Abstract
γ-Secretase is a membrane protein complex that proteolyzes within the transmembrane domain of >100 substrates, including those derived from the amyloid precursor protein and the Notch family of cell surface receptors. The nine-transmembrane presenilin is the catalytic component of this aspartyl protease complex that carries out hydrolysis in the lipid bilayer. Advances in cryoelectron microscopy have led to elucidation of the structure of the γ-secretase complex at atomic resolution. Recently, structures of the enzyme have been determined with bound APP- or Notch-derived substrates, providing insight into the nature of substrate recognition and processing. Molecular dynamics simulations of substrate-bound enzyme suggest dynamic mechanisms of intramembrane proteolysis. Structures of the enzyme bound to small-molecule inhibitors and modulators have also been solved, setting the stage for rational structure-based drug discovery targeting γ-secretase.
γ-Secretase is a membrane-embedded protease complex [1], a founding member of intramembrane proteases [2] that carry out hydrolysis of substrate transmembrane domains (TMDs) within the hydrophobic environment of the lipid bilayer. Although the catalytic component of this complex—presenilin—contains two conserved transmembrane aspartates that activate water for proteolysis [3], this multi-pass membrane protein bears no resemblance to classical water-soluble aspartyl proteases such as renin, cathepsin D and HIV protease. Along with presenilin, γ-secretase is composed of three other membrane proteins: nicastrin, Aph-1 and Pen-2 [4] (Fig. 1A). These four proteins come together in the endoplasmic reticulum, whereupon presenilin cleaves itself [3] into an N-terminal fragment (NTF) and C-terminal fragment [5], with the entire assembly becoming the active γ-secretase complex [6-8]. Each presenilin subunit contributes one of the catalytic aspartates to the active site, which resides at the NTF-CTF interface.
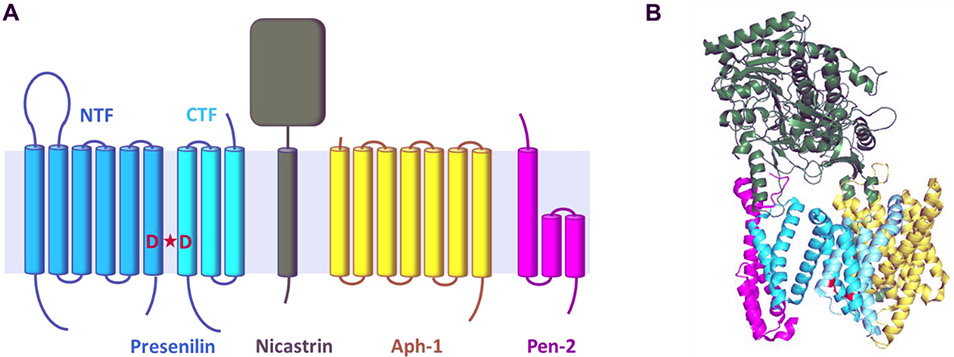
Figure 1.
(A) Components of the γ-secretase complex and their membrane topology. Presenilin is a nine-TMD protein and the catalytic component of the aspartyl protease complex. Active site aspartic acids denoted in red. Upon assembly with the other three members of the complex (Nicastrin, Aph-1 and Pen-2), presenilin undergoes autoproteolysis to N-terminal fragment (NTF) and C-terminal fragment (CTF) to become the active protease complex. (B) Structure of the γ-secretase complex as determined by cryoelectron microscopy (PDB: 5A63). In this structure, TM2 was not visible, and the active site appears open (active site aspartic acids D257 and D385 in PSEN1 shown in red).
The γ-secretase complex is found in all metazoans and is essential for development [9]. Arguably the most important of its >100 known substrates is the Notch family of cell-surface receptors. Notch undergoes successive proteolysis upon interaction with cognate ligand on the surface of a neighboring cell, with the final cut by γ-secretase within the Notch TMD releasing the Notch intracellular domain (NICD)[10,11]. Nuclear translocation of the NICD leads to activation of transcription factors that control cell differentiation [12]. Knockout of presenilin or other γ-secretase components is embryonic lethal and resembles the phenotype seen upon knockout of Notch genes [13,14].
γ-Secretase also processes the TMD of the amyloid precursor protein (APP) in producing the amyloid β-peptide (Aβ) that deposits in the brain in Alzheimer’s disease (AD) [15]. Dominant missense mutations in APP and presenilin genes cause familial Alzheimer’s disease (FAD) [16]. A single mutant allele fates carriers to FAD in mid-life. These mutations can elevate the proportion of aggregation-prone 42-residue Aβ (Aβ42) relative to the major 40-residue variant (Aβ40). However, APP processing by γ-secretase is complex: initial endoproteolysis produces intermediates Aβ48 or Aβ49, which are subsequently trimmed in increments of roughly three amino acids to form shorter secreted peptides such as Aβ40 and Aβ42 [17]. How FAD mutations affect all these cleavage events is still being determined [18].
Initial approaches to elucidate the structure and mechanism of the enzyme involved mutagenesis and designed chemical probes [1]. Substrate-based peptidomimetic transition-state analogs were key to identifying presenilin as the catalytic component [19,20], for characterizing the active site (e.g., [21]), and for enzyme purification (e.g.,[8]). Other substrate-based peptides that assume a helical conformation were found to bind to an exosite distinct from the active site [22]. Cysteine mutagenesis coupled with thiol-reactive reagents helped identify water-accessible pores and determine whether these pores were cytosolic or lumenal/extracellular [23-26]. This approach also suggested the location of inhibitor binding sites. In addition, systematic scanning of APP substrate with a photoactivatable residue allowed mapping the substrate contacts with γ-secretase, identified novel exosites in the complex and provided insights into their role in the translocation of the substrate to the active site [27]. In a recent study, this approach was leveraged to show that FAD mutations in PSEN1 alter enzyme-substrate interactions [28].
Initial structures of the γ-secretase complex derived using cryo-electron microscopy (cryo-EM) were poorly resolved, showing what appeared to be a globular structure with several pores (e.g., [29,30]). Advances in cryo-EM technology, however, quickly led to the first atomic-resolution structure [31], collaboratively solved by the laboratories of Yigong Shi and Sjors Scheres. The structure revealed the horseshoe-like arrangement of the 19 total TMDs of the complex, with the extracellular ectodomain of the nicastrin subunit hovering over the concave side. Because a previous study showed this nicastrin domain had homology with aminopeptidases (but without catalytic activity) and could bind to the Notch substrate [32], the location of this domain in the cryo-EM structure suggested substrate approaches the active site via the concave side. Soon though, a revised TMD assignment of the protease complex [33] (Fig. 1B) and a biochemical study [34] revealed that the active site is accessed from the convex side and that nicastrin acts as a steric block on potential transmembrane substrates with long ectodomains. In addition, choice of detergent for solubilization was found to influence the structure and activity of γ-secretase, with CHAPSO substantially increasing activity and stabilizing oligomeric complexes [35].
Structures with co-bound substrates
In 2019, the Shi lab published two groundbreaking reports on atomic structures of the γ-secretase complex bound to Notch and APP substrates [36,37]. In both structures, the active site of the protease was inactivated by alanine mutation of one of the catalytic aspartates, to prevent substrate cleavage. Mutation to cysteine was also performed both in presenilin and in the substrate to allow oxidation and disulfide bond formation between substrate and enzyme. Through these two mutagenesis strategies, isolation of intact substrate-enzyme complexes could be maximized. The structures of the Notch- and APP-bound complexes were solved by cryo-EM to 2.7 and 2.6 Å resolution, respectively.
Both structures showed the N-terminal half of substrate TMD in an α-helical conformation, surrounded by presenilin NTF helices (shown for APP substrate in Fig. 2A). The C-terminal half of the substrate TMD was in an extended conformation, bound in the active site and interacting with β-strands found in both presenilin NTF and CTF (Fig. 2B). Multiple regions of presenilin that were not observed in the unbound structure were now resolved in the substrate-bound structures. This included loop 1 (L1), TM2, and part of TM6. The only path for lateral substrate entry appears to be between TM2 and TM6, with TM2 acting as a gate that then closes upon substrate binding in the active site. TM7 in the presenilin CTF assumes a β-strand conformation in interacting with the C-terminal half of substrate TMD (Fig. 2B), while also interacting with a new β-strand at the C-terminus of the presenilin NTF. Interestingly, all these changes in the presenilin conformation can be seen in a structure of the enzyme bound to a dipeptide inhibitor called DAPT, reported several years earlier [38]. Thus, interaction with the small inhibitor was sufficient to induce all these conformational effects on the enzyme.
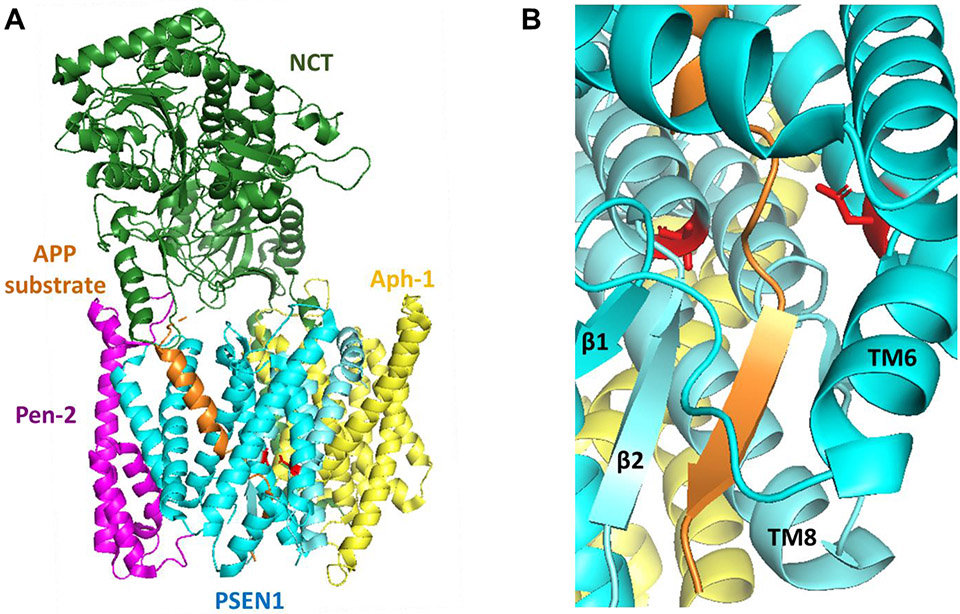
Figure 2.
(A) Structure of the γ-secretase complex bound with APP-derived substrate (PDB: 6IYC). Colors of γ-secretase components as in Fig. 1; APP substrate in orange. Solving the structure required disabling the active site through PSEN1 D385A mutation and crosslinking substrate to PSEN1 by cysteine mutagenesis and disulfide bond formation. Note that the N-terminal part of the APP TMD is in a helical conformation and enveloped by presenilin. (B) Close-up of the active site (not actually active due to D385A mutation) showing unwinding of the C-terminal part of the APP TMD, with β-sheet formation with presenilin.
Although the active site had been disabled through mutation, both Notch and APP substrates appeared to have the correct backbone amides in proper register for cleavage. For Notch, this is a Gly-Val amide, previously identified as the cleavage site by N-terminal sequencing of the Notch intracellular domain [12]. For APP, the exact cleavage site was unclear between a Leu-Val that would give Aβ49 and the nearby Thr-Leu bond that would give Aβ48, consistent with mass spectrometric analysis of the APP intracellular domain showing γ-secretase cleaves at either sites [39]. Meanwhile, the N-terminus of both substrates in the bound structures appears to be interacting with the nicastrin ectodomain, although this may be due to the artificial cysteine mutagenesis and crosslinking of this part of the substrate to presenilin.
Structures with co-bound compounds
The Shi lab also reported structures of the γ-secretase complex bound to small-molecule inhibitors and modulators. The first, discussed above, solved in collaboration with the Scheres lab, was the protease bound to the dipeptide inhibitor DAPT [38]. While the structure of the bound compound itself could not be discerned, electron density for the inhibitor showed binding near the active site. Intriguingly, the compound induced conformational changes in the enzyme to resolve regions not seen in previous apo-enzyme structures, including L1, TMD2 and part of TMD6. In the same study, the researchers resolved three different structural classes of apo-enzyme, including one that contained an unidentified helical density. The DAPT-bound protease structure closely overlapped with that of the enzyme bound to this helical density. Later, similar conformational changes were seen with APP- and Notch-bound enzyme [36,37], supporting the unidentified helical density as a composite of bound substrates that had co-purified. Apparently, DAPT works as a substrate mimic, inducing the enzyme into a “closed” conformation and effectively blocking lateral entry of substrate molecules into the active site.
Most recently, the Shi lab reported structures of γ-secretase bound to two former clinical candidate inhibitors as well as a transition-state analog peptidomimetic inhibitor and a modulator that stimulates Aβ42-to-Aβ38 carboxypeptidase trimming [40]. The two clinical candidates, semagacestat and avagacestat, were developed as potent inhibitors of γ-secretase activity in vivo, effectively lowering Aβ production in the brain [41,42]. However, the two compounds differ in their substrate selectivity: semagacestat inhibits APP and Notch processing by γ-secretase with equal potencies, while avagacestat displays selectivity for inhibiting cleavage of APP over that of Notch [43] (although this has been challenged [44]). Despite this apparent difference between the two compounds, both failed in late-stage clinical trials, due in part to serious side effects attributable to inhibition of Notch signaling [45,46].
Surprisingly, both of these former clinical candidates were found to bind to the same general site on presenilin where the C-terminal region of substrate TMD interacts to form a β-sheet; inhibition is apparently due to blocking this particular substrate-enzyme interaction. Avagacestat, however, binds to this region of presenilin primarily through hydrophobic interactions, while semagacestat forms four hydrogen bonds with the presenilin backbone. The bound structure of avagacestat suggests sites on this molecule that might be chemically modified to enhance potency and selectivity. Thus, elucidation of how these two compounds interact with the protease may facilitate structure-based design to improve selectivity for inhibiting APP proteolysis.
The structure of γ-secretase bound to a transition-state analog inhibitor (Fig. 3A) showed direct interaction with the two catalytic aspartates (Fig. 3B), as such inhibitors are designed to do. A key hydroxyl group that replaces the scissile amide bond of substrate mimics a gem-diol intermediate that forms upon water addition to the amide carbonyl carbon. In the cryo-EM structure, this hydroxyl group is a hydrogen bond donor to one catalytic aspartate and a hydrogen bond acceptor to the other active site aspartate. The hydrophobic side chains of the inhibitor presumably interact with active site pockets for corresponding residues of substrate. Indeed, the structure of the transition-state analog bound to the protease confirmed the identity of these key pockets in the enzyme active site observed with bound substrate [37].
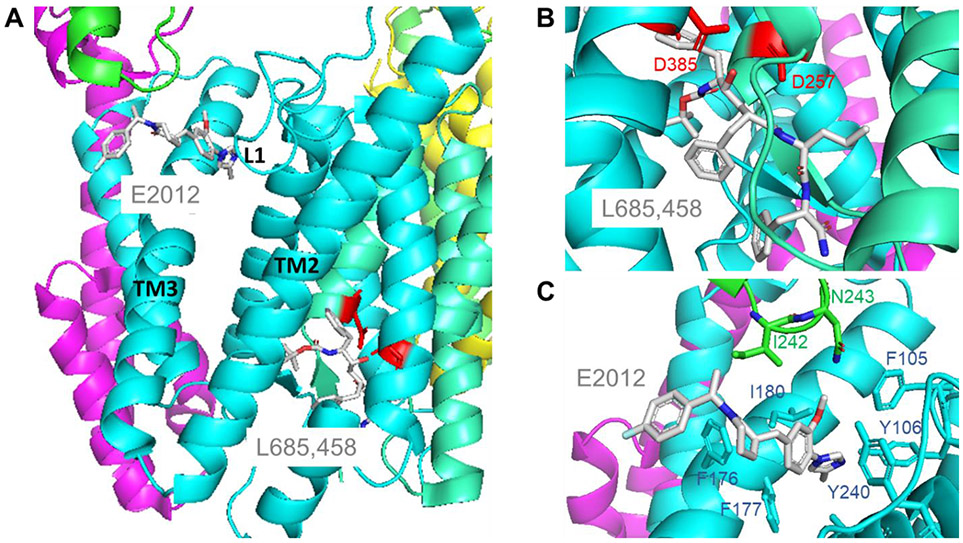
Figure 3.
(A) Structure of the γ-secretase complex bound to transition-state analog inhibitor L685,458 and to modulator E2012 (PDB: 7D8X). Colors of γ-secretase components as in Figs. 1 and 2. Bound compounds are sticks colored by atom type. Note that L685,458 binds near the catalytic aspartic acids, while E2012 binds distal to the active site. (B) Close-up of L685,458 binding. The transition-state mimicking hydroxyl group of the inhibitor is coordinated with the two catalytic aspartic acids. (C) E2012 is nestled in a binding site that becomes available upon L685,458 binding to the active site. The modulator binding site includes presenilin Loop 1 residues F105 and Y106 and TM3 residues F176, F177 and I180 and Nicastrin loop residues I242 and N243.
The transition-state analog inhibitor also proved useful for determining the structure of the protease complex bound to an allosteric modulator called E2012. This modulator compound lowers levels of the putatively pathogenic Aβ42 peptide by stimulating its trimming by γ-secretase to less aggregation-prone Aβ peptides [47]. The cryo-EM structure of bound E2012 alone could not be determined. However, because a transition-state analog inhibitor enhances binding of E2012 [48], an atomic-resolution structure of γ-secretase bound to both E2012 and the active site-directed inhibitor could be determined (Fig. 3A). E2012 was found to bind to a hydrophobic pocket formed by L1, TM3 and TM5 of presenilin and a nearby loop in the ectodomain of nicastrin (Fig. 3C). Interaction of E2012 with L1 of presenilin is consistent with a recent scanning mutagenesis studies [49]. Intriguingly, an exposed part of bound E2012 has been chemically modified for use in identifying a γ-secretase modulatory protein [50].
Molecular Dynamics Simulations
The recent cryo-EM structures of γ-secretase have provided excellent starting points for conducting all-atom molecular dynamics (MD) simulations of its intramembrane proteolytic action. In these simulations, the enzyme complex is embedded in a lipid bilayer and solvated in an aqueous medium (Figure 4A). MD simulations have provided valuable insights into the conformational changes [51,52], substrate binding [53,54], water distribution [51], lipid interactions [51] and ligand binding [54-56] of γ-secretase. However, most of the simulation studies have been based on the earlier cryo-EM structures of γ-secretase without the substrate bound. Moreover, the very slow proteolysis of APP substrate by γ-secretase (kcat ~ 2 h−1; e.g., [18,57]) has presented a challenge for MD simulations with limited timescales (typically microseconds).
Figure 4.
(A) Computational model of γ-secretase that is embedded in a lipid bilayer and solvated in 0.15 M NaCl aqueous solution. (B) All-atom GaMD simulations captured spontaneous activation of γ-secretase, during which the enzyme active site was poised for proteolysis of the APP substrate at the ε cleavage site. (C) Active (wildtype) and (D) shifted active (M51F) conformational states of the APP substrate-bound γ-secretase. Distinct APP intracellular domain (AICD) products were generated from the wildtype and M51F mutant APP.
To address the above challenge, Bhattarai et al. [58] have performed Gaussian accelerated molecular dynamics (GaMD) simulations to investigate substrate cleavage of both wildtype and mutant APP by γ-secretase (Fig. 4). GaMD is an enhanced sampling computational technique that works by applying a harmonic boost potential to reduce system energy barriers and accelerate molecular dynamics simulations by orders of magnitude [59,60]. It does not require predefined collective variables, being advantageous for unconstrained enhanced sampling of complex biological systems. Moreover, because the boost potential exhibits a Gaussian distribution, the original free energy profiles can be properly recovered for large biomolecules. GaMD has thus been applied in enhanced simulations of γ-secretase. The latest cryo-EM structure of APP-bound γ-secretase (PDB: 6IYC) was used with the catalytic aspartates restored and the artificial Cys mutagenesis and disulfide crosslinking undone. GaMD simulations captured spontaneous activation of γ-secretase (Fig. 4B). The protonated Asp257 formed a hydrogen bond with the carbonyl oxygen in Leu49 of the scissile amide bond in APP. Water molecules entered the presenilin active site. One water molecule was trapped between the two catalytic Asp residues through stable hydrogen bonds. This would induce nucleophilic attack of carbonyl carbon in Leu49 by the activated water molecule, which is a key step for enzyme proteolysis. The enzyme active site was well poised for proteolysis of wildtype APP substrate at the major endoproteolytic (ε) cleavage site to generate Aβ49 and APP intracellular domain (AICD) 50-99 (Fig. 4C). Moreover, GaMD simulations revealed that APP FAD mutations I45F and T48P preferred ε cleavage between residues Leu49-Val50, while M51F mutation shifted ε cleavage site to Thr48-Leu49, generating Aβ48 and AICD49-99 (Fig. 4D). The GaMD simulations thus successfully predicted the effects of FAD mutations on ε cleavage of the APP substrate, being highly consistent with biochemical experimental analyses of APP proteolytic products using mass spectroscopy and western blotting [58].
In another recent study, MD model was developed for FAD mutations in APP (V717I) and presenilin (E280A, G384A, A434C, and L435F) [61]. The simulations suggested that the enzyme-substrate complexes were less stable when substrate or enzyme carried an FAD mutation. However, in these simulations, both catalytic aspartates were kept in the negatively charged state, resulting in increased distance due to charge repulsion. Moreover, a single water molecule was not recruited between these aspartates in a manner seen with other aspartyl proteases.
Future Directions
Multiple atomic-resolution cryo-EM structures of the γ-secretase complex have been determined in recent years, as an apo-enzyme as well as bound to substrates and small-molecule ligands. Such structures have been used as starting points to apply sophisticated computational methods for all-atom MD simulations, and these dynamic models suggest structural mechanisms for intramembrane proteolysis by γ-secretase that are supported by biochemical experiments. Further validation of structural mechanisms could come from trapping the protease complex at the transition-state of cleaving substrate TMD. Full TMD peptidomimetics have been recently developed for this purpose [62]. MD modeling of tripeptide trimming would help elucidate the structural mechanism of the processive proteolysis that takes place after initial endoproteolysis of substrate. Understanding the normal mechanism of carboxypeptidase activity of γ-secretase is especially important, as this trimming process is deficient with FAD-mutant enzyme or APP substrate [18]. The new structures and dynamic models should also facilitate drug design, to find selective inhibitors and modulators of γ-secretase for the treatment of Alzheimer’s disease. In this context, a new report showing that high Aβ38 levels are associated with less cognitive decline and conversion to Alzheimer’s disease in clinical cohorts [63] provides important validation for γ-secretase as a viable therapeutic target, particularly modulation that stimulates trimming.
Acknowledgment
This work was supported by U.S. National Institutes of Health grants GM122894 and AG66986 to M.S.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts statement
Nothing to declare.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• Of special interest
•• Of outstanding interest
- 1.Wolfe MS: Structure and Function of the γ-Secretase Complex. Biochemistry 2019, 58:2953–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun L, Li X, Shi Y: Structural biology of intramembrane proteases: mechanistic insights from rhomboid and S2P to γ-secretase. Curr Opin Struct Biol 2016, 37:97–107. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ: Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 1999, 398:513–517. [DOI] [PubMed] [Google Scholar]
- 4.De Strooper B: Aph-1, Pen-2, and nicastrin with presenilin generate an active γ-secretase complex. Neuron 2003, 38:9–12. [DOI] [PubMed] [Google Scholar]
- 5.Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, et al. : Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 1996, 17:181–190. [DOI] [PubMed] [Google Scholar]
- 6.Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T: The role of presenilin cofactors in the γ-secretase complex. Nature 2003, 422:438–441. [DOI] [PubMed] [Google Scholar]
- 7.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C: Reconstitution of γ-secretase activity. Nat Cell Biol 2003, 5:486–488. [DOI] [PubMed] [Google Scholar]
- 8.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ: γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proc Natl Acad Sci U S A 2003, 100:6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichrath J, Reichrath S: Notch Signaling and Embryonic Development: An Ancient Friend, Revisited. Adv Exp Med Biol 2020, 1218:9–37. [DOI] [PubMed] [Google Scholar]
- 10.Sprinzak D, Blacklow SC: Biophysics of Notch Signaling. Annu Rev Biophys 2021, 50:157–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, et al. : A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 1999, 398:518–522. [DOI] [PubMed] [Google Scholar]
- 12.Schroeter EH, Kisslinger JA, Kopan R: Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 1998, 393:382–386. [DOI] [PubMed] [Google Scholar]
- 13.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S: Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 1997, 89:629–639. [DOI] [PubMed] [Google Scholar]
- 14.Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LH, Sisodia SS: Presenilin 1 is required for Notchl and DII1 expression in the paraxial mesoderm. Nature 1997, 387:288–292. [DOI] [PubMed] [Google Scholar]
- 15.Selkoe DJ, Hardy J: The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 2016, 8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanzi RE: The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2012, 2:pii: a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y: γ-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci 2009, 29:13042–13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••18. Devkota S, Williams TD, Wolfe MS: Familial Alzheimer's disease mutations in amyloid protein precursor alter proteolysis by γ-secretase to increase amyloid β-peptides of >45 residues. J Biol Chem. 2021, 296:100281. First comprehensive and quantitative analysis of effects of FAD mutations on all proteolytic processing events on APP substrate by γ-secretase.
- 19.Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, et al. : Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 2000, 405:689–694. [DOI] [PubMed] [Google Scholar]
- 20.Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai J-Y, Rahmati T, Xia W, Selkoe DJ, Wolfe MS: Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nature Cell Biology 2000, 2:428–434. [DOI] [PubMed] [Google Scholar]
- 21.Esler WP, Das C, Wolfe MS: Probing pockets S2-S4' of the γ-secretase active site with (hydroxyethyl)urea peptidomimetics. Bioorg Med Chem Lett 2004, 14:1935–1938. [DOI] [PubMed] [Google Scholar]
- 22.Kornilova AY, Bihel F, Das C, Wolfe MS: The initial substrate-binding site of γ-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A 2005, 102:3230–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato C, Morohashi Y, Tomita T, Iwatsubo T: Structure of the catalytic pore of γ-secretase probed by the accessibility of substituted cysteines. J Neurosci 2006, 26:12081–12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolia A, Chavez-Gutierrez L, De Strooper B: Contribution of presenilin transmembrane domains 6 and 7 to a water-containing cavity in the γ-secretase complex. J Biol Chem 2006, 281:27633–27642. [DOI] [PubMed] [Google Scholar]
- 25.Sato C, Takagi S, Tomita T, Iwatsubo T: The C-terminal PAL motif and transmembrane domain 9 of presenilin 1 are involved in the formation of the catalytic pore of the γ-secretase. J Neurosci 2008, 28:6264–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita T: Probing the Structure and Function Relationships of Presenilin by Substituted-Cysteine Accessibility Method. Methods Enzymoi 2017, 584:185–205. [DOI] [PubMed] [Google Scholar]
- 27.Fukumori A, Steiner H: Substrate recruitment of γ-secretase and mechanism of clinical presenilin mutations revealed by photoaffinity mapping. EMBO J 2016, 35:1628–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••28. Trambauer J, Rodríguez Sarmiento RM, Fukumori A, Feederle R, Baumann K, Steiner H: Aβ43-producing PS1 FAD mutants cause altered substrate interactions and respond to γ-secretase modulation. EMBO Rep 2020, 21:e47996. Provides evidence from mutagenesis and photo-activated crosslinking for altered substrate-enzyme interactions with PSEN1 FAD mutations.
- 29.Lazarov VK, Fraering PC, Ye W, Wolfe MS, Selkoe DJ, Li H: Electron microscopic structure of purified, active γ-secretase reveals an aqueous intramembrane chamber and two pores. Proc Natl Acad Sci U S A 2006, 103:6889–6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elad N, De Strooper B, Lismont S, Hagen W, Veugelen S, Arimon M, Horre K, Berezovska O, Sachse C, Chavez-Gutierrez L: The dynamic conformational landscape of γ-secretase. J Cell Sci 2015, 128:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu P, Bai XC, Ma D, Xie T, Yan C, Sun L, Yang G, Zhao Y, Zhou R, Scheres SH, et al. : Three-dimensional structure of human γ-secretase. Nature 2014, 512:166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE 3rd, Sudhof T, Yu G: Nicastrin functions as a γ-secretase-substrate receptor. Cell 2005, 122:435–447. [DOI] [PubMed] [Google Scholar]
- 33.Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y: An atomic structure of human γ-secretase. Nature 2015, 525:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolduc DM, Montagna DR, Gu Y, Selkoe DJ, Wolfe MS: Nicastrin functions to sterically hinder γ-secretase-substrate interactions driven by substrate transmembrane domain. Proc Natl Acad Sci U S A 2016, 113E509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou R, Yang G, Shi Y: Dominant negative effect of the Ioss-of-function γ-secretase mutants on the wild-type enzyme through heterooligomerization. Proc Natl Acad Sci U S A 2017, 114:12731–12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••36. Yang G, Zhou R, Zhou Q, Guo X, Yan C, Ke M, Lei J, Shi Y: Structural basis of Notch recognition by human γ-secretase. Nature 2019, 565:192–197. First atomic-resolution structure of γ-secretase bound to substrate.
- ••37. Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y: Recognition of the amyloid precursor protein by human γ-secretase. Science 2019, 363:eaaw0930. First atomic-resolution structure of γ-secretase bound to APP substrate, with implications for Alzheimer's disease biology and treatment.
- 38.Bai XC, Rajendra E, Yang G, Shi Y, Scheres SH: Sampling the conformational space of the catalytic subunit of human γ-secretase. Elite 2015, 4:pii: e11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu Y, Misonou H, Sato T, Dohmae N, Takio K, Ihara Y: Distinct intramembrane cleavage of the beta-amyloid precursor protein family resembling γ-secretase-like cleavage of Notch. J Biol Chem 2001, 276:35235–35238. [DOI] [PubMed] [Google Scholar]
- ••40. Yang G, Zhou R, Guo X, Yan C, Lei J, Shi Y: Structural basis of γ-secretase inhibition and modulation by small molecule drugs. Cell. 2021, 184:521–533. First atomic-resolution structures of γ-secretase bound to drug candidates for Alzheimer's disease.
- 41.Albright CF, Dockens RC, Meredith JE Jr., Olson RE, Slemmon R, Lentz KA, Wang JS, Denton RR, Pilcher G, Rhyne PW, et al. : Pharmacodynamics of selective inhibition of γ-secretase by avagacestat. J Pharmacol Exp Ther 2013, 344:686–695. [DOI] [PubMed] [Google Scholar]
- 42.Lanz TA, Hosley JD, Adams WJ, Merchant KM: Studies of Aβ pharmacodynamics in the brain, cerebrospinal fluid, and plasma in young (plaque-free) Tg2576 mice using the γ-secretase inhibitor N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo-6,7-di hydro-5H-dibenzo[b,d]azepin-7-yl]-L-alaninamide (LY-411575). J Pharmacol Exp Ther 2004, 309:49–55. [DOI] [PubMed] [Google Scholar]
- 43.Gillman KW, Starrett JE, Parker MF, Xie K, Bronson JJ, Marcin LR, McElhone KE, Bergstrom CP, Mate RA, Williams R, et al. : Discovery and Evaluation of BMS-708163, a Potent, Selective and Orally Bioavailable γ-Secretase Inhibitor. ACS Medicinal Chemistry Letters 2010, 1:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crump CJ, Castro SV, Wang F, Pozdnyakov N, Ballard TE, Sisodia SS, Bales KR, Johnson DS, Li YM: BMS-708,163 targets presenilin and lacks notch-sparing activity. Biochemistry 2012, 51:7209–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, He F, Sun X, Thomas RG, et al. : A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med 2013, 369:341–350. [DOI] [PubMed] [Google Scholar]
- 46.Coric V, Salloway S, van Dyck CH, Dubois B, Andreasen N, Brody M, Curtis C, Soininen H, Thein S, Shiovitz T, et al. : Targeting Prodromal Alzheimer Disease With Avagacestat: A Randomized Clinical Trial. JAMA Neurol 2015, 72:1324–1333. [DOI] [PubMed] [Google Scholar]
- 47.Borgegard T, Jureus A, Olsson F, Rosqvist S, Sabirsh A, Rotticci D, Paulsen K, Klintenberg R, Yan H, Waldman M, et al. : First and second generation γ-secretase modulators (GSMs) modulate amyloid-beta (Aβ) peptide production through different mechanisms. J Biol Chem 2012, 287:11810–11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pozdnyakov N, Murrey HE, Crump CJ, Pettersson M, Ballard TE, Am Ende CW, Ahn K, Li YM, Bales KR, Johnson DS: γ-Secretase modulator (GSM) photoaffinity probes reveal distinct allosteric binding sites on presenilin. J Biol Chem 2013, 288:9710–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L, Lauro BM, Wolfe MS, Selkoe DJ: Hydrophilic loop 1 of Presenilin-1 and the APP GxxxG transmembrane motif regulate γ-secretase function in generating Alzheimer-causing Aβ peptides. J Biol Chem. 2021, 296:100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •50. Hur JY, Frost GR, Wu X, Crump C, Pan SJ, Wong E, Barros M, Li T, Nie P, Zhai Y, et al. : The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer's disease. Nature 2020, 586:735–740. Describes the use of chemical probes for discovery of a modulatory protein of the γ-secretase complex.
- 51.Hitzenberger M, Zacharias M: γ-Secretase Studied by Atomistic Molecular Dynamics Simulations: Global Dynamics, Enzyme Activation, Water Distribution and Lipid Binding. Front Chem 2019, 6:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aguayo-Ortiz R, Chavez-Garcia C, Straub JE, Dominguez L: Characterizing the structural ensemble of γ-secretase using a multiscale molecular dynamics approach. Chem Sci 2017, 8:5576–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Somavarapu AK, Kepp KP: Membrane dynamics of γ-secretase provides a molecular basis for β-amyloid binding and processing. ACS Chem Neurosci 2017, 8:2424–2436. [DOI] [PubMed] [Google Scholar]
- 54.Petit D, Hitzenberger M, Lismont S, Zoltowska KM, Ryan NS, Mercken M, Bischoff F, Zacharias M, Chávez-Gutiérrez L: Extracellular interface between APP and Nicastrin regulates Aβ length and response to γ-secretase modulators. EMBO J 2019, 38:e101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gertsik N, Ende CWA, Geoghegan KF, Nguyen C, Mukherjee P, Mente S, Seneviratne U, Johnson DS, Li YM: Mapping the Binding Site of BMS-708163 on γ-Secretase with Cleavable Photoprobes. Cell Chem Biol 2017, 24:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hitzenberger M, Zacharias M: Uncovering the Binding Mode of γ-Secretase Inhibitors. ACS Chem Neurosci 2019, 10:3398–3403. [DOI] [PubMed] [Google Scholar]
- 57.Kamp F, Winkler E, Trambauer J, Ebke A, Fluhrer R, Steiner H: Intramembrane proteolysis of β-amyloid precursor protein by γ-secretase is an unusually slow process. Biophys J 2015, 108:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••58. Bhattarai A, Devkota S, Bhattarai S, Wolfe MS, Miao Y: Mechanisms of γ-Secretase Activation and Substrate Processing. ACS Cent Sci 2020, 6:969–983. First all-atom molecular dynamics model for γ-secretase catalysis of APP substrate proteolysis.
- 59.Miao Y, Feher VA, McCammon JA: Gaussian Accelerated Molecular Dynamics: Unconstrained Enhanced Sampling and Free Energy Calculation. J Chem Theory Comput 2015, 11:3584–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Arantes PR, Bhattarai A, Hsu RV, Pawnikar S, Huang YM, Palermo G, Miao Y: Gaussian accelerated molecular dynamics (GaMD): principles and applications. Wiley Interdiscip Rev Comput Mol Sci 2021, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dehury B, Somavarapu AK, Kepp KP: A computer-simulated mechanism of familial Alzheimer's disease: Mutations enhance thermal dynamics and favor looser substrate-binding to γ-secretase. J Struct Biol 2020, 212:107648. [DOI] [PubMed] [Google Scholar]
- •62. Bhattarai S, Devkota S, Wolfe MS: Design of Transmembrane Mimetic Structural Probes to Trap Different Stages of γ-Secretase-Substrate Interaction. J Med Chem 2021, 64:15367–15378. Describes stoichiometric peptidomimetic inhibitors of γ-secretase developed as structural probes to capture transition states of intramembrane proteolysis.
- •63. Cullen N, Janelidze S, Palmqvist S, Stomrud E, Mattsson-Carlgren N, Hansson O: Association of CSF Aβ38 Levels With Risk of Alzheimer Disease-Related Decline. Neurology 2021, online ahead of print. Provides important new clinical evidence supporting γ-secretase modulation as a viable therapeutic strategy for the potential prevention of Alzheimer's disease.