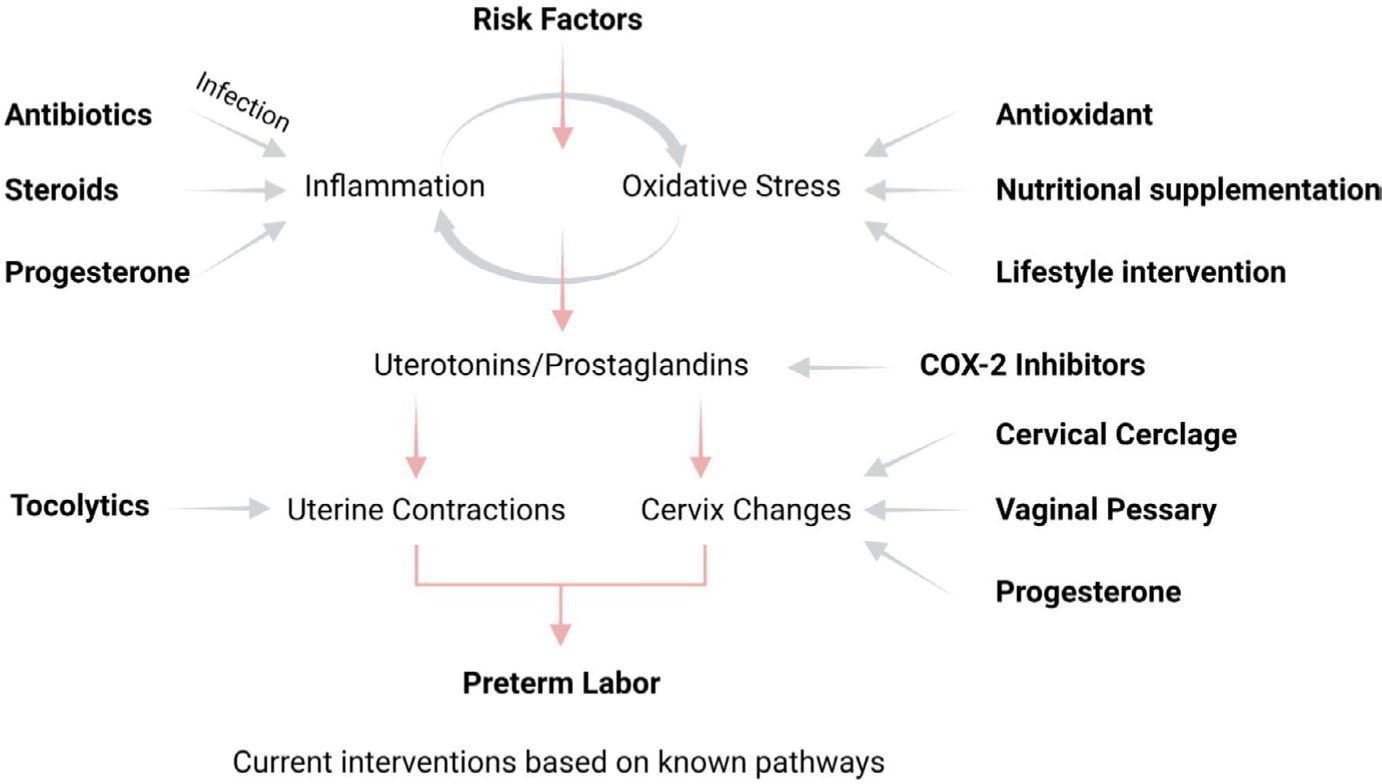
Pathways of preterm birth and current intervention strategies. During pregnancy, oxidative stress and inflammation generated in response to various risk factors lead to preterm labor-inducing pathways. Current interventions are designed primarily to reduce uterine contractions and cervical changes predisposing to preterm labor
3 |. FETOMATERNAL INTERFACES AND IMMUNOLOGIC HOMEOSTASIS
There are two distinct fetomaternal interfaces: (a) between the placenta and decidua basalis and (b) between fetal membranes and the decidua parietalis. Immune tolerance at these interfaces maintains pregnancy, protects the semi-allogenic graft (the fetus), and promotes its growth and development.78 The former one is well studied and reported. A review by Racicot and Mor detailed the various mechanisms that maintain the immunologic balance between the fetomaternal units.79 The placenta is the primary barrier between the fetus and the mother and interfaces with the decidua basalis. The placental cells establish an environment immunologically supportive of pregnancy with immune cells capable of supporting fetoplacental growth and defending the mother and the fetus from microbial and other exogenous challenges during pregnancy.79 Several classic reviews on the immunologic balance between the placenta and the maternal environment can be seen in the following references.56,59,80–89 Various cell types–innate and adaptive immune cells as well as placental and decidual cells–contribute to a mutually tolerogenic environment that allows fetal survival. The pathologic consequences of immune cell activation, including changes in tolerogenic HLA class molecules (HLA-G) and the damage they cause to the interface, which promotes inflammatory activation as seen in preterm birth, have been reported extensively in the literature.50,63,84,90–94 Besides referencing their importance when appropriate, this review will not focus on the cellular or immune response at the placental/decidual interface and its contribution to parturition at term or preterm. On the contrary, the relative contributions of fetal membranes and the decidua parietalis are largely unclear.13 The primary focus of this review is to introduce this interface and how it contributes immunologically to the parturition process that can also constitute the fetal inflammatory response (FIR).
The human fetal membranes (amniochorionic membranes) and decidua parietalis cover the entire surface structure of the uterine cavity. The fetal membranes comprise a single layer of amnion epithelial cells (AEC) and an extracellular matrix region rich in various collagen types and sprinkled with amnion mesenchymal cells (AMC), chorion mesenchymal cells (CMC), and chorionic trophoblast cells (CTC). Amnion epithelial cells are in direct contact with the amniotic fluid and the fetus, whereas chorionic trophoblast cells line up with the maternal decidua.95,96 One of the reasons for not considering the fetal membrane decidual interface as a serious contributor to immunologic tolerance associated with pregnancy is that fetal membranes are avascular compared to the placenta, the site of interface between the fetal and maternal blood. Unlike the placenta, fetal membranes do not have resident immune cells.97 However, this does not undermine the immunologic properties of fetal membranes and their contribution to immune tolerance at the fetomaternal interface. This cellular-level tolerance is unique, and the rest of the review will discuss how the fetal membrane generates an FIR, a major determinant of pregnancy outcome by causing immune intolerance due to cellular derangements.
4 |. THE FETAL INFLAMMATORY RESPONSE (FIR)
Preterm birth is a syndrome involving a multitude of overlapping factors and various underlying pathophysiologic conditions. Although tremendous heterogeneity exists in the definition of the FIR, inflammation of the intrauterine cavity can determine mortality and morbidity in premature babies.98–107 A majority of the studies referenced here describe increased inflammatory cytokines and chemokines,100,102,108–110 immune cell migration into fetal tissues (fetal membranes [histologic chorioamnionitis] and the umbilical cord [funisitis]),43,111–118 increased reactive oxygen species in the amniotic fluid46,116,117,119 and fetal tissues,45,46,120,121 changes to antimicrobial defense mechanisms,122–125 and HLA molecules.92,126–12 The FIR can respond to an infectious104,130 or non-infectious (sterile) process,131–133 either an endogenous or an exogenous exposure by the fetal tissue.134 Measurement of biochemical factors in biological fluids does not always indicate their origin; however, it is evident that the fetus and fetal tissues are immunocompetent to respond to changes in the uterine environment. Sterile inflammation and its mediators are essential in both term and preterm birth.104,120,133,135–137 Studies aiming to understand the generation of sterile inflammation have shed light on the mechanisms of involvement of fetal tissues in normal term parturition, specifically fetal membranes, in the process of initiation of normal term labor and delivery.66,138,139 The FIR is not always an innate and adaptive immune-cell- and or interleukin-mediated inflammation but includes intracellular and extracellular biological processes, products of these various processes and changes produced locally and distantly.130,139,140 This review will examine this from the fetal membrane's perspective.
5 |. THE FETUS OR THE MOTHER
Parturition results when the maternal uterine tissues transition to an active state of labor. This is an inflammatory process, and both fetal-and maternal-derived inflammatory mediators contribute to it. This inflammatory overload can cause endocrine and immune-mediated functional changes that maintain pregnancy in each uterine tissue. For example, functional progesterone withdrawal in the myometrium and similar changes in the cervix have been reported.141–148 Similarly, decidual activation is also an inflammatory process where both stromal cells and resident immune cells provide immunohomeostasis that maintains pregnancy.30,33,149 Decidual activation involves immune cell migration into other fetomaternal uterine tissues,33,63,81,145,150 whereas the inflammatory process involves endocrine mediators, immune cells, and paracrine factors such as extracellular vesicles151 and other secreted factors (cytokines, chemokines, and growth factors). Multiple theories exist on the timing of inflammatory signals arriving in maternal uterine tissues and the beginning of parturition; however, the dogma is that maternal activation of the delivery process is a response to various fetomaternal signals.5,6,33,152 Maternal uterine activation is the terminal and essential event in ensuring the delivery of the fetus. Hence, the theory that controlling the premature activation of inflammatory overload that overrides maternal uterine tissue homeostasis can reduce the incidence of preterm birth. However, failure to reduce the preterm birth rate suggests that mere intervention of terminal events like myometrial contractions and cervical ripening is insufficient to stop the various fetomaternal signals that initiate the terminal process (Figure 1).
The FIR, as mentioned above, is often discussed in association with preterm birth, as it is a major cause of fetal morbidity and mortality.103,153–155 However, the current dogma that maternal signals are the sole initiators of human term parturition needs to be reconsidered, and the FIR should be discussed along with maternal inflammatory initiators. The FIR is not always a response to infectious or etiologic stimuli but may be a natural and physiologic response from the fetus and fetal tissues that maintain and protect pregnancy. The dogma of the FIR as a signaling mechanism that can communicate with the mother to initiate parturition at term and preterm is further discussed. What is the FIR? How is the FIR generated as a signal to compromise fetomaternal interface integrity? This review is necessary for the following reasons:
The FIR as a mechanistic proparturition signal at term and preterm has not been investigated. However, its usefulness as a biomarker for various underlying pathologies in preterm birth has been discussed extensively.
The FIR as a paracrine mediator and its mechanistic role in compromising the chorio-decidual interface is not fully elucidated.
Non-immune cell and extracellular mediators such as extracellular vesicles that contribute to the FIR at the chorio-decidual interface are not discussed in the literature.
The remainder of this review will explain FIR generation by fetal membranes based on cellular- and extracellular-level changes and the capability of a fetal-membrane-derived FIR to compromise chorio-decidual interface immune homeostasis.
6 |. FETAL MEMBRANES AS A MODEL TISSUE FOR STUDIES OF THE FIR
Current and ongoing studies have indicated that fetal membrane dysfunction is a major contributing factor to the FIR and initiation of parturition.47 Fetal membranes are the innermost lining of the intrauterine cavity and provide structural stability as well as mechanical, antimicrobial, immune, and endocrine functions to maintain pregnancy and protect the fetus.11,95,156 The amnion epithelial layer and the chorion trophoblast layer of the fetal membranes exist as independent tissues until the early 2nd trimester of pregnancy, when they fuse to form the amniochorionic membrane. The combined life of the amniochorion begins at this stage, and as emphasized by John Moore et al, it is not a mere appendage or an extension of the placenta.13 The amniochorion is distinct from the placenta and performs unique functions. Like the fetus and the placenta, fetal membranes undergo massive cellular replication and growth to accommodate the growing fetus and increase in amniotic fluid volume. Cellular replication during gestation leads to senescence of the fetal membrane cells. Cellular senescence is indicated by progressive attrition of telomeres, the cap structures on chromosomes, considered as a biologic marker of aging.157,158 Telomere reduction is seen as a progressive process in human fetal membranes that continues until the term where the shortest telomers are recorded.159 Bonney et al reported similar progressive telomere reduction in the fetal membranes and placenta of murine pregnancies.160 The life expectancy of fetal membranes is correlated with gestational length, and therefore, telomere-associated aging is a physiologic mechanism.47 Telomere reduction generates cell-free fetal DNA (cffDNA) fragments that are damage-associated molecular pattern markers (DAMPs).161 cffDNA triggers an innate response from the cells that can determine cell fate.161 However, DNA fragmentation is a normal process in replicating cells and is repaired by multitudes of DNA repair enzymes, such as 8-oxoguanine glycosylase (OGG1).162 Our studies have indicated that fragmented DNA, including telomere fragments, and reduced expression of OGG1 cause increased activation of the stress signaler p38 mitogen-activated kinase (MAPK) in human fetal membrane cells as pregnancy progresses to term.163 p38MPAK is a constitutively expressed signaler in fetal membrane cells, facilitating cellular replication through cyclin-dependent kinase (CDKs); however, p38MAPK overactivation in response to either endogenous (eg, cffDNA) or exogenous (risk factors like cigarette smoke or infection)134 stressors can cause cellular senescence that stops cell replication and minimizes normal functions.164,165 At term, p38MAPK overexpression is also induced by intrauterine oxidative stress (OS) that can accelerate DNA damage and cffDNA release and reduce the cell's capacity to repair damaged DNA.161 These changes trigger a vicious cycle of events that can further damage the cells, and secreted products from these cells damage neighboring healthy cells.
7 |. FETAL MEMBRANE CELLULAR SENESCENCE GENERATES FIR
Senescent cells are not cleared from the tissue environment like apoptotic or autophagic cells, and their presence is associated with localized inflammation. Senescence-associated inflammation (senescence-associated secretory phenotype–SASP) is characterized by multiple proinflammatory cytokines, chemokines, growth factors, matrix-degrading enzymes, cytokine/chemokine receptors, and various other markers.166,167 Besides, cffDNA, DMAPs such as HMGB1,120 IL-33,168,169 heat shock proteins,170 and uric acid171 are also released from damaged cells due to senescence at term, creating a unique inflammatory environment. DAMPs are generated in response to a unique cellular response (eg, senescence and necrosis) to a specific event, unlike the generation of non-specific responders such as CRP or IL-6.172 DAMPs are potent mediators of inflammation, and they can cause more damage to cells and their surroundings.
8 |. THE EPITHELIAL-MESENCHYMAL TRANSITION (EMT) OF CELLS GENERATES AN FIR
Overactivation of p38MAPK is not restricted to cell senescence. A subset of amnion epithelial cells that do not undergo senescence can still transition to mesenchymal cells via p38MAPK-mediated signaling and activation of a set of transcription factors (eg, snail1 and 2, zeb, Twist, and smad).173 Kalluri and Weinberg, in their review, described epithelial-mesenchymal transition (EMT) as a biologic process that allows epithelial cells embedded in the basement membrane to undergo multiple biochemical changes, enabling them to assume a mesenchymal cell phenotype. EMT enables enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, greatly increased production of ECM components, and localized inflammation174 (Figure 2). The factors that determine cell fate, that is, whether they undergo senescence or EMT, is unclear. p38MAPK causes EMT of amnion epithelial cells in response to stressors. The generation of mesenchymal cells aids their migration and invasion of the extracellular matrix region while inducing localized inflammation. MMP9 is one of the inflammatory mediators generated during EMT capable of degrading the Type IV basement region that connects amnion epithelial cells to the fetal membrane extracellular matrix scaffold.173 An increased number of mesenchymal cells that are more vulnerable to oxidative stress and inflammation than epithelial cells in the fetal membrane matrix along with localized inflammation weakens the membranes. Mesenchymal cells are recycled back to amnion epithelial cells (mesenchymal-epithelial transition [MET]) during normal pregnancy by progesterone and a membrane progesterone receptor-mediated mechanism173,175 (Figure 2). This cellular recycling is inhibited by increased OS (at term), which causes enhanced p38MAPK activity. Although the roles of DAMPs and SASPs are not clear with EMT-associated inflammation, localized cellular derangements and matrix-degrading inflammatory activity can structurally and mechanically weaken the membrane, rendering it dysfunctional.
FIGURE 2.
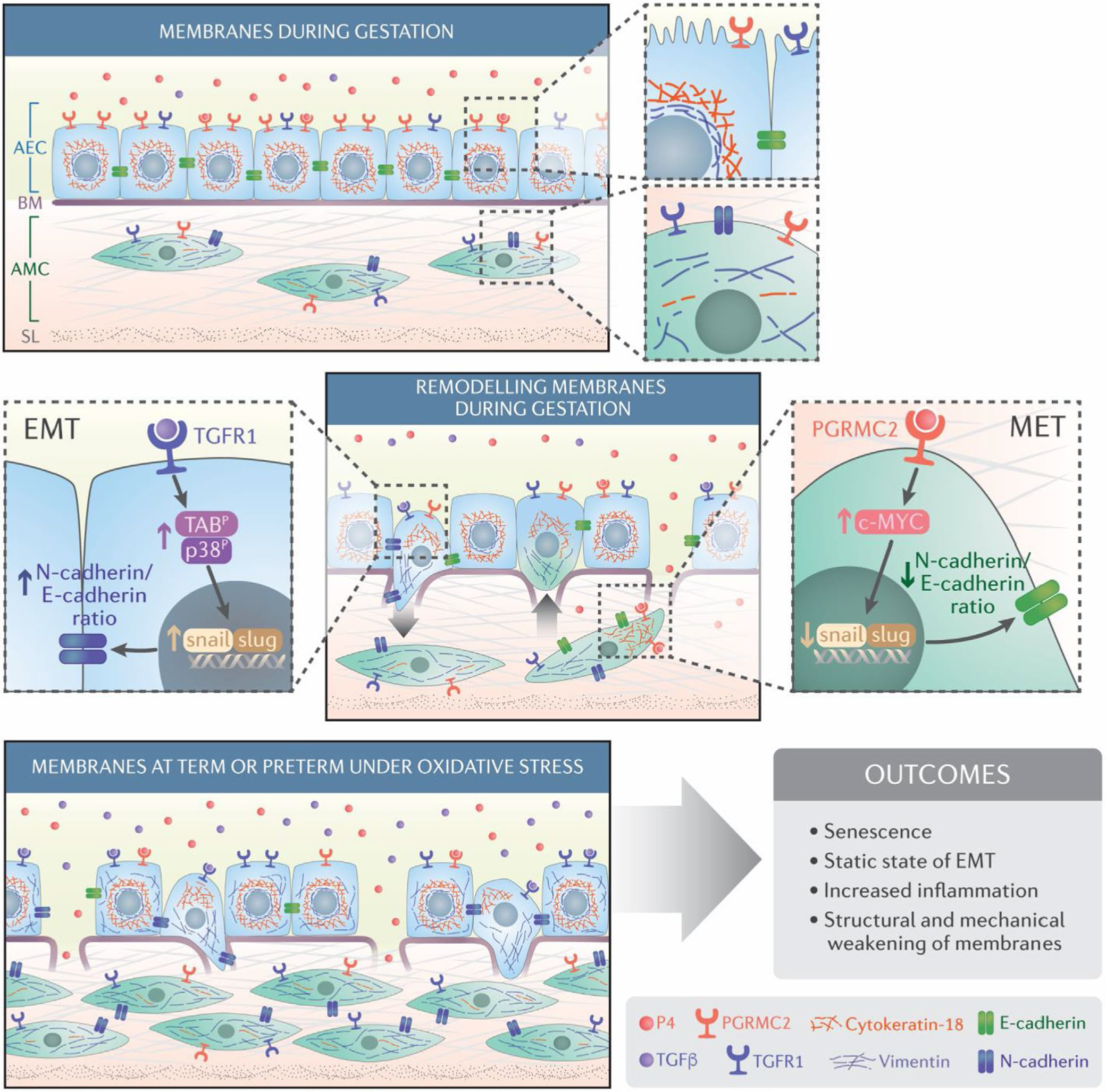
Schematic representation of maintenance of the fetal membrane integrity and its disruption due to cellular transitions: The Amnion membrane is comprised of amnion epithelial cells (AEC) and amnion mesenchymal cells (AMC) separated by a Type 4 basement membrane collagen (BM) and extracellular matrix (ECM). During gestation, progesterone (P4) helps to maintain AEC epithelial state. AMCs, located in the extracellular matrix, express a fibroblastoid morphology. The ratio between AEC and AMC is 10:1 in normal gestation. EMT is mediated by TGF-β/TGF-β receptor-mediated mechanisms regulated by p38MAPK (middle panel; left). P4 and progesterone membrane receptor component (PGRMC2) mediated MET recycles AMC back to AEC to maintain tissue homeostasis and prevent accumulation of AMCs in the membrane extracellular matrix p38MAPK (middle panel; right). Oxidative stress causes increased activation of p38MAPK at term or preterm leading to increased EMT and a reduction in the function of progesterone as oxidative stress down-regulates PGRMC2 in AMCs. As the recycling of cells is stalled, AMC accumulates in the ECM and causes inflammatory changes (bottom panel). This figure was originally publsihed in Sci Signaling Richardson et al.173 reproduced here with permission
9 |. PHYSIOLOGIC AND PATHOLOGIC FIR AT TERM AND PRETERM PREGNANCIES
The FIR is always discussed in the context of infection and preterm birth,98,102,176 placental pathology,154 fetal brain injury,177 and higher neonatal morbidity.178 The discussion on this topic will resume after discussion of the role of FIR in normal pregnancy and parturition. Senescence and EMT-associated FIR from fetal membranes are naturally occurring and normal physiologic responses at term to promote labor. The intrauterine oxygen environment changes during pregnancy,179,180 which is a major determinant of a balanced FIR in the membranes and the placenta during gestation. A localized FIR helps to remodel fetal membranes and the placenta and assists the developing fetus during gestation. It is unlikely that this localized FIR is propagated as fetal signals during gestation. Even if these signals reach the maternal tissues, an FIR derived from normal physiologic changes is unlikely to be strong enough to unbalance maternal uterine tissue homeostasis. Kang Sun's group has identified a unique inflammatory response mediated by endocrine mediators in the fetal membrane cells, specifically ECM fibroblasts. Studies from this group have shown that CCAAT/enhancer-binding protein δ (C/EBPδ), a transcription factor involved in growth arrest and differentiation, provides a feedforward induction of COX-2 and 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) expression to endogenous PGE2 and cortisol that can increase membrane inflammation, and subsequent propagation of PGE2 can cause labor.181 Multiple reports by Kang Sun's group have shown that the cyclic process of cortisol generation by 11β-HSD1 in membrane fibroblasts is also an endocrine-mediated FIR-generating event. Data suggest that ECM denaturation through excessive MMP activity can be enabled by 11β-HSD1 in the human fetus,182 thus weakening the membranes.183,184
Besides the FIR mechanisms initiated by fetal membrane cells, immune cells at the fetomaternal interface are well reported as facilitators of initiation of pregnancy and its maintenance. Mor et al reported that a balanced FIR during normal gestation helps remodel tissues and aid fetal growth.185,186 Immunomodulation in early pregnancy, maintained by a balanced ratio between IL-10 (anti-inflammatory) and TNF-α (proinflammatory), reported in maternal blood samples supports this argument.186 We postulate that cellular-level changes and the generation of inflammatory mediators by fetal tissues are part of the FIR, and thus, the FIR is not restricted to immune-cell-mediated activities. Cellular-level changes eliciting chemotactic responses can bring immune cells to membranes187 and the placenta.90 This influx of immune cells is a normal response required to amplify the uterine tissues' inflammatory load to promote parturition. An immune cell-associated FIR is also reported in the amniotic fluid of term pregnancies and is equally represented by fetal and maternal immune cells.113,188 A review of the literature by Miller et al from Gomez-Lopez's group has summarized that maternal (uterine and decidual) lymphoid cells are involved in the initiation and maintenance of pregnancy.189 In summary, the FIR is a response of fetal cell-fate-related changes to in utero conditions at different gestational periods and not necessarily initiated by immune cells. However, coordinated activities between the fetal cells and fetomaternal immune cells constitute the complete FIR response and one of the fetal signals to induce parturition.
A pathologic FIR is the most reported concept during pregnancy. As seen in term labor and delivery, a physiological FIR from fetal membranes can be induced pathologically in response to various risk factors often associated with adverse pregnancy outcomes. Untimely oxidative stress and inflammation in response to risk factors cause telomere reduction, premature activation of fetal membrane senescence and EMT, 11β-HSD1 activation, and chemotactic responses causing infiltration of immune cells (histologic chorioamnionitis).
10 |. PROPAGATION OF FIR
SASP and DAMP responses originating from fetal-derived tissues should be considered as an FIR. The FIR can propagate to various fetomaternal tissues to transition them to a labor phenotype. Propagation of these markers is primarily hematogenous115,190,191; however, their half-life may pose a challenge in reaching specific maternal destinations, even if they are constantly secreted from the cells.192 Cells also use extracellular vesicles, specifically exosomes 30–160 nm in diameter in size, to transport DAMPS and other inflammatory mediators in a paracrine way to overcome the limitations to prevent their degradation193 (Figure 3). Most importantly, pathological changes in cells cause the generation of DAMPs, SASP, and other inflammatory mediator-enriched exosomes. These exosomes are fetomaternal paracrine signalers and induce uterine activation and preterm labor.70 Besides exosomes, fetal cells (immune and non-immune cells) also reach the maternal uterine and other tissues (Figure 4). Both fetal cells and fetal tissue-derived exosomes constitute the FIR. Experimental evidence generated from our laboratory to demonstrate that the FIR can initiate preterm birth is shown below.
FIGURE 3.
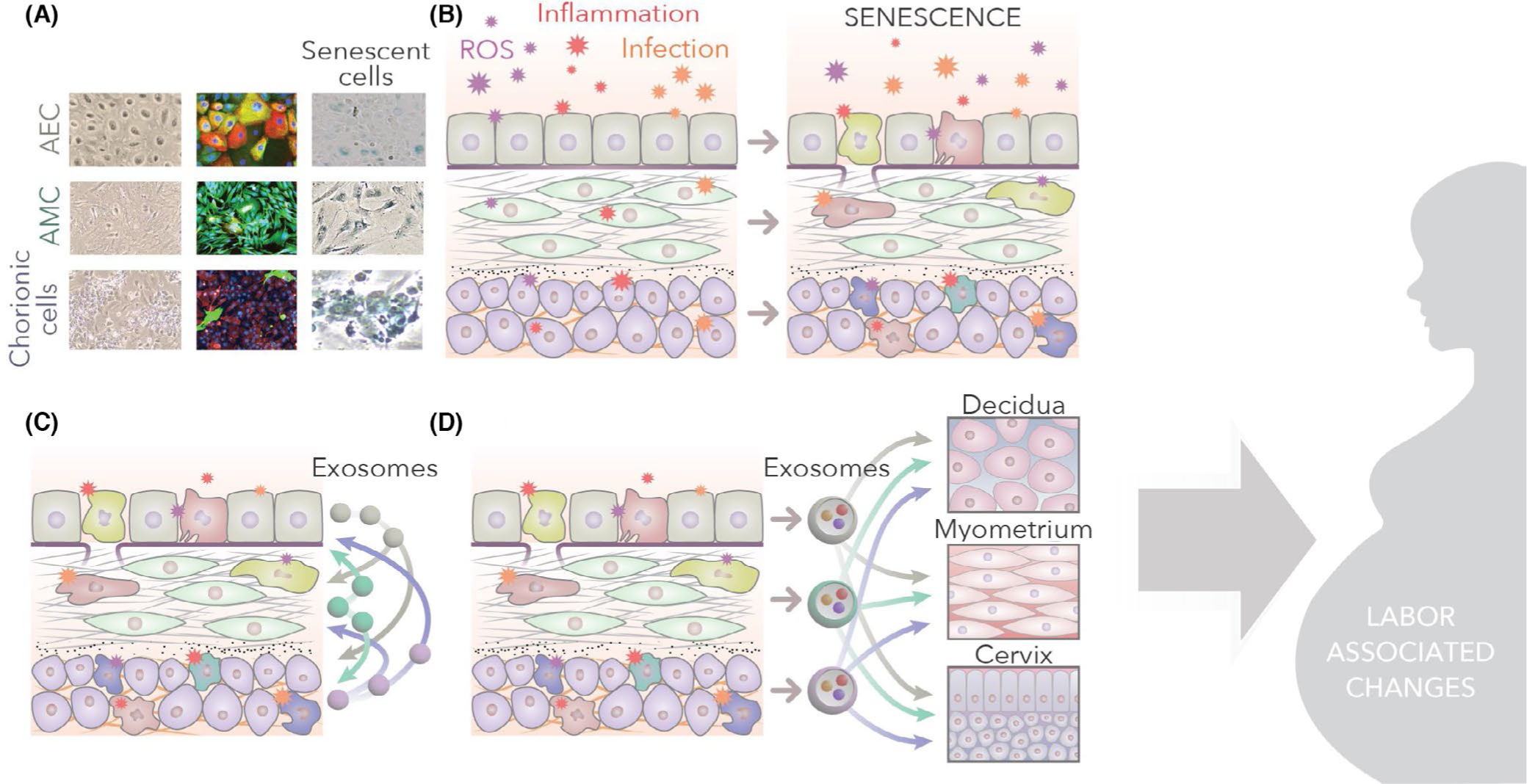
Senescence and paracrine signaling: A. Amnion epithelial (AEC), amnion mesenchymal (AMC), and chorion cells (CTC) (normal morphology under light microscopy), immunofluorescent staining of cytokeratin (CK)-18 (red), vimentin (green) staining show a meta-state of AEC, vimentin-positive AMC, and predominantly CK-18 positive (red) CTCs. Sensecence in AEC, AMC and CTC in response to oxidative stress is shown (blue staining cells) B. Infection, inflammation and other mechanisms of reactive oxygen species (ROS) increase can cause senescence of membrane cells. C. Senescence cause the release of senescence associated secretory phenotype (SASPs) and damage associated molecular pattern (DAMPs). SASPs and DAMPs are packaged inside the exosomes, which can cause paracrine signaling within the membrane cells. D. Senescence and inflammation increase within the fetal membrane cells and environment, leading to the propagation of exosomes to maternal decidua, myometrium, and cervix
FIGURE 4.

Tracking fetal-derived exosomes and cells during pregnancy. A. Generation of transgenic mouse model containing TDTomato construct. A cre-reporter transgenic mouse model with membrane-targeted tdTomato (mT) A: A 2-color fluorescent Cre-reporter allele (mT/ mG construct) which has a membrane-targeted tandem dimer Tomato (mT) red fluorescent protein expressed in all cells and tissues, while enhanced green fluorescent protein (mG) is not expressed. Mating strategy to producing mT+expressing progeny. B. Immunohistology shows fetal exosomes (mT+, red staining), and flow cytometry data show 20% of maternal blood during gestation contains fetal (mT+) exosomes. C. Left panel: mT+cells are localized in various maternal organs. The placenta (fetal in origin) is expected to contain mT+in all the cells, as shown in the figure. mT+cells are seen in the uterus and even in maternal lungs. Right panel: Fetal Macrophage (φ) can be detected in maternal tissues. Confocal microscopy was used to colocalize mT expressing cells (red membrane fluorescence) with Mφ marker F4/80 (green membrane fluorescence) in the maternal cervix and uterus. 3D models were generated to confirm the expression of mT signal throughout the membrane of the cell. Scale bars represent 10 μm. See white arrows indicating mT signals surrounding cell membranes (n = 4) (these figures are reproduced with permission from Science family of journals/AAAS)
The above paragraphs highlight the mechanistic pathways in a cell that can generate inflammation (FIR). These are independent of immune cells and are extensively studied. Figures 2 and 3 demonstrate schematically how the FIR can be generated by transitioning cells or senescent cells (Figures 2 and 3). Both senescence and EMT generate various markers of inflammation that can be packaged inside exosomes. As shown in Figure 3C–D, exosomes from the fetal membranes can cause intracellular and intercellular signaling as well as function as paracrine mediators. This paracrine mediation by exosomes should be considered as one of the fetal signals for parturition.
11 |. THE FIR AS A CAUSE OR CONSEQUENCE OF INFECTION AND PRETERM BIRTH: A MODEL TO TEST THE FIR
As most biological sample analyses are performed after clinical symptoms appear in pregnant women, the question remains whether the FIR is a cause or a consequence of preterm labor. Some of our recent work was designed to partly answer this question. To this end, we established a mouse model where the fetal tissues express a marker not expressed in maternal tissues194 (Figure 4A). Therefore, fetal cells and exosomes generated from these tissues are expected to carry these markers (Figure 4B), and their trafficking to the maternal side can be determined using various approaches. Our model utilized a transgenic mouse with a membrane-targeted, two-color fluorescent cyclic recombinase (Cre)-reporter allele where tandem dimer Tomato (mT+) fluorescence is expressed in the plasma membrane of all cells and tissues. In the presence of Cre, mT is excised, and cells express a membrane-localized enhanced green fluorescent protein (mG+) that replaces mT.194 When a transgenic male was mated with a wild-type female, the fetal tissues expressed this construct, whereas it was absent in the maternal tissues. Using this approach, we developed and validated a mouse model to determine the trafficking of mT+ fetal cells and exosomes between maternal and fetal tissues194 (Figure 4A).
12 |. EXPERIMENTAL EVIDENCE DEMONSTRATED THE PRESENCE OF MT+ (FETAL DERIVED) EXOSOMES IN MATERNAL PLASMA AND FETAL IMMUNE CELLS IN THE UTERUS AND CERVIX
The maternal plasma of WT mice mated with mT/mG males were collected, and exosomes expressing mT analyzed using flow cytometry showed that ~20% of exosomes were fetal in origin on day 13 of mouse gestation (Figure 4B).194 mT+were also localized in maternal reproductive tissues, specifically the cervix and uterus (Figure 4B right panel). Exosomes expressing mT+ were localized in the maternal uterus and cervix, as indicated by the expression of both mT+ and the exosome marker CD63 (Figure 4B). Besides exosomes, we noted that fetal immune cells migrated to maternal tissues and were localized in the maternal uterus and cervix (Figure 4C left panel). In Figure 4C, placental tissue was used as a positive control where all placental cells are expected to show mT+ expression. mT+ fetal cells (red cells) were shown to migrate toward the maternal uterine tissues as well as other tissues, including maternal lungs. Our primary focus was on innate immune cell trafficking, and as shown in the 3D view (Figure 4C. right panel), fetal cells are surrounded by red fluorescence due to the membrane-targeted tdtomato label. Some of these fetal cells (red) express macrophage (φ) marker F4/80 (green), indicating that fetal macrophages can traffic to the maternal uterus and cervix during pregnancy. Trafficking of exosomes and fetal cells does not necessarily imply an FIR but can only indicate that fetal-specific exosomes and cells migrate to the maternal side. Fetal cell trafficking to the maternal tissues (microchimerism) and their long-term presence is not a new phenomenon; however, their functional relevance and the type of cells that cross over to the maternal side and persist there for an extended period of time are still unclear.195,196
To confirm that the FIR increases in response to a maternal infection, an LPS-induced preterm model was used. To determine the propagation of fetal inflammatory cells, studies were conducted where animals were sacrificed after 12 hours, and the F-M tissues were collected and analyzed for (fetal) cells expressing mT+ (Figure 5). In this study (Table 1; data on neutrophils are shown here), we determined that the fetal innate cell response in the fetal and maternal tissue was higher in PTB. After treatment with LPS, an increased number of fetal neutrophils in the fetal and maternal tissues suggested a buildup of FIR prior to PTB. Maternal neutrophils did not change in the fetal (fetal membranes Figure 5A) and maternal tissues (decidua; Figure 5B), whereas an increase in fetal neutrophils contributed to HCA after LPS treatment. The table (Figure 5C) shows the significance level. This is the first report to show a fetal-specific neutrophil response associated with HCA, a condition that contributes to neonatal morbidity. Our data also supported a postulated role for HCA in PTB.111,118,197
FIGURE 5.

Determination of fetal neutrophils in the fetal membrane (FM) and the decidua: mT+neutrophils population was quantitated in tissue homogenates from the fetal membranes and decidua after an LPS challenge of animals (red bars). A. In the fetal membranes, the total neutrophis (maternal+fetal) was increased significantly after an LPS challenge, whereas mT-(maternal) was not changed. Fetal (mT+) was significantly higher in the membranes after the LPS challenge compared to PBS (blue bars). B. In the decidua, LPS challenge decreased neutrophils slightly compared to total (regardless of fetal vs. maternal)
TABLE 1.
Oxidative stress inducedamnion cell derived exosomes cause increased production of uterotonins in maternal uterine cells
| Normal AEC-derived exosomes |
Oxidative stress induced AEC-derived exosomes |
|||||
|---|---|---|---|---|---|---|
| Tissue type | IL-6 | IL-8 | PGE2 | IL-6 | IL-8 | PGE2 |
| Myometrium | ↑ | ↑ | ↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ |
| Decidua | ↑ | ↑ | ↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ |
| BeWo | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
Note: ↑ – < 0.05; ↑↑↑ – < 0.001; ↔ No change.
13 |. EXOSOMAL PROPAGATION OF THE FIR AND ITS FUNCTIONAL ROLE IN INDUCING BIRTH
To determine that the FIR is propagated via exosomes, exosomes derived from fetal membrane cells under ordinary and specific environments have been characterized.198 Unique cargos, including DAMPs such as HMGB1 and cffDNA, were packaged under specific environmental conditions when cells were exposed to oxidative stress or inflammatory conditions.193 Bioinformatics analysis of cargo contents represented enrichment of various inflammatory mechanisms in the cells at the time of exosome release.193,199 Histological analysis showed that fetal amnion-cell-derived exosomes in myometrial tissue sections at term are higher in specimens taken during labor vs. before labor.69 Furthermore, functional studies showed that inflammatory-cargo-enriched exosomes cause inflammatory changes associated with parturition in the cervix and myometrial cells.69 These in vitro data were further recapitulated using animal models of pregnancy. As reported above and shown in Figure 4, fetal exosomes reach the maternal circulation during pregnancy and can be isolated and characterized.200 When characterized, exosomes isolated from maternal plasma showed a gestational-age-dependent change in their cargo content in murine pregnancies. Pro- and anti-inflammatory mediators are balanced until day 15 (gestational period 19.5 days) in normal murine pregnancy. Proinflammatory cargo is increased on day 18, and the contents and the pathway represented by this cargo were different than on days 5 and 9, when there was a more anti-inflammatory type. A proinflammatory shift was observed on day 15 and peaked on day 18 of pregnancy. This observation of exosome cargo led us to test the hypothesis that inflammatory responses encoded in the exosomes may function as parturition-inducing signals. To test this hypothesis, day 18 exosomes enriched in inflammatory cargo were isolated and injected into animals on day 15 of their pregnancy, which led to preterm birth. In contrast, injection of exosomes from day nine did not cause preterm birth.200 Preterm birth after the injection of day 18 exosomes was associated with inflammation in both fetomaternal compartments.200 This suggests that exosomes can function as signalers of parturition and trigger an inflammatory process.
Although similar functional studies are not possible in humans, a similar trend in fetal exosomes was observed.201,202 Maternal blood samples from a longitudinal pregnancy cohort were used to isolate placental-specific exosomes (placental alkaline phosphatase as a marker) at different gestational periods and at term. miRNA and protein cargo analyses revealed that fetal exosomes exhibit unique cargo profiles in each trimester.201,202 Third-trimester and term fetal exosomes were enriched in biomarkers indicative of an inflammatory state that supported animal model data and indicated that the FIR was propagated via exosomes. In vitro studies have shown that inflammatory exosomes from fetal membrane cells undergoing senescence or EMT-induced functional changes in uterine tissues increase inflammation. As shown in Table 1, inflammatory cytokines and prostaglandin E2 were increased in myometrial decidual cells, but no effect was seen in placental cells. These data suggest that fetal exosomes can cause cell-type-specific responses.
We also noted that immune cells react to inflammatory-cargo-enriched exosomes from senescent cells. When decidual cells, along with CD45+ cells, were exposed to senescent fetal membrane cell-derived exosomes, an increase in CD45+CD66b+ cells (from 35.57% to 51.78%) was observed.
14 |. EXOSOMAL FIR CAUSES IMMUNE IMBALANCE AT THE FETOMATERNAL INTERFACE (CHORIO-DECIDUAL INTERFACE)
Immune homeostatic mechanisms at the placental/decidual interface are well studied, but the least reported is that of choriodecidual interface (Figure 6A) homeostasis. Several reports have shown the pathologic impact of the choriodecidual interface regarding its contribution to adverse pregnancy events. Gomez-Lopez et al demonstrated two T-cell subsets (effector and activated T cells), and memory-like T cells in the choriodecidua of women with spontaneous labor, and chemokines, cytokines, and matrix-degrading enzymes.203 Transcriptome analysis by Arenas-Hernandez et al compared maternal circulating and choriodecidual leukocytes, revealing distinct differences. Biological pathways indicate that leukocytes are likely to be involved in the parturition process at the choriodecidua.204 Neutrophil extracellular trap (NET)osis-forming neutrophil recruitment has been reported at the choriodecidual interface in animal models of ascending GBS infection.205 The role of immune cells in pregnancy maintenance is supported by reports showing that choriodecidual B produces progesterone-induced blocking factor 1 (PIBF1), an immunoregulatory pregnancy maintenance protein.206 B-cell deficiency and subsequent PIBF1 deficiency are associated with preterm birth in animal models, which is related to choriodecidual inflammatory imbalnce.206 Adrian Erlebacher's review on the role of decidual immune cells in embryogenesis, placentation, and placental pathologies highlights the tolerogenic activities that occur at the fetomaternal interface but primarily at the placental/decidual interface.90 Although trophoblast cells of the placenta and chorion trophoblast cells of the fetal membranes may have the same lineage and share specific characteristics features and cell markers, they are topographically and functionally distinct cell types. The decidual lining of the chorion and its resident immune cells can have unique functions at this interface, and this area of research is still largely untapped. Besides immune cells and inflammatory markers, endocrine factors such as Relaxin produced by choriodecidual cells are increased in pPROM and preterm birth. Relaxin induces chorionic trophoblast and decidual cell production of uterotonic inflammatory cytokines.207 Similarly, in vitro models have shown that prolactin causes increased chemotaxis at the choriodecidual junction.208 Elevation of proinflammatory gene expression has also been reported in the choriodecidual region in spontaneous labor; however, increases in anti-inflammatory IL-10 production in response to various infectious agents have also been reported at the choriodecidual junction.209,210 The role of IL-10 as an immunomodulatory cytokine at the choriodecidual interface has also been reported by others.211 These data suggest a well-balanced immunomodulatory function at this interface to maintain pregnancy and a proinflammatory switch, likely in response to an FIR or a combination of maternal and fetal inflammatory responses causing immune imbalance and disturbance to cellular homeostasis.
FIGURE 6.
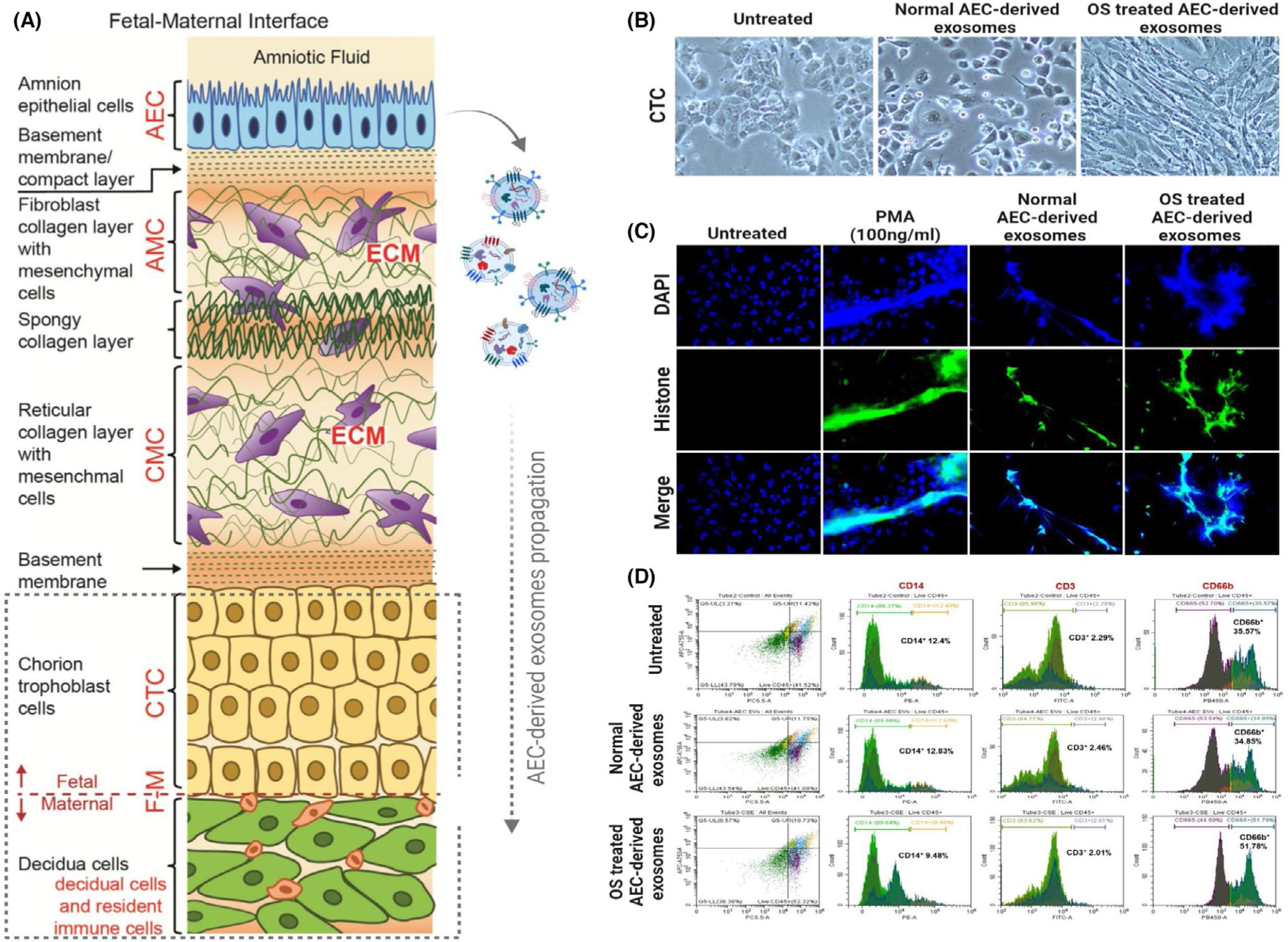
A. Representation of fetal membranes. The chorio-decidual region is shown in the box with broken lines. B. Chorion trophoblast cells (CTC) in culture for 48 h (left), treated with exosomes from amnion epithelial cells (AEC) grown under normal cell culture conditions (middle), or treated with cigarette smoke extract (CSE) treated oxidatively stressed (OS) AEC-derived exosomes. Exosomes from OS stressed AECs transitioned CTCs to CMCs. C. NETosis induction by exosomes: Representative images from a study using neutrophils from healthy pregnant subjects. Control cells do not show NETosis. Phorbol 12-myristate 13-acetate (PMA) and CSE-treated AEC-derived exosomes induced a higher number of NETosis compared to control AEC-derived exosomes. D. Flow cytometry. Decidual CD45+ cells were examined after treatment with senescent AEC exosomes. CD14, CD3, and CD66b antibodies were tested for NK cells, neutrophils, and monocytes. Compared to normal AEC-derived exosomes, CSE-treated AEC exosomes increased CD66b+ cells, whereas no change in CD14+ and CD3+ cells were seen. Figure 6A is a drawing by Dr. Lauren Richardson (Assistant Professor, The University of Texas Medical Branch at Galveston)
Despite the current knowledge on immune cells and inflammatory mediators, there are multiple reasons for the lack of knowledge on the impact of the FIR on the choriodecidual interface.
Studies conducted after a term or preterm delivery of the placenta are generally confounded by various labor- and delivery-associated factors or pathological factors of preterm labor.
Chorion trophoblast cell isolation has been problematic, and several studies have used placental choriocarcinoma cell lines to understand chorion function or preparations containing chorionic extracellular matrix or decidual stromal cells.
Choriodecidual interphases and immune cell populations in animal models differ from those in humans.
Current experimental models do not allow testing of intercellular interactions, a key component of cellular homeostasis.
15 |. FIR-INDUCED CHANGES IN CHORIN TROPHOBLAST CELLS (CHORION) THAT CAN IMPACT FETOMATERNAL IMMUNE TOLERANCE AT THE CHORIODECIDUAL INTERFACE
Recent success in isolating chorion trophoblast cells and their characterization enabled several studies212 that helped us to understand the type of compromise experienced at the choriodecidua interface in response to FIR mediated by senescent fetal-cell-derived exosomes. We have conducted various experiments to test for changes in chorion properties in response to a paracrine FIR. In our model, the FIR was represented by exosomes derived from senescent amnion epithelial cells, and their impact on the choriodecidual interface, specifically on chorion trophoblast cells, was tested. Chorion trophoblast cells were used, as they function as the barrier between the maternal decidua and fetal membranes. We posit that compromise to this barrier is an essential mechanism in the further destabilization of this interface. We report the following changes in chorion cells or markers released from chorion cells in response to exposure to senescent amnion cell exosomes compared to exosomes derived from normal amnion cells:
Senescent-cell-derived exosomes induced EMT in chorion trophoblast cells: As mentioned above, mesenchymal cells at the choriodecidual junction represent an unstable state due to their higher vulnerability to inflammatory and oxidative stress mediators (Figure 6B).
Senescent-cell-derived exosomes induce NETosis: As shown in Figure 6B, neutrophils are one of the innate immune cell populations that increase after a challenge. Therefore, we examined any changes to these neutrophils reaching the choriodecidual interface when they are exposed to inflammatory-cargo-enriched exosomes derived from amnion cells. In an in vitro model study, the generation of NETosis was examined (Figure 6C) using neutrophils isolated from blood samples. Both oxidative-stress-stimulated amnion-derived exosomes that carry inflammatory cargo and phorbol 12-myristate 13-acetate (PMA; a known inducer of NETosis) induced NETosis, whereas this was not evident in control neutrophils treated with amnion cell exosomes. These data suggest that an exosomal FIR can cause neutrophil activation and immune amplification.
Senescent-cell-derived exosomes activate decidual CD45+ cells: A flow cytometric analysis was performed using decidual cells, along with CD45+ cells isolated from normal term fetal membranes. About 30% of decidual cells were CD45+, and exosomes derived from senescent amnion epithelial cells increased the proportion of CD45+CD66b+ cells from 35.57% to 51.78% (Figure 6D).
Senescent-cell-derived exosomes cause changes in other secretory molecules: The expression of multitudes of other secretory factors and cellular HLA-G was also examined, and a summary of significant changes are displayed in Table 2. As mentioned above, the immunomodulatory cytokine IL-10 plays a major role at the choriodecidual interface, where the chorion is a major producer of IL-10. The FIR, indicated by exosomes in our in vitro model, downregulated IL-10. Fetal membrane cells and decidua respond to various endogenous and exogenous challenges by producing inflammatory cytokines. However, this cytokine signature is not the same for all cell types. Chorion trophoblast cells react to an insult by producing high levels of TNFα. When exposed to senescent cell-derived exosomes, chorion cells increased TNFα production compared to control AEC-derived exosomes. Chorion cells are a major source of the propregnancy and anti-inflammatory hormone progesterone (P4) in the fetal membrane/decidual region. Senescent cell-derived exosomes caused a decrease in P4 production compared to normal amnion cell exosomes. A schematic of exosome-induced changes at the choriodecidual interface is shown in Figure 7.
Chorion HLAG expression is not altered by senescent-cell-derived exosomes: Chorion cells are a significant producer of HLAG in fetal membranes, and they provide a barrier function between decidual immune cells and chorion cells. We did not observe any changes in HLAG expression in chorion cells; however, the soluble form of HLAG was reduced in response to inflammatory exosomes. Although the descriptive nature of these studies does not provide any mechanistic knowledge of how changes in chorion cells impact the choriodecidual junction, it generates a hypothesis that factors that can contribute to immune tolerance at the fetomaternal interface may be compromised by an incoming FIR. Oxidative stress and inflammation can create a vicious cycle of events to destabilize the structural, immune, mechanical, and endocrine functions of membranes. Cellular changes that reduce immune resistance and inflammation may allow decidual immune cell migration and activation, leading to inflammation enhancement.
TABLE 2.
Oxidative stress inducedamnion cell derived exosomes cause proinflammatory changes at the choriodecidual interface
| Normal AEC-derived exosomes | Oxidative stress induced AEC-derived exosomes | |
|---|---|---|
| IL-10 | ↔ | ↓ |
| TNF-α | ↔ | ↑↑↑ |
| Progesterone | ↔ | ↓↓ |
| HLA-G | ↔ | ↔ |
| sHLA-G | ↔ | ↓ |
FIGURE 7.

Propagation of exosomes and expected changes at the choriodecidual interface. Chorion trophoblast cells are rich sources of progesterone and IL-10 with anti-inflammatory properties and help to maintain choriodecidual homeostasis. Fetal cell exosomes generated during pregnancy can carry fetal cellular signals (shown in green circles) (top panel); however, they are natural and physiologic responses and insufficient to change the choriodecidual interface's homeostasis. In response to intrauterine infection or inflammation, oxidative stress generated can accelerate membrane cell EMT and senescence. These pathologic events can generate inflammatory cargo enriched exosomes (shown in red circles) (bottom panel) that can increase TNF-α production decrease in P4, and IL-10 release. Immune cell infiltration in response to this event can bring neutrophils to the fetal membranes that can cause NETosis. These inflammatory changes can compromise the integrity of the choriodecidual interface
Several elegant animal model studies by Kallapur's group have provided substantial evidence to support the hypothesis of the FIR as a key mediator of infection-associated preterm birth. Intrauterine administration of lipopolysaccharide (LPS) or live Escherichia coli (E coli) induced PTB, but the outcome was determined by the intensity of the FIR at the choriodecidual interface.213,214
16 |. MATERNAL UTERINE TISSUEDERIVED INFLAMMATORY SIGNALING AND THE FIR
Exosomal cargo delivery of inflammatory mediators can occur in both directions. Decidual, cervical, and myometrial cell exosomes exposed to various inflammatory and oxidative stress environments generated exosomes with distinct cargo, all of which indicated enrichment of inflammation. Treatment of these exosomes on fetal cell membranes caused inflammation; however, some of the cells (CTC) also increased IL-10. These studies are interesting, as maternal inflammation can also be propagated via exosomes, and unlike in the FIR, fetal cells tend to balance the response.151 Of note, these responses are dependent on the dose of exosomes and their cargo contents. During pregnancy, it is obvious that a normal uterine environment constantly propagates a maternal inflammatory response that can reach the fetus and prime tissues but is insufficient to generate an FIR. Even if an FIR is induced, it will likely be a milder version to enhance localized inflammation for fetoplacental and membrane growth. Thus, it can be speculated that exosomes enriched with maternal inflammatory cargo may function as paracrine mediators to deliver materials that can support pregnancy. However, a maternal infection or oxidative stress can be detrimental to the generation of an FIR that, in a feedforward, fashion can contribute to preterm birth or pPROM. Exosomes are also known to carry microbial antigens. Therefore, pathologic pregnancies can also be triggered by exosomes carrying either microbes or microbial antigens to generate an FIR. The latter situation is interesting, as pathologic outcomes in such scenarios may present as a sterile infection where the culture of microbes from biological specimens is likely negative.
17 |. SUMMARY AND CONCLUSIONS
Fetal membrane cells can generate inflammation through either physiologic or pathologic activation of p38MAPK in human fetal membrane cells, mostly in response to a stressful environment in the uterine cavity (Figure 8). P38MAPK can accelerate the senescence of amnion and chorion cells, and EMT occurs in cells that are not senescent. Both these conditions cause NF-kB activation and generate inflammation characterized by SASP and DAMPs that are packaged in exosomes for propagation. Inflammation also causes chemoattraction to the fetal membranes, all of which further enhance the fetal membrane inflammatory load. Propagated exosomes enriched with inflammatory mediators can reach the maternal uterine compartment (decidua, myometrium, and cervix), where they deliver fetal signals. In summary, when term approaches, fetal membrane structure, function, and mechanics are compromised due to cellular-level changes, and this is correlated with fetal growth. Thus, a mature fetus, ready for its independent life, creates an environment to generate fetal signals to indicate it readiness for delivery that can be considered as an FIR. Similarly, pregnancy-associated risk factors cause premature activation of the FIR leading to preterm birth. This is the FIR contribution of fetal membranes toward labor at term and preterm. Cellular-level changes that are independent of immune cells and endocrine factors may occur in other fetomaternal units as well. Both immune and endocrine mediators may contribute to this process or produce complementary functions. A coordinated activity between various mediators ensures parturition. Regardless, the FIR is a major contributor to this process (Figure 8). This review introduces a new dogma that fetal derived signals (or fetal signaling) are a critical mechanistic contributor to the parturition process. Addressing maternal inflammation-associated changes alone is insufficient to reduce the risk of preterm birth, and designing strategies to mitigate FIR is essential.
FIGURE 8.
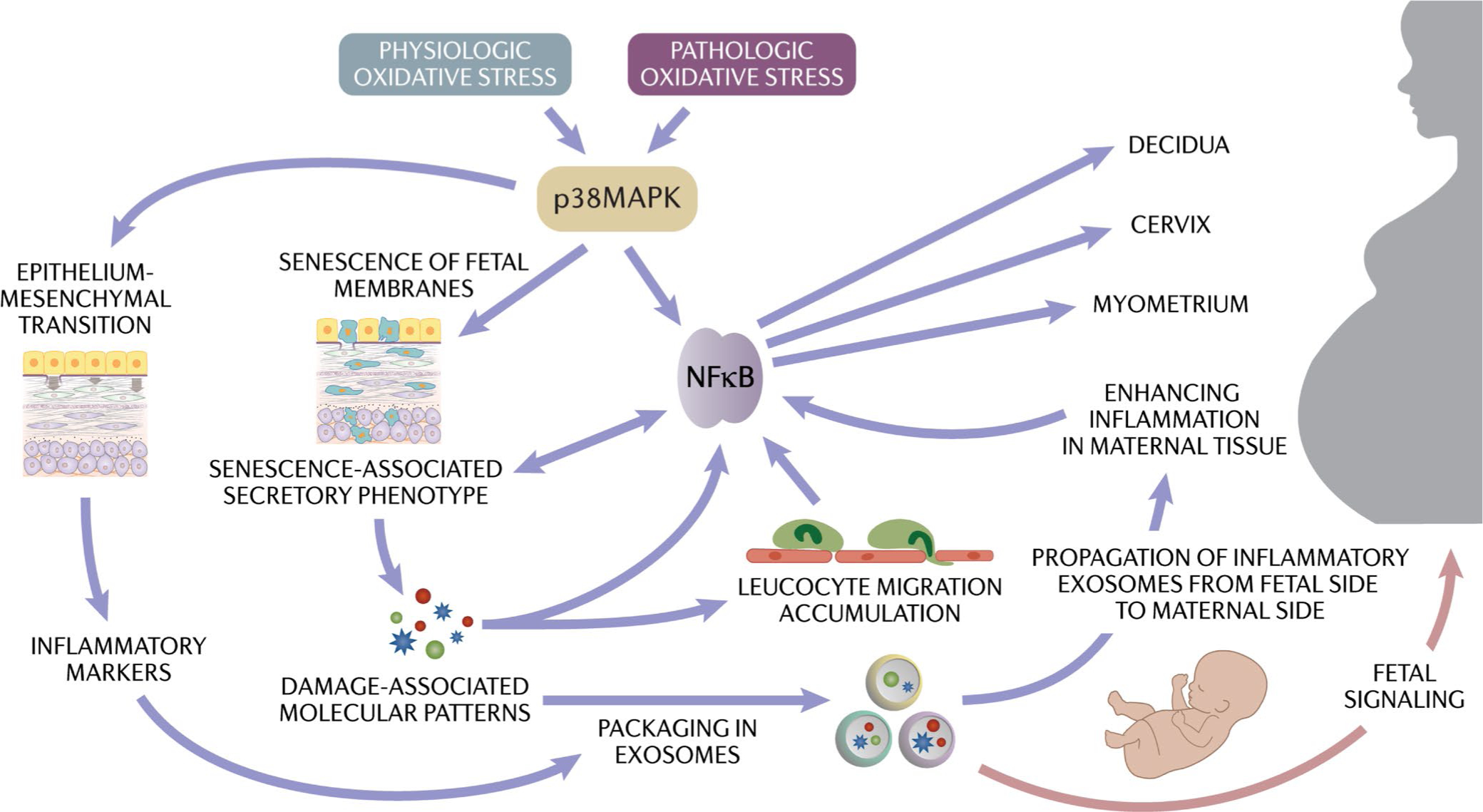
Physiologic (term) and pathologic (preterm) activation of oxidative stress and inflammation and the cascade of events resulting in parturition. Generation of the fetal inflammatory response (FIR) due to the cellular level changes can generate exosomes packaged in signalers that can transition quiescent maternal uterine tissues to an active state of labor (fetal signaling generated by changes to the fetal membrane cells)
ACKNOWLEDGEMENTS
I like to thank Dr Enkhtuya Radnaa, Assistant Professor, Division of the Basic Research and Translational Division, Dept of OBGYN, The University of Texas Medical Branch at Galveston, TX for all the help with several reported and ongoing studies in The Menon laboratory that led to this review as well as developing several figures for this manuscript. I also like to thank other faculty members of the division (Dr Ananth Kumar Kammala, and Dr Lauren Richardson; both Assistant Professors) for their help with various experiments on FIR in the lab. I also like to thank some outstanding past students (Ourlad Tantengco) and post-doctoral fellows (Samantha Sheller-Miller, and Jossimara Polettini), MFM Fellow (Faranak Behnia) for their major contributions in various topics discussed in this review.
Funding information
This study is funded partly by 1R01HD100729 (NIH/NICHD) to R Menon
Footnotes
CONFLICT OF INTEREST
Dr. Menon's research work was partly supported by ILIAS Biologics, Daejeon, S Korea between 2019–2021.
This article introduces a series of reviews covering Immunity at the Maternal/Fetal interface appearing in Volume 308 of Immunological Reviews.
DATA AVAILABILITY STATEMENT
This is a review article. Data included are either published or available in author's lab.
REFERENCES
- 1.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tal R, Taylor HS. Endocrinology of pregnancy. In: Feingold KR, Anawalt B, Boyce A, et al. , Endotext. MDText.com, Inc.; 2000.
- 3.Rokas A, Mesiano S, Tamam O, LaBella A, Zhang G, Muglia L. Developing a theoretical evolutionary framework to solve the mystery of parturition initiation. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith R Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol. 1998;39(1–2):215–220. [DOI] [PubMed] [Google Scholar]
- 5.Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. 2016;22(5):535–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendelson CR, Montalbano AP, Gao L. Fetal-to-maternal signaling in the timing of birth. J Steroid Biochem Mol Biol. 2017;170:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro-Mendoza CK, Lackritz EM. Epidemiology of late and moderate preterm birth. Semin Fetal Neonatal Med. 2012;17(3):120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol. 2010;34(6):408–415. [DOI] [PubMed] [Google Scholar]
- 10.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. [DOI] [PubMed] [Google Scholar]
- 11.Menon R, Richardson LS. Preterm prelabor rupture of the membranes: A disease of the fetal membranes. Semin Perinatol. 2017;41(7):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murtha AP, Menon R. Regulation of fetal membrane inflammation: a critical step in reducing adverse pregnancy outcome. Am J Obstet Gynecol. 2015;213(4):447–448. [DOI] [PubMed] [Google Scholar]
- 13.Menon R, Moore JJ. Fetal membranes, not a mere appendage of the placenta, but a critical part of the fetal-maternal interface controlling parturition. Obstet Gynecol Clin North Am. 2020;47(1):147–162. [DOI] [PubMed] [Google Scholar]
- 14.Villar J, Papageorghiou AT, Knight HE, et al. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol. 2012;206(2):119–123. [DOI] [PubMed] [Google Scholar]
- 15.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362(6):529–535. [DOI] [PubMed] [Google Scholar]
- 16.Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Ann Med. 2008;40(3):167–195. [DOI] [PubMed] [Google Scholar]
- 17.Lockwood CJ, Kuczynski E. Markers of risk for preterm delivery. J Perinat Med. 1999;27(1):5–20. [DOI] [PubMed] [Google Scholar]
- 18.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of O, Gynecologists' Committee on Practice B-O. Prediction and Prevention of Spontaneous Preterm Birth: ACOG Practice Bulletin, Number 234. Obstet Gynecol. 2021;138(2):e65–e90. [DOI] [PubMed] [Google Scholar]
- 20.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42(1):1–8. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsson B Infectious and inflammatory mechanisms in preterm birth and cerebral palsy. Eur J Obstet Gynecol Reprod Biol. 2004;115(2):159–160. [DOI] [PubMed] [Google Scholar]
- 22.Bersani I, Thomas W, Speer CP. Chorioamnionitis–the good or the evil for neonatal outcome? J Matern Fetal Neonatal Med. 2012;25(Suppl 1):12–16. [DOI] [PubMed] [Google Scholar]
- 23.Johanzon M, Odesjo H, Jacobsson B, Sandberg K, Wennerholm UB. Extreme preterm birth: onset of delivery and its effect on infant survival and morbidity. Obstet Gynecol. 2008;111(1):42–50. [DOI] [PubMed] [Google Scholar]
- 24.McCabe ER, Carrino GE, Russell RB, Howse JL. Fighting for the next generation: US Prematurity in 2030. Pediatrics. 2014;134(6):1193–1199. [DOI] [PubMed] [Google Scholar]
- 25.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206–215. [DOI] [PubMed] [Google Scholar]
- 26.Armistead B, Oler E, Adams Waldorf K, Rajagopal L. The double life of group B streptococcus: asymptomatic colonizer and potent pathogen. J Mol Biol. 2019;431(16):2914–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15(10):479–487. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan SP, Ananth CV. Periviable births: epidemiology and obstetrical antecedents. Semin Perinatol. 2013;37(6):382–388. [DOI] [PubMed] [Google Scholar]
- 29.Lawn JE, Kinney MV, Belizan JM, et al. Born too soon: accelerating actions for prevention and care of 15 million newborns born too soon. Reprod Health. 2013;10(Suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlicev M, Norwitz ER. Human parturition: nothing more than a delayed menstruation. Reprod Sci. 2018;25(2):166–173. [DOI] [PubMed] [Google Scholar]
- 31.Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23(7):947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey ML, MacDonald PC. Endocrinology of preterm birth. Clin Obstet Gynecol. 1984;27(3):562–571. [DOI] [PubMed] [Google Scholar]
- 33.Norwitz ER, Bonney EA, Snegovskikh VV, et al. Molecular regulation of parturition: the role of the decidual clock. Cold Spring Harb Perspect Med. 2015;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87(6):2924–2930. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell MD, Romero RJ, Edwin SS, Trautman MS. Prostaglandins and parturition. Reprod Fertil Dev. 1995;7(3):623–632. [DOI] [PubMed] [Google Scholar]
- 36.Reinl EL, England SK. Fetal-to-maternal signaling to initiate parturition 1. J Clin Invest. 2015;125(7):2569–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montalbano AP, Hawgood S, Mendelson CR. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154(1):483–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA. 2004;101(14):4978–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casey ML, Brown CE, Peters M, MacDonald PC. Endothelin levels in human amniotic fluid at mid-trimester and at term before and during spontaneous labor. J Clin Endocrinol Metab. 1993;76(6):1647–1650. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Avila C, Edwin SS, Mitchell MD. Endothelin-1,2 levels are increased in the amniotic fluid of women with preterm labor and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1992;166(1 Pt 1):95–99. [DOI] [PubMed] [Google Scholar]
- 41.Brady R, Zaidi SI, Mayer C, Katz DM. BDNF is a target-derived survival factor for arterial baroreceptor and chemoafferent primary sensory neurons. J Neurosci. 1999;19(6):2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman DR, Romero R, Johnston JM. Detection of platelet-activating factor in amniotic fluid of complicated pregnancies. Am J Obstet Gynecol. 1990;162(2):525–528. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65(12 Pt 2):S194–202. [DOI] [PubMed] [Google Scholar]
- 44.Richardson L, Menon R. Proliferative, migratory, and transition properties reveal metastate of human amnion cells. Am J Pathol. 2018;188(9):2004–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polettini J, Richardson LS, Menon R. Oxidative stress induces senescence and sterile inflammation in murine amniotic cavity. Placenta. 2018;63:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods JR Jr. Reactive oxygen species and preterm premature rupture of membranes-a review. Placenta. 2001;22(Suppl A):S38–S44. [DOI] [PubMed] [Google Scholar]
- 47.Menon R Human fetal membranes at term: Dead tissue or signalers of parturition? Placenta. 2016;44:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saroyo YB, Wibowo N, Irwinda R, et al. Oxidative stress induced damage and early senescence in preterm placenta. J Pregnancy. 2021;2021:9923761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chai M, Barker G, Menon R, Lappas M. Increased oxidative stress in human fetal membranes overlying the cervix from term non-labouring and post labour deliveries. Placenta. 2012;33(8):604–610. [DOI] [PubMed] [Google Scholar]
- 50.Bukowski R, Sadovsky Y, Goodarzi H, et al. Onset of human preterm and term birth is related to unique inflammatory transcriptome profiles at the maternal fetal interface. PeerJ. 2017;5:e3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen BW, Bonney EA, Pearce BD, Donahue LR, Sarkar IN, Preterm birth international C. A cross-species analysis of animal models for the investigation of preterm birth mechanisms. Reprod Sci. 2016;23(4):482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semmes EC, Coyne CB. Innate immune defenses at the maternal-fetal interface. Curr Opin Immunol. 2021;74:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quinn KH, Parast MM. Decidual regulatory T cells in placental pathology and pregnancy complications. Am J Reprod Immunol. 2013;69(6):533–538. [DOI] [PubMed] [Google Scholar]
- 54.Ticconi C, Di Simone N, Campagnolo L, Fazleabas A. Clinical consequences of defective decidualization. Tissue Cell. 2021;72: 101586. [DOI] [PubMed] [Google Scholar]
- 55.Yang F, Zheng Q, Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front Immunol. 2019;10:2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak-Kim JYH. The role of decidual immune cells on human pregnancy. J Reprod Immunol. 2017;124:44–53. [DOI] [PubMed] [Google Scholar]
- 57.Nancy P, Erlebacher A. T cell behavior at the maternal-fetal interface. Int J Dev Biol. 2014;58(2–4):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X, Zhou Y, Wei H. Roles of HLA-G in the maternal-fetal immune microenvironment. Front Immunol. 2020;11:592010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol. 2019;4(31):eaat6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lash GE, Ernerudh J. Decidual cytokines and pregnancy complications: focus on spontaneous miscarriage. J Reprod Immunol. 2015;108:83–89. [DOI] [PubMed] [Google Scholar]
- 61.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golos TG. Pregnancy initiation in the rhesus macaque: towards functional manipulation of the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonney EA, Johnson MR. The role of maternal T cell and macrophage activation in preterm birth: Cause or consequence? Placenta. 2019;79:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hadley EE, Richardson LS, Torloni MR, Menon R. Gestational tissue inflammatory biomarkers at term labor: A systematic review of literature. Am J Reprod Immunol. 2018;79(2). 10.1111/aji.12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menon R Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87(6):590–600. [DOI] [PubMed] [Google Scholar]
- 66.Menon R, Behnia F, Polettini J, Richardson LS. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin Immunopathol. 2020;42(4):431–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menon R Oxidative stress damage as a detrimental factor in preterm birth pathology 14. Front Immunol. 2014;5:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menon R Initiation of human parturition: signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet Gynecol Sci. 2019;62(4):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hadley EE, Sheller-Miller S, Saade G, et al. Amnion epithelial cell derived exosomes induce inflammatory changes in uterine cells. Am J Obstet Gynecol. 2018;219(5):478.e1–478.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Menon R, Shahin H. Extracellular vesicles in spontaneous preterm birth. Am J Reprod Immunol. 2020;85:e13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shahin HI, Radnaa E, Tantengco OAG, et al. Microvesicles and exosomes released by amnion epithelial cells under oxidative stress cause inflammatory changes in uterine cellsdagger. Biol Reprod. 2021;105(2):464–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tantengco OAG, Radnaa E, Shahin H, Kechichian T, Menon R. Cross talk: Trafficking and functional impact of maternal exosomes at the Feto-maternal Interface under normal and pathologic states. Biol Reprod. 2021;105(6):1562–1576. [DOI] [PubMed] [Google Scholar]
- 73.Medley N, Vogel JP, Care A, Alfirevic Z. Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2018;11:CD012505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyazaki C, Moreno RG, Ota E, Swa T, Oladapo OT, Mori R. Tocolysis for inhibiting preterm birth in extremely preterm birth, multiple gestations and in growth-restricted fetuses: a systematic review and meta-analysis. Reprod Health. 2016;13(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grimes DA, Nanda K. Magnesium sulfate tocolysis: time to quit. Obstet Gynecol. 2006;108(4):986–989. [DOI] [PubMed] [Google Scholar]
- 76.Thangaratinam S, Rogozinska E, Jolly K, et al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technol Assess. 2012;16(31):iii–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conde-Agudelo A, Romero R, Nicolaides K, et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod Update. 2003;9(4):347–357. [DOI] [PubMed] [Google Scholar]
- 79.Racicot K, Kwon JY, Aldo P, Silasi M, Mor G. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol. 2014;72(2):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta. 2014;35(4):241–248. [DOI] [PubMed] [Google Scholar]
- 81.Dietl J, Honig A, Kammerer U, Rieger L. Natural killer cells and dendritic cells at the human feto-maternal interface: an effective cooperation? Placenta. 2006;27(4–5):341–347. [DOI] [PubMed] [Google Scholar]
- 82.Harris LK, Benagiano M, D'Elios MM, Brosens I, Benagiano G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am J Obstet Gynecol. 2019;221(5):457–469. [DOI] [PubMed] [Google Scholar]
- 83.Kaminski VL, Ellwanger JH, Chies JAB. Extracellular vesicles in host-pathogen interactions and immune regulation - exosomes as emerging actors in the immunological theater of pregnancy. Heliyon. 2019;5(8):e02355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mor G, Kwon JY. Trophoblast-microbiome interaction: a new paradigm on immune regulation. Am J Obstet Gynecol. 2015;213(4 Suppl):S131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nancy P, Erlebacher A. Epigenetic repression of chemokine expression at the maternal-fetal interface as a mechanism of feto-maternal tolerance. Med Sci (Paris). 2012;28(12):1037–1039. [DOI] [PubMed] [Google Scholar]
- 86.PrabhuDas M, Bonney E, Caron K, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16(4):328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schumacher A, Sharkey DJ, Robertson SA, Zenclussen AC. Immune cells at the fetomaternal interface: how the microenvironment modulates immune cells to foster fetal development. J Immunol. 2018;201(2):325–334. [DOI] [PubMed] [Google Scholar]
- 88.Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol. 2009;82(1):24–31. [DOI] [PubMed] [Google Scholar]
- 89.Zhang X, Wei H. Role of decidual natural killer cells in human pregnancy and related pregnancy complications. Front Immunol. 2021;12: 728291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Erlebacher A Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. [DOI] [PubMed] [Google Scholar]
- 91.Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009;183(2):1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stout MJ, Cao B, Landeau M, French J, Macones GA, Mysorekar IU. Increased human leukocyte antigen-G expression at the maternal-fetal interface is associated with preterm birth. J Matern Fetal Neonatal Med. 2015;28(4):454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gomez-Lopez N, Arenas-Hernandez M, Romero R, et al. Regulatory T cells play a role in a subset of idiopathic preterm labor/birth and adverse neonatal outcomes. Cell Rep. 2020;32(1): 107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci USA. 2009;106(14):5767–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Menon R, Richardson LS, Lappas M. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta. 2019;79:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richardson L, Vargas G, Brown T, et al. Redefining 3Dimensional placental membrane microarchitecture using multiphoton microscopy and optical clearing. Placenta. 2017;53:66–75. [DOI] [PubMed] [Google Scholar]
- 97.Jacobs SO, Sheller-Miller S, Richardson LS, Urrabaz-Garza R, Radnaa E, Menon R. Characterizing the immune cell population in the human fetal membrane. Am J Reprod Immunol. 2021;85(5):e13368. [DOI] [PubMed] [Google Scholar]
- 98.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8(1):3–13. [DOI] [PubMed] [Google Scholar]
- 99.Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11(1):18–25. [DOI] [PubMed] [Google Scholar]
- 100.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014;11(6):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Faro J, Romero R, Schwenkel G, et al. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasomedagger. Biol Reprod. 2019;100(5):1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50(3):652–683. [DOI] [PubMed] [Google Scholar]
- 103.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chalupska M, Kacerovsky M, Stranik J, et al. Intra-amniotic infection and sterile intra-amniotic inflammation in cervical insufficiency with prolapsed fetal membranes: clinical implications. Fetal Diagn Ther. 2021;48(1):58–69. [DOI] [PubMed] [Google Scholar]
- 105.Cobo T, Kacerovsky M, Jacobsson B. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;211(6):708. [DOI] [PubMed] [Google Scholar]
- 106.Cobo T, Kacerovsky M, Holst R-M, et al. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet Gynecol Scand. 2012;91(8):930–935. [DOI] [PubMed] [Google Scholar]
- 107.Kacerovsky M, Musilova I, Khatibi A, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2012;25(10):2014–2019. [DOI] [PubMed] [Google Scholar]
- 108.Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med. 2012;17(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang L, Saito M, Jobe A, et al. Intra-amniotic administration of E coli lipopolysaccharides causes sustained inflammation of the fetal skin in sheep. Reprod Sci. 2012;19(11):1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Edey LF, O'Dea KP, Herbert BR, et al. The local and systemic immune response to intrauterine LPS in the prepartum mouse. Biol Reprod. 2016;95(6):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chaiworapongsa T, Romero R, Kim JC, et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol. 2002;186(6):1178–1182. [DOI] [PubMed] [Google Scholar]
- 112.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24(9):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gomez-Lopez N, Romero R, Xu YI, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol. 2017;217(6):693. e1–693.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Musilova I, Kacerovsky M, Hornychova H, Kostal M, Jacobsson B. Pulsation of the fetal splenic vein–a potential ultrasound marker of histological chorioamnionitis and funisitis in women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2012;91(9):1119–1123. [DOI] [PubMed] [Google Scholar]
- 115.Menon R, Taylor BD. Exploring inflammatory mediators in fetal and maternal compartments during human parturition. Obstet Gynecol. 2019;134(4):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Longini M, Perrone S, Vezzosi P, et al. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin Biochem. 2007;40(11):793–797. [DOI] [PubMed] [Google Scholar]
- 117.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24(12):1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis–a complex pathophysiologic syndrome. Placenta. 2010;31(2):113–120. [DOI] [PubMed] [Google Scholar]
- 119.Dutta EH, Behnia F, Boldogh I, et al. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes.8. Mol Hum Reprod. 2016;22(2):143–157. [DOI] [PubMed] [Google Scholar]
- 120.Bredeson S, Papaconstantinou J, Deford JH, et al. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One. 2014;9(12):e113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Menon R, Boldogh I, Hawkins HK, et al. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol. 2014;184(6):1740–1751. [DOI] [PubMed] [Google Scholar]
- 122.Erez O, Romero R, Tarca AL, et al. Differential expression pattern of genes encoding for anti-microbial peptides in the fetal membranes of patients with spontaneous preterm labor and intact membranes and those with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2009;22(12):1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tambor V, Kacerovsky M, Lenco J, Bhat G, Menon R. Proteomics and bioinformatics analysis reveal underlying pathways of infection associated histologic chorioamnionitis in pPROM. Placenta. 2013;34(2):155–161. [DOI] [PubMed] [Google Scholar]
- 124.Lim R, Barker G, Lappas M. Human cathelicidin antimicrobial protein 18 (hCAP18/LL-37) is increased in foetal membranes and myometrium after spontaneous labour and delivery. J Reprod Immunol. 2015;107:31–42. [DOI] [PubMed] [Google Scholar]
- 125.King AE, Paltoo A, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta. 2007;28(2–3):161–169. [DOI] [PubMed] [Google Scholar]
- 126.Rizzo R, Andersen AS, Lassen MR, et al. Soluble human leukocyte antigen-G isoforms in maternal plasma in early and late pregnancy. Am J Reprod Immunol. 2009;62(5):320–338. [DOI] [PubMed] [Google Scholar]
- 127.Biyik I Maternal serum soluble HLA-G in complicated pregnancies. J Matern Fetal Neonatal Med. 2014;27(4):381–384. [DOI] [PubMed] [Google Scholar]
- 128.Cao B, Mysorekar IU. Intracellular bacteria in placental basal plate localize to extravillous trophoblasts. Placenta. 2014;35(2):139–142. [DOI] [PubMed] [Google Scholar]
- 129.Tantengco OAG, Richardson L, Lee A, et al. Histocompatibility Antigen, Class I, G (HLA-G)'s role during pregnancy and parturition: a systematic review of the literature. Life (Basel). 2021;11(10):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parris KM, Amabebe E, Cohen MC, Anumba DO. Placental microbial-metabolite profiles and inflammatory mechanisms associated with preterm birth. J Clin Pathol. 2021;74(1):10–18. [DOI] [PubMed] [Google Scholar]
- 131.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keelan JA. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J Reprod Immunol. 2018;125:89–99. [DOI] [PubMed] [Google Scholar]
- 133.Nadeau-Vallée M, Obari D, Palacios J, et al. Sterile inflammation and pregnancy complications: a review. Reproduction. 2016;152(6):R277–R292. [DOI] [PubMed] [Google Scholar]
- 134.Behnia F, Sheller S, Menon R. Mechanistic differences leading to infectious and sterile inflammation 3. Am J Reprod Immunol. 2016;75(5):505–518. [DOI] [PubMed] [Google Scholar]
- 135.Padron JG, Saito Reis CA, Kendal-Wright CE. The role of danger associated molecular patterns in human fetal membrane weakening. Front Physiol. 2020;11:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gomez-Lopez N, Romero R, Plazyo O, et al. Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol. 2017;217(5):592.e1–592.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kacerovsky M, Romero R, Stepan M, et al. Antibiotic administration reduces the rate of intraamniotic inflammation in preterm prelabor rupture of the membranes. Am J Obstet Gynecol. 2020;223(1):114. e1–114.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Menon R, Mesiano S, Taylor RN. Programmed fetal membrane senescence and exosome-mediated signaling: a mechanism associated with timing of human parturition. Front Endocrinol (Lausanne). 2017;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Polettini J, Dutta EH, Behnia F, Saade GR, Torloni MR, Menon R. Aging of intrauterine tissues in spontaneous preterm birth and preterm premature rupture of the membranes: A systematic review of the literature. Placenta. 2015;36(9):969–973. [DOI] [PubMed] [Google Scholar]
- 140.Iliodromiti Z, Antonakopoulos N, Sifakis S, et al. Endocrine, paracrine, and autocrine placental mediators in labor 9. Hormones (Athens). 2012;11(4):397–409. [DOI] [PubMed] [Google Scholar]
- 141.Mesiano S, Welsh TN. Steroid hormone control of myometrial contractility and parturition. Semin Cell Dev Biol. 2007;18(3):321–331. [DOI] [PubMed] [Google Scholar]
- 142.Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97(5):E719–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brubaker D, Barbaro A, Chance MR, Mesiano S. A dynamical systems model of progesterone receptor interactions with inflammation in human parturition. BMC Syst Biol. 2016;10(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci. 2011;18(1):6–19. [DOI] [PubMed] [Google Scholar]
- 145.Yellon SM. Immunobiology of cervix ripening. Front Immunol. 2019;10:3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25(1):69–79. [DOI] [PubMed] [Google Scholar]
- 147.Tantengco OAG, Menon R. Contractile function of the cervix plays a role in normal and pathological pregnancy and parturition. Med Hypotheses. 2020;145: 110336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tattersall M, Engineer N, Khanjani S, et al. Pro-labour myometrial gene expression: are preterm labour and term labour the same? Reproduction. 2008;135(4):569–579. [DOI] [PubMed] [Google Scholar]
- 149.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–45. [DOI] [PubMed] [Google Scholar]
- 150.Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig. 2003;10(6):323–338. [DOI] [PubMed] [Google Scholar]
- 151.Shepherd MC, Radnaa E, Tantengco OA, et al. Extracellular vesicles from maternal uterine cells exposed to risk factors cause fetal inflammatory response. Cell Commun Signal. 2021;19(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Front Biosci. 2007;12:912–918. [DOI] [PubMed] [Google Scholar]
- 153.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202. [DOI] [PubMed] [Google Scholar]
- 154.Sato Y Inflammatory lesions in placental pathology. J Obstet Gynaecol Res. 2022;48(1):58–65. [DOI] [PubMed] [Google Scholar]
- 155.Sarno L, Della Corte L, Saccone G, et al. Histological chorioamnionitis and risk of pulmonary complications in preterm births: a systematic review and Meta-analysis. J Matern Fetal Neonatal Med. 2021;34(22):3803–3812. [DOI] [PubMed] [Google Scholar]
- 156.Menon R, Lappas M, Zakar T. Editorial: the role of the fetal membranes in pregnancy and birth. Front Physiol. 2021;12: 653084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fagagna FDD, Reaper PM, Clay-Farrace L, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426(6963):194–198. [DOI] [PubMed] [Google Scholar]
- 158.Bekaert S, De MT, Van OP. Telomere attrition as ageing biomarker. Anticancer Res. 2005;25(4):3011–3021. [PubMed] [Google Scholar]
- 159.Menon R, Yu J, Basanta-Henry P, et al. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One. 2012;7(2):e31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bonney EA, Krebs K, Saade G, et al. Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta. 2016;43:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere fragment induced amnion cell senescence: a contributor to parturition? PLoS One. 2015;10(9):e0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Menon R, Polettini J, Syed TA, Saade GR, Boldogh I. Expression of 8-oxoguanine glycosylase in human fetal membranes. Am J Reprod Immunol. 2014;72(1):75–84. [DOI] [PubMed] [Google Scholar]
- 163.Behnia F, Taylor BD, Woodson M, et al. Chorioamniotic Membrane senescence: a signal for parturition? Am J Obstet Gynecol. 2015;213(3):359.e1–359.e16. [DOI] [PubMed] [Google Scholar]
- 164.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30(8):1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Menon R, Boldogh I, Urrabaz-Garza R, et al. Senescence of primary amniotic cells via oxidative DNA damage. PLoS One. 2013;8(12):e83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Scott LM, Bryant AH, Rees A, Down B, Jones RH, Thornton CA. Production and regulation of interleukin-1 family cytokines at the materno-fetal interface. Cytokine. 2017;99:194–202. [DOI] [PubMed] [Google Scholar]
- 169.Çekmez Y, Çekmez F, Özkaya E, et al. uPAR, IL-33, and ST2 values as a predictor of subclinical chorioamnionitis in preterm premature rupture of membranes. J Interferon Cytokine Res. 2013;33(12):778–782. [DOI] [PubMed] [Google Scholar]
- 170.Liong S, Lappas M. Endoplasmic reticulum stress is increased after spontaneous labor in human fetal membranes and myometrium where it regulates the expression of prolabor mediators. Biol Reprod. 2014;91(3):70. [DOI] [PubMed] [Google Scholar]
- 171.Mulla MJ, Myrtolli K, Potter J, et al. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia 3. Am J Reprod Immunol. 2011;65(6):542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Omere C, Richardson L, Saade GR, Bonney EA, Kechichian T, Menon R. Interleukin (IL)-6: A friend or foe of pregnancy and parturition? evidence from functional studies in fetal membrane cells. Front Physiol. 2020;11:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Richardson LS, Taylor RN, Menon R. Reversible EMT and MET mediate amnion remodeling during pregnancy and labor. Sci Signal. 2020;13(618):eaay1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lozovyy V, Richardson L, Saade G, Menon R. Progesterone receptor membrane components: key regulators of fetal membrane integrity. Biol Reprod. 2021;104(2):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Musilova I, Kacerovsky M, Andrys C, Kostal M, Slaba K, Jacobsson B. The fetal splenic vein flow pattern and fetal inflammatory response in the preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2013;27(8):770–774. [DOI] [PubMed] [Google Scholar]
- 177.Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012;67(4):287–294. [DOI] [PubMed] [Google Scholar]
- 178.Arad I, Ergaz Z. The fetal inflammatory response syndrome and associated infant morbidity. Isr Med Assoc J. 2004;6(12):766–769. [PubMed] [Google Scholar]
- 179.Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lu JW, Wang WS, Zhou Q, et al. C/EBPdelta drives key endocrine signals in the human amnion at parturition. Clin Transl Med. 2021;11(6):e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mi Y, Wang W, Lu J, et al. Proteasome-mediated degradation of collagen III by cortisol in amnion fibroblasts. J Mol Endocrinol. 2018;60(2):45–54. [DOI] [PubMed] [Google Scholar]
- 183.Wang WS, Guo CM, Sun K. Cortisol regeneration in the fetal membranes, a coincidental or requisite event in human parturition? Front Physiol. 2020;11:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li W, Gao L, Wang Y, Duan T, Myatt L, Sun K. Enhancement of cortisol-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by interleukin 1beta in cultured human chorionic trophoblast cells. Endocrinology. 2006;147(5):2490–2495. [DOI] [PubMed] [Google Scholar]
- 185.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaislasuo J, Simpson S, Petersen JF, et al. IL-10 to TNFalpha ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am J Reprod Immunol. 2020;83(1):e13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jacobs SO, Sheller-Miller S, Richardson LS, Urrabaz-Garza R, Radnaa E, Menon R. Characterizing the immune cell population in the human fetal membrane. Am J Reprod Immunol. 2020;85:e13368. [DOI] [PubMed] [Google Scholar]
- 188.Gomez-Lopez N, Romero R, Xu YI, et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol. 2018;79(4):e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Miller D, Motomura K, Garcia-Flores V, Romero R, Gomez-Lopez N. Innate lymphoid cells in the maternal and fetal compartments. Front Immunol. 2018;9:2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hill AV, Menon R, Perez-Patron M, Carrillo G, Xu X, Taylor BD. High-mobility group box 1 at the time of parturition in women with gestational diabetes mellitus. Am J Reprod Immunol. 2019;82(5):e13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hawkins TL, Roberts JM, Mangos GJ, Davis GK, Roberts LM, Brown MA. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484–492. [DOI] [PubMed] [Google Scholar]
- 192.Zandarashvili L, Sahu D, Lee K, et al. Real-time kinetics of high-mobility group box 1 (HMGB1) oxidation in extracellular fluids studied by in situ protein NMR spectroscopy. J Biol Chem. 2013;288(17):11621–11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sheller-Miller S, Urrabaz-Garza R, Saade G, Menon R. Damage-Associated molecular pattern markers HMGB1 and cell-Free fetal telomere fragments in oxidative-Stressed amnion epithelial cell-Derived exosomes. J Reprod Immunol. 2017;123:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sheller-Miller S, Choi K, Choi C, Menon R. Cyclic-recombinasereporter mouse model to determine exosome communication and function during pregnancy. Am J Obstet Gynecol. 2019;221(5):502;e501–502 e512. [DOI] [PubMed] [Google Scholar]
- 195.Khosrotehrani K, Bianchi DW. Fetal cell microchimerism: helpful or harmful to the parous woman? Curr Opin Obstet Gynecol. 2003;15(2):195–199. [DOI] [PubMed] [Google Scholar]
- 196.Bianchi DW, Khosrotehrani K, Way SS, MacKenzie TC, Bajema I, O'Donoghue K. Forever connected: the lifelong biological consequences of fetomaternal and maternofetal microchimerism. Clin Chem. 2021;67(2):351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gomez-Lopez N, Romero R, Xu YI, et al. A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci. 2017;24(6):934–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Monsivais LA, Sheller-Miller S, Russell W, et al. Fetal membrane extracellular vesicle profiling reveals distinct pathways induced by infection and inflammation in vitro. Am J Reprod Immunol. 2020;84(3):e13282. [DOI] [PubMed] [Google Scholar]
- 199.Sheller-Miller S, Radnaa E, Arita Y, et al. Environmental pollutant induced cellular injury is reflected in exosomes from placental explants. Placenta. 2020;89:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sheller-Miller S, Trivedi J, Yellon SM, Menon R. Exosomes cause preterm birth in mice: evidence for paracrine signaling in pregnancy. Sci Rep. 2019;9(1):608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Menon R, Debnath C, Lai A, et al. Protein profile changes in circulating placental extracellular vesicles in term and preterm births: a longitudinal study. Endocrinology. 2020;161(4):bqaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Menon R, Debnath C, Lai A, et al. Circulating exosomal miRNA profile during term and preterm birth pregnancies: a longitudinal study. Endocrinology. 2019;160(2):249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69(3):212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arenas-Hernandez M, Gomez-Lopez N, Garcia-Flores V, et al. Choriodecidual leukocytes display a unique gene expression signature in spontaneous labor at term. Genes Immun. 2019;20(1):56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kothary V, Doster RS, Rogers LM, et al. Group B streptococcus induces neutrophil recruitment to gestational tissues and elaboration of extracellular traps and nutritional immunity. Front Cell Infect Microbiol. 2017;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Huang B, Faucette AN, Pawlitz MD, et al. Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat Med. 2017;23(1):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Horton JS, Yamamoto SY, Bryant-Greenwood GD. Relaxin augments the inflammatory IL6 response in the choriodecidua. Placenta. 2012;33(5):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nunez-Sanchez E, Flores-Espinosa MDP, Mancilla-Herrera I, et al. Prolactin modifies the in vitro LPS-induced chemotactic capabilities in human fetal membranes at the term of gestation. Am J Reprod Immunol. 2021;86(2):e13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lui S, Duval C, Farrokhnia F, et al. Delineating differential regulatory signatures of the human transcriptome in the choriodecidua and myometrium at term labor. Biol Reprod. 2018;98(3):422–436. [DOI] [PubMed] [Google Scholar]
- 210.Zaga-Clavellina V, Flores-Espinosa P, Pineda-Torres M, et al. Tissue-specific IL-10 secretion profile from term human fetal membranes stimulated with pathogenic microorganisms associated with preterm labor in a two-compartment tissue culture system. J Matern Fetal Neonatal Med. 2014;27(13):1320–1327. [DOI] [PubMed] [Google Scholar]
- 211.Simpson KL, Keelan JA, Mitchell MD. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. J Clin Endocrinol Metab. 1998;83(12):4332–4337. [DOI] [PubMed] [Google Scholar]
- 212.Radnaa E, Urrabaz-Garza R, Elrod ND, et al. Generation and characterization of human Fetal membrane and Decidual cell lines for reproductive biology experimentsdagger. Biol Reprod. 2021; 10.1093/biolre/ioab231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Presicce P, Senthamaraikannan P, Alvarez M, et al. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod. 2015;92(2):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cappelletti M, Presicce P, Feiyang M, et al. The induction of preterm labor in rhesus macaques is determined by the strength of immune response to intrauterine infection. PLoS Biol. 2021;19(9):e3001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article. Data included are either published or available in author's lab.


