Abstract
Background
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication after abdominal surgery. The incidence of VTE after colorectal malignancy is higher than that after general surgery. Although more attention has been paid to the prevention of VTE, there is still a large gap between clinical practice and guideline recommendation.
Methods
The Venous ThromboEmbolism incidence in patients with ColoRectal Cancer (CRC-VTE trial) will be a prospective, multicenter, cohort study to determine the current status of the incidence, diagnosis, treatment, and prevention of VTE after colorectal cancer surgery in China, as well as to further improve the level of prevention and treatment of VTE events in these fragile patients. In this study, 1,217 patients will be enrolled at 40 centers in China and evaluated on VTE events and adverse events related to VTE prevention at 5–9 and 21–28 days after surgery. The primary outcome is the incidence of VTE events during the follow-up, and secondary outcome is the incidence of adverse events associated with VTE prevention.
Discussion
This study will comprehensively evaluate the incidence and prevention of VTE after colorectal cancer surgery in China, balance the relationship between VTE prevention and bleeding adverse events, and the formulate a guideline for the prevention of VTE after colorectal surgery that might suitable for national conditions.
Trial Registration
Clinical trial registration number NCT04588805 (The CRC-VTE trial).
Keywords: Colorectal cancer, operation, venous thromboembolism (VTE), anticoagulation, low molecular weight heparin, cohort
Introduction
Pulmonary embolism (PE) and deep vein thrombosis (DVT) are two clinical manifestations of the same disease at different stages and locations, which are collectively known as venous thromboembolism (VTE) (1,2). Surgical patients have a variety of risk factors that can easily lead to an increase in the incidence of VTE. Preoperative reduced activity, intraoperative immobilization and long-term postoperative bed rest all lead to significantly slower venous blood flow (3). Anesthesia and surgical trauma can promote the release of tissue factors and directly activate the exogenous coagulation system, leading to hypercoagulability or thrombosis (4). Cancer patients are prone to the development of VTE because of their higher levels of leukocytes, platelets, and tissue factor–positive microvesicles (5). Malignant tumors are an important cause of the increased incidence of VTE, while thrombotic diseases, especially PE, are one of the most common causes of early postoperative death in patients with malignant tumors (6).
Without preventive measures, the incidence of perioperative DVT post general surgery is 10–40% (7), and the incidence of thrombosis after abdominal surgery is 15–40% (8). The incidence of DVT after colorectal surgery is 2 times higher and the incidence of PE is 3 times higher than that after abdominal surgery (9). Studies have shown that surgery is an independent risk factor for the occurrence of VTE. Compared with other abdominal surgery, patients with colorectal surgery are more likely to develop VTE after surgery due to special position and long operation time (10), especially after radical colorectal surgery, which has been reported as high as 37–46% (11). Colorectal cancer is an independent predictor of postoperative VTE (12). Meanwhile, VTE also significantly affected the prognosis of surgery patients and reduced the life quality of patients (6). In the short term, VTE is the most important cause of death in oncological surgical patients within one month after surgery. In the long-term, about 1/3 of DVT patients will have post-thrombotic syndrome (PTS), and 30% of VTE patients will have recurrence within 8 years after surgery (9).
Based on the serious harm of VTE in perioperative patients, both domestic and foreign guidelines [such as American College of Chest Physicians, 9th edition (ACCP-9), National Comprehensive Cancer Network 2018 (NCCN 2018), Guidelines for the Prevention and Management of Perioperative Thrombosis in Chinese General Surgery, etc.] recommend the mechanical and pharmacological prophylaxis for perioperative patients with medium to high risk of VTE (10,13,14). It is emphasized that the period of drug prophylaxis should be extended to 4 weeks postoperatively for cancer patients undergoing abdominal and pelvic surgery. Although perioperative VTE prevention has received more and more attention, there are still large gaps between clinical practice and guideline recommendations, as well as between physicians' concepts and clinical standards. The Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting (ENDORSE) study which is a multinational cross-sectional study, found that 64% of all surgical patients are at risk for VTE, and only an average of 59% of them have actually received VTE prophylaxis in accordance with the ACCP guidelines (15). The percentage of surgical patients in Europe and the United States receiving standard VTE prophylaxis is about 60–80%, while in Asia, although the risk of VTE is not lower than that in Europe and America, the proportion of accepting normative preventive measures is far lower than that in Europe and America (15). According to Identification of Chinese Hospitalized Patients’ Risk Profile for Venous Thromboembolism (DISSOLVE 2) study which is a multinational cross-sectional study, the proportion of Chinese inpatients receiving any prevention of VTE (no matter drug, device or other methods) was only 14.2%, while the proportion referring to ACCP guidelines and receiving appropriate prevention methods was 9.0%, and the proportion completely following the guidelines and receiving standard prevention was only 3.1% (16). Even in China’s economically developed areas such as Beijing, Shanghai and Guangzhou, the situation of perioperative VTE prevention is not optimistic. Therefore, this study aims to further clarify the current situation of the incidence, diagnosis, treatment and prevention of perioperative VTE in colorectal cancer patients in China, as well as to understand the gap between domestic clinical practice and the recommended opinions of the guidelines.
Methods
Study design and setting
The Venous ThromboEmbolism incidence in patients with ColoRectal Cancer (CRC-VTE) trial will be a prospective, multicenter, cohort study to determine the current incidence of postoperative VTE events in Chinese colorectal cancer patients and the incidence of bleeding and other adverse events associated with the chemical prophylaxis against VTE events. Patients will be enrolled consecutively from 40 centers in China from July 2021 to December 2021. The trial flowchart is illustrated in Figure 1. The incidence of VTE events within 1 month in patients undergoing colorectal cancer surgery was significantly higher than that before surgery. Therefore, VTE events will be evaluated in 5–9 and 21–28 days after surgery, and the relevant physical, imaging, and laboratory examinations will be completed to evaluate whether patients have suffered a DVT or/and PE. This study will be conducted according to the principles of the Declaration of Helsinki (as revised in 2013). Ethics committee of Beijing Friendship Hospital, Capital Medical University has approved the study protocol (Approval number: 2020-P2-183-02). All participants will sign written informed consent.
Figure 1.
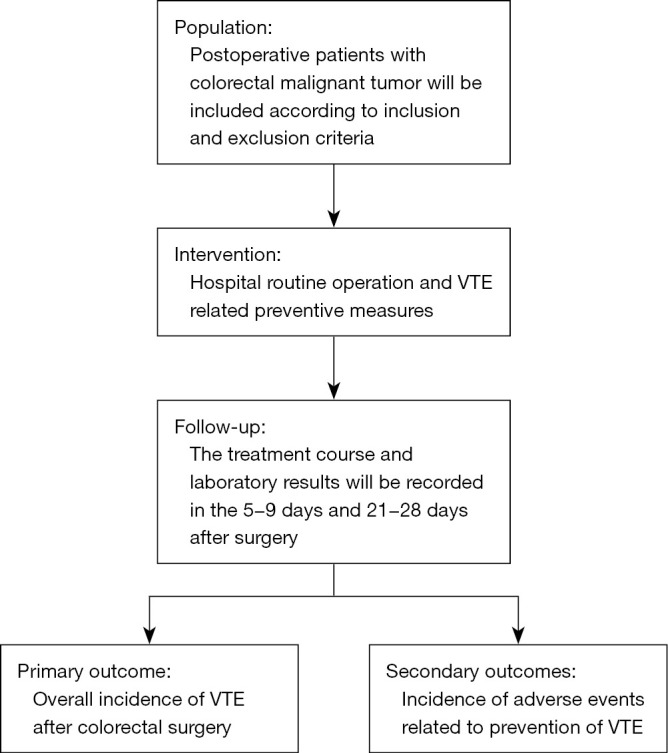
Study flowchart. VTE, venous thromboembolism.
Population
Consecutively enrolled patients who were clinically diagnosed as colorectal cancer and hospitalized in participating centers and received surgical treatment, including radical surgery, palliative surgery, and other limited surgery for colorectal cancer, will be eligible to participate in this study. The inclusion and exclusion criteria are listed in Table 1.
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria |
| (I) No age limit |
| (II) Preoperative colonoscopy confirmed colorectal cancer |
| (III) Hospitalized and operated in the participating centers |
| (IV) Radical operation |
| (V) Palliative surgery |
| (VI) Other limited operation for colorectal cancer |
| Exclusion criteria |
| (I) Patients undergoing emergency surgery |
| (II) Patients diagnosed with benign diseases |
| (III) Patients who had DVT or PE of both lower limbs before operation |
| (IV) Patients who have been enrolled in other studies or cannot obtain informed consent |
| (V) Patients who are already on anticoagulation drugs |
DVT, deep vein thrombosis; PE, pulmonary embolism.
Study procedure and treatment
This study will be an observational study. The investigators do not interfere with the clinical decision-making and treatment process of the patients. Only clinical data of the patients are collected in a targeted manner, and routine follow-up is performed around 21–28 days after surgery. The specific study process of patients is broken into three periods: screening, perioperative, follow-up. In the screening period, we will screen suitable patients according to inclusion and exclusion criteria and sign informed consent with patients. After admission, patients with a confirmed colorectal malignancy will be evaluated by CAPRINI score within 24 hours. Baseline data including general information, general condition and CAPRINI score, previous related medical history and disease history, treatment history, preoperative laboratory tests, and tumor related conditions will be collected; during the perioperative period, intervention will be determined based on VTE risk stratification and corresponding guideline recommendation, and the interventions used will follow the guideline “patients diagnosed with abdominal and malignant tumors are recommended to use low molecular weight heparin for anticoagulation for 4 weeks, or use drugs according to the existing anticoagulant treatment habits”. We will collect brief surgical data of patients, including surgical method, time, and bleeding situation, and fill in postoperative VTE prevention, relevant diagnosis and treatment information and postoperative patient routine 5–9 days before discharge; as for the follow-up period, information about the occurrence of VTE, VTE prevention-related events, related laboratory tests, examinations, diagnosis, and treatment will be collected during the routine 21–28 days follow-up after surgery. All the collected data will be filled in through the network filling system. The specific information collected is shown in Table 2.
Table 2. Study timelines and schedule.
| Tasks | Screening period | Follow-up | |
|---|---|---|---|
| 5–9 days after surgery | 21–28 days after surgery | ||
| Informed consent | √ | ||
| Demographic data | √ | ||
| Surgical information | √ | ||
| VTE related | |||
| Symptom assessment | √ | √ | √ |
| Physical examination | √ | √ | √ |
| Laboratory examination1 | √ | √ | √ |
| Imaging examination2 | √ | √ | √ |
| Caprini score | √ | √ | |
| Prophylactic use of VTE | √ | √ | √ |
| Evaluation for concomitant treatments | √ | √ | √ |
| Evaluation for bleeding risk | √ | √ | √ |
| Treatment compliance | √ | √ | √ |
| Prevention of VTE related adverse events | √ | √ | √ |
| Other adverse events | √ | √ | √ |
1, DIC examination is an important examination item in laboratory examination, which should be examined 5–9 and 21–28 days after operation; 2, imaging examination should be based on color Doppler ultrasound, if DVT or PE occurs, further venography or CTPA should be performed. DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism; DIC, disseminated intravascular coagulation.
Study outcomes
The primary study outcome is the total incidence of VTE events, including DVT and PE: (I) DVT is diagnosed by combining the clinical symptoms of swelling and pain of the lower limbs, tenderness behind the lower leg and/or medial thigh and auxiliary examination of color ultrasound of the veins of both lower limbs, and lower extremity venography is the gold standard for the diagnosis of DVT; (II) PE should be initially diagnosed in combination with related clinical manifestations such as dyspnea and shortness of breath and laboratory tests such as plasma D-dimer. CT pulmonary arteriography (CTPA) is the preferred examination method for the diagnosis of PE (17). The secondary outcomes are individual PE and DVT, major bleeding, minor bleeding, heparin induced thrombocytopenia (HIT), abnormal liver function during VTE prevention. A major bleeding is defined as any of the following situations according to the International Society on Thrombosis and Hemostasis (ISTH) criteria:
A ≥20 g/L fall in hemoglobin;
A transfusion of ≥2 units of red blood cells or whole blood;
Bleeding at critical sites including intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome and retroperitoneal syndrome;
Sample size and statistical analysis
In this prospective cohort study design, we assumed that the perioperative incidence rate of VTE in colorectal cancer patients was 24% (P) with an allowable error of 0.1P and α=0.05 (20-22). According to the sample size estimation formula (23), the sample size required for this study is 1,217 cases. The primary analysis will describe the characteristics of the patients and mainly calculate the total incidence of VTE events in colorectal cancer patients within 5–7 and 21–28 days after surgery. The incidence of various adverse events arising from VTE prevention in patients will also be calculated separately. Consecutive data (patients’ VTE prevention time, D-dimer, platelet, etc.) will be presented as the mean standard deviation or median in the quartile range. The classified data (patients’ basic characteristics, surgical methods, complications, etc.) will be presented as counts and percentages. Statistical analysis will be carried out from the following points: (I) according to the prevention of VTE, patients can be divided into no prevention group, intermediate group (with prevention but not according to the guidelines) and prevention group (with prevention and meeting the guideline), Groups will be determined according to guidelines issued by the Chinese Medical Association (12); (II) patients can be divided into low risk, medium risk, and high risk groups according to the CAPRINI score. All P values are double-sided and the statistical significance is set at the 5% level. Proportion and 95% confidence interval will be calculated. SPSS (IBM) will be used for statistical analysis of all data.
Discussion
Prevention of VTE in patients with colorectal malignancy should begin with risk assessment. The CAPRINI score is the worldwide consensus for evaluating the risk of VTE in the perioperative period (14,24,25). According to domestic and foreign studies, the CAPRINI score can effectively identify people with high incidence of VTE, and the higher the score, the higher the risk of VTE (26-28). The preventive measures for VTE mainly include mechanical prophylaxis and drug prophylaxis, the purpose of the former is to increase venous return of the lower limbs through external pressure to reduce the occurrence of VTE. As for drug prophylaxis, low molecular weight heparin is the first-line choice according to the guideline proposed by the American Association for Colorectal Surgery (22). Some novel anticoagulant drugs, such as rivaroxaban and dabigatran, are used as preventive drugs for postoperative VTE in colorectal cancer. Although some clinical studies such as Rivaroxaban for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer (PROLAPS II) are currently under way, large-scale evidence-based evidence is still lacking (29). Of note, there is clear evidence that duration prolongation of prophylactic use of low molecular weight heparin after major abdominal and pelvic tumor surgery can effectively reduce the incidence of VTE without increasing bleeding events (30). Therefore, for patients with colorectal cancer, the application of anticoagulants for 4 weeks after surgery may bring about a better trade-off property (31). Individualized prevention of VTE is recommended in the design of this study based on the risk level of VTE assessed twice by CAPRINI system after admission and surgery. Certainly, as an observational study, we do not limit the prevention measures. Bleeding is the most common complication in the prevention of VTE in patients with colorectal cancer. A meta-analysis of 24 studies involving 804,003 patients found that thromboprophylaxis in perioperative patients with colorectal cancer reduced the incidence of VTE, while may increase the chance of bleeding (32). Therefore, in this study, bleeding events are taken as the main safety outcome, and postoperative bleeding events related to VTE prevention will be recorded in detail, and the corresponding measures will be taken for patients with adverse bleeding events in time. Although continued use of anticoagulants after discharge can reduce the incidence of VTE events in patients, these recommendations for adoption rates have been statistically low, with only 1.5% of patients undergoing colorectal cancer resection receiving post-discharge prophylaxis (33). Schlick has developed a post-hospital risk generator for colorectal cancer, which can be used to quickly calculate the predictive probability of post-hospital VTE in an individual patient, to identify a group of patients at high risk of post-hospital VTE events, and to target them for post-hospital prevention (34). However, it also has the disadvantages of low predictive probability and require larger sample data, thus the risk-benefit ratio of post-discharge prevention of VTE is still difficult to explain. We will make statistics on medication compliance of Chinese patients discharged after colorectal cancer surgery, and analyze the necessity and feasibility of prolonging postoperative VTE prevention. We analyzed the results of a large questionnaire on the status of VTE after colorectal surgery in China prior to the commencement of this study (35). The result showed that VTE events are common in colorectal malignant tumors, but there is still a lack of specific data on the prevention and treatment of VTE in colorectal surgery patients in China. Therefore, in order to further understand the current situation of perioperative anticoagulation and the incidence of postoperative VTE in colorectal patients, we designed such a study. The results of this study will help us to establish the prevention norms and guidelines for VTE in colorectal surgery suitable for China’s national conditions.
Acknowledgments
We are grateful to Mr. Miao Che for his generous help in the statistical design.
Funding: This work was supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (No. ZYLX201504), Clinical Center for Colorectal Cancer, Capital Medical University (No. 1192070313), Research Foundation of Beijing Friendship Hospital, Capital Medical University (No. YYQDKT2016-5), and Shanghai “Rising Stars of Medical Talent” Youth Development Program—Youth Medical Talents—Clinical Pharmacist Program [SHWJRS (2019) 072].
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study will be conducted according to the principles of the Declaration of Helsinki (as revised in 2013). Ethics committee of Beijing Friendship Hospital, Capital Medical University has approved the study protocol (Approval number: 2020-P2-183-02). All participants will sign written informed consent.
Footnotes
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1860/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-21-1860/coif). The authors have no conflicts of interest to declare.
References
- 1.Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334-49. 10.1002/cncr.25714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandén P, Svensson PJ, Själander A. Venous thromboembolism and cancer risk. J Thromb Thrombolysis 2017;43:68-73. 10.1007/s11239-016-1411-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Previtali E, Bucciarelli P, Passamonti SM, et al. Risk factors for venous and arterial thrombosis. Blood Transfus 2011;9:120-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Martino RR, Goodney PP, Spangler EL, et al. Variation in thromboembolic complications among patients undergoing commonly performed cancer operations. J Vasc Surg 2012;55:1035-1040.e4. 10.1016/j.jvs.2011.10.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood 2017;130:1499-506. 10.1182/blood-2017-03-743211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006;243:89-95. 10.1097/01.sla.0000193959.44677.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:381S-453S. [DOI] [PubMed] [Google Scholar]
- 8.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:338S-400S. 10.1378/chest.126.3_suppl.338S [DOI] [PubMed] [Google Scholar]
- 9.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996;125:1-7. 10.7326/0003-4819-125-1-199607010-00001 [DOI] [PubMed] [Google Scholar]
- 10.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e227S-77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum 2010;53:1355-60. 10.1007/DCR.0b013e3181eb9b0e [DOI] [PubMed] [Google Scholar]
- 12.Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res 2012;129:568-72. 10.1016/j.thromres.2011.07.047 [DOI] [PubMed] [Google Scholar]
- 13.Streiff MB, Holmstrom B, Angelini D, et al. NCCN Guidelines Insights: Cancer-Associated Venous Thromboembolic Disease, Version 2.2018. J Natl Compr Canc Netw 2018;16:1289-303. 10.6004/jnccn.2018.0084 [DOI] [PubMed] [Google Scholar]
- 14.Liu FL, Zhang TP. Chinese guidelines for prevention and management of perioperative thrombosis in general surgery. Chinese Journal of Practical Surgery 2016;36:469-74. [Google Scholar]
- 15.Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008;371:387-94. 10.1016/S0140-6736(08)60202-0 [DOI] [PubMed] [Google Scholar]
- 16.Zhai Z, Kan Q, Li W, et al. VTE Risk Profiles and Prophylaxis in Medical and Surgical Inpatients: The Identification of Chinese Hospitalized Patients' Risk Profile for Venous Thromboembolism (DissolVE-2)-A Cross-sectional Study. Chest 2019;155:114-22. 10.1016/j.chest.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 17.Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317-27. 10.1056/NEJMoa052367 [DOI] [PubMed] [Google Scholar]
- 18.Schulman S, Angerås U, Bergqvist D, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010;8:202-4. 10.1111/j.1538-7836.2009.03678.x [DOI] [PubMed] [Google Scholar]
- 19.Kaatz S, Ahmad D, Spyropoulos AC, et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:2119-26. 10.1111/jth.13140 [DOI] [PubMed] [Google Scholar]
- 20.Sakon M, Maehara Y, Yoshikawa H, et al. Incidence of venous thromboembolism following major abdominal surgery: a multi-center, prospective epidemiological study in Japan. J Thromb Haemost 2006;4:581-6. 10.1111/j.1538-7836.2006.01786.x [DOI] [PubMed] [Google Scholar]
- 21.McLeod RS, Geerts WH, Sniderman KW, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the canadian colorectal DVT prophylaxis trial: a randomized, double-blind trial. Ann Surg 2001;233:438-44. 10.1097/00000658-200103000-00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleming F, Gaertner W, Ternent CA, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guideline for the Prevention of Venous Thromboembolic Disease in Colorectal Surgery. Dis Colon Rectum 2018;61:14-20. 10.1097/DCR.0000000000000982 [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Shen Y, Ning J, et al. Sample size calculations for prevalent cohort designs. Stat Methods Med Res 2017;26:280-91. 10.1177/0962280214544730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e326S-50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang SM, Jang MJ, Kim KH, et al. Prevention of venous thromboembolism, 2nd edition: Korean Society of Thrombosis and Hemostasis Evidence-based Clinical Practice Guidelines. J Korean Med Sci 2014;29:164-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahl V, Hu HM, Henke PK, et al. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg 2010;251:344-50. 10.1097/SLA.0b013e3181b7fca6 [DOI] [PubMed] [Google Scholar]
- 27.Zhou H, Wang L, Wu X, et al. Validation of a venous thromboembolism risk assessment model in hospitalized chinese patients: a case-control study. J Atheroscler Thromb 2014;21:261-72. 10.5551/jat.20891 [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Liu C, Chen X, et al. Comparison between Caprini and Padua risk assessment models for hospitalized medical patients at risk for venous thromboembolism: a retrospective study. Interact Cardiovasc Thorac Surg 2016;23:538-43. 10.1093/icvts/ivw158 [DOI] [PubMed] [Google Scholar]
- 29.Becattini C, Pace U, Rondelli F, et al. Rivaroxaban for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer. Design of the PRO-LAPS II STUDY. Eur J Intern Med 2020;72:53-9. 10.1016/j.ejim.2019.11.015 [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen MS, Jørgensen LN, Wille-Jørgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev 2009;(1):CD004318. 10.1002/14651858.CD004318.pub2 [DOI] [PubMed] [Google Scholar]
- 31.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2189-204. 10.1200/JCO.2013.49.1118 [DOI] [PubMed] [Google Scholar]
- 32.Li YD, Li HD, Zhang SX. Effect of thromboprophylaxis on the incidence of venous thromboembolism in surgical patients with colorectal cancer: a meta-analysis. Int Angiol 2020;39:353-60. 10.23736/S0392-9590.20.04321-7 [DOI] [PubMed] [Google Scholar]
- 33.Merkow RP, Bilimoria KY, Sohn MW, et al. Adherence with postdischarge venous thromboembolism chemoprophylaxis recommendations after colorectal cancer surgery among elderly Medicare beneficiaries. Ann Surg 2014;260:103-8. 10.1097/SLA.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 34.Schlick CJR, Liu JY, Yang AD, et al. Pre-Operative, Intra-Operative, and Post-Operative Factors Associated with Post-Discharge Venous Thromboembolism Following Colorectal Cancer Resection. J Gastrointest Surg 2020;24:144-54. 10.1007/s11605-019-04354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao HW, Han JG, Zhang ZT. Questioniare analysis of the status of venous thromboembolism after colorectal surgery in China. Chinese Journal of Practical Surgery 2020;40:551-6. [Google Scholar]


