Summary
Pregnancy is a unique condition where the maternal immune system is continuously adapting in response to the stages of fetal development and signals from the environment. The placenta is a key mediator of the fetal/maternal interaction by providing signals that regulate the function of the maternal immune system as well as provides protective mechanisms to prevent the exposure of the fetus to dangerous signals. Bacterial and/or viral infection during pregnancy induce a unique immunological response by the placenta, and type I interferon is one of the crucial signaling pathways in the trophoblast cells. Basal expression of type I interferon-β and downstream ISGs harbors physiological functions to maintain the homeostasis of pregnancy, more importantly, provides the placenta with the adequate awareness to respond to infections. The disruption of type I interferon signaling in the placenta will lead to pregnancy complications and can compromise fetal development. In this review, we focus the important role of placenta derived type I interferon and its downstream ISGs in the regulation of maternal immune homeostasis and protection against viral infection. These studies are helping us to better understand placental immunological functions and provide a new perspective for developing better approaches to protect mother and fetus during infections.
Keywords: Interferon stimulated genes, ISG, placenta, trophoblast, type I interferon, pregnancy
1. Introduction
The challenge of managing pregnant women during pandemics and their exclusion from early vaccine clinical trials, has, once more, revealed the importance of understanding the function and the role of the immune system during pregnancy. In the last decade, our knowledge of the adaptational changes occurring to the maternal immune system throughout pregnancy, especially during early pregnancy, has advanced significantly. From being focused on the process of tolerance to paternal antigens 1 and the breach of this tolerance being considered as the main cause of pregnancy loses and other clinical complications; we now have a better comprehension of the immunological functions on reproductive organs and their cellular immune component. We have achieved a better comprehension associated with the specific role of the immune cells present at the implantation site and their critical role in supporting many of the physiological changes that take place at the uterus, which are necessary for implantation, placentation, and fetal growth. In addition, we know that pregnant women can controll viral infections in most cases, and vaccination has beneficial effects to both the mother and the fetus. But pregnancy is not a static process, it is an evolving and continuously changing process; accordingly, the maternal immune system changes in response to the environment and signals originated from the placenta and the fetus. In this review we will discuss first the normal role of the immune system during early pregnancy, its interaction with the fetal/placenta interface; and second, we will review the mechanism by which the placenta provides an immune modulatory function to protect the fetus and the mother against infections.
2. Immune Homeostasis of Pregnancy
Pregnancy is a unique period that requires major adaptational processes that involve complex interactions between fetus and mother. During the different trimesters of pregnancy, the maternal immune system must undergo highly dynamic and regulated transformation and adaptation throughout gestation, as it must avoid rejecting a semiallogeneic fetus and remain competent to fight against infections. In general, pregnancy can be divided into four critical periods that are associated with implantation, placentation, fetal growth, and parturition. In each of these periods the immunological milieu changes in response to the developmental stages of the fetus; or, as discussed below, it can also change in response to environmental factors, primarily infections, which can consequently impact the outcome of the pregnancy.
Embryo implantation is the critical first step for the establishment of pregnancy. Interestingly, inflammation is required for successful implantation. Starting with the apposition and attachment of blastocyst to the endometrial surface epithelium, the uterus must be receptive to embrace the implanting blastocyst during a specific period named the implantation window. Therefore, the uterus is primed to activate pro-inflammatory signals, such as prostaglandin E2 (PGE2) 2 and produce a range of cytokines, including interleukin-6 (IL-6), Leukemia Inhibitory Factor (LIF), IL-15, tumor necrosis factor (TNF), and chemokines, including CXC-chemokine ligand 8 (CXCL8), CXCL10 and CC-chemokine ligand 2 (CCL2), which can be secreted by endometrial stromal cells and immune cells that are recruited to the implantation site 3–6. For the role of these pro-inflammatory cytokines, it has been demonstrated that IL-15 and IL-18 can stimulate the activation of uterine natural killer (uNK) cells and regulate the inflammation process during implantation. In addition, TNF-α, IL-1β, IL-1RA, and IL-12 are involved in the extracellular remodeling and trophoblast invasion, which are critical for implantation and placentation 7–9.
Using in vitro models of implantation, we demonstrated that TNFα promotes the expression of inflammatory factors from endometrial stroma cells, and these inflammatory factors foster trophoblast differentiation, which grants them the capacity to migrate and invade into the uterine compartment 10. One of the cytokines induced by TNFα in human endometrial stroma cells (hESC) was IL-17. We found that the IL-17 pathway is enriched in trophoblasts during the time of implantation. IL-17 interacts with trophoblast cells and induces the expression of genes responsible for promoting trophoblast migration and invasion 10 (Figure 1). The findings from this study demonstrated the presence of an inflammatory network led by TNFα that promotes the expression and secretion of pro-inflammatory cytokines, such as IL-17 in hESC.
Figure 1. Immune network responsible for embryo implantation and placentation.
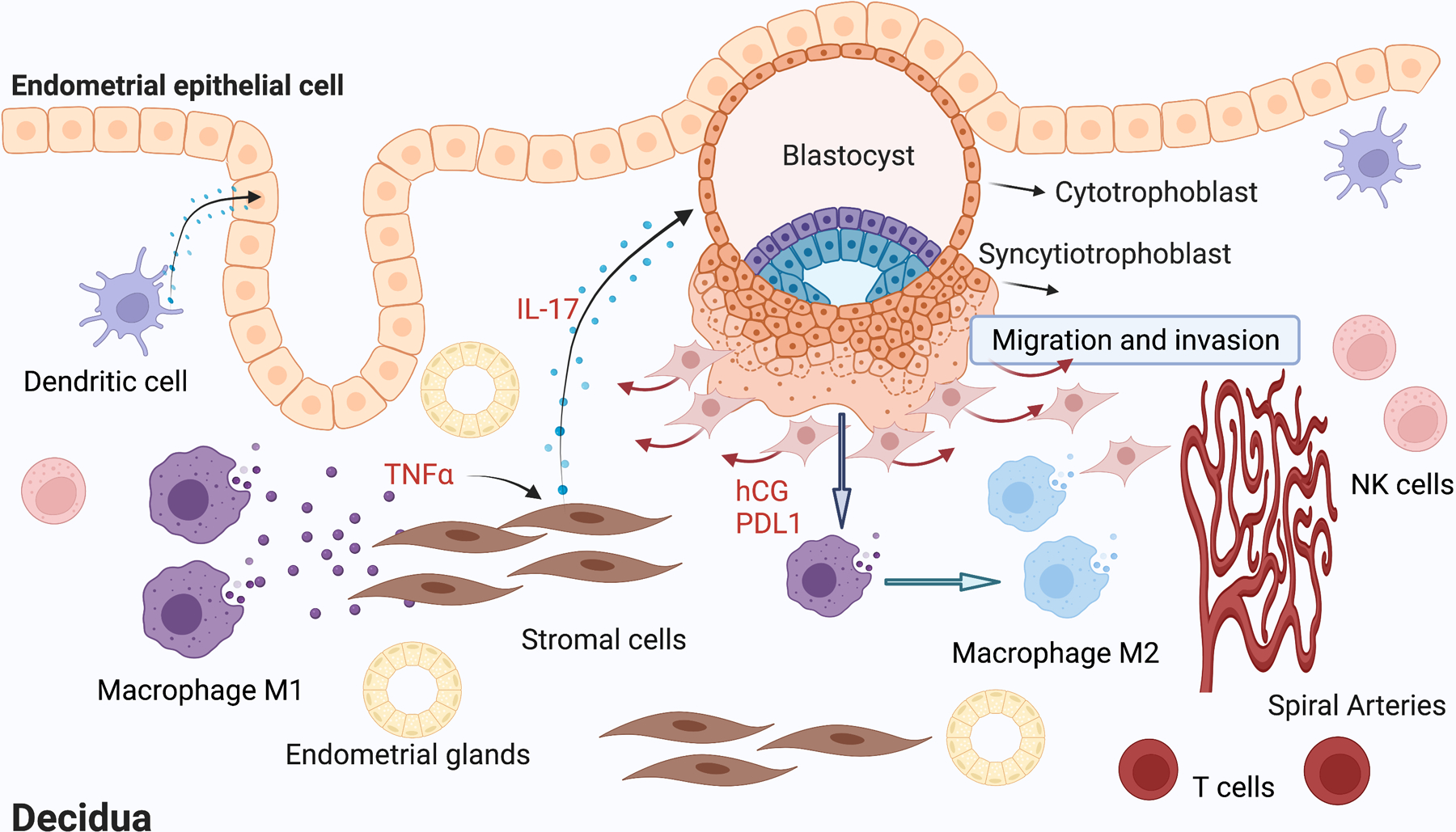
During the peri-implantation period, activated macrophages (M1 phenotype) secret TNFα, which induces the expression and secretion of pro-inflammatory cytokines such as IL-17 in human endometrial stroma cells. IL-17 by acting on trophoblast cells promotes the expression of factors necessary for trophoblast migration and invasion. As the trophoblasts invade deeply into the endometrial stroma and form the placenta, increasing levels of hCG inhibit the expression of pro-inflammatory cytokines by endometrial stroma cells and prevents the recruitment of CD8+ T cells, which is necessary for preventing fetal rejection. Moreover, PD-L1 secreted by trophoblast cells promotes the shift of decidual macrophages into M2 phenotype that decreases inflammation and supports tolerance. IL-17, interleukin-17; hCG, human chorionic gonadotropin; TNFα, tumor necrosis factor α; NK, natural killer; PDL1, programmed cell death ligand 1. Figure created with BioRender.com
These in vitro findings are supported by clinical observations. In cases of infertility or implantation failure, local injury, as result of endometrial biopsy, before in vitro fertilization can significantly increase implantation rates 11,12, which is suggested to be caused by the induction of the pro-inflammatory molecules necessary for implantation 13,14. Indeed, the mechanism by which the biopsy treatment increases endometrial receptivity demonstrates that the local injury induces an inflammatory response that is characterized by elevated levels of pro-inflammatory cytokines/chemokines, such as macrophage inflammatory protein-1β (MIP-1β), TNFα, growth regulated oncogene α (GROα), osteopontin (OPN) and IL-15 13. In addition, there is an increase in the number of innate immune cells, particularly macrophages, dendritic cells (DCs), and a unique population of natural killer (NK) cells with a CD56hiCD57lo phenotype. Moreover, it has been shown that the use of non-steroidal anti-inflammatory drugs around conception is associated with increased risk of miscarriage 15. Taken together, these findings emphasize the requirement for a pro-inflammatory environment for a successful embryo implantation. However, the localized inflammation during implantation must be tightly controlled, otherwise embryo implantation will be impaired due to the disturbance of the dynamic balance between pro- and anti-inflammatory mediators 16,17.
Interestingly, recent findings from our group have shown that, during early pregnancy the IL-10 to TNFα ratio in normal pregnancies was significantly higher than in patients with pregnancy loss 18. Furthermore, the early pro-inflammatory profile is followed by a clear shift toward an anti-inflammatory profile immediately after implantation; characterized by an increase in IL-10, decrease in TNFα, and increasing IL-10/ TNFα ratio. Contrary to successful pregnancies, pregnancy loss was associated with a failure in this shift, with no increase in neither IL-10 alone nor in the IL-10/ TNFα ratio. These findings demonstrate that an inflammatory condition is necessary for embryo implantation, however, once implantation is complete, the shift from inflammation to anti-inflammation is necessary for the maintenance of the pregnancy. Furthermore, the role of inflammation during implantation is evolutionary conserved between multiple species. Griffith et al. showed that inflammation is observed in Marsupials as well as Eutherians 19. While in the marsupials, the pro-inflammatory process continues after the early implantation leading to detachment from the epithelium of the lumen, in eutherians, there is a shift to anti-inflammatory cytokines that allow the continuation of the pregnancy. The lack of the inflammatory shift observed in the marsupials may be associated with the inadequate trophoblast migration and invasion necessary for placentation 19. Consequently, although inflammation is essential for embryo attachment and early implantation, the shift towards an anti-inflammatory condition is essential for the successful maintenance of the pregnancy 20. A major unanswered question is what are the factor(s) regulating or promoting the shift from pro-inflammatory to anti-inflammatory?
The answer to this question may exist in the signals that originate from the fetus; many of which have immune modulatory properties. We postulate that embryo-derived factors need to communicate with stromal and immune cells at the maternal/fetal immune interface, which helps to prevent a continuous inflammatory condition. One of the earliest immune modulatory factors secreted by the embryo is human chorionic gonadotropin (hCG), which can determine the function and differentiation of T cells 21–23, B cells, and dendritic cells 24,25. hCG has been suggested to induce the immune modulatory changes seen in the phenotype of B-cells and also modulate the function of immune cells either through a direct pathway that involves the direct binding of hCG to its receptor on T and B cells 23,26 or indirect pathways by inducing changes in regulatory cell populations, such as dendritic cells 27–29. In our study, we observed that endometrial stroma cells are an earlier target of hCG. The stroma initially supports inflammation under the influence of TNFα 10 by the secretion of pro-inflammatory cytokines and chemokines, which are required for blastocyst attachment and early trophoblast invasion 30,31. As the placenta forms, increasing levels of hCG inhibit the expression of those pro-inflammatory cytokines by endometrial stroma cells and prevents the recruitment of immune cells, such as CD8+ T cells, that could reject the placenta 30.
In addition, macrophages are another important cellular component modulating the inflammatory milieu at the implantation site 32. During early implantation, macrophages present at the decidua are M1 type 33,34 and produce inflammatory cytokines such as TNFα 35; necessary for the modulation of cytokines and chemokines produced by the stroma 36. Consequently, the modification of the type of macrophages present in the second phase of the implantation process is critical for the inflammatory shift. Again, signals derived from the embryo are critical for this shift. We reported that first trimester trophoblast cells secret Interferon Stimulated Genes (ISGs) 37, such as programmed death-ligand 1 (PDL1), which can induce the polarization of M1 into M2 macrophages, consequently decreasing inflammation 38,39 (Figure 1).
The last immunological phase of pregnancy is associated with the process of parturition. The maturing fetus has completed most of its development. Now, fetal signals will promote the switch to a pro-inflammatory in various feto-maternal tissue to ensure proper delivery. Many biochemical and endocrine factors are involved in the parturition event, such as platelet activation factor 40, endothelins 41–43, transforming growth factor 44, and platelet-derived growth factor, which will contribute to the pro-inflammatory environment. Apart from these factors, fetal membrane senescence and the inflammation induced by this process acts as a paracrine signaling system during parturition, as it has been shown that fetal cell senescence promotes the release of inflammatory senescence-associated secretory phenotype (SASP); a set of inflammatory biomarkers associated with term labor 45,46. However, the premature activation of fetal membrane senescence is a characteristic observed in preterm birth (PTB) and preterm premature rupture of the fetal membranes (pPROM) 46. Therefore, parturition is a timed event characterized by the recrudescence of an inflammatory process, which promotes uterine contraction, expulsion of fetus, and rejection of the placenta 5.
In summary, pregnancy is a unique immunologic condition, which requires the precise switching between different immune statuses during the three developmental trimesters, any disturbance to the dynamic balance will be detrimental to pregnancy progression, resulting in poor pregnancy outcomes and complications. Thus, the success of pregnancy greatly depends on the capability of the maternal immune system and the local signals from the maternal-fetal interface to control and regulate the immune response during pregnancy.
3. The Role of the Placenta as an Immune Modulatory Organ
Infections pose a significant threat to pregnancy and to the well-being of the fetus. Bacterial and viral infections are considered as the two types of microorganisms that have been proven to be of major risk for the normal success of the pregnancy. There are strong clinical links between bacterial infection and preterm birth 47–50. Indeed, infections have been reported to be responsible for up to 40% of preterm birth cases 51,52. Furthermore, 80% of preterm deliveries occurring at less than 30 weeks of gestation have evidence of some types of infection 49,50. Various experimental in vivo models have demonstrated that delivery of infectious components triggers preterm delivery, fetal damage, and even maternal death 53–55. Clinical studies have also correlated placental infection and inflammation with prematurity 56,57. Maternal Immune Activation (MIA) is associated with the higher risk for developmental problems in the offspring, such as autism spectrum disorders (ASD), schizophrenia, and allergies 58, which is supported by many experimental studies 59–63.
Growing literature suggests that the way in which a microorganism can induce a pregnancy complication, such as preterm birth, involves the dysregulation of the innate immune responses towards the pathogen, leading to excessive inflammation and apoptosis at the maternal-fetal interface 64,65. Meaning the same cells under normal conditions that promote fetal acceptance may initiate a detrimental immune response, triggered by infection, that promote fetal rejection 66–68. However, a new paradigm is being established in terms of the immunological response of the mother to micro-organisms. Contrary to previous thoughts that would focus only on the maternal immunologic response to the pathogens, it is now evident that the overall response during pregnancy is determined and influenced by a specific and active responses from the fetal/placental unit. In other words, the immunological response to infections during pregnancy is the result of the combination of signals originated from the maternal immune system and the fetal-placental unit, leading to a unique immunological response (Figure 2). Although the fetal immune system can recognize and respond, it is mainly the signals originating from the placenta that will modulate how the maternal immune system will behave in the presence of different pathogens. Therefore, it is crucial to understand how the placenta responds to dangerous signals and the modulatory role of placental signaling on maternal immune response.
Figure 2. Unique immunological response to infection during pregnancy.
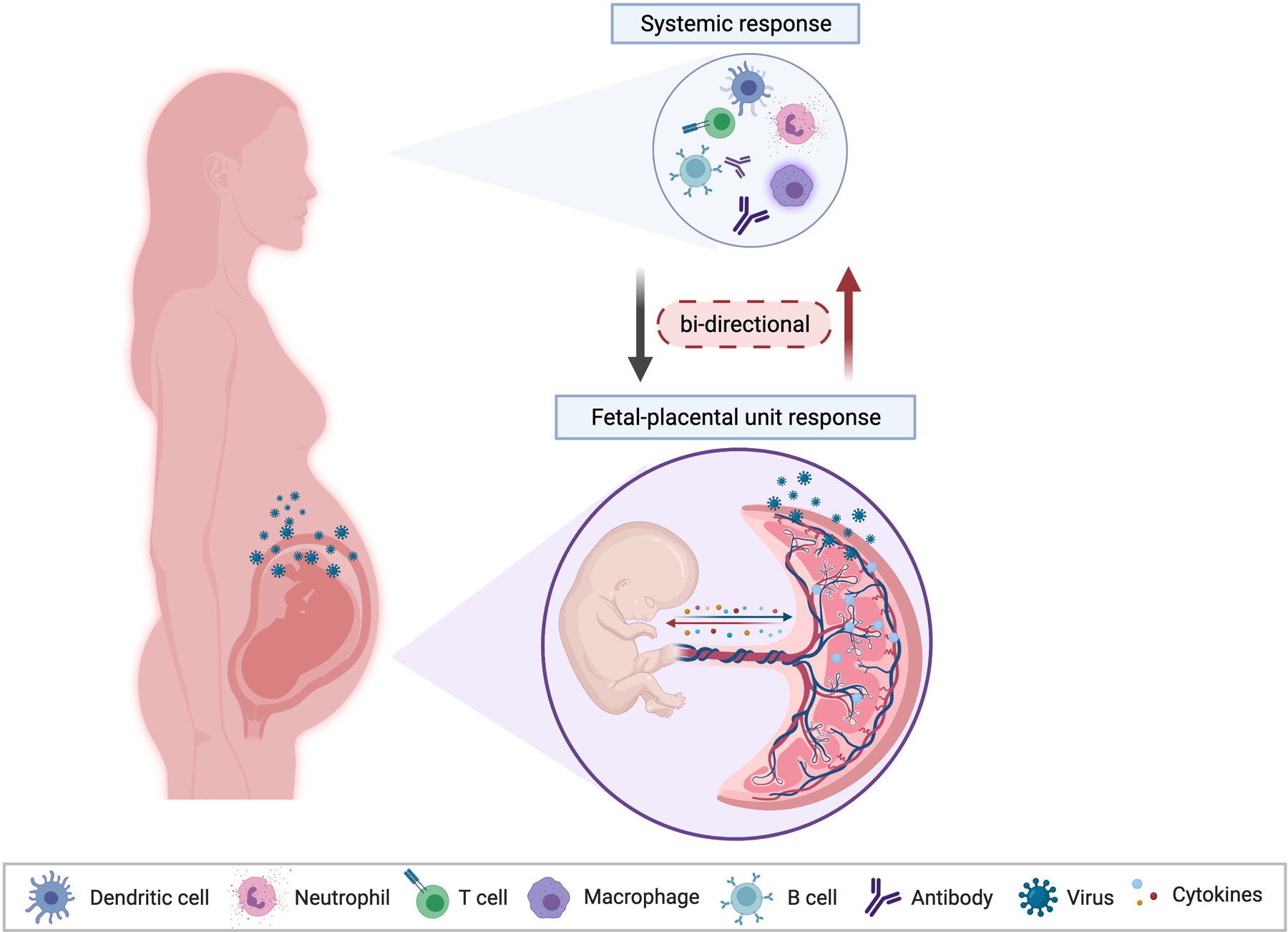
Maternal immunological responses to infection during pregnancy is complex and is strongly influenced by signals originated from the fetus and the placenta. Fetal and placenta signals have a selective effect on the type of immune cells recruited to the site of the infection. In a two-way direction, the maternal immune response will also impact the development of placenta and fetus. Therefore, the immunological response to infections during pregnancy is the result of the combination of signals originated from the maternal immune system and the fetal-placental unit. Figure created with BioRender.com
Following implantation and early placentation, the placenta functions as the primary organ to exchange nutrients, gases, and metabolic waste products between maternal and fetal blood. In this stage, there is rapid growth and development of fetus, which requires an undisturbed environment that, otherwise, will impact fetal development. The trophoblast represents the first point of contact between the blastocyst and the maternal decidua and, as discussed above, has an active role in shaping the immunological milieu at the implantation site 69. Trophoblast cells of human placenta, which are derived from the outer cell mass of the blastocyst, not only serving as the means for nutrient exchange, but also protecting the fetus from foreign pathogen attack. As early as nine days after implantation, the embryo is surrounded by two layers of trophoblast cells: the inner mononuclear cytotrophoblasts (CTBs) and the outer multinucleated syncytiotrophoblasts (STBs) layer. As the outermost layer of human placenta, STBs are highly resistant to infections, including Listeria monocytogenes, Toxoplasma gondii, HCMV, HSV1, and ZIKV 70–77. However, when compared to the low susceptibility of STBs, CTBs are more susceptible to infections. For example, STBs of first-trimester chorionic villi are mostly resistant to HCMV infection, while CTBs and other cells of the villous core are more susceptible 73. Consistently, the primitive trophoblasts, which represent the early phase of trophoblast cell development during implantation, are sensitive to ZIKV infection but become resistant after the formation of syncytium 78. Therefore, CTBs, which are considered as placental stem cells, are more susceptible to infections when compared with STBs.
Trophoblast cells can attract and educate immune cells, and in addition, respond to signals from the microenvironment in a unique way that supports decidual differentiation, angiogenesis, spiral artery remodeling, placental, and fetal development 3,79–89. Trophoblast have the ability to sense and respond to their microenvironment through the expression of Pattern Recognition Receptors (PRRs), such as Toll-Like Receptors (TLRs) 62,68,86,90–100 and NOD-like receptors (NLRs) 101–106, which can recognize specific molecular patterns in the microenvironment. These receptors are able to recognize damage-associated molecular patterns (DAMPs) 107,108, as well as, pathogen-associated molecular patterns (PAMPs) from bacteria, viruses, and other microbes 68,93,100,109–111.
The classical response to bacterial lipopolysaccharides (LPS) involves the recognition by TLR4, subsequent activation of nuclear factor-kappa-B (NF-κB) through the myeloid differentiation factor 88 (MyD88) dependent pathway, and finally the production of chemokines and cytokines (e.g. IL-1β and TNFα) 112–120. A second major group of cytokines that are induced by LPS signals through TLR4 is type I IFN-β and IFN-α, which is mediated by the MyD88 independent pathway via TIR domain-containing adaptor-inducing interferon-β (TRIF) and interferon regulatory factor 3 (IRF3) 121,122. We found that in the trophoblast, TLR4 ligation by LPS, at low concentrations, is mainly associated with the expression of IFN-β through the MyD88 independent pathway 37,123. TLR4 ligation induces IFN-β expression by the phosphorylation of tank-binding kinase (TBK) and IRF3, which are the main regulators of type I IFN expression 122,124,125. Exposure of trophoblast cells to LPS in the presence or absence of the TBK inhibitor BX795 126,127, prevents LPS/TLR4-induced IFN-β expression. Furthermore, IFN-β basal levels in the placenta from Tlr4−/− or Trif−/− mice are significantly reduced 37. These findings emphasize a potential role of commensal bacteria-derived factors maintaining IFN-β basal expression through the TLR4/TBK/IRF3 pathway in trophoblasts. We postulate that this basal IFN-β expression is essential for maintaining immune homeostasis and to prevent viral replication to reach the fetus (Figure 3).
Figure 3. Regulation of IFN-β basal expression through TLR4/TBK/IRF3 pathway.
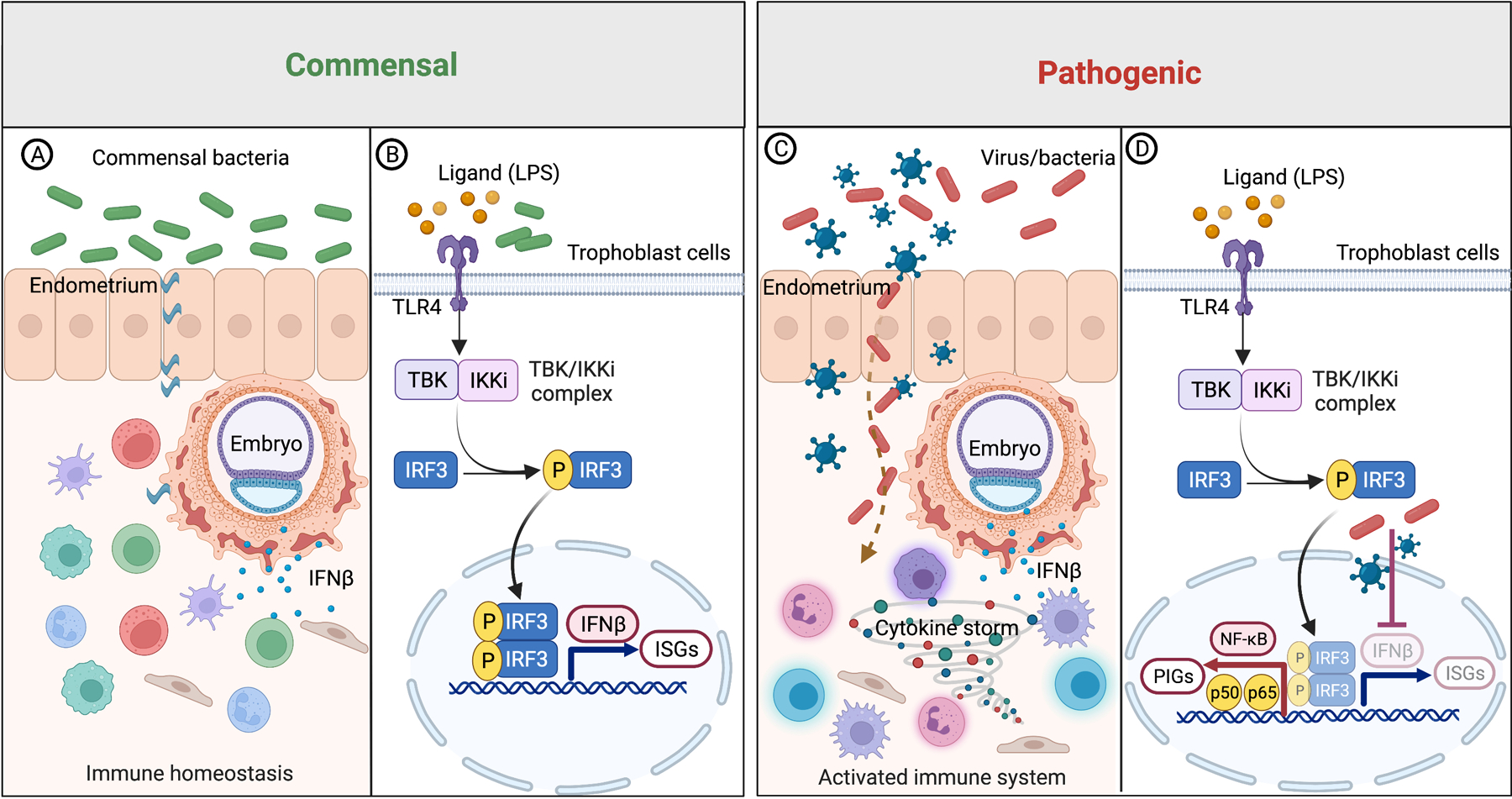
(A-B) Signals derived from commensal bacteria maintains IFN-β basal expression through TLR4/TBK/IRF3 pathway. Basal IFN-β promotes the expression of immune modulatory ISGs, as well as provide awareness and protection against microbial (bacteria & viruses) infections.
(C-D) Disruption of the TLR4/TBK/IRF3 pathway, e.g., viral infections, blocks IFN-β basal expression in trophoblast cells, leading to decreased protection against pathogenic bacteria and a shift towards the NF-κB pathway and induction of a cytokine storm of pro-inflammatory factors such as IL-1β and TNFα.
LPS, lipopolysaccharides; TLR4, toll like receptor 4; TBK1, TANK binding kinase 1; IKKi, inhibitor of nuclear factor kappa B kinase subunit epsilon; IRF, interferon regulatory factor; IFN-β, interferon-β; ISGs, interferon stimulated genes; NF-κB, nuclear factor-kappa-B; PIGs, pro-inflammatory genes. P, phosphorylation. Figure created with BioRender.com
4. Type I Interferon-Beta Protection and Immune Modulation During Pregnancy
Type I IFNs (IFN-α and IFN-β) are polypeptides able to induce an anti-microbial state, modulate innate immune responses, and induce the activation of the adaptive immune system 128,129. There are three IFN types: type I IFN, type II IFN, and type III IFN. Type I IFN, is the largest IFN class, including IFN-α, IFN-β, IFN-ε, IFN-κ, and IFN-ω. IFN-α and IFN-β are the most well-defined type I IFNs. IFN-β is encoded by a single gene, whereas IFN-α comprises 13 subtypes in human 130,131. Type I IFN responses can be induced by both viral and bacterial pathogens. These pathogens are detected by TLRs, NLRs, RIG-I-like receptors (RLRs), and cGAS which promote signaling through Stimulator of IFN Genes (STING) 132,133. Constitutive, baseline expression of type I interferon is very low in most tissues and can rapidly be triggered by viral or bacterial infection 134. Following secretion from cells, type I IFNs mediate their effects by binding to its cell surface receptor, the type I Interferon associated receptor (IFNAR), and activating members of the janus kinase (JAK) family 135,136. Activated JAK kinases phosphorylate the signal transducers and activators of transcription (STAT) family of transcription factors that homo-or heterodimerize and form complexes (GAS; ISGF3) with other transcription factors to activate transcription of ISGs (Figure 4A). ISGs are the primary effectors of type I IFN mediated biological responses 128,137, which act as downstream functional proteins of IFN signaling. They exert multiple effects in many different aspects. Depending on cell type, IFN doses and time of treatment, 50–1000 ISGs have been identified by microarrays 138–140. ISGs profiles induced by three different types of IFN are unique but partially overlapped 141. However, some ISGs can be directly induced by viral infection independent of IFN production 142.
Figure 4. Regulation of IFN-β expression and function in trophoblast cells.
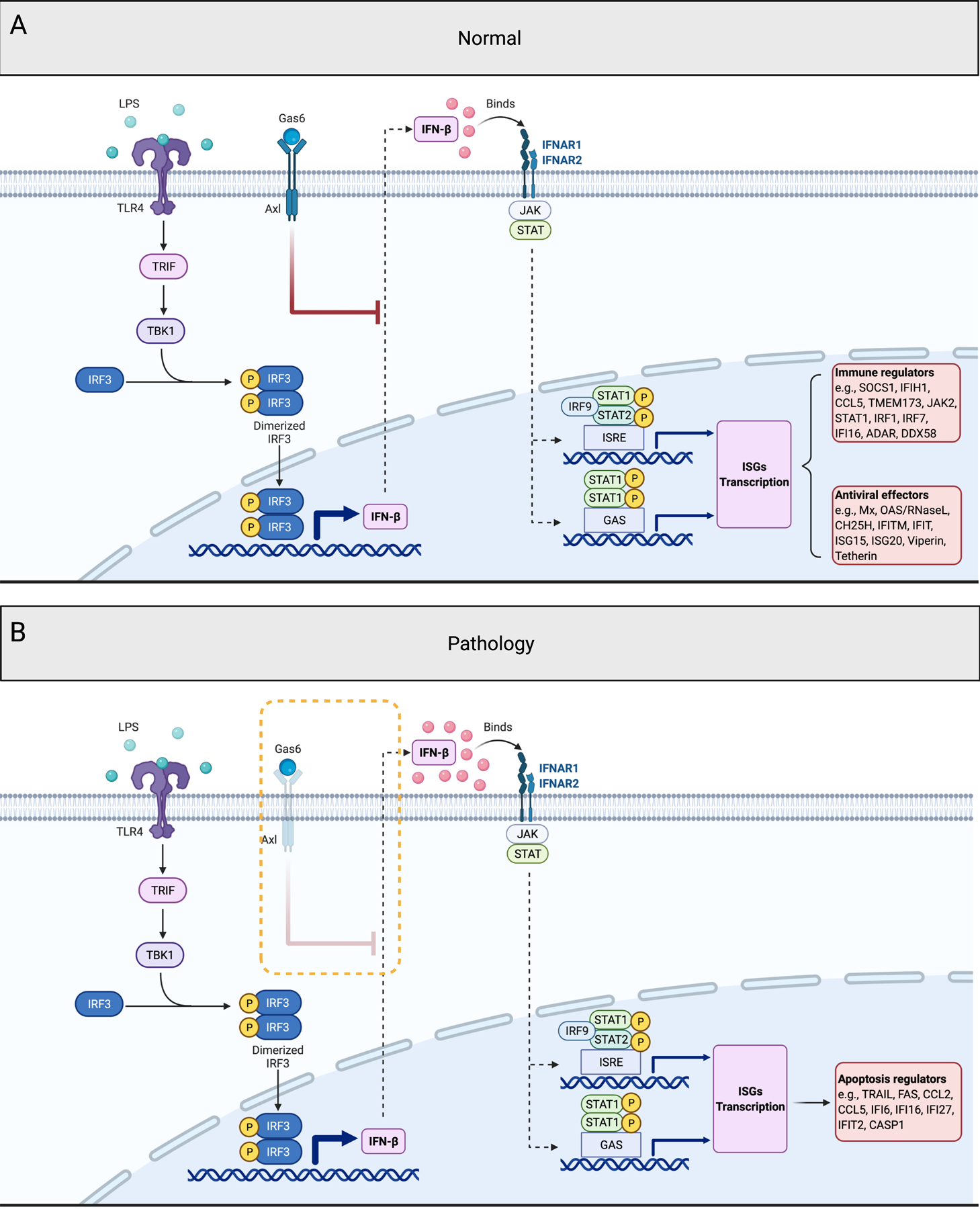
(A) Normal regulation of IFN-β expression and function in trophoblast cells. In normal condition, LPS binds to TLR4 induces IFN-β expression by the phosphorylation of TRIF/TBK1/IRF3, and secreted IFN-β binds to IFNAR and stimulates numerous ISGs expression. These ISGs function as the immune regulators and anti-viral effectors to maintain cellular homeostasis and to inhibit viral replication. Axl and Axl ligand Gas6, inhibits chronic IFN-β expression and its potential toxic effects.
(B) Effect of deletion of Axl on trophoblast function. Lack of Axl expression in trophoblast cells leads to the loss of negative regulation on IFNβ expression, followed by enhanced IFN-β expression and the selective induction of apoptosis related ISGs, such as TRAIL, FAS and CASP1.
LPS, lipopolysaccharides; TLR4, toll like receptor 4; TRIF, TIR-domain-containing adapter-inducing interferon-β; TBK1, TANK binding kinase 1; IRF, interferon regulatory factor; IFN-β, interferon-β; Gas6, growth arrest specific 6; Axl, AXL receptor tyrosine kinase; IFNAR, interferon alpha and beta receptor subunit; JAK, janus kinase; STAT, signal transducer and activator of transcription; ISRE, interferon-stimulated response element; GAS, gamma-activated sequence; SOCS1, suppressor of cytokine signaling 1; IFIH1, interferon induced with helicase c domain 1; CCL, C-C motif chemokine ligand; TMEM173, transmembrane protein 173; IFI, interferon inducible protein; ADAR, adenosine deaminase RNA specific; DDX58, DExD/H-box helicase 58; Mx, MX dynamin like GTPase; OAS, 2’−5’-oligoadenylate synthetase; CH25H, cholesterol 25-hydroxylase; IFITM, interferon induced transmembrane protein; IFIT, interferon induced protein with tetratricopeptide repeats; ISG20, interferon stimulated exonuclease gene 20; ISG15, ISG15 ubiquitin like modifier; Viperin/RSAD2, radical S-adenosyl methionine domain containing 2; Tetherin/BST2, bone marrow stromal cell antigen 2; TRAIL/TNFSF10, TNF superfamily member 10; FAS, Fas cell surface death receptor; CASP1, caspase 1; P, phosphorylation. Figure created with BioRender.com
While identified and named for their ability to interfere with viral replication in infected cells, IFNs, through specific ISGs, have immunomodulatory, cell differentiation, anti-angiogenic, and pro-apoptotic effects 143. However, in addition to the protective effect, type I IFNs can have deleterious effects and promote complications, such as autoimmune diseases that are associated with excessive or chronic type I IFN responses. Therefore, regulation of their expression and function is critical for an efficient immune response and maintenance of tissue homeostasis.
In the placenta, Type I IFN expression is known to be a characteristic in several species, including humans 71,144–153; and IFN-β is the predominant class, especially during the first trimester 6,154–161. In the context of pregnancy, we have shown that loss of IFN-β signaling in the placenta leads to: 1) uncontrolled viral replication and fetal viral infection 123, 2) maternal mortality 123 and 3) hypersensitivity to bacterial products 162; suggesting a critical role of IFN-β signaling in the protection of pregnancy. Therefore, it is very important to better understand the function of type I IFN during gestation and the downstream regulatory genes for the development of therapeutic approaches against viral infections during pregnancy.
5. Basal Type I Interferon Signaling
Many cell types produce IFN-β, and due to the widespread expression of IFNAR, JAK1, STAT1, STAT2, and IRF9, type I IFN signaling pathway is inducible in most cell types. Constitutive IFN-β is an essential component in healthy animals, and is necessary for multiple biological functions, including maintenance of the hematopoietic stem cell niche, bone remodeling, and numerous immune cell functions. Disturbance of IFN-β signaling contributes to the pathology of antiviral immunity, autoimmune disease, and cancer 135. A number of studies suggested that the basal expression of IFN-β under homeostatic conditions partly depends on the commensal microbial flora, which can systemically calibrate type I IFN responses 163 164 165,166, and the basal IFN-β production by commensal microbiota might be mediated through TLR signaling 163,167.
Basal IFN-β expression and the attendant tonic IFNAR signaling enables immune cells to rapidly mobilize an anti-microbial response 128. In macrophages, for example, constitutive IFN-β is important for maintaining their phagocytic potential 168,169, and elimination of endogenous IFN-β impairs bacterial clearance by macrophage 170. Furthermore, constitutive IFN-β expression shows great importance for NK cell homeostasis and antitumoral function as well. It has been demonstrated that IL-15 expression, which is a type I IFN-regulated gene, is maintained by basal IFN-β expression and is necessary for NK cell proliferation 171–174 as the number of NK cells is significantly lower in IFNAR1−/− or IFNAR2−/− mice compared with wild type mice. In addition, the lack of basal IFN-β secretion and decreased NK activity is associated with increased susceptibility to tumor formation 175. Thus, these findings highlight the critical role of basal IFN-β signaling as a physiological pathway to maintain cell homeostasis and prime cells to exert a rapid and potent innate and adaptive immune response to pathogenic challenges. Conversely, loss of basal IFN-β signaling results in aberrant immune cell homeostasis and function, bone remodeling, and impaired antiviral and antitumor immunity.
During pregnancy, the normal IFN-β signaling is necessary for the maintenance of a healthy pregnancy, therefore, women with dysregulated type I IFN signaling exhibit adverse pregnancy outcomes, including preeclampsia, preterm birth, and neurodevelopmental defects in the fetus 176–180. Noteworthy, high levels of type I IFN during pregnancy can be pathogenic, as it has been shown that pregnant lupus patients who have preeclampsia and other fetal complications express high level of ISG expression in their peripheral blood mononuclear cells (PBMCs). Moreover, it has been demonstrated that PAMP-driven activation of type I IFN is sufficient to prime for systemic and uterine proinflammatory cytokine and chemokine production which leads to preterm birth. In response to TLR3 ligation by Poly(I:C) or viral infection in in vitro and in vivo models, IFN signaling can lead to trophoblast apoptosis and fetal demise 62,68,181,182. Therefore, these findings suggest the importance of a balanced state of Type I IFN expression during pregnancy. Noteworthy, this type I IFN priming effects are conserved from mice to nonhuman primates and humans 177.
During pregnancy the placenta is continuously exposed to bacterial products either through the maternal blood or originating from the lower reproductive track. Similar as the data shown for NK or macrophages, we observed that activation of TLR4 in trophoblast cells is associated with the expression of IFN-β in a cyclic manner. Exposure of pregnant mice or trophoblast cells to LPS is characterized by phosphorylation of TBK and IRF3, leading to increased expression of IFN-β and downstream ISGs by the trophoblast (Figure 4A) 155. Interestingly, shortly after IFN-β exposure, cells enter a state known as “IFN desensitization” that allows cells to recover from IFN signaling.
In the placenta, IFN-β expression is regulated by the expression of Axl receptor tyrosine kinase (Axl) and suppressor of cytokine signaling 1 (SOCS1) protein; two ISGs that can negatively regulate IFN signaling 183,184. TYRO3, AXL, and MER (TAM) receptors are membrane tyrosine kinase receptors found in high abundance in immune cells and have been reported to regulate innate immune responses by dampening TLR signaling 185. Loss of TAM receptor signaling has been associated with stages of hypersensitive inflammatory responses implicated in sepsis, chronic inflammatory disease, and autoimmune diseases 183,184,186–189. SOCS1 has been characterized as a negative feedback loop for type I IFN signaling 188; however, the induction of SOCS1 proteins by IFNAR activation has been shown to progress through and be contingent on TAM receptor activation 187. Trophoblast cells express Axl and Mer mRNA, as well as the Axl ligand growth arrest specific 6 (Gas6). Their activation is associated with increased expression of SOCS1 and downregulation of IFN-β expression. Knockdown of Axl receptor in trophoblast cells results in dysregulation of IFN-β expression leading to apoptosis and fetal death 37 (Figure 4B). These findings demonstrate a central role for TAM receptors during pregnancy as negative feedback loop regulators of type I IFN signaling, and their absence is associated with an augmentation of IFN-β/IFNAR signaling. Infections that either suppress IFN-β response or blocks the negative regulatory mechanism leading to exacerbated expression of type I IFN-β will be detrimental for fetal development, the overall outcome of the pregnancy, or even maternal survival.
In conclusion, we ascertain the signals derived from the normal microbiota as positive inducers of basal IFNβ expression that provides an adequate platform to respond to bacterial and/or viral infections, and the TAM receptors as negative inhibitory regulators that prevent a chronic condition characterized by exacerbated expression of type I IFN-β which leads to cell apoptosis (Figure 4).
6. Role of Trophoblasts as an Immunological Barrier during Viral Infections
The immune homeostasis of pregnancy can be disrupted by infections from different pathogens (bacteria, parasites and viruses), leading to the impairment of fetal growth and development 190. In addition, pregnancy complications, such as miscarriage, preeclampsia, and preterm birth are associated with the disruption of the immunological milieu by either prolonging the inflammatory state (miscarriage) or shifting to inflammation at an early stage (preterm birth) 36,111,191. In the long term, maternal infection has been shown to increase the risk for neuropsychiatric disorders including autism spectrum disorders 192 and schizophrenia in the offspring 193,194. Among those threatening pathogens during pregnancy, the traditional ‘TORCH’ pathogens, Toxoplasma gondii, other, rubella virus, cytomegalovirus (CMV), and herpes simplex virus (HSV), have been considered as major causes of fetal morbidity and mortality. Unexpectedly, the new emerging ZIKA virus (ZIKV) is found to fulfill all the requirements of a ‘TORCH’ pathogen. It causes mild or absent symptoms in infected mothers but can be transmitted through placenta to fetus, which causes microcephaly and other fetal malformations 195–197. Therefore, ZIKA virus is recognized as the newest ‘TORCH’ pathogen 197,198.
As indicated above, the placenta is able to recognize and respond to dangerous signals originating from microorganisms and damaged tissue. One of the outcomes from trophoblast cells responding to TLRs ligands is the recruitment and differentiation of immune cells. Ligation of the TLR3 agonist poly(I:C) and TLR4 ligand LPS induces the expression of chemokines, which in turn promotes monocyte, NK cells, and neutrophil chemotaxis 109. Moreover, viral single stranded RNA (ssRNA), a TLR8 agonist, triggers restricted pro-inflammatory cytokine response by upregulating the secretion of IL-6, IL-8, and IFN-β 199,200. Therefore, trophoblasts response against various pathogens is TLR specific, eliciting specific production and secretion of cytokines and chemokines according to the pathogen, which will also exert specific influences on the immune environment during pregnancy (Details on TLR responses have been recently reviewed by Koga et al.) 201
A critical role of the placenta is to prevent viral vertical transmission, which can be lethal to fetus or can induce major developmental problems. During the first trimester of pregnancy, IFN-β functions as a central modulator for this protection. During early pregnancy, first trimester trophoblast cells can mount a strong type I IFN-β response, which is characterized by the expression of multiple ISGs 37. This response is characteristic of first trimester trophoblast cells, which differs from third trimester placentas, during which type III interferons are highly expressed 71. Compared to the constitutive release of type III interferons from full-term human trophoblast cells 71,202, we did not detect IFN-β in the culture media from human first-trimester trophoblast cell line and human primary culture cells 203, however, both the human first-trimester trophoblast cell line and primary culture cells can robustly induce IFN-β signaling when infected with Zika Virus infection. The increase of IFN-β secretion leads to the induction of several anti-viral ISGs 203. We have found IFN-β protein expression in both human 37 and mouse placenta (unpublished data), suggestive of an evolutionarily conserved trend. Interestingly, although we detect constitutive protein expression at the placenta, we are unable to detect secreted levels in the blood or supernatant of cultured trophoblast cells. Secreted levels are detectable only following viral infection or TLR ligation, such as ZIKV, ligation of the TLR3 agonist poly(I:C) or TLR4 ligand LPS. These findings suggest that IFN-β is stored within first-trimester trophoblast cells, which allows for a rapid and potent response to viral infection. This is critical to prevent the toxicity of high levels of IFN-β to the placenta and fetus 37,182, and this expression pattern is very different from the constitutive release of type III interferons from full-term human trophoblast cells 153.
This differential expression of type I and type III interferon may be associated with the different stages of placenta/trophoblast differentiation and function. Although early studies had suggested that type III interferons were functionally redundant with type I interferons 204, several reports indicate that IFN-λ has specific functions, which differ from type I interferons 205. Another difference between the type I IFNs and type III IFN pathways is the use of distinct receptors. IFN-λ specifically interact with a heterodimeric receptor composed of two chains: a specific ligand-binding chain IFN-λR1 (or IL-28Rα) and the IL-10R2 (or IL-10Rβ) chain 206. In contrast, type I interferons are ligands of the IFNAR receptor 207. The evolutionary adaptation that confers type I IFN-β signaling in the developing placenta may reflect the need for immune regulation and tolerance, which is required for the establishment of the pregnancy 69.
In the animal models, when WT or Ifnar−/− pregnant mice are challenged with DNA viruses (MHV68 or HSV-2) or RNA virus (ZIKA virus), there is a significant increase in viral titers in the Ifnar−/− placenta and fetus, suggesting that in the absence of overall IFN signaling, there is a lack of adequate defense against viral infection. Compared to the global Ifnar−/− mouse model, the importance of placental type I IFN is exemplified by experiments consisting of embryo transfers that distinguish between placental and maternal type I IFN signaling. Breeding Ifnar−/− males with Ifnar+/− females results in litters with half of the embryos/placentas lacking a functional IFNAR (homozygous Ifnar−/−), in a mother with functional IFNAR (Ifnar+/−), and half embryos/placenta with a functional IFNAR (heterozygous Ifnar+/−), in a heterozygous (Ifnar+/−) mother. Pregnant females infected with a DNA virus (MHV68) on E8.5 showed that although the mothers had a functional IFNAR, viral titers were significantly higher in placenta and fetus lacking IFNAR signaling (homozygous Ifnar−/−) compared to those with a functional IFNAR (heterozygous Ifnar+/−). Therefore, despite sharing the same maternal phenotype (functional IFNAR), the susceptibility to infection was increased in fetal/placenta units lacking a functional IFNAR 123. Furthermore, when WT embryos (i.e., WT placenta, can produce and respond to type I IFN) were transferred into pseudopregnant Ifnar−/− dams (dam can produce type I IFN but can not activate the IFN signaling pathway due to lack of receptor), there were less viral titers in the placenta and absent fetal viral infection compared to mice with Ifnar−/− embryo and Ifnar−/− dam. Interestingly, the presence of a WT placenta prevented maternal mortality 123.
These results highlight the importance of IFN signaling in the placenta during viral infection, which suggest that: 1) placental type I IFN is critical in the control of viral replication; and 2) in the absence of placental type I IFN signaling, the resulting uncontrolled viral replication can transform the placenta into a viral reservoir with detrimental effects not only to the fetus but to the mother as well. Thus, in the setting of viral infection, placental type I IFN signaling has protective effects to both the mother and fetus.
7. Placenta Derived ISGs-Immune Modulators and Protection Against Virus
The viral life cycle involves multiple steps, including attachment, uncoating, genome replication, transcription, translation, particle assembly, and egress. The final step is to generate more virions that will be released to infect neighboring cells. Every stage of the viral life cycle is a potential target for ISGs. Thus, murine myxovirus resistance 1 (Mx1) targets viral entry by trapping viral components, such as nucleocapsids, and preventing the virus from entering cells 208. Cholesterol-25-hydroxylase (CH25H), an enzyme that oxidizes cholesterol into 25-hydroxycholesterol (25HC) and can block ZIKV entry providing protection against ZIKV infection in mice and rhesus macaques 209. The IFN-inducible transmembrane (IFITM) family has been shown to block viral entry as well. In humans, there are four members in the IFITM family: IFITM1, IFITM2, IFITM3 and IFITM5 210. They display specific anti-viral effects to different viruses 211. ISGs that inhibit viral translation, include zinc-finger antiviral protein (ZAP), the IFN-induced protein with tetratricopeptide repeats (IFIT) family, the OAS-RNaseL pathway, protein kinase R (PKR) 212–215, and the exonuclease Interferon stimulated gene 20 (ISG20). ISG20 inhibits HBV replication by directly binding to the epsilon stem-loop structure of viral RNA and degrades the viral RNA. This effect is dependent on its Exo III domain 216. There are few ISGs shown to inhibit viral assembly and egress. Viperin inhibits HIV-1 and influenza A virus budding at the cell membrane by restraining farnesyl diphosphate synthase (FPPS), an enzyme required for isoprenoid biosynthesis 217,218. However, Viperin also shows anti-viral effect in early stage of viral life cycle, as it is suggested that Viperin inhibits HCV viral RNA replication 219. Moreover, Viperin expression decreases ZIKV infection in vitro and this anti-ZIKV activity depends on the C-terminal region 220. Therefore, these ISGs target at different stages of viral cycle to exhibit their anti-viral effects.
Evaluation of the placental response to SARS-CoV-2 reveled a significant increase in the expression of interferon signaling pathways and ISGs in individuals with COVID-19 compared to healthy individuals 221,222. Single-cell RNA sequencing of placenta cells revealed significant upregulation of ISG15, a central player in host antiviral responses 222. Moreover, it was found that ISGs, including IFITM1 and IFITM3, were upregulated in the placenta of pregnant patients with severe disease when compared to asymptomatic/mild COVID-19-positive pregnant women 223. Interestingly, a recent study reported that maternal SARS-CoV-2 infection was associated with sex-specific alteration in placental ISGs response 224. In male SARS-CoV-2-exposed placentas, there was a significantly increased expression of the classical ISGs, such as IFI6, CXCL10, CCL2, MX1, and OAS1. This induction was not observed in female SARS-CoV-2-exposed placentas 224. This data suggests robust interferon signaling and ISGs induction specifically in the male placental infected with SARS-CoV-2. These new findings further strengthen the critical role of IFN signaling and ISGs in placental antiviral response 182,225,226.
During pregnancy, ISGs are important not only for their anti-viral function, but also are essential immune modulators that can influence multiple aspects of endometrial function. ISGs, such as ISG15 227, MX2, and OAS1, play a crucial role in endometrial cell differentiation, implantation, and conceptus development during early pregnancy 228. In the mice uterus, ISG15 mRNA was detected between embryonic-day 3.5 (E3.5) and E9.5 of pregnancy. ISG15 mRNA levels were very low on E3.5 and 4.5 (the start of implantation) but significantly increased (11-fold) on E9.5 229. In humans, ISG15 expression was detected in cytotrophoblast progenitor cells in the placental villi and the cell column with a maximum expression levels observed in the first trimester, less in the second trimester, and no expression in the third trimester 230. ISG20 has also been studied for its role during the implantation window in mice 231. ISG20 mRNA was present in the uterine luminal and glandular epithelium on E3 and E4, which then decreased rapidly and disappeared completely on E5–6 231. This tightly regulated expression pattern of ISG20 during early pregnancy in mice and humans suggest a very specific role of ISG20 during the early stages of implantation and placentation; however, its specific role is still not clearly understood. We described the characterization of ISG20 in first-trimester trophoblast cells in response to ZIKV infection and demonstrated that ISG20 is essential for trophoblast protection again ZIKV replication 203.
In terms of immune regulation, trophoblast cells can modulate macrophage polarization through the secretion of ISGs, such as PDL1. Decidual macrophages have a high degree of plasticity that allows them to change their phenotypes based on the signals present at the implantation site. During the pre-implantation period, macrophages are mainly the M1 phenotype, they then change to M2 phenotype following trophoblast attachment and invasion and then revert to M1 phenotype at the time of delivery 232. The polarization of M2 macrophages from M1 is mediated in part by the secretion of PD-L1 by trophoblast cells and is regulated by IFNβ 38. Overall, we know now that trophoblast cells, in response to IFNβ, secrete multiple ISGs that are associated with the regulation of specific immune cells and their local immune functions (Figure 5). Immune cells present at the maternal-fetal interface display unique characteristics necessary for the support of the pregnancy, such as NK cells and macrophages 36. We postulate that the unique phenotype of these immune cells is determined by trophoblast derived ISGs. Trophoblast derived IL-15 in response to IFNβ modulates NK cell activation and homeostasis 233,234. Nicotinamide phosphoribosyltransferase (NAMPT), another trophoblast derived ISG, is known to be a key modulator of macrophages differentiation, polarization, and migration 235. Despite all these observations, the role of ISGs in modulating immune cell functions during pregnancy remains poorly understood and needs further investigation.
Figure 5. The role of ISGs at the maternal/fetal interface.
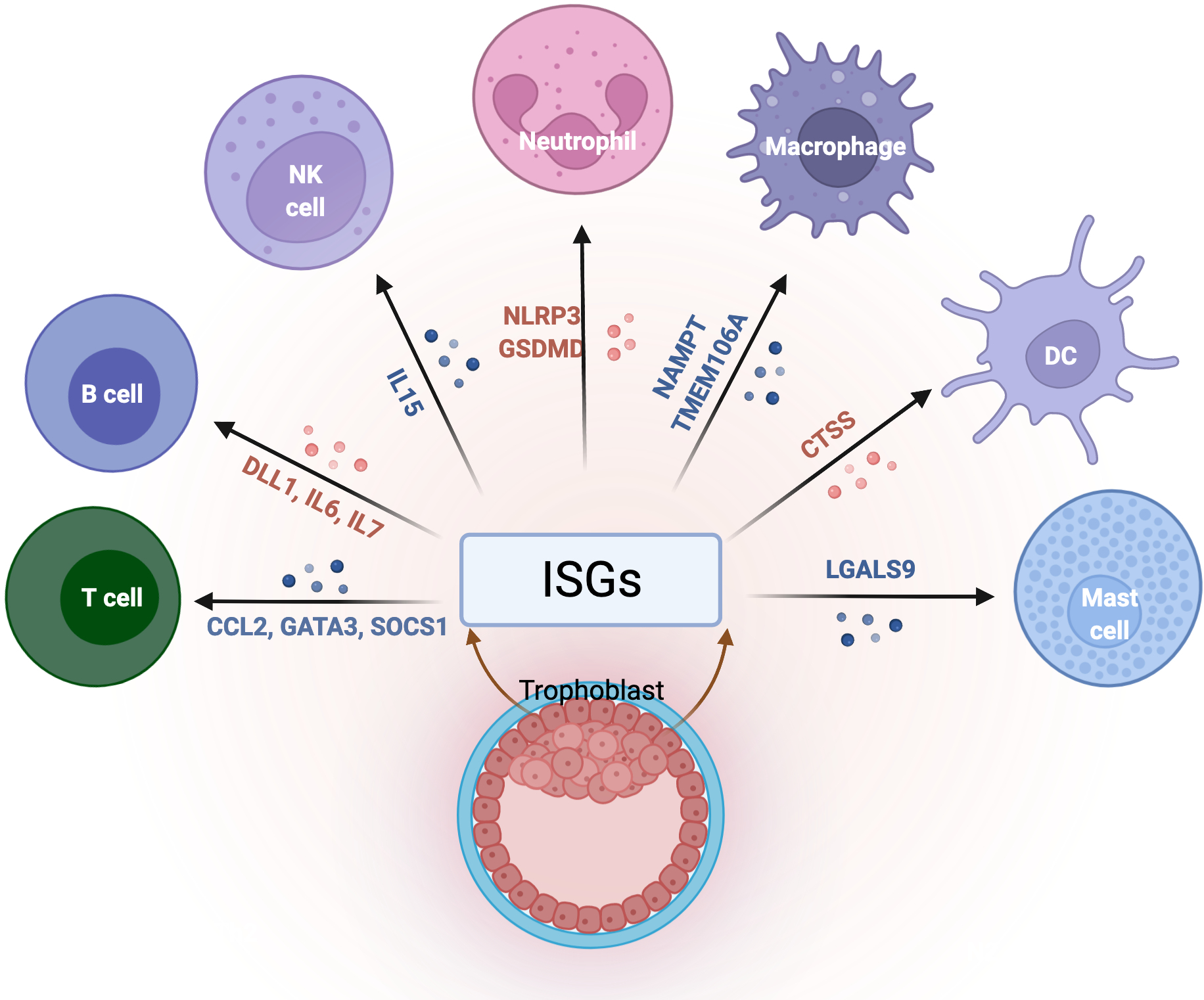
Immune cells present at the maternal-fetal interface display unique characteristics necessary for the support of pregnancy, and the trophoblasts plays a critical role in the regulation of their function and differentiation. Trophoblast secreted ISGs modulate immune cells functions and maintains tissue homeostasis at the maternal-fetal interface by preserving tolerance to paternal antigens as well as protection against infections.
Infections that affect ISGs expression in trophoblast cells will disrupt the immunological balance, leading to pregnancy complications. </p/> NK, natural killer; DC, dendritic cells; ISGs, interferon stimulated genes; IL, interleukin; CCL2, C-C motif chemokine ligand 2; GATA3, GATA binding protein 3; SOCS1, suppressor of cytokine signaling 1; DLL1, delta like canonical notch ligand 1; NLRP3, NLR family pyrin domain containing 3; GSDMD, gasdermin D; NAMPT, nicotinamide phosphoribosyltransferase; TMEM106A, transmembrane protein 106A; CTSS, cathepsin S; LGALS9, galectin 9. Figure created with BioRender.com
8. Conclusion
Type I IFN signaling is critical for the modulation of the immune system and provides protection against viral infection, especially during pregnancy. To achieve a successful pregnancy, the maternal immune system must be tightly controlled and be equipped with the capability to rapidly respond to pathogens. Type I IFN signaling and the derived ISGs are necessary for numerous physiological processes during pregnancy. As the potent effectors of Type I IFN signaling, ISGs have shown various functions, including anti-viral, immunomodulatory, and pro-apoptotic. Unfortunately, the regulation of IFNβ expression and function, as well as the immunomodulatory function of its derived ISGs during pregnancy are still poorly understood. Furthermore, the impact of these ISGs on fetal development is also unknown. Thus, a better understanding of individual ISG function will facilitate the development of ISG-based therapeutics and identification of ISGs as potential clinical biomarkers.
ACKNOWLEDGMENTS
The authors and the research cited in this review were supported in part by NIH grant NIAID 1R01Al145829-01.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–606. [DOI] [PubMed] [Google Scholar]
- 2.Vilella F, Ramirez L, Berlanga O, et al. PGE2 and PGF2alpha concentrations in human endometrial fluid as biomarkers for embryonic implantation. J Clin Endocrinol Metab. 2013;98(10):4123–4132. [DOI] [PubMed] [Google Scholar]
- 3.Erlebacher A Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. [DOI] [PubMed] [Google Scholar]
- 4.Kover K, Liang L, Andrews GK, Dey SK. Differential expression and regulation of cytokine genes in the mouse uterus. Endocrinology. 1995;136(4):1666–1673. [DOI] [PubMed] [Google Scholar]
- 5.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Racicot K, Kwon JY, Aldo P, Silasi M, Mor G. Understanding the Complexity of the Immune System during Pregnancy. Am J Reprod Immunol. 2014;72(2):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11(6):613–630. [DOI] [PubMed] [Google Scholar]
- 8.Karmakar S, Dhar R, Das C. Inhibition of cytotrophoblastic (JEG-3) cell invasion by interleukin 12 involves an interferon gamma-mediated pathway. J Biol Chem. 2004;279(53):55297–55307. [DOI] [PubMed] [Google Scholar]
- 9.Ledee N, Petitbarat M, Rahmati M, et al. New pre-conception immune biomarkers for clinical practice: interleukin-18, interleukin-15 and TWEAK on the endometrial side, G-CSF on the follicular side. J Reprod Immunol. 2011;88(2):118–123. [DOI] [PubMed] [Google Scholar]
- 10.You Y, Stelzl P, Joseph DN, et al. TNF-α Regulated Endometrial Stroma Secretome Promotes Trophoblast Invasion. Frontiers in immunology. 2021;12:737401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril. 2003;79(6):1317–1322. [DOI] [PubMed] [Google Scholar]
- 12.Raziel A, Schachter M, Strassburger D, Bern O, Ron-El R, Friedler S. Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure. Fertil Steril. 2007;87(1):198–201. [DOI] [PubMed] [Google Scholar]
- 13.Gnainsky Y, Granot I, Aldo PB, et al. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertility and Sterility. 2010;94(6):2030–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekel N, Gnainsky Y, Granot I, Mor G. Inflammation and implantation. AJRI. 2010;63(1):17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li DK, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ. 2003;327(7411):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledee N, Petitbarat M, Chevrier L, et al. The Uterine Immune Profile May Help Women With Repeated Unexplained Embryo Implantation Failure After In Vitro Fertilization. Am J Reprod Immunol. 2016;75(3):388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SK, Kim JY, Hur SE, et al. An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod. 2011;26(11):2964–2971. [DOI] [PubMed] [Google Scholar]
- 18.Kaislasuo J, Simpson S, Petersen JF, et al. IL-10 to TNFalpha ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am J Reprod Immunol. 2020;83(1):e13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci U S A. 2017;114(32):E6566–e6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaislasuo J, Simpson S, Petersen JF, et al. IL-10 to TNFalpha ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am J Reprod Immunol. 2019:e13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauss K, Ehrentraut S, Zenclussen AC, Schumacher A. The pregnancy hormone human chorionic gonadotropin differentially regulates plasmacytoid and myeloid blood dendritic cell subsets. Am J Reprod Immunol. 2018;79(4):e12837. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher A, Brachwitz N, Sohr S, et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182(9):5488–5497. [DOI] [PubMed] [Google Scholar]
- 23.Schumacher A, Heinze K, Witte J, et al. Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol. 2013;190(6):2650–2658. [DOI] [PubMed] [Google Scholar]
- 24.Dauven D, Ehrentraut S, Langwisch S, Zenclussen AC, Schumacher A. Immune Modulatory Effects of Human Chorionic Gonadotropin on Dendritic Cells Supporting Fetal Survival in Murine Pregnancy. Front Endocrinol (Lausanne). 2016;7:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fettke F, Schumacher A, Canellada A, et al. Maternal and Fetal Mechanisms of B Cell Regulation during Pregnancy: Human Chorionic Gonadotropin Stimulates B Cells to Produce IL-10 While Alpha-Fetoprotein Drives Them into Apoptosis. Frontiers in immunology. 2016;7:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher A, Poloski E, Sporke D, Zenclussen AC. Luteinizing hormone contributes to fetal tolerance by regulating adaptive immune responses. Am J Reprod Immunol. 2014;71(5):434–440. [DOI] [PubMed] [Google Scholar]
- 27.Bourdiec A, Bedard D, Rao CV, Akoum A. Human chorionic gonadotropin regulates endothelial cell responsiveness to interleukin 1 and amplifies the cytokine-mediated effect on cell proliferation, migration and the release of angiogenic factors. Am J Reprod Immunol. 2013;70(2):127–138. [DOI] [PubMed] [Google Scholar]
- 28.Carbone F, Procaccini C, De Rosa V, et al. Divergent immunomodulatory effects of recombinant and urinary-derived FSH, LH, and hCG on human CD4+ T cells. J Reprod Immunol. 2010;85(2):172–179. [DOI] [PubMed] [Google Scholar]
- 29.Schafer A, Pauli G, Friedmann W, Dudenhausen JW. Human choriogonadotropin (hCG) and placental lactogen (hPL) inhibit interleukin-2 (IL-2) and increase interleukin-1 beta (IL-1 beta), −6 (IL-6) and tumor necrosis factor (TNF-alpha) expression in monocyte cell cultures. J Perinat Med. 1992;20(3):233–240. [DOI] [PubMed] [Google Scholar]
- 30.Silasi M, You Y, Simpson S, et al. Human Chorionic Gonadotropin modulates CXCL10 Expression through Histone Methylation in human decidua. Scientific reports. 2020;10(1):5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osokine I, Erlebacher A. Chapter 8 - Immunology of the decidua. In: Mor G, ed. Reproductive Immunology. Academic Press; 2021:129–145. [Google Scholar]
- 32.Mor G, Abrahams VM. Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol. 2003;1:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi Y, Ning F, Lash GE. Chapter 3 - Uterine macrophages: Essential roles for a successful human pregnancy. In: Mor G, ed. Reproductive Immunology. Academic Press; 2021:39–53. [Google Scholar]
- 34.Smith AL, Bole Aldo P, Racicot KE. Chapter 17 - Placental regulation of immune functions. In: Mor G, ed. Reproductive Immunology. Academic Press; 2021:335–348. [Google Scholar]
- 35.Simpson S, Kaislasuo J, Peng G, et al. Peri-implantation cytokine profile differs between singleton and twin IVF pregnancies. Am J Reprod Immunol. 2020:e13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maxwell AJ, You Y, Aldo PB, Zhang Y, Ding J, Mor G. Chapter 1 - The role of the immune system during pregnancy: General concepts. In: Mor G, ed. Reproductive Immunology. Academic Press; 2021:1–21. [Google Scholar]
- 37.Kwon JY, Aldo P, You Y, et al. Relevance of placental type I interferon beta regulation for pregnancy success. Cell Mol Immunol. 2018;15(12):1010–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang YH, Aldo P, You Y, et al. Trophoblast-secreted soluble-PD-L1 modulates macrophage polarization and function. J Leukoc Biol. 2020;108(3):983–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu XH, Li ZH, Muyayalo KP, et al. A newly intervention strategy in preeclampsia: Targeting PD-1/Tim-3 signaling pathways to modulate the polarization of decidual macrophages. Faseb j. 2022;36(1):e22073. [DOI] [PubMed] [Google Scholar]
- 40.Sugano T, Narahara H, Nasu K, Arima K, Fujisawa K, Miyakawa I. Effects of platelet-activating factor on cytokine production by human uterine cervical fibroblasts. Mol Hum Reprod. 2001;7(5):475–481. [DOI] [PubMed] [Google Scholar]
- 41.Casey ML, Brown CE, Peters M, MacDonald PC. Endothelin levels in human amniotic fluid at mid-trimester and at term before and during spontaneous labor. J Clin Endocrinol Metab. 1993;76(6):1647–1650. [DOI] [PubMed] [Google Scholar]
- 42.Schrey MP, Hare A. Endothelin-1 stimulates phospholipid hydrolysis and prostaglandin F2 alpha production in primary human decidua cell cultures. Prostaglandins Leukot Essent Fatty Acids. 1992;47(4):321–325. [DOI] [PubMed] [Google Scholar]
- 43.Usuki S, Saitoh T, Sawamura T, et al. Increased maternal plasma concentration of endothelin-1 during labor pain or on delivery and the existence of a large amount of endothelin-1 in amniotic fluid. Gynecol Endocrinol. 1990;4(2):85–97. [DOI] [PubMed] [Google Scholar]
- 44.Romero R, Wu YK, Oyarzun E, Hobbins JC, Mitchell MD. A potential role for epidermal growth factor/alpha-transforming growth factor in human parturition. Eur J Obstet Gynecol Reprod Biol. 1989;33(1):55–60. [DOI] [PubMed] [Google Scholar]
- 45.Behnia F, Taylor BD, Woodson M, et al. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol. 2015;213(3):359 e351–316. [DOI] [PubMed] [Google Scholar]
- 46.Menon R Initiation of human parturition: signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet Gynecol Sci. 2019;62(4):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero R, Chaiworapongsa T, Espinoza J. Micronutrients and intrauterine infection, preterm birth and the fetal inflammatory response syndrome. J Nutr. 2003;133(5 Suppl 2):1668S–1673S. [DOI] [PubMed] [Google Scholar]
- 48.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194(3):630–637. [DOI] [PubMed] [Google Scholar]
- 49.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. [DOI] [PubMed] [Google Scholar]
- 50.Lamont RF. The role of infection in preterm labour and birth. Hosp Med. 2003;64(11):644–647. [DOI] [PubMed] [Google Scholar]
- 51.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200(4):374 e371–375. [DOI] [PubMed] [Google Scholar]
- 52.Peltier MR, Klimova NG, Arita Y, et al. Polybrominated diphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta. 2012;33(9):745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15(10):479–487. [DOI] [PubMed] [Google Scholar]
- 54.Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. Bjog. 2011;118(2):136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pettker CM, Buhimschi IA, Magloire LK, Sfakianaki AK, Hamar BD, Buhimschi CS. Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstet Gynecol. 2007;109(3):739–749. [DOI] [PubMed] [Google Scholar]
- 57.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Seminars in fetal & neonatal medicine. 2006;11(5):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry. 2013;3:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res. 2006;59(1):50–55. [DOI] [PubMed] [Google Scholar]
- 60.Burd I, Bentz AI, Chai J, et al. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res. 2010;88(9):1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pirianov G, Waddington SN, Lindstrom TM, Terzidou V, Mehmet H, Bennett PR. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J(2) delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology. 2009;150(2):699–706. [DOI] [PubMed] [Google Scholar]
- 62.Koga K, Cardenas I, Aldo P, et al. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61(3):196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cardenas VA, Chao LL, Studholme C, et al. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging. 2011;32(4):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26(7):877–897. [DOI] [PubMed] [Google Scholar]
- 66.Mor G Pregnancy Reconceived. Natural History. 2007;116(4):36–41. [Google Scholar]
- 67.Mor G, Cardenas I. The immune system in pregnancy: A unique complexity. AJRI. 2010;63(6):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175(12):8096–8104. [DOI] [PubMed] [Google Scholar]
- 69.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17(8):469–482. [DOI] [PubMed] [Google Scholar]
- 70.Bayer A, Delorme-Axford E, Sleigher C, et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am J Obstet Gynecol. 2015;212(1):71 e71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bayer A, Lennemann NJ, Ouyang Y, et al. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell host & microbe. 2016;19(5):705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delorme-Axford E, Donker RB, Mouillet JF, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A. 2013;110(29):12048–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol. 2000;74(15):6808–6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koi H, Zhang J, Makrigiannakis A, et al. Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol Reprod. 2002;67(5):1572–1579. [DOI] [PubMed] [Google Scholar]
- 75.Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol. 2006;168(4):1210–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maidji E, Nigro G, Tabata T, et al. Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am J Pathol. 2010;177(3):1298–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 2010;6(1):e1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheridan MA, Yunusov D, Balaraman V, et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A. 2017;114(9):E1587–E1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramhorst R, Fraccaroli L, Aldo P, et al. Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am J Reprod Immunol. 2012;67(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manaster I, Mizrahi S, Goldman-Wohl D, et al. Endometrial NK cells are special immature cells that await pregnancy. J Immunol. 2008;181(3):1869–1876. [DOI] [PubMed] [Google Scholar]
- 81.Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065–1074. [DOI] [PubMed] [Google Scholar]
- 82.Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2011;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aldo PB, Racicot K, Craviero V, Guller S, Romero R, Mor G. Trophoblast Induces Monocyte Differentiation Into CD14+/CD16+ Macrophages. Am J Reprod Immunol. 2014;72(3):270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Svensson-Arvelund J, Mehta RB, Lindau R, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015;194(4):1534–1544. [DOI] [PubMed] [Google Scholar]
- 85.Repnik U, Tilburgs T, Roelen DL, et al. Comparison of macrophage phenotype between decidua basalis and decidua parietalis by flow cytometry. Placenta. 2008;29(5):405–412. [DOI] [PubMed] [Google Scholar]
- 86.Abrahams VM, Bole-Aldo P, Kim YM, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173(7):4286–4296. [DOI] [PubMed] [Google Scholar]
- 87.Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL. Two unique human decidual macrophage populations. J Immunol. 2011;186(4):2633–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119(7):2062–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erlebacher A Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13(1):23–33. [DOI] [PubMed] [Google Scholar]
- 90.Medzhitov R, Janeway CA Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300. [DOI] [PubMed] [Google Scholar]
- 91.Abrahams VM, Aldo PB, Murphy SP, et al. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180(9):6035–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abrahams VM, Schaefer TM, Fahey JV, et al. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I : C). Hum Reprod. 2006;21(9):2432–2439. [DOI] [PubMed] [Google Scholar]
- 93.Abrahams VM, Romero R, Mor G. TLR-3 and TLR-4 mediate differential chemokine production and immune cell recruitment by first trimester trophoblast cells. AJRI. 2005;53(6):279. [Google Scholar]
- 94.Beijar EC, Mallard C, Powell TL. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta. 2006;27(2–3):322–326. [DOI] [PubMed] [Google Scholar]
- 95.Chan G, Guilbert LJ. Ultraviolet-inactivated human cytomegalovirus induces placental syncytiotrophoblast apoptosis in a Toll-like receptor-2 and tumour necrosis factor-alpha dependent manner. J Pathol. 2006;210(1):111–120. [DOI] [PubMed] [Google Scholar]
- 96.Chatterjee P, Weaver LE, Doersch KM, et al. Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS One. 2012;7(7):e41884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Equils O, Lu D, Gatter M, et al. Chlamydia heat shock protein 60 induces trophoblast apoptosis through TLR4. J Immunol. 2006;177(2):1257–1263. [DOI] [PubMed] [Google Scholar]
- 98.Holmlund U, Cebers G, Dahlfors AR, et al. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107(1):145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koga K, Aldo PB, Mor G. Toll-like receptors and pregnancy: trophoblast as modulators of the immune response. J Obstet Gynaecol Res. 2009;35(2):191–202. [DOI] [PubMed] [Google Scholar]
- 100.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator?: the role of toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25(5):375–388. [DOI] [PubMed] [Google Scholar]
- 101.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7(12):1250–1257. [DOI] [PubMed] [Google Scholar]
- 102.Hoang M, Potter JA, Gysler SM, et al. Human fetal membranes generate distinct cytokine profiles in response to bacterial Toll-like receptor and nod-like receptor agonists. Biol Reprod. 2014;90(2):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kavathas PB, Boeras CM, Mulla MJ, Abrahams VM. Nod1, but not the ASC inflammasome, contributes to induction of IL-1beta secretion in human trophoblasts after sensing of Chlamydia trachomatis. Mucosal immunology. 2013;6(2):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med. 2016;44(1):33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yokota S, Okabayashi T, Fujii N. The battle between virus and host: modulation of Toll-like receptor signaling pathways by virus infection. Mediators Inflamm. 2010;2010:184328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mulla MJ, Salmon JE, Chamley LW, et al. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1beta production by human first trimester trophoblast. PLoS One. 2013;8(6):e65237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Janeway CA Jr., Medzhitov R Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. [DOI] [PubMed] [Google Scholar]
- 108.Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343(5):338–344. [DOI] [PubMed] [Google Scholar]
- 109.Abrahams VM, Fahey JV, Schaefer TM, Wright JA, Wira CR, Mor G. Stimulation of first trimester trophoblast cells with Poly(I:C) induces SLPI secretion. AJRI. 2005;53(6):280 ASRI205–204. [Google Scholar]
- 110.Costello MJ, Joyce SK, Abrahams VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57(1):67–80. [DOI] [PubMed] [Google Scholar]
- 111.Kim YM, Romero R, Oh SY, et al. Toll-like receptor 4: A potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005;193(3 Suppl):921 e921–928. [DOI] [PubMed] [Google Scholar]
- 112.Medzhitov R, Preston-Hurlburt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2(2):253–258. [DOI] [PubMed] [Google Scholar]
- 113.Bjorkbacka H, Fitzgerald KA, Huet F, et al. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol Genomics. 2004;19(3):319–330. [DOI] [PubMed] [Google Scholar]
- 114.Chelvarajan RL, Collins SM, Doubinskaia IE, et al. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J Leukoc Biol. 2004;75(6):982–994. [DOI] [PubMed] [Google Scholar]
- 115.Coats SR, Reife RA, Bainbridge BW, Pham TT, Darveau RP. Porphyromonas gingivalis lipopolysaccharide antagonizes Escherichia coli lipopolysaccharide at toll-like receptor 4 in human endothelial cells. Infect Immun. 2003;71(12):6799–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Doyle SL, Jefferies CA, O’Neill LA. Bruton’s tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFkappaB activation by lipopolysaccharide. J Biol Chem. 2005;280(25):23496–23501. [DOI] [PubMed] [Google Scholar]
- 117.Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198(7):1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fu Y, Xie C, Chen J, et al. Innate stimuli accentuate end-organ damage by nephrotoxic antibodies via Fc receptor and TLR stimulation and IL-1/TNF-alpha production. J Immunol. 2006;176(1):632–639. [DOI] [PubMed] [Google Scholar]
- 119.Hirschfeld AF, Jiang R, Robinson WP, McFadden DE, Turvey SE. Toll-like receptor 4 polymorphisms and idiopathic chromosomally normal miscarriage. Hum Reprod. 2007;22(2):440–443. [DOI] [PubMed] [Google Scholar]
- 120.Hirschfeld M, Kirschning CJ, Schwandner R, et al. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163(5):2382–2386. [PubMed] [Google Scholar]
- 121.Akira S Toll-like receptor signaling. J Biol Chem. 2003;278(40):38105–38108. [DOI] [PubMed] [Google Scholar]
- 122.Kolb JP, Casella CR, SenGupta S, Chilton PM, Mitchell TC. Type I interferon signaling contributes to the bias that Toll-like receptor 4 exhibits for signaling mediated by the adaptor protein TRIF. Sci Signal. 2014;7(351):ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Racicot K, Aldo P, El-Guindy A, Kwon JY, Romero R, Mor G. Cutting Edge: Fetal/Placental Type I IFN Can Affect Maternal Survival and Fetal Viral Load during Viral Infection. J Immunol. 2017;198(8):3029–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Charrel-Dennis M, Latz E, Halmen KA, et al. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell host & microbe. 2008;4(6):543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Doyle S, Vaidya S, O’Connell R, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17(3):251–263. [DOI] [PubMed] [Google Scholar]
- 126.Bai LY, Chiu CF, Kapuriya NP, et al. BX795, a TBK1 inhibitor, exhibits antitumor activity in human oral squamous cell carcinoma through apoptosis induction and mitotic phase arrest. European journal of pharmacology. 2015;769:287–296. [DOI] [PubMed] [Google Scholar]
- 127.Yu T, Yang Y, Yin DQ, et al. TBK1 inhibitors: a review of patent literature (2011 – 2014). Expert Opin Ther Pat. 2015;25(12):1385–1396. [DOI] [PubMed] [Google Scholar]
- 128.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dutia BM, Allen DJ, Dyson H, Nash AA. Type I interferons and IRF-1 play a critical role in the control of a gammaherpesvirus infection. Virology. 1999;261(2):173–179. [DOI] [PubMed] [Google Scholar]
- 130.Lopez de Padilla CM, Niewold TB. The type I interferons: Basic concepts and clinical relevance in immune-mediated inflammatory diseases. Gene. 2016;576(1 Pt 1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. [DOI] [PubMed] [Google Scholar]
- 132.Cavlar T, Ablasser A, Hornung V. Induction of type I IFNs by intracellular DNA-sensing pathways. Immunol Cell Biol. 2012;90(5):474–482. [DOI] [PubMed] [Google Scholar]
- 133.Kim S, Kaiser V, Beier E, et al. Self-priming determines high type I IFN production by plasmacytoid dendritic cells. Eur J Immunol. 2014;44(3):807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kotredes KP, Thomas B, Gamero AM. The Protective Role of Type I Interferons in the Gastrointestinal Tract. Frontiers in immunology. 2017;8:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang WB, Levy DE, Lee CK. STAT3 negatively regulates type I IFN-mediated antiviral response. J Immunol. 2011;187(5):2578–2585. [DOI] [PubMed] [Google Scholar]
- 137.Wong MT, Chen SS. Emerging roles of interferon-stimulated genes in the innate immune response to hepatitis C virus infection. Cell Mol Immunol. 2016;13(1):11–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Veer MJ, Holko M, Frevel M, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69(6):912–920. [PubMed] [Google Scholar]
- 139.Lanford RE, Guerra B, Lee H, Chavez D, Brasky KM, Bigger CB. Genomic response to interferon-alpha in chimpanzees: implications of rapid downregulation for hepatitis C kinetics. Hepatology. 2006;43(5):961–972. [DOI] [PubMed] [Google Scholar]
- 140.Sarasin-Filipowicz M, Oakeley EJ, Duong FH, et al. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci U S A. 2008;105(19):7034–7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95(26):15623–15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sen GC, Peters GA. Viral stress-inducible genes. Adv Virus Res. 2007;70:233–263. [DOI] [PubMed] [Google Scholar]
- 143.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312(5775):879–882. [DOI] [PubMed] [Google Scholar]
- 144.Chard T, Craig PH, Menabawey M, Lee C. Alpha interferon in human pregnancy. Br J Obstet Gynaecol. 1986;93(11):1145–1149. [DOI] [PubMed] [Google Scholar]
- 145.Howatson AG, Farquharson M, Meager A, McNicol AM, Foulis AK. Localization of alpha-interferon in the human feto-placental unit. J Endocrinol. 1988;119(3):531–534. [DOI] [PubMed] [Google Scholar]
- 146.Aboagye-Mathiesen G, Toth FD, Hager H, et al. Human trophoblast interferons. Antiviral research. 1993;22(2–3):91–105. [DOI] [PubMed] [Google Scholar]
- 147.Aboagye-Mathiesen G, Toth FD, Petersen PM, et al. Differential interferon production in human first and third trimester trophoblast cultures stimulated with viruses. Placenta. 1993;14(2):225–234. [DOI] [PubMed] [Google Scholar]
- 148.Aboagye-Mathiesen G, Toth FD, Zdravkovic M, Ebbesen P. Functional characteristics of human trophoblast interferons. Am J Reprod Immunol. 1996;35(4):309–317. [DOI] [PubMed] [Google Scholar]
- 149.Yockey LJ, Iwasaki A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity. 2018;49(3):397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ander SE, Rudzki EN, Arora N, Sadovsky Y, Coyne CB, Boyle JP. Human Placental Syncytiotrophoblasts Restrict Toxoplasma gondii Attachment and Replication and Respond to Infection by Producing Immunomodulatory Chemokines. mBio. 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chang G, Mouillet JF, Mishima T, et al. Expression and trafficking of placental microRNAs at the feto-maternal interface. Faseb j. 2017;31(7):2760–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Corry J, Arora N, Good CA, Sadovsky Y, Coyne CB. Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal-fetal interface. Proc Natl Acad Sci U S A. 2017;114(35):9433–9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wells AI, Coyne CB. Type III Interferons in Antiviral Defenses at Barrier Surfaces. Trends Immunol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Racicot K, Mor G. Risks associated with viral infections during pregnancy. J Clin Invest. 2017;127(5):1591–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Racicot K, Kwon JY, Aldo P, et al. Type I Interferon Regulates the Placental Inflammatory Response to Bacteria and is Targeted by Virus: Mechanism of Polymicrobial Infection-Induced Preterm Birth. Am J Reprod Immunol. 2016;75(4):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aboagye-Mathiesen G, Toth FD, Zdravkovic M, Ebbesen P. Human trophoblast interferons: production and possible roles in early pregnancy. Early Pregnancy. 1995;1(1):41–53. [PubMed] [Google Scholar]
- 158.Lee BN, Hammill H, Popek EJ, et al. Production of interferons and beta-chemokines by placental trophoblasts of HIV-1-infected women. Infectious diseases in obstetrics and gynecology. 2001;9(2):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee BN, Ordonez N, Popek EJ, et al. Inflammatory cytokine expression is correlated with the level of human immunodeficiency virus (HIV) transcripts in HIV-infected placental trophoblastic cells. J Virol. 1997;71(5):3628–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reuben JM, Gonik B, Li S, Loo L, Turpin J. Induction of cytokines in normal placental cells by the human immunodeficiency virus. Lymphokine and cytokine research. 1991;10(3):195–199. [PubMed] [Google Scholar]
- 161.Reuben JM, Turpin JA, Lee BN, et al. Induction of inflammatory cytokines in placental monocytes of gravidae infected with the human immunodeficiency virus type 1. J Interferon Cytokine Res. 1996;16(11):963–971. [DOI] [PubMed] [Google Scholar]
- 162.Cardenas I, Means RE, Aldo P, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185(2):1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Abt MC, Pamer EG. Commensal bacteria mediated defenses against pathogens. Curr Opin Immunol. 2014;29:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108(13):5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ganal SC, Sanos SL, Kallfass C, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37(1):171–186. [DOI] [PubMed] [Google Scholar]
- 166.Kawashima T, Kosaka A, Yan H, et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-beta. Immunity. 2013;38(6):1187–1197. [DOI] [PubMed] [Google Scholar]
- 167.Abt MC, Artis D. The intestinal microbiota in health and disease: the influence of microbial products on immune cell homeostasis. Curr Opin Gastroenterol. 2009;25(6):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. [DOI] [PubMed] [Google Scholar]
- 169.Vogel SN, Fertsch D. Endogenous interferon production by endotoxin-responsive macrophages provides an autostimulatory differentiation signal. Infect Immun. 1984;45(2):417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kumaran Satyanarayanan S, El Kebir D, Soboh S, et al. IFN-beta is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nature communications. 2019;10(1):3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fehniger TA, Suzuki K, Ponnappan A, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193(2):219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. [DOI] [PubMed] [Google Scholar]
- 174.Montoya M, Schiavoni G, Mattei F, et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99(9):3263–3271. [DOI] [PubMed] [Google Scholar]
- 175.Swann JB, Hayakawa Y, Zerafa N, et al. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol. 2007;178(12):7540–7549. [DOI] [PubMed] [Google Scholar]
- 176.Andrade D, Kim M, Blanco LP, et al. Interferon-alpha and angiogenic dysregulation in pregnant lupus patients who develop preeclampsia. Arthritis & rheumatology (Hoboken, NJ). 2015;67(4):977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cappelletti M, Presicce P, Lawson MJ, et al. Type I interferons regulate susceptibility to inflammation-induced preterm birth. JCI Insight. 2017;2(5):e91288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Crow YJ, Manel N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15(7):429–440. [DOI] [PubMed] [Google Scholar]
- 179.Meuwissen ME, Schot R, Buta S, et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J Exp Med. 2016;213(7):1163–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sanchis A, Cervero L, Bataller A, et al. Genetic syndromes mimic congenital infections. J Pediatr. 2005;146(5):701–705. [DOI] [PubMed] [Google Scholar]
- 181.Bakaysa SL, Potter JA, Hoang M, et al. Single- and double-stranded viral RNA generate distinct cytokine and antiviral responses in human fetal membranes. Mol Hum Reprod. 2014;20(7):701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yockey LJ, Jurado KA, Arora N, et al. Type I interferons instigate fetal demise after Zika virus infection. Sci Immunol. 2018;3(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. [DOI] [PubMed] [Google Scholar]
- 185.Kwon J-Y, Park Y, Kwon H-Y, Maeng Y-S, Aldo PB, Kim SH. Chapter 18 - TAM receptors in pregnancy. In: Mor G, ed. Reproductive Immunology. Academic Press; 2021:349–363. [Google Scholar]
- 186.Chan PY, Carrera Silva EA, De Kouchkovsky D, et al. The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science. 2016;352(6281):99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fourgeaud L, Traves PG, Tufail Y, et al. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532(7598):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rothlin CV, Leighton JA, Ghosh S. Tyro3, Axl, and Mertk receptor signaling in inflammatory bowel disease and colitis-associated cancer. Inflammatory bowel diseases. 2014;20(8):1472–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146(5):R151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and −4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191(4):1346–1355. [DOI] [PubMed] [Google Scholar]
- 192.Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2015;45(12):4015–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61(8):774–780. [DOI] [PubMed] [Google Scholar]
- 194.Brown AS, Schaefer CA, Quesenberry CP Jr., Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162(4):767–773. [DOI] [PubMed] [Google Scholar]
- 195.Alvarado MG, Schwartz DA. Zika Virus Infection in Pregnancy, Microcephaly, and Maternal and Fetal Health: What We Think, What We Know, and What We Think We Know. Arch Pathol Lab Med. 2017;141(1):26–32. [DOI] [PubMed] [Google Scholar]
- 196.Schwartz DA. Autopsy and Postmortem Studies Are Concordant: Pathology of Zika Virus Infection Is Neurotropic in Fetuses and Infants With Microcephaly Following Transplacental Transmission. Arch Pathol Lab Med. 2017;141(1):68–72. [DOI] [PubMed] [Google Scholar]
- 197.Schwartz DA. The Origins and Emergence of Zika Virus, the Newest TORCH Infection: What’s Old Is New Again. Arch Pathol Lab Med. 2017;141(1):18–25. [DOI] [PubMed] [Google Scholar]
- 198.Coyne CB, Lazear HM. Zika virus - reigniting the TORCH. Nat Rev Microbiol. 2016;14(11):707–715. [DOI] [PubMed] [Google Scholar]
- 199.Aldo PB, Mulla MJ, Romero R, Mor G, Abrahams VM. Viral ssRNA induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am J Reprod Immunol. 2010;64(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Potter JA, Garg M, Girard S, Abrahams VM. Viral single stranded RNA induces a trophoblast pro-inflammatory and antiviral response in a TLR8-dependent and -independent manner. Biol Reprod. 2015;92(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shigeta N, Kumasawa K, Koga K. Chapter 12 - Autoimmunity, regulatory T cells, and pregnancy: Maintaining the balance. In: Mor G, ed. Reproductive Immunology. Academic Press; 2021:239–251. [Google Scholar]
- 202.Bayer A, Delorme-Axford E, Sleigher C, et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am J Obstet Gynecol. 2015;212(1):71 e71–71 e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ding J, Aldo P, Roberts CM, et al. Placenta-derived interferon-stimulated gene 20 controls ZIKA virus infection. EMBO Rep. 2021;22(10):e52450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bierne H, Travier L, Mahlakõiv T, et al. Activation of Type III Interferon Genes by Pathogenic Bacteria in Infected Epithelial Cells and Mouse Placenta. PLOS ONE. 2012;7(6):e39080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-Lambda (IFN-λ) Is Expressed in a Tissue-Dependent Fashion and Primarily Acts on Epithelial Cells In Vivo. PLOS Pathogens. 2008;4(3):e1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Marcello T, Grakoui A, Barba-Spaeth G, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131(6):1887–1898. [DOI] [PubMed] [Google Scholar]
- 207.Nan J, Wang Y, Yang J, Stark GR. IRF9 and unphosphorylated STAT2 cooperate with NF-κB to drive IL6 expression. ProcNatlAcadSciUSA. 2018;115(15):3906–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gao S, von der Malsburg A, Paeschke S, et al. Structural basis of oligomerization in the stalk region of dynamin-like MxA. Nature. 2010;465(7297):502–506. [DOI] [PubMed] [Google Scholar]
- 209.Li C, Deng YQ, Wang S, et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity. 2017;46(3):446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brass AL, Huang IC, Benita Y, et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139(7):1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Huang IC, Bailey CC, Weyer JL, et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7(1):e1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol. 2013;13(1):46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Goff SP. Retrovirus restriction factors. Mol Cell. 2004;16(6):849–859. [DOI] [PubMed] [Google Scholar]
- 214.Kok KH, Jin DY. Balance of power in host-virus arms races. Cell Host Microbe. 2013;14(1):5–6. [DOI] [PubMed] [Google Scholar]
- 215.Munir M, Berg M. The multiple faces of proteinkinase R in antiviral defense. Virulence. 2013;4(1):85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu Y, Nie H, Mao R, et al. Interferon-inducible ribonuclease ISG20 inhibits hepatitis B virus replication through directly binding to the epsilon stem-loop structure of viral RNA. PLoS Pathog. 2017;13(4):e1006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nasr N, Maddocks S, Turville SG, et al. HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood. 2012;120(4):778–788. [DOI] [PubMed] [Google Scholar]
- 218.Wang X, Hinson ER, Cresswell P. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe. 2007;2(2):96–105. [DOI] [PubMed] [Google Scholar]
- 219.Helbig KJ, Eyre NS, Yip E, et al. The antiviral protein viperin inhibits hepatitis C virus replication via interaction with nonstructural protein 5A. Hepatology. 2011;54(5):1506–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Van der Hoek KH, Eyre NS, Shue B, et al. Viperin is an important host restriction factor in control of Zika virus infection. Sci Rep. 2017;7(1):4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Garcia-Flores V, Romero R, Xu Y, et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun. 2022;13(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lu-Culligan A, Chavan AR, Vijayakumar P, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y). 2021;2(5):591–610 e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mourad M, Jacob T, Sadovsky E, et al. Placental response to maternal SARS-CoV-2 infection. Sci Rep. 2021;11(1):14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bordt EA, Shook LL, Atyeo C, et al. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci Transl Med. 2021;13(617):eabi7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Casazza RL, Lazear HM, Miner JJ. Protective and Pathogenic Effects of Interferon Signaling During Pregnancy. Viral immunology. 2020;33(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yockey LJ, Lucas C, Iwasaki A. Contributions of maternal and fetal antiviral immunity in congenital disease. Science. 2020;368(6491):608–612. [DOI] [PubMed] [Google Scholar]
- 227.Bott RC, Ashley RL, Henkes LE, et al. Uterine vein infusion of interferon tau (IFNT) extends luteal life span in ewes. Biol Reprod. 2010;82(4):725–735. [DOI] [PubMed] [Google Scholar]
- 228.Spencer TE, Burghardt RC, Johnson GA, Bazer FW. Conceptus signals for establishment and maintenance of pregnancy. Anim Reprod Sci. 2004;82–83:537–550. [DOI] [PubMed] [Google Scholar]
- 229.Austin KJ, Bany BM, Belden EL, Rempel LA, Cross JC, Hansen TR. Interferon-stimulated gene-15 (Isg15) expression is up-regulated in the mouse uterus in response to the implanting conceptus. Endocrinology. 2003;144(7):3107–3113. [DOI] [PubMed] [Google Scholar]
- 230.Schanz A, Baston-Bust DM, Heiss C, Beyer IM, Krussel JS, Hess AP. Interferon stimulated gene 15 expression at the human embryo-maternal interface. Arch Gynecol Obstet. 2014;290(4):783–789. [DOI] [PubMed] [Google Scholar]
- 231.Pan H, Zhu L, Deng Y, Pollard JW. Microarray analysis of uterine epithelial gene expression during the implantation window in the mouse. Endocrinology. 2006;147(10):4904–4916. [DOI] [PubMed] [Google Scholar]
- 232.Zhang Y, Ma L, Hu X, Ji J, Mor G, Liao A. The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum Reprod. 2019;34(1):25–36. [DOI] [PubMed] [Google Scholar]
- 233.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180(4):1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101(12):4887–4893. [DOI] [PubMed] [Google Scholar]
- 235.Travelli C, Colombo G, Mola S, Genazzani AA, Porta C. NAMPT: A pleiotropic modulator of monocytes and macrophages. Pharmacol Res. 2018;135:25–36. [DOI] [PubMed] [Google Scholar]


