Abstract
Background
This international study aimed to characterise the impact of acute SARS-CoV-2 infection in people with cystic fibrosis and investigate factors associated with severe outcomes.
Methods Data from 22 countries prior to 13th December 2020 and the introduction of vaccines were included. It was de-identified and included patient demographics, clinical characteristics, treatments, outcomes and sequalae following SARS-CoV-2 infection. Multivariable logistic regression was used to investigate factors associated with clinical progression to severe COVID-19, using the primary outcome of hospitalisation with supplemental oxygen.
Results
SARS-CoV-2 was reported in 1555 people with CF, 1452 were included in the analysis. One third were aged <18 years, and 9.4% were solid-organ transplant recipients. 74.5% were symptomatic and 22% were admitted to hospital. In the non-transplanted cohort, 39.5% of patients with ppFEV1<40% were hospitalised with oxygen verses 3.2% with ppFEV >70%: a 17-fold increase in odds. Worse outcomes were independently associated with older age, non-white race, underweight body mass index, and CF-related diabetes. Prescription of highly effective CFTR modulator therapies was associated with a significantly reduced odds of being hospitalised with oxygen (AOR 0.43 95%CI 0.31-0.60 p<0.001). Transplanted patients were hospitalised with supplemental oxygen therapy (21.9%) more often than non-transplanted (8.8%) and was independently associated with the primary outcome (Adjusted OR 2.45 95%CI 1.27-4.71 p=0.007).
Conclusions
This is the first study to show that there is a protective effect from the use of CFTR modulator therapy and that people with CF from an ethnic minority are at more risk of severe infection with SARS-CoV-2.
Key words: COVID-19, Coronavirus, Cystic fibrosis, SARS-CoV-2, Transplant
Key messages.
What is already known on this topic
The literature on the impact of COVID-19 in people with cystic fibrosis (CF) has expanded from initial case reports early in the pandemic to larger descriptive studies. A recent publication of the European experience reported higher incidence rates of SARS-CoV-2 infection in the CF population compared to age-matched non-CF controls. Emerging themes from the existing literature suggest there is a wide spectrum of disease severity after infection while advancing age, severe lung disease and lung transplantation have been associated with worse outcomes and death. Studies to date, however, are primarily descriptive in nature and limited by small sample sizes, thus making it difficult to quantify the risk for severe COVID-19 disease while adjusting for confounding factors.
What this study adds
This global study of 1452 individuals represents the largest cohort of people with CF in an analysis for the impact of SARS-CoV-2 infection prior to the licensing of SARS-CoV-2 vaccines. While adjusting for confounding covariates, factors associated with an increased risk for hospitalization with supplemental oxygen following SARS-CoV-2 infection included older age, non-white race, low lung function, malnutrition, organ transplantation, and CF-related diabetes. A protective effect was noted in those individuals who were on highly effective modulator therapy at the time of SARS-CoV-2 infection in this global cohort.
The implications of this study
Several clinical and demographic risk factors were associated with poor SARS-CoV-2 outcomes in people with CF. We present evidence confirming that being post-transplant and having lower lung function is strongly associated with more severe COVID-19 outcomes. Notably, highly effective modulator therapy was associated with a protective effect. This supports the policy that patients eligible for these medications should have immediate access to improve health and potentially protect against severe COVID-19 disease. Overall, these results could help guide clinicians and care teams when recommending mitigation strategies to the CF community.
Alt-text: Unlabelled box
1. Introduction
Cystic fibrosis (CF) is a rare inherited multi-organ condition affecting people from around the globe [1]. It is life limiting with a progressive decline in lung health characterised by recurrent respiratory infections and pulmonary exacerbations, which can be triggered by respiratory viruses. These require frequent hospital admissions for aggressive antibiotic therapy in up to 50% of adults with CF each year [2]. In recent years new disease modifying drugs in the form of CF transmembrane conductance regulator modulator therapies (CFTRm) have been introduced that appear to have an impact on the disease course of CF [3].
Definitions of severe SARS-CoV-2 infection are generally denoted as clinical sequalae such as need for supplemental oxygen therapy, intensive care, ventilation, and death [4]. Defining severe SARS-CoV-2 is challenging in people with CF. They are often hospitalised during pulmonary exacerbations for intensive treatment with antibiotics. Ten percent will use non-invasive ventilation (NIV) over the course of their lifetime because of their underlying chronic illness [5] with 2.4% currently reported as using it in the US and between 7 and 11% using long term oxygen therapy over the last few years [6].
Due to the relatively low absolute number of infections reported in people with CF during the first nine months of the pandemic, existing literature is largely descriptive [7,8,9]. The first wave of the pandemic in Europe showed that people with CF are more frequently hospitalised due to SARS-CoV-2 infection than the age-matched general population infected with SARS-CoV-2 [10]. Within the CF population infected with SARS-CoV-2, higher proportions with advanced lung disease, CF related diabetes (CFRD) or recipients of a solid organ transplant were admitted to hospital [11]. There remains a need to characterise SARS-CoV-2 infection in people with CF globally, and to better understand factors influencing more severe outcomes and more clinically vulnerable sub-groups within this population.
This study aims to characterise the impact of acute SARS-CoV-2 infection in people with CF and investigate possible risk and protective factors associated with severe SARS-CoV-2 infection. As indications for hospitalization may vary in different healthcare systems, in this study severe infection is defined as hospitalisation requiring additional or new supplemental oxygen.
2. Methods
2.1. Study design and population
The 22 countries currently forming the CF Registry Global Collaboration (Supplementary table 1) cover a total global CF population of over 88,000 people. Data on cases of SARS-CoV-2 were collected through established CF registries or through de-identified case reports in adherence to a standardised data collection template agreed upon by the CF Registry Global Collaboration. Data were collected according to each individual nation's CF registry ethics approval or national guidelines.
All confirmed diagnoses of SARS-CoV-2 infection diagnosed between 1st February 2020 and 13th December 2020 were eligible for inclusion in the study, including the 181 previously reported by the collaboration [8]. December 2020 was when the first Covid-19 vaccinations were approved and began being rolled out in some countries and was also when the Alpha variant was first designated a ‘variant of concern’ by WHO. The Covid-19 cohort described in this study includes several large countries outside of Europe that contributed over half of the cohort. All but one participating country in the study reported cases. SARS-CoV-2 cases known to CF teams and diagnosed by RT-PCR, antigen/lateral flow test, or confirmed by medical team consensus were included.
2.2. Variables
Demographic characteristics defined at diagnosis of SARS-CoV-2 included age, sex, race, CFTR genotype, pregnancy and transplant status. Baseline clinical characteristics in the year prior to diagnosis of SARS-CoV-2 included lung function as recorded by the best forced expiratory volume in one second percent predicted (ppFEV1, Global Lung Function Initiative (GLI) equations), body mass index (BMI), CFRD as defined by prescription of insulin therapy, Pseudomonas aeruginosa infection (intermittent or chronic infection), pancreatic insufficiency, systemic hypertension, and prescription of CFTRm at the time of SARS-CoV-2 diagnosis. The four available CFTRm, which are subject to varying access across countries, were categorised into highly effective (ivacaftor or combination elexacaftor/tezacaftor/ivacaftor) and effective (combination lumacaftor/ivacaftor or combination tezacaftor/ivacaftor).
BMI values were converted to percentiles in patients aged ≤18 years according to Centers for Disease Control and Prevention (CDC) reference values. BMI was then reported and analysed as a categorical variable to allow the paediatric and adult patients to be analysed together. People aged <19 years were classified as underweight (percentile ≤12%), normal (percentile 13-84%) or overweight (percentile ≥85%). People aged ≥19 years were classified as underweight (BMI <18.5 kg/m2), normal (18.5-24.9 kg/m2) or overweight ( (≥ 25.0 kg/m2), according to WHO guidelines [12].
Race was collected in 4 categories: white, black, Asian, and other. Due to variations in how racial groups are defined across countries and low numbers in non-white groups, the multivariable analysis combined categories to white and non-white. Those of Hispanic ethnicity were recorded as white race, which in the US made up 13% of their cohort. In recognition of the clinical differences between the pre- and post-transplant CF condition, summary statistics were stratified by solid organ transplant status.
2.3. Outcomes
The primary outcome of severe SARS-CoV-2 infection, as defined by hospitalisation with requirement for new or additional supplemental oxygen therapy, corresponds to level 4 in the WHO COVID-19 clinical progression matrix [13]. Secondary outcomes of interest included hospitalisation, additional NIV use, intensive care unit (ICU) admission, intubation with mechanical ventilation, extracorporeal membrane oxygenation (ECMO) and death. Deaths were reported as all-cause mortality within 3 months of SARS-CoV-2 diagnosis, and further stratified as COVID-related or un-related as reported by the patient's clinical team.
2.4. Statistical analysis
Demographic, clinical characteristics and outcomes were summarised using frequencies and proportions for categorical variables and median and inter-quartile range (IQR) for continuous variables. We investigated associations between key baseline characteristics and the primary outcome of hospitalisation with supplemental oxygen using multivariable logistic regression. We derived adjusted odds ratios (AOR), 95% confidence intervals (CI) and two-sided p-values for the associations. Correlation between patients within countries was accounted for using robust standard errors in multivariable analyses [14]. Model covariates were selected a priori based on evidence from previous studies and were checked for multicollinearity before inclusion in the models. Model assumptions were assessed graphically.
The primary multi-variable analysis included all patients aged ≥6 years, as ppFEV1 measurements are not typically reported in CF registries in children <6 years of age. A secondary analysis was conducted for the non-transplanted patient cohort. Recognising post-transplant patients were likely to have a different lung disease process and medication history, this included additional variables; Pseudomonas aeruginosa infection status, BMI and CFTR modulator (CFTRm) use. The third planned fully adjusted multivariable analysis was not conducted for the outcome of admission to ICU due to low numbers of patients with this outcome.
Missing data were dealt with using clinical imputation rules then multiple imputation with chained equations (MICE) using fully conditional specification applied to all variables in the analysis models, and adjusted model estimates were approximated by combining Rubin's rules [15]. More details on missing data analysis can be found in Supplementary statement 1.
We conducted an ad-hoc sensitivity analysis of the non-transplant model in US/UK patients only. In 2020 the highly effective Elex/Tez/Iva were more commonly available in these countries.
Study reporting and missing data analysis were guided by the STROBE [16] and STRATOS [17] frameworks. Data management and statistical analyses were done using Stata 15 and Stata 17. All tests were two-sided and p<0.05 was considered statistically significant.
3. Results
3.1. Participants
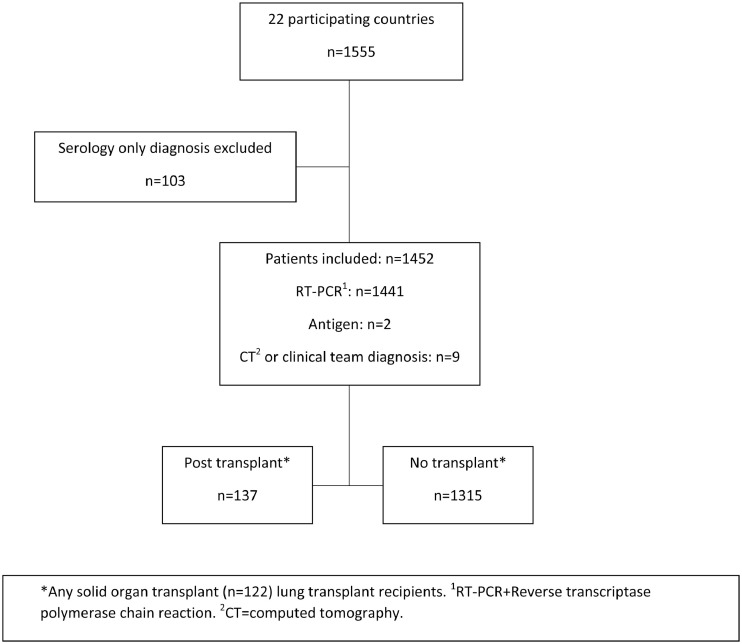
Twenty-two countries participated in this study, reporting 1555 people with CF diagnosed with SARS-CoV-2 infection between 1 February and 13 December 2020. These results suggest that at least 1.8% of the CF population in these countries was infected in this first year of the pandemic. 103 cases were excluded due to receiving a serology antibody only diagnosis rather than a test for acute SARS-CoV-2 infection, leaving 1,452 in the analysis cohort (Fig. 1 ). Only 9 were included with a clinical or CT diagnosis from early in the pandemic, when RT-PCR testing was less available in some countries. The cases across countries ranged from zero cases in New Zealand to 751 in the USA.
Fig. 1.
Study flow diagram.
3.2. Descriptive data
A total of 1452 people with CF were included in the analysis (Table 1 ), four hundred and twenty-one (29%) were children (<18 years) and no cases of repeat infection were reported. More cases were reported during the final three months of the year than in the first 8 months of the study (882 vs. 570). The median age at SARS-CoV-2 infection was 24 years (IQR 16-33). The post-transplant group had a higher median age than the non-transplant group (34 years vs. 23 years). Overall, 50.5% of the cohort were male, 72.9% of the cohort were white race and 82.5% had at least one copy of the F508del CFTR variant.
Table 1.
Baseline demographic and clinical characteristics by transplant status.
| Overall | Non-transplant | Post-transplant | |
|---|---|---|---|
| N=1452 | N=1315 | N=137 | |
| Sex; n (%) | |||
| Male | 733 (50.5) | 659 (50.1) | 74 (54.0) |
| Female | 719 (49.5) | 656 (49.9) | 63 (46.0) |
| Age; Median (IQR) | 24 (16-33) | 23 (15-32) | 34 (27-45) |
| Age; n (%) | |||
| <18 | 421 (29.0) | 415 (31.6) | 6 (4.4) |
| 18-39 | 806 (55.5) | 722 (54.9) | 84 (61.3) |
| ≥40 | 225 (15.5) | 178 (13.5) | 47 (34.3) |
| Best ppFEV1; Median (IQR) | 79.9 (59.0-96.4) | 80.0 (59.5-97.0) | 78.0 (57.1-91.0) |
| Best ppFEV1; n (%) | |||
| <40% | 102 (7.0) | 89 (6.8) | 13 (9.5) |
| 40-70% | 314 (21.6) | 283 (21.5) | 31 (22.6) |
| >70% | 694 (47.8) | 630 (47.9) | 64 (46.7) |
| N/A (age <6 years) | 90 (6.2) | 89 (6.8) | 1 (0.7) |
| Missing | 252 (17.4) | 224 (17.0) | 28 (20.4) |
| BMI category1; n (%) | |||
| Underweight | 134 (9.2) | 109 (8.3) | 25 (18.2) |
| Normal | 765 (52.7) | 671 (51.0) | 94 (68.6) |
| Overweight | 294 (20.2) | 280 (21.3) | 14 (10.2) |
| Missing | 259 (17.8) | 255 (19.4) | 4 (2.9) |
| Race; n (%) | |||
| White | 1059 (72.9) | 973 (74.0) | 86 (62.8) |
| Non-white | 87 | 82 (6.2) | <6 (-) |
| Black | - | 27 (2.1) | <6 (-) |
| Asian | - | 17 (1.3) | <6 (-) |
| Any other race | - | 38 (2.9) | <6 (-) |
| Missing | 306 (21.1) | 260 (19.8) | 46 (33.6) |
| CFTR Genotype; n (%) | |||
| Heterozygous F508del | 620 (42.7) | 565 (43.0) | 55 (40.1) |
| Homozygous F508del | 578 (39.8) | 510 (38.8) | 68 (49.6) |
| Other | 252 (17.4) | 238 (18.1) | 14 (10.2) |
| Missing | 2 (0.1) | 2 (0.2) | 0 (0.0) |
| CF-related diabetes; n (%) | |||
| Yes | 362 (24.9) | 268 (20.4) | 94 (68.6) |
| Missing | 87 (6.0) | 77 (5.9) | 10 (7.3) |
| P. aeruginosa infection2; n (%) | |||
| Yes | 655 (45.1) | 600 (45.6) | 55 (40.1) |
| Missing | 96 (6.6) | 52 (4.0) | 44 (32.1) |
| Pancreatic insufficiency; n (%) | |||
| Yes | 1167 (80.4) | 1037 (78.9) | 130 (94.9) |
| Missing | 39 (2.7) | 34 (2.6) | 5 (3.6) |
| Systemic Hypertension; n (%) | |||
| Yes | 77 (5.3) | 37 (2.8) | 40 (29.2) |
| Missing | 283 (19.5) | 255 (19.4) | 28 (20.4) |
| CFTR modulators3; n (%) | |||
| Any CFTR modulator | 751 (51.7) | 738 (56.1) | 13 (9.5) |
| Iva or Elex/Tez/Iva | 556 (38.3) | 544 (41.4) | 12 (8.8) |
| Lum/Iva or Tez/Iva | 195 (13.4) | 194 (14.8) | 1 (0.7) |
| No CFTR modulator | 685 (47.2) | 563 (42.8) | 122 (89.1) |
| Missing | 16 (1.1) | 14 (1.1) | 2 (1.5) |
| Pregnant; n (%) | |||
| Yes | 32 (2.2) | 32 (2.4) | 0 (0.0) |
| Missing | 353 (24.3) | 328 (24.9) | 25 (18.2) |
| Time of diagnosis | |||
| Feb-May | 198 (13.6) | 161 (12.2) | 37 (27.0) |
| June-Sept | 372 (25.6) | 351 (26.7) | 21 (15.3) |
| Oct-Dec | 882 (60.7) | 803 (61.1) | 79 (57.7) |
Proportions are calculated from column totals (n/N). Where no 'Missing' row is included, variables are 100% complete. 1 BMI categories are defined according to WHO guidelines. People aged <19 years classified as underweight (percentile ≤12%), normal (percentile 13-84%) or overweight (percentile ≥85%). People aged ≥19 years classified as underweight (BMI <18.5 kg/m2), normal (18.5-24.9 kg/m2) or overweight ( (≥ 25.0 kg/m2). 2 Chronic or intermittent infection in the year prior to SARS-CoV-2 diagnosis. 3 Using CFTR modulators at the time of SARS-CoV-2 diagnosis.
Iva=Ivacaftor.
Elex/Tez/Iva=combination Elexacaftor/Tezacaftor/Ivacaftor.
Lum/Iva=combination Lumacaftor/Ivacaftor.
Tez/Iva=combination Tezacaftor/Ivacaftor.
IQR=interquartile range.
CFTR=cystic fibrosis transmembrane conductance regulator.
BMI=body mass index.
ppFEV1=percent predicted forced expiratory volume in 1 second.
The median best ppFEV1 pre-COVID-19 was 80%, with the distribution of lung function being comparable for non-transplant and post-transplant patients. The post-transplant cohort was small (n=137), some of the characteristics did however differ as shown in Table 1, of note for pancreatic insufficiency, BMI, CFRD and use of CFTRm drugs, which are not widely prescribed post-transplant. Thirty-two individuals (11.1% of females aged >14 years in the cohort) were pregnant when they acquired the infection.
3.3. Clinical Course
The presenting symptoms of SARS-CoV-2 infection are described in Table 2 . Fever was the commonest symptom, present in 38% of people. A similar number (36%) reported increased cough. There were slight differences between the cohorts, notably for fatigue and fever. The proportion with fever and cough did not change across the age ranges (Supplementary table 2).
Table 2.
Infection management and disease course by transplant status.
| Overall | Non-transplant | Post-transplant | |
|---|---|---|---|
| n (%) | N=1452 | N=1315 | N=137 |
| Hospitalisation | |||
| No | 1086 (74.8) | 1030 (78.3) | 56 (40.9) |
| Yes | 316 (21.8) | 236 (17.9) | 80 (58.4) |
| Missing | 50 (3.4) | 49 (3.7) | 1 (0.7) |
| Hospitalisation with supplemental oxygen1 | |||
| No | 1241 (85.5) | 1145 (87.1) | 96 (70.1) |
| Yes | 128 (8.8) | 98 (7.5) | 30 (21.9) |
| Missing | 83 (5.7) | 72 (5.5) | 11 (8.0) |
| Intensive care unit admission | |||
| No | 1373 (94.6) | 1263 (96.0) | 110 (80.3) |
| Yes | 46 (3.2) | 27 (2.1) | 19 (13.9) |
| Missing | 33 (2.3) | 25 (1.9) | 8 (5.8) |
| Non-invasive ventilation | |||
| No | 1361 (93.7) | 1242 (94.4) | 119 (86.9) |
| Yes | 29 (2.0) | 21 (1.6) | 8 (5.8) |
| Missing | 62 (4.3) | 52 (4.0) | 10 (7.3) |
| Invasive Mechanical ventilation | |||
| No | 1363 (93.9) | 1245 (94.7) | 118 (86.1) |
| Yes | 20 (1.4) | 11 (0.8) | 9 (6.6) |
| Missing | 69 (4.8) | 59 (4.5) | 10 (7.3) |
| ECMO | |||
| No | 1357 (93.5) | 1233 (93.8) | 124 (90.5) |
| Yes | 4 (0.3) | 2 (0.2) | 2 (1.5) |
| Missing | 91 (6.3) | 80 (6.1) | 11 (8.0) |
| Vital status2 | |||
| Alive | 1432 (98.6) | 1305 (99.2) | 127 (92.7) |
| Died (any cause) | 20 (1.4) | 10 (0.8) | 10 (7.3) |
| Died (COVID-19) | 17 (1.2) | 9 (0.7) | 8 (5.8) |
| Symptoms3 | |||
| Fever | 558 (38.4) | 489 (37.2) | 69 (50.4) |
| Myalgia (joint pain) | 141 (9.7) | 117 (8.9) | 24 (17.5) |
| Dyspnea (shortness of breath) | 266 (18.3) | 229 (17.4) | 37 (27.0) |
| Increased cough | 522 (36.0) | 480 (36.5) | 42 (30.7) |
| Fatigue | 234 (16.1) | 199 (15.1) | 35 (25.5) |
| Other | 467 (32.2) | 427 (32.5) | 40 (29.2) |
| Any symptoms | 1082 (74.5) | 968 (73.6) | 114 (83.2) |
| Missing | 38 (2.6) | 34 (2.6) | 4 (2.9) |
Proportions are calculated from column totals (n/N).
New or additional supplemental oxygen.
As of 15th January 2021 3 Symptom categories are not mutually exclusive, with patients able to experience >1.
Additional oxygen, ICU admission, NIV, invasive mechanical ventilation and ECMO are not mutually exclusive and people can appear in more than one group.
ECMO=Extracorporeal membrane oxygenation.
Three hundred and sixteen people (22%) were admitted to hospital in the overall cohort (Table 2) and 128 (41%) of the 316 met the primary outcome of interest requirement for supplemental oxygen with 46 (15%) requiring intensive care. Of these 316 there was a marked difference in the proportion admitted from the post-transplant group (58% vs 18%). Indications for hospitalisation were not standardised, relying on the local physicians to determine need. Once hospitalised the post-transplant group were also more commonly admitted to ICU and required invasive ventilation. Similar proportions in each cohort received NIV and supplemental oxygen after admission to hospital. 10 people died in each group following SARS-CoV-2 infection with 3 of these felt not to be related to SARS-CoV-2 as assessed by the treating physician.
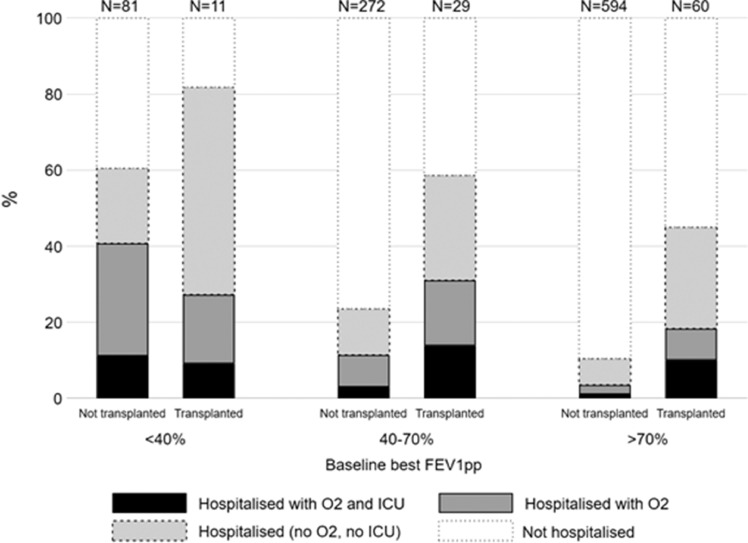
Irrespective of transplant status, higher proportions of people with lower baseline lung function were hospitalised. The distribution for the use of supplemental oxygen and ICU admission within hospitalised patients by lung function and transplant status is displayed in Fig. 2 . Rates of hospital admission, with supplemental oxygen and intensive care admission, also increased with older age in the non-transplant cohort (Supplementary Figure 1). Scatter plots showing lung function and age are shown for both cohorts in Supplementary Figures 2a&b.
Fig. 2.
Disease course by transplant status and baseline Best ppFEV1. People >6 years, with non-missing outcome and baseline best ppFEV1 data are included in this graph (N=1047). Total non-missing for each group are represented as bar labels – e.g., N=81 non-transplanted patients with <40% ppFEV1 had non-missing outcome values. Outcomes are coded as mutually exclusive – note that this differs from how the data are presented in Table 2, Table 3.
The demographic and clinical characteristics by outcomes in the non-transplanted cohort are shown in Table 3 . In the non-transplant cohort, 16.0% patients aged ≥40 years with non-missing outcome data were hospitalised with supplemental oxygen, compared with 4.3% aged <18 years. A higher proportion of people with baseline best ppFEV1 <40% (39.5%) were also hospitalised with oxygen, than those with ppFEV1 >70% (3.2%). Similarly, a higher proportion of people in the underweight BMI category were reported with the primary outcome – 22.1% compared with 8.4% of those with a normal BMI. . Higher rates of hospitalisation with oxygen were observed in people with CFRD (12.9%) compared with those without (6.3%). Lower rates of hospitalisation with oxygen were observed in patients prescribed any CFTRm therapies (5.9%) compared with those with no modulators (10.6%).
Table 3.
Demographic and clinical characteristics by disease course (non-transplant cohort, N=1315).
| Hospitalisation with supplemental oxygen | Hospitalisation | Intensive care unit | ||||
|---|---|---|---|---|---|---|
| N=1243 | N=1266 | N=1290 | ||||
| n/N | % | n/N** | % | n/N | % | |
| Sex; n (%) | ||||||
| Male | 51/619 | 8.2 | 115/632 | 18.2 | 16/645 | 2.5 |
| Female | 47/624 | 7.5 | 121/634 | 19.1 | 11/645 | 1.7 |
| Age; n (%) | ||||||
| <18 | 17/394 | 4.3 | 62/401 | 15.5 | <6/408 | - |
| 18-39 | 54/680 | 7.9 | 127/696 | 18.2 | 12/706 | 1.7 |
| ≥40 | 27/169 | 16.0 | 47/169 | 27.8 | 10/176 | 5.7 |
| Best FEV1; n (%) | ||||||
| <40% | 32/81 | 39.5 | 51/83 | 61.4 | 9/87 | 10.3 |
| 40-70% | 30/271 | 11.1 | 68/275 | 24.7 | 8/280 | 2.9 |
| >70% | 19/591 | 3.2 | 70/599 | 11.7 | 6/620 | 1 |
| BMI category; n (%) | ||||||
| Underweight | 23/104 | 22.1 | 42/105 | 40.0 | <6/108 | - |
| Normal | 52/624 | 8.3 | 126/637 | 19.8 | 14/657 | 2.1 |
| Overweight | 15/264 | 5.7 | 42/269 | 15.6 | 6/275 | 2.2 |
| Race; n (%) | ||||||
| White | 58/951 | 6.1 | 153/972 | 15.7 | 21/951 | 2.2 |
| Non-white | 14/81 | 17.3 | 26/82 | 31.7 | <6/81 | - |
| Black | 6/26 | 23.1 | 10/27 | 37.0 | <6/26 | - |
| Asian | 0/17 | 0.0 | 4/17 | 23.5 | 0/17 | 0 |
| Any other race | 8/38 | 21.1 | 12/38 | 31.6 | <6/38 | - |
| Genotype; n (%) | ||||||
| Heterozygous F508del | 41/516 | 7.9 | 90/527 | 17.1 | 10/553 | 1.8 |
| Homozygous F508del | 33/500 | 6.6 | 91/510 | 17.8 | 12/500 | 2.4 |
| Other | 24/225 | 10.7 | 55/227 | 24.2 | <6/235 | - |
| CF-related diabetes; n (%) | ||||||
| No | 60/945 | 6.3 | 152/959 | 15.8 | 15/955 | 1.6 |
| Yes | 34/264 | 12.9 | 75/268 | 28.0 | 11/264 | 4.2 |
| P. aeruginosa infection; n (%) | ||||||
| No | 32/636 | 5.0 | 88/643 | 13.7 | 11/656 | 1.7 |
| Yes | 64/565 | 11.3 | 141/576 | 24.5 | 15/587 | 2.6 |
| Pancreatic insufficiency; n (%) | ||||||
| No | 11/225 | 4.9 | 26/227 | 11.5 | 3/243 | 1.2 |
| Yes | 87/989 | 8.8 | 204/1005 | 20.3 | 24/1018 | 2.4 |
| CFTR modulators; n (%) | ||||||
| Any CFTR modulator | 42/713 | 5.9 | 105/726 | 14.5 | 14/725 | 1.9 |
| Iva or Elex/Tez/Iva | 27/528 | 5.1 | 68/539 | 12.6 | 11/533 | 2.1 |
| Lum/Iva or Tez/Iva | 15/185 | 8.1 | 37/187 | 19.8 | 3/192 | 1.6 |
| No modulator | 55/520 | 10.6 | 124/527 | 23.5 | 13/555 | 2.3 |
| Time of diagnosis; n (%) | ||||||
| Feb-May | 30/157 | 19.1 | 69/161 | 42.9 | 9/157 | 5.7 |
| June-Sept | 27/340 | 7.9 | 60/348 | 17.2 | 9/343 | 2.6 |
| Oct-Dec | 41/746 | 5.5 | 107/757 | 14.1 | 9/790 | 1.1 |
Column row totals represent non missing data for the column outcome. n=number admitted N=total number reported in the cohort. Row proportions are calculated from the total non-missing in each outcome (See Table 1 for missing data). CFTR=cystic fibrosis transmembrane conductance regulator. FEV1=forced expiratory volume in 1 second, BMI=body mass index. Iva=ivacaftor, elex=elexacaftor, tez=tezacaftor.
Of the 236 that were hospitalised in the non-transplant group, 98 (42%) required supplemental oxygen, 27 (11.4%) were admitted to an intensive care unit, and 21 (8.9%) had NIV. Descriptive data for the whole cohort, including transplanted patients, is shown in the Supplementary Table 3. Of the 126 post-transplant patients with non-missing outcome data, 23.8% were hospitalised with supplemental oxygen, compared with 7.9% of the non-transplanted patients. Similarly, a higher proportion of post-transplant patients (14.7%) were admitted to ICU following SARS-CoV-2 infection than non-transplanted patients (2.1%).
3.4. Multivariable analysis
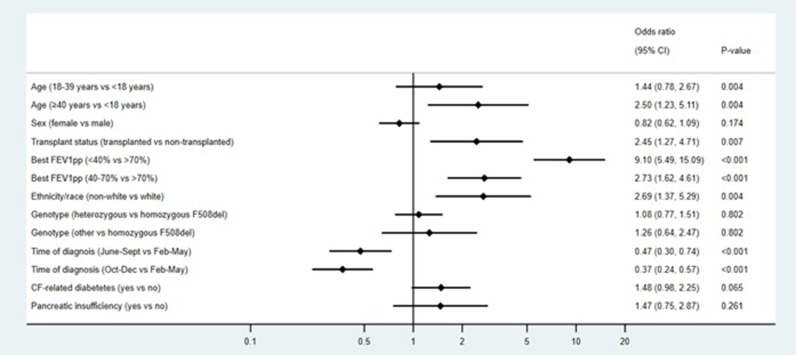
For those over 6 years of age the results of the primary multivariable analysis for hospitalisation with new or additional supplemental oxygen across the whole cohort of people with CF diagnosed with SARS-CoV-2 is shown in Fig. 3 (n=1362). Having a pre-pandemic baseline best ppFEV1 <40% compared with >70% was associated with a 9-times higher risk of being hospitalised with oxygen (AOR 9.10 95%CI 5.49-15.09 p<0.001). Other factors associated with the primary outcome are being of an older age (AOR 2.50 95%CI 1.23-5.11 p=0.004), having received a transplant (AOR 2.45 95%CI 1.27-4.71 p=0.007), being of non-white race (AOR 2.69 95%CI 1.37-5.29 p=0.004), and a diagnosis earlier in the pandemic (Supplementary table 4).
Fig. 3.
Multivariable analysis for hospitalisation with supplemental oxygen in all patients (n=1362, m10). All analyses are adjusted for clustering on country using robust standard errors. Excluding people aged <6 years as ppFEV1 measurements are not recommended in this age group. p values shown represent the overall p values for the variable and are not associated with the level-to-level comparisons within these variables, which are represented by the 95% CIs. CI=Confidence interval ppFEV1=forced expiratory volume in 1 second percent predicted, BMI=body mass index, Iva=ivacaftor, elex=elexacaftor, tez=tezacaftor m10=multiple imputed dataset with 10 iterations.
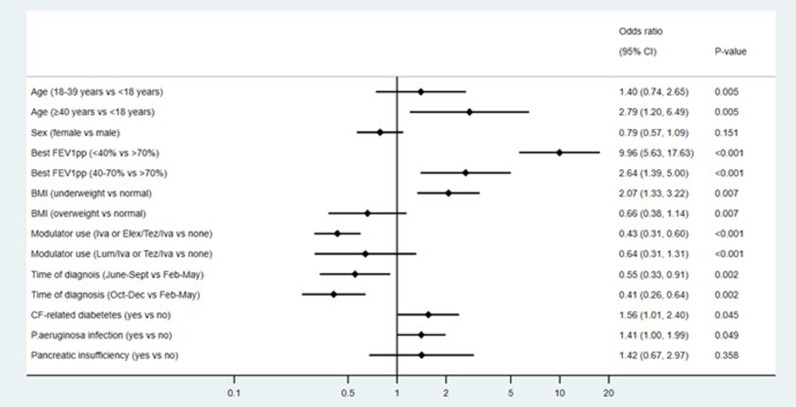
Multivariable analysis of the 1315 non-transplant patients included the additional variables of BMI, Pseudomonas aeruginosa infection status and use of a CFTRm therapy (Fig. 4 ). The associations observed in the primary analysis remained, with additional factors showing evidence of independent associations including underweight BMI (AOR 2.07 95%CI 1.33-3.22 p=0.007), CFRD (AOR 1.56 95%CI 1.01-2.40 p=0.045) and Pseudomonas aeruginosa infection (AOR 1.41 95%CI 1.00-1.99 p=0.049). CFTRm use showed a negative association with the outcome, specifically for highly effective modulators compared with no modulators (AOR 0.43 95%CI 0.31-0.60 p<0.001) (Supplementary table 5). A limited multivariable analysis was performed for the outcome of ICU, because of the small number admitted to ICU (n=46). The age and sex adjusted odds ratios are shown in Supplementary table 6, with female showing a protective effect AOR 0.58 (0.42-0.81) and a transplant AOR of 6.13 (3.05-12.34).
Fig. 4.
Multivariable analysis for hospitalisation with supplemental oxygen in the non-transplant cohort (N=1226, m=10). All analyses are adjusted for clustering on country using robust standard errors. Excluding people aged <6 years as ppFEV1 measurements are not recommended in this age group. p values shown represent the overall p values for the variable and are not associated with the level-to-level comparisons within these variables, which are represented by the 95% CIs. CI=Confidence interval ppFEV1=forced expiratory volume in 1 second percent predicted, BMI=body mass index, Iva=ivacaftor, elex=elexacaftor, tez=tezacaftor m10=multiple imputed dataset with 10 iterations.
The sensitivity analysis in the non-transplant cohort, with patients from the US and UK only, showed that the evidence of effect of highly effective CFTRm therapies remained, showing a protective effect with a 59% reduction in risk of the primary outcome compared with no modulators (AOR 0.41 95%CI 0.18-0.90 p=0.079) (Supplementary Table 8).
4. Discussion
This global study of 1452 individuals represents the largest cohort of people with CF in an analysis for the impact of SARS-CoV-2 infection prior to the licensing of SARS-CoV-2 vaccines. We used a composite end point of hospitalisation with additional oxygen supplementation to indicate severe infection.
The study has confirmed that transplant remains an independent risk factor for more severe SARS-CoV-2. The study included 137 people with CF who had received a solid organ transplant (122 lung transplants). They showed a greater proportion requiring hospitalisation, supplemental oxygen, being admitted to ICU, requiring invasive ventilation, and dying than in the non-transplant population. Once hospitalised, transplant patients had a 1 in 8 chance of all cause death, the overall mortality for pwCF was low at less than 0.8% and stood at 4% once admitted to hospital. This is lower than the mortality in lung transplant groups reported elsewhere [18]. With low numbers of deaths within this study interpretation of mortality rates must be made with caution.
Poor lung function, especially in those with ppFEV1 <40 has emerged as key potential risk factor for severe SARS-CoV-2. Our results show this is the case in both non-transplant and post-transplant groups. When looking at the post-transplant group in more detail, the proportions across the three lung function categories are similar to those in the non-transplant group (Fig. 2). Severe post-transplant lung disease is often due to chronic lung allograft dysfunction, this group are likely to be on more immunosuppression which is a known risk factor.
Older age is known to be a risk factor for admission and death with SARS-CoV-2 in the general population. The median age at admission to hospital for this cohort was 28 years, with those having been transplanted slightly older (36 years) as would be expected given CF disease progression over time leads to transplant. When removing the post-transplant group from the analysis, older age remained an independent risk factor for severe disease, even though this group is likely to also have a lower lung function. For both groups this was much younger than reported across the general population.
An important new finding of this study that has not previously been reported is that being of non-white race appears to be associated with a higher risk of severe infection for people with CF. This is in-line with results for the general population, which may be associated with socioeconomic or genetic factors as well as pathophysiological differences [19]. It is worth noting this was a significant finding despite our study classifying people of Hispanic race/ethnicity as white. Evidence from the USA confirm Hispanics populations have worse outcomes with SARS-CoV-2 [20].
Prescription of CFTRm therapy, especially so-called ‘highly effective modulators’ ivacaftor and elaxacaftor/tezacaftor/ivacaftor, was shown to have a protective effect against severe SARS-CoV-2 infection in non-transplanted patients. During the period of this study the modulator Elex/Tez/Iva was only widely available for prescription in the USA and UK, these also were also two of the countries with the highest rates of SARS-CoV-2 infection per capita in 2020 [21]. The robust standard error analysis method should have accounted for any clustering for example within countries however a sensitivity analysis on cases in only the two countries with highly effective CFTRm available provided further evidence that the association between highly effective modulators and outcome remained and confirmed it was not just an effect of different health care systems in these countries (Supplementary table 5). The use of CFTRm may also have impacted the finding that genotype group did not appear to be a risk factor, although a recent paper shows that CFTR function which is related to genotype correlates well with lung disease and other clinical features [22].
In the general population the risk of having SARS-CoV-2 whilst pregnant is a concern with high numbers having severe infection and what appears to be worse outcomes [23]. Our study included 32 people with CF who were pregnant at the time of SARS-CoV-2 infection, no deaths were reported in this group, although small numbers prevent analysis for association with more severe disease course in this group.
Hospitalisation alone and hospitalisation with additional supplemental oxygen occurred at higher rates across the entire cohort during first three months of the pandemic. This is likely to be linked to the evolution of testing strategies as the pandemic progressed, in the early phases many countries were unable to test for suspected cases in the community meaning only those hospitalised will have been tested. Later in the pandemic, more robust testing regimens will have resulted in higher numbers of mild cases being definitively diagnosed with a positive RT-PCR in the community. There may also have been an evolution of knowledge about the effects of SARS-CoV-2 that meant that people with CF and infection were not automatically admitted if infection appeared mild.
CF-related diabetes was significantly associated with severe SARS-CoV-2 infection in the multivariate analysis of the non-transplant group. Similarly, Pseudomonas aeruginosa was associated with severe infection in the non-transplant group. Unlike SARS-CoV-2 in the general population, female sex does not appear to have a protective effect in the CF cohort. This may be related to females with CF tending to have poorer health outcomes in the first place [24].
In the multivariable analysis of the non-transplant group only, being underweight is associated with more severe infection. Being overweight may have a protective effect, in contrast to the linear increase in risk of hospital admission starting from BMI > 23 kg/m2 observed in a large cohort of 6.9 million UK adults [25]. However, given people with CF are often only mildly overweight, this finding is in line with current nutritional guidelines, which recommend BMI values above 23 kg/m2 for male patients and above 22 kg/m2 for female patients [26].
4.1. Strengths and Limitations
This study has the strength of including a very diverse range of countries across the globe, all with very different healthcare systems and different populations united by a common pandemic.
SARS-CoV-2 infection continues to be a rapidly evolving global situation with participating countries at different phases of the pandemic. Different countries have had different availability for testing at different stages. Many countries had limited testing for SARS-CoV-2 in people in the first few months of the pandemic, meaning that the overall CF population prevalence of 1.8% may be an under-estimate. While varying availability of testing was adjusted for in the multivariable analysis by using the time of diagnosis of a positive result, this risk of ascertainment bias should still be noted.
Whilst adjustments were made and sensitivity analysis conducted to account for differences between countries, the large proportion of cases coming from a small number of countries should be noted. Countries with larger CF populations, and higher absolute numbers of SARS-CoV-2 cases amongst them, also tend to have access to advanced healthcare systems, including CFTRm therapy and lung transplantation.
Missing data was addressed with modern techniques to impute the data for modelling purposes. Data was collected for acute treatment of SARS-CoV-2, however these have not been reported due to large amounts of missing data for IV antibiotics (47.4% missing), oral antibiotics (49.2% missing) and steroids (66.9% missing).
Different countries and CF registries define and capture ethnicity in subtly different ways; there was no differentiation available in some countries between Hispanic white and Hispanic non-white thus this group was categorised as white. Small numbers of non-white people also meant analysis was done with just two categories of white and non-white, even with these limitations the non-white group were shown to be at greater risk of severe SARS-CoV-2.
4.2. Interpretation
The disease course of SARS-CoV-2 amongst people with CF is varied and can be mild or even asymptomatic. However, the outcomes can be severe, resulting in hospitalisation, intensive care admission or even death in a small number of cases. People who are over 40 years old, have advanced lung disease (ppFEV1 <40), or have received a solid-organ transplant are at higher risk of severe infection requiring hospitalisation and new or additional supplemental oxygen. New and successful treatments for SARS-CoV-2 have now become established, meaning high risk groups such as those with CF are eligible for early treatment, but it should be noted equality of access to these treatments and indeed vaccination continues to be variable across the world.
People with CF should continue to follow guidance from medical experts to guard against infection, including ensuring that they and their families receive and keep up-to-date with COVID-19 vaccination.
The finding that highly effective modulator therapy may be protective against severe disease and hospitalisation caused by SARS-Cov-2 supports the policy that all people with CF who are likely to benefit should have immediate access to these medicines.
4.3. Generalisability
The risk for severe disease in those with low lung function in both the post-transplant and non-transplant group, who effectively have different underlying mechanisms for their lung disease, means that an absolute reduction in lung volume is likely to be a risk factor rather than just the mechanism behind the lung disease.
Credit Author statement
All authors were involved in conceptualization and methodology. Authors from individual countries were responsible for data collection and validation for their country. RC and EMcC were responsible for Data Curation. EMcC perfomed the formal analysis with supervision from SBC. RC, SBC and EMcC were responsible for the writing of the original draft, all authors were involved with review and editing of the paper.
Funding
AS, RC, have funding from Canadian Institutes of Health Research to support the Global Registry Collaboration on Covid and CF.
COI Statement
All authors declare no conflicts of interest in relationship to this work. Outside of this work the following authors declare payments or honoraria to them or their institution for a combination of lectures, presentations, educational events, advisory boards, steering groups, grants or consultancy fees: SC-Vertex, Chiesi, Profile, Zambon. RC- Vertex, P-RB – Astra-Zeneca, Boehringer Ingelheim, GSK, Insmed, Chiesi, Pfizer, Vertex, Zambon. I deM – Vertex,LN – Vertex, Boehringer,LVS-F – Vertex, AS -Vertex.
Acknowledgements
We would like to thank the people with cystic fibrosis around the world that have consented to have their anonymised data collected by their respective CF Registries. The local and National CF teams that have worked hard to collect and clean the data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcf.2022.06.006.
Contributor Information
CF Registry Global Collaboration:
Scott C Bell, David Reid, Peter Wark, Eva Van Braeckel, Sophie Gohy, Christiane Knoop, Jessica Pirson, Elke De Wachter, Lieven Dupont, Laurence Hanssens, Vicky Nowé, Monique Lequesne, Rodrigo A Athanazio, Daniela G Meneses, Véronique Boussaud, Graziella Brinchault, Emmanuelle Coirier-Duet, Jean-Christophe Dubus, Dominique Grenet, Sandra de Miranda, Laurence Beaumont, Reem Kanaan, Muriel Lauraens, Clémence Martin, Marie Mittaine, Anne Prévotat, Martine Reynaud-Gaubert, Isabelle Sermet-Gaudelus, Aurelie Tatopoulos, Raphael Chiron, Marie-Laure Dalphin, Michele Gerardin, Laurence Weiss, Nathalie Wizla, Sophie Ramel, Barry Plant, Cedric Gunaratnam, Abaigeal Jackson, Karin de Winter- de Groot, Bart Luijk, Geertjan Wesseling, Elena Kondratyeva, Elena Zhekayte, Elena Amelina, Mariya Mukhina, Olga Simonova, Antonio Alvarez-Fernandez, Amparo Sole-Jover, Isidoro Cortell-Aznar, Rosa Girón-Moreno, Alejandro López-Neyra, Isabel Ramos-Cancelo, Maite Lázaro-Carrasco, Dolores Pastor Vivero, Marta Ruiz de Valbuena, Concepción Prados-Sanchez, Jordi Costa-Colomer, Silvia Gartner, Layla Diab-Caceres, Marita Gilljam, Ulrika Lindberg, Stefanie Diemer, Mark Allenby, Stephen J Bourke, Susan C Charman, Janet Collinson, Owen Dempsey, Sarah Denniston, Maya Desai, Jamie Duckers, Christine Etherington, Elaine Gunn, Alex Higton, Timothy Ho, Jeremy Hull, Andrew Jones, Robert Ian Ketchell, Susan L. Madge, Anirban Maitra, Ghulam Mujtaba, Edward Nash, Dilip Nazareth, Christopher O'Brien, Claire Onyon, Christopher Orchard, Daniel Peckham, Helen Rodgers, Nadia Shafi, Nicholas Simmonds, Kevin Southern, Martin Walshaw, Danie Watson, Joanna L. Whitehouse, Annalisa Orenti, Basil Elnazir, and Des Cox
Appendix. Supplementary materials
References
- 1.Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, Burgel PR, Tullis E, Castaños C, Castellani C, Byrnes CA, Cathcart F, Chotirmall SH, Cosgriff R, Eichler I, Fajac I, Goss CH, Drevinek P, Farrell PM, Gravelle AM, Havermans T, Mayer-Hamblett N. K RFT future of cystic fibrosis care: a global perspective. LRMed. 2020 doi: 10.1016/S2213-2600(19)30337-6. J 124. doi: 10. 1016/S2213 2600 (19)30337 6. E 2019 S 27. E in: LRMed 2019 DP 31570318. The future of cystic fibrosis care: a global perspective. Lancet Respiratory Medicine. 2020;8 (1):65–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charman Susan, Lee Andrew, Cosgriff Rebecca, McClenaghan Elliot CS. Annual Report - UK CF Registry [Internet]. UK CF Registry Annual Report. 2019. Available from: https://www.cysticfibrosis.org.uk/the-work-we-do/uk-cf-registry/reporting-and-resources
- 3.Balfour-Lynn IM, King JA. CFTR modulator therapies - Effect on life expectancy in people with cystic fibrosis. Paediatr Respir Rev. 2020 doi: 10.1016/j.prrv.2020.05.002. S1526-0542 (20)30081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health. No Title [Internet]. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available from: https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 5.Archangelidi O, Carr SB, Simmonds NJ, Bilton D, Banya W, Cullinan P. Non-invasive ventilation and clinical outcomes in cystic fibrosis: Findings from the UK CF registry. J Cystic Fibrosis. 2018 doi: 10.1016/j.jcf.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 6.No Title [Internet]. CFF Patient Registry Report. 2020 [cited 2020 Nov 22]. Available from: https://www.cff.org/sites/default/files/2021-11/Patient-Registry-Annual-Data-Report.pdf
- 7.Cosgriff R, Ahern S, Bell SC, Brownlee K, Burgel PR, Byrnes C, et al. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cystic Fibrosis. 2020;19(3):355–358. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClenaghan E, Cosgriff R, Brownlee K, Ahern S, Burgel PR, Byrnes CA, et al. The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J Cystic Fibrosis. 2020 doi: 10.1016/j.jcf.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bain R, Cosgriff R, Zampoli M, Elbert A, Burgel PR, Carr SB, Castaños C, Colombo C, Corvol H, Faro A, Goss CH, Gutierrez H, Jung A, Kashirskaya N, Marshall BC, Melo J, Mondejar-Lopez P, de Monestrol I, Naehrlich L, Padoan R, Pastor-Vivero MD, Rizvi S, Sal BM. Clinical characteristics of SARS-CoV-2 infection in children with cystic fibrosis: An international observational study. J Cystic Fibrosis. 2021;20(1):25–30. doi: 10.1016/j.jcf.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naehrlich L, Orenti A, Dunlevy F, Kasmi I, Harutyunyan S, Pfleger A, et al. Incidence of SARS-CoV-2 in people with cystic fibrosis in Europe between February and June 2020. J Cystic Fibrosis. 2021;20(4):566–577. doi: 10.1016/j.jcf.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung A, Orenti A, Dunlevy F, Aleksejeva E, Bakkeheim E, Carr SB, et al. Early View Factors for severe outcomes following SARS- CoV-2 infection in people with cystic fibrosis in Europe Factors for severe outcomes following SARS-CoV-2 infection in people with cystic fibrosis in Europe. 2021; [DOI] [PMC free article] [PubMed]
- 12.World Health Organisation. BMI [Internet]. cited 2021 Oct 2. Available from: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi
- 13.Infection WWG on the CC and M of C 19. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8) doi: 10.1016/S1473-3099(20)30483-7. e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817–838. [Google Scholar]
- 15.White IR, Royston P WA. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC VJSI. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85(11):867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KJ, Tilling KM, Cornish RP, Little RJA, Bell ML, Goetghebeur E, initiative Hogan JW CJS. Framework for the treatment and reporting of missing data in observational studies: The Treatment And Reporting of Missing data in Observational Studies framework. J Clin Epidemiol. 2021;135:79–88. doi: 10.1016/j.jclinepi.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamp JC, Hinrichs JB, Fuge J, Ewen R, Gottlieb J. COVID-19 in lung transplant recipients—Risk prediction and outcomes. PLOS One. 2021;16(10) doi: 10.1371/journal.pone.0257807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khunti K, Singh AK, Pareek M, Hanif W. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369(April):1–2. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 20.Xu JJ, Chen JT, Belin TR, Brookmeyer RS, Suchard MA, Ramirez CM. Racial and ethnic disparities in years of potential life lost attributable to COVID-19 in the United States: an analysis of 45 states and the District of Columbia. Int J Environ Res Public Health. 2021;18(6):2921. doi: 10.3390/ijerph18062921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John Hopkins Coronavirus Resource Centre [Internet]. cited 2021. Available from: Tracking - Johns Hopkins Coronavirus Resource Center (jhu.edu)
- 22.McCague AF, Raraigh KS, Pellicore MJ, Davis-Marcisak EF, Evans TA, Han ST, et al. Correlating Cystic Fibrosis Transmembrane Conductance Regulator Function with Clinical Features to Inform Precision Treatment of Cystic Fibrosis. Am J Respir Critical Care Med. 2019;199(9):1116–1126. doi: 10.1164/rccm.201901-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatrics. 2021;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keogh RH, Szczesniak R, Taylor-Robinson D BD. Up-to-date and projected estimates of survival for people with cystic fibrosis using baseline characteristics: A longitudinal study using UK patient registry data. J Cyst Fibros. 2018;17(2):218–227. doi: 10.1016/j.jcf.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O'Rahilly S, Aveyard P JS. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turck D., Braegger C.P., Colombo C., Declercq D., Morton A., Pancheva R., Robberecht E., Stern M., Strandvik B., Wolfe S., Schneider S.M., Wilschanski M. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin Nutrit. 2016;35(3):557–577. doi: 10.1016/j.clnu.2016.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.