Abstract
Background
Rapid evolution of the therapeutic management of prostate cancer, especially in in second-generation androgen inhibitors, has increased the opportunity of transformation from prostate cancer (PCa) to neuroendocrine prostate cancer (NEPC). NEPC still lacks effective diagnostic and therapeutic interventions. Researches into the molecular characteristics of neuroendocrine differentiation is undoubtedly crucial to the discovery of new target genes for accurate diagnostic and therapeutic targets.
Purpose
In this review, we focus on the relevant genes and molecular mechanisms that have contributed to the transformation in the progression of PCa and discuss the potential targeted molecule that might improve diagnostic accuracy and therapeutic effectiveness.
Methods
The relevant literatures from PubMed have been reviewed for this article.
Conclusion
Several molecular characteristics influence the progression of neuroendocrine differentiation of prostate cancer which will provide a novel sight for accurate diagnosis and target therapeutic intervention for patients with NEPC.
Keywords: Androgen deprivation therapy, NEPC, Neuroendocrine differentiation, Molecular mechanism, Prostate cancer
Introduction
Androgen deprivation therapy (ADT) has become the standard treatment for patients with PCa, owing to the unique role of the androgen in the growth and progression in prostate cancer (Ren et al. 2013; Wong et al. 2014). Tumors receiving ADT eventually progress to an androgen-resistant state, known as CRPC or more lethal NEPC (Attard et al. 2016). NEPC is an aggressive variant of prostate cancer, characterized by the negative expression of AR and lower level of PSA (Sternberg 2019; Beltran et al. 2014). Patients with NEPC have more frequent distant metastases, more frequent RB1 and TP53 gene loss and express characteristic neuroendocrine markers, such as enolase 2 (eno2), chromogranin A (CHGA), synaptophysin (SYP), etc. (Conteduca et al. 2019; Sagnak et al. 2011).
Emerging researches on molecular mechanisms of neuroendocrine differentiation provide a robust and reliable strategy to understand the molecular features, which identify sensitive biological makers and novel therapeutic targets to improve the lives of the patients with NEPC. Unfortunately, NEPC patients still survive less than one year after diagnosis (Wang et al. 2014). Identification of new targeted genes remains a critical challenge.
NMYC
The aberrant overexpression and amplifications of N-Myc and Aurka in NEPC contribute to the progression of neuroendocrine differentiation in a synergistic manner (Beltran et al. 2011). Beltran H et al. reported the first paper regarding concurrent AURKA and NMYC gene in NEPC while a potential relationship between AURKA and NMYC has been demonstrated in neuroblastoma (Otto et al. 2009). N-Myc (encoded by MYCN), a transcription factor, is responsible for the growth of brain during embryogenesis and is a key oncogene in oncogenesis of neuroblastoma (Schwab 1993). Medulloblastoma (Thomas et al. 2009), and glioblastoma multiforme (Tateishi et al. 2016). They further validated the results and indicated that N-Myc improve the stabilization of Aurka by inhibiting the interaction with the E3 ubiquitin ligase FBXW7, and blinds to the promoters of target genes including NSE, Syn and AR to regulate their expression, eventually leading to a neuroendocrine phenotype (Beltran et al. 2011). Additionally, the application of Aurora A inhibitor for NEPC models shows great sensitivity and result in the tumor shrinkage and reversal of the phenotype, which identify new therapeutic targets for patients with NEPC (Mosquera et al. 2013).
Concurrent ALK and MYCN gene amplifications contribute to the activation of Wnt/β-catenin signaling pathway in a synergistic manner, leading to the progression of prostate cancer to NEPC. (Unno et al. 2021) (Fig. 1). Anaplastic lymphoma kinase (ALK) is a member of receptor tyrosine kinase family, and the most prevalent alterations of ALK are chromosomal rearrangements leading to fusion genes, which play an oncogenic role in a variety of malignancies, such as non-small cell lung cancer (NSCLC) and anaplastic large cell lymphoma (ALCL) (Du et al. 2018).
Fig. 1.
ALK-NMYC and AURKA-NMYC
Wnt/β-catenin signaling is a canonical pathway in the Wnt family which is dependent on the presence of β-catenin (Wodarz and Nusse 1998).For understanding the underlying mechanism of activation of the canonical Wnt signaling, UnnoK et al. further found that the amplifications of ALK and MYCN gene have a specific impact on the expression of Csnk1e (tyrosine kinase 1) and Ddx3x (DEAD-box RNA helicase) using a lentivirus-mediated gene transfer and tissue recombination model, which contribute to Dvl2 phosphorylation and polymerization in a synergistic manner, leading to activation of β-catenin driving neuroendocrine differentiation(Unno et al. 2021).
EZH2
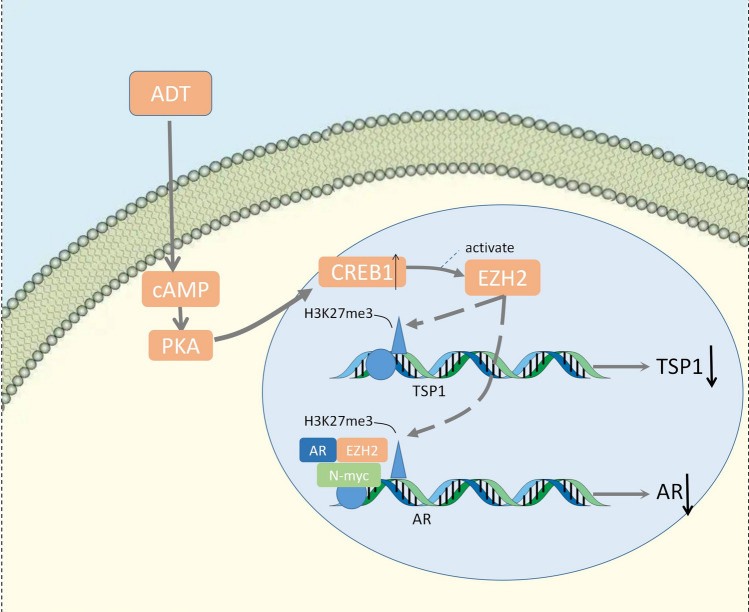
The synthesis of N-Myc/AR/EZH2-PRC2 complex is notably dependent on the presence of EZH2, and N-MYC shows a remarkable synergistic effect with EZH2 and AR to inhibit AR signaling by methylation, leading to neuroendocrine transformation of prostate cancer (Dardenne et al. 2016) (Fig. 2). The EZH2 gene is located on chromosome 7q35 (Cardoso et al. 2000) and is a member of the polycomb group gene (PcGs) family. Polycomb repressive complex 2 (PRC2) is a PcG protein core complex that contributes to gene silence through methylation in the promoter of downstream genes (Duan et al. 2020). EZH2, as the catalytic subunit of PRC2, possesses histone methyltransferase activity and have a repressive impact on targeted gene through histone 3 lysine 3 methylation at position 27 (H3K27me3) (Simon and Lange 2008).
Fig. 2.
NMYC-EZH2 and CREB1-EZH2-TSP1
Recent researches have found that the expression of EZH2 is significantly upregulated in advanced prostate cancer with distant metastases, supporting the clinical relevance of poor diagnosis the emergence of NEPC in enzalutamide-induced models (Luo et al. 2019; Nadal et al. 2014; Varambally et al. 2002). PCa with ADT elevated cAMP levels and induce the expression of EZH2 by activation of PKA-CREB1 signaling, resulting in the neuroendocrine differentiation and preferentially repressing the expression of TSP1 (Zhang et al. 2018) (Fig. 2). TSP1, as an endogenous angiogenesis inhibitor inhibits proliferation of endothelial cell and induces the apoptosis of endothelial cell (Taraboletti et al. 1990). CREB1 is a 43 kDa transcription factor (TF) (Steven et al. 2020). It functions as a second messenger and activates PKA-CREB signaling which is responsible for overexpression of a variety of downstream genes including the oncogene cyclin D1, leading to tumorigenesis and proliferation of several tumors. (Zhang et al. 2020).
Currently, the molecular features between CREB1 and EZH2 remains controversial. HDAC1 / HDAC2, a downstream target gene of CREB, has attracted attention for its combination with EZH2 in nasopharyngeal carcinoma, suggesting the similar biological role in NEPC (Tong et al. 2012). Collectively, CREB1-EZH2-TSP axis is inevitably involved in the regulation of NE phenotype in prostate cancer progression, which guide a potential determinant for therapeutic strategy in NEPC patients.
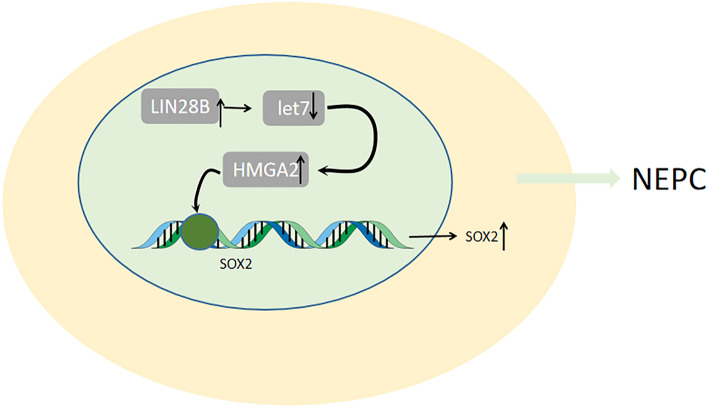
LIN28B
Expression of HMGA2 is significantly elevated by LIN28B due to the negative expression of let-7 miRNA. The SOX2 expression is also considerably high with the upregulation of HMGA2, which has been demonstrated to be involved in lineage plasticity and lead to neuroendocrine differentiation by regulating stem cell-like gene networks in PCa (Lovnicki et al. 2020) (Fig. 3). SOX2 belongs to SOX family, located on chromosome 3q26.3-Q27, and is a group of transcription factors (Stevanovic et al. 1994). Recent researches in SOX2 have put emphasis on its key role in stem cell maintenance, lineage fate determination and reprogramming of somatic cells(Sarkar and Hochedlinger 2013).LIN28B, as an RNA-binding protein, selectively inhibit the expression of let-7 miRNA which performs as tumor suppressors and are associated with the downregulation of oncogenes and regulate mitotic pathways including RAS, MYC and HMGA2(Heo et al. 2009; Büssing et al. 2008). Recent improvements provide a novel insight in relationship between LIN28B and various tumor types, including colon cancer(King et al. 2011), ovarian cancer (Lin et al. 2018), liver cancer (Nguyen et al. 2014), neuroblastoma (Chen et al. 2020).
Fig. 3.
LIN28B/let7/HMGA2/SOX2
The loss of ESE3/EH downregulated the let-7 microRNAs, an inhibitor of Lin28, leading to the imbalance of Lin28/let-7 and is involved in transformation and Stem-like Phenotype of PCa (Albino et al. 2016). In a word, the Lin28/let-7 axis plays a key role in the induction of transformation of prostate cancer to NEPC which might be conducive to the identification of target gene for existing therapies.
ONECUT2
The aberrant expression of ONECUT2 in PCa contribute to the upregulation of SMAD3, leading to the activation of hypoxia signaling by HIF1α.The concurrent amplifications of ONECUT2 and hypoxia signaling play an important role in driving neuroendocrine differentiation and the overexpression of NE marker genes (Guo et al. 2019).ONECUT2 is a novel member of the ONECUT family, located on human chromosome 18, and usually functions as a transcription factor, which have fundamental roles during tumorigenesis proliferation, migration and differentiation in hepatocellular carcinoma (Zhang et al. 2015), ovarian cancer(Lu et al. 2018) and lung adenocarcinoma(Ma et al. 2019). The aberrant overexpression of ONECUT2 in CRPC downregulate AR signaling and FOXA1 which are responsible for the neuroendocrine features of CRPC (Rotinen et al. 2018). Conceivably, FOXA1 functions as an inhibitor of neuroendocrine differentiation (Kim et al. 2017).
Cellular plasticity and upregulation of hypoxia response genes in prostate cancer mediated by the overexpression of ONECUT2 are involved in the progression of NE differentiation. It is suggested that treatment directed by inhibition of hypoxia or ONECUT2 might provide a novel insight of therapies to inhibit the progression and occurrence of NEPC.
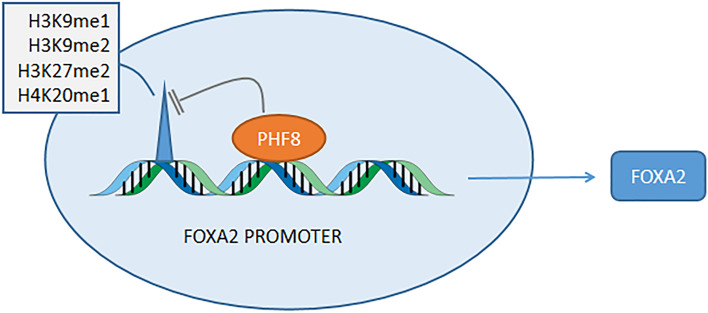
PHF8
FOXA2 is incredibly overexpressed due to the removement of repressive methylated proteins in the FOXA2 promoter region by PHF8, which allows for elicitation of aggressive phenotype (Liu et al. 2021) (Fig. 4). The aberrant amplifications of PHF8 are involved in the occurrence, progression and invasion in PCa and intensive researches regarding unique role of PHF8 imply PHF8 as a regulator of neuroendocrine differentiation (Ma et al. 2015; Tong et al. 2016). PHF8 (KDM7B), as a histone demethylase, is responsible for demethylation of H3K9me1/2, H3K27me2 and H4K20me1, allowing transcription of downstream genes (Liu et al. 2010), and is involved in hepatocellular carcinoma (Ye et al. 2019), X-chromosome-linked intellectual disability (XLID) (Chen et al. 2018), melanoma (Moubarak et al. 2022), esophageal cancer (Sun et al. 2013) and so on. FOXA2 is a specific biomarker in NEPC (Park et al. 2017) and can combine with the Siah2-dependent regulation of HIF suggesting the important roles in driving neuroendocrine phenotype (Qi et al. 2010).
Fig. 4.
PHF8 upregulates the expression of FOXA2
Further studies are warranted regarding the relationship between PHF8 and FOXA2 which is still unknown and it might lead to sensitive biological targets for patients with NEPC.
MUC1-C
MYC-BRN2 is directly or indirectly activated by negative expression of AR axis, in which MUC1-C plays a unique role (Lin et al. 2017; Yasumizu et al. 2020). BRN2, as a neural transcription factor, is negatively correlated with AR activity and identified as an emerging biologic activator of NEPC (Bishop et al. 2017). In addition, MUC1 is a heterodimeric protein that is aberrantly overexpressed in multiple tumors, which contribute to immune evasion in aggressive breast cancer (Maeda et al. 2018; Li et al. 2020a). To date, MUC1-C features the function of EMT, drug resistance, malignant phenotype maintenance (Rajabi and Kufe 2017).
MUC1-C plays a notable role in expression of MYCN, EZH2, and specific biomarkers which have been identified as drivers of NEPC. The molecular features of MYCN and EZH2 in NED have been reported above. Furthermore, repression of the p53 pathway shows a remarkable effect on OCT4, SOX2, KLF4 and MYC pluripotency and drives stemness (Yasumizu et al. 2020). Cell stemness driven by MUC1-C mainly depend on the presence of p53 and SOX2, which regulates lineage plasticity through the LIN28B/HMGA2/SOX axis as described above (Lovnicki et al. 2020). P53 performs as a tumor suppressor and features its alterations in mutations and deletions in a variety of tumors (Khemlina et al. 2015; Akamatsu et al. 2015).
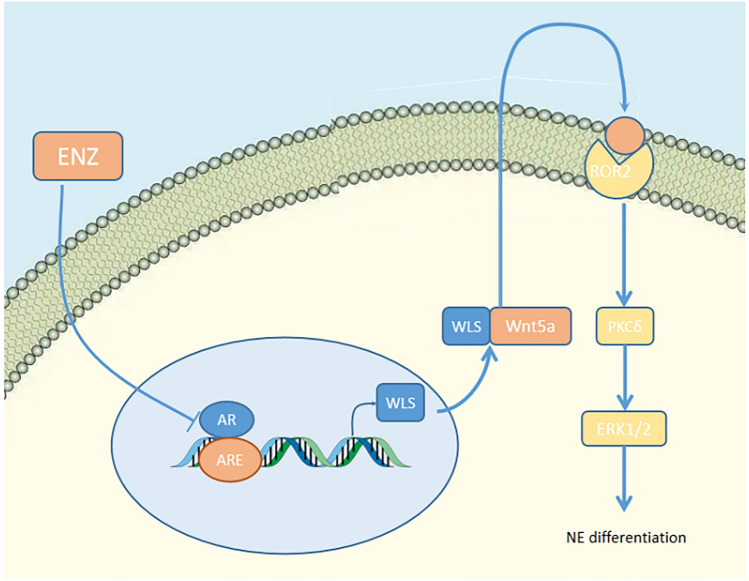
WLS
The downregulation of AR by application of androgen potent inhibitor acts as an activator of WLS, leading to the activation of a non-canonical Wnt pathway. Concurrent overexpression of WLS and Wnt5a contribute to downstream ROR2/PKCδ/ERK signaling in a synergistic manner so as to drive neuroendocrine phenotype (Bland et al. 2021) (Fig. 5). ROR2 is an orphan receptor tyrosine kinase and function as a non-canonical Wnt signaling receptor. Wnt5A enhances the chemotaxis and proliferation of leukemia by the activation of ROR1/ROR2 (Yu et al. 2016). Extracellular signal-regulated kinase 1/2 (ERK) belongs to the mitogen-activated protein kinase (MAPK) family and recent improvements emphasize on its effect on signal transmission in signaling cascades (Guo et al. 2020). MAPK/ERK pathway is associated with neuroendocrine phenotype due to the presence of IL-8 (Kim et al. 2017).
Fig. 5.
WLS/Wnt
WLS (wntless) is a conserved multi-channel endoplasmic reticulum (ER) transmembrane protein.
WLS carrier protein plays a fundamental role in transport and secretion of the Wnt protein after glycosylated and palmitolated in ER into extracellular medium (Bänziger et al. 2006; Smolich et al. 1993). The non-canonical signal consists of Wnt-PCP and Wnt-Ca and features its independence on β-catenin (Komiya and Habas 2008). Wnt4, Wnt5a, and Wnt11 have be reported to be ligands of non-canonical signal (Veeman et al. 2003). Both the canonical pathway and the non-canonical pathway play a vital role in NE differentiation (Unno et al. 2021; Uysal-Onganer et al. 2010). Based on this, inhibition of all WNT signaling may potentially have a unique role in reversing NE transformation, in which WLS is indispensable.
TROP2
Expression of Trop2 is aberrantly upregulated in NEPC and influence tumor growth, metastasis and neuroendocrine differentiation. Recent studies have identified Trop2 as a driver of aggressive neuroendocrine phenotype, in which elevated PARP1 plays a key role. Furthermore, Trop2-induced NE phenotype can be reversed by PARP1 inhibitors (Hsu et al. 2020). PARP1 is a DNA-dependent ADP- ribosyltransferase has several biologic functions in DNA replication, chromatin remodeling and apoptosis (Ray Chaudhuri and Nussenzweig 2017; Schiewer and Knudsen 2014; Fujimoto et al. 2017). Trophoblast surface antigen 2 (Trop2), also known as tumor-associated calcium signal transducer, is a class of cell surface glycoproteins (Lipinski et al. 1981), elevated levels of Trop2 is closely interrelated to poor prognosis and higher risk of metastasis in various tumors, such as in oral, gastric, thyroid and pancreatic cancer (Fong et al. 2008a, b; Mühlmann et al. 2009; Sun et al. 2021). Additionally, in prostate cancer it consistently enhances tumor growth, migration, metastasis and lineage plasticity (Trerotola et al. 2013).
Elevated levels of Trop2 also contribute to the expression of SOX2 and EZH2 (Hsu et al. 2020), which are associated with lineage plasticity and drug resistance (Li et al. 2020b). To date, although PARP1 is a key factor for TROP2 to act as a NED driver, the underlying mechanism by which TROP2 regulates PARP1 is still unknown. In previous report, c-Myc bind to the promoter of PARP1 and activate its expression to promotes the generation of IPSC and pluripotency maintenance. Similarly, Trop2 may regulate PARP1 through c-Myc to participate in cell reprogramming and NE differentiation (Hsu et al. 2020).
ZBTB46
Downregulation of AR signaling in PCa with ADT leads to elevated levels of LIF and activate LIF-STAT3 pathway to allows for elicitation of aggressive neuroendocrine phenotype. ZBTB46 was involved in the transformation by inactivation of AR signaling (Liu et al. 2019) (Fig. 6). Leukemia inhibitory factor (LIF) is a member of the IL-6 cytokine family. It is a pleiotropic cytokine with a wide range of activities (Nicola and Babon 2015) such as survival, proliferation and metastasis in various tumors through three pathways: JAK/STAT (Stahl et al. 1994), MAP kinase (Williams et al. 2009) and PI (3) kinase (Oh et al. 1998) pathways.
Fig. 6.
ZBTB46/LIF/STAT and ZBTB46/PTGS1
ZBTB46 is a novel tumorigenic factor in prostate cancer, and is negatively correlated with the AR signaling (Chen et al. 2017). It acts as an upstream regulator of Snail and enhance the tumorigenic capacity, progression, invasion and metastasis by regulating the expression of E-cadherin and RKIP in prostate cancer (Beach et al. 2008; Poblete et al. 2014). The inhibition of AR contributes to the upregulation of ZBTB46 and elevated ZBTB46 binds to the promoter of PTGS1 to improve its expression, which is responsible for the malignant transformation and tumors progressing to NEPC (Chen et al. 2019). The androgen-responsive gene SPDEF is prominently down-regulated due to the negative expression of AR signaling during androgen deprivation therapy, resulting in the aberrant elevation of ZBTB46 which is identified as a transcriptional coactivator (Tsai et al. 2018). SPDEF is an E26-specific (ETS) transcription factor (Oettgen et al. 2000), and is considered as a tumor suppressor of PCa (Gu et al. 2007). Downregulation of SPDEF is closely related to the occurrence, growth and metastasis in prostate cancer (Steffan et al. 2016). Prostaglandin G/H synthase 1 (PTGS1) is a physiologically important prostaglandin synthase that plays an important role in the progression of bone diseases, tumors and inflammatory diseases (Smith et al. 2000; Choi et al. 2008; Kargman et al. 1995; Wang et al. 2019).
EGFR–LIFR-SUCLG2
PCa with ADT induces upregulation of EGFR and acts as a transcriptional regulator that binds to the LIFR promoter to stimulate LIFR expression. The upregulation of LIFR is associated with suclg2. ADT upregulates EGFR-LIFR signaling, activates suclg2, and in turn promotes metabolic changes associated with NE differentiation and an aggressive prostate cancer phenotype (Lin et al. 2020). ADT induced upregulation of the succinate-CoA ligase GDP-forming beta subunit (SUCLG2). Succinate-CoA ligase (SUCL) is a heterodimeric enzyme composed of a Suclg1 α-subunit and a substrate-specific Suclg2 β-subunit, which generates ATP or GTP respectively, this subunit is able to regulate succinate metabolism and NE differentiation in prostate cancer (Kacso et al. 2016).
Others
Mammalian target of rapamycin (mTOR) acts as an activator of AKT signaling, and aberrant amplifications of constitutively active mTOR leads to NE differentiation and the expression of NSE in LNCaP cells (Kanayama et al. 2017). The level of FOXB2 is significantly upregulated in aggressive PCa which are responsible for NE differentiation by inducing agonistic ligands (mainly WNT7B) to activate the Wnt pathway (Moparthi et al. 2019). It is obvious that the Wnt signaling pathway, both canonical and non-canonical, plays a critical role in inducing the neuroendocrine differentiation process. Heterochromatin protein 1α (HP1α) downregulates the expression of androgen receptor and RE1-silencing transcription factors, and enriches repressive trimethylated histone H3 at Lys9 marks on their respective gene promoters, significantly stimulating NE differentiation and enhanced invasiveness (Ci et al. 2018). Protocadherin-PC (PCDH-PC), encoded on the human Y chromosome, is aberrantly upregulated in prostate cancer tumor cells after hormone deprivation. The upregulation of PCDH-PC results in the upregulation of nuclear β-catenin and induce the NE transformation (Yang et al. 2005; Terry et al. 2006). Interleukin (IL)—6 is associated with progression and differentiation of a variety of tumors (Spiotto and Chung 2000), and STAT3 act as an IL-6-acting mediator to inhibit growth and induce neurite extension and the amplification of NSE expression (Spiotto and Chung 2000). Some cytokines including IL-6 and IL-8 are closely related to the transformation from prostate cancer to NEPC. IL-6 induces NED through SATA3 as described above. IL-8 has direct oncogenicity and can significantly induce cell proliferation, mediate NED and inhibit apoptosis through the STAT3/AKT/NF-κB pathway (Guo et al. 2017).
Conclusion
NEPC is usually diagnosed at an advanced stage and shows great increase in incidence due to the rapid development of drug resistance. Understanding the driving factors of this kind of highly invasive tumor may provide a theoretical basis for developing the effective treatment strategy. In this review, aimed to better understanding of NEPC progression, we summarize the literature on several genes and pathways that contribute to the development of NEPC. Furthermore, we would like to discuss some others potential and complicate mechanisms in this process to provide some references for further research.
Androgen, as a steroid hormone, maintains the growth and development of PCa by combining with AR. Nevertheless, AR signaling is inhibited in NEPC and how do NEPC survive catches our attention. Further studies are warranted regarding this alternative survival pathway. In the relevant literatures, we found that the upregulation of SREBPs influence the synthesis of enzymes that is critical for lipogenesis and cholesterol synthesis during the progression to androgen independence and the outcome seems to be consistent with our conjecture. Interestingly, CREB interacts with CRTC2 mediating mTOR signaling to regulate the expression of SREBP1 in the liver and PCa with ADT elevated cAMP levels to activate PKA-CREB signaling (Han et al. 2015). On the basis of CREB-SREBPs relationship in liver, we put forward a conjecture that the regulatory mechanisms in NEPC is similar to that in the liver and infer that the upregulation of CREB by elevated cAMP levels in NPEC could interact with CRTC2, a critical mediator of mTOR, to regulate COPII, leading to the upregulation of SREBP-1 and function as an alternative pathway of AR signal. Furthermore, MYC, as an oncogene, activate and cooperate with SREBP-1 to regulate the synthesis of fatty acid and lipogenesis (Gouw et al. 2019). Therefore, we propose the further inference that the upregulation of SREBP-1 by CREB-SREBPs interact with MYC to regulate the lipogenesis and cholesterol synthesis in NEPC and maintain the occurrence and development of NEPC.
The canonical Wnt signal stabilizes β- Catenin and allows its translocation into the nucleus to interact with (TCF/LEF) and coactivators to trigger Wnt target genes and lead to the neuroendocrine differentiation (Ciarlo et al. 2012). During the oviduct development, the canonical WNT cascade increases NR5A2 binding to the CYP11A1 and 3β-HSD gene promoters to facilitate steroidogenesis and regulate oviductal epithelial secretion (Tan et al. 2021). CYP11A1 and 3β-HSD take part in almost all steroidogenic processes and NR5A2 has also been identified as a critical regulator of steroidogenesis (Miller and Auchus 2011). Meanwhile, in the progression of gastrointestinal tumor, the synergy between NR5A21 and β-catenin/TCF4 signaling upregulate the expression of cyclin D1, cyclin E1, and c-Myc which is the downstream targets of the canonical Wnt signaling (Schoonjans et al. 2005). C-Myc is an oncogene that contributes to the genesis of many human cancers and are responsible for proliferation, inflammation, and self-renewing (Dang 2012). On basis of these reports, it can be inferred that the mediation of the canonical Wnt signal might activate the transcription factor NR5A2 and regulate transcriptional control of steroidogenesis by CTNNB1(β-catenin)/NR5A2 signaling in NEPC to replace androgen as a main driver of NEPC growth and progression. Meanwhile, the synergy of β-catenin/TCF4/ NR5A2/c-Myc might play an inevitably role in proliferation and differentiation of NEPC and ALK and MYCN gene amplifications described above might function as the triggering factor to mediate the β-catenin/TCF4/ NR5A2/c-Myc (Unno et al. 2021).
SREBPs, CYP11A1 and 3β-HSD described above are responsible for the formation of steroid hormones, cholesterol and fatty acid and they are inextricably associated with the genes involved in neuroendocrine differentiation like Wnt and CREB, and MYC might to be the bridge between SREBPs, CYP11A1 and 3β-HSD. Therefore, we have every reason to suspect that there is a certain connection between them, although it still remains largely elusive. We believe that the potential connection between them will be made known to the public in the near future to bring hope to patients with NEPC.
Author contributions
All authors contributed to the study conception and design.
Funding
The study was supported by General Project of Jiangsu Provincial Health Commission (M2021038) and Zhenjiang Social Development Project (SH2020025).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akamatsu S et al (2015) The placental gene PEG10 promotes progression of neuroendocrine prostate cancer. Cell Rep 12(6):922–936. 10.1016/j.celrep.2015.07.012 [DOI] [PubMed] [Google Scholar]
- Albino D et al (2016) Activation of the Lin28/let-7 axis by loss of ESE3/EHF promotes a tumorigenic and stem-like phenotype in prostate cancer. Cancer Res 76(12):3629–3643. 10.1158/0008-5472.Can-15-2665 [DOI] [PubMed] [Google Scholar]
- Attard G et al (2016) Prostate cancer. Lancet 387(10013):70–82. 10.1016/s0140-6736(14)61947-4 [DOI] [PubMed] [Google Scholar]
- Bänziger C et al (2006) Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125(3):509–522. 10.1016/j.cell.2006.02.049 [DOI] [PubMed] [Google Scholar]
- Beach S et al (2008) Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene 27(15):2243–2248. 10.1038/sj.onc.1210860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H et al (2011) Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 1(6):487–495. 10.1158/2159-8290.Cd-11-0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H et al (2014) Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res 20(11):2846–2850. 10.1158/1078-0432.Ccr-13-3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JL et al (2017) The master neural transcription factor BRN2 is an androgen receptor-suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov 7(1):54–71. 10.1158/2159-8290.Cd-15-1263 [DOI] [PubMed] [Google Scholar]
- Bland T et al (2021) WLS-Wnt signaling promotes neuroendocrine prostate cancer. iScience 24(1):101970. 10.1016/j.isci.2020.101970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büssing I, Slack FJ, Grosshans H (2008) let-7 microRNAs in development, stem cells and cancer. Trends Mol Med 14(9):400–409. 10.1016/j.molmed.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Cardoso C et al (2000) The human EZH2 gene: genomic organisation and revised mapping in 7q35 within the critical region for malignant myeloid disorders. Eur J Hum Genet 8(3):174–180. 10.1038/sj.ejhg.5200439 [DOI] [PubMed] [Google Scholar]
- Chen WY et al (2017) Inhibition of the androgen receptor induces a novel tumor promoter, ZBTB46, for prostate cancer metastasis. Oncogene 36(45):6213–6224. 10.1038/onc.2017.226 [DOI] [PubMed] [Google Scholar]
- Chen X et al (2018) Phf8 histone demethylase deficiency causes cognitive impairments through the mTOR pathway. Nat Commun 9(1):114. 10.1038/s41467-017-02531-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY et al (2019) Androgen deprivation-induced ZBTB46-PTGS1 signaling promotes neuroendocrine differentiation of prostate cancer. Cancer Lett 440–441:35–46. 10.1016/j.canlet.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Chen D et al (2020) LIN28B promotes neuroblastoma metastasis and regulates PDZ binding kinase. Neoplasia 22(6):231–241. 10.1016/j.neo.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Langenbach R, Bosetti F (2008) Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. Faseb J 22(5):1491–1501. 10.1096/fj.07-9411com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ci X et al (2018) Heterochromatin protein 1α mediates development and aggressiveness of neuroendocrine prostate cancer. Cancer Res 78(10):2691–2704. 10.1158/0008-5472.Can-17-3677 [DOI] [PubMed] [Google Scholar]
- Ciarlo M et al (2012) Regulation of neuroendocrine differentiation by AKT/hnRNPK/AR/β-catenin signaling in prostate cancer cells. Int J Cancer 131(3):582–590. 10.1002/ijc.26402 [DOI] [PubMed] [Google Scholar]
- Conteduca V et al (2019) Clinical features of neuroendocrine prostate cancer. Eur J Cancer 121:7–18. 10.1016/j.ejca.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV (2012) MYC on the path to cancer. Cell 149(1):22–35. 10.1016/j.cell.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne E et al (2016) N-Myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell 30(4):563–577. 10.1016/j.ccell.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X et al (2018) ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer 9(4):423–430. 10.1111/1759-7714.12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Du W, Guo W (2020) EZH2: a novel target for cancer treatment. J Hematol Oncol 13(1):104. 10.1186/s13045-020-00937-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong D et al (2008a) TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol 21(2):186–191. 10.1038/modpathol.3801001 [DOI] [PubMed] [Google Scholar]
- Fong D et al (2008b) High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer 99(8):1290–1295. 10.1038/sj.bjc.6604677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M et al (2017) The HSF1-PARP13-PARP1 complex facilitates DNA repair and promotes mammary tumorigenesis. Nat Commun 8(1):1638. 10.1038/s41467-017-01807-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AM, Margulis K, Liu NS et al (2019) The MYC oncogene cooperates with sterol-regulated element-binding protein to regulate lipogenesis essential for neoplastic growth. Cell Metab 30(3):556-572.e5. 10.1016/j.cmet.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X et al (2007) Reduced PDEF expression increases invasion and expression of mesenchymal genes in prostate cancer cells. Cancer Res 67(9):4219–4226. 10.1158/0008-5472.Can-06-3689 [DOI] [PubMed] [Google Scholar]
- Guo Y et al (2017) IL-8 promotes proliferation and inhibition of apoptosis via STAT3/AKT/NF-κB pathway in prostate cancer. Mol Med Rep 16(6):9035–9042. 10.3892/mmr.2017.7747 [DOI] [PubMed] [Google Scholar]
- Guo H et al (2019) ONECUT2 is a driver of neuroendocrine prostate cancer. Nat Commun 10(1):278. 10.1038/s41467-018-08133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YJ et al (2020) ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med 19(3):1997–2007. 10.3892/etm.2020.8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J et al (2015) The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature 524(7564):243–246. 10.1038/nature14557 [DOI] [PubMed] [Google Scholar]
- Heo I et al (2009) TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138(4):696–708. 10.1016/j.cell.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Hsu EC et al (2020) Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc Natl Acad Sci U S A 117(4):2032–2042. 10.1073/pnas.1905384117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacso G et al (2016) Two transgenic mouse models for β-subunit components of succinate-CoA ligase yielding pleiotropic metabolic alterations. Biochem J 473(20):3463–3485. 10.1042/bcj20160594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama M et al (2017) Hyperactive mTOR induces neuroendocrine differentiation in prostate cancer cell with concurrent up-regulation of IRF1. Prostate 77(15):1489–1498. 10.1002/pros.23425 [DOI] [PubMed] [Google Scholar]
- Kargman SL et al (1995) Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res 55(12):2556–2559 [PubMed] [Google Scholar]
- Khemlina G, Ikeda S, Kurzrock R (2015) Molecular landscape of prostate cancer: implications for current clinical trials. Cancer Treat Rev 41(9):761–766. 10.1016/j.ctrv.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Kim J et al (2017) FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 36(28):4072–4080. 10.1038/onc.2017.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE et al (2011) LIN28B promotes colon cancer progression and metastasis. Cancer Res 71(12):4260–4268. 10.1158/0008-5472.Can-10-4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4(2):68–75. 10.4161/org.4.2.5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W et al (2020a) MUC1-C drives stemness in progression of colitis to colorectal cancer. JCI Insight. 10.1172/jci.insight.137112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H et al (2020b) SOX2 has dual functions as a regulator in the progression of neuroendocrine prostate cancer. Lab Invest 100(4):570–582. 10.1038/s41374-019-0343-5 [DOI] [PubMed] [Google Scholar]
- Lin X et al (2017) Overexpression of MUC1 and genomic alterations in its network associate with prostate cancer progression. Neoplasia 19(11):857–867. 10.1016/j.neo.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X et al (2018) RNA-binding protein LIN28B inhibits apoptosis through regulation of the AKT2/FOXO3A/BIM axis in ovarian cancer cells. Signal Transduct Target Ther 3:23. 10.1038/s41392-018-0026-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SR et al (2020) EGFR-upregulated LIFR promotes SUCLG2-dependent castration resistance and neuroendocrine differentiation of prostate cancer. Oncogene 39(44):6757–6775. 10.1038/s41388-020-01468-9 [DOI] [PubMed] [Google Scholar]
- Lipinski M et al (1981) Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci U S A 78(8):5147–5150. 10.1073/pnas.78.8.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W et al (2010) PHF8 mediates histone H4 lysine 20 demethylation events involved in cell cycle progression. Nature 466(7305):508–512. 10.1038/nature09272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YN et al (2019) Leukemia inhibitory factor promotes castration-resistant prostate cancer and neuroendocrine differentiation by activated ZBTB46. Clin Cancer Res 25(13):4128–4140. 10.1158/1078-0432.Ccr-18-3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q et al (2021) Histone demethylase PHF8 drives neuroendocrine prostate cancer progression by epigenetically upregulating FOXA2. J Pathol 253(1):106–118. 10.1002/path.5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovnicki J et al (2020) LIN28B promotes the development of neuroendocrine prostate cancer. J Clin Invest 130(10):5338–5348. 10.1172/jci135373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T et al (2018) Blockade of ONECUT2 expression in ovarian cancer inhibited tumor cell proliferation, migration, invasion and angiogenesis. Cancer Sci 109(7):2221–2234. 10.1111/cas.13633 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Luo J et al (2019) LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat Commun 10(1):2571. 10.1038/s41467-019-09784-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q et al (2015) The histone demethylase PHF8 promotes prostate cancer cell growth by activating the oncomiR miR-125b. Onco Targets Ther 8:1979–1988. 10.2147/ott.S85443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q et al (2019) ONECUT2 overexpression promotes RAS-driven lung adenocarcinoma progression. Sci Rep 9(1):20021. 10.1038/s41598-019-56277-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T et al (2018) MUC1-C induces PD-L1 and immune evasion in triple-negative breast cancer. Cancer Res 78(1):205–215. 10.1158/0008-5472.Can-17-1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ (2011) The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32(1):81–151. 10.1210/er.2010-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moparthi L, Pizzolato G, Koch S (2019) Wnt activator FOXB2 drives the neuroendocrine differentiation of prostate cancer. Proc Natl Acad Sci U S A 116(44):22189–22195. 10.1073/pnas.1906484116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera JM et al (2013) Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 15(1):1–10. 10.1593/neo.121550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubarak RS et al (2022) The histone demethylase PHF8 regulates TGFβ signaling and promotes melanoma metastasis. Sci Adv 8(7):e7127. 10.1126/sciadv.abi7127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlmann G et al (2009) TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol 62(2):152–158. 10.1136/jcp.2008.060590 [DOI] [PubMed] [Google Scholar]
- Nadal R et al (2014) Small cell carcinoma of the prostate. Nat Rev Urol 11(4):213–219. 10.1038/nrurol.2014.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH et al (2014) Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell 26(2):248–261. 10.1016/j.ccr.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola NA, Babon JJ (2015) Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev 26(5):533–544. 10.1016/j.cytogfr.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen P et al (2000) PDEF, a novel prostate epithelium-specific ets transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J Biol Chem 275(2):1216–1225. 10.1074/jbc.275.2.1216 [DOI] [PubMed] [Google Scholar]
- Oh H et al (1998) Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem 273(16):9703–9710. 10.1074/jbc.273.16.9703 [DOI] [PubMed] [Google Scholar]
- Otto T et al (2009) Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 15(1):67–78. 10.1016/j.ccr.2008.12.005 [DOI] [PubMed] [Google Scholar]
- Park JW et al (2017) FOXA2 is a sensitive and specific marker for small cell neuroendocrine carcinoma of the prostate. Mod Pathol 30(9):1262–1272. 10.1038/modpathol.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblete CE et al (2014) Increased SNAIL expression and low syndecan levels are associated with high Gleason grade in prostate cancer. Int J Oncol 44(3):647–654. 10.3892/ijo.2014.2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J et al (2010) Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell 18(1):23–38. 10.1016/j.ccr.2010.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi H, Kufe D (2017) MUC1-C oncoprotein integrates a program of EMT, epigenetic reprogramming and immune evasion in human carcinomas. Biochim Biophys Acta Rev Cancer 1868(1):117–122. 10.1016/j.bbcan.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Chaudhuri A, Nussenzweig A (2017) The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol 18(10):610–621. 10.1038/nrm.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren SC, Chen R, Sun YH (2013) Prostate cancer research in China. Asian J Androl 15(3):350–353. 10.1038/aja.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotinen M et al (2018) ONECUT2 is a targetable master regulator of lethal prostate cancer that suppresses the androgen axis. Nat Med 24(12):1887–1898. 10.1038/s41591-018-0241-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagnak L et al (2011) Prognostic significance of neuroendocrine differentiation in prostate adenocarcinoma. Clin Genitourin Cancer 9(2):73–80. 10.1016/j.clgc.2011.07.003 [DOI] [PubMed] [Google Scholar]
- Sarkar A, Hochedlinger K (2013) The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12(1):15–30. 10.1016/j.stem.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiewer MJ, Knudsen KE (2014) Transcriptional roles of PARP1 in cancer. Mol Cancer Res 12(8):1069–1080. 10.1158/1541-7786.Mcr-13-0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonjans K et al (2005) Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation. Proc Natl Acad Sci U S A 102(6):2058–2062. 10.1073/pnas.0409756102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M (1993) Amplification of N-myc as a prognostic marker for patients with neuroblastoma. Semin Cancer Biol 4(1):13–18 [PubMed] [Google Scholar]
- Simon JA, Lange CA (2008) Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res 647(1–2):21–29. 10.1016/j.mrfmmm.2008.07.010 [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182. 10.1146/annurev.biochem.69.1.145 [DOI] [PubMed] [Google Scholar]
- Smolich BD et al (1993) Wnt family proteins are secreted and associated with the cell surface. Mol Biol Cell 4(12):1267–1275. 10.1091/mbc.4.12.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiotto MT, Chung TD (2000) STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate 42(3):186–195. 10.1002/(sici)1097-0045(20000215)42:3%3c186::aid-pros4%3e3.0.co;2-e [DOI] [PubMed] [Google Scholar]
- Stahl N et al (1994) Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science 263(5143):92–95. 10.1126/science.8272873 [DOI] [PubMed] [Google Scholar]
- Steffan JJ et al (2016) The transcription factor SPDEF suppresses prostate tumor metastasis. J Biol Chem 291(39):20826. 10.1074/jbc.A112.379396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg CN (2019) Enzalutamide, an oral androgen receptor inhibitor for treatment of castration-resistant prostate cancer. Future Oncol 15(13):1437–1457. 10.2217/fon-2018-0940 [DOI] [PubMed] [Google Scholar]
- Stevanovic M et al (1994) The cDNA sequence and chromosomal location of the human SOX2 gene. Mamm Genome 5(10):640–642. 10.1007/bf00411460 [DOI] [PubMed] [Google Scholar]
- Steven A et al (2020) What turns CREB on? And off? And why does it matter? Cell Mol Life Sci 77(20):4049–4067. 10.1007/s00018-020-03525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X et al (2013) Oncogenic features of PHF8 histone demethylase in esophageal squamous cell carcinoma. PLoS ONE 8(10):e77353. 10.1371/journal.pone.0077353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H et al (2021) TROP2 modulates the progression in papillary thyroid carcinoma. J Cancer 12(22):6883–6893. 10.7150/jca.62461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Zhang L, Li T et al (2021) Lgr4 regulates oviductal epithelial secretion through the WNT signaling pathway. Front Cell Dev Biol. 9:666303. 10.3389/fcell.2021.666303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G et al (1990) Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol 111(2):765–772. 10.1083/jcb.111.2.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K et al (2016) Myc-driven glycolysis is a therapeutic target in glioblastoma. Clin Cancer Res 22(17):4452–4465. 10.1158/1078-0432.Ccr-15-2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry S et al (2006) Protocadherin-PC promotes androgen-independent prostate cancer cell growth. Prostate 66(10):1100–1113. 10.1002/pros.20446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WD et al (2009) Patched1 deletion increases N-Myc protein stability as a mechanism of medulloblastoma initiation and progression. Oncogene 28(13):1605–1615. 10.1038/onc.2009.3 [DOI] [PubMed] [Google Scholar]
- Tong ZT et al (2012) EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene 31(5):583–594. 10.1038/onc.2011.254 [DOI] [PubMed] [Google Scholar]
- Tong D et al (2016) The HIF/PHF8/AR axis promotes prostate cancer progression. Oncogenesis 5(12):e283. 10.1038/oncsis.2016.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trerotola M et al (2013) Trop-2 promotes prostate cancer metastasis by modulating β(1) integrin functions. Cancer Res 73(10):3155–3167. 10.1158/0008-5472.Can-12-3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC et al (2018) Androgen deprivation therapy-induced epithelial-mesenchymal transition of prostate cancer through downregulating SPDEF and activating CCL2. Biochim Biophys Acta Mol Basis Dis 1864(5 Pt A):1717–1727. 10.1016/j.bbadis.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Unno K et al (2021) Activated ALK cooperates with N-Myc via Wnt/β-catenin signaling to induce neuroendocrine prostate cancer. Cancer Res 81(8):2157–2170. 10.1158/0008-5472.Can-20-3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal-Onganer P et al (2010) Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol Cancer 9:55. 10.1186/1476-4598-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varambally S et al (2002) The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419(6907):624–629. 10.1038/nature01075 [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5(3):367–377. 10.1016/s1534-5807(03)00266-1 [DOI] [PubMed] [Google Scholar]
- Wang HT et al (2014) Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol 32(30):3383–3390. 10.1200/jco.2013.54.3553 [DOI] [PubMed] [Google Scholar]
- Wang Y et al (2019) Inhibition of PTGS1 promotes osteogenic differentiation of adipose-derived stem cells by suppressing NF-kB signaling. Stem Cell Res Ther 10(1):57. 10.1186/s13287-019-1167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NK et al (2009) Dissecting specificity in the Janus kinases: the structures of JAK-specific inhibitors complexed to the JAK1 and JAK2 protein tyrosine kinase domains. J Mol Biol 387(1):219–232. 10.1016/j.jmb.2009.01.041 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R (1998) Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14:59–88. 10.1146/annurev.cellbio.14.1.59 [DOI] [PubMed] [Google Scholar]
- Wong YN et al (2014) Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat Rev Clin Oncol 11(6):365–376. 10.1038/nrclinonc.2014.72 [DOI] [PubMed] [Google Scholar]
- Yang X et al (2005) A human- and male-specific protocadherin that acts through the wnt signaling pathway to induce neuroendocrine transdifferentiation of prostate cancer cells. Cancer Res 65(12):5263–5271. 10.1158/0008-5472.Can-05-0162 [DOI] [PubMed] [Google Scholar]
- Yasumizu Y et al (2020) Author Correction: MUC1-C regulates lineage plasticity driving progression to neuroendocrine prostate cancer. Nat Commun 11(1):1095. 10.1038/s41467-020-14808-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H et al (2019) PHF8 plays an oncogene function in hepatocellular carcinoma formation. Oncol Res 27(5):613–621. 10.3727/096504018x15410353669149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J et al (2016) Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation. J Clin Invest 126(2):585–598. 10.1172/jci83535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J et al (2015) Comprehensive profiling of novel microRNA-9 targets and a tumor suppressor role of microRNA-9 via targeting IGF2BP1 in hepatocellular carcinoma. Oncotarget 6(39):42040–42052. 10.18632/oncotarget.5969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y et al (2018) Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat Commun 9(1):4080. 10.1038/s41467-018-06177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H et al (2020) Complex roles of cAMP-PKA-CREB signaling in cancer. Exp Hematol Oncol 9(1):32. 10.1186/s40164-020-00191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.