Abstract
Background
The most common type of primary liver cancer is hepatocellular carcinoma (HCC), and hepatitis B virus (HBV)-related HCC accounts for many HCC cases and has a high mortality rate. The goal of our study was to investigate the efficacy and safety of lenvatinib plus sintilimab therapy in real-world practice and identify factors affecting long-term prognosis.
Methods
A retrospective study was conducted with 139 consecutive patients with unresectable HCC treated with lenvatinib or lenvatinib plus sintilimab at the Fifth Medical Center of PLA General Hospital from June 2018 to June 2021. The 139 patients were divided into the control group (85 patients) and the combined treatment group (54 patients) according to the antitumour drugs used for treatment. Efficacy was determined using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 and the HCC-specific modified RECIST (mRECIST) for 139 patients who completed the 1st and second tumour assessments. Safety was evaluated in 60 patients in the combined treatment group and 90 patients in the control group using the Common Terminology Criteria for Adverse Events version 5.0.
Results
A total of 139 male Chinese patients (49.6% ≥ 55 years old) were included in the efficacy analysis. The median overall survival in the combined treatment group was 21.7 months, and the median progression-free survival was 11.3 months. According to the mRECIST criteria, the objective response rate was 38.9%, and the disease control rate was 92.6%. The median overall survival (mOS), median progression-free survival (mPFS), overall response rate (ORR) and disease control rate (DCR) in the lenvatinib monotherapy group were 12.8 months, 6.6 months, 24.7%, and 74.1%, respectively. Hypertension was the most common adverse event in both groups. Some immune-related adverse events, such as hypothyroidism (n = 5), elevated blood creatinine (n = 3), elevated cardiac enzymes (n = 1), elevated amylase (n = 1) and increased fasting glucose (n = 1), occurred only in the combined therapy group. Five patients in the lenvatinib monotherapy group and six patients in the lenvatinib plus sintilimab group discontinued therapy due to severe adverse events (AEs) (grade 3). No ≥ 4-grade AEs occurred in any patients.
Conclusion
The TKI lenvatinib combined with PD-1-targeted immunotherapy sintilimab is efficacious and safe in real-world practice and may lead to better long-term outcomes than lenvatinib alone.
Keywords: HBV-related hepatocellular carcinoma (HBV-HCC), Tyrosine kinase inhibitor (TKI), Immune checkpoint inhibitor (ICI), Lenvatinib, Sintilimab, Male
HBV-related hepatocellular carcinoma (HBV-HCC); Tyrosine kinase inhibitor (TKI); Immune checkpoint inhibitor (ICI); Lenvatinib; Sintilimab; Male.
1. Introduction
Globally, hepatocellular carcinoma (HCC) is the sixth most common cancer and the fourth leading cause of cancer-related death, according to 2020 global cancer statistics [1]. Unlike in developed countries, in China, the most common infectious disease is hepatitis B virus (HBV), ranking first in the number of people living with HBV globally [2]. Patients with HBV-related HCC are defined as those who are seropositive for hepatitis B surface antigen (HBsAg), are seronegative for anti-HCV antibodies and have no history of alcoholism [3]. China's HBV-related HCC incidence and mortality rates are higher than the world average [4, 5]. HCC associated with HBV is heterogeneous, with a wide range of clinical outcomes. In terms of the clinical characteristics, and in addition to incidence and mortality rates that outnumber those in America, Europe and Japan, HCC has several different features in China [6, 7]. They are mainly manifested in the following aspects: (1) In most instances, HCC is diagnosed at an advanced disease stage on initial examination, which indicates a trend toward younger onset. (2) Chronic HBV infection is a major risk factor for HCC, and HBV infection is believed to contribute to 80% of HCC cases. (3) In Europe and North America, hepatitis C and excessive alcohol consumption are the leading causes of HCC. Male Chinese patients typically experience a greater tumour burden (number and size of tumours) and a much more complicated situation regarding clinical features, such as having more macrovascular invasion. Surgical resection represents an effective protocol for treating liver tumours [8]. However, most patients miss the chance to undergo surgery due to late diagnosis and limited treatment options. Therefore, systemic treatments, including locoregional therapies (LRTs), molecularly targeted therapy and immunotherapy, are recommended for patients at an advanced stage [9]. The only VEGFR inhibitor available for the first-line treatment of advanced HCC between 2008 and 2017 was sorafenib [10]. Despite the improved progression-free survival achieved by lenvatinib, there was no evident difference in the overall survival compared to that associated with sorafenib [11]. Recent advancements in molecularly targeted therapy and immunotherapy have yielded remarkable improvements in liver cancer outcomes. The clinical trial of CheckMate 459 demonstrated that nivolumab may be considered a therapeutic option for patients who are not responding to tyrosine kinase inhibitors or antiangiogenic drugs [12]. The results of early clinical trials indicate that lenvatinib plus pembrolizumab could be a promising treatment option [13, 14]. Sintilimab, developed by Innovent Biologics and Eli-Lilly, is a fully human IgG4 monoclonal antibody similar to pembrolizumab [15]. The National Medical Products Administration of China has approved its use in the treatment of several advanced tumours, including HCC [16]. Even with increased options for extending overall survival, it is still necessary to develop new therapeutic models for HCC that cannot be resected. Whether the aetiology of HCC and clinical characteristics or interindividual variations in response to targeted therapy and/or immunotherapy in Chinese patients are different from those in American, European and Japanese populations remains to be explored [6]. As described by Professor Rizzo, in the past, no single biomarker has been able to predict which HCC patients are likely to benefit from immunotherapy; today, each biomarker and combinations of biomarkers can indicate which patients are likely to benefit from immunotherapy [17]. Despite this, it is important to note that there are a number of treatment problems for liver cancer that urgently need to be resolved, including the need for better and less toxic therapies that have fewer side effects [18]. Hence, we aimed to determine whether lenvatinib plus sintilimab-dominated treatment would be effective and safe in Chinese patients with unresectable HBV-related HCC and to identify prognostic factors affecting the long-term prognosis.
2. Patients and methods
2.1. Study design and patients
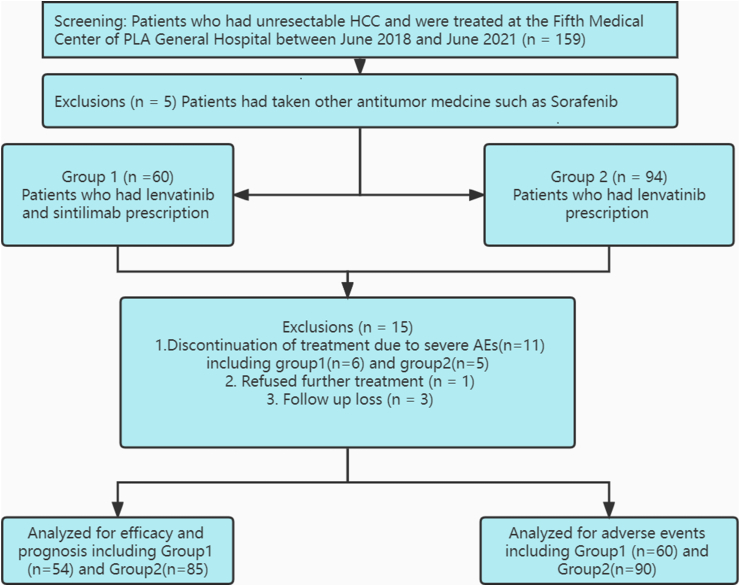
A total of 159 eligible patients (≥18 years) with advanced HBV-related HCC were enrolled in this hospital-based retrospective study and treated at the Fifth Medical Center of PLA General Hospital from June 2018 to June 2021. Some of these patients were excluded for the following reasons: they had received previous therapy, such as the antitumour drug sorafenib (n = 5), had discontinued treatment before the 1st tumour response assessment due to severe adverse events (AEs) (n = 11), had refused further therapy (n = 1), or were lost to follow-up (n = 3). Finally, 54 patients were divided into the combined treatment group (lenvatinib plus sintilimab, that is, group 1), and 85 patients were included in the control group (lenvatinib monotherapy, that is, group 2), as demonstrated in Figure 1. Other key inclusion criteria were as follows: (1) an ECOG scale performance score of 0–2; (2) liver function of Child–Pugh class A or B; (3) Barcelona Clinic Liver Cancer (BCLC) stages B or C; (4) an estimated life expectancy over 3 months; and (5) as defined by the Modified Response Evaluation Criteria in Solid Tumors (mRECIST), at least one measurable tumour lesion must exist. The exclusion criteria included (1) the combination of other primary malignancies; (2) the combination of other organ dysfunction, such as acute myocardial infarction, malignant hypertension, heart failure, or acute renal failure; (3) end-stage HCC; (4) the presence of other known infectious diseases; and (5) incomplete data.
Figure 1.
Patient flow. HCC, hepatocellular carcinoma.
2.2. Efficacy and safety assessments
An assessment of treatment response was scheduled at weeks 4–8 using magnetic resonance imaging (MRI) or dynamic computed tomography (CT). The imaging information was evaluated by 2 independent and experienced imaging experts.
In addition to the objective response rate (ORR), the disease control rate (DCR) of solid tumours was assessed according to the Response Evaluation Criteria in Solid Tumors, version 1 (RECIST 1.1) and HCC-specific modified RECIST (mRECIST). During the course of the study, vital signs and clinical laboratory results, including blood cell content, routine urinalysis, liver and kidney function, blood electrolytes, coagulation function and tumour markers such as alpha-fetoprotein, AFP or gamma-glutamyl transpeptidase, and GGT, were continuously evaluated for safety. Furthermore, a comprehensive evaluation of the incidence and severity of adverse events was undertaken during the antitumour procedure using the National Cancer Institute Common Terminology criteria for adverse events version 5.0 [19].
2.3. Lenvatinib and sintilimab treatment
Lenvatinib was administered orally at a starting dose of 12 mg/day for patients with ≥60 kg body weight or 8 mg/day for patients with <60 kg body weight in the lenvatinib monotherapy group [20]. The combined treatment group received the same doses of lenvatinib as the lenvatinib monotherapy group combined with sintilimab at a fixed dose of 200 mg every 3 weeks. All patients continued treatment until disease progression, unacceptable toxicity, death, or discontinuation for any reason.
2.4. Primary and secondary endpoints
Overall survival (OS) and progression-free survival (PFS) were the primary endpoints, and ORR, DCR, and adverse events (AEs) were the secondary endpoints.
2.5. Evaluation of antitumour responses
The antitumour response was assessed using RECIST 1.1 and mRECIST. In the fourth week of treatment, the first on-study radiographic examination was conducted, and subsequent examinations were taken every four weeks until the disease progressed. A confirmation of complete or partial responses were made within four weeks of the initial response. As patients experienced disease progression or stopped the treatment, their overall survival was monitored every 30 days until they died, lost contact, or the study ended.
2.6. Statistical analyses
In this study, continuous variables were converted into categorical variables. For categorical variables, the frequencies (%) are shown, and Fisher's exact or chi-square test was used to compare them. SPSS v26.0 (IBM Corp.) was used to perform statistical analyses, and survival curves were generated with GraphPad Prism v9.0 (GraphPad Software, Inc.). The PFS and OS were all estimated by the Kaplan–Meier method. Prognostic factors were first evaluated using univariate Cox regression analysis. Multivariate Cox regression analysis was used to evaluate the significant factors (p < 0.2) identified in the univariate Cox regression analysis. Descriptive statistics were used to describe the AE outcomes.
3. Results
3.1. Patient characteristics and laboratory findings according to baseline data
Our retrospective analysis included 139 patients with unresectable HBV-related HCC between June 2018 and June 2021. The baseline patient characteristics are listed in Table 1. All patients were male. Age was stratified as under 55 and over 55 years. There were 24 and 45 patients ≥55 years old in the treatment and control groups, respectively. A total of 66.7% of patients in the treatment group and 80% in the control group had Child–Pugh class A liver function reserve. A total of 16/54 (29.60%) and 16/85 (18.80%) patients were at BCLC stages B and C, respectively. A substantial proportion of these HBV-related HCC patients received locoregional therapies (LRTs), such as argon-helium cryosurgical treatment, ultrasound-guided microwave ablation of liver cancer, and hepatic artery interventional embolization treatment, within 1 month before or after taking antitumour drugs. The remaining 9 patients in the combined treatment group and 11 patients in the control group did not receive any local or regional therapies, as depicted in Table 1. In terms of age, ECOG-PS score, Child–Pugh classification, BCLC stage, history of hypertension and diabetes, and LRT use, there was no significant difference between the two groups. The baseline laboratory results of the two groups did not differ significantly, as shown in Table 2.
Table 1.
Baseline data of 139 HBV-related HCC patients.
| Item | Group 1 (n = 54) | Group 2 (n = 85) | χ2 | P value |
|---|---|---|---|---|
| Age (≥55) | 24/54 (44.40%) | 45/85 (52.90%) | 0.954 | 0.329 |
| Child-Pugh Score(A) | 36/54 (66.70%) | 68/85 (80.00%) | 3.116 | 0.078 |
| PS Score (≤1’) | 41/54 (75.90%) | 72/85 (84.70%) | 1.674 | 0.196 |
| History of hypertension | 9/54 (16.70%) | 19/85 (22.40%) | 0.664 | 0.415 |
| History of diabetes | 4/54 (7.40%) | 14/85 (16.50%) | 2.406 | 0.121 |
| Ascites | 46/54 (85.20%) | 73/85 (84.90%) | 0.130 | 0.909 |
| Loco-regional therapies | ||||
| Argon–Helium cryosurgical | 22/54 (40.70%) | 37/85 (43.50%) | 0.484 | 0.922 |
| microwave ablation | 9/54 (16.70%) | 16/85 (18.80%) | ||
| interventional therapy | 14/54 (25.90%) | 21/85 (24.70%) | ||
| none | 9/54 (16.70%) | 11/85 (12.90%) | ||
| BCLC Stage (B) | 40/54 (74.10%) | 66/85 (77.60%) | 0.233 | 0.620 |
Group1, lenvatinib plus sintilimab treatment group; Group2, lenvatinib monotherapy group.
Hepatocellular Carcinoma (HCC); Performance status (PS); Barcelona Clinic Liver Cancer (BCLC); Loco-regional therapies (LRT).
Table 2.
Laboratory data of 139 patients with HBV-related HCC at baseline.
| Group 1 (n = 54) | Group 2 (n = 85) | χ2 | P value | |
|---|---|---|---|---|
| Albumin | ||||
| (<35 g/L) | 22/54 (40.70%) | 33/85 (38.80%) | 0.051 | 0.822 |
| (≥35 g/L) | 32/54 (59.30%) | 52/85 (61.20%) | ||
| TBIL | ||||
| (<20.5umol/L) | 20/54 (37.00%) | 23/85 (27.00%) | 1.539 | 0.215 |
| (≥20.5umol/L) | 34/54 (63.00%) | 62/85 (73.00%) | ||
| PTA | ||||
| (<75%) | 29/54 (53.70%) | 33/85 (38.80%) | 2.959 | 0.085 |
| (≥75%) | 25/54 (46.30%) | 52/85 (61.20%) | ||
| AFP | ||||
| (<400 ng/ml) | 27/54 (50.00%) | 36/85 (42.40%) | 0.779 | 0.337 |
| (≥400 ng/ml) | 27/54 (50.00%) | 49/85 (57.60%) | ||
| GGT | ||||
| (<33 U/L) | 51/54 (94.40%) | 72/85 (84.70%) | 3.075 | 0.080 |
| (≥33 U/L) | 3/54 (5.60%) | 13/85 (15.30%) | ||
| PLT | ||||
| (<100∗109/L) | 15/54 (27.80%) | 21/85 (24.70%) | 0.162 | 0.687 |
| (≥100∗109/L) | 39/54 (72.20%) | 64/85 (75.30%) | ||
Group1, lenvatinib plus sintilimab treatment group; Group2, lenvatinib monotherapy group.
Total bilirubin (TBIL); Prothrombin time activity (PTA); Alpha fetoprotein (AFP); Gamma-glutamyl transpeptidase (GGT); Platelets (PLT).
3.2. Effectiveness and safety assessment
3.2.1. Effectiveness
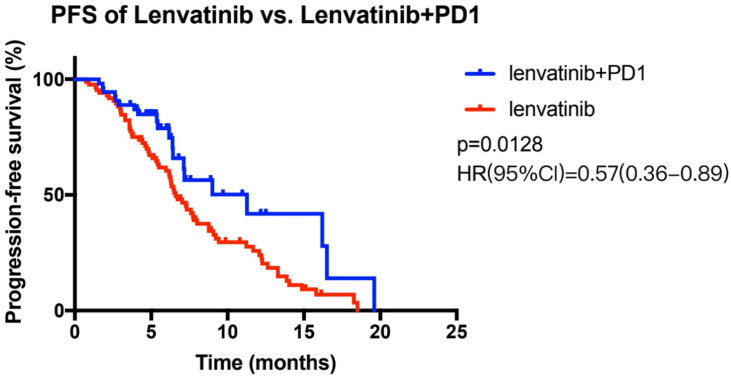
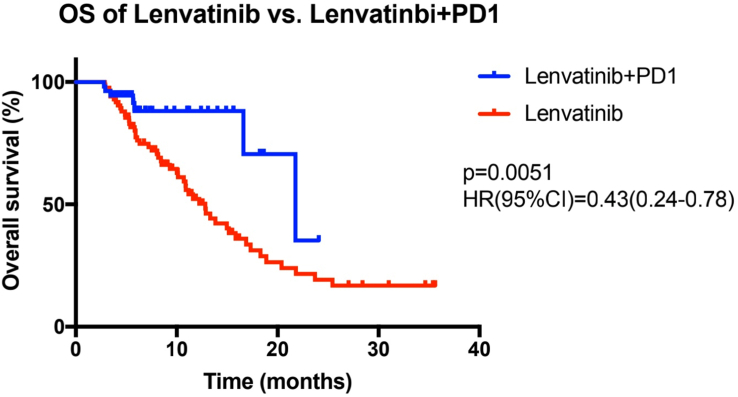
In this study, from baseline to the end of the follow-up, the median PFS for the combined therapy group and the lenvatinib monotherapy group was 11.3 and 6.6 months, respectively. Figure 2 shows a statistically significant difference between these two groups (p = 0.0128, HR = 0.57 (95% CI: 0.36–0.89)). Furthermore, the combined therapy group had a median OS of 21.7 months, while the OS of the lenvatinib monotherapy group was 12.8 months (p = 0.0051, HR = 0.43 (95% CI: 0.24–0.78)) (Figure 3). Regarding the secondary assessment criteria, the ORR and DCR of the combined therapy group were 33.3% and 87.0%, respectively, according to the RECIST 1.1 criteria. The ORR and DCR in the control group and the lenvatinib monotherapy group were 24.7% and 74.1%, respectively. There was no statistically significant difference between the combined therapy group and the lenvatinib monotherapy group in ORR or DCR (p > 0.05) (Table 3). However, the ORR and DCR of the combined therapy group were 38.9% and 92.6%, respectively, according to the mRECIST criteria. There was a marked difference between the two groups in terms of the DCR (92.6% vs. 74.1%, p = 0.006) (Table 4).
Figure 2.
The survival curves of all patients with HBV-related advanced hepatocellular carcinoma treated with lenvatinib plus sintilimab and with lenvatinib alone. Cumulative progression-free survival curves, in which the blue lines show the patients in the combined therapy group and the red lines show the patients in the lenvatinib monotherapy group.
Figure 3.
The survival curve of all patients with advanced HCC related to HBV who received lenvatinib and sintilimab treatment and lenvatinib monotherapy. Cumulative overall survival curves, in which the blue lines show the patients in the combined therapy group and the red lines show the patients in the lenvatinib monotherapy group.
Table 3.
Clinical response according to RECIST1.1
| Group1 (n = 54) | Group2 (n = 85) | χ2 | P value | |
|---|---|---|---|---|
| ORR | 18/54 (33.30%) | 21/85 (24.70%) | 1.110 | 0.292 |
| DCR | 47/54 (87.00%) | 63/85 (74.10%) | 0.089 | 0.765 |
Group1, lenvatinib plus sintilimab treatment group; Group2, lenvatinib monotherapy group.
Objective response rate (ORR); Disease Control Rate (DCR).
Table 4.
Clinical response according to mREFLECT eligibility criteria.
| Group1 (n = 54) | Group2 (n = 85) | χ2 | P value | |
|---|---|---|---|---|
| ORR | 21/54 (38.90%) | 21/85 (24.70%) | 3.150 | 0.076 |
| DCR | 50/54 (92.60%) | 63/85 (74.10%) | 7.412 | 0.006 |
Group1, lenvatinib plus sintilimab treatment group; Group2, lenvatinib monotherapy group.
Objective response rate (ORR); Disease Control Rate (DCR).
3.2.2. Safety assessment
Part 1. Adverse events (AEs) in the lenvatinib monotherapy group. As depicted in Table 5, a total of 289 AEs occurred in 90 patients. Seven grade 3 adverse events occurred in the lenvatinib monotherapy group. The overlapping counts of AEs are worth noting. The following AEs were identified as having the highest frequency of any grade: hypertension, asthenia, diarrhoea, hand-foot skin reaction (HFSR), abdominal pain, nausea and loss of appetite. Grade 3 adverse reactions, including 4 cases of hypertension, 1 case of diarrhoea, 1 case of HFSR and 1 case of leukopenia, were observed in 5 cases, with one having concomitant hypertension/diarrhoea. Part 2. Adverse events in the combined therapy group. Similarly, the most common AE was hypertension (45%) among the 60 HBV-related HCC patients. Asthenia (n = 19), low-grade fever (n = 16), and diarrhoea (n = 15) were also common AEs. However, some AEs, including hypothyroidism (n = 5), elevated blood creatinine (n = 3), elevated cardiac enzymes (n = 1), elevated amylase (n = 1) and increased fasting glucose (n = 1), occurred only in the combined therapy group (lenvatinib plus sintilimab) (Table 6). Six patients had grade 3 adverse effects, including hypertension (n = 2), diarrhoea (n = 3) and leukopenia (n = 1). Most AEs in this study were reversible or manageable by dose modification and/or symptomatic treatment rather than treatment discontinuation. However, 5 patients in the lenvatinib monotherapy group and 6 patients in the lenvatinib plus sintilimab group discontinued therapy due to severe AEs (grade 3). No adverse reactions (grade≥4) occurred in any patients.
Table 5.
Adverse events of lenvatinib monotherapy (n = 90).
| AEs∗ | Not happened | Grade 1 | Grade 2 | Grade 3 | Grade 4 | happened | Any grade |
|---|---|---|---|---|---|---|---|
| hypertension | 56 (62.22) | 18 | 12 | 4 | 0 | 34 (37.78) | 4 (4.44) |
| HFSR | 73 (81.11) | 11 | 5 | 1 | 0 | 17 (18.89) | 1 (1.11) |
| Asthenia | 60 (66.67) | 17 | 13 | 0 | 0 | 30 (33.33) | 0 (0.00) |
| diarrhoea | 66 (73.33) | 11 | 12 | 1 | 0 | 24 (26.67) | 1 (1.11) |
| Constipation | 77 (85.56) | 10 | 3 | 0 | 0 | 13 (14.44) | 0 (0.00) |
| Abdominal pain | 69 (76.67) | 15 | 6 | 0 | 0 | 21 (23.33) | 0 (0.00) |
| Bloating | 72 (80.00) | 11 | 7 | 0 | 0 | 18 (20.00) | 0 (0.00) |
| Nausea | 69 (76.67) | 17 | 4 | 0 | 0 | 21 (23.33) | 0 (0.00) |
| Vomiting | 75 (83.33) | 8 | 7 | 0 | 0 | 15 (16.67) | 0 (0.00) |
| Loss of appetite | 68 (75.56) | 14 | 8 | 0 | 0 | 22 (24.44) | 0 (0.00) |
| OM | 85 (94.44) | 5 | 0 | 0 | 0 | 5 (5.56) | 0 (0.00) |
| Joint pain | 74 (82.22) | 15 | 1 | 0 | 0 | 16 (17.78) | 0 (0.00) |
| peripheral edema | 82 (91.11) | 8 | 0 | 0 | 0 | 8 (8.89) | 0 (0.00) |
| Proteinuria | 67 (74.44) | 18 | 5 | 0 | 0 | 23 (25.56) | 0 (0.00) |
| Leukopenia | 80 (88.89) | 7 | 2 | 1 | 0 | 10 (11.11) | 1 (1.11) |
| Thrombocytopenia | 83 (92.22) | 6 | 1 | 0 | 0 | 7 (7.78) | 0 (0.00) |
Data are presented as n (%); ∗ counting overlapping; oral mucositis (OM); hand-foot skin reaction (HFSR).
Table 6.
Adverse events of lenvatinib plus sintilimab therapy (n = 60).
| AEs∗ | Not happened | Grade 1 | Grade 2 | Grade 3 | Grade 4 | happened | Any grade |
|---|---|---|---|---|---|---|---|
| hypertension | 33 (55.00) | 18 | 7 | 2 | 0 | 27 (45.00) | 2 (3.33) |
| HFSR | 54 (90.00) | 5 | 1 | 0 | 0 | 6 (10.00) | 0 (0.00) |
| Asthenia | 41 (68.33) | 16 | 3 | 0 | 0 | 19 (31.67) | 0 (0.00) |
| Fever | 44 (73.33) | 13 | 3 | 0 | 0 | 16 (26.67) | 0 (0.00) |
| diarrhoea | 45 (75.00) | 9 | 3 | 3 | 0 | 15 (25.00) | 3 (5.00) |
| Bloating | 50 (83.33) | 6 | 4 | 0 | 0 | 10 (16.67) | 0 (0.00) |
| Nausea | 50 (83.33) | 6 | 4 | 0 | 0 | 10 (16.67) | 0 (0.00) |
| Loss of appetite | 52 (86.77) | 8 | 0 | 0 | 0 | 8 (13.33) | 0 (0.00) |
| Joint pain | 52 (86.77) | 7 | 1 | 0 | 0 | 8 (13.33) | 0 (0.00) |
| Proteinuria | 53 (88.33) | 5 | 2 | 0 | 0 | 7 (11.67) | 0 (0.00) |
| Hypothyroidism | 55 (91.77) | 5 | 0 | 0 | 0 | 5 (8.33) | 0 (0.00) |
| Leukopenia | 54 (90.00) | 4 | 1 | 1 | 0 | 6 (10.00) | 1 (1.67) |
| Thrombocytopenia | 57 (95.00) | 3 | 0 | 0 | 0 | 3 (5.00) | 0 (0.00) |
| Elevated creatinine | 57 (95.00) | 3 | 0 | 0 | 0 | 3 (5.00) | 0 (0.00) |
| Elevated serum amylase | 59 (98.33) | 1 | 0 | 0 | 0 | 1 (1.67) | 0 (0.00) |
| Elevated cardiac enzyme | 59 (98.33) | 1 | 0 | 0 | 0 | 1 (1.67) | 0 (0.00) |
| elevated blood glucose | 59 (98.33) | 1 | 0 | 0 | 0 | 1 (1.67) | 0 (0.00) |
Data are presented as n (%); ∗ counting overlapping; oral mucositis (OM); hand-foot skin reaction (HFSR).
3.3. Prognostic factors for overall survival of HBV-related HCC patients
The BCLC stage (HR = 0.37, 95% CI = 0.21–0.64, p = 0.003) and lenvatinib plus sintilimab treatment (HR = 0.35, 95% CI = 0.20–0.62, p = 0.005) were significantly associated with OS in the univariate analysis of all 139 patients. Factors with a p value < 0.20 were alpha-fetoprotein (AFP) (HR = 1.48, 95% CI = 0.88–2.51, p = 0.133) and albumin (HR = 1.45, 95% CI = 0.86–2.50, p = 0.156). The BCLC stage and sintilimab plus lenvatinib treatment remained significant independent predictors of survival in the multivariate analysis (HR = 2.73, 95% CI = 1.32–5.61, p = 0.006; HR = 0.35, 95% CI = 0.16–0.78, p = 0.01, respectively) (Table 7).
Table 7.
Multivariable Cox regression analysis for overall survival (n = 139).
| Variables | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Lenvatinib plus sintilimab treatment | 0.35 (0.20–0.62) | 0.005 | 0.35 (0.16–0.78) | 0.010 |
| Child-pugh class (A) | 0.74 (0.40–1.37) | 0.297 | ||
| PS score (≤1′) | 0.88 (0.43–1.79) | 0.708 | ||
| AFP (≥400 μg/L) | 1.48 (0.88–2.51) | 0.133 | 2.96 (1.34–6.58) | 0.118 |
| Albumin (<35 g/L) | 1.45 (0.86–2.50) | 0.156 | 2.96 (1.34–6.58) | 0.187 |
| TBIL (<20.5 μmol/L) | 0.73 (0.41–1.30) | 0.286 | ||
| GGT (≥33 U/L) | 1.50 (0.68–3.49) | 0.304 | ||
| PTA (<75%) | 1.20 (0.72–2.12) | 0.423 | ||
| PLT (<100∗109/L) | 0.88 (0.50–1.54) | 0.650 | ||
| BCLC stage (C) | 0.37 (0.21–0.64) | 0.003 | 2.73 (1.32–5.61) | 0.006 |
Hazard ratio (HR); Confidence interval (CI); Performance status (PS); Barcelona Clinic Liver Cancer (BCLC); Total bilirubin (TBIL); Prothrombin time activity (PTA); Alpha fetoprotein (AFP); Gamma-glutamyl transpeptidase (GGT); Platelets (PLT).
4. Discussion
In this retrospective study, lenvatinib plus sintilimab and lenvatinib monotherapy for patients with HBV-related advanced HCC were compared in terms of efficacy and safety. According to the mRECIST criteria, the DCR in the lenvatinib plus sintilimab group was significantly higher than that in the lenvatinib group. Additionally, both PFS and OS were significantly longer in the lenvatinib plus sintilimab group than in the lenvatinib group.
In China, more than 90% of primary liver cancers are HCC, with a high incidence and mortality because of HBV infection [21]. Molecular targeted therapies for HCC with antiangiogenic effects have been the first-line treatment for several years [22]. Antiangiogenic drugs, such as sorafenib and lenvatinib, remain the backbone of systemic therapy for advanced HCC, but their effectiveness is limited [23]. In a recent study, it was found that combining lenvatinib and pembrolizumab provides highly promising results for advanced HCC patients, with an ORR of 41% and a DCR of 56%, respectively [24]. In this study, lenvatinib plus sintilimab proved to be a safe and efficacious therapy for unresectable HCC associated with HBV. Specifically, it was found that patients who received lenvatinib plus sintilimab as the first-line antitumour drugs showed better DCR, OS and PFS than patients who took lenvatinib monotherapy (DCR: 92.6% vs. 74.1% p value 0.045, median OS: 21.7 months vs. 12.8 months p = 0.0051, median PFS: 11.3 months vs. 6.6 months p = 0.0128), although no significant differences in ORR (p > 0.05) existed between the groups. The possible reasons for the differences in efficacy between the lenvatinib plus sintilimab group and the lenvatinib monotherapy group are as follows: (1) First, previous studies suggested that lenvatinib could improve the tumour microenvironment and thus act in synergy with certain immune checkpoint inhibitors [25, 26]. In our study, lenvatinib demonstrated a synergistic effect when used in combination with sintilimab. By blocking FGFR4, a recent study showed that lenvatinib decreased tumour PD-L1 levels and Treg differentiation and improved anti-PD-1 efficacy [27]. (2) Second, sintilimab, which is similar to pembrolizumab, has a higher binding affinity towards PD-1 on the surface of T cells than pembrolizumab. This could be another reason why the PFS and OS were significantly longer in our combination therapy group. (3) A large portion of the patients received a certain LRT within 1 month before or after taking the antitumour drugs, although no pronounced difference was found between the lenvatinib plus sintilimab group and the lenvatinib monotherapy group. Some studies have demonstrated that LRT reduces the local tumour load and stimulates the immune response by releasing tumour-associated antigens [28]. LRT may prolong OS and PFS in HBV-related HCC patients.
The findings of this study are in line with those results of combined targeted and immune therapy in previous studies [29, 30]. It is worth mentioning that a study indicated that Chinese advanced HCC patients have a better response to drugs than those from other countries, which was described in a phase 3, multicentre, noninferiority trial published in The Lancet [10]. Moreover, a short-term prognostic study from China revealed that TKI (lenvatinib) plus anti-PD-1 (camrelizumab) as postprogression treatment is both effective and safe [31]. In this study, a substantial proportion of patients in the combined therapy group adhered to the regimen of lenvatinib plus sintilimab after tumour progression. This may be another reason why the OS and PFS in the combined therapy group were longer than those in the lenvatinib monotherapy group.
In regard to the AEs in our study, hypertension remained the most commonly reported adverse reaction, followed by hand-foot syndrome and diarrhoea in both groups. Moreover, another common adverse reaction recorded in the combined group was asthenia, which may be induced by PD-1 (sintilimab). Similar findings have been reported previously [32, 33]. Moreover, it is worth noting that 4 patients in the lenvatinib monotherapy group and 2 patients in the combined therapy group discontinued lenvatinib treatment due to grade 3 hypertension. There is no doubt that hypertension is the most common AE of TKI treatment. This phenomenon explains why tumour vascular epithelial cells were damaged, leading to upregulation of nitric oxide synthase and decreasing nitric oxide production, which ultimately leads to hypertension [34]. The results of a multicentre retrospective study showed that hypertension was significantly associated with better survival rates and was the earliest indicator of a better prognosis [35]. Compared with the monotherapy group, the combined therapy group had a higher incidence of hypertension. The most common reason for dose reductions and treatment halts is a subjective condition referred to as systemic fatigue. Approximately one-third of patients experienced fatigue in the lenvatinib monotherapy group in our study. Some differences in AEs were seen in the combined therapy group compared to the lenvatinib monotherapy group. Specifically, hypothyroidism (grade 1) occurred in 5 patients. Increased creatinine, fasting blood glucose, serum amylase and myocardial enzymes were found in some cases. It was judged that these adverse reactions might be caused by PD-1 (sintilimab). Similar results were reported in other studies [36, 37]. Lenvatinib has also been associated with thyroid dysfunction, which is a frequent side effect of TKI treatment [38]. In our study, 5 patients developed hypothyroidism in the combined therapy group. In another group, however, there was no hypothyroidism. The relevance of thyroid dysfunction to clinical outcome remains controversial. Shomura and colleagues prospectively enrolled and treated 46 patients with advanced hepatocellular carcinoma and observed that hypothyroidism grade 2/3 was associated with better outcomes [39]. In contrast, a retrospective study showed that thyroid dysfunction was an independent factor associated with shorter PFS [40]. In previous studies of a mouse model of HCC, lenvatinib altered tumour blood vessels and normal organ blood vessels [41, 42]. The causes of hypothyroidism include the direct destructive effects of chemotherapeutic agents on the thyroid gland and reduced blood flow caused by the inhibitory effect of VEGF binding to thyroid cells [43].
In terms of prognostic features, in the multivariate regression analysis, lenvatinib plus sintilimab therapy and BCLC stage were independent factors predicting prognosis in the patients in our study. On the one hand, the findings confirmed the effectiveness of lenvatinib plus sintilimab therapy in addition to a longer OS and PFS; on the other hand, the findings demonstrated that those patients in BCLC stage B with better liver function reserve could have longer survival than those patients in BCLC stage C. Lenvatinib plus sintilimab is more effective than lenvatinib alone in treating HBV-related advanced HCC in Chinese patients, according to these results. Many studies have shown that the Child–Pugh score of HCC patients is a useful prognostic factor affecting prognosis [44, 45]. However, a similar result was not found in this study. There are a couple of possible reasons. First, the Child–Pugh score is not adequate to provide sufficient objectivity for evaluating liver function since the presence of ascites is accounted for in the Child–Pugh score. Moreover, there are certainly overlaps between ascites and albumin, which are associated with osmotic pressure. It should be noted that the ECOG-PS score was not a prognostic factor in this study. Clinical practitioners should be aware that the ECOG-PS score has a certain level of subjectivity. Calculation of the ECOG-PS score was based on the degree of decline in physical activity associated with the tumour load, not other causes.
5. Highlights and limitations
This retrospective study has several strengths compared to previous studies. First, all the subjects included in the study were male patients with HBV-related HCC, which reflects the peculiarity of the current liver tumour situation in China and is significant for clinical practice. Second, the follow-up period was longer than those of other studies. Most importantly, as an important complement to the therapeutic regimens of HCC, the data of this real-world study could fill the gap between clinical guidelines and real-world practice. Several limitations exist, however. First, the sample size of this retrospective single-centre study was small; therefore, selection bias could not be avoided. Moreover, female patients were not included in our study; therefore, efficacy and safety need to be studied in females in the future. Some data on post-line treatment for patients with liver cancer were not obtained during the follow-up period. Thus, the effects of second-line treatment on patients with HCC could not be determined. We did not assess the impact of anti-HBV treatment on antitumour therapy since the data on HBV DNA load, serum HBsAg level and HBeAg status were not collected prospectively. The results of this study need to be confirmed by further prospective studies. Nevertheless, we found that the OS and PFS of patients who had taken lenvatinib plus sintilimab were longer than those of patients who had received lenvatinib monotherapy as first-line treatment. This finding would be useful in informing the choice of antitumour drugs among Chinese male HBV-related HCC patients in real-world clinical practice.
6. Conclusion
Treatment with lenvatinib plus sintilimab is an effective and safe first-line treatment option for Chinese men with advanced HCC related to HBV and may result in better long-term outcomes than lenvatinib alone.
7. Ethics approval and consent to participate
This study was approved by the director of the Fifth Medical Center of PLA General Hospital (Beijing, China). The authors reviewed the patients' medical records and collected relevant information. Written informed consent was obtained from patients/participants. Any potentially identifying images or data contained in this article were published with the written consent of the individual(s).
Declarations
Author contribution statement
Lei Zhao; Niajia Chang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Lei Shi; Fengyi Li; Fanglin Meng; Xiaohui Xie: Performed the experiments; Wrote the paper.
Zhe Xu: Performed the experiments; Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Fusheng Wang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research was supported by the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (81721002).
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Zhe Xu, Email: xuzhe302@139.com.
Fusheng Wang, Email: Fswang302@163.com.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen M.H., Wong G., Gane E., Kao J.H., Dusheiko G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin. Microbiol. Rev. 2020;33 doi: 10.1128/CMR.00046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levrero M., Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 2016;64:S84–s101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Xue X., Liao W., Xing Y. Comparison of clinical features and outcomes between HBV-related and non-B non-C hepatocellular carcinoma. Infect. Agents Cancer. 2020;15:11. doi: 10.1186/s13027-020-0273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EASL Clinical Practice Guidelines Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak L.Y., Cruz-Ramón V., Chinchilla-López P., Torres H.A., LoConte N.K., Rice J.P., Foxhall L.E., Sturgis E.M., Merrill J.K., Bailey H.H., Méndez-Sánchez N., Yuen M.F., Hwang J.P. Global epidemiology, prevention, and management of hepatocellular carcinoma. Am. Soc. Clin. Oncol. Educ. Book. 2018;38:262–279. doi: 10.1200/EDBK_200939. [DOI] [PubMed] [Google Scholar]
- 8.Jia L., Gao Y., He Y., Hooper J.D., Yang P. HBV induced hepatocellular carcinoma and related potential immunotherapy. Pharmacol. Res. 2020;159:104992. doi: 10.1016/j.phrs.2020.104992. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z., Lin Y., Zhang J., Zhang Y., Li Y., Liu Z., Li Q., Luo M., Liang R., Ye J. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J. Exp. Clin. Cancer Res.: CR (Clim. Res.) 2019;38:447. doi: 10.1186/s13046-019-1412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., Blanc J.F., Vogel A., Komov D., Evans T.R.J., Lopez C., Dutcus C., Guo M., Saito K., Kraljevic S., Tamai T., Ren M., Cheng A.L. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (London, England) 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 11.Tomonari T., Sato Y., Tani J., Hirose A., Ogawa C., Morishita A., Tanaka H., Tanaka T., Taniguchi T., Okamoto K., Sogabe M., Miyamoto H., Muguruma N., Uchida K., Masaki T., Takayama T. Comparison of therapeutic outcomes of sorafenib and lenvatinib as primary treatments for hepatocellular carcinoma with a focus on molecular-targeted agent sequential therapy: a propensity score-matched analysis. Hepatol. Res.: Off. J. Jpn. Soc. Hepatol. 2021;51:472–481. doi: 10.1111/hepr.13597. [DOI] [PubMed] [Google Scholar]
- 12.Yau T., Park J.W., Finn R.S., Cheng A.L., Mathurin P., Edeline J., Kudo M., Harding J.J., Merle P., Rosmorduc O., Wyrwicz L., Schott E., Choo S.P., Kelley R.K., Sieghart W., Assenat E., Zaucha R., Furuse J., Abou-Alfa G.K., El-Khoueiry A.B., Melero I., Begic D., Chen G., Neely J., Wisniewski T., Tschaika M., Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial, the Lancet. Oncology. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo A., Dadduzio V., Ricci A.D., Massari F., Di Federico A., Gadaleta-Caldarola G., Brandi G. Lenvatinib plus pembrolizumab: the next frontier for the treatment of hepatocellular carcinoma? Expet Opin. Invest. Drugs. 2021:1–8. doi: 10.1080/13543784.2021.1948532. [DOI] [PubMed] [Google Scholar]
- 14.Schulte N., Li M., Zhan T., Dreikhausen L., Sollors J., Antoni C., Diehl S., Schoenberg S.O., Rahbari N., Reissfelder C., Giordano F.A., Ebert M.P., Teufel A. Response of advanced HCC to pembrolizumab and lenvatinib combination therapy despite monotherapy failure. Zeitschrift fur Gastroenterol. 2020;58:773–777. doi: 10.1055/a-1190-5681. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L., Mai W., Jiang W., Geng Q. Sintilimab: a promising anti-tumor PD-1 antibody. Front. Oncol. 2020;10:594558. doi: 10.3389/fonc.2020.594558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie D.Y., Ren Z.G., Zhou J., Fan J., Gao Q. Chinese clinical guidelines for managing hepatocellular carcinoma: updates and insights. Hepatobiliary Surg. Nutr. 2019;9(2020):452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzo A., Brandi G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021;27:100328. doi: 10.1016/j.ctarc.2021.100328. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo A., Ricci A.D., Brandi G. Immune-based combinations for advanced hepatocellular carcinoma: shaping the direction of first-line therapy. Future Oncol. 2021;17:755–757. doi: 10.2217/fon-2020-0986. [DOI] [PubMed] [Google Scholar]
- 19.Freites-Martinez A., Santana N., Arias-Santiago S., Viera A. Using the common Terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermo-Sifiliográficas. 2021;112:90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Gordan J.D., Kennedy E.B., Abou-Alfa G.K., Beg M.S., Brower S.T., Gade T.P., Goff L., Gupta S., Guy J., Harris W.P., Iyer R., Jaiyesimi I., Jhawer M., Karippot A., Kaseb A.O., Kelley R.K., Knox J.J., Kortmansky J., Leaf A., Remak W.M., Shroff R.T., Sohal D.P.S., Taddei T.H., Venepalli N.K., Wilson A., Zhu A.X., Rose M.G. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2020;38:4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 21.Yip T.C., Wong V.W., Chan H.L., Tse Y.K., Lui G.C., Wong G.L. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology. 2020;158:215–225. doi: 10.1053/j.gastro.2019.09.025. e216. [DOI] [PubMed] [Google Scholar]
- 22.Sonbol M.B., Riaz I.B., Naqvi S.A.A., Almquist D.R., Mina S., Almasri J., Shah S., Almader-Douglas D., Uson Junior P.L.S., Mahipal A., Ma W.W., Jin Z., Mody K., Starr J., Borad M.J., Ahn D.H., Murad M.H., Bekaii-Saab T. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol. 2020;6 doi: 10.1001/jamaoncol.2020.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilmi M., Neuzillet C., Calderaro J., Lafdil F., Pawlotsky J.M., Rousseau B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: current knowledge and future research directions. J. Immunother. Cancer. 2019;7:333. doi: 10.1186/s40425-019-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finn R.S., Ikeda M., Zhu A.X., Sung M.W., Baron A.D., Kudo M., Okusaka T., Kobayashi M., Kumada H., Kaneko S., Pracht M., Mamontov K., Meyer T., Kubota T., Dutcus C.E., Saito K., Siegel A.B., Dubrovsky L., Mody K., Llovet J.M. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato Y., Tabata K., Kimura T., Yachie-Kinoshita A., Ozawa Y., Yamada K., Ito J., Tachino S., Hori Y., Matsuki M., Matsuoka Y., Ghosh S., Kitano H., Nomoto K., Matsui J., Funahashi Y. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson L. Targeted therapies: lenvatinib SELECTs survival benefit, Nature reviews. Endocrinology. 2017;13:500. doi: 10.1038/nrendo.2017.96. [DOI] [PubMed] [Google Scholar]
- 27.Yi C., Chen L., Lin Z., Liu L., Shao W., Zhang R., Lin J., Zhang J., Zhu W., Jia H., Qin L., Lu L., Chen J. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology (Baltimore, Md.) 2021;74:2544–2560. doi: 10.1002/hep.31921. [DOI] [PubMed] [Google Scholar]
- 28.Kloeckner R., Galle P.R., Bruix J. Local and regional therapies for hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):137–149. doi: 10.1002/hep.31424. [DOI] [PubMed] [Google Scholar]
- 29.Yuan G., Cheng X., Li Q., Zang M., Huang W., Fan W., Wu T., Ruan J., Dai W., Yu W., Chen M., Guo Y., Hu X., Chen J. Safety and efficacy of camrelizumab combined with apatinib for advanced hepatocellular carcinoma with portal vein tumor thrombus: a multicenter retrospective study. OncoTargets Ther. 2020;13:12683–12693. doi: 10.2147/OTT.S286169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craft D.V., Goss N.H., Chandramouli N., Wood H.G. Purification of biotinidase from human plasma and its activity on biotinyl peptides. Biochemistry. 1985;24:2471–2476. doi: 10.1021/bi00331a012. [DOI] [PubMed] [Google Scholar]
- 31.Xu J., Shen J., Gu S., Zhang Y., Wu L., Wu J., Shao G., Zhang Y., Xu L., Yin T., Liu J., Ren Z., Xiong J., Mao X., Zhang L., Yang J., Li L., Chen X., Wang Z., Gu K., Chen X., Pan Z., Ma K., Zhou X., Yu Z., Li E., Yin G., Zhang X., Wang S., Wang Q. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin. Cancer Res. : Off. J. Am. Asso. Cancer Res. 2021;27:1003–1011. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 32.Wei F., Huang Q., He J., Luo L., Zeng Y. Lenvatinib plus camrelizumab versus lenvatinib monotherapy as post-progression treatment for advanced hepatocellular carcinoma: a short-term prognostic study. Cancer Manag. Res. 2021;13:4233–4240. doi: 10.2147/CMAR.S304820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., Li D., Verret W., Xu D.Z., Hernandez S., Liu J., Huang C., Mulla S., Wang Y., Lim H.Y., Zhu A.X., Cheng A.L. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda M., Okusaka T., Mitsunaga S., Ueno H., Tamai T., Suzuki T., Hayato S., Kadowaki T., Okita K., Kumada H. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. : Off. J. Am. Asso. Cancer Res. 2016;22:1385–1394. doi: 10.1158/1078-0432.CCR-15-1354. [DOI] [PubMed] [Google Scholar]
- 35.Shimose S., Iwamoto H., Niizeki T., Shirono T., Noda Y., Kamachi N., Okamura S., Nakano M., Suga H., Kuromatsu R., Yamaguchi T., Kawaguchi T., Tanaka M., Noguchi K., Koga H., Torimura T. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Cancers. 2020;12 doi: 10.3390/cancers12071867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee M.S., Ryoo B.Y., Hsu C.H., Numata K., Stein S., Verret W., Hack S.P., Spahn J., Liu B., Abdullah H., Wang Y., He A.R., Lee K.H. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Cannon B.A., Castro-Sanchez A., Barragan-Carrillo R., de la Rosa Pacheco S., Platas A., Fonseca A., Vega Y., Bojorquez-Velazquez K., Bargallo-Rocha J.E., Mohar A., Villarreal-Garza C. Adherence to adjuvant tamoxifen in Mexican young women with breast cancer. Patient Prefer. Adherence. 2021;15:1039–1049. doi: 10.2147/PPA.S296747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirooka M., Ochi H., Hiraoka A., Koizumi Y., Matsuura B., Joko K., Michitaka K., Abe M., Hiasa Y. Destructive thyroiditis induced by lenvatinib in three patients with hepatocellular carcinoma. Intern. Med. (Tokyo, Japan) 2019;58:791–795. doi: 10.2169/internalmedicine.1874-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shomura M., Okabe H., Sato E., Fukai K., Shiraishi K., Hirose S., Tsuruya K., Arase Y., Anzai K., Kagawa T. Hypothyroidism is a predictive factor for better clinical outcomes in patients with advanced hepatocellular carcinoma undergoing lenvatinib therapy. Cancers. 2020;12 doi: 10.3390/cancers12113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohki T., Sato K., Kondo M., Goto E., Sato T., Kondo Y., Akamatsu M., Sato S., Yoshida H., Koike Y., Obi S. Impact of adverse events on the progression-free survival of patients with advanced hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Drugs - Real World Outcome. 2020;7:141–149. doi: 10.1007/s40801-020-00179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faivre S., Rimassa L., Finn R.S. Molecular therapies for HCC: looking outside the box. J. Hepatol. 2020;72:342–352. doi: 10.1016/j.jhep.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Liu X., Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. The Oncologist. 2019;24:S3–s10. doi: 10.1634/theoncologist.2019-IO-S1-s01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torino F., Corsello S.M., Longo R., Barnabei A., Gasparini G. Hypothyroidism related to tyrosine kinase inhibitors: an emerging toxic effect of targeted therapy. Nat. Rev. Clin. Oncol. 2009;6:219–228. doi: 10.1038/nrclinonc.2009.4. [DOI] [PubMed] [Google Scholar]
- 44.Kim K.M., Shim S.G., Sinn D.H., Song J.E., Kim B.S., Kim H.G. Child-Pugh, MELD, MELD-Na, and ALBI scores: which liver function models best predicts prognosis for HCC patient with ascites? Scand. J. Gastroenterol. 2020;55:951–957. doi: 10.1080/00365521.2020.1788139. [DOI] [PubMed] [Google Scholar]
- 45.Huang F., Gao J. Modified Child-Pugh grade vs albumin-bilirubin grade for predicting prognosis of hepatocellular carcinoma patients after hepatectomy. World J. Gastroenterol. 2020;26:749–758. doi: 10.3748/wjg.v26.i7.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.