Summary
Prevention of premalignant lesion progression is a promising approach to reducing lung cancer burden in high-risk populations. Substantial preclinical and clinical evidence has demonstrated efficacy of the prostacyclin analogue iloprost for lung cancer chemoprevention. Iloprost activates peroxisome proliferator-activated receptor gamma (PPARG) to initiate chemopreventive signaling and in vitro, which requires the transmembrane receptor Frizzled9 (FZD9). We hypothesized a Fzd9−/− mouse would not be protected by iloprost in a lung cancer model. Fzd9−/− mice were treated with inhaled iloprost in a urethane model of lung adenoma. We found that Fzd9−/− mice treated with iloprost were not protected from adenoma development compared to wild-type mice nor did they demonstrate increased activation of iloprost signaling pathways. Our results established that iloprost requires FZD9in vivo for lung cancer chemoprevention. This work represents a critical advancement in defining iloprost’s chemopreventive mechanisms and identifies a potential response marker for future clinical trials.
Subject areas: Biological sciences, Biochemistry, Cancer
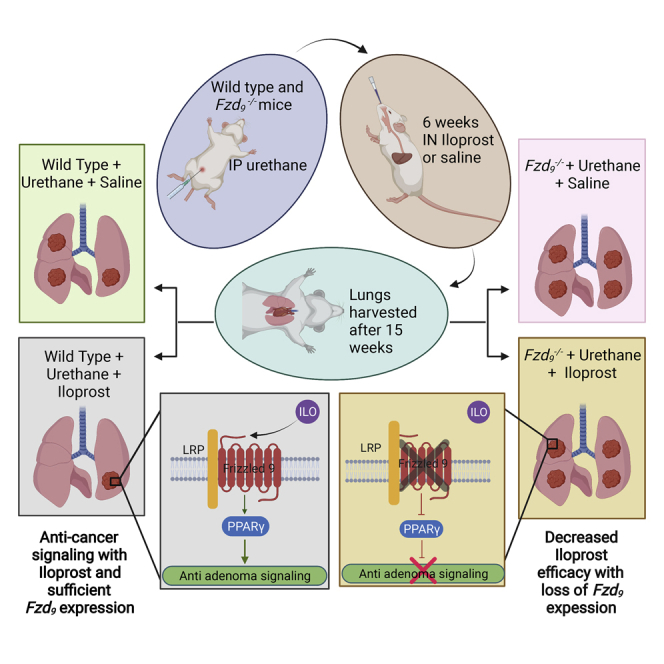
Graphical abstract
Highlights
-
•
Loss of Frizzled 9 reduces the lung cancer chemopreventive effect of iloprost in mice
-
•
Lung cancer prevention by prostacyclin synthase in mice does not require Frizzled 9
-
•
Activation of iloprost downstream targets is reduced with loss of Frizzled 9
Biological sciences; Biochemistry; Cancer
Introduction
Iloprost is a prostacyclin analogue and an FDA approved pulmonary hypertension therapy that has been repurposed as a lung cancer chemoprevention agent (clinical trials: NCT00084409, NCT02237183). In the oral iloprost clinical trial, former smokers had reduced endobronchial dysplasia with six months of iloprost compared to placebo (Keith et al., 2011). Increased understanding of iloprost’s mechanism of action is the key to moving this prevention strategy to the clinic for high-risk patients. Prostacyclins activate peroxisome proliferator- activated receptor gamma (PPARG) through the lone prostacyclin receptor IP and PPARG transgenic mice develop fewer lung tumors after urethane exposure compared to wild-type mice, an effect that is not enhanced by treatment with iloprost (Nemenoff et al., 2008). When IP null mice are crossed with lung epithelial cell-specific prostacyclin synthase overexpression (PgisTg) mice, they are still protected from lung cancer (Nemenoff et al., 2008). Because an alternative to IP is involved in prostacyclin protection in these models, Frizzled9 (FZD9) was hypothesized to be involved because of its role in similar signaling through PPARG. FZD9 is a seven-transmembrane domain G protein-coupled receptor that binds WNT7A in the lung and signals to PPARG, leading to normal lung epithelial maintenance (Winn et al., 2005, 2006). This contrasts with most WNT/FZD binding that activates oncogenic β-catenin signaling, where, for example, WNT signaling can enhance proliferation of KRAS mutant mouse adenocarcinoma cells or where blocking WNT signaling can cause apoptosis in squamous carcinoma cells (He et al., 2004; Pacheco-Pinedo et al., 2011). WNTs and FZDs are variably expressed across different NSCLC histology cell lines, but FZD9 is rarely expressed in these lines (Winn et al., 2005). In vitro experiments demonstrate that iloprost inhibits transformed growth of NSCLC cell lines, but only in cells with FZD9 expression (Tennis et al., 2010). Knockdown of FZD9 expression in NSCLC cells blocks response to iloprost and prevents activation of PPARG (Tennis et al., 2010). Of the ten FZDs, only FZD9 activates PPARG with iloprost treatment, supporting a specific relationship between iloprost, FZD9, and PPARG (Tennis et al., 2010).
In vitro and in vivo models have demonstrated downstream effects of iloprost on markers of epithelial to mesenchymal transition (EMT). Iloprost decreases expression of snail and vimentin and increases e-cadherin and crumbs3 in a human bronchial epithelial cell line, and mice with increased levels of prostacyclin have similar expression changes (New et al., 2018). Iloprost also alters EMT gene expression in cultured dysplastic cells (New et al., 2018). Both oral and inhaled iloprost reduce multiplicity of urethane-induced adenomas and inhaled iloprost affects expression of EMT genes (Nemenoff et al., 2008; Tennis et al., 2021). With preclinical and clinical evidence for effectiveness of iloprost against premalignant lung lesions, we aim to increase understanding of mechanisms of action and potential response markers for implementation of iloprost chemoprevention in high-risk patients. Here, we present data from an in vivo model of inhaled iloprost lung cancer chemoprevention with Fzd9−/− mice confirming that FZD9 is required for activity of iloprost and reduced adenoma multiplicity.
Results
Loss of Fzd9 decreases intranasal iloprost efficacy in vivo
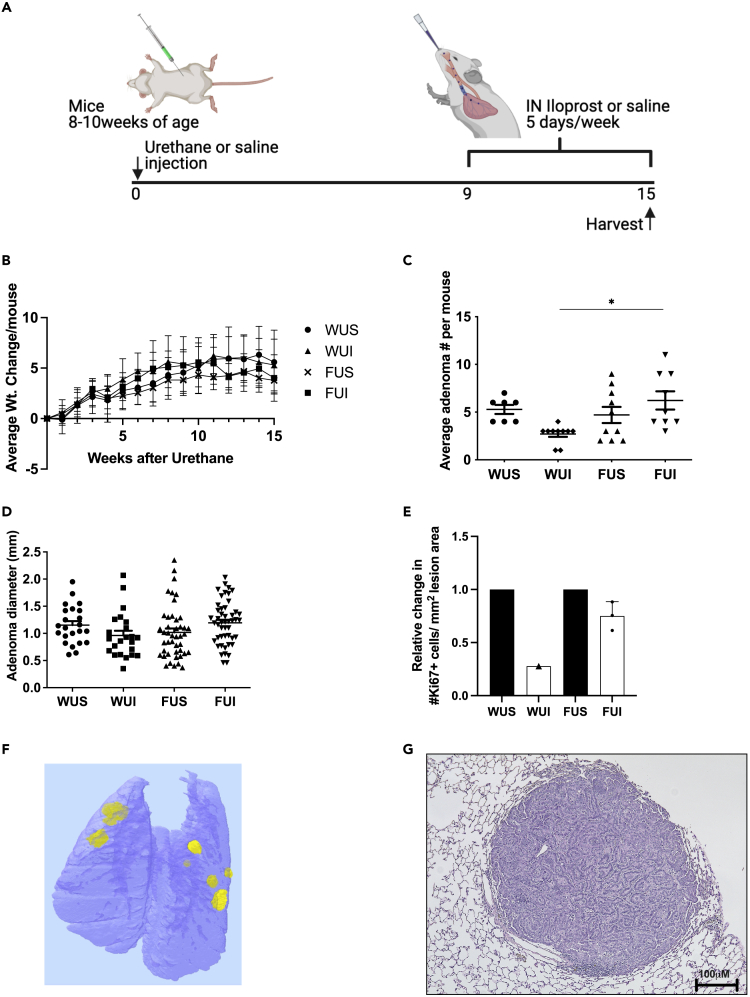
We previously identified both a dependence on FZD9 signaling for iloprost activity and a decrease in FZD9 expression following cigarette smoke exposure in a human cultured lung epithelial cell line (Tennis et al., 2010, 2016). We generated the first FVBN Fzd9−/− mouse using a CRISPR approach in collaboration with the Regional Mouse Genetics Core Facility at National Jewish Health (manuscript under review). After sequence confirmed knockout, animals were bred and genotyped to confirm transmission of the knockout to subsequent generations. To further analyze the effect of Fzd9 expression on iloprost chemoprevention treatment, Fzd9−/− mice and wild-type (WT) mice were administered a single dose of urethane and after 9 weeks, treated with intranasal iloprost 5 days/week for 6 weeks (Figure 1A). After 15 weeks, no group of mice had a significant difference in weight (Figure 1B). WT mice treated with intranasal iloprost in this study had a 50% decrease in adenoma multiplicity compared to the wild-type control group (Figure 1C) (though not significant in this experiment (p = 0.09), this has been previously reported as significant) (Nemenoff et al., 2008; Tennis et al., 2021). In contrast, Fzd9−/− mice treated with Iloprost had a slightly increased number of adenomas compared to Fzd9−/− saline controls (Figure 1C). Fzd9−/− iloprost-treated mice had a significant increase of 130% more adenomas compared to WT iloprost-treated mice (Figure 1C). There were no differences in adenoma diameter (Figure 1D). The effect of iloprost on adenoma proliferation state in each group was determined by Ki67 immunohistochemistry. Relative to their genotype saline treated controls, WT urethane mice had a larger decrease in Ki67 expression with iloprost treatment than Fzd9−/− urethane mice with iloprost treatment (Figure 1E, see also Table S1). However, these data represent only a trend, as statistical analysis could not be completed on the entire group because although three samples were collected for Ki67 staining from each group, only one WUI section had a lesion, likely because of the preventive effects of iloprost. Figure 1F shows a microCT image and Figure 1G shows an H&E stain representative of adenoma development in the 14-week urethane model in the FVB strain. Decreased lesion multiplicity and a trend toward decreased Ki67 expression demonstrate that the chemopreventive activity of iloprost is reduced with loss of Fzd9 expression in vivo.
Figure 1.
Iloprost requires Fzd9 to prevent murine adenoma development
(A) Schematic of urethane and inhaled iloprost lung cancer chemoprevention model.
(B) Change in weights of mice in the urethane and inhaled iloprost model.
(C) Average adenoma number per mouse in the urethane exposed groups.
(D) Diameter of adenomas in urethane exposed groups.
(E) Average number of Ki67 expressing cells/mm2 adenoma area per mouse exposed to urethane. Urethane mice are compared to their genotype saline control.
(F) microCT image of Fzd9−/− urethane iloprost mouse lung.
(G) H&E of a representative adenoma generated by the 15-week urethane model. WUS, wild type urethane saline; WUI, wild type urethane iloprost; FUS, Fzd9−/− urethane saline; FUI, Fzd9−/− urethane iloprost. Data are represented as mean ± SEM. One-way ANOVA with Tukey’s post hoc test was used to measure significance. ∗p < 0.05. Scale bar is 100uM.
Prostacyclin does not require Fzd9 for protective effects
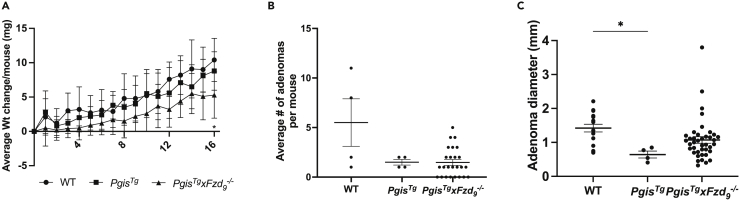
Preclinical lung cancer prevention studies using FVBN prostacyclin synthase transgenic mice (PgisTg), demonstrated that increased prostacyclin reduces lung adenoma formation in vivo, but this does not require the traditional prostacyclin receptor IP (Keith et al., 2002, 2004; Nemenoff et al., 2008). We found that iloprost requires Fzd9 for anticancer signaling in vitro and in vivo, so we were interested in whether Fzd9 was required for the in vivo protection seen in PgisTg mice. We crossed PgisTg mice with Fzd9−/− mice to generate PgisTgxFzd9−/− mice, confirmed PgisTg and Fzd9−/− by genotyping, and used these mice in the urethane model of lung adenomas (Figure 2A). After 16 weeks, PgisTgxFzd9−/− urethane mice had statistically significant lower weight than the WT urethane mice (p = 0.03), but there was no difference in weight compared to PgisTg urethane mice (Figure 2B). The PgisTgxFzd9−/− mice had no other indications of poor health upon evaluation by the facility veterinarian. Saline mice had no weight differences and no adenomas. Average adenoma multiplicity per group 16 weeks after urethane exposure was decreased in PgisTg mice compared to WT mice, but there was no difference between PgisTg g and PgisTg xFzd9−/− (Figure 2C). Adenoma size was significantly different between WT and PgisTg mice, but not different between PgisTg xFzd9−/− and other groups (Figure 2C). Prostacyclin protection from lung adenomas does not appear to rely on Fzd9 expression; however, the study was limited by lower-than-expected overall lesion numbers with urethane exposure.
Figure 2.
Transgenic prostacyclin does not require Fzd9 expression for preventive effects in the mouse lung
(A) Change in weights of mice in the urethane model.
(B) Average adenoma number per mouse in the urethane exposed groups.
(C) Diameter of adenomas in urethane exposed groups. WT, wild type; PgisTg, prostacyclin synthase transgenic; PgisTg x Fzd9−/− prostacyclin synthase transgenic with Fzd9 knockout. Data are represented as mean ± SEM. A one-way ANOVA with Tukey’s post-hoc test was used to measure significance. ∗p < 0.05.
Loss of Fzd9 reverses the effect of iloprost on downstream target expression and activity
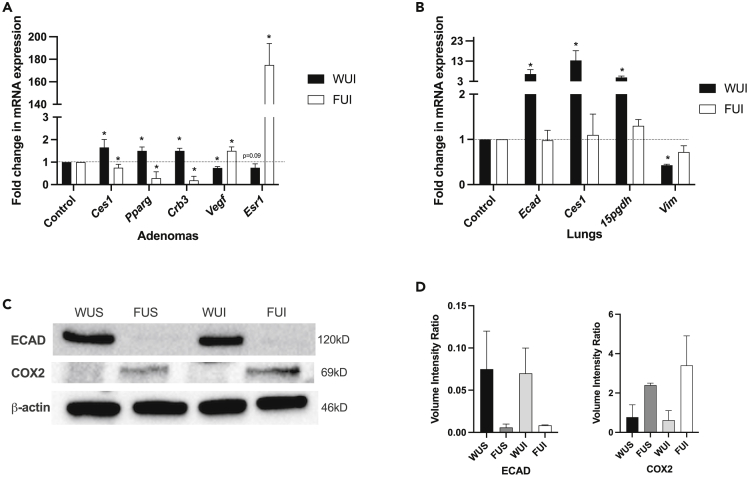
Prostacyclin reverses EMT gene expression and downstream targets of PPARG in the lung in vitro and in vivo, so we measured expression changes in these genes in the Fzd9−/− urethane/iloprost mouse study (New et al., 2018). Adenomas from urethane exposed WT and Fzd9−/− mice were dissected from lung tissue and analyzed by qPCR. In WT mice exposed to urethane, iloprost treatment led to higher levels of prostacyclin targets carboxylesterase 1 (Ces1), Pparg, and epithelial marker crumbs3 (Crb3) compared to urethane exposed saline controls (Figure 3A). WT urethane iloprost mice also expressed lower levels of lung tumor-associated genes vascular endothelial growth factor (Vegf) and estrogen receptor 1 (Esr1) compared to saline treated controls (Figure 3A). In contrast, in Fzd9−/− mice exposed to urethane, iloprost treatment led to adenomas with lower Ces1, Pparg, and Crb3 and higher Vegf and Esr1 compared to saline controls (Figure 3A). A similar trend was observed in whole lung tissue from the same mice. In lungs from WT urethane exposed mice, iloprost increased expression of e-cadherin (Ecad), Ces1, and prostacyclin target 15-pgdh and decreased expression of vimentin (Vim) (Figure 3B). In lungs from Fzd9−/− mice, iloprost had no significant effect on these genes (Figure 3B). Protein expression by western blot in Fzd9−/− urethane mice had less e-cadherin (ECAD) than WT urethane mice and no increase with iloprost treatment (Figures 3C and 3D, see also Figure S3). COX2 protein, which is decreased by PPARG signaling in the lung, was higher in Fzd9−/− urethane mice compared to WT urethane mice (Figures 3C and 3D). COX2 expression decreased slightly in WT urethane mice after iloprost treatment but increased in Fzd9−/− urethane mice treated with iloprost (Figures 3C and 3D). These alterations in downstream targets support the hypothesis that Fzd9−/− loss rescues iloprost chemopreventive activity.
Figure 3.
Loss of Fzd9in vivo prevents iloprost-induced expression changes
(A) mRNA expression in adenomas from urethane exposed WT and Fzd9−/− mice treated with iloprost or saline control.
(B) Whole lung mRNA expression from the same mice. PCR expression was normalized to RPS18, and relative change and p-value calculated relative to each genotype’s urethane/saline control. Data are represented as mean ± SEM. Student’s t-tests were used to measure significance. ∗p < 0.05.
(C) Western blots of mouse whole lung tissue for ECAD, COX2, and β-actin loading control (see Figure S4 for full blot).
(D) Quantification of western blot band density (N = 2). Data are represented as mean ± SEM. A one-way ANOVA with Tukey’s post hoc test was used to measure significance. ∗p < 0.05 WUS, wild type urethane saline; WUI, wild type urethane iloprost; FUS, Fzd9−/− urethane saline; FUI, Fzd9−/−, urethane iloprost.
To investigate whether loss of Fzd9 expression influences iloprost’s activation of PPARG, serum collected from WT and Fzd9−/− mice was added to 293T cells and its effect on a peroxisome proliferator-activated receptor gamma (PPRE) luciferase was analyzed by dual luciferase reporter assay (Edwards et al., 2019). Serum from WT urethane mice treated with iloprost stimulated significantly higher PPRE activity than the WT urethane saline treated group (Figure 4). There was no difference between the Fzd9−/− urethane iloprost and urethane saline groups (Figure 4). This demonstrates that loss of Fzd9 prevents the activation of PPARG that is characteristic of iloprost chemoprevention.
Figure 4.
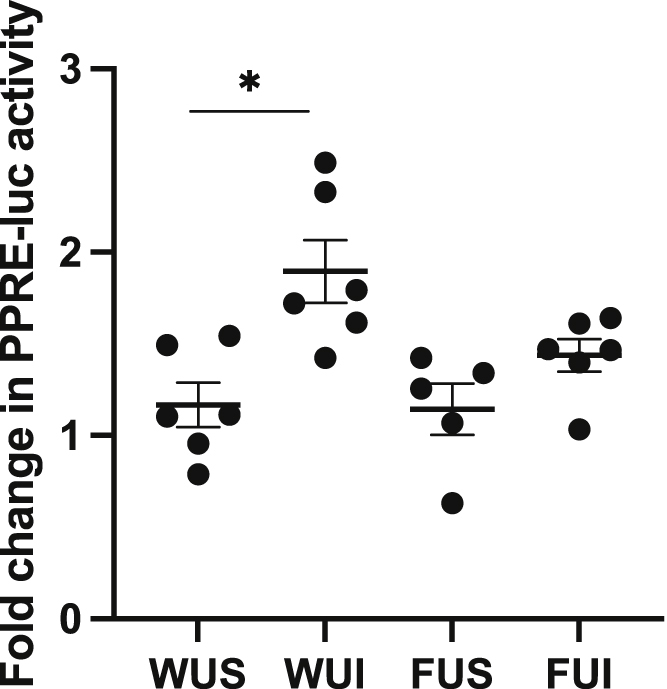
Loss of Fzd9in vivo reduces active PPARG in serum
Serum was collected from wild type urethane saline (WUS), wildtype urethane iloprost (WUI), Fzd9−/− urethane saline (FUS), and Fzd9−/− urethane iloprost (FUI) mice. 293t cells were transfected with PPRE and mouse serum was added to the media, followed by luciferase measurement at 48 h (N = 3). Fold change is relative to a luciferase control. Data are represented as mean ± SEM. One-way ANOVA with Tukey’s post hoc test was used to measure significance. ∗p < 0.05.
FZD9 is a potential receptor for iloprost
The prostacyclin receptors are seven transmembrane spanning receptors; similar to FZD9 and the prostacyclin receptor, IP is not required for the preventive effects of prostacyclin or iloprost in the lung, suggesting an alternative receptor may mediate iloprost’s effects. We are interested in whether iloprost can bind to FZD9, so we performed computational-based molecular modeling studies to predict the binding mode of iloprost in FZD9. Small molecule docking studies indicated that iloprost has a good potential to bind the ligand binding pocket of FZD9 by virtue of extensive favorable contact between the carboxylic acid moiety and residue Y277 at the bottom of the pocket. This includes strong electrostatic and hydrogen bond interactions, as well as hydrophobic interactions involving the ring system (Figure S5A). Indeed, all of the top scoring poses in the docking results occupied the same region of the protein and presented a similar interaction pattern. The solvent-corrected binding energy of the presented iloprost binding mode was −8.9 kcal/mol, likewise indicating the overall binding favorability. Based on these potential interactions, a plasmid was generated by site-directed mutagenesis with a point mutation leading to an amino acid substitution—Y277S—in the FZD9 binding pocket (Figure S5B). A549 (adenocarcinoma) and Human Bronchial Epithelial Cells (HBEC) cell lines were transfected with the mutant plasmid, and its effect on iloprost activity was evaluated using PPRE luciferase, a measure of downstream iloprost signaling. Both FZD9 and iloprost activate PPRE luciferase, and this is used as a readout of downstream activity (Smith et al., 2022; Tennis et al., 2010, 2016). Following two 10uM iloprost doses, PPRE luciferase activity increased in both A549 and HBEC cell lines. When iloprost was combined with transfection of the mutant plasmid, PPRE activity did not increase in either cell line, suggesting that the Fzd9 mutant plasmid may prevent iloprost signaling (Figures S5C and S5D). This supports further investigation into whether iloprost functions as a FZD9 ligand and activator of FZD9 downstream signaling.
Discussion
Iloprost is an FDA approved drug that was repurposed for lung cancer chemoprevention and has positive clinical trial results with minimal side effects (Keith et al., 2011). Previous in vitro work demonstrated that iloprost requires FZD9 expression to activate PPARG cancer preventive signaling (Tennis et al., 2010). Our current study presents the critical finding that in vivo, the chemopreventive effect of iloprost on pulmonary adenomas is dependent on Fzd9 expression. To investigate the role of FZD9 in iloprost activity, we compared wild type and Fzd9−/− mice with exposure to urethane and treatment with intranasal iloprost. Iloprost decreased adenoma burden in wild type mice, whereas no significant difference was observed in Fzd9−/− mice treated with iloprost. We also compared adenomas from wild type and Fzd9−/− iloprost-treated mice and saw no change in proliferation by Ki67 staining, suggesting that Fzd9 is important for an effect of iloprost on proliferation. However, no change in adenoma size with iloprost in our study suggests that inhibition of proliferation may not be the main effect of iloprost, which is supported by data from a clinical trial of iloprost lung cancer chemoprevention that found only a nonsignificant decreased in Ki67 staining (Keith et al., 2011). Expression and activity data demonstrate that iloprost decreases proliferation and EMT in lesions but is not effective without the presence of Fzd9. Studies using the PgisTg mouse in lung cancer models have demonstrated the chemoprotective effect of endogenously produced prostacyclin; however, our results demonstrated that Fzd9 is not required for this effect. This suggests that the relevance of FZD9 for prostacyclin chemoprevention is limited to iloprost. If prostacyclin does not require its traditional receptor IP or FZD9 to exert chemoprotective effects, there must be alternative receptors or pathways that stimulate preventive signaling with prostacyclin binding (Whittle et al., 2012). Additional studies will be required to investigate the protective mechanisms of prostacyclin overexpression.
Differences in preventive pathway signaling component expression after iloprost treatment confirm the importance of FZD9 for activation or suppression of iloprost targets. Changes in EMT are associated with early lesion development in the lung and EMT is often a target of chemopreventive agents (New et al., 2018; Tennis et al., 2016; Jaromy and Miller, 2021; Liu et al., 2019). Reversal of EMT gene expression is associated with iloprost treatment in vitro and in vivo (New et al., 2018). In the whole lung, we found expression of epithelial genes increased, and expression of mesenchymal genes decreased in iloprost-treated wild-type mice but not in Fzd9−/− mice. In adenomas, epithelial marker Crb3 was upregulated with iloprost in wild-type mice but not in Fzd9−/− mice. Ces1 and Pparg, which are associated with activation of prostacyclin signaling in the lung, were not increased in adenomas from Fzd9−/− mice after iloprost (New et al., 2018; Keith et al., 2004). Ces1 also increased in whole lung tissue from wild-type mice treated with iloprost but not Fzd9−/− mice. 15pgdh, a tumor suppressive enzyme that inactivates prostaglandin E2 (PGE2) and is increased by PPARG activation, was increased with iloprost in wild-type mice but not in Fzd9−/− mice (Kim et al., 2015). The decrease observed in COX2 protein in the whole lung between wild type control and wild type iloprost, though minimal, suggests that iloprost may alter inflammatory signaling. Increased COX2 in Fzd9−/− iloprost-treated mice indicates that, without Fzd9, iloprost may activate an alternative pathway that increases inflammatory signals. Decreased inflammatory signaling in biopsies of squamous lung dysplasia is associated with high-grade lesion persistence, suggesting a conflict between the ability of iloprost to reduce high-grade lesions and its potential anti-inflammatory role (Keith et al., 2011; Merrick et al., 2018). Further work will need to delineate the context and timing of effects of iloprost on inflammation during the progression of premalignant lung lesions.
Vegf, which is associated with reduced survival in lung adenocarcinoma patients, was decreased in adenomas with iloprost in wild type mice but increased in Fzd9−/− iloprost-treated mice (Jung et al., 2021; Naikoo et al., 2017). Although Esr2 is predominantly expressed in lung tissue and lung tumors, we only detected changes in Esr1 in adenomas from our mouse model (Liau et al., 2021). Esr1 was decreased in adenomas from wild-type mice treated with iloprost but significantly elevated in adenomas from Fzd9−/− mice treated with iloprost. The increased expression of Vegf and Esr1 with iloprost treatment in Fzd9−/− mice suggests that tumor promoting signals may be caused by the combination of iloprost and Fzd9 loss. Without Fzd9, iloprost may target other receptors that do not stimulate chemopreventive signaling but promote lesion development. We observed a small but nonsignificant increase in adenomas after urethane with iloprost treatment in Fzd9−/− mice compared to Fzd9−/− saline treated mice, suggesting that a larger or longer study may have detected increased lesion number in Fzd9−/− mice treated with iloprost. Overall, these expression changes paint a picture of iloprost’s reduced capacity to activate protective signaling pathways in the lung epithelium and the potential for tumor promotion without the presence of Fzd9.
Efficacy of iloprost was measured in the oral and inhaled iloprost clinical trials by bronchoscopy with biopsy at baseline and follow up. This method identifies histologic changes in lung cells and the presence of persistent dysplasia detected by bronchoscopy is associated with increased risk of developing squamous carcinoma (Merrick et al., 2016). Additional measures of iloprost response are needed to bring iloprost chemoprevention to the clinic, especially noninvasive molecular tests. Edwards et al. recently described an assay that measured the activation of PPARG in mouse and human serum (Edwards et al., 2019). We used this assay to measure activity of PPARG in serum samples from animals in our study. This experiment verified that the activation of PPARG was decreased in Fzd9−/− mice treated with iloprost compared to wild-type mice treated with iloprost. If PPARG activation can be correlated with histologic response to iloprost, this assay could inform a potential noninvasive response test for future clinical applications of iloprost. This type of assay could allow providers to monitor patients receiving iloprost chemoprevention at multiple time points to determine if they should continue treatment, reducing the potential for side effects in patients who are not benefiting from the intervention.
A greater understanding of the mechanism of activity for iloprost chemoprevention will speed its clinical application. Studies have demonstrated that FZD9 is required for iloprost activity; however, it is still unknown if they interact directly. The concept that FZDs could be direct targets for small molecule drugs has been challenged in the past. Recently, however, smoothened agonist SAG1.3 was shown to bind FZD6 to induce conformational changes in the receptor and stimulate activation and interactions in the FZD6 signaling pathway (Kozielewicz et al., 2020). This work supports our interest in defining the specific relationship between FZD9 and iloprost and suggests opportunities to target FZDs in cancer (Sompel et al., 2021). No crystal structure of FZD9 exists, but by using structures of homologous proteins, we generated an in silico molecular model for iloprost and FZD9 interaction that suggested favorable structures for binding. With knowledge of specific residues in the FZD9 binding pocket that might influence iloprost binding, we mutated FZD9 at a single residue. This mutation in the potential binding pocket decreased iloprost’s ability to activate PPARG, suggesting interaction with FZD9 may be required for iloprost’s chemopreventive function. These data support further investigation of the hypothesis that iloprost agonizes FZD9 and encourages additional in vitro and structural studies to explore this critical piece of iloprost’s mechanism.
In summary, substantial in vitro and now in vivo data support the essential role of FZD9 in the activity of iloprost in lung epithelium, adding to our understanding of iloprost’s chemopreventive mechanisms. Because it is required for iloprost activity, FZD9 expression may be a mechanistically relevant marker for predicting response to iloprost. Further testing of our ex vivo PPARG activity assay may provide the first noninvasive measure of iloprost activity in patients. Chemoprevention is an essential tool for reducing the burden of cancer in high-risk populations and this study adds to mounting evidence for the use of iloprost as lung cancer chemoprevention in former smokers and suggests new directions for future research.
Limitations of the study
Models using chemical carcinogens to induce cancer in mice are essential for chemoprevention studies but do have some challenges. Experimental groups often have expected but unpredictable death, which can lead to uneven group sizes to include all surviving animals in final analyses. We can try to compensate for this with larger group sizes for mice with unknown reactions to new treatments. We included larger groups for the Fzd9−/− mice, because the combination of urethane and iloprost was untested in this genotype and we wanted to ensure sufficient animals survived for statistical analysis of lesion numbers. Urethane in wild-type animals has been tested repeatedly, so we could use smaller numbers for control animals, which is important for addressing the three R’s of animal research. Also because of unpredictability in carcinogen models, the wild type iloprost group in this study had a nonsignificant decrease in adenoma multiplicity (p = 0.09). Reduced premalignant lesions with iloprost treatment have achieved statistical significance in previous studies (Tennis et al., 2021; Nemenoff et al., 2008). For proliferation analysis of adenomas with Ki67 staining, we collected our standard of three fixed specimens per group, but statistical analysis was limited by the presence of a lesion in only one of the wild type urethane iloprost lungs selected for fixation. With no crystal structure of FZD9 available for our in silico binding analysis, we had to use structures of homologous proteins to generate a molecular model for a possible iloprost and FZD9 interaction (Figure S5A). The utility of existing structures of other FZDs is limited because of low-resolution and unsuitability for docking analysis, so using the SMO structure is the best approach at this time.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| ECAD (CD324) | Protein Tech | #RRID: AB_10697811, #20847-1-AP |
| COX2 (PTGS2) | Protein Tech | #RRID: AB_2881731, #663511Ig |
| Ki67 | Abcam | #RRID: AB_443209, #15580 |
| β-actin | BioRad | #RRID: AB_2571580, #MCA5775GA |
| Precision Protein Streptactin-HRP | BioRad | #1610380 |
| Immun-Star Goat Anti-Mouse (GAM)-HRP Conjugate | BioRad | #1705047 |
| Immun-Star Goat Anti-Rabbit (GAR)-HRP Conjugate | Biorad | #1705046 |
| Protein Precision Ladder | BioRad | #1610375 |
| Chemicals, peptides, and recombinant proteins | ||
| Urethane | Sigma-Aldrich | #943-50g, Lot #WXBC8654V |
| Iloprost | Cayman Chemicals | #18215 |
| Methyl Acetate | Sigma Aldrich | 45999-200ML-F |
| 2-mercaptoethanol | ThermoFisher Scientific | #21985023 |
| 10X Tris-Glycine Buffer | BioRad | #1610771 |
| Ponceau S | Fisher Bioreagents | #BP103-10 |
| Non-Fat Dry Milk | LabScientific | #M0841 |
| 2X Laemmeli Buffer | BioRad | #1610737 |
| Clarity Western ECL substrate | Biorad | #1705060 |
| Diva Decloaker RTU | Biocare Medical | #DV2004G1 |
| Background Punisher | Biocare medical | #BP974M |
| 2.5% Normal Horse Serum | Vector laboratories | S-2012-50 |
| TransIT-X2 Dynamic Delivery System | Mirus | #MIR6004 |
| GoTaq | Promega | #M7132 |
| PierceTM RIPA buffer | Thermo Scientific | #89900 |
| Tween-20 | Fisher BioReagents | #BP337-500 |
| Sodium Dodecyl Sulfate | Fisher BioReagents | #BP166-500 |
| Tris | Fisher BioReagents | #BP152-1 |
| Glycine Electrophoresis grade | MP Biomedicals, LLC | #808822 |
| RNA Later | Sigma Life Sciences | #R0901 |
| Red Blood Cell Lysing Buffer Hybri-Max | Sigma Life Sciences | #R7757 |
| Critical commercial assays | ||
| Dual-Luciferase Reporter Assay | Promega | #E1960 |
| PierceTM BCA Assay | Thermo Scientific | #23225 |
| Betazoid Dab Chromagen Kit | Fisher Scientific | #BDB2004L |
| Sso Advanced Universal SYBR | BioRad | #1725274 |
| High Capacity cDNA Reverse Transcription Kit | Fisher Scientific | #43-688-13 |
| Vectastain Elite ABC anti-mouse/Rabbit RTU HRP immunodetection kit | Novus Biologicals | PK-7200 |
| Experimental models: Cell lines | ||
| HBEC2KT and HBEC3KT | Dr. John Minna | RRID: CVCL_X491, #CRL-4051 |
| A549 | ATCC | RRID: CVCL_0023, #CRM-CCL-185 |
| Experimental models: Organisms/strains | ||
| FVB/N Fzd9−/− | National Jewish Hospital Regional Mouse Genomics Core Facility | N/A |
| FVB/N PgisTg+ | University of Colorado | N/A |
| FVB/N PgisTg+xFzd9−/− | Rocky Mountain Regional VA Medical Center Veterinary Medical Unit | N/A |
| Recombinant DNA | ||
| PPRE | Addgene | RRID: Addgene_1015, #1050 |
| Renilla | Promega | #E2231 |
| Y277S_FZD9 C-term his tag | Genescript | Lot: U279VGC020-1/Q87680 |
| Oligonucleotides | ||
| Ces1 - Mouse | BioRad | qMmuCID0026391 |
| 15pgdh - Mouse | BioRad | qMmuCID0024412 |
| Pparg - Mouse | BioRad | qMmuCID0018821 |
| Crb3- Mouse | BioRad | qMmuCID0008889 |
| Vegf – Mouse | BioRad | qMmuCED0040260 |
| Esr1 – Mouse | BioRad | qMmuCED0044294 |
| Rps18 - Mouse | Biorad | qMmuCED0045430 |
| Vim – Mouse | BioRad | qMmuCED0046651 |
| Ecad - Mouse | BioRad | qMmuCED0044197 |
| Fzd9 Forward Primer (5′-TGCACATACAGATAGACAAGC-3′) | Integrated DNA technologies | #207267468 |
| Fzd9 Reverse Knockout Primer (5′-GCCAGCCCGGACCTTATTTG-3′) | Integrated DNA technologies | #207267470 |
| Fzd9 Reverse Wildtype Primer (5′-CACTCACTGTAGCTGTCTTCAG-3′) | Integrated DNA technologies | #207267469 |
|
PgisTg Forward Primer (Small Ts) (5′-TGTGAAGGAACCTTACTTCTGTGG-3′) |
Integrated DNA technologies | #287949701 |
|
PgisTg Reverse Transgenic Primer (Small Ta) (5′-TGGACAAACCACAACTAGAATGCA-3′) |
Integrated DNA technologies | #287949702 |
| Software and algorithms | ||
| Graphpad Prism version 9.0.2 | Graphad Prism | RRID: SCR_002798, version 9.0.2 |
| Bio-Rad ChemiDoc Imager | BioRad | RRID: SCR_019037 |
| MicroCT- ctAN, CTvol (VGStudio) | Bruker | RRID; SCR_021338, RRID: SCR_017997 |
| CFX Maestro | BioRad | RRID: SCR_018064, #12013758 |
| FlashGelTM System | Lonza | #57062 |
| Other | ||
| RPMI | Thermo Scientific | #11875093 |
| Keratinocyte-SFM | Thermo Scientific | #17005042 |
| Opti-MEM | Thermo Scientific | #31985062 |
| 2.2% precast agarose Gels | Lonza | #57032 |
| Mini-PROTEAN Tris/Tricine precast gels | BioRad | #4568094 |
Resource availability
Lead contact
Further questions should be directed to the lead contact, Meredith Tennis (Meredith.tennis@cuanschutz.edu).
Materials availability
-
•
This study did not generate new or unique reagents.
Experimental model and subject details
Animals: Mice
Fzd9−/− mice were developed by the Regional Mouse Genetics Core Facility at National Jewish Health and the University of Colorado. Guide design was done using CRISPR and the Broad Institute sgRNA Design software, both of which have been refined to better identify off target events. Results from each software were compared, and the guide which performed best using both algorithms was chosen. Guide activity was verified by incubating guide RNA and Cas9 protein with a PCR product containing the target sequence and comparing the ratio of cut to uncut PCR product. Zygotes were injected with guide RNA(s), Cas9, and DNA template if appropriate. Zygotes were then transferred into pseudopregnant recipients. F0 pups were genotyped by PCR using primers outside the region to be modified to identify putative positive founders. These mice were then bred to FVB/N wildtype mice. Once germline transmission was established, F1 mice were sequenced to confirm the modification and intercrossed to generate homozygous knockout mice. Two genotyping assays were established to identify Fzd9−/− mice for experiments that include a common forward primer (5-TGCACATACAGATAGACAAGC-3′) and separate wildtype reverse primer (5-CACTCACTGTAGCTGTCTTCAG-3′) and knockout reverse primer (5-GCCAGCCCGGACCTTATTTG-3′). Fzd9−/− mice were transferred to the Rocky Mountain Regional Veterans Affairs Medical Veterinary Care Unit (RMRVAMC VMU) and animals for experiments were generated by breeding Fzd9−/− males with Fzd9−/− females to generate 100% knockout litters.
FVBN prostacyclin synthase transgenic (PgisTg) mice were developed using a construct containing the human surfactant protein-C (SP-C) promoter and full-length rat Pgis cDNA (Nemenoff et al., 2008). PgisTg heterozygote mice are bred with FVB/N wildtype mice to generate the heterozygotes used in experiments. To generate PgisTg xFzd9−/− mice, PGISTg (het) mice were bred with Fzd9−/− mice, resulting in 50% PgisTg (het)xFzd9−/+ mice. PgisTg (het)xFzd9−/+ mice were then crossed, resulting in 50% PgisTg (het) xFzd9−/−. Subsequent PgisTg (het)xFzd9−/− animals were generated by breeding PgisTg (het)xFzd9−/− mice with Fzd9−/− mice, for 50% PgisTg (het)xFzd9−/− litters. All animals for experiments were bred at the RVRVAMC VMU. All procedures were performed with IACUC approved protocols at the RVRMAF VMU.
Mouse genotyping
To confirm continued genotypes in mice used for experiments, genomic DNA was extracted from ear clips of each mouse with a DNeasy Blood & Tissue kit (Qiagen). The DNA was amplified by RT-PCR with forward and reverse DNA primers and GoTaq Green Master mix (Promega) on a CFX96 Touch (Biorad). To confirm Fzd9−/− genotype, a knockout gel and wildtype gel were run. The same forward primer was used for both reactions (5′-TGCACATACAGATAGACAAGC-3′), while the knockout reverse primer (5′-GCCAGCCCGGACCTTATTTG-3′) identified a sequence left by the CRISPR manipulation and the wildtype reverse primer (5′-CACTCACTGTAGCTGTCTTCAG-3′) identified WT Fzd9. PgisTg genotyping used the published protocol with forward primer (5′-TGGACAAACCACAACTAGAAT-3′) and reverse primer (5′-TGTGAAGGAACCTTACTTCTGTGG-3′) (Nemenoff et al., 2008). PCR product was analyzed by electrophoresis in precast 2.2% agarose gels at 275V (Lonza). Gels were imaged with the FlashGel™ Dock and the FlashGel™ camera using the FlashGel™ Camera and Capture software.
Fzd9−/− inhaled iloprost in vivo study
Male and female mice (8 weeks old) were housed in a pathogen-free facility in the RMRVAMC VMU in four groups: wild type saline, wild type urethane, Fzd9−/− saline, and Fzd9−/− urethane. Nine mice were included in each wild type group and 12 in each Fzd9−/− group. Mice were injected IP with 100 μL of 1 mg urethane/g body weight dissolved in the 0.9% saline vehicle or 100ul saline vehicle only (Fritz et al., 2014). Mice were weighed daily for 7 days after urethane injection and weekly for the remainder of the experiment. Nine weeks after urethane exposure, iloprost (Cayman Chemicals) or the saline vehicle was administered intranasally at a dose of 5 μg/mouse/100 μL (50 μL per nare with a P200 pipette), five days/week for 6 weeks (Figure 1A). Mice were anesthetized with isofluorane, half the iloprost was delivered to one nare, followed by a pause to observe inhalation, and then the second half of the dose was delivered to the second nare (Lammi et al., 2016). This iloprost treatment regimen was used because small pre-malignant adenomas are detectable in mice after 6 weeks of urethane exposure and mice can tolerate iloprost intranasal treatment (Lammi et al., 2016; Fritz et al., 2014). Following the last iloprost treatment at 15 weeks post urethane, mice were sacrificed by a lethal dose of Fatal Plus. Serum was collected by cardiac puncture. Lesions were dissected from surrounding lung tissue and diameters measured with digital calipers. Surrounding lung tissue was saved for RNA extraction. Studies were carried out in accordance with the recommendations in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the RMRVAMC Animal Care and Use Committee.
PgisTg x Fzd9−/− urethane in vivo study
Females and males (8 weeks old) were housed in a pathogen-free facility in the RMRVAMC VMU in six groups: wild type saline, wild type urethane, PgisTg saline, PgisTg urethane, PgisTgxFzd9−/− saline, and PgisTgx Fzd9−/− urethane. The Pgis transgene leads to increased levels of prostacyclin and development of the model has been published (Keith et al., 2002). Mice received an IP injection of 100uL of 1 mg urethane/g body weight dissolved in the 0.9% saline vehicle or saline vehicle alone. Mice were weighted daily for 7 days after urethane injection and weekly for the remainder of the experiment. 16 weeks after the urethane injection, the mice were sacrificed by a lethal dose of Fatal Plus. Lesions were dissected from surrounding lung tissue using a dissecting microscope and diameter measured with digital calipers. Studies were carried out in accordance with the recommendations in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the RMRVAMC Animal Care and Use Committee.
Cells
Non-transformed human bronchial epithelial cells (HBEC3KT (male) and HBEC2KT (female)) (gifts from the lab of Dr. John Minna, UT Southwestern) were cultured in Keratinocyte Serum Free Medium (GIBCO). All HBEC cell cultures were grown and handled in a dedicated incubator. A549 human lung cancer cell line (male, purchased from the Cell Technologies shared resource at the University of Colorado Cancer Center), was cultured in RPMI (GIBCO) with 10% fetal bovine serum. 293T human embryonic kidney cell line (ATCC) was cultured in DMEM (GIBCO) with 10% fetal bovine serum. All cell lines were carried at 37°C in a humidified 5% CO2 incubator and passaged twice per week. All in vitro studies were approved by the University of Colorado Institutional Biosafety Committee.
Method details
MicroCT imaging
Three mice/group were imaged immediately before harvest. Mice were anesthetized with isoflurane and imaged in Bruker 1276 Skyscan microCT by placing mice prone in a heated mouse bed with breathing cone and supplied with oxygen and isoflurane to maintain anesthesia. Image parameters were as follows: X-ray tube voltage of 70 kV, current of 200 μA, pixel size of 40.79 μm, exposure time of 167 ms with 0.5 mm aluminum filter and 0.7 rotation stepping. Using the computer program CTan by Bruker, the CT scan was turned into a binary image. The threshold for what was considered tumor and vessel, which are approximately the same density vs what is considered aerated lung tissue is chosen by the person performing the data analysis. This threshold is chosen based on closely matching the binary image of the scan to the actual scan. The same threshold was then applied to each scan. With CTan, an automatic series of computer operations separates the aerated lung tissue from the rest of the mouse. The lungs are then selected as a region of interest and the tumors and vessels are selected from this lung region. A 3D reconstruction was then made of the vessels and tumors and tumors were identified manually and differentiated from vessels. Vessels were removed through a combination of manual deletion and automatic computer operations. Data analysis was then performed to obtain tumor count and tumor volume. The 3D models were then generated in CTan and visualized in the program CTvol, also by Bruker.
Reverse transcriptase-quantitative PCR
Mouse lung tissue and lesions were collected in RNA Later (Qiagen) at the time of harvest. qPCR Prime PCR Assays (Bio-Rad) for mouse included: Crb3, Vegf, Esr1, 15pgdh, Ecad, Ces1, Pparg, Vim, and Rps18. qPCR was conducted using standard protocol for Sso Advanced SYBR Green Master Mix (Bio-Rad) on a CFX96 Touch (Bio-Rad).
Western blots
Protein was extracted using Pierce RIPA buffer (Thermo Scientific) and protease inhibitor (Thermo Scientific). The samples were quantified by BCA assay (Thermo Scientific). 20ug of protein was mixed with 2X laemmeli buffer and BME for a 1:1 sample, denatured at 56°C for five minutes and electrophoresed and transferred with a molecular marker according to the manufacturer’s protocol (Mini-Protean Tetra Cell, BioRad). Membranes were stained with 0.1% Ponceau S to confirm sufficient protein transfer. The membrane was blocked in 10 mL of 5% Non-Fat dry milk in 1X TBS-T for 1 hour, followed by three 15-minute washes with 1X TBS-T. Blots were incubated in primary antibody (COX2 1:500 Protein tech #663511Ig, ECAD 1:3,000 Protein Tech #20874-1-AP) overnight at 4°C. Membranes washed and incubated in diluted 1:5,000 secondary anti-rabbit IgG antibody (BioRad) and 1:5000 StrepTactin HRP conjugate (Biorad) for 1 hour. Membranes were washed and exposed to 2 mL of Clarity Western ECL substrate (Biorad) for five minutes. Images were captured and quantified with a ChemiDoc imager (BioRad). To confirm protein loading, the membrane was stripped, rinsed, blocked, and probed for B-actin (Biorad #MCA5775GA).
Immunohistochemistry
5 μm lung sections from urethane, iloprost, and saline groups were deparaffinized, blocked with 0.3% hydrogen peroxide, and antigen retrieval performed in boiling Diva Decloaker (Biocare Medical, DV2004G1) under pressure for 5 minutes. Sections were blocked with Background Punisher (Biocare Medical BP974M) and 2.5% Normal Horse serum (Vector Labs S-2012-50). Sections were incubated in Ki67 primary Ab (1:2000 dilution, Abcam 15580) for 1 hour at room temperature, followed by anti-rabbit universal antibody for 30 minutes and ABC reagent for 30 minutes at room temperature (Vector Laboratories PK-7200). Ki67+ nuclei were detected using Betazoid DAB chromagen kit (Vector Laboratories BDB2004). Tumor area was measured and Ki67-positive nuclei/mm2 were counted in each tumor. Replicate blinded counts were conducted. The Ki67+ nuclei/mm2 tumor area for each tumor was averaged by group (Dwyer-Nield et al., 2017). H&E stains were done on 5 μm lung sections from urethane, iloprost, and saline groups and were used to confirm adenoma presence and structure.
Serum PPARG activity assay
Serum was collected by intracardiac puncture immediately after euthanizing each mouse. 293t cells were seeded at 2,000 cells/well in a 96 well plate. After twenty-four hours, the cells were transfected with 45 ng PPARG response element firefly luciferase (PPRE was a gift from Bruce Spiegelman; Addgene plasmid #1015) and 5 ng renilla control reporter vector (Promega) using TransiIT-X2 transfection reagent (Mirus Bio) and Opti-MEM media (Thermo Fisher) per the manufacturer’s protocol. Cells were treated with 10ul mouse serum at 24 hours and 48 hours post transfection (Edwards et al., 2019). Serum treatments were conducted in triplicate and included empty and mock transfection controls. Luciferase activity was measured after 48 hours using the Dual-Luciferase Reporter assay kit (Promega) on the Glomax instrument (Promega).
In silico binding prediction
The homology model of human FZD9 was generated using Biovia Discovery Studio 2018 (Biovia, Inc) using the structure of the human smoothened receptor as a template (Wang et al., 2013). YASARA Structure 19.1 (Yasara Biosciences GmbH, Vienna, Austria) was used to evaluate the quality of the resulting homology models, which were subjected to 1 ns of explicit solvent-based MD simulation utilizing the YASARA2 force field (Krieger et al., 2002, 2009). The snapshots of the MD simulation (taken every 25 ps) were evaluated using the WHAT_IF and WHAT_CHECK structure validation tools to quantitatively evaluate the overall quality of each predicted structure (Hooft et al., 1996; Vriend, 1990). The highest scoring structure snapshot had a Z-score of −0.15, indicating the homology model had a high degree of compatibility with the protein sequence and was of similar quality to that of the average of ∼30,000 high-resolution crystal structures contained in the Protein Data Bank. This structure was used for the subsequent docking studies. The binding mode of Iloprost was predicted using the flexible docking protocol within Discovery Studio coupled with binding energy analysis utilizing the Generalized Born with Simple Switching (GBSW) implicit solvent model (Koska et al., 2008; Feig et al., 2004). The binding site sphere was set large enough to encompass the vast majority of the solvent accessible surface of the protein. The final ranking of the docked poses was performed via consensus scoring, combining the predicted binding energy with overall number of favorable intermolecular contacts and the Jain,29 PLP2,30 and Ludi331 scoring functions. Figures were rendered using Lightwave 2018 (Lightwave3D Group) and Photoshop CC 2018 (Adobe Systems, Inc.).
Mutant plasmid transfections
A human FZD9 mutant plasmid with a serine substitution for tyrosine at amino acid 277 (Y277S) was generated by GenScript (Piscataway, NJ) using site-directed mutagenesis. A549 and HBEC cells were transfected with 45 ng PPRE-luciferase, 5 ng renilla control reporter vector, plasmid control vector, and/or FZD9 Y277S plasmid using TransIT-X2 transfection reagent and Opti-MEM media per the manufacturer’s protocol. Cells were treated at 24 and 48 hours with 10uM of Iloprost or methyl acetate vehicle and analyzed for PPRE luciferase activity as described above.
Quantification and statistical analysis
Fzd9−/− adenoma counts
The number of adenomas per mouse in urethane exposed wildtype or Fzd9−/− mice treated with iloprost or a saline control were averaged for each group (Figure 1C). Statistical analysis one-way ANOVA with Tukey post-hoc analysis was conducted using GraphPad Prism (RRID: SCR_002798, version 9.0.2). ∗p < 0.05 Error bars represent SEM for each group.
Mice
WUS: n = 7
WUI: n = 10
FUS: n = 10
FUI: n = 9
PgisTgx Fzd9−/− adenoma counts
The number of adenomas in urethane treated wildtype, PgisTg, and PgisTgx Fzd9−/− were averaged for each group (Figure 2B). Statistical analysis using a one-way ANOVA with Tukey post-hoc analysis was conducted using GraphPad Prism. ∗p < 0.05 Error bars represent SEM for each group.
Mice
WT: n = 4
PgisTg: n = 4
PgisTgxFzd9−/−: n = 25
Adenoma diameter measurements
The diameter of each adenoma was measured with a digital caliper (Figures 1D and 2C). One-way ANOVAs with Tukey post-hoc analysis were performed in Graphpad Prism to determine statistical significance between groups in each experiment. ∗p < 0.05 Error bars represent SEM for each group.
Inhaled Iloprost Fzd9−/− Study:
WUS: n = 23
WUI: n = 23
FUS: n = 44
FUI: n = 48
PgisTgxFzd9−/− Study:
WT: n = 17
PgisTg: n=4
PgisTgxFzd9−/−: n = 19
Average weight change in mouse studies
The weight change per mouse was measured weekly for each group until the end of the study (Figures 1B and 2A). The weight changes for each mouse were averaged by group to show the average weight change every week per group. One-way ANOVAs with Tukey pos-hoc analysis in Graph-Pad Prism was performed to determine significance between groups. ∗p < 0.05 Error bars represent SEM.
Mice.
Inhaled Iloprost Fzd9−/− Study:
WUS: n = 9
WUI: n = 9
FUS: n = 12
FUI: n = 14
PgisTgxFzd9−/− Study:
WT: n = 9
PgisTg: n = 9
PgisTgx Fzd9−/−: n = 29
Reverse transcriptase-quantitative PCR
All gene expression data was normalized to the reference gene Rps18 and fold changes were calculated using the 2−ΔΔCt method. PCR analysis was conducted in triplicate and statistical analysis was done by Student’s t-test comparing the urethane/iloprost mice to their genotype urethane/saline control (Figures 3A and 3B). ∗p < 0.05 For extraction of adenoma RNA, all lesions dissected from an individual mouse were pooled. Error bars represent SEM.
Adenoma qPCR:
WUI: n = 5
FUI: n = 8
Lung qPCR
WUI: n = 12
FUI: n = 8
Western Blot quantitative analysis
Quantitative analysis was performed by creating a ratio between the band intensity for the protein of interest and the sample’s corresponding β-actin band intensity. Blots were repeated in duplicate and band intensity ratios were averaged (Figure 3D). A one-way ANOVA with Tukey post-hoc analysis in GraphPad Prism was used to measure significance. ∗p < 0.05 Error bars represent SEM.
Ki67 proliferative index
Lesion area was measured and Ki67-positive nuclei/mm2 were counted in each lesion. Replicate blinded counts were conducted. The Ki67+ nuclei/mm2 lesion area was averaged by group (Dwyer-Nield et al., 2017). A one-way ANOVA with Tukey post-hoc analysis in GraphPad Prism was used to measure significance (Figure 1E). ∗p < 0.05Error bars represent SEM.
Adenomas stained
WUS: n = 4
WUI: n = 1
FUS: n = 3
FUI: n = 4
Serum PPARG activity assay
PPRE-luciferase activity was normalized to renilla activity and analyzed relative to the wild type saline group. Each serum sample was performed in triplicate and averaged. N = 3 for each sample group. Significance was assessed by one-way ANOVA with Tukey post-hoc analysis in GraphPad Prism (Figure 4). ∗p < 0.05 Error bars represent SEM.
Site directed mutagenesis
PPRE-luciferase activity was normalized to renilla activity and analyzed relative to empty controls. Each transfection was performed with technical triplicates and averaged. N = 3 for each sample group. Significance was assessed by one-way ANOVA with Tukey post-hoc analysis in GraphPad Prism (Figures S2C and S2D). ∗p < 0.05 Error bars represent SEM.
Acknowledgments
This work was supported by the National Cancer Institute (R01CA214531) (MT), an NIH CURE Diversity Supplement (AS), and the University of Colorado Cancer Center Cell Technologies shared resource funded by the National Cancer Institute through a Cancer Center Support Grant (P30CA046934).)
Author contributions
Conceptualization, M.T., L.N., and R.K.; Methodology, J.S., B.Z., J.G., K.T., and K.K.; Investigation, K.S., A.S., A.E., L.N., and D.S.; Funding Acquisition, M.T.; Project Administration, K.S., E.A., and M.T.; Supervision, M.T. and L.N.; Writing – Original Draft, KS and MT.; Writing – Review & Editing, K.S., E.A., M.T., L.N., R.K., and D.S.
Declaration of interests
The authors declare no competing interests.
Published: June 17, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104442.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This data does not report original code.
-
•
Any information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Dwyer-Nield L., Hickey G.A., Friedman M., Choo K., Mcarthur D.G., Tennis M.A., New M.L., Geraci M., Keith R.L. The second-generation PGI2 analogue treprostinil fails to chemoprevent tumors in a murine lung adenocarcinoma model. Cancer Prev. Res. 2017;10:671–679. doi: 10.1158/1940-6207.capr-17-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards L., Watt J., Webster T.F., Schlezinger J.J. Assessment of total, ligand-induced peroxisome proliferator activated receptor gamma ligand activity in serum. Environ. Health. 2019;18:45. doi: 10.1186/s12940-019-0486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig M., Onufriev A., Lee M.S., Im W., Case D.A., Brooks C.L.,3R.D. Performance comparison of generalized born and Poisson methods in the calculation of electrostatic solvation energies for protein structures. J. Comput. Chem. 2004;25:265–284. doi: 10.1002/jcc.10378. [DOI] [PubMed] [Google Scholar]
- Fritz J.M., Tennis M.A., Orlicky D.J., Lin H., Ju C., Redente E.F., Choo K.S., Staab T.A., Bouchard R.J., Merrick D.T., et al. Depletion of tumor-associated macrophages slows the growth of chemically induced mouse lung adenocarcinomas. Front. Immunol. 2014;5:587. doi: 10.3389/fimmu.2014.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., You L., Uematsu K., Xu Z., Lee A.Y., Matsangou M., Mccormick F., Jablons D.M. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6:7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooft R.W.W., Vriend G., Sander C., Abola E.E. Errors in protein structures. Nature. 1996;381:272. doi: 10.1038/381272a0. [DOI] [PubMed] [Google Scholar]
- Jaromy M., Miller J.D. Pharmacologic mechanisms underlying antidiabetic drug metformin's chemopreventive effect against colorectal cancer. Eur. J. Pharmacol. 2021;897:173956. doi: 10.1016/j.ejphar.2021.173956. [DOI] [PubMed] [Google Scholar]
- Jung W.Y., Min K.W., Oh Y.H. Increased VEGF-A in solid type of lung adenocarcinoma reduces the patients' survival. Sci. Rep. 2021;11:1321. doi: 10.1038/s41598-020-79907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith R.L., Blatchford P.J., Kittelson J., Minna J.D., Kelly K., Massion P.P., Franklin W.A., Mao J., Wilson D.O., Merrick D.T., et al. Oral iloprost improves endobronchial dysplasia in former smokers. Cancer Prev. Res. 2011;4:793–802. doi: 10.1158/1940-6207.capr-11-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith R.L., Miller Y.E., Hoshikawa Y., Moore M.D., Gesell T.L., Gao B., Malkinson A.M., Golpon H.A., Nemenoff R.A., Geraci M.W. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 2002;62:734–740. [PubMed] [Google Scholar]
- Keith R.L., Miller Y.E., Hudish T.M., Girod C.E., Sotto-Santiago S., Franklin W.A., Nemenoff R.A., March T.H., Nana-Sinkam S.P., Geraci M.W. Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Res. 2004;64:5897–5904. doi: 10.1158/0008-5472.can-04-1070. [DOI] [PubMed] [Google Scholar]
- Kim J., Sato M., Choi J.W., Kim H.W., Yeh B.I., Larsen J.E., Minna J.D., Cha J.H., Jeong Y. Nuclear receptor expression and function in human lung cancer pathogenesis. PLoS One. 2015;10:e0134842. doi: 10.1371/journal.pone.0134842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koska J., Spassov V.Z., Maynard A.J., Yan L., Austin N., Flook P.K., Venkatachalam C.M. Fully automated molecular mechanics based induced fit protein-ligand docking method. J. Chem. Inf. Model. 2008;48:1965–1973. doi: 10.1021/ci800081s. [DOI] [PubMed] [Google Scholar]
- Kozielewicz P., Turku A., Bowin C.F., Petersen J., Valnohova J., Canizal M.C.A., Ono Y., Inoue A., Hoffmann C., Schulte G. Structural insight into small molecule action on Frizzleds. Nat. Commun. 2020;11:414. doi: 10.1038/s41467-019-14149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger E., Joo K., Lee J., Lee J., Raman S., Thompson J., Tyka M., Baker D., Karplus K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: four approaches that performed well in CASP8. Proteins. 2009;77(Suppl 9):114–122. doi: 10.1002/prot.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger E., Koraimann G., Vriend G. Increasing the precision of comparative models with YASARA NOVA--a self-parameterizing force field. Proteins. 2002;47:393–402. doi: 10.1002/prot.10104. [DOI] [PubMed] [Google Scholar]
- Lammi M.R., Ghonim M.A., Pyakurel K., Naura A.S., Ibba S.V., Davis C.J., Okpechi S.C., Happel K.I., Deboisblanc B.P., Shellito J., Boulares A.H. Treatment with intranasal iloprost reduces disease manifestations in a murine model of previously established COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L630–L638. doi: 10.1152/ajplung.00297.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau C.S., Mogan P., Thomas W. Oestrogen actions contribute to female gender-specific risks in the development of lung carcinoma. J. Steroid Biochem. Mol. Biol. 2021;208:105786. doi: 10.1016/j.jsbmb.2020.105786. [DOI] [PubMed] [Google Scholar]
- Liu X., Wu Y., Zhou Z., Huang M., Deng W., Wang Y., Zhou X., Chen L., Li Y., Zeng T., et al. Celecoxib inhibits the epithelial-to-mesenchymal transition in bladder cancer via the miRNA-145/TGFBR2/Smad3 axis. Int. J. Mol. Med. 2019;44:683–693. doi: 10.3892/ijmm.2019.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D.T., Edwards M.G., Franklin W.A., Sugita M., Keith R.L., Miller Y.E., Friedman M.B., Dwyer-Nield L.D., Tennis M.A., O'keefe M.C., et al. Altered cell-cycle control, inflammation and adhesion in high-risk persistent bronchial dysplasia. Cancer Res. 2018;78:4971–4983. doi: 10.1158/0008-5472.can-17-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D.T., Gao D., Miller Y.E., Keith R.L., Baron A.E., Feser W., Kennedy T.C., Blatchford P.J., Braudrick S., Hirsch F.R., et al. Persistence of bronchial dysplasia is associated with development of invasive squamous cell carcinoma. Cancer Prev. Res. 2016;9:96–104. doi: 10.1158/1940-6207.capr-15-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naikoo N.A., Dil A., Rasool R., Shah S., Ahangar A.G., Siddiqi M.A., Shah Z.A. Upregulation of vascular endothelial growth factor (VEGF), its role in progression and prognosis of non-small cell lung carcinoma. Cancer Genet. 2017;216–217:67–73. doi: 10.1016/j.cancergen.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Nemenoff R., Meyer A.M., Hudish T.M., Mozer A.B., Snee A., Narumiya S., Stearman R.S., Winn R.A., Weiser-Evans M., Geraci M.W., Keith R.L. Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator–activated receptor γ. Cancer Prev. Res. 2008;1:349–356. doi: 10.1158/1940-6207.capr-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New M.L., White C.M., Mcgonigle P., Mcarthur D.G., Dwyer-Nield L.D., Merrick D.T., Keith R.L., Tennis M.A. Prostacyclin and EMT pathway markers for monitoring response to lung cancer chemoprevention. Cancer Prev. Res. 2018;11:643–654. doi: 10.1158/1940-6207.capr-18-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Pinedo E.C., Durham A.C., Stewart K.M., Goss A.M., Lu M.M., Demayo F.J., Morrisey E.E. Wnt/β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J. Clin. Invest. 2011;121:1935–1945. doi: 10.1172/jci44871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.J., Do P., Sompel K., Elango A., Tennis M.A. miR-520a-5p regulates Frizzled 9 expression and mediates effects of cigarette smoke and iloprost chemoprevention. Sci. Rep. 2022;12:2388. doi: 10.1038/s41598-022-06292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompel K.,E.A., Elango A., Smith A.J., Tennis M.A. Cancer chemoprevention through Frizzled receptors and EMT. Discover Oncol. 2021;12:32. doi: 10.1007/s12672-021-00429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennis M.A., New M.L., Mcarthur D.G., Merrick D.T., Dwyer-Nield L.D., Keith R.L. Prostacyclin reverses the cigarette smoke-induced decrease in pulmonary Frizzled 9 expression through miR-31. Sci. Rep. 2016;6:28519. doi: 10.1038/srep28519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennis M.A., Smith A.J., Dwyer-Nield L.D., Keith R.L. Intranasal iloprost prevents tumors in a murine lung carcinogenesis model. Cancer Prev. Res. 2021;15:11–16. doi: 10.1158/1940-6207.capr-21-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennis M.A., Van Scoyk M., Heasley L.E., Vandervest K., Weiser-Evans M., Freeman S., Keith R.L., Simpson P., Nemenoff R.A., Winn R.A. Prostacyclin inhibits non-small cell lung cancer growth by a frizzled 9-dependent pathway that is blocked by secreted frizzled-related protein 1. Neoplasia. 2010;12:244–253. doi: 10.1593/neo.91690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend G. What IF: a molecular modeling and drug design program. J. Mol. Graph. 1990;8:52–56. doi: 10.1016/0263-7855(90)80070-v. [DOI] [PubMed] [Google Scholar]
- Wang C., Wu H., Katritch V., Han G.W., Huang X.P., Liu W., Siu F.Y., Roth B.L., Cherezov V., Stevens R.C. Structure of the human smoothened receptor bound to an antitumour agent. Nature. 2013;497:338–343. doi: 10.1038/nature12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle B.J., Silverstein A.M., Mottola D.M., Clapp L.H. Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: treprostinil is a potent DP1 and EP2 agonist. Biochem. Pharmacol. 2012;84:68–75. doi: 10.1016/j.bcp.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Winn R.A., Marek L., Han S.Y., Rodriguez K., Rodriguez N., Hammond M., Van Scoyk M., Acosta H., Mirus J., Barry N., et al. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J. Biol. Chem. 2005;280:19625–19634. doi: 10.1074/jbc.m409392200. [DOI] [PubMed] [Google Scholar]
- Winn R.A., Van Scoyk M., Hammond M., Rodriguez K., Crossno J.T., Jr., Heasley L.E., Nemenoff R.A. Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2006;281:26943–26950. doi: 10.1074/jbc.m604145200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This data does not report original code.
-
•
Any information required to reanalyze the data reported in this paper is available from the lead contact upon request.