Abstract
The distinctive flavor of wine, brandy, and other grape-derived alcoholic beverages is affected by many compounds, including esters produced during alcoholic fermentation. The characteristic fruity odors of the fermentation bouquet are primarily due to a mixture of hexyl acetate, ethyl caproate (apple-like aroma), iso-amyl acetate (banana-like aroma), ethyl caprylate (apple-like aroma), and 2-phenylethyl acetate (fruity, flowery flavor with a honey note). The objective of this study was to investigate the feasibility of improving the aroma of wine and distillates by overexpressing one of the endogenous yeast genes that controls acetate ester production during fermentation. The synthesis of acetate esters by the wine yeast Saccharomyces cerevisiae during fermentation is ascribed to at least three acetyltransferase activities, namely, alcohol acetyltransferase (AAT), ethanol acetyltransferase, and iso-amyl AAT. To investigate the effect of increased AAT activity on the sensory quality of Chenin blanc wines and distillates from Colombar base wines, we have overexpressed the alcohol acetyltransferase gene (ATF1) of S. cerevisiae. The ATF1 gene, located on chromosome XV, was cloned from a widely used commercial wine yeast strain of S. cerevisiae, VIN13, and placed under the control of the constitutive yeast phosphoglycerate kinase gene (PGK1) promoter and terminator. Chromoblot analysis confirmed the integration of the modified copy of ATF1 into the genome of three commercial wine yeast strains (VIN7, VIN13, and WE228). Northern blot analysis indicated constitutive expression of ATF1 at high levels in these yeast transformants. The levels of ethyl acetate, iso-amyl acetate, and 2-phenylethyl acetate increased 3- to 10-fold, 3.8- to 12-fold, and 2- to 10-fold, respectively, depending on the fermentation temperature, cultivar, and yeast strain used. The concentrations of ethyl caprate, ethyl caprylate, and hexyl acetate only showed minor changes, whereas the acetic acid concentration decreased by more than half. These changes in the wine and distillate composition had a pronounced effect on the solvent or chemical aroma (associated with ethyl acetate and iso-amyl acetate) and the herbaceous and heads-associated aromas of the final distillate and the solvent or chemical and fruity or flowery characters of the Chenin blanc wines. This study establishes the concept that the overexpression of acetyltransferase genes such as ATF1 could profoundly affect the flavor profiles of wines and distillates deficient in aroma, thereby paving the way for the production of products maintaining a fruitier character for longer periods after bottling.
The distinctive flavor of wine, brandy, and other grape-derived alcoholic beverages is affected by many variables. More specifically, grape variety, viticultural practices, and soil affect vine development and berry composition and exert major influences on the distinctiveness of wine and brandy flavor, as evaluated by sensory descriptive analyses (5, 30). In addition, enological practices, including yeast and fermentation conditions, have a prominent effect on the primary flavors of Vitis vinifera wines. In this regard, it is well documented that the volatile profile of wines is dominated by those components that are formed during fermentation, since these volatile compounds are present in the highest concentrations (22, 32, 33). Moreover, the character of brandy is further changed as the absolute and relative amounts of volatiles are altered by distillation. Furthermore, the flavor of both wine and brandy immediately after fermentation or distillation only approximates that of the finished product (5). After the sudden and dramatic changes in composition during fermentation and distillation, chemical constituents generally react slowly during aging to move to their equilibria, resulting in gradual changes in flavor (2, 5, 27). The harmonious complexity of wine and brandy can subsequently be further increased by volatile extraction during oak barrel aging.
Despite the extensive information published on flavor chemistry, odor thresholds, and aroma descriptions, the flavor of complex products such as wine and brandy cannot be predicted. With a few exceptions (e.g., terpenes in the aromatic varieties and alkoxypyrazines in the vegetative or herbaceous cultivars), perceived flavor is the result of specific ratios of many compounds rather than being attributable to a single impact compound (5). In wines and brandies, the major products of yeast fermentation, esters and alcohols, contribute to a generic background flavor (22, 32, 33), whereas subtle combinations of trace components derived from the grapes usually elicit the characteristic aroma notes of these complex beverages (5, 30).
During the primary or alcoholic fermentation of grape sugars, the wine yeast, Saccharomyces cerevisiae, produces ethanol, carbon dioxide, and a number of by-products, including esters, of which alcohol acetates and C4 to C10 fatty acid ethyl esters are found in the highest concentrations in wine and brandy (33, 38). Although these compounds are ubiquitous to all wines and brandies, the level of esters formed varies significantly. Apart from being dependent on factors such as grape cultivar and rootstocks (34), as well as grape maturity (17, 18), the concentration of esters produced during fermentation is dependent on the yeast strain (28), fermentation temperature (8, 36), insoluble material in the grape must (7), vinification methods (15), skin contact (9, 26), must pH (25), the amount of sulfur dioxide (6), amino acids present in the must (16), and malolactic fermentation (20). Furthermore, the ester content of distilled beverages is greatly dependent on whether the yeast lees are present at the time of distillation (33).
The characteristic fruity odors of wine, brandy, and other grape-derived alcoholic beverages are primarily due to a mixture of hexyl acetate, ethyl caproate (apple-like aroma), iso-amyl acetate (banana-like aroma), ethyl caprylate (apple-like aroma) and 2-phenylethyl acetate (fruity, flowery flavor with a honey note) (11). The synthesis of acetate esters such as iso-amyl acetate and ethyl acetate in S. cerevisiae is ascribed to at least three acetyltransferase activities, namely alcohol acetyltransferase (AAT), ethanol acetyltransferase, and iso-amyl AAT (24, 29). These acetyltransferases are sulfhydryl enzymes which react with acetyl coenzyme A (acetyl-CoA) and, depending on the degree of affinity, with various higher alcohols to produce esters (31, 35, 42, 43). It has also been shown that these enzymatic activities are strongly repressed under aerobic conditions and by the addition of unsaturated fatty acids to a culture (10, 23).
The ATF1-encoded AAT activity is the best-studied acetyltransferase activity in S. cerevisiae (10, 11, 12, 23, 24). It has been reported that the 61-kDa ATF1 gene product (Atf1p) is located within the yeast's cellular vacuomes (24) and plays a major role in the production of iso-amyl acetate and to a lesser extent ethyl acetate during beer fermentation (12). To investigate the role of AAT in wine and brandy composition, we have cloned, characterized, and mapped the ATF1 gene from a widely used commercial wine yeast strain, VIN13. The aim of this study was to overexpress the ATF1 gene during fermentation to determine its effect on the yeast metabolism, acetate ester formation, and flavor profiles of Chenin blanc wines and distillates from Colombar base wines. This study could ultimately lead to the development of a variety of wine yeast strains for the improvement of the flavor profiles of different types and styles of wines and distillates, especially of those products deficient in aroma and lacking a long, fruity shelf life.
MATERIALS AND METHODS
Microbial strains, media, and genetic methods.
All yeast and bacterial strains used in this study and their relevant genotypes are listed in Table 1. Escherichia coli cells were grown in Luria-Bertani broth at 37°C (37). S. cerevisiae cells were grown at 30°C in synthetic media SCD and SCDSM (containing 0.67% yeast nitrogen base without amino acids [Difco], supplemented with either the required amino acids and 2% glucose [for SCD] or 0.5% glucose and 60 μg of sulfometuron methyl [Dupont] per ml dissolved in N-,N-,dimethylformamide [for SCDSM]) as well as in a rich medium, YPD (containing 1% yeast extract, 2% peptone and 2% glucose). Laboratory strains were also grown in synthetic medium SCDD (containing 0.67% yeast nitrogen base without amino acids, supplemented with 10% glucose and the appropriate amino acids). Synthetic media containing all the required amino acids except leucin were designated SCD−Leu and SCDD−Leu. Solid media contained 2% agar (Difco). All bacterial transformations and isolation of DNA were carried out according to standard protocols (37). Laboratory yeast strains were also transformed according to standard protocols (1). Industrial wine yeast strains were transformed by means of electroporation. YPD (10 ml) was inoculated with yeast cells, and the cells were incubated at 30°C until stationary phase. A prewarmed 100-ml volume of YPD was then inoculated with 10 ml of the preculture and incubated until the mid-logarithmic growth phase was reached (absorbance at 600 nm [A600] of 1). The cells were then harvested, washed with 50 ml of sterile water, resuspended in 50 ml of a 0.025 M 1,4-dithiothreitol solution, and incubated at room temperature for 10 min. Thereafter, the cells were harvested again and washed in 50 ml Tris-EDTA disodium salt buffer (pH 7.5). Finally, the cells were resuspended in 10 ml of Tris-EDTA disodium salt buffer. Linear DNA (10 to 15 μg) in a maximum volume of 20 μl was added to a 400-μl cell suspension in a microcentrifuge tube and incubated on ice for 10 min. Thereafter, 400 μl of a 70% polyethylene glycol solution was added and mixed in thoroughly but carefully. The mixture was transferred to electroporation cuvettes and incubated on ice for 5 to 10 min. The EasyjecT + 450 V Twin pulse apparatus (EquiBio) was used for electroporation. The pulse program was as follows: voltage, 1,300 V; capacity, 25 μF; shunt, 329 Ω; and pulse, 8.2 ms. The yeast cells were then immediately plated on SCDSM and incubated at 30°C for at least 7 days.
TABLE 1.
Microbial strains and plasmids used in this study
| Strain or plasmid | Genotype or construct | Reference or source |
|---|---|---|
| Escherichia coli DH5α | F′ endA1 hsdR17 (rK− mK+) supE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(laclZYA-argF)U169 deoR [F80dlac DE(lacZ)M15] | GIBCO-BRL/Life Technologies |
| Saccharomyces cerevisiae | ||
| Laboratory strains | ||
| ISP15 | MATa his3 leu2 trp1 ura3 STA2 | 19 |
| FY10 | MATa leu2 ura3 | 41 |
| Industrial strains | ||
| VIN7 | Commercial wine yeast strain | Anchor Yeast, Cape Town, South Africa |
| VIN13 | Commercial wine yeast strain | Anchor Yeast, Cape Town, South Africa |
| WE228 | Commercial wine yeast strain | Anchor Yeast, Cape Town, South Africa |
| Transformants | ||
| ISP15(pATF1-m) | PGK1P-ATF1-PGK1T | This study |
| FY10(pATF1-m) | PGK1P-ATF1-PGK1T | This study |
| VIN7(pATF1-s) | SMR1-410 PGK1P-ATF1-PGK1T | This study |
| VIN13(pATF1-s) | SMR1-410 PGK1P-ATF1-PGK1T | This study |
| WE228(pATF1-s) | SMR1-410 PGK1P-ATF1-PGK1T | This study |
| Plasmids | ||
| pHVX2 | bla LEU2 PGK1P-PGK1T | 40 |
| pPGK1 | bla LEU2 PGK1P-PGK1T | This study |
| pPATX2 | bla LEU2 PGK1P-ATF1-PGK1T | This study |
| pATF1-m | bla LEU2 PGK1P-ATF1-PGK1T | This study |
| pATF1-s | bla LEU2 SMR1-410 PGK1P-ATF1-PGK1T | This study |
| pYIpLac128 | bla LEU2 | 14 |
| pWX509 | bla SMR1-410 | 4 |
| pBR322-ACT1 | bla ACT1 | 13 |
Plasmid construction and recombinant DNA methods.
Standard procedures for isolation and manipulation of DNA were used throughout this study (1). Restriction enzymes, T4 DNA ligase, and Expand Hi-Fidelity DNA polymerase (Boehringer Mannheim) were used in the enzymatic manipulation of DNA according to the specifications of the supplier.
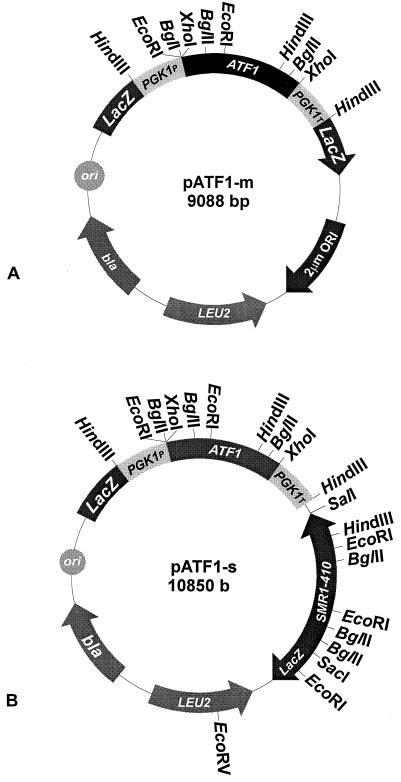
The restriction maps of the gene constructs and plasmids are shown in Fig. 1. The following two primers were synthesized to amplify the coding region of ATF1 by means of the PCR technique: ATF′F (5′-GATCCTCGAGATGAATGAAATCGATGAGAA-3′) and ATF′R (5′-GATCCTCGAGGTAAGGGCCTAAAAGGAGAG-3′). Both the forward (ATF′F) and reverse (ATF′R) primers contain an XhoI site (boldface). Twenty of the bases in each primer are homologous to ATF1 (underlined). Genomic DNA from the commercial wine yeast strain VIN13 was used as a template to amplify the coding sequence of the ATF1 gene.
FIG. 1.
(A) Restriction map of episomal plasmid pATF1-m. (B) Restriction map of integrating plasmid pATF1-s. In both plasmids the ATF1 gene is inserted between the yeast phosphoglycerate kinase gene (PGK1) promoter (PGK1P) and terminator (PGK1T).
A multicopy, episomal S. cerevisiae-E. coli shuttle plasmid containing the ATF1 gene under the control of the regulatory sequences of the yeast phosphoglycerate kinase gene (PGK1), pATF1-m (“m” refers to multicopy) (Fig. 1A), was constructed as follows: the PCR-generated 1,580-bp fragment was digested with XhoI, subcloned into plasmid pHVX2 (40), and digested with XhoI, thereby generating plasmid pATF1-m. Plasmid pHVX2 contains the PGK1 gene promoter (PGK1P) and terminator (PGK1T), with the unique restriction sites EcoRI, XhoI, and BglII located in between them. Plasmid pATF1-m was transformed into laboratory strains of S. cerevisiae, ISP15 and FY10, and maintained as an episomal plasmid in SCD−Leu and SCDD−Leu selection media.
Similarly, a single-copy integrating S. cerevisiae-E. coli shuttle plasmid containing the PGK1P-ATF1-PGK1T gene cassette, pATF1-s (“s” refers to single copy) (Fig. 1B), was constructed as follows: the HindIII-HindIII fragment containing the PGK1P and PGK1T sequences was obtained from plasmid pHVX2 (40) and inserted into the unique HindIII site of plasmid YIpLac128 (14), generating plasmid pPGK1. The 1,580-bp XhoI-XhoI PCR fragment, containing the coding region of the ATF1 gene, was then inserted into the unique XhoI site of plasmid pPGK1, thereby generating plasmid pPATX2. A 3,200-bp SacI-SalI fragment containing the dominant selectable SMR1-410 marker gene (a mutant allele of an endogenous gene of S. cerevisiae conferring resistance to the herbicide sulfometuron methyl, i.e., Smr) was obtained from plasmid pWX509 (4) and subcloned into the unique SacI and SalI sites of plasmid pPATX2, thereby generating plasmid pATF1-s. Plasmid pATF1-s was linearized with EcoRV in the LEU2 gene for integration into the genomes of laboratory strains ISP15 and FY10 as well as wine yeast strains VIN7, VIN13, and WE228.
Hybridization probes.
All blots were performed using a 1,580-bp XhoI-XhoI DNA fragment from plasmid pATF1-m as a probe for the ATF1 gene. ATF1, ACT1, and lambda DNA probes were labeled with [32P]dATP, using the Prime-It II randomly primed labeling kit (Stratagene). The actin-encoding gene (ACT1) was used as the internal control for Northern blotting and a 563-bp ClaI fragment isolated from pBR322-ACT1 (13) was used to probe for ACT1 transcripts.
Southern blot analysis.
Genomic DNA was isolated from the control yeast strains (VIN7, VIN13, and WE228) as well as the corresponding transformed S. cerevisiae strains [VIN7(pATF1-s), VIN13(pATF1-s), and WE228(pATF1-s)], using the standard mechanical method (1), and digested with EcoRV. The DNA fragments were separated by agarose gel electrophoresis and transferred to a Hybond-N nylon membrane (Amersham), and Southern blot hybridization was performed.
Northern blot analysis.
Total RNA was isolated from cells by using the FastRNA Kit-RED product (BIO 101). Cells were precultured in YPD and then inoculated into Chenin blanc grape juice. Total RNA was isolated on days 3, 5, 7, 9, and 11 after inoculation of the juice. The RNA (10 μg) from each culture was subjected to formamide gel electrophoresis. The RNA was then transferred to Hybond-N nylon membranes, and Northern blotting was performed according to standard procedures (1).
Preparation of intact chromosomal DNA for pulsed-field gel electrophoresis and chromoblotting.
Chromosomal DNA samples were prepared according to the embedded-agarose procedure (3). Intact chromosomal DNAs were separated using contour-clamped homogeneous electric field (CHEF) electrophoresis. The apparatus used was the CHEF-MAPPER (Bio-Rad Laboratories, Richmond, Va.). All CHEF separations were carried out according to the method of Van der Westhuizen and Pretorius (39). The chromosomes were transferred to Hybond-N nylon membranes, and Southern blotting was performed according to standard procedures (1).
Fermentation trials with laboratory yeasts.
Laboratory strains of S. cerevisiae, ISP15, ISP15(pATF1-m), ISP15(pATF1-s), FY10, FY10(pATF1-m), and FY10(pATF1-s) (Table 1), were each inoculated into 100 ml of SCDD−Leu medium and incubated at 30°C. The yeast cells were then harvested and resuspended in 10 ml of grape juice and incubated for 30 min at 30°C; samples of the suspension were then inoculated (2 × 106 cells/ml) into 400 ml of Colombar juice and left to ferment for 18 days at 15°C. Routine analysis, sugar and volatile acid determination, and gas chromatographic (GC) analysis were done on the wines (results not shown).
White wine production.
The wine yeast strains VIN7, VIN7(pATF1-s), VIN13, and VIN13(pATF1-s) were each inoculated (2 × 106 cells/ml) into 4.5 liters of Chenin blanc grape juice and fermented at 15°C until dry. The wines were then cold stabilized, filtered, and bottled according to standard practices for white wine production. All fermentations were done in triplicate.
Base wine production and small-scale distillation.
Wine yeast strains VIN13, VIN13(pATF1-s), WE228, and WE228(pATF1-s) were each inoculated into 10 liters of Colombar grape juice, to which no sulfur dioxide was added, and fermented at 15°C until dry. These fermentations were also done in triplicate. Two 5-liter round-bottom flasks were each filled with 4.5 liters of base wine and yeast lees derived from the original 10-liter base wine fermentation volume. Two copper plates and 3 g of copper-sulfate were added to the base wine and heated in heating mantles. The distillation flow rate was maintained at 5 ml/min, and the distillate was collected until 30% (vol/vol) alcohol was reached. The same procedure was followed with the second distillation, except that the first 40 ml of distillate collected at a flow rate of 2 ml/min was discarded. The flow rate was then adjusted to 5 ml/min, and the heart was collected until 70% (vol/vol) alcohol was reached.
GC analysis.
To a 50-ml volume of each Chenin blanc wine or Colombar base wine were added 4 ml of a solution (2.2 mg/liter) of 4-methyl-2-pentanol (internal standard) and 30 ml of diethyl ether. The flask was then mechanically rotated at 60 rpm for 30 min. The top ether layer was separated and the extracts were then analyzed. The internal standard was added directly to the distilled samples and injected into the gas chromatograph.
Analyses were done on a Hewlett-Packard model 5890 series II gas chromatograph with a Lab Alliance capillary column (length, 60 m; inside diameter, 0.32 μm; film width, 0.5 μm). The injection block and detector temperatures were kept constant at 200 and 250°C, respectively. Hydrogen was used as the carrier gas, with an injection volume of 3 μl (split, 20 ml/min). A Hewlett-Packard 3396A integrator was used to quantify the peaks by using standard solutions. The oven temperature was programmed as follows: 35°C (10 min) to 230°C at 3°C/min. For the distillate analysis (4 μl was injected), the conditions were as described above, except that a different oven program was used, as follows: 30°C (5 min) to 80°C at 2°C/min, and 80 to 230°C at 3°C/min.
Chenin blanc extractions were done after alcoholic fermentation and 6 months after bottling. Extractions from the Colombar base wine were made after alcoholic fermentation. Samples from the distillate were taken after the second distillation.
Sensory evaluations.
The Chenin blanc wines were sensorially evaluated for fruity or flowery and solvent or chemical intensity and the distillates were evaluated for solvent or chemical, heads-associated, and herbaceous intensity by a panel of five experienced judges. The wines and distillates were evaluated on a scale from 1 to 5, where 1 represented the absence or very low intensity of a specific flavor and 5 represented a very high intensity of the flavor.
Statistical analysis.
Statistical differences between the results for wines produced by the control yeasts and those for wines produced by the modified yeasts were determined by applying standard analysis of variance methods to the data. The significant differences between values were determined by a two-tailed test.
RESULTS
Cloning and constitutive expression of ATF1 in laboratory and industrial yeast strains.
With the aim to produce increased levels of AAT throughout fermentation, the ATF1 gene was cloned from a widely used commercial wine yeast strain, VIN13, and placed under the constitutive regulatory sequences of the phosphoglycerate kinase gene (PGK1) of S. cerevisiae to generate plasmids pATF1-m (Fig. 1A) and pATF1-s (Fig. 1B).
Both plasmids pATF1-m and pATF1-s were transformed into two laboratory strains of S. cerevisiae, ISP15 and FY10, and Leu+ transformants were screened on SCD−Leu agar plates. Plasmid pATF1-m was maintained as an episomal plasmid in selection media, and transformants ISP15(pATF1-m) and FY10(pATF1-m) yielded a strong fruity or pineapple- or banana-like aroma on the selective SCD−Leu agar plates. Colombar juice was fermented with these transformed laboratory strains. Although these ethanol-sensitive transformants did not ferment the juice until dry, the results indicated increased levels of ethyl- and iso-amyl acetate production (data not shown).
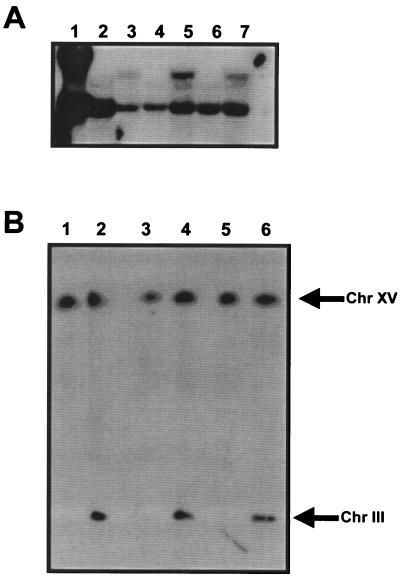
To allow for the stable maintenance of the PGK1P-ATF1-PGK1T gene construct in nonselective grape juice medium, the commercial wine yeast strains VIN7, VIN13, and WE228 were transformed with linearized (in the LEU2 gene) plasmid pATF1-s to facilitate direct integration into the LEU2 gene located on chromosome III. Integration of pATF1-s into the genomes of Smr transformants VIN7(pATF1-s), VIN13(pATF1-s), and WE228(pATF1-s) was confirmed by Southern blot and chromoblot analysis (Fig. 2). A single ATF1 hybridization band of 4,361 bp was obtained with the control host yeast strains (VIN7, VIN13, and WE228), whereas two hybridization bands of 4,361 and 9,416 bp, corresponding to the wild-type ATF1 gene and the integrated PGK1P-ATF1-PGK1T gene cassette, respectively, were obtained with the recombinant wine yeast strains [VIN7(pATF1-s), VIN13(pATF1-s), and WE228(pATF1-s)] (Fig. 2A). To avoid any chromosomal positional effect on the regulation of the PGK1P-ATF1-PGK1T gene cassette, a chromoblot was performed to determine whether integration occurred at the targeted LEU2-site on chromosome III. Figure 2B clearly shows that all untransformed strains (VIN7, VIN13, and WE228) contain a single hybridization band corresponding to chromosome XV, on which the wild-type ATF1 gene is located. Furthermore, only the transformed wine yeast strains [VIN7(pATF1-s), VIN13(pATF1-s), and WE228(pATF1-s)] contain an additional hybridization band corresponding to chromosome III, thereby confirming an integration event (presumably by homologous recombination) at the LEU2 target site (Fig. 2B).
FIG. 2.
(A) Genomic DNA analysis of ATF1. Lanes were loaded with EcoRI-HindIII-digested lambda DNA (lane 1) or EcoRV-digested genomic DNA of the yeast strains WE228, WE228(pATF1-s), VIN13, VIN13(pATF1-s), VIN7, and VIN7(pATF1-s) (lanes 2 to 7, respectively). (B) Chromoblot analysis of ATF1. The chromosomes of wine yeast strains WE228 (lane 1), WE228(pATF1-s) (lane 2), VIN13 (lane 3), VIN13(pATF1-s) (lane 4), VIN7 (lane 5), and VIN7(pATF1-s) (lane 6) were transferred to nylon membranes and probed.
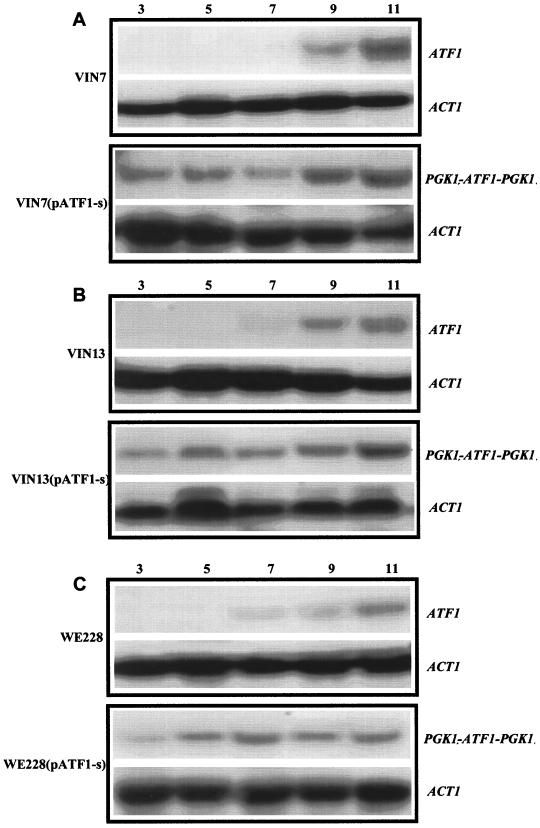
Northern blotting was performed with the control and transformed industrial yeast strains to study the expression levels of the ATF1 gene during fermentation. Intense ATF1 mRNA hybridization bands on the Northern blots were observed for transformants VIN7(pATF1-s), VIN13(pATF1-s), and WE228(pATF1-s) at all the sampling time points, whereas intense ATF1 transcript bands in the wild types were only detectable from day 7 of the fermentation (Fig. 3). These results indicate that expression of the integrated ATF1 gene under the control of the PGK1 promoter and terminator sequences in the transformed strains was high throughout the fermentation, whereas in the untransformed strains it was only high during the later stages of fermentation.
FIG. 3.
Northern blot analysis of ATF1 transcripts prepared from yeast cells during fermentation. RNA from commercial wine yeast strains VIN7, VIN13, and WE228 together with their corresponding transformants carrying the constitutively expressed PGK1P-ATF1-PGK1T gene cassette, VIN7(pATF1-s), VIN13(pATF1-s), and WE228(pATF1-s), was isolated on days 3, 5, 7, 9, and 11, subjected to formamide gel electrophoresis, and probed for ATF1 and ACT1. The sizes of the ATF1 and ACT1 transcripts were determined relative to a Bio-Rad Laboratories RNA molecular size marker.
GC analyses of wines and distillates.
Concentrations of certain esters, higher alcohols, and acids were determined for the wines and distillates (Table 2). The GC analysis results confirmed high levels of AAT activity. Specifically, 3- to 10-fold and 3.5- to 12-fold increases in the production of ethyl acetate and iso-amyl acetate, respectively, were observed for both the Chenin blanc and Colombar base wines fermented with VIN7(pATF1-s), VIN13(pATF1-s), and WE228(pATF1-s) compared to the wines fermented with VIN7, VIN13, and WE228. Hexyl acetate and 2-phenylethyl acetate concentrations increased from 1.4- to 2-fold and from 2.4- to 10.8-fold, respectively, in the wines fermented with VIN7(pATF1-s), VIN13(pATF1-s), and WE228(pATF1-s). The concentrations of ethyl caproate were 1.4-fold greater in both of the Chenin blanc wines fermented with VIN7(pATF1-s) and VIN13(pATF1-s) than in the wines fermented with VIN7 and VIN13. The concentrations of ethyl caprate and ethyl caprylate were not significantly different for the Chenin blanc wines fermented with VIN7 and VIN7(pATF1-s), or for those fermented with VIN13 and VIN13(pATF1-s). The ethyl caprylate concentration was similar for the Colombar wines fermented with VIN13, VIN13(pATF1-s), WE228, and WE228(pATF1-s), but the ethyl caprate concentration showed a slight decrease in the Colombar wines fermented with VIN13(pATF1-s) compared to that in the wine fermented with VIN13. The total ester concentrations showed 9.8- and 6.1-fold increases in the Chenin blanc wines fermented with VIN7(pATF1-s) and VIN13(pATF1-s), respectively, compared to the wines fermented with VIN7 and VIN13. The total ester concentrations of the Colombar wines fermented with VIN13(pATF1-s) and WE228(pATF1-s) showed 3.9- and 3.2-fold increases, respectively.
TABLE 2.
Yeast strain effect on the concentrations of major volatiles in Chenin blanc wines as well as Colombar base wines and the respective 70% distillates
| Component | Concn (mg/liter)
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chenin blanc
|
Colombar base wine after alcoholic fermentation
|
70% Distillate after second distillation
|
||||||||||||||
| After alcoholic fermentation
|
After 6-mo bottle aging
|
|||||||||||||||
| VIN7 | VIN7 (pATF1-s) | VIN13 | VIN13 (pATF1-s) | VIN7 | VIN7 (pATF1-s) | VIN13 | VIN13 (pATF1-s) | WE228 | WE228 (pATF1-s) | VIN13 | VIN13 (pATF1-s) | WE228 | WE228 (pATF1-s) | VIN13 | VIN13 (pATF1-s) | |
| Acetic acid | 511.0 | 205.1 | 114.5 | 37.7 | 895.1 | 702.7 | 247.0 | 205.3 | 116.5 | 49.7 | 44.9 | 22.9 | 21.5 | 12.7 | 8.3 | 6.3 |
| Decanoic acid | 5.2 | 5.2 | 4.0 | 5.1 | 4.4 | 8.3 | 3.5 | 5.2 | 5.1 | 4.9 | 4.7 | 5.0 | 19.1 | 18.3 | 16.4 | 17.4 |
| Hexanoic acid | 6.5 | 6.6 | 5.3 | 6.8 | 7.5 | 7.2 | 7.4 | 9.1 | 7.3 | 7.9 | 7.2 | 7.0 | 12.2 | 14.3 | 11.4 | 11.3 |
| i-Butyric acid | 0.9 | 1.4 | 0.5 | 0.5 | 1.0 | 1.8 | 1.6 | 1.5 | 0.9 | 1.1 | 0.6 | 0.6 | 1.8 | 2.1 | 1.2 | 1.1 |
| n-Butyric acid | 1.1 | 1.2 | 0.8 | 1.1 | 2.4 | 2.6 | 1.9 | 2.9 | 1.3 | 1.2 | 0.9 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| n-Valeric acid | 0.4 | 0.4 | 0.7 | 0.5 | 0.4 | 0.4 | 0.6 | 0.5 | 0.3 | 0.3 | 0.5 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| Octanoic acid | 9.9 | 10.6 | 9.2 | 10.8 | 9.7 | 8.9 | 9.6 | 11.3 | 12.5 | 12.9 | 13.1 | 12.1 | 34.4 | 33.2 | 31.2 | 31.2 |
| Propionic acid | 0.8 | 0.7 | 1.0 | 1.3 | 8.9 | 10.6 | 16.0 | 12.7 | 0.8 | 0.8 | 1.0 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2-Phenylethyl acetate | 0.5 | 5.4 | 0.4 | 2.1 | 0.3 | 2.7 | 0.3 | 1.3 | 0.8 | 1.9 | 0.8 | 2.2 | 1.1 | 4.2 | 2.2 | 5.4 |
| Diethyl succinate | 1.5 | 1.7 | 1.6 | 1.5 | 1.2 | 1.4 | 1.2 | 1.1 | 1.3 | 1.3 | 1.1 | 1.1 | 2.4 | 1.9 | 0.5 | 0.3 |
| Ethyl acetate | 68.5 | 724.2 | 93.0 | 607.0 | 35.9 | 142.2 | 32.0 | 182.1 | 133.5 | 446.0 | 208.1 | 868.6 | 589.4 | 1,792.6 | 723.2 | 2,668.8 |
| Ethyl butyrate | 0.6 | 0.5 | 0.5 | 0.6 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 | 0.6 | 0.5 | 0.2 | 2.4 | 2.0 | 2.1 | 1.3 |
| Ethyl caprate | 1.7 | 1.6 | 1.7 | 1.8 | 2.0 | 1.9 | 2.3 | 2.2 | 2.2 | 2.3 | 2.3 | 1.9 | 10.2 | 10.2 | 11.2 | 13.5 |
| Ethyl caproate | 1.6 | 2.2 | 1.4 | 1.9 | 12.3 | 11.7 | 10.1 | 14.1 | 1.4 | 1.7 | 1.6 | 1.9 | 10.2 | 10.1 | 11.0 | 11.2 |
| Ethyl caprylate | 1.9 | 1.9 | 1.9 | 2.1 | 2.7 | 2.4 | 2.7 | 3.1 | 2.4 | 2.4 | 2.1 | 2.2 | 10.6 | 10.2 | 11.3 | 10.5 |
| Ethyl lactate | 1.3 | 1.6 | 1.2 | 1.4 | 6.9 | 6.8 | 8.4 | 7.4 | 1.6 | 1.5 | 1.6 | 1.4 | 33.9 | 27.5 | 10.9 | 8.1 |
| Hexyl acetate | 2.4 | 5.0 | 2.9 | 4.6 | 0.5 | 1.0 | 0.6 | 0.9 | 2.3 | 3.9 | 2.9 | 4.1 | 0.9 | 1.3 | 1.2 | 1.7 |
| Iso-amyl acetate | 5.3 | 65.1 | 8.6 | 53.5 | 3.7 | 29.2 | 6.0 | 32.3 | 9.2 | 35.4 | 13.1 | 45.6 | 22.2 | 81.5 | 39.2 | 140.3 |
| 2-Phenylethyl alcohol | 13.9 | 10.6 | 7.9 | 6.0 | 15.2 | 15.0 | 9.7 | 8.0 | 9.4 | 7.6 | 8.1 | 5.4 | 6.3 | 7.4 | 5.3 | 5.4 |
| Hexanol | 1.7 | 0.4 | 1.4 | 0.5 | 1.8 | 0.8 | 1.7 | 0.9 | 1.7 | 0.8 | 1.3 | 0.5 | 11.5 | 6.7 | 9.7 | 5.8 |
| Iso-amyl alcohol | 139.0 | 101.2 | 119.5 | 73.2 | 141.9 | 125.7 | 129.5 | 86.0 | 135.0 | 105.0 | 131.0 | 73.2 | 820.1 | 621.7 | 778.7 | 474.8 |
| Iso-butanol | 25.9 | 32.0 | 13.2 | 12.5 | 26.9 | 34.1 | 14.1 | 13.3 | 24.6 | 22.7 | 16.1 | 13.9 | 151.8 | 128.2 | 91.9 | 77.6 |
| Methanol | 325.0 | 304.7 | 351.0 | 331.5 | 490.1 | 485.1 | 521.0 | 513.3 | 32.3 | 30.0 | 47.8 | 36.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| n-Butanol | 0.4 | 0.3 | 0.8 | 0.5 | 0.4 | 0.3 | 0.8 | 0.4 | 0.6 | 0.5 | 1.3 | 0.6 | 4.6 | 3.3 | 9.0 | 4.4 |
| Propanol | 19.1 | 17.2 | 55.1 | 41.3 | 22.0 | 22.2 | 64.1 | 49.8 | 28.7 | 27.8 | 58.9 | 44.6 | 247.9 | 225.7 | 414.4 | 312.4 |
| Total esters | 82.5 | 805.9 | 110.1 | 673.4 | 58.0 | 191.5 | 54.6 | 236.5 | 152.4 | 493.2 | 242.1 | 938.4 | 647.0 | 1,912.1 | 801.4 | 2,852.6 |
| Total higher alcohols | 525.0 | 466.4 | 548.9 | 465.5 | 698.3 | 683.3 | 740.9 | 671.8 | 232.3 | 194.4 | 265.1 | 176.4 | 1,242.1 | 993.0 | 1,308.4 | 880.3 |
In all of the experimental wines the methanol concentration decreased as a result of the use of the modified strains. This is considered to be a positive tendency, since excessive levels of methanol could be considered toxic. The propanol, hexanol, iso-amyl alcohol, and 2-phenylethyl alcohol concentrations decreased in the wines fermented with the modified strains compared to those fermented with VIN7, VIN13, and WE228. These decreases in the alcohol concentrations corresponded with the increase in the ester concentrations. AAT uses the different alcohols and acetyl-CoA as substrates for the production of the corresponding esters. The iso-butanol levels differed between the different yeast strains. The concentrations of iso-butanol in the Chenin blanc and Colombar wines fermented with VIN13(pATF1-s) and WE228(pATF1-s) were decreased compared to those in the control wines, but they were increased in the Chenin blanc wines fermented with VIN7 and VIN7(pATF1-s).
The acetic acid concentrations in the wines produced by the transformed strains were drastically decreased compared to those in wines produced by the control strains, which is a positive result, since the volatile acidity of the wine was therefore decreased. Acid concentrations, excluding acetic acid, appear to have remained constant.
To determine the effect of bottle-aging on the ester concentrations and aroma profiles, the Chenin blanc wines were bottle aged for 6 months at 8°C and then subjected to GC analysis (Table 2). Ethyl acetate, iso-amyl acetate, and hexyl acetate levels decreased drastically in all the wines during this storage period, but their concentrations still remained 4- to 5.7-fold, 5.4- to 7.9-fold, and 1.5- to 2-fold higher in the wines fermented with VIN7(pATF1-s) and VIN13(pATF1-s) than in the control wines. The concentration of 2-phenylethyl acetate also decreased during this period, but the concentration in the Chenin blanc and Colombar wines fermented with VIN7(pATF1-s) (9-fold) and VIN13(pATF1-s) (4.3-fold) and WE228(pATF1-s) (2.4-fold) and VIN13(pATF1-s) (2.8-fold), respectively, remained higher. The concentrations of ethyl caprate, ethyl caproate, and ethyl caprylate showed an overall increase during this storage period, but the difference in the concentrations between the wines fermented with the control yeast strains and those fermented with the modified yeast strains were not significant. The acetic acid concentration increased drastically due to ester hydrolysis in both the VIN7(pATF1-s)- and VIN7-fermented wines, but still was lower in the wine fermented with VIN7(pATF1-s) than in the VIN7-fermented Chenin blanc. Similar results were obtained for the Chenin blanc wines fermented with VIN13 and VIN13(pATF1-s) (Table 2).
The analysis of the low wine (first distillate of base wine) indicated that all of the measured compounds were concentrated during the first distillation (data not shown). After the second distillation the content of the distillate changed, since only the aroma compounds present in the heart were concentrated while the rest of the components, such as some acids, were discarded in the heads and tails. The spirit produced by the recombinant strains WE228(pATF1-s) and VIN13(pATF1-s) contained higher concentrations of total esters and lower concentrations of total higher alcohols than the respective control strains (Table 2). Concentrations of ethyl acetate, iso-amyl acetate, hexyl acetate, and 2-phenylethyl acetate were elevated in the distillates fermented with recombinant yeast strains VIN13(pATF1-s) and WE228(pATF1-s). The concentrations of propanol, iso-butanol, iso-amyl alcohol, hexanol, and acetic acid were lower in the same distillates.
The GC results were statistically evaluated by a two-tailed test (Table 3). The results indicated that the values obtained for all the wines and distillates for ethyl acetate, iso-amyl acetate, iso-amyl alcohol and acetic acid differed significantly (P ≤ 0.05). The values for 2-phenylethyl acetate, 2-phenylethyl alcohol, propanol, hexyl acetate, hexanol, iso-butanol, and n-butanol differed significantly for the 70% spirit (P ≤ 0.05), but only in some cases for the Chenin blanc wines.
TABLE 3.
Statistical differences between wines and distillates produced by control and modified yeast strains with respect to certain fermentation bouquet volatilesa
| Component | Statistical difference
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Chenin blanc
|
Colombar base wine after alcoholic fermentation
|
70% Distillate after second distillation
|
||||||
| After alcoholic fermentation
|
After 6-mo bottle aging
|
|||||||
| VIN7 vs VIN7 (pATF1-s) | VIN13 vs VIN13 (pATF1-s) | VIN7 vs VIN7 (pATF1-s) | VIN13 vs VIN13 (pATF1-s) | WE228 vs WE228 (pATF1-s) | VIN13 vs VIN13 (pATF1-s) | WE228 vs WE228 (pATF1-s) | VIN13 vs VIN13 (pATF1-s) | |
| Ethyl acetate | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Iso-amyl acetate | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Iso-amyl alcohol | 0.0001 | 0.0004 | 0.0039 | 0.0013 | 0.0006 | 0.0002 | 0.0006 | 0.0001 |
| Acetic acid | 0.0001 | 0.0001 | 0.0022 | 0.0399 | 0.0001 | 0.0001 | 0.0001 | 0.0006 |
| 2-Phenylethyl acetate | 0.0001 | 0.1161 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 |
| 2-Phenylethyl alcohol | 0.0013 | 0.1161 | 0.6433 | 0.0022 | 0.0022 | 0.0039 | 0.0039 | 0.0161 |
| Propanol | 0.3739 | 0.0022 | 0.9999 | 0.0006 | 0.0001 | 0.0001 | 0.0075 | 0.0039 |
| Iso-butanol | 0.0006 | 0.9999 | 0.0022 | 0.3739 | 0.0001 | 0.0001 | 0.0039 | 0.0008 |
| n-Butanol | 0.0002 | 0.9999 | 0.0039 | 0.0013 | 0.0006 | 0.0002 | 0.0161 | 0.0001 |
| Hexyl acetate | 0.0001 | 0.1161 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0006 | 0.0013 |
| Hexanol | 0.0001 | 0.3739 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0004 |
P ≤ 0.05.
Sensory analyses.
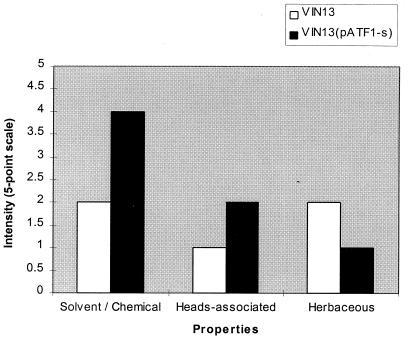
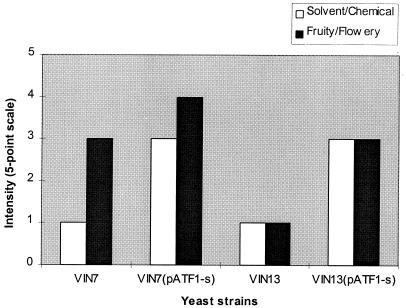
The solvent or chemical aroma, associated with ethyl- and iso-amyl acetate, was detected in all the distillates, as were herbaceous and heads-associated aromas (Fig. 4). The aroma which differed the most between the wines fermented with the industrial strains and those fermented with the genetically modified strains was the solvent or chemical aroma. The fruity or flowery character was stronger in the Chenin blanc wines fermented with VIN7(pATF1-s) and VIN13(pATF1-s) than in the control wines (Fig. 5). However, these wines were also dominated by the solvent/chemical aroma. Nevertheless, the wines fermented with the modified wine yeast strains had a much more intense and complex aroma than the control wines.
FIG. 4.
The aroma property intensities of distillate, distilled from Colombar base wines fermented with VIN13 (control) and VIN13(pATF1-s) (modified yeast strain).
FIG. 5.
The aroma property intensities of Chenin blanc wine fermented with yeast strains VIN7 and VIN13 (controls) and VIN7(pATF1-s) and VIN13(pATF1-s) (modified yeast strains).
The sensory evaluation data were also statistically evaluated (Table 4). The results indicated that the solvent or chemical property differed significantly between the control and modified yeast strains for both the distillate and the Chenin blanc wines. The fruity or flowery property differed significantly between the control and modified yeast strains for the Chenin blanc wines, and the herbaceous and heads-associated properties differed significantly for the distillate. Only those properties with a significant difference are shown in Fig. 4 and 5.
TABLE 4.
Statistical differences between wines and distillates, produced by control and modified yeast strains, with respect to sensory evaluations
| Wine or distillate, property, and effect | Degrees of freedom | Deviance | Approx significance level |
|---|---|---|---|
| Chenin blanc (after 6-mo bottle aging) | |||
| Solvent or chemical | |||
| VIN13 vs VIN13(pATF1-s) | 1 | 3.469839 | 0.0625 |
| Remainder | 2 | 1.569997 | 0.4561 |
| VIN7 vs VIN7(pATF1-s) | 1 | 8.456209 | 0.0036 |
| Remainder | 2 | 0 | 0.9999 |
| Fruity or flowery | |||
| VIN13 vs VIN13(pATF1-s) | 1 | 2.85073 | 0.0913 |
| Remainder | 2 | 3.374044 | 0.1851 |
| 70% Distillate | |||
| Solvent or chemical | |||
| VIN13 vs VIN13(pATF1-s) | 1 | 12.64813 | 0.0004 |
| Remainder | 2 | 7.126894 | 0.68 |
| Herbaceous | |||
| VIN13 vs VIN13(pATF1-s) | 1 | 5.137426 | 0.0234 |
| Remainder | 2 | 0.351211 | 0.8389 |
| Heads associated | |||
| VIN13 vs VIN13(pATF1-s) | 1 | 4.444999 | 0.035 |
| Remainder | 2 | 0.907532 | 0.6352 |
Only the properties that showed a statistically significant difference are shown in the table. P ≤ 0.05.
DISCUSSION
The absolute and relative amounts of specific fermentation-derived alcohols and esters, such as alcohol acetates, contribute significantly to the fruity odors and fermentation bouquet of wines and brandies (11, 22, 32, 33). AAT, ethanol acetyltransferase, and iso-amyl AAT represent the key AATs in yeast strains that play a pivotal role in the production of alcohol acetates during fermentation (24, 29). However, it was previously shown that the AAT-encoding gene (ATF1) of brewer's yeast is not transcribed in the presence of oxygen or unsaturated fatty acids and that it is only expressed during the later stages of the fermentation (10). At the beginning of fermentation, ester synthesis is very slow due to the high metabolic demand for acetyl-CoA for yeast growth (43). During this phase of the fermentation curve, oxygen and acetyl-CoA are rapidly consumed to support the production of unsaturated fatty acids and sterols. Immediately following this, an equilibrium is established between acetyl-CoA consumption for fatty acid and sterol synthesis and that for ester production. This represents the first induction for ester synthesis and occurs after about 8 h of beer wort fermentation. When fatty acid and sterol synthesis finally stops, there is a peak in cellular acetyl-CoA levels, and at this point the second induction of ester synthesis occurs. This happens near the midpoint of the fermentation curve and is relatively short-lived. However, this contributes significantly to the overall ester level in beer (43).
In an attempt to increase the yeast's AAT production throughout the fermentation of grape juice, and thereby enhance the synthesis of important acetate esters and presumably the fruitiness of Chenin blanc wine and Colombar base wine, the ATF1 gene was constitutively expressed (PGK1 promoter and terminator) in three commercial wine yeast strains VIN7, VIN13, and WE228. Northern blot analyses showed that the PGK1P-ATF1-PGK1T gene cassette was transcribed at high levels throughout the fermentation, resulting in higher concentrations of several esters in the wines and distillates. The drastic increases in the levels of ethyl acetate and iso-amyl acetate had a pronounced effect on the aroma of the fermented and distilled products. Some of the other esters whose concentrations were also increased as a result of the overexpression of ATF1 include hexyl acetate, ethyl caproate, and 2-phenylethyl acetate, presenting a flowery and fruity aroma. By comparison, the overexpression of ATF1 in brewing yeast strains resulted in beer with increased levels of ethyl acetate and iso-amyl acetate and decreased concentrations of the corresponding alcohols (21). As can be expected, the sensorial evaluation of the wines and distillates in this study indicated that excessively high concentrations of ethyl acetate, however, did not improve the fermentation bouquet and aroma of the young wines. A more controlled increase of the level of this ester should have a more positive effect on aroma. However, an important fact is that the ethyl acetate/iso-amyl acetate ratio decreased from 11.1 just after the alcoholic fermentation to 4.9 after 6 months of bottle aging. Therefore, the hydrolysis of ethyl acetate occurs at a faster rate than that of iso-amyl acetate. This is also true for 2-phenylethyl acetate (twofold reduction). Therefore, further bottle aging might reduce the negative effect of the initial high levels of ethyl acetate. These products may also be used for blending purposes to improve the aroma of a neutral wine or distillate. Distillates are normally matured in wooden barrels for a minimum of 3 years, during which the concentrations of the compounds are changed. New aroma compounds can be extracted from the wood, and the concentrations of others can increase or decrease. Therefore, when the spirit is matured, ethyl acetate and iso-amyl acetate concentrations would likely decrease due to hydrolysis and evaporation. Apart from cultivar-specific wines, white wine also has a generic fruity character, which often disappears during bottle aging. Therefore, higher initial levels of esters (including ethyl acetate) could lead to a more complex brandy and to white wines with a more fruity character. This is substantiated by the statistical differences found between the wines and distillates fermented by industrial and genetically modified yeast strains, with respect to both composition (fermentation-produced volatiles) and quality (flavor properties).
In conclusion, this study has clearly demonstrated that the manipulation of the expression of a single yeast gene such as ATF1 could alter the ester production significantly during wine fermentation, thereby adjusting the aroma profiles of wine and distillates considerably. This paper indicates the enormous effect that gene technology is likely to have on our understanding of flavor chemistry, paving the way for the production of higher quality wines and brandies with fruitier and even novel aromas.
ACKNOWLEDGMENTS
We thank Distillers Corporation for performing the GC analyses. We also express our sincere gratitude to the ARC-Nietvoorbij Centre for Vine and Wine for the use of their distillation equipment. We are also grateful to Johan Marais (Nietvoorbij) and Enzo D'Aguanno for the critical reading of the manuscript and to William Lilly for the drawing of the graphics and tables.
We express our sincere gratitude to the South African wine industry (Winetech) and the National Research Foundation (NRF) for financial support.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Truhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1994. [Google Scholar]
- 2.Cantagrel R, Lurton L, Vidal J P, Galy B. From vine to Cognac. In: Piggott J R, Patterson A, editors. Understanding natural flavours. Glasgow, United Kingdom: Blackie Academic and Professional; 1995. pp. 208–228. [Google Scholar]
- 3.Carle G F, Olson M V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci USA. 1985;82:3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey G P, Xiao W, Rank G H. A convenient dominant selection marker for gene transfer in industrial strains of Saccharomyces yeast: SMR1 encoded resistance to the herbicide sulfometuron methyl. J Inst Brew. 1988;94:93–97. [Google Scholar]
- 5.Cole V C, Noble A C. Flavor chemistry and assessment. In: Lea A G H, Piggott J R, editors. Fermented beverage production. London, United Kingdom: Blackie Academic and Professional; 1995. pp. 361–385. [Google Scholar]
- 6.Daudt C E, Ough C S. Variations in some volatile acetate esters formed during grape juice fermentation. Effects of fermentation temperature, SO2, yeast strain, and grape variety. Am J Enol Vitic. 1973;24:130–135. [Google Scholar]
- 7.Edwards C G, Beelman R B, Bartley C E, McConnell A L. Production of decanoic acid and other volatile compounds and the growth of yeast and malolactic bacteria during vinification. Am J Enol Vitic. 1990;41:48–56. [Google Scholar]
- 8.Engan S. Esters in beer. Brew Dig. 1974;49:40–48. [Google Scholar]
- 9.Falqué E, Fernández E. Effects of different skin contact times on Treixadura wine composition. Am J Enol Vitic. 1996;47:309–312. [Google Scholar]
- 10.Fujii T, Kobayashi O, Yoshomoto H, Furukawa S, Tamai Y. Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces cerevisiae alcohol acetyltransferase gene. Appl Environ Microbiol. 1997;63:910–915. doi: 10.1128/aem.63.3.910-915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii T, Nagasawa N, Iwamatsu A, Bogaki T, Tamai Y, Hamachi M. Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl Environ Microbiol. 1994;60:2786–2792. doi: 10.1128/aem.60.8.2786-2792.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii T, Yoshimoto H, Tamai Y. Acetate ester production by Saccharomyces cerevisiae lacking the ATF1 gene encoding the alcohol acetyltransferase. J Ferment Bioeng. 1996;81:538–542. [Google Scholar]
- 13.Gallwitz J F J. Site-directed mutagenesis of the yeast actin gene: a test for actin function in vivo. EMBO J. 1991;10:3951–3958. doi: 10.1002/j.1460-2075.1991.tb04965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenised yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 15.Gómez E, Laencina J, Martinez A. Vinification effects on changes in volatile compounds of wine. J Food Sci. 1994;59:406–409. [Google Scholar]
- 16.Herraiz T, Ough C S. Formation of ethyl esters from amino acids by yeasts during the alcoholic fermentation of grape juice. Am J Enol Vitic. 1993;44:41–48. [Google Scholar]
- 17.Houtman A C, Marais J, Du Plessis C S. The possibilities of applying present-day knowledge of wine aroma components: influence of several juice factors on fermentation rate and ester production during fermentation. S Afr J Enol Vitic. 1980;1:27–33. [Google Scholar]
- 18.Houtman A C, Marais J, Du Plessis C S. Factors affecting the reproducibility of fermentation of grape juice and of the aroma composition of wines. I. Grape maturity, sugar, inoculum concentration, aeration, juice turbidity and ergosterol. Vitis. 1980;19:37–54. [Google Scholar]
- 19.Lambrechts M G, Bauer F F, Marmur J, Pretorius I S. Muc1, a mucin-like protein that is regulated by Mss10, is critical for pseudohyphal differentiation in yeast. Proc Natl Acad Sci USA. 1996;93:8419–8424. doi: 10.1073/pnas.93.16.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurent M H, Henick-Kling T, Acree T E. Changes in the aroma and odor of Chardonnay wine due to malolactic fermentation. Vitic Enol Sci. 1994;49:3–10. [Google Scholar]
- 21.Lee S, Villa K, Patino H. Yeast strain development for enhanced production of desirable alcohols/esters in beer. J Am Soc Brew Chem. 1995;53:153–156. [Google Scholar]
- 22.Maarse H, Van Den Berg F. Flavour of distilled products. In: Piggott J R, Patterson A, editors. Understanding natural flavours. Glasgow, United Kingdom: Blackie Academic and Professional; 1994. pp. 243–267. [Google Scholar]
- 23.Malcorps P, Cheval J M, Jamil S, Dufour J P. A new model for the regulation of ester synthesis by alcohol acetyltransferase in Saccharomyces cerevisiae during fermentation. J Am Soc Brew Chem. 1991;49:47–53. [Google Scholar]
- 24.Malcorps P, Dufour J P. Ester synthesis by Saccharomyces cerevisiae: localisation of acetyl-CoA: iso-amylalcohol acetyltransferase (“AT”) Proc Eur Brew Conv. 1987;21:377–384. [Google Scholar]
- 25.Marais J. The effect of pH on esters and quality of Colombar wine during maturation. Vitis. 1978;17:396–403. [Google Scholar]
- 26.Marais J. Effect of grape temperature, oxidation and skin contact time on Sauvignon blanc juice and wine composition and wine quality. S Afr J Enol Vitic. 1998;19:10–16. [Google Scholar]
- 27.Marais J, Pool H J. Effect of storage time and temperature on the volatile composition and quality of dry white table wines. Vitis. 1980;19:151–164. [Google Scholar]
- 28.Mateo J, Jimenez M, Herta T, Pastor A. Comparison of volatiles produced by four Saccharomyces cerevisiae strains isolated from Monastrell musts. Am J Enol Vitic. 1992;43:206–209. [Google Scholar]
- 29.Minetoki T, Bogaki T, Iwamatsu A, Fujii T, Hamachi M. The purification, properties and internal peptide sequences of alcohol acetyltransferase from Saccharomyces cerevisiae Kyokai no. 7. Biosci Biotechnol Biochem. 1993;57:2094–2098. doi: 10.1271/bbb.57.2094. [DOI] [PubMed] [Google Scholar]
- 30.Noble A C. Wine flavour. In: Piggott J R, Patterson A, editors. Understanding natural flavours. Glasgow, United Kingdom: Blackie Academic and Professional; 1994. pp. 228–242. [Google Scholar]
- 31.Nordström K. Formation of ethyl acetate in fermentation with brewer's yeast. III. Participation of coenzyme A. J Inst Brew. 1962;68:398–407. [Google Scholar]
- 32.Nykänen L. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am J Enol Vitic. 1986;37:84–96. [Google Scholar]
- 33.Nykänen L, Suomalainen H. Formation of aroma compounds by yeast. In: Nykänen L, Suomalainen H, editors. Aroma of beer, wine and distilled alcoholic beverages. Dordrecht, The Netherlands: Reidel; 1983. pp. 3–16. [Google Scholar]
- 34.Ough C S, Cook J A, Lider L A. Rootstock-scion interactions concerning wine making. II. Wine compositional and sensory changes attributed to rootstock and fertilizer differences. Am J Enol Vitic. 1968;19:254–265. [Google Scholar]
- 35.Peddie H A B. Ester formation in brewery fermentations. J Inst Brew. 1990;96:327–331. [Google Scholar]
- 36.Piendl A, Geiger E. Technological factors in the formation of esters during fermentation. Brew Dig. 1980;55:26–35. [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Schreier P. Flavour composition of wines: a review. Crit Rev Food Sci Nutr. 1979;12:59–111. doi: 10.1080/10408397909527273. [DOI] [PubMed] [Google Scholar]
- 39.Van der Westhuizen T J, Pretorius I S. The value of electrophoretic fingerprinting and karyotyping in wine yeast breeding programmes. Antonie Leeuwenhoek. 1992;61:249–257. doi: 10.1007/BF00713932. [DOI] [PubMed] [Google Scholar]
- 40.Volschenk H, Viljoen M, Grobler J, Petzold B, Bauer F F, Subden R, Young R A, Lonvaud A, Denayrolles M, Van Vuuren H J J. Engineering pathways for malate degradation in Saccharomyces cerevisiae. Nat Biotechnol. 1997;15:253–257. doi: 10.1038/nbt0397-253. [DOI] [PubMed] [Google Scholar]
- 41.Winston F, Dollard C, Ricurpeco-Hovasse S L. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]
- 42.Yoshioka K, Hashimoto N. Ester formation by alcohol acetyltransferase from brewers' yeast. Agric Biol Chem. 1981;45:2183–2190. [Google Scholar]
- 43.Yoshioka K, Hashimoto N. Cellular fatty acid and ester formation by brewers' yeast. Agric Biol Chem. 1983;47:2287–2294. [Google Scholar]