Abstract
Aggregation of the microtubule associated protein tau plays a major role in Alzheimer’s disease and several other neurodegenerative disorders. An exciting recent development is the finding that, akin to some other proteins associated with neurodegenerative disease, tau has a high propensity to condensate via the mechanism of liquid-liquid phase separation (LLPS). Here, we discuss the evidence for tau LLPS in vitro, the molecular mechanisms of this reaction, and the role of post-translational modifications and pathogenic mutations in tau phase separation. We also discuss recent studies on tau LLPS in cells, and the insights these studies provided regarding the link between LLPS and neurodegeneration in tauopathies.
Keywords: tau, liquid-liquid phase separation, protein condensation, protein aggregation, neurodegenerative diseases
Tau pathology in neurodegenerative diseases
Tau, the microtubule associated protein (Box 1), plays a crucial role in the pathogenesis of a wide range of neurodegenerative disorders collectively classified as tauopathies. These include Alzheimer’s disease (AD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), agyrophilic grain disease (AGD), Pick’s disease (PiD), some forms of frontotemporal dementia (FTD), and chronic traumatic encephalopathy (CTE). One of the key histopathological signatures of all these disorders is accumulation of insoluble cytoplasmic tau inclusions in brain [1–5]. The main type of these inclusions are neurofibrillary tangles (NFTs), which typically are composed of paired helical filaments, even though structures with alternative morphologies can also be present [3–5]. The abundance of NFTs in AD brains appears to correlate with the progression of the disease. However, smaller oligomeric species are also accumulating during the pathogenic process, and some of these oligomers appear to be more neurotoxic than mature filaments [3–5]. Regardless of specific morphology, tau filaments have properties of amyloid fibrils, self-propagating by a seeded polymerization mechanism similar to that involved in replication of prions responsible for transmissible spongiform encephalopathies. Remarkably, rapidly growing data suggest that, akin to prions, tau aggregates can exist as structurally distinct “strains”, and that prion-like mechanisms may be involved in spreading of tau aggregates from cell to cell, and from one brain region to another [6, 7].
Box 1. Tau protein.
Tau is encoded by the MAPT gene which comprises 16 exons on chromosome 17q21 in the human genome. Alternative splicing of MAPT results in the expression of six major tau isoforms in the central nervous system (Fig. IA). These isoforms differ with respect to the number of N-terminal inserts and the number of 31–32-residue pseudo-repeat sequences in the C-terminal part of the protein [3–6]. Structurally, tau consists of four distinct regions, including the N-terminal projection domain (NTD), the proline-rich domain (PRD), microtubule-binding domain (MTBD), and the C-terminal domain (CTD) (Fig. IA) [3–6]. PRD and MTBD are responsible for tau binding to microtubules. An important characteristic of tau is highly polarized distribution of charges, where the NTD and CTD are negatively charged, whereas PRD and MTBD contain large proportion of basic residues and carry net positive charge (Fig. IB). Both the predictive algorithms (Fig. IC) as well as experimental data indicate that full-length tau is largely an intrinsically disordered protein. Nevertheless, elements of local secondary structure appear to exist within the MTBD [3, 73] and potentially also in the C-terminal segment (Fig. IC).
Multiple point mutations in the MAPT gene are associated with familial cases of a neurodegenerative disorder known as frontotemporal dementia with parkinsonism-17 (FTDP-17) (Fig. ID) [1, 2]. These mutations can contribute to disease pathogenesis via mechanisms such as reduction of tau ability to bind to microtubules and/or enhancement of protein’s aggregation propensity [74–76].
Tau is known to undergo extensive post-translational modifications including, among others, phosphorylation, acetylation, ubiquitination, glycation, glycosylation, SUMOylation, and methylation [3, 4, 53, 77, 78]. These modifications play an important role both in tau biology as well as pathology. Perhaps the most consequential of these is phosphorylation, as it not only plays a regulatory role in microtubule assembly, but is also believed to greatly affect pathological tau aggregation [3, 4, 53, 77, 78]. Tau in AD is hyperphosphorylated, with over 45 distinct phosphorylation sites identified to date.
The landscape of this phosphorylation is very complex, with great many subpopulations of molecules phosphorylated at different residues [79]. Most of these phosphorylation sites are at multiple Ser and Thr residues in the proline-rich region and the C-terminal part. A whole range of protein kinases is involved in tau Ser/Thr phosphorylation in brain, including proline-directed kinases and those of different selectivities [53, 77]. Another type of tau phosphorylation found in AD brain is phosphorylation of several Tyr residues by tyrosine kinases [53, 77]. It is not fully clear, however, which of these sites are phosphorylated before and which after pathological protein aggregation.
Box 1 Fig. I. The structure of tau.
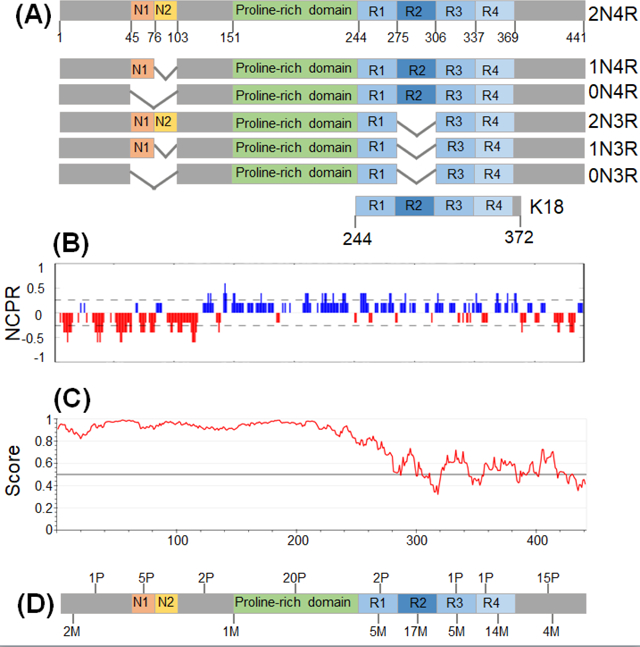
(A) Structural domains in different isoforms of tau. (B) Polarized charge distribution as illustrated by the analysis of net charge per residue (NCPR) [24]. (C) Prediction for intrinsically disordered regions in tau using IUPred2A algorithm [80]. Residues with scores above 0.5 are predicted as disorderd. (D) The number of phosphorylation sites found in AD (P) and disease-associated mutations (M) in different tau domains.
Tau pathology is believed to be initiated by the detachment of tau from microtubules, likely as a result of phosphorylation and/or decreased rate of dephosphorylation [3–5, 8]. In response to stress, phosphorylated free tau mislocalizes and accumulates in dendrites and soma [3–5, 8]. This eventually leads to protein aggregation, even though individual steps involved in this reaction chain are still not fully understood.
Liquid-liquid phase separation as an emerging player in tauopathies
While spatial organization within cells typically revolves around membrane-encapsulated organelles such as the nucleus, Golgi apparatus or endoplasmic reticulum, some cellular compartments within the cytoplasm and the nucleus are not surrounded by lipid bilayers. Examples of such membraneless organelles include P-bodies, stress granules, Cajal bodies, or the nucleolus. Rapidly growing observations indicate that the latter type of cellular compartmentalization is driven by liquid-liquid phase separation (LLPS) of proteins and protein-RNA acid complexes, a process resulting in the formation of granules (or droplets) in which macromolecules are highly condensed. Initially, protein droplets are highly dynamic, but with time their material properties often change through the process referred to as aging (Box 2 and Fig. 1).
Box 2. Liquid-liquid phase separation (LLPS).
LLPS occurs when macromolecules and the solvent demix into condensed and dilute phases, forming liquid droplets [9–12]. Concentration of macromolecules at which they start to demix is defined as a saturation concentration (Fig. 1B) [51]. LLPS of proteins can be either homotypic (i.e., involving just one component) or heterotypic, where multiple proteins and/or RNA molecules are present [9–12]. Proteins with high propensity for LLPS typically contain repetitive modular domains or intrinsically disordered regions with weakly adhesive motifs. The latter regions are often of low complexity with regard to the amino acid composition, a feature that increases their capacity for LLPS by promoting multivalent intermolecular interactions. The types of weak adhesive interactions involved in protein LLPS are schematically shown in Fig. 1A. The most common of these appear to be electrostatic interactions (which are especially important in heterotypic LLPS of protein/RNA mixtures), cation-π interactions between Arg or Lys residues and aromatic side chains,π-π stacking of aromatic residues, dipole-dipole interactions, and broadly defined hydrophobic interactions [10, 12, 18, 81, 82]. The nature of these interactions in a particular system can be probed by changing ionic strength and/or by addition of agents such as 1,6-hexanediol that often abrogate LLPS, presumably by interfering with hydrophobic and/or other non-electrostatic interactions [83, 84]. It has been proposed that protein LLPS may also be driven by steric zipper-type interactions between β-strands [85–87], even though the evidence for this is limited.
Liquid droplets formed via LLPS evolve with time. The first step in this evolution is growth in size (Fig. 1B). This may occur through fusion events and/or Ostwald ripening, a process in which proteins from inherently unstable small droplets diffuses into larger, more stable droplets [12, 88]. After longer incubation, droplets often (though not always) change their material properties, becoming increasingly less dynamic (Fig. 1C). This process, known as droplet “aging”, has been initially described as gelation in which molecules become increasingly crosslinked [86]. However, a recent study demonstrates that, at least for some proteins, droplets aging involves a transition to “Maxwell glasses” [89]. The latter state is characterized by both viscous and elastic properties, with elasticity remaining unchanged and viscosity increasing with time due to protein jamming. For some proteins, the final stage of the aging process is a transition to solid-like amyloid fibrils [16–20, 57]. In principle, the latter transition could occur from gel, Maxwell glass, or directly from the liquid state. Which of these mechanisms is operational in fibrillation of specific proteins within the droplets is at present unknown.
Fig. 1. Physical properties of protein liquid droplets formed through LLPS.
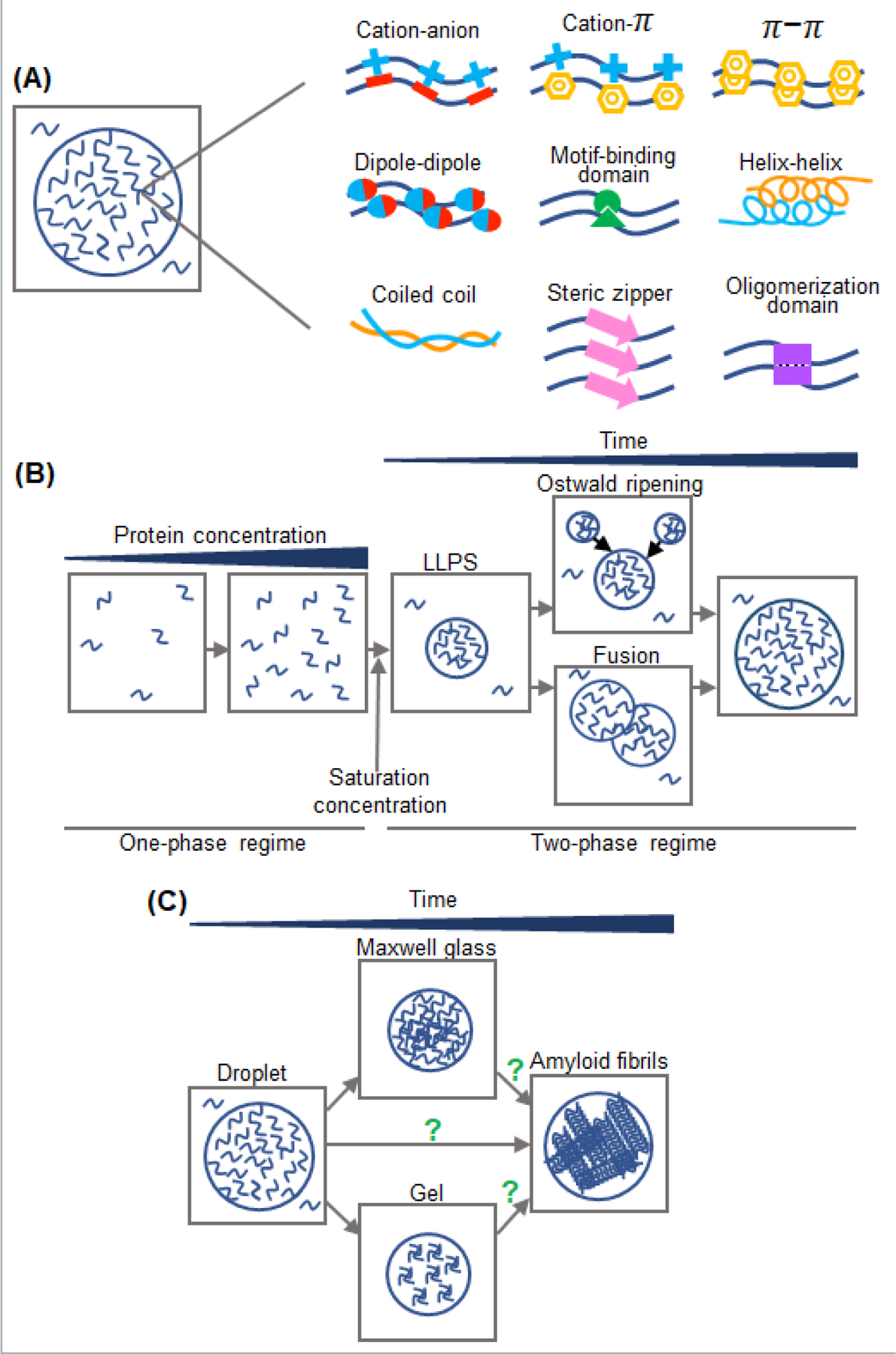
(A) Types of interactions known to drive protein LLPS. (B) Left, A transition of protein solution from a single phase to a two-phase system in which liquid droplets with highly concentrated protein coexist with a dilute protein solution. The concentration of protein at which LLPS starts to occur is known as a saturation concentration. Right, Highly dynamic droplets grow in size with time due to fusion (coalescence) and Ostwald ripening. (C) Upon aging, initially dynamic droplets change their material properties, undergoing a transition to gel-like structures or Maxwell glasses. For some proteins, this is followed (or accompanied) by formation of amyloid fibrils.
While the rapidly growing body of evidence indicates that cellular compartmentalization via LLPS may play a key role in numerous biological and biochemical processes [9–12], there is also a darker side of this phenomenon. Indeed, LLPS appears to be associated with the pathogenesis of some of the most devastating neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS), AD, and some forms of FTD. This was first suggested by the observations that some proteins associated with these disorders not only form amyloid fibrils but also undergo LLPS, and that the properties of the resulting droplets are affected by disease-causing mutations [13–15]. Since these initial observations, a rapidly proliferating body of work has further strengthened the link between LLPS and neurodegenerative diseases [16–20]. Here, we focus on LLPS of tau. We first describe the insights into the mechanism of tau LLPS and consequences of this process as revealed by studies in vitro. This is followed by a discussion of recent studies on tau condensation in a cellular context and the potential role LLPS may play in tau biology and pathology.
Homotypic LLPS of tau
Initial studies on tau LLPS were performed with a phosphorylated protein expressed in insect cells (p-tau441), and these studies demonstrated that p-tau441 has the capacity to form liquid droplets in the presence of crowding agents (see Glossary) [21, 22]. One of these early studies also suggested that phosphorylation is a major driver (if not a prerequisite) for tau LLPS and that this reaction is driven by a complex combination of electrostatic and hydrophobic interactions [22]. However, a series of subsequent reports clearly showed that bacterially-expressed non-phosphorylated full-length tau also undergoes robust LLPS under physiologically relevant conditions [23–25]. The latter reaction was found to be highly dependent on the ionic strength of the buffer, with a decreased tendency to form liquid droplets with increased salt concentration [24]. This, together tau droplet’s low sensitivity to 1,6-hexanediol, as well as tau’s low content of aromatic residues and its polarized charged distribution, strongly suggest that LLPS of full-length tau is largely driven by electrostatic forces, with only minor contributions of other interactions known to promote LLPS of other proteins (Box 2 and Fig. 1A). The above mechanism was further supported by studies with a series of deletion tau variants, which led to the conclusion that LLPS of full-length tau at physiological pH is largely driven by attractive intermolecular electrostatic interactions between the negatively charged N-terminal and positively charged middle/C-terminal domains of the protein [24]. Of note, tau LLPS has also been reported to occur in the presence of extremely high (4–5 M) concentrations of NaCl, where it is driven by hydrophobic interactions [26]. Even though such conditions are far from physiological, it has been speculated that similar hydrophobic interactions could become important upon hyperacetylation of tau that eliminates positive charges on numerous Lys residues [26].
LLPS was also observed for the internal K18 fragment of tau (Fig. IA), but only at high protein concentrations [27–30]. Biophysical studies identified hot spots in K18 condensation and revealed that LLPS of this fragment is associated with a transition of residues within the KXGS motifs of the repeats from an extended conformation to transient β-hairpin-like state [29], even though another study suggested an overall expansion of the molecule [28]. Interesting as they are, it is not clear whether the lessons from studies with the non-physiological K18 fragment could apply to the full-length tau. Indeed, the mechanisms of homotypic condensation of these two proteins appear to be quite different, with LLPS of K18 being largely driven by hydrophobic rather than electrostatic interactions [27].
Tau condensation in the presence of RNA and other molecules
One of the first observations regarding the ability of tau and its fragments to form liquid droplets was made in the context of heterotypic LLPS (or complex coacervation; see Box 2) in the presence of RNA [31, 32]. The maximum condensation of tau/RNA mixtures occurs at ~1:1 ratio of net positive and negative charges on tau and RNA, respectively; at higher RNA concentrations droplets start to dissolve [31]. Droplet dissolution under such conditions, also observed for RNA mixtures with other proteins, is often referred to as a reentrant phase transition [33, 34]. Complex coacervation of tau diminishes rapidly at higher salt concentrations and shows little sensitivity to 1,6-hexandiol [31, 35]. Thus, it appears that, akin to homotypic tau LLPS, complex coacervation is largely driven by electrostatic interactions. However, the molecular nature of these interactions is likely different, with complex coacervation being driven not only by electrostatic tau-tau interactions but also (and likely predominantly) tau-RNA interactions. This is consistent with the higher microviscosity observed within tau-RNA droplets as compared to homotypic droplets, suggesting a higher density of multivalent interactions in complex coacervates [35]. Furthermore, experiments in the presence of molecular crowders indicate a potential for coexistence of homotypic and heterotypic tau droplets, with the equilibrium depending on RNA concentration [35].
The findings regarding tau LLPS in the presence of RNA bring up questions regarding the specificity of tau for different types of RNA. This issue has been addressed in several reports, leading to different conclusions. While an early study using a crosslinking approach indicated that tau in hiPSC-derived neurons and HEK293 cells associates preferentially with tRNAs [31], studies focused on nuclear speckles in tau-expressing HEK293 cells and rTg4510 mice reported preferential association of tau aggregates with small nuclear and nucleolar RNAs [36]. In yet another study, it was found that oligomeric tau species can bind to m6A modified RNA directly or through its interaction with HNRNPA2B1 [37]. Clearly, this important issue requires further studies.
Tau condensation has also been shown to be strongly enhanced by another polyanion, heparin [38, 39]. Even though heparin is unlikely to be a physiological partner of tau, this observation is of significance as this polyanion is widely used as a cofactor in studies on tau fibrillation [3, 4, 40]. Furthermore, given that hyperphosphorylation results in the reversion of the overall charge of tau from positive to negative, based on molecular dynamics simulation, it was recently suggested that the abundant positively charged polyamine, spermine, may play an important role in condensation of hyperphosphorylated tau in cells [41]. However, this possibility is still to be tested experimentally.
Finally, tau LLPS in vitro has been shown to be modulated by certain metal ions [42], small molecules [43–45] and proteinaceous partners [46–48]. Zinc appears to have a particularly strong effect, promoting tau condensation in vitro at micromolar concentrations [42]. Even though the concentration of free zinc in cells is normally much lower, this observation may be of relevance to AD pathogenesis, as the level of zinc in AD brains is highly elevated [49, 50].
The effect of disease-associated mutation on tau LLPS
A number of point mutations in MAPT is associated with familial cases of FTDP-17 (Box 1). Therefore, it was of great interest to assess the impact of these mutations on tau LLPS. The first study addressing this issue reported that several variants of tau441 with pathogenic mutations (P301L, P301S, ΔK280, A152T) formed droplets under conditions where no LLPS was observed for the wild-type protein, suggesting that an enhanced LLPS propensity of mutant protein may contribute to their increased aggregation potential [22]. However, a subsequent study using a variety of approaches demonstrated that the pathogenic tau variants tested (P301L, ΔK280 and G272V) have essentially identical saturation concentrations and phase diagrams as the wild-type protein [39]. Given that saturation concentration provides an objective measure of a protein’s intrinsic propensity for LLPS [51], it appears that point mutations do not significantly affect tau’s capacity to undergo condensation under physiologically relevant buffer conditions. This is consistent with the electrostatic interactions model of tau LLPS in which intramolecular attractive electrostatic interactions between the negatively charged N-terminal and positively charged middle/C-terminal regions involve a great many residues [24]; therefore, point mutations should have a minimal effect.
Posttranslational modifications as modulators of tau LLPS
Tau undergoes numerous posttranslational modifications (PTMs) (Box 1) and at least some of them appear to have a regulatory effect on LLPS. This may be due to their effect on protein charge, its conformation, interactions with other macromolecules, or a combination of these factors. Arguably the most consequential of tau PTMs is phosphorylation (Box 1). Even though the initial assertion that phosphorylation is a prerequisite of tau LLPS [22] has not been confirmed in subsequent studies [23–25], there is considerable evidence that phosphorylation may regulate this process and/or its functional consequences. This is indicated by the increased LLPS propensity of full-length tau upon introduction of phosphomimetic substitutions [22, 25, 52] or that of K18 upon phosphorylation by MARK2 [27], and by the observation that phosphorylation can modulate the assembly of microtubules within the context of tau condensates (see below) [52]. Given the importance of electrostatic interactions in LLPS of full-length tau, the modulatory action of phosphorylation is likely strongly dependent on the identity of sites phosphorylated by different kinases, as this would affect the degree of polarized charge distribution. Molecular details of this putative regulatory mechanism remain, however, largely unexplored.
Another important PTM of tau is acetylation of abundant Lys residues [3, 4, 53]. Since acetylation eliminates positive charge on Lys residues, one would expect it to reduce the propensity of tau to undergo heterotypic LLPS in the presence of RNA, and such an effect has indeed been observed for K18 [54]. Acetylation was also shown to inhibit homotypic LLPS of full-length tau [23, 54], likely due to the disruption of polarized charge distribution and the resulting weakening of intermolecular electrostatic interactions.
The role of LLPS in microtubule formation
Tau in neurons is associated with microtubules, playing an important role in their assembly [3, 4, 55]. However, there are major gaps in our understanding of how microtubule bundles form, and the mechanism involved in local nucleation believed to drive this process. Therefore, it is perhaps not surprising that one of the first observations regarding tau LLPS were made in the context of studies on the mechanism of tubulin bundles formation [21]. It was found that, in the presence of a crowding agent, α/β dimers of tubulin partitioned into tau droplets, with a large increase in the local concentration of tubulin in comparison to that outside droplets. Within minutes, tubulin in the condensed phase started to polymerize into stable microtubule bundles that eventually formed a very dense network, with tau molecules encapsulating microtubules remaining in a liquid-like state. A subsequent study revealed that phosphorylation may play a regulatory role in microtubule bundles assembly within tau droplets, with the extent of this effect depending on specific phosphorylation sites [52]. Another aspect of tau-microtubule interactions was revealed in a study showing that recruitment to microtubule surface may, in fact, induce tau LLPS at concentrations far below its saturation concentration in the absence of microtubules [56]. This effect was observed both in the test tube and in neurons, although the liquid-like character of surface induced condensates in neurons has not been directly confirmed.
The regulatory role of LLPS in tau aggregation
Studies with other LLPS-prone proteins revealed that the properties of liquid droplets change with time, a process often referred to as droplet aging (see Box 2 and Fig. 1C). For some proteins, the final stage of this aging process is the formation of a network of amyloid fibrils [16–20, 57] (see Box 2 and Fig. 1C). Thus, given that the hallmark of AD and other tauopathies is the accumulation of intracellular tau filaments [3–6], it is of great interest to determine the effect of LLPS on the aggregation properties of tau.
The first study exploring this relationship reported that droplets formed by insect cells-expressed phosphorylated tau (p-tau441) quickly lost their initially dynamic character and, after prolonged incubation, there was a transition of tau within droplets into amorphous aggregates that bind a fluorescent dye, ThioS [22]. Since this dye can stain amyloid fibrils, it was suggested that p-tau441 aggregates formed within droplets are rich in β-sheet structure similar to that found in amyloids. However, a subsequent study (using non-phosphorylated tau) found that the aggregates formed within droplets in the absence of any cofactors react with an antibody that specifically recognizes small tau oligomers, but not mature fibrils [25]. The latter oligomers are associated with tau toxicity independent of fibril formation [3–6]. The notion that, akin to the situation in bulk solution, fibrillation of tau within droplets requires anionic cofactors is consistent with studies using the K18 fragment [27]. In this case, robust fibrillation of the protein was observed within the droplets in the presence (but not absence) of heparin. The authors proposed a model, in which recruitment of polyanionic heparin to highly concentrated K18 within droplets increases local concentration of protein/polyanion complexes, thereby promoting amyloid formation [27].
The link between tau LLPS and amyloid fibril formation was further explored using non-phosphorylated full-length protein [39]. This study found that pathogenic mutations within the repeat region greatly accelerate the transition of droplets into less dynamic assemblies. This loss of protein dynamicity within droplets was followed by formation of fibrillar aggregates, with the rate of fibrillation for each protein variant being much faster compared to that in bulk solution. Moreover, consistent with their effect on droplet dynamicity, disease-related mutations were found to dramatically increase the rate of protein fibrillation within droplets [39]. The apparent lack of correlation between the effect of pathogenic mutations on the intrinsic propensity of tau for LLPS (which is essentially identical for wild-type and mutant proteins; see above) and mutation-dependent acceleration of droplet maturation and fibril formation within them strongly indicates that different types of interactions drive LLPS and subsequent protein aggregation/fibrillation within the condensed phase.
The study on tau fibrillation using the mixtures of 2N4R and 2N3R tau isoforms (that have intrinsically different aggregation propensities) has also revealed a unique regulatory mechanism of tau fibrillation under the conditions of LLPS [39]. In this mechanism, the presence of slowly aggregating 2N3R isoform leads to the decrease in fibrillation rate of normally faster aggregating 2N4R isoform by effectively lowering the concentration of the latter isoform within the droplets (Fig. 2) [39]. Different types of tauopathies appear to be associated with the presence of different tau isoforms in the filaments. While 4R tau is the predominant (if not sole) isoform in filaments associated with PSP, globular glial tauopathy, CBD, and ageing-related tau astrogliopathy [1, 2, 58], 3R tau filaments are exclusively present in PiD [59]. AD, CTE, primary age-related tauopathy and some familial forms of dementia, on the other hand, are associated with filaments formed by both tau isoforms [1, 2]. Thus, such a regulatory mechanism may be of direct relevance to the molecular basis of phenotypic variability in tauopathies. Furthermore, a similar regulatory mechanism could potentially apply to other LLPS- and aggregation-prone proteins that, due to alternative splicing, may exist in several isoforms with distinct aggregation propensities.
Fig. 2. Fibrillation of tau within the droplets.
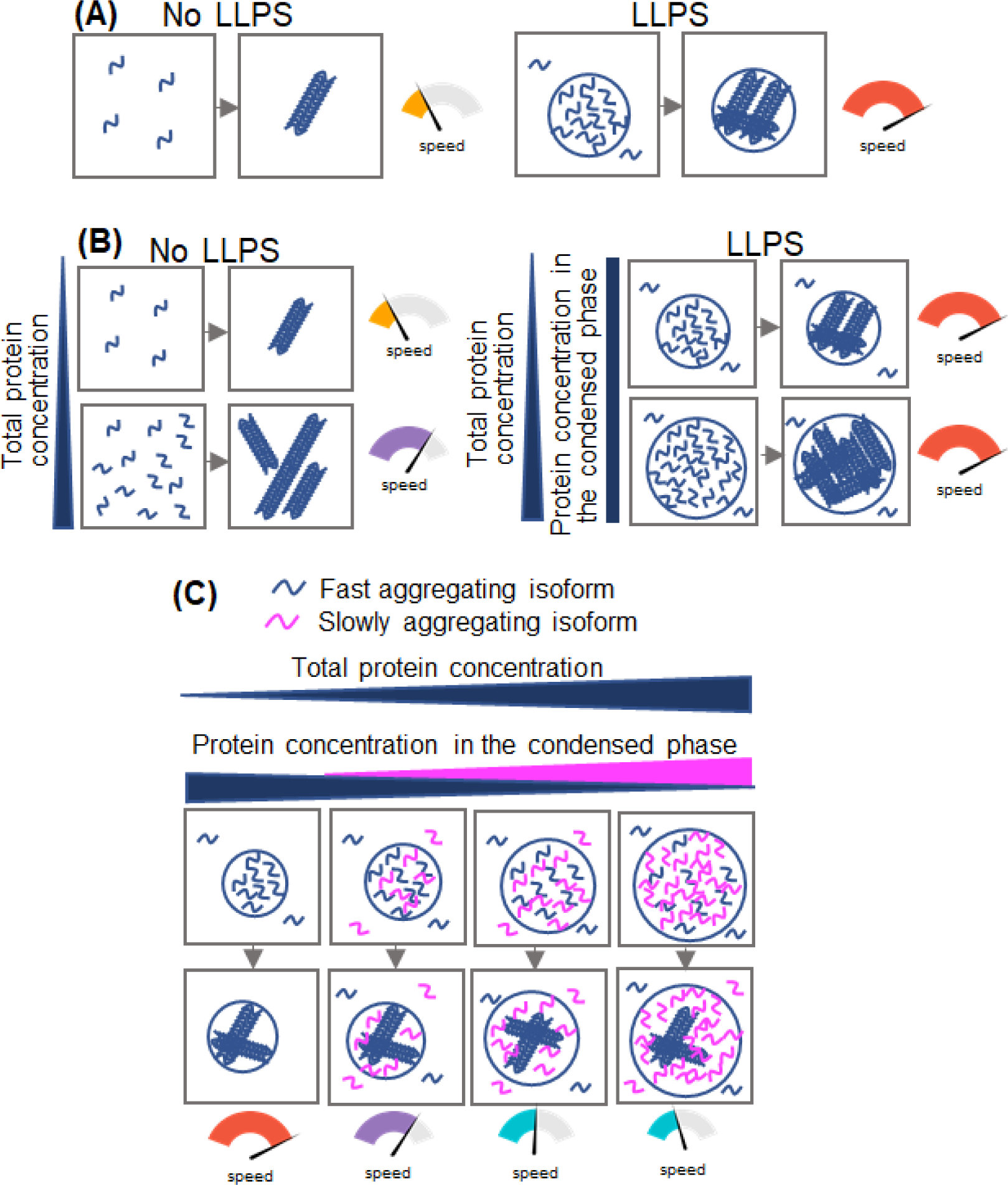
(A) The rate of tau fibrillation within droplets is much higher than that in bulk solution in the absence of LLPS. (B) In the absence of LLPS, the rate of tau fibrillation accelerates with increasing protein concentration (left). By contrast, in the presence of LLPS the concentration of tau in the condensed (droplets) and dilute phases is constant regardless of total protein concentration. Thus, under these conditions the rate of protein fibrillation is also constant (right). (C) When two tau isoforms with different aggregation propensities (but similar LLPS capacity) are recruited into droplets, the fibrillation rate of the rapidly aggregating species (e.g., 2N4R isoform) is gradually decreased in the presence of increasing proportions of the slowly aggregating species (e.g., 2N3R isoform). This is because, under given conditions, total concentration of all tau isoforms within the droplets remains constant, resulting in the “dilution” of the fast aggregating isoform within the condensed phase (for details see [39])
LLPS of tau in a cellular context
Apart from well-documented observations regarding the ability of tau to form liquid condensates in the test tube, there is growing evidence that tau LLPS occurs in cells. The first study in this regard used GFP-fused tau, finding that expression of this protein in primary cortical mouse neurons led to the formation of tau-rich cytoplasmic droplets that have a dynamic, liquid-like character [22]. The ability of tau to undergo LLPS in a cellular environment was confirmed in subsequent studies using a stable murine hippocampal neuronal cell line HT22 [60]. Interestingly, GFP-tau droplets in these cells appeared hollow, with much larger concentration of tau in the shell than within droplet interior [60]. Furthermore, the negatively charged N-terminal inserts were found to play a regulatory role in tau LLPS in HT22 cells, with 2N-tau undergoing more efficient condensation compared to the 1N- and 0N isoforms [60]. The latter observation is generally consistent with the results of experiments in the test tube, apparently supporting the notion that attractive electrostatic interactions between oppositely charged regions play an important role in tau LLPS [24].
The mechanisms and consequences of tau condensation in cells were further explored by an optogenetic approaches using tau fused to cryptochrome-2 protein, Cry2 [61]. This protein undergoes light-sensitive self-association upon exposure to blue light and has been used as a fusion partner in studying LLPS of other proteins [10]. Studies with different domains of tau fused to cry2 in neuroblastoma SH SY5Y cells revealed that the polypeptide corresponding to the Pro-rich domain alone could undergo extensive LLPS upon light activation, and that the transition from the condensed to dilute phase became increasingly irreversible upon multiple illumination cycles. By contrast, no LLPS was observed in similar experiments with the pseudo-repeat polypeptide, suggesting to the authors the importance of the proline-rich domain in this process. Furthermore, it was reported that introduction of phosphomimetic mutations in the Pro-rich polypeptide, or the addition of the N-terminal domain to this segment, abrogate light-induced LLPS. The latter finding is in apparent contrast to the conclusions reached in the above described study with more physiologically relevant naturally occurring tau isoforms containing different numbers of N-terminal inserts [60].
The picture is further complicated by another study using the same optogenetic approach, in which formation of light-induced cytoplasmic granules in SH-SY5Y cells by full-length tau (1N4R isoform) was found to be rare [37]. In contrast, robust formation of such granules was observed in cultured cortical neurons. While largely reversible upon short exposure to light, these granules became stable when induced by longer or repeated light exposure, and it was found that tau within these inclusions formed stable oligomers that were able to seed aggregation of the pseudo-repeat polypeptide in a tau K18 sensor cell line [37]. The authors also found that tau oligomerization leads to cytoplasmic translocation of the RNA-binding protein HNRNPA2B1 associated with N6-methyladenosine RNA (m6A), proposing that the oligomeric tau complex with HNRNPA2B1 and m6A regulates RNA translational stress response and promotes stress granules formation. This finding is of potential relevance to the pathogenic process in tauopathies, as the level of m6A-HNRNPA2B1 complex with oligomeric tau is elevated in AD brains [37].
Apart from HNRNPA2B1, hyperphosphorylated tau has been shown to co-localize in neuronal inclusions with several other RNA binding proteins that are markers for stress granules [62–65]. The most important of these appears to be T-cell intracellular antigen 1 (TIA1). Different aspects of tau-TIA1 interactions and the role of these interactions in neurodegenerative diseases have been explored in a series of studies [46, 62–64], revealing that the level of tau expression and pathogenic mutations regulate formation of tau- and TIA1-positive stress granules and suggesting that TIA1-tau interactions within the granules promotes tau misfolding and toxicity [63] This notion is consistent with the finding that reduced TIA1 expression provides neuroprotection and reduces tau pathology [64]. Importantly, this tau pathology-promoting effect of TIA1 appears to be related to its ability to facilitate formation of toxic tau oligomers, as observed in brains of P301S tau-expressing PS19 mice [64] and further confirmed in studies in vitro using purified proteins [46]. The observations that tau colocalizes with TIA1 and other stress granule marker proteins per se do not prove that these proteins are indeed localized within dynamic stress granules. Nevertheless, the notion that tau can be recruited to such granules is directly supported by the evidence for its incorporation into sodium arsenite-induced stress granules in HEK293T cells [66].
An interesting twist in studies on tau LLPS in cells was the recent finding that the uptake of extracellular tau aggregates derived from aged P301L tau mice led to condensation of tau on the nuclear envelope of cells expressing either P301L 0N4R tau or P301L/V377M K18 tau fragment [67]. These condensates were highly dynamic, indicating that they were formed through the LLPS mechanism. Importantly, tau condensation on nuclear envelope was found to trigger sequestration of nucleoporins from the nuclear pore complex, disruption of molecular trafficking across the nuclear envelope and, eventually, apoptotic cell death, suggesting an alternative mechanism for LLPS-mediated tau toxicity [67]. The involvement of nuclear protein condensates in tau toxicity is also suggested by the finding that tau aggregates are concentrated in nuclear speckles, altering the organization and dynamics of these membraneless organelles normally involved in pre-mRNA splicing [36]. These effects could contribute to the neurodegenerative mechanisms in AD and related disorders by causing alterations in nuclear RNA processing [36]. However, it is not yet clear whether tau LLPS per se is involved in this process.
Concluding remarks
Tau, a protein involved in several neurodegenerative diseases, has an intrinsic propensity to undergo LLPS, both on its own and in the presence of RNA and/or proteinaceous partners. This leads to the formation of liquid droplets in which protein concentration is greatly increased. The main driving force for both homotypic and heterotypic LLPS of tau appears to be electrostatic interactions that can be modulated by post-translational modifications, even though details of this regulatory mechanism are still poorly understood. One of the consequences of tau condensation within initially highly dynamic droplets is the acceleration of protein aggregation into amyloid fibrils, a process that can be regulated in a unique way by the presence of different tau isoforms with distinct intrinsic aggregation propensities. Even though studies on tau LLPS in neurons are still at early stages, these studies have clearly demonstrated the capacity of tau to undergo condensation in different cellular compartments (Fig. 3), identified several proteinaceous partners that co-localize with tau in cellular granules, and provided intriguing hints regarding potential mechanisms by which tau condensation could contribute to neurodegeneration. However, many fundamentally important questions regarding the role of tau LLPS in disease pathogenesis still remain unanswered (see Outstanding Questions). One such yet unexplored issue pertains to recent groundbreaking findings that different phenotypes of tauopathies such as Alzheimer’s disease, Pick’s disease or corticobasal degeneration are associated with brain accumulation of structurally distinct strains of tau filaments [68]. Could LLPS-induced compartmentalization of tau and/or compartment-specific cofactors facilitate tau polymerization into structurally distinct filaments? This is an intriguing possibility that needs to be explored. Furthermore, if membraneless organelles are indeed the site (or one of the sites) of tau aggregation, the question arises how tau filaments and/or oligomers could be released from these compartments to act as “seeds” that facilitate prion-like spread of tau pathology within brain.
Fig. 3. Illustration of different sites of tau condensation via LLPS in neurons.
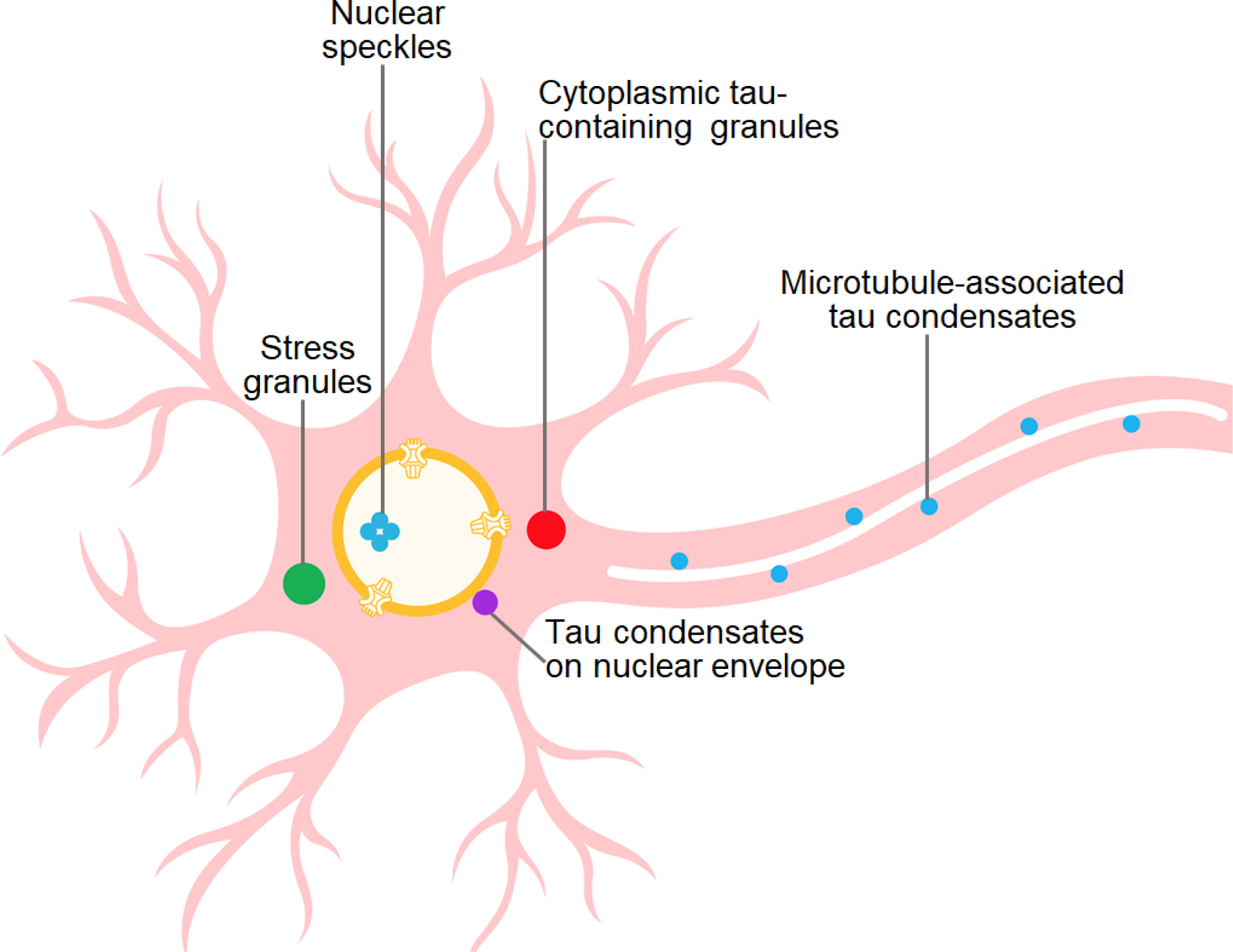
With regard to nuclear speckles, tau aggregates were reported to concentrate in these membraneless organelles [36]. It is not clear whether this is directly related to tau LLPS per se.
Studies on structural and functional properties of protein condensates in living cells present enormous technical challenges. While these challenges have led to rapid development of innovative methodologies and research strategies [69], most of these novel approaches are yet to be utilized in studies on cellular aspects of tau LLPS. For example, application of the emerging methods of proximity labeling, such as proximity-dependent biotin identification (BioID) [70], could potentially provide wealth of information regarding the dynamic network of interacting partners in tau-containing membraneless organelles. Furthermore, the use of powerful optogenetic approaches to interrogate tau-containing condensates has been so far limited to the Cry2-based OptoDroplet systems, the limitation of which is poorly understood nature of Cry2-Cry2 interactions. Expansion of these studies to emerging next-generation optogenetic systems, such as Corelets [71], should overcome these limitations, allowing better mechanistic understanding of tau condensation and its cellular consequences. Finally, additional insights into the structure and biophysical properties of tau-containing condensates will likely be provided by in situ cryo-electron tomography, a technique of an great potential for studying intracellular sub-structures [72].
Highlights.
Tau alone or in the presence of RNA has a high propensity to undergo LLPS, forming liquid droplets.
LLPS of full-length tau under physiologically relevant buffer conditions is largely driven by intermolecular electrostatic interactions between oppositely charged protein regions or between tau and RNA.
Phosphorylation and other posttranslational modifications appear to play a regulatory role in tau LLPS.
LLPS has a major impact on tau aggregation into amyloid fibrils. It also leads to unique regulatory mechanisms of fibrillation when multiple tau isoforms are present within the condensates.
Tau can undergo LLPS in neurons and this may contribute to normal biological functions of the protein as well as to tau pathology in neurodegenerative diseases.
Outstanding Questions.
What are the mechanisms by which specific post-translational modifications within different protein domains regulate tau LLPS and what are the physiological and pathological consequences of this regulation?
Can LLPS regulate tau phosphorylation by co-recruitment of specific kinases to the granules, especially those containing SH3 domains?
Studies in vitro indicate that LLPS of tau can promote microtubule formation. Does this effect contribute to microtubule formation in vivo? If it does, how do cells under normal physiological conditions protect themselves from an increased tendency of tau to aggregate within the condensates?
Emerging evidence indicates that tau LLPS in neurons can occur in several different compartments. Is tau a major driver of this process as opposed to being “passively” co-recruited to membraneless organelles formed by other proteins or protein/RNA complexes?
Cellular studies suggest several potential mechanisms by which LLPS could contribute to tau pathology. Are other mechanism also possible, and which of them plays a key role in the pathogenesis of neurodegenerative diseases?
What is the role of LLPS in prion-like propagation of structurally distinct strains of tau filaments?
Acknowledgements
We thank Benjamin Dumm for comments on the manuscript and Sofia Rybytska for help with the illustrations. This work was supported in part by National Institutes of Health grant RF1 AG061797.
Glossary
- Complex coacervation
a heterotypic LLPS of protein/RNA mixtures driven by attractive electrostatic interactions between protein’s positively charged Arg/Lys residues and negatively charged phosphate groups of RNA
- Crowding agents
polymers such as dextran or polyethylene glycol that are used to increase the effective protein concentration and, therefore, mimic the conditions of the macromolecules in the crowded cytoplasm
- Heterotypic LLPS
demixing of a two or multiple component system (e.g., several proteins, protein-RNA mixture)
- Homotypic LLPS
demixing of a single component system
- Saturation concentration
concentration of a given macromolecule at which it starts to undergo demixing into condensed and dilute phases
- 1,6-Hexanediol
an aliphatic alcohol that disrupts hydrophobic interactions. It is commonly used to probe the nature of interactions within liquid-like condensates
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ingram EM and Spillantini MG (2002) Tau gene mutations: dissecting the pathogenesis of FTDP-17. Trends Mol Med 8 (12), 555–62. [DOI] [PubMed] [Google Scholar]
- 2.Strang KH et al. (2019) MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab Invest 99 (7), 912–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandelkow EM and Mandelkow E (2012) Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med 2 (7), a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y and Mandelkow E (2016) Tau in physiology and pathology. Nat Rev Neurosci 17 (1), 5–21. [DOI] [PubMed] [Google Scholar]
- 5.Guo T et al. (2017) Roles of tau protein in health and disease. Acta Neuropathol 133 (5), 665–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goedert M et al. (2017) Propagation of Tau Aggregates and Neurodegeneration. Annu Rev Neurosci 40, 189–210. [DOI] [PubMed] [Google Scholar]
- 7.Guo JL and Lee VM (2014) Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 20 (2), 130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal K et al. (2016) Tau and neurodegenerative disease: the story so far. Nat Rev Neurol 12 (1), 15–27. [DOI] [PubMed] [Google Scholar]
- 9.Banani SF et al. (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18 (5), 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin Y and Brangwynne CP (2017) Liquid phase condensation in cell physiology and disease. Science 357 (6357). [DOI] [PubMed] [Google Scholar]
- 11.Uversky VN (2017) Protein intrinsic disorder-based liquid-liquid phase transitions in biological systems: Complex coacervates and membrane-less organelles. Adv Colloid Interface Sci 239, 97–114. [DOI] [PubMed] [Google Scholar]
- 12.Boeynaems S et al. (2018) Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol 28 (6), 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y et al. (2015) Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol Cell 60 (2), 208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel A et al. (2015) A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 162 (5), 1066–77. [DOI] [PubMed] [Google Scholar]
- 15.Murakami T et al. (2015) ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron 88 (4), 678–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elbaum-Garfinkle S (2019) Matter over mind: Liquid phase separation and neurodegeneration. J Biol Chem 294 (18), 7160–7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan VH and Fawzi NL (2019) Physiological, Pathological, and Targetable Membraneless Organelles in Neurons. Trends Neurosci 42 (10), 693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedelsky NB and Taylor JP (2019) Bridging biophysics and neurology: aberrant phase transitions in neurodegenerative disease. Nat Rev Neurol 15 (5), 272–286. [DOI] [PubMed] [Google Scholar]
- 19.Babinchak WM and Surewicz WK (2020) Liquid-Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation. J Mol Biol 432 (7), 1910–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zbinden A et al. (2020) Phase Separation and Neurodegenerative Diseases: A Disturbance in the Force. Dev Cell 55 (1), 45–68. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Vega A et al. (2017) Local Nucleation of Microtubule Bundles through Tubulin Concentration into a Condensed Tau Phase. Cell Rep 20 (10), 2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wegmann S et al. (2018) Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J 37 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreon JC et al. (2018) Acetylation Disfavors Tau Phase Separation. Int J Mol Sci 19 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyko S et al. (2019) Liquid-liquid phase separation of tau protein: The crucial role of electrostatic interactions. J Biol Chem 294 (29), 11054–11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanaan NM et al. (2020) Liquid-liquid phase separation induces pathogenic tau conformations in vitro. Nat Commun 11 (1), 2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y et al. (2021) Liquid-Liquid Phase Separation of Tau Driven by Hydrophobic Interaction Facilitates Fibrillization of Tau. J Mol Biol 433 (2), 166731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambadipudi S et al. (2017) Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nat Commun 8 (1), 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majumdar A et al. (2019) Liquid-Liquid Phase Separation Is Driven by Large-Scale Conformational Unwinding and Fluctuations of Intrinsically Disordered Protein Molecules. J Phys Chem Lett 10 (14), 3929–3936. [DOI] [PubMed] [Google Scholar]
- 29.Ambadipudi S et al. (2019) Residue-specific identification of phase separation hot spots of Alzheimer’s-related protein tau. Chem Sci 10 (26), 6503–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong X et al. (2021) Liquid-Liquid Phase Separation of Tau Protein Is Encoded at the Monomeric Level. J Phys Chem Lett 12 (10), 2576–2586. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X et al. (2017) RNA stores tau reversibly in complex coacervates. PLoS Biol 15 (7), e2002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y et al. (2019) Narrow equilibrium window for complex coacervation of tau and RNA under cellular conditions. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee PR et al. (2017) Reentrant Phase Transition Drives Dynamic Substructure Formation in Ribonucleoprotein Droplets. Angew Chem Int Ed Engl 56 (38), 11354–11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milin AN and Deniz AA (2018) Reentrant Phase Transitions and Non-Equilibrium Dynamics in Membraneless Organelles. Biochemistry 57 (17), 2470–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Najafi S et al. (2021) Liquid-liquid phase separation of Tau by self and complex coacervation. Protein Sci 30 (7), 1393–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lester E et al. (2021) Tau aggregates are RNA-protein assemblies that mislocalize multiple nuclear speckle components. Neuron 109 (10), 1675–1691 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang L et al. (2021) Interaction of tau with HNRNPA2B1 and N(6)-methyladenosine RNA mediates the progression of tauopathy. Mol Cell 81 (20), 4209–4227 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y et al. (2020) Electrostatically Driven Complex Coacervation and Amyloid Aggregation of Tau Are Independent Processes with Overlapping Conditions. ACS Chem Neurosci 11 (4), 615–627. [DOI] [PubMed] [Google Scholar]
- 39.Boyko S et al. (2020) Regulatory mechanisms of tau protein fibrillation under the conditions of liquid-liquid phase separation. Proc Natl Acad Sci U S A 117 (50), 31882–31890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goedert M et al. (1996) Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature 383 (6600), 550–3. [DOI] [PubMed] [Google Scholar]
- 41.Ivanov SM et al. (2020) Cellular polyamines condense hyperphosphorylated Tau, triggering Alzheimer’s disease. Sci Rep 10 (1), 10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh V et al. (2020) Zinc promotes liquid-liquid phase separation of tau protein. J Biol Chem 295 (18), 5850–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babinchak WM et al. (2020) Small molecules as potent biphasic modulators of protein liquid-liquid phase separation. Nat Commun 11 (1), 5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai B et al. (2021) Myricetin slows liquid-liquid phase separation of Tau and activates ATG5-dependent autophagy to suppress Tau toxicity. J Biol Chem 297 (4), 101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pradhan A et al. (2021) C1 Inhibits Liquid-Liquid Phase Separation and Oligomerization of Tau and Protects Neuroblastoma Cells against Toxic Tau Oligomers. ACS Chem Neurosci 12 (11), 1989–2002. [DOI] [PubMed] [Google Scholar]
- 46.Ash PEA et al. (2021) TIA1 potentiates tau phase separation and promotes generation of toxic oligomeric tau. Proc Natl Acad Sci U S A 118 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siegert A et al. (2021) Interplay between tau and alpha-synuclein liquid-liquid phase separation. Protein Sci 30 (7), 1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darling AL et al. (2021) Small heat shock protein 22 kDa can modulate the aggregation and liquid-liquid phase separation behavior of tau. Protein Sci 30 (7), 1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Religa D et al. (2006) Elevated cortical zinc in Alzheimer disease. Neurology 67 (1), 69–75. [DOI] [PubMed] [Google Scholar]
- 50.Portbury SD and Adlard PA (2017) Zinc Signal in Brain Diseases. Int J Mol Sci 18 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alberti S et al. (2019) Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 176 (3), 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savastano A et al. (2021) Disease-Associated Tau Phosphorylation Hinders Tubulin Assembly within Tau Condensates. Angew Chem Int Ed Engl 60 (2), 726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alquezar C et al. (2020) Tau Post-translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation. Front Neurol 11, 595532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ukmar-Godec T et al. (2019) Lysine/RNA-interactions drive and regulate biomolecular condensation. Nat Commun 10 (1), 2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baas PW and Qiang L (2019) Tau: It’s Not What You Think. Trends Cell Biol 29 (6), 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan R et al. (2019) Microtubules gate tau condensation to spatially regulate microtubule functions. Nat Cell Biol 21 (9), 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathieu C et al. (2020) Beyond aggregation: Pathological phase transitions in neurodegenerative disease. Science 370 (6512), 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flament S et al. (1991) Abnormal Tau proteins in progressive supranuclear palsy. Similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol 81 (6), 591–6. [DOI] [PubMed] [Google Scholar]
- 59.Delacourte A et al. (1996) Specific pathological Tau protein variants characterize Pick’s disease. J Neuropathol Exp Neurol 55 (2), 159–68. [DOI] [PubMed] [Google Scholar]
- 60.Wu C et al. (2021) Tau N-Terminal Inserts Regulate Tau Liquid-Liquid Phase Separation and Condensates Maturation in a Neuronal Cell Model. Int J Mol Sci 22 (18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X et al. (2020) The proline-rich domain promotes Tau liquid-liquid phase separation in cells. J Cell Biol 219 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanderweyde T et al. (2012) Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies. J Neurosci 32 (24), 8270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanderweyde T et al. (2016) Interaction of tau with the RNA-Binding Protein TIA1 Regulates tau Pathophysiology and Toxicity. Cell Rep 15 (7), 1455–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apicco DJ et al. (2018) Reducing the RNA binding protein TIA1 protects against tau-mediated neurodegeneration in vivo. Nat Neurosci 21 (1), 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolozin B and Ivanov P (2019) Stress granules and neurodegeneration. Nat Rev Neurosci 20 (11), 649–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brunello CA et al. (2016) Internalized Tau sensitizes cells to stress by promoting formation and stability of stress granules. Sci Rep 6, 30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang SG et al. (2021) Pathologic tau conformer ensembles induce dynamic, liquid-liquid phase separation events at the nuclear envelope. BMC Biol 19 (1), 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi Y et al. (2021) Structure-based classification of tauopathies. Nature 598 (7880), 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bracha D et al. (2019) Probing and engineering liquid-phase organelles. Nat Biotechnol 37 (12), 1435–1445. [DOI] [PubMed] [Google Scholar]
- 70.Roux KJ et al. (2012) A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol 196 (6), 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bracha D et al. (2018) Mapping Local and Global Liquid Phase Behavior in Living Cells Using Photo-Oligomerizable Seeds. Cell 175 (6), 1467–1480 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turk M and Baumeister W (2020) The promise and the challenges of cryo-electron tomography. FEBS Lett 594 (20), 3243–3261. [DOI] [PubMed] [Google Scholar]
- 73.Mukrasch MD et al. (2009) Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol 7 (2), e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barghorn S et al. (2000) Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry 39 (38), 11714–21. [DOI] [PubMed] [Google Scholar]
- 75.von Bergen M et al. (2001) Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. J Biol Chem 276 (51), 48165–74. [DOI] [PubMed] [Google Scholar]
- 76.Combs B and Gamblin TC (2012) FTDP-17 tau mutations induce distinct effects on aggregation and microtubule interactions. Biochemistry 51 (43), 8597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noble W et al. (2013) The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol 4, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wegmann S et al. (2021) A current view on Tau protein phosphorylation in Alzheimer’s disease. Curr Opin Neurobiol 69, 131–138. [DOI] [PubMed] [Google Scholar]
- 79.Mair W et al. (2016) FLEXITau: Quantifying Post-translational Modifications of Tau Protein in Vitro and in Human Disease. Anal Chem 88 (7), 3704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meszaros B et al. (2018) IUPred2A: context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res 46 (W1), W329–W337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomes E and Shorter J (2019) The molecular language of membraneless organelles. J Biol Chem 294 (18), 7115–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J et al. (2018) A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 174 (3), 688–699 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molliex A et al. (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163 (1), 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kroschwald S et al. (2015) Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. Elife 4, e06807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiang S et al. (2015) The LC Domain of hnRNPA2 Adopts Similar Conformations in Hydrogel Polymers, Liquid-like Droplets, and Nuclei. Cell 163 (4), 829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kato M et al. (2012) Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149 (4), 753–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kato M and McKnight SL (2017) Cross-beta Polymerization of Low Complexity Sequence Domains. Cold Spring Harb Perspect Biol 9 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brangwynne CP (2013) Phase transitions and size scaling of membrane-less organelles. J Cell Biol 203 (6), 875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jawerth L et al. (2020) Protein condensates as aging Maxwell fluids. Science 370 (6522), 1317–1323. [DOI] [PubMed] [Google Scholar]


