Abstract
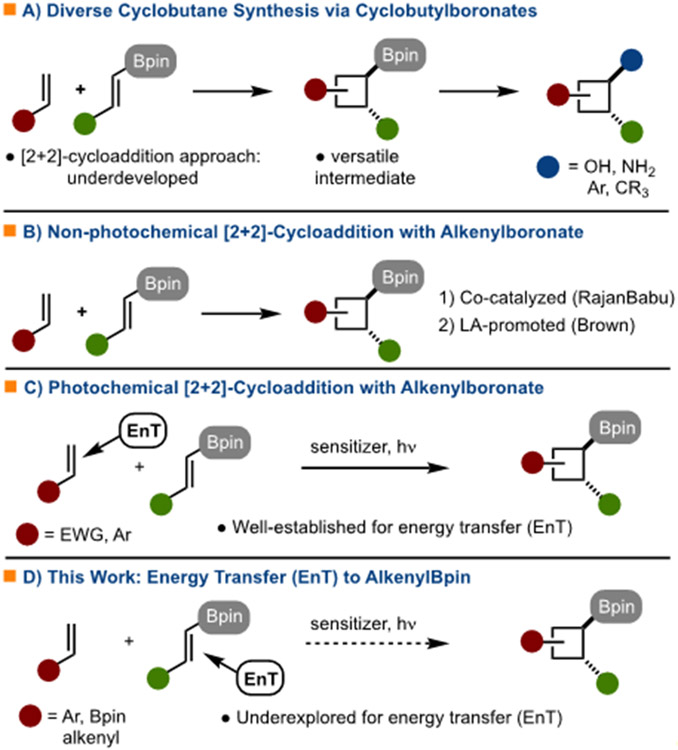
A new strategy for the synthesis of highly versatile cyclobutylboronates via the photosensitized [2+2]-cycloaddition of alkenylboronates and alkenes is presented. The process is mechanistically different from other processes in that energy transfer occurs with the alkenylboronate as opposed to the other alkene. This strategy allows for the synthesis of an array of diverse cyclobutylboronates. The conversion of these adducts to other compounds as well as their utility in the synthesis of melicodenine C is demonstrated.
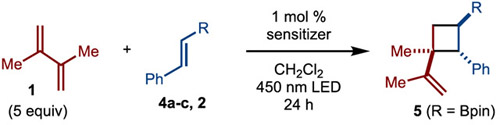
Graphical Abstract
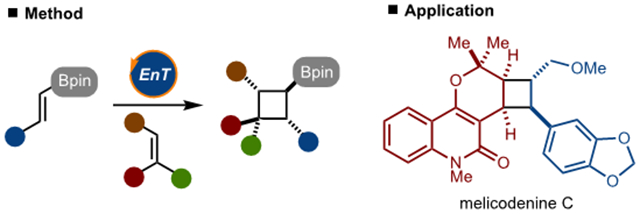
A process for the synthesis of cyclobutylboronates by [2+2]-cycloaddition is presented. The reaction is enabled by triplet energy transfer to an alkenylboronate, which is an underexplored photochemical process. The process operates with a wide array of alkenylboronates and alkenes to generate a diverse range of products. The utility of the products is demonstrated in the synthesis of melicodenine C.
Cyclobutanes are important intermediates due to their ease of functionalization, presence in many natural products and bioactive molecules, and crucial role in medicinal chemistry. While numerous methods are available for their synthesis, [2+2]-cycloadditions have been established as a principal method for synthesis of cyclobutanes because readily available alkenes serve as the starting materials.1
To aid in the synthesis of diverse cyclobutanes, cyclobutylboronates are versatile intermediates due to the ease with which the C-B bond can be elaborated to other functional groups (Scheme 1A). 2 Established methods for cyclobutylboronate synthesis include, hydro- di- or carboboration of cyclobutenes, 3 , 4 ring expansion of cyclopropanes, 5 electrocyclization, 6 C-H functionalization, 7 and [2+2]-cycloaddition.8,9,10,11 The final strategy is particularly attractive as readily available alkenylboronates and alkenes can be used (Scheme 1A). To date, several approaches have been described, including Co-catalyzed,8 Lewis-acid promoted,9 or photochemical-[2+2]-cycloadditions10 (Scheme 1B/C). Known photochemical [2+2]-cycloadditions operate by energy transfer from an excited state sensitizer to the alkene not bearing the boron, which is typically an electron deficient alkene (Scheme 1C).10,12 [2+2]-Cycloaddition reactions that operate by energy transfer to the alkenylboronate are not known (Scheme 1D). Development of such a process would be enabling as entirely different sets of cyclobutylboronates could be accessed. For these reasons, and based on the general interests of our lab in [4+2]/[2+2]-cycloadditions 13 and borylation reactions, 14 we targeted [2+2]-cycloadditions with alkenylboronates for development.
Scheme 1.
Synthesis of Cyclobutylboronates by [2+2]-Cycloaddition.
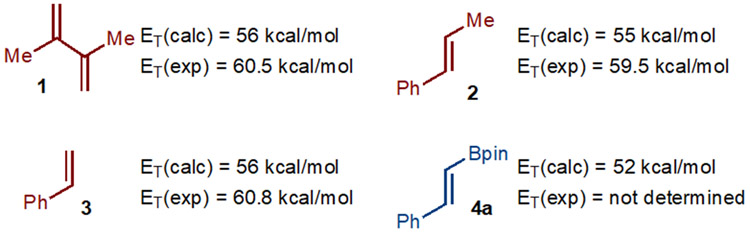
In 2018, Watson and Gilmour established that energy transfer to alkenylboronates is possible (in the context of alkene isomerization reactions).15 However, for [2+2]-cycloaddition to proceed by the design outlined in Scheme 1D, bimolecular quenching of the triplet state of the alkenylboronate is necessary.16,17 One factor that could be important is that the Bpin unit alters the photophysical properties of the attached alkene such that energy transfer is more facile relative to the other alkene component. In the absence of selective energy transfer, complex mixtures of products might be expected that result from different photoexcited substrates undergoing various non-desired product forming reactions. Based on calculations, it was determined that the Bpin unit lowers the triplet energy (52 kcal/mol for 4a) relative to other alkenes (55-56 kcal/mol for 1-3) that could be used in the [2+2]-cycloaddition (Scheme 2). It should be noted that the calculated triplet energies are lower than the experimentally determined values.18 While the triplet energy of 4a has not been experimentally determined, it is likely greater than 52 kcal/mol. While many factors influence the efficiency of energy transfer, based on triplet energy alone, it was deemed plausible that a sensitizer could be identified to allow for energy transfer to the alkenylboronate in preference to other alkene.
Scheme 2.
Experimental and Calculated Triplet Energies (ET) of Various Alkenes. ET(calc): Calculated triplet energies, CH2Cl2,electronic energy, ωB97XD/aug-cc-pVTZ. ET(exp): experimentally determined triplet energies.18
An initial evaluation of various photosensitizers in the presence of 2,3-dimethylbutadiene (1) and alkenylboronate 4a led to encouraging initial results (Table 1, entries 1-7). In particular, fac-Ir(ppy)3 emerged as a promising photosensitizer (Table 1, entry 7). Optimization of the solvent and concentration allowed for synthesis of 5 in 82% yield (10:1 dr). While in these reactions, photoisomerization was detected by the observation that Z-4a was formed, it does not appear to affect the outcome, as Z-4a is equally efficient in providing the same products (Table 1, entry 11). Finally, other substituents were explored such as BMIDA (4b), SiMe3 (4c), and Me (2), which only led to product formation in low yield, thus demonstrating the importance of the Bpin unit. In all of these cases, photoisomerization was detected, thus indicating that quenching can occur, yet productive cycloaddition reaction does not proceed.
Table 1.
Optimization of Reaction Conditions.
| |||||
|---|---|---|---|---|---|
| entry | R | sensitizer | ET (kcal/mol) | Conc. (M) | yielda |
| 1 | Bpin (4a) | (Ir[dF(CF3)ppy]2(bpy))PF6 | 60.4 | 0.4 | 38% |
| 2 | Bpin (4a) | (Ir[dF(CF3)ppy]2(dtbbpy))PF6 | 60.2 | 0.4 | 45% |
| 3 | Bpin (4a) | fac-Ir(dFppy)3 | 60.1 | 0.4 | 49% |
| 4 | Bpin (4a) | fac-Ir(p-Fppy)3 | 58.6 | 0.4 | 60% |
| 5 | Bpin (4a) | fac-Ir(p-CF3ppy)3 | 56.4 | 0.4 | 62% |
| 6 | Bpin (4a) | [Ir(dFppy)2dtbbpy]PF6 | 55.4 | 0.4 | 41% |
| 7 | Bpin (4a) | fac-Ir(ppy)3 | 55.2 | 0.4 | 71% |
| 8 | Bpin (4a) | [Ir(ppy)2dtbbpy]PF6 | 49.2 | 0.4 | 28% |
| 9 | Bpin (4a) | fac-Ir(ppy)3 | 55.2 | 0.1 | 56% |
| 10b | Bpin (4a) | fac-Ir(ppy)3 | 55.2 | 0.8 | 82% |
| 11b | Bpin (Z-4a) | fac-Ir(ppy)3 | 55.2 | 0.8 | 82% |
| 12 | BMIDA (4b) | fac-Ir(ppy)3 | 55.2 | 0.8 | <10% |
| 13 | SiMe3 (4c) | fac-Ir(ppy)3 | 55.2 | 0.8 | <10% |
| 14 | Me (2) | fac-Ir(ppy)3 | 55.2 | 0.8 | <10% |
Yield was determined by 1H NMR analysis of the unpurified reaction mixture with an internal standard (0.1 mmol scale).
0.2 mmol scale.
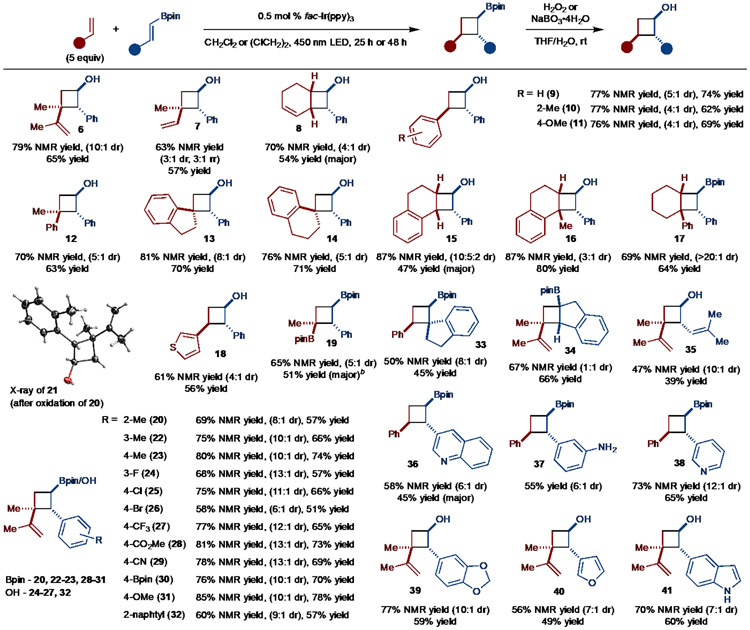
Evaluation of the alkene scope revealed that other dienes were permitted such as isoprene (product 7) and 1,3-cyclohexadiene (product 8) (Scheme 3). The reaction also tolerates the use of alkenyl arenes. For example, use of styrene allowed for the formation of 9 in good yield and selectivity. Other derivatives resulted in the formation of products with similar levels of selectivity (products 10 and 11). Disubstituted and trisubstituted alkenyl arenes also functioned well in the reaction. For example, α-substituted styrenes allowed for the synthesis of a quaternary carbon (products 12-14). In the case of 1,2-dihydronaphthalene, product 15 was formed in low selectivity; however, use of 1-methyl-1,2-dihydronapthalene restored high levels of selectivity (product 16). Particularly noteworthy is the example that led to the formation of 17 as a single observable diastereomer. Finally, isopropenyl-Bpin could also be coupled to generate 19 in good selectivity and yield, thus allowing for the synthesis of polyborylated products. In addition, reactions with unactivated alkenes or alkenes that bear electron-withdrawing substituents failed to deliver the products.
Scheme 3.
Substrate Scope. aNMR yield refers to yield determined by 1H NMR analysis of the unpurified reaction mixture of the Bpin product except for 8-11, 13, 15, 16, which was determined after oxidation. Diastereomeric ratio (dr) determined of the unpurified reaction mixture by 1H NMR analysis. Yield is of isolated, purified product. In some cases, oxidation of the Bpin was conducted to facilitate purification. bIsolated in >20:1 dr.
In the case of alkenylboronates, broad tolerance of various functional groups on the aryl unit was observed. In particular, halogens (products 24-26), esters (product 28), boronic esters (product 30), nitriles (product 29), and unprotected amines (product 37) all allowed for product formation. Sterically demanding substituents also did not impede the reaction (product 20). Various heterocycles such as pyridine (product 38), quinoline (product 36), indole (product 41), and furan (product 40) were also tolerated, thus demonstrating the applicability of this method to medicinal chemistry. Substitution of the alkenylboronate was also tolerated, as evidenced by the formation of 32 and 33. In the case of the former, the yield suffered due to dimerization, whereas poor diastereoselectivity was observed in the latter. Finally, the aryl group was not necessary as a borylsubstituted diene could be used to generate 35. However, the efficiency of this reaction is lower, likely due to a higher triplet energy of the diene (relative to 4a). Attempted reaction with only alkyl-substituted alkenylboronates did not result in product formation, likely due to a high triplet energy.
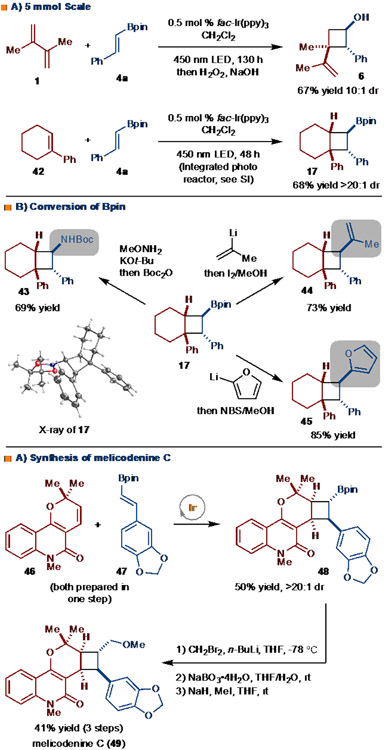
The reaction could be carried out on 5 mmol. In the synthesis of 6, prolonged reaction time (130 hrs) was necessary achieve full conversion with our standard reaction setup (Scheme 4A). However, for more challenging examples (e.g., synthesis of 17) that required 48 hrs of reaction time on small scale (0.2 mmol) the use of the Integrated Photochemical Reactor (ICR)19 was employed. On a 5 mmol scale, preparative amounts of product 17 can be easily obtained in 48 hrs (Scheme 4A).
Scheme 4.
Application in Synthesis
The products generated by this method are useful intermediates for chemical synthesis, primarily due to the ability to functionalize the C-B bond. As illustrated in Scheme 4B, application of amination,20 Zweifel,21 and cross-coupling 22 protocols allowed for conversion to amine (43), alkene (44), and furan (45) containing products, respectively. To further underscore the utility of the method, a short synthesis of melicodenine C (49) was undertaken (Scheme 4B).23 The requisite starting materials (46 and 47) were prepared in one step from commercially available materials. Subjection of 46 and 47 to the standard reaction conditions (fac-Ir(ppy)3, 450 nm LED, CH2Cl2) resulted in the formation of 48 in 50% yield as a single observable diastereomer, demonstrating that the use of a complex and sterically demanding alkene (47) was tolerated. Simple elaboration of the Bpin unit to the methyl ether by application of a Matteson homologation,24 oxidation, and etherification sequence allowed for the synthesis of melicodenine C in five steps. A notable aspect of this route is that the Bpin unit can serve as a handle for further transformation in the construction of derivatives. Closely related structures to melicodenine C have shown promise as agonists of the human TLR4 receptor.23c
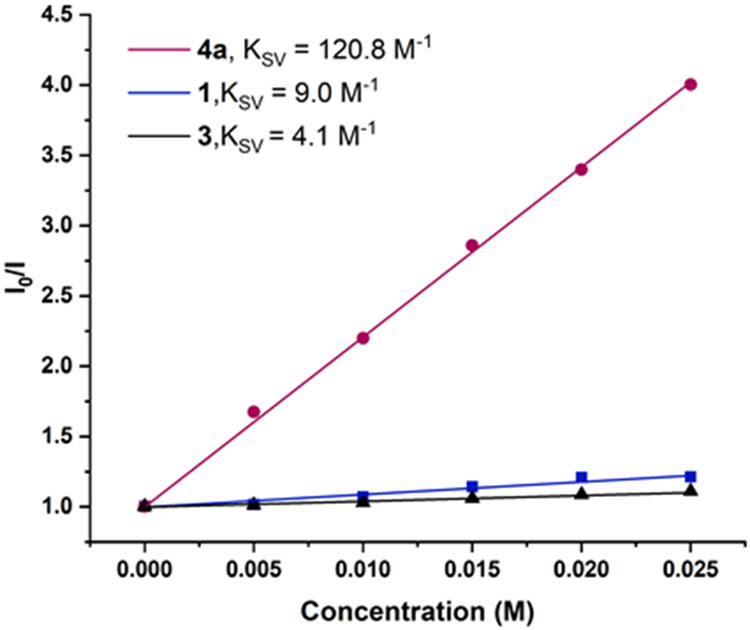
To probe the mechanism of the reaction, Stern-Volmer luminescence quenching experiment was first conducted. A linear correlation with respect to alkenylBpin concentration was observed, thus demonstrating that 4a is an effective quencher (Ksv = 120.8 M−1, Figure 1). Additional quenching studies with 2,3-dimethyl butadiene (1, Ksv = 9.0 M−1), styrene (3, Ksv = 4.1 M−1) and other coupling partners (Figure S2) confirmed that they are less effective quenchers of fac-Ir(ppy)3(T1). Even given the use of 5 equiv of 2,3-dimethyl butadiene, alkenylBpin (4a) is still a more effective quencher, when considering initial concentrations (See the Supporting Information for details).
Figure 1.
Stern-Volmer Luminescence Quenching of fac-Ir(ppy)3
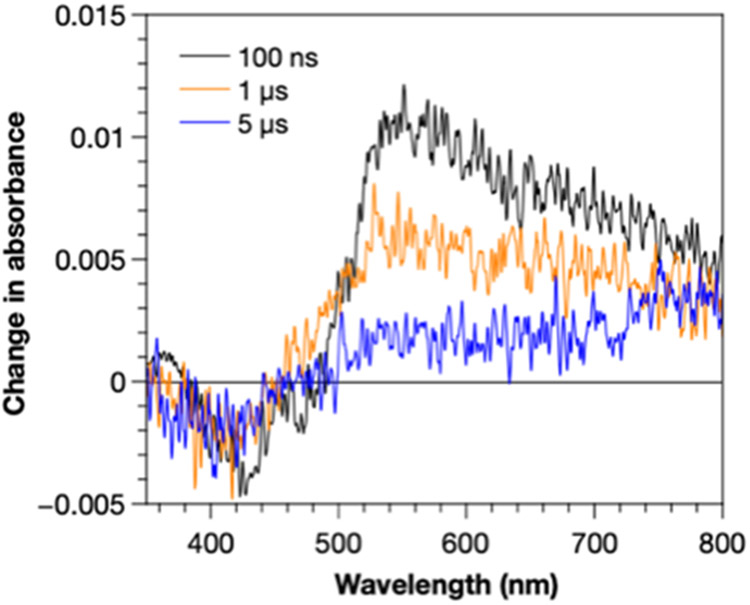
To look for evidence of electron transfer versus triplet energy transfer, transient absorption spectra of fac-Ir(ppy)3 in the presence of 4a were measured at 100 ns, 1 μs, and 5 μs (Figure 2). At 100 ns, there is a broad transient absorption extending from 484 nm to beyond 800 nm. There is also a small bleach centered at 408 nm. Both of these features decay over the subsequent 5 μs. The transient spectra of excited fac-Ir(ppy)3 in the absence of any quencher displays the same features (Figure S7). In addition, when compared to the spectrum for [Ir(ppy)3]+ we do not observe the strong bleach at 380 nm or the large, positive transient absorbance at 350 nm that are characteristic of the Ir(IV) species. 25 Taken together, this strongly supports an energy transfer mechanism instead of an electron transfer mechanism.
Figure 2.
Transient spectra at 100 ns (black), 1 μs (orange), and 5 μs (blue) for 100 mM of 4a and 35 μM of Ir(ppy)3. The transient absorption at 520 nm and bleach at 408 nm are consistent with excited Ir(ppy)3 and inconsistent with [Ir(ppy)3]+.
In addition, quenching experiments were conducted with fac-Ir(dFppy)3, which has a higher triplet energy than fac-Ir(ppy)3 and results in a lower yielding cycloaddition (Table 1, entry 3). In this case, quenching with diene 1 and boronate 4a were closer to unity (Ksv (4a)/ Ksv (1) = 2.1, Table 2, Figure S3). Based on simple kinetic modelling with the initial concentrations, diene 1 quenches the triplet state of fac-Ir(dFppy)3 ~2x more effectively than alkenylBpin 4a (See the Supporting Information for details). Compare this with the case of fac-Ir(ppy)3 in which Ksv (4a)/ Ksv (1) = 13.5 (Table 2) and when considering the initial concentrations, alkenylBpin 4a quenches the excited state of fac-Ir(ppy)3(T1) ~3x more effectively compared to diene 1. Finally, quenching studies with [Ir(dFppy)2dtbpy]PF6 were also conducted, because it has a similar triplet energy as fac-Ir(ppy)3 yet results in a lower yield (Table 1, compare entries 6-7). Despite the similar triplet energies, the quenching between 4a and 1 only slightly prefers 4a (Ksv (4a)/ Ksv (1) = 3.5, Table 2, Figure S3). In addition, the efficacy of energy transfer is not purely linked to the triplet energy of the respective components as size of sensitizer26 and homoleptic versus cationic complexes likely play a major role. Based on the data shown in Table 2, selective quenching of the alkenylBpin relative to the other alkene component appears to be important for high yielding [2+2]-cycloaddition to occur. Increased quenching of the diene leads to polymerization, which results in lower yield.
Table 2.
Comparison of Stern-Volmer Luminescence Quenching
| fac-Ir(ppy)3 | [Ir(dFppy)2dtbbpy]PF6 | fac-Ir(dFppy)3 | |
|---|---|---|---|
| yield of [2+2]a | 71% | 41% | 49% |
| ET (kcal/mol)b | 55.2 | 55.4 | 60.1 |
| KSV (4a) (M−1) | 120.8 | 232.1 | 999.7 |
| KSV (1) (M−1) | 9.0 | 67.1 | 476.8 |
| KSV (4a) /KSV (1) | 13.4 | 3.5 | 2.1 |
Condition: 4a (0.2 mmol, 1.0 equiv), 1 (5.0 equiv), [Ir] (1.0 mol %) in CH2Cl2 (0.4 M), 450 nm, 24 h.
ref. (27)
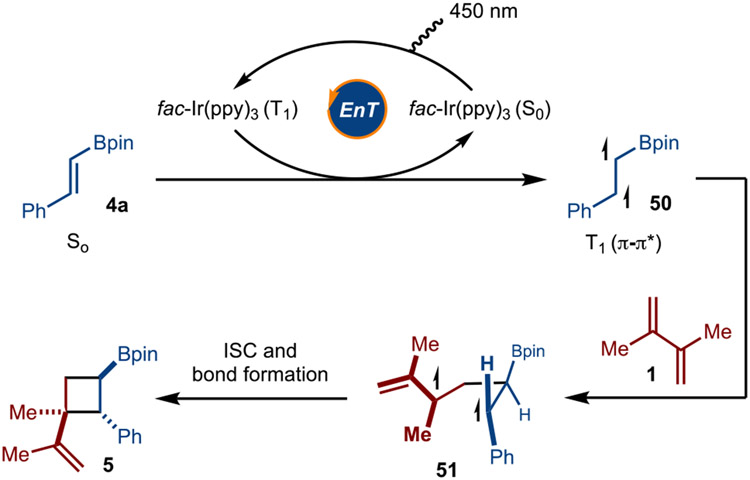
Based on the mechanism studies, the reaction is proposed to occur by Dexter energy transfer from the photoexcited triplet state of fac-Ir(ppy)3 to alkenylboronate 4a to result in the T1 state 50 (Scheme 5). 15-16 Addition of the α-boryl radical (50) to the alkene (1) results in formation of diradical 51. Intersystem crossing and bond formation then occurs to generate product 5.
Scheme 5.
Proposed pathway of the reaction.
In conclusion, a versatile method for the synthesis of cyclobutylboranates is demonstrated. The [2+2]-cycloaddition reaction likely operates by energy transfer to the alkenylboronate, which is an underexplored pathway in photochemistry. By harnessing this type of reactivity, a range of synthetically useful cylobutylboronates can be prepared, which has been demonstrated in the synthesis of various functionalized cyclobutanes and the natural product melicodenine C.28
Supplementary Material
Acknowledgements
We thank Indiana University and the NIH (R35GM131755) and NSF CAREER awards (CHE-1554760 and CHE-2047492) for financial support. This project was partially funded by the Vice Provost for Research through the Research Equipment Fund and the NSF MRI program, CHE-1726633 and CHE-1920026. We thank Dr. Maren Pink and Dr. Veronica Carta of the IU Molecular Structure Center for acquisition of X-ray crystal structure data. Support for the acquisition of the Bruker Venture D8 diffractometer through the Major Scientific Research Equipment Fund from the President of Indiana University and the Office of the Vice President for Research is gratefully acknowledged. We thank Ryan James for experimental assistance.
References
- 1).(a) Poplata S, Tröster A, Zou Y-Q, Bach T, T. Chem. Rev 2016, 116, 9748–9815. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brimioulle R, Lenhart D, Maturi MM, Bach T, Angew. Chem. Int. Ed. Engl 2015, 54, 3872–3890. [DOI] [PubMed] [Google Scholar]; (c) Xu Y, Conner ML, Brown MK, Angew. Chem. Int. Ed 2015, 54, 11918–11928. [DOI] [PubMed] [Google Scholar]
- 2).(a) Armstrong R, Aggarwal V, Synthesis 2017, 49, 3323–3336. [Google Scholar]; (b) Sandford C, Aggarwal VK, Chem. Commun 2017, 53, 5481–5494 [DOI] [PubMed] [Google Scholar]
- 3).(a) For hydroboration/protoboration/diboration reactions, see: Novoa L, Trulli L, Fernandez I, Parra A, Tortosa M, Org. Lett 2021, 23, 7434–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Novoa L, Trulli L, Parra A, Tortosa M, Angew. Chem. Int. Ed 2021, 21, 11763–11768. [DOI] [PubMed] [Google Scholar]; (c) Clement HA, Boghi M, McDonald RM, Bernier L, Coe JW, Farrell W, Helal CJ, Reese MR, Sach NW, Lee JC, Hall DG Angew. Chem. Int. Ed 2019, 58, 18405–18409. [DOI] [PubMed] [Google Scholar]; (d) Mercer JAM, Cohen CM, Shuken SR, Wagner AM, Smith MW, Moss FR, III MD; Smith R Vahala A Gonzalez-Martinez SG Boxer N Burns Z, J. Am. Chem. Soc 2016, 138, 15845–15848. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Guisán-Ceinos M, Parra A, Martín-Heras V, Tortosa M, Angew. Chem. Int. Ed 2016, 55, 6969. [DOI] [PubMed] [Google Scholar]; (f) Brener L, Brown HC, J. Org. Chem 1977, 42, 2702. [Google Scholar]
- 4).(a) For carboboration reactions, see: Simlandy AK, Lyu M-Y, Brown MK, ACS. Catal 2021, 11, 12815–12820. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hancock EN, Kuker EL, Tantillo DJ, Brown MK, Angew. Chem. Int. Ed 2020, 59, 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Logan KM, Brown MK, Angew. Chem. Int. Ed 2017, 56, 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).(a) For example, see: Silvi M, Aggarwal VK, J. Am. Chem. Soc 2019, 141, 9511–9515. [DOI] [PubMed] [Google Scholar]; (b) Fawcett A, Biberger T, Aggarwal VK, Nat. Chem 2019, 117–122. [DOI] [PubMed] [Google Scholar]; (c) Hari DP, Abell JC, Fasano V, Aggarwal VK. J. Am. Chem. Soc 2020, 142, 5515–5520 [DOI] [PubMed] [Google Scholar]
- 6).Giustra ZX, Yang X, Chen M, Bettinger HF, Liu S-Y, Angew. Chem. Int. Ed. Engl 2019, 58, 18918–18922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).For example, see: He J, Shao Q, Wu Q, Yu J-Q, J. Am. Chem. Soc 2017, 139, 3344–3347. [DOI] [PubMed] [Google Scholar]; (b) Murakami R, Tsunoda K, Iwai T, Sawamura M, Chem. Eur. J 2014, 20, 13127–13131. [DOI] [PubMed] [Google Scholar]
- 8).Parsutkar MM, Pagar VV, RajanBabu TV, J. Am. Chem. Soc 2019, 141, 15367–15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Conner ML, Brown MK, J. Org. Chem 2016, 81, 8050–8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). For selected examples, see: Scholz SO, Kidd JB, Capaldo L, Flikweert NE, Littlefield RM, Yoon TP, Org. Lett 2021, 23, 3496. Demchuk OP, Hryshchuk OV, Vashchenko BV, Kozytskiy AV, Tymtsunik AV, Komarov IV, Grygorenko OO, J. Org. Chem 2020, 85, 5927–5940. Coote SC, Bach T, J. Am. Chem. Soc 2013, 135, 14948–14951. Kleinnijenhuis RA, Timmer BJJ, Lutteke G, Smits JMM, de Gelder R, van Maarseveen JH, Hiemstra H, Chem. Eur. J 2016, 22, 1266–1269. Hollis WG, Lappenbusch WC Jr, Everberg KA, Woleben CM, Tetrahedron Lett. 1993, 34, 7517. 16n
- 11).For reviews regarding cycloadditions of alkenylboron, see: a) Hilt G, Bolze P, Synthesis 2005, 2005, 2091–2115. [Google Scholar]; b) Welker ME, Tetrahedron 2008, 64, 11529–11539. [Google Scholar]; c) Eberlin L, Tripoteau F, Carreaux F, Whiting A, Carboni B, Beilstein J Org Chem 2014, 10, 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Pyziak J, Walkowiak J, Marciniec B, Chem. Eur. J 2017, 23, 3502–3541. [DOI] [PubMed] [Google Scholar]; e) Grygorenko OO, Moskvina VS, Hryshchuk OV, Tymtsunik AV, Synthesis 2020, 52, 2761–2780. [Google Scholar]
- 12).(a) For reviews regarding energy transfer, see: Strieth-Kalthoff F, Glorius F, Chem. 2020, 6, 1888. [Google Scholar]; (b) Zhou Q-Q, Zou Y-Q, Lu L-Q, Xiao W-J, Angew. Chem. Int. Ed 2019, 58, 1586–1604. [DOI] [PubMed] [Google Scholar]; (c) Strieth-Kalthoff F, James MJ, Teders M, Pitzer L, Glorius F, Chem. Soc. Rev 2018, 47, 7190–7202. [DOI] [PubMed] [Google Scholar]
- 13). For example, see: Ni D, Witherspoon BP, Zhang H, Zhou C, Houk KN, Brown MK, Angew. Chem. Int. Ed 2020, 59, 11432–11439. 4a.
- 14).For example, see: Chen L-A, Lear AR, Gao P, Brown MK, Angew. Chem. Int. Ed 2019, 52, 11206–11206. [Google Scholar]
- 15).(a) Molloy JJ, Metternich JB, Daniliuc CG, Watson AJB, Gilmour R, Angew. Chem. Int. Ed 2018, 57, 3168–3172. [DOI] [PubMed] [Google Scholar]; (b) Molloy JJ, Schäfer M, Wienhold M, Morack T, Daniliuc CG, Gilmour R, Science 2020, 369, 302–306. [DOI] [PubMed] [Google Scholar]
- 16).(a) For selected example of sensitized [2+2]-cycloaddition involving alkenylarenes, see: Bach T, Pelkmann C, Harms K, Tetrahedron Lett. 1999, 40, 2103 [Google Scholar]; (b) Lu Z, Yoon TP, Angew. Chem. Int. Ed 2012, 51, 10329–10332. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Blum TR, Miller VD, Bates DM, Guzei IA, Yoon TP, Science 2016, 354, 1391–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tröster A, Alonso R, Bauer A, Bach T, J. Am. Chem. Soc 2016, 138, 7808–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Huang X, Quinn TR, Harms K, Webster RD, Zhang L, Wiest O, Meggers E, J. Am. Chem. Soc 2017, 139, 9120–9123. [DOI] [PubMed] [Google Scholar]; (f) Zhao J, Brosmer JL, Tang Q, Yang Z, Houk KN, Diaconescu PL, Kwon O, J. Am. Chem. Soc 2017, 139, 9807–9810. [DOI] [PubMed] [Google Scholar]; (g) Daub ME, Jung H, Lee BJ, Won J, Baik M-H, Yoon TP, J. Am. Chem. Soc 2019, 141, 9543–9547. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zhu M, Zheng C, Zhang X, You S-L, J. Am. Chem. Soc 2019, 141, 2636. [DOI] [PubMed] [Google Scholar]; (i) Becker MR, Richardson AD, Schindler CS, Nat. Commun 2019, 10, 5095. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Oderinde MS, Mao E, Ramirez A, Pawluczyk J, Jorge C, Cornelius LAM, Kempson J, Vetrichelvan M, Pitchai M, Gupta A, Gupta AK, Meanwell NA, Mathur A, Dhar TGM, J. Am. Chem. Soc 2020, 142, 3094–3103. [DOI] [PubMed] [Google Scholar]; (k) Hörmann FM, Kerzig C, Chung TS, Bauer A, Wenger OS, Bach T, Angew. Chem. Int. Ed 2020, 59, 9659–9668. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Sherbrook EM, Jung H, Cho D, Baik M-H, Yoon TP, Chem. Sci 2020, 11, 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Oderinde MS, Ramirez A, Dhar TGM, Cornelius LAM, Jorge C, Aulakh D, Sandhu B, Pawluczyk J, Sarjeant AA, Meanwell NA, Mathur A, Kempson J, J. Org. Chem 2021, 86, 1730–1747. [DOI] [PubMed] [Google Scholar]; (n) Murray PRD, Bussink WMM, Davies GHM, van der Mei FW, Antropow AH, Edwards JT, D’Agostino LA, Ellis JM, Hamann LG, Romanov-Michailidis F, Knowles RR, J. Am. Chem. Soc 2021, 143, 4055. [DOI] [PubMed] [Google Scholar]
- 17). For a review regarding sensitized alkene isomerization of alkenylarenes, see: Molloy JJ, Morack T, Gilmour R, Angew. Chem. Int. Ed. Engl 2019, 58, 13654–13664. For selected examples, see: (b) Singh K, Staig SJ, Weaver JD, Facile Synthesis of Z-Alkenes via Uphill Catalysis. J. Am. Chem. Soc 2014, 136, 5275–5278. Day JI, Singh K, Trinh W, Weaver JD, J. Am. Chem. Soc 2018, 140, 9934–9941. Ref 15
- 18).Ni T, Caldwell RA, Melton LA, J. Am. Chem. Soc 1989, 111, 457–464. [Google Scholar]
- 19).Le C, Wismer MK, Shi Z-C, Zhang R, Conway DV, Li G, Vachal P, Davies IW, MacMillan DWC, ACS. Central Sci 2017, 3, 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Edelstein EK, Grote AC, Palkowitz MD, Morken JP, Synlett. 2018, 29, 1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Armstrong RJ, Niwetmarin W, Aggarwal VK, Org. Lett 2017, 19, 2762. [DOI] [PubMed] [Google Scholar]
- 22).Odachowski M, Bonet A, Essafi S, Conti-Ramsden P, Harvey JN, Leonori D, Aggarwal VK, J. Am. Chem. Soc 2016, 138, 9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).(a) Nakashima K.-i, Oyama M, Ito T, Akao Y, Witono JR, Darnaedi D, Tanaka T, Murata J, Iinuma M, Tetrahedron. 2012, 68, 2421. [DOI] [PubMed] [Google Scholar]; (b) Holla H, Jenkins ID, Neve JE, Pouwer RH, Pham N, Teague SJ, Quinn RJ, Tetrahedron Lett 2012, 53, 7101. [Google Scholar]; (c) Neve JE, Wijesekera HP, Duffy S, Jenkins ID, Ripper JA, Teague SJ, Campitelli M, Garavelas A, Nikolakopoulos G, Le PV, de A Leone P, Pham NB, Shelton P, Fraser N, Carroll AR, Avery VM, McCrae C, Williams N, Quinn RJ, J. Med. Chem 2014, 57, 1252–1275. [DOI] [PubMed] [Google Scholar]
- 24).Sonawane RP, Jheengut V, Rabalakos C, Larouche-Gauthier R, Scott HK, Aggarwal VK, Angew. Chem. Int. Ed 2011, 50, 3760. [DOI] [PubMed] [Google Scholar]
- 25).Stevenson BG, Spielvogel EH, Loiaconi EA, Wambua VM, Nakhamiyayev RV, and Swierk J JR. Am. Chem. Soc 2021, 143, 8878–8885 [DOI] [PubMed] [Google Scholar]
- 26).Singh A, Fennell CJ, Weaver JD, Chem. Sci 2016, 7, 6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Singh A, Teegardin K, Kelly M, Prasad KS, Krishnan S, Weaver JD, J. Organomet. Chem 2015, 776, 51–59 [Google Scholar]
- 28).CCDC for 21 (2070506). CCDC for 17 (2070507)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.