Abstract
Melatonin, a pleiotropic hormone, has many regulatory effects on the circadian and seasonal rhythms, sleep and body immune system. It is used in the treatment of blind circadian rhythm sleep disorders, delayed sleep phase and insomnia. It is a potent antioxidant, anti-inflammatory, free radical scavenger, helpful in fighting infectious disease and cancer treatment. Decreased level of circulating melatonin was associated with an increased blood glucose level, losing the anti-oxidant protection and anti-inflammatory responses. We aimed to evaluate the effect of melatonin administration, in streptozotocin (STZ) induced diabetic rats, on blood glucose level and pancreatic beta (β) cells. Diabetes mellitus was induced in Sprague dawley male rats by the intravenous (i.v) injection of 65 mg/kg of STZ. Diabetic rats received melatonin at a dose of 10 mg/kg daily for 8 weeks by oral routes. The results showed, after 8 weeks of melatonin administration, a reduction in: 1- fasting blood glucose (FBG) and fructosamine (FTA) levels, 2- kidney and liver function parameters, 3- levels of serum triglycerides, cholesterol and LDL-C, 4- malondialdehyde (MDA), 5- NF-κB expression in treated group, 6- pro-inflammatory cytokines (IL-1β and IL-12) and immunoglobulins (IgA, IgE and IgG). Furthermore, an elevation in insulin secretion was noticed in melatonin treated group that indicated β cells regeneration. Therefore, melatonin administration, in STZ induced diabetic rats; reduced hyperglycemia, hyperlipidemia and oxidative stress. Melatonin acted as an anti-inflammatory agent that reduced pro-inflammatory cytokines (IL-1β and IL-12) and oxidative stress biomarkers (MDA). Melatonin succeeded in protecting β cells under severe inflammatory situations, which was apparent by the regeneration of islets of Langerhans in treated diabetic rats. Moreover, these results can open a gate for diabetes management and treatment.
Keywords: β-cells, Diabetes mellitus, IL-1β, IL-12, Inflammation
1. Introduction
Melatonin (5-methoxy-N-acetyltryptamine), a tryptophan indolic molecule, is a hormone that is secreted from the pineal gland with a daily rhythm. Where, its secretion increases in darkness and hence it is named as the darkness hormone. Several researches demonstrated the relation among melatonin, insulin secretion and glucose homeostasis (Heo et al., 2018, Oliveira et al., 2018, Xu et al., 2019). Yavuz et al. (2003) reported a reduction in malondialdehyde (MDA) and an elevation in glutathione peroxidase (GPx) activity after melatonin administration in diabetic rats; in addition to the preservation of integrity of pancreatic beta (β) cells. The results of Klepac et al. (2006) proofed the scavenging effects of melatonin and reported the reduction in oxidative stress after 24 h of its administration in diabetic rats. Also, Hajam and Rai (2020) showed that melatonin revived the changes in biochemical and haematological parameters in diabetic rats. Moreover, Hajam et al. (2021) administrated diabetic rats with external melatonin in addition to insulin; and reported an enhancement in renal functions. Patients with diabetes showed low level of serum melatonin and an upregulated mRNA expression of membrane melatonin receptors (Peschke et al., 2007). Diabetes occurrence was linked to the polymorphisms in melatonin receptor genes (Zhang et al. 2020). Also, patients with type I diabetes (T1DM), with mean blood glucose level > 154 mg/dl, had less urinary melatonin than healthy ones (Amaral et al., 2014). Another study showed that sleep restriction, which result in low melatonin secretion, in patients with T1DM weaken insulin sensitivity and disrupted glycemic control on the next day (Donga et al., 2010). Melatonin is safe when used as a supplement (Xie et al., 2017), easily absorbed via any administration route and can pass through all morphophysiological barriers as blood brain barrier and placenta (Dong et al., 2016). Moreover, Kanter et al. (2006) showed that melatonin administration decreased fasting glucose level and slightly increased insulin level; the authors retained these to the action of melatonin on pancreatic β cells and stimulation of insulin secretion. Also, Ahmad Hajam et al. (2018) showed the recovery of pancreas of STZ diabetic rats, treated with melatonin, which in turn regulated the pancreatic function.
T1DM is considered as an autoimmune disease in which the destruction of pancreatic β cells by patient's immune system occurs, resulting in a reduction of insulin production and an elevation of blood glucose level (Saberzadeh-Ardestani et al., 2018). Pancreatic histopathological features of this type of diabetes include a decrease in β cell mass and mononuclear cells infiltration that is known as insulitis. Insulin administration is the traditional treatment to avoid hyperglycemia and diabetic ketoacidosis. Atkinson et al. (2014) reported that 10 to 20% of the β cells continue to function at the time of diagnosis.
Many researchers has induced T1DM in rodents by a single STZ injection (Lenzen, 2008, Yin et al., 2006, Furman, 2015, Wu and Yan, 2015). STZ, an antibiotic synthesized by Streptomyces achromogens, is composed of deoxyglucose molecule connected to a methylnitrosourea molecule, which is responsible for its cytotoxicity; while the glucose molecule targets STZ to pancreatic β cells through the glucose transporter 2 (GLUT2) receptor (Lenzen, 2008). Because GLUT2 is found in the kidney and liver to a lower scale, excessive dosages of STZ might affect kidney and liver functions (Bouwens and Rooman, 2005). STZ is fastly digested in the liver and excreted rapidly by the kidney; as a result, STZ has a very short half-life (15 min in the serum after its injection) (Eleazu et al., 2013) and its cytotoxic effect to the kidney and liver can be ignored (Tesch and Allen, 2007). Therefore, any functional deterioration of the kidney and liver is related to the consequences of diabetic hyperglycemia and not to the STZ effect (Wu et al., 2015). Accordingly, STZ induced T1DM animal model is a deep-rooted and widely used method for studying diabetes development and consequences. Although the STZ induced T1DM animal model is not in any way comparable to that in human, the single STZ injection model remains useful in studying diabetic glucotoxicity-induced β cell derangement due to its low cost, saving time, and easy methodology.
Our model to induce Type I diabetes in rats included the intravenous (i.v) injection of 65 mg/kg of STZ. STZ led to the destruction of pancreatic β cells inducing hyperglycemia, insulin deficiency, thirsty and an increase in urination, all of that were features of T1DM. Therefore, the present study was designed to: 1- test the possibility that melatonin can regenerate pancreatic β cells and increase insulin sensitivity, 2- apply immunological (cytokines and immunoglobulins) and immunohistochemical (for NF-κB and insulin) examination for the explanation of the melatonin action on pancreas, 3- study the effect of melatonin administration on metabolic abnormalities associated with diabetes like oxidative stress, hyperglycemia, abnormal lipid profile, altered kidney and liver function. This was performed on STZ diabetic rats; where melatonin was orally administrated, after the induction of hyperglycemia, at a dose of 10 mg/kg daily for 8 weeks.
2. Materials and methods
2.1. Chemicals
STZ (S0130), 2-deoxy-2(3-methyl-3-nitrosoureido)-D-glucopyranose, and melatonin (M5250) was purchased from Sigma Company, USA. 50 mM sodium citrate buffer with pH 4.5 (Fisher, USA) was prepared just before use.
2.2. Animals
Sprague dawley male rats (10 weeks old; weighting; 170–220 g) were purchased from National Organization for Drug Control and Research (NODCAR, Cairo, Egypt). On arrival, rats were housed in conventional cages for one week before the experiment for acclimatization (Ahmed et al., 2022, Farid et al., 2013, Farid et al., 2021). Rats were maintained at controlled temperature (21 ± 2 °C) and on a 12 h dark–light cycle. Rats received standard pellets, containing all nutritive elements. Drinking water and food were provided ad libitum. All the experimental procedures were performed according to the international care and use of laboratory animals’ guidelines; and were approved by the Institutional Animal Care and Use Committee (CU-IACUC), Cairo University, Egypt.
2.3. Diabetes induction
On the first experimental day, all rats were fasted for 6–8 h prior to STZ administration. 32.5 mg of STZ was dissolved in 50 mM sodium citrate buffer to a final concentration of 32.5 mg/ml. Rats were i.v. injected by the prepared STZ solution at 65 mg/kg (2.0 ml/kg) according to Furman (2015). Control group was i.v. injected with citrate buffer only. Rats were administrated normal food and 10% sucrose water in the first day, only, of STZ administration. On the 10th day, all rats were fasted for 6–8 h and blood samples from tail vein were collected for measuring fasting blood glucose (FBG) level. Rats with FBG level > 150 mg/dl in comparison to control group were considered diabetic. Serum fructosamine (FTA) was determined at the end of experiment, to measure average blood glucose level over the past 3 weeks, using Rat FTA ELISA Kit (MBS2601586, MyBioSource, USA).
2.4. Experimental design
Forty Sprague dawley male rats were divided equally into four groups. Group I: healthy control rats that received sodium citrate buffer only and vehicle (6% alcohol (w/v) aqueous solution), group II: melatonin administrated non diabetic control rats, group III: STZ diabetic rats that received vehicle only and group IV: melatonin treated STZ diabetic rats. Melatonin was dissolved in 6% alcohol solution and orally administrated at 10 mg/kg of body weight daily after induction of hyperglycemia for 8 weeks between 5 and 7 pm daily.
2.5. Oral glucose tolerance test (OGTT)
Rats were fasted for 6 h, then 2 gm/kg of glucose was orally administrated as a bolus at 9 am. FBG was monitored at 0, 30, 60, 90 and 120 min after receiving glucose. Blood samples were taken from the rats’ tail tip using 27G needle according to Pedro et al. (2020); where small blood drop (<5 µl) was positioned on a glucose strip in the glucometer.
2.6. Samples collection
Animals were terminally anesthetized with 80 mg/kg pentobarbital, at the end of the study; where blood samples were collected through heart puncture using 19G needle according to Parasuraman et al. (2010). This method is recommended to collect a high-quality single large blood amount from the animal. Blood was allowed to clot at room temperature followed by centrifugation. Serum was divided into aliquots and stored at −20 °C. Organs (liver, kidney and pancreas) were also collected from different experimental groups for histopathological and immunohistochemical examination.
2.7. Biochemical analysis
Serum insulin was measured by Insulin Rat ELISA Kit (Invitrogen ERINS, Thermo Fisher, USA). Serum and liver cholesterol and triglycerides were measured by Cholesterol Assay Kit - HDL and LDL/VLDL (ab65390, Abcam, UK) and Serum Triglyceride Assay Kit (STG-1-NC, ZenBio, USA). Liver function enzymes were determined by Rat ALT ELISA Kit (ab234579, Abcam, UK) and Rat AST ELISA Kit (MBS264975, MyBioSource, USA). Kidney function enzymes were measured by rat urea ELISA kit (MBS2600001, MyBioSource, USA) and rat creatinine ELISA kit (MBS007289, MyBioSource, USA). Serum Malondialdehyde (MDA) was determined by rat MDA ELISA Kit (MBS268427, MyBioSource, USA). Superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity was determined by rat SOD ELISA Kit (MBS266897, MyBioSource, USA) and rat GPx ELISA kit (MBS727547, MyBioSource, USA). Bio-Rad protein assay was used to measure total protein concentration in tissue homogenates.
2.8. Immunological analysis
Serum interleukin (IL)-1β and IL-12 levels were determined by Rat IL-1β ELISA Kit (MBS843359, MyBioSource, USA) and Rat IL-12 ELISA Kit (MBS2507061, MyBioSource, USA). Serum immunoglobulin (Ig) levels were determined by Rat IgG ELISA Kit (ab189578, Abcam, UK), Rat IgE ELISA Kit (CSB-E07984r, Cusabio, USA) and Rat IgA ELISA Kit (ab157735, Abcam, UK).
2.9. Histopathological studies
Organs from different experimental groups were fixed in 10% buffered formalin for 24 h (Farid et al., 2020). After fixation, tissues were washed in tab water; then dehydrated in ascending series of ethanol 70, 90, 95 and 100%, respectively, followed by clearing in xylene and embedding in paraffin wax at 55C°(Farid et al., 2022). Five sections (4 µm thickness) were cut from each tissue followed by staining with hematoxylin and eosin. Briefly, slides were put in xylene to dissolve the paraffin wax, then in absolute alcohol for two min, 95% alcohol for one min, 70% alcohol for one min. Slides were stained with hematoxylin for five min then washed in tab water. Excess stain was removed in acid alcohol for few seconds. Sections were stained with eosin for four to five min and washed in water then absolute alcohol (95%) and xylene. Sections were mounted by DPX (Hegazy et al., 2015).
2.10. Immunhistochemical staining
Formalin- fixed rat pancreatic sections, 4 μm, were deparaffinized, rehydrated and washed by phosphate buffered saline (PBS). 3% hydrogen peroxide in methanol was applied to pancreatic sections for blocking endogenous peroxidase activity followed by washing with PBS then blocking with 5% bovine serum albumin (BSA). Anti- (nuclear factor) NF-kB p65 antibody (phospho S536, ab86299, Abcam, UK) and monoclonal anti-insulin antibody (I2018, Sigma, USA) was used, individually, as a primary antibody for NF-kB and insulin in pancreatic sections, respectively. Sections were incubated for an hour, washed and incubated with HRP conjugated rabbit anti-rat IgG (ab6734, Abcam, UK) as a secondary antibody for two hours. 3, 3-diaminobenzidine (DAB) was used to visualize the reaction until brown colour appears. Mayer's hematoxyline was used to counterstain pancreatic sections. Brown colour indicated positive reaction.
2.11. Statistical analysis
The results were evaluated by One Way ANOVA test and compared with Tukey test (Abdel-Monaem et al., 2015, Kamel et al., 2013, Mohie El-Dinn et al., 2018). Results were expressed as mean ± SD and values were considered significant at P < 0.05 (Farid et al., 2022, Shater et al., 2022).
3. Results
3.1. Body weight and biochemical measurement
In all measured parameters, melatonin administration in control group II did not show any significant changes in comparison to healthy control group I. Non-treated diabetic control group III showed a significant reduction in body weight (145.50 ± 0.32) in comparison to healthy control group I (220.12 ± 0.53); melatonin administration in diabetic group IV improved body weight of rats to record a 201.53 ± 0.54 gm. Insulin level of melatonin treated group IV (2.8 ± 3.21) was significantly elevated than that of non-treated group III (0.91 ± 1.96); and nearly equal to that of normal control (3.01 ± 1.25).
Both of FBG and FTA levels increased significantly in non-treated diabetic group III, 244.18 ± 1.23 and 298.83 ± 0.75, respectively, in comparison to healthy controls (89.08 ± 0.65 and 157.51 ± 0.69 for FBG and FTA, respectively (Table 1.)). A significant decline in FBG level was observed in melatonin treated diabetic group IV (91.93 ± 0.25) in comparison to non-treated diabetic group III (244.18 ± 1.23). Also, level of FTA in group IV decreased to reach 168.54 ± 1.59 after melatonin administration, which is comparable to that of healthy controls (157.51 ± 0.69).
Table 1.
the effect of melatonin administration in different experimental groups.
| Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Body weight (gm) | 220.12 ± 0.53 | 225.65 ± 2.41 | 145.50 ± 0.32a | 201.53 ± 0.54a,b |
| Insulin (ng/ml) | 3.01 ± 1.25 | 3.91 ± 0.47 | 0.91 ± 1.96 a | 2.8 ± 3.21b |
| FBG level (mg/dl) | 89.08 ± 0.65 | 80.11 ± 4.21 | 244.18 ± 1.23a | 91.93 ± 0.25b |
| FTA (µmol/L) | 157.51 ± 0.69 | 149.15 ± 1.01 | 298.83 ± 0.75 a | 168.54 ± 1.59a,b |
| Liver function | ||||
| ALT (U/L) | 25.07 ± 1.65 | 26.01 ± 2.01 | 45.56 ± 2.50a | 29.44 ± 0.65b |
| AST (U/L) | 21.69 ± 0.87 | 20.31 ± 1.54 | 35.55 ± 1.80a | 23.85 ± 0.43a |
| Kidney function | ||||
| Urea (mg/dl) | 37.64 ± 0.59 | 35.74 ± 5.40 | 83.53 ± 1.43a | 39.65 ± 1.92a,b |
| Creatinine (mg/dl) | 0.43 ± 2.57 | 0.44 ± 0.48 | 1.43 ± 2.46 a | 0.73 ± 1.75b |
In each raw, results were expressed as mean ± SD, arepresents significance compared with the corresponding healthy control group I and brepresents significance compared with the corresponding diabetic group III (P < 0.05). Group I: healthy control rats, group II: melatonin administrated control rats, group III: non-treated STZ diabetic rats and group IV: melatonin treated STZ diabetic rats
Level of Urea (39.65 ± 1.92) in treated group IV decreased significantly when compared to that of diabetic group III (83.53 ± 1.43), but it was still higher than that of healthy control group I (37.64 ± 0.59). Creatinine level in diabetic group III (1.43 ± 2.46) was higher than those of healthy controls (0.43 ± 2.57) and treated group IV (0.73 ± 1.75).
Induction of diabetes in group III elevates liver enzymes ALT and AST to reach 45.56 ± 2.50 and 35.55 ± 1.80, respectively, when compared to those of control group I (25.07 ± 1.65 and 21.69 ± 0.87 for ALT and AST, respectively). A significant reduction in ALT and AST level (29.44 ± 0.65 and 23.85 ± 0.43, respectively) was observed in melatonin treated diabetic group IV in comparison to diabetes induced group III (Table 1.).
3.2. Serum and liver lipid profile
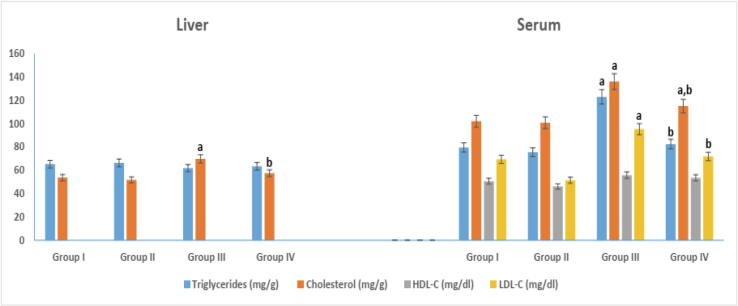
Melatonin administration in group IV caused a significant reduction in serum triglycerides and cholesterol level, 82.45 ± 0.82 and 115.06 ± 0.76, respectively, in comparison to non-treated diabetic group III (Fig. 1). However, group IV serum cholesterol level (115.06 ± 0.76) remains higher than that of healthy control (102.01 ± 0.93) in a significant way. No significance difference in serum HDL-C levels was noticed between different experimental groups. On the other hand, serum LDL-C level was significantly elevated in non-treated diabetic group III (95.24 ± 2.09) in comparison to healthy controls. Melatonin administration in group IV succeeded in decreasing LDL-C level to reach 71.89 ± 1.48 that was similar to healthy control's level (69.45 ± 2.49). Liver cholesterol in group III (69.84 ± 0.16) showed a significant elevation when compared to that (53.74 ± 1.57) of control group I. No significant change in liver triglycerides was observed.
Fig. 1.
Serum and liver lipid profile in different experimental groups. arepresents significance compared with the corresponding healthy control group I and brepresents significance compared with the corresponding diabetic group III (P < 0.05). Group I: healthy control rats, group II: melatonin administrated control rats, group III: non-treated STZ diabetic rats and group IV: melatonin treated STZ diabetic rats.
3.3. Glucose homeostasis
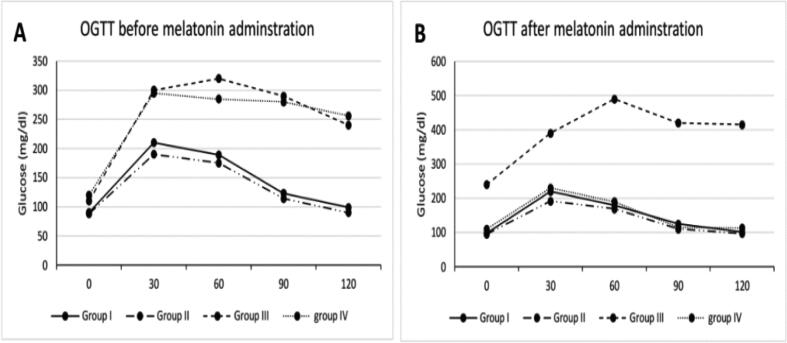
Fig. 2 shows OGTT results before and after melatonin administration. No significant difference, before melatonin administration (week 0), was recorded between different groups at 0 min. After 30 min of glucose administration, group III and IV recorded a high blood glucose level than groups I and II. This behaviour continued till 120 min (Fig. 2A). After 8 weeks of melatonin administration, a high blood glucose level in non– treated diabetic group III was observed at 0 till 120 min in comparison to control groups I and II. Melatonin treated diabetic group IV showed a blood glucose level resembled to that of control groups I and II (Fig. 2B). Table 2 showed values of area under curve (AUC). At 0 week, a significant elevation in AUC was noticed in group III (32550 ± 3547) in comparison to those of groups I and II (18495 ± 2530 and 17040 ± 1981, respectively). No significant difference was observed between diabetic group III and treated group IV. After 8 weeks, AUC value of both healthy control I and treated group IV were nearly the same.
Fig. 2.
Oral glucose tolerance test (OGTT): (A) before; and (B) after 8-week of melatonin administration. Group I: healthy control rats, group II: melatonin administrated control rats, group III: non-treated STZ diabetic rats and group IV: melatonin treated STZ diabetic rats.
Table 2.
Area under curve (AUC) for OGTT before and after 8 weeks of melatonin administration.
| Time | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Week 0 | 18495 ± 2530 | 17040 ± 1981 | 32550 ± 3547a | 31440 ± 1245a |
| Week 8 | 18735 ± 6578 | 17010 ± 2491 | 48825 ± 4235a | 21045 ± 1478b |
In each raw, results were expressed as mean ± SD, arepresents significance compared with the corresponding healthy control group I and brepresents significance compared with the corresponding diabetic group III (P < 0.05). Group I: healthy control rats, group II: melatonin administrated control rats, group III: non-treated STZ diabetic rats and group IV: melatonin treated STZ diabetic rats.
3.4. Oxidative stress parameters
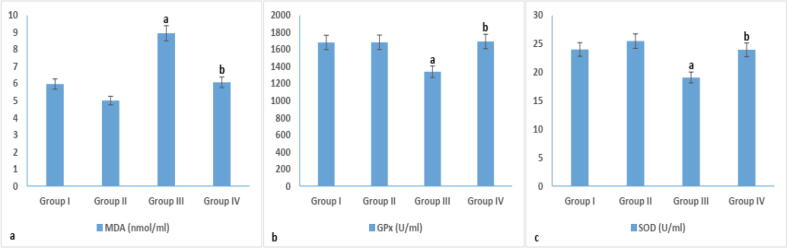
Diabetes induction by STZ in group III caused a reduction in serum SOD and GPx levels (19.09 ± 2.41 and 1340.52 ± 5.69, respectively) and an elevation in MDA level, 8.95 ± 1.46, when compared to control groups I and II (Fig. 3). However, a reduction in MDA level (6.08 ± 2.06) was observed in melatonin treated group IV in comparison to group III. On the other hand, an elevation in SOD level (23.97 ± 0.67) was recorded in group IV in comparison to that of group III (19.09 ± 2.41) (Fig. 3).
Fig. 3.
Oxidative stress in different experimental groups. arepresents significance compared with the corresponding healthy control group I and brepresents significance compared with the corresponding diabetic group III (P < 0.05). Group I: healthy control rats, group II: melatonin administrated control rats, group III: non-treated STZ diabetic rats and group IV: melatonin treated STZ diabetic rats.
3.5. Immunological parameters
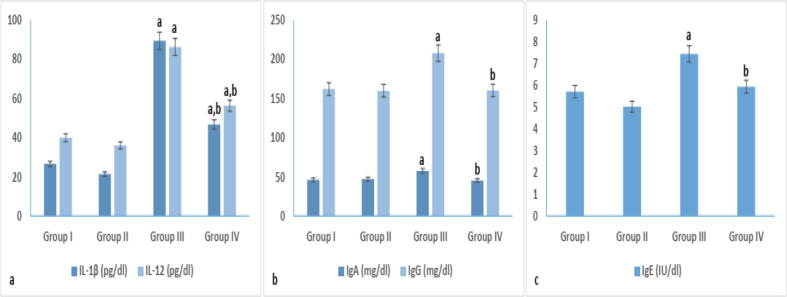
According to the immunological results in Fig. 4, immunoglobulin levels (57.51 ± 0.81, 7.44 ± 0.49 and 207.66 ± 1.63 for IgA, IgE and IgG, respectively) were significantly elevated in non-treated diabetic group III in comparison to control groups I and II. In melatonin treated diabetic group IV, Immunoglobulin levels showed a significant reduction to reach 45.45 ± 1.59 (IgA), 5.94 ± 0.94 (IgE) and 160.21 ± 2.06 (IgG) when compared to those of non-treated diabetic group III.
Fig. 4.
Immunological parameters (cytokines and immunoglobulin) in different experimental groups. arepresents significance compared with the corresponding healthy control group I and brepresents significance compared with the corresponding diabetic group III (P < 0.05). Group I: healthy control rats, group II: melatonin administrated control rats, group III: non-treated STZ diabetic rats and group IV: melatonin treated STZ diabetic rats.
A significant elevation in level of pro-inflammatory cytokine IL-1β (89.34 ± 0.87) and IL-12 (86.21 ± 2.40) was observed upon diabetes induction in group III in comparison to healthy controls. Melatonin administration in group IV succeeded in reducing pro-inflammatory cytokines to reach 46.72 ± 0.68 and 56.30 ± 0.59 for IL-1β and IL-12, respectively, when compared to non-treated diabetic group III. However, levels of cytokines in group IV remains significantly higher than those of healthy controls.
3.6. Histopathological and immunhistochemical studies
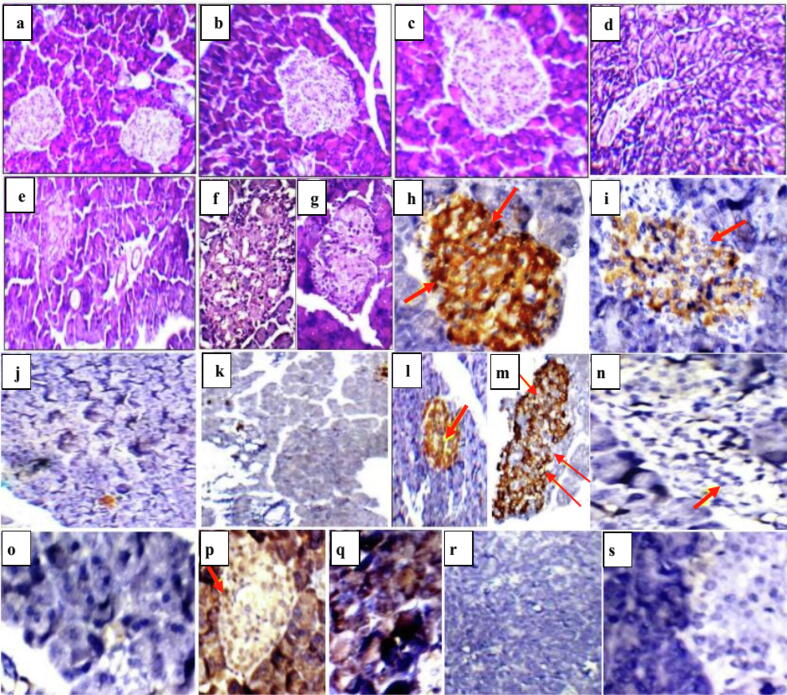
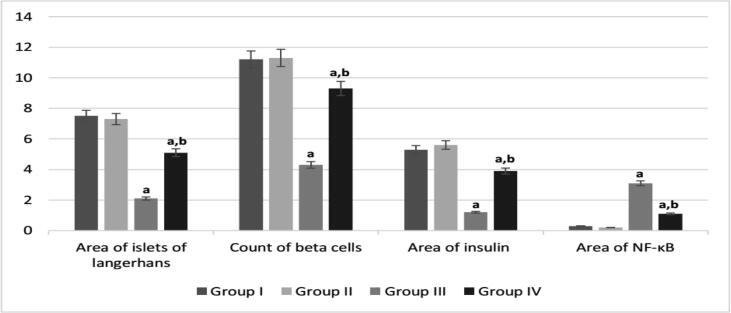
Pancreatic sections of healthy control groups I and II revealed average-sized pale-staining islets of Langerhans. Induction of diabetes by STZ in group III caused a size reduction and deformation of pancreatic islet as a result of destruction of the β cells. On the other hand, melatonin administration in diabetic group IV helped in restoring the islets of Langerhans as shown in Fig. 5. Immunhistochemical staining of pancreatic sections of healthy control groups I and II demonstrated normal brown coloured areas of insulin-immunoreactive β cells and no expression of nuclear transcription factor NF-κB (Fig. 5). Diabetic group III showed small areas of insulin-secreting β cells and high expression of NF-κB as compared to control groups I and II. Larger areas of β cells were observed in melatonin administrated diabetic group IV accompanied with a reduction in NF-κB expression when compared to diabetic group III. According to Fig. 6, diabetic group III showed a significant reduction in area of islets, β cell count and insulin area; and a significant elevation in NF-κB expression. Melatonin administration in group IV, reduced NF-κB expression, enhanced β cells regeneration, increased area of islets and secreted insulin.
Fig. 5.
Pancreatic sections of rat showing a], b] and c] normal sized stained islets of Langerhans in healthy control group I and melatonin administrated control group II (H&E; x200&400, respectively), d] and e] small-sized hypocellular pale-staining islets of Langerhans in non-treated diabetic group III (H&E; x200&400, respectively), f] and g] restored islets of Langerhans in melatonin treated diabetic group IV (H&E; x400), h] and i] positive anti-insulin antibody reaction as indicated by brown colour (group I and II; x400), j] and k] negative anti-insulin antibody reaction in non-treated diabetic group III (x200), l] and m] positive anti-insulin antibody reaction in melatonin treated diabetic group IV (x200&x400, respectively), n] and o] no NF- κB expression in group I and II (x400), p] and q] significant elevation of NF- κB expression as brown colour indicates positive immunstaining in non treated diabetic group III, r] and s] no NF- κB expression in melatonin treated diabetic group IV (x200&400, respectively).
Fig. 6.
Quantitative analysis of histopathological and immunohistochemial results. arepresents significance compared with the corresponding healthy control group I and brepresents significance compared with the corresponding diabetic group III (P < 0.05). Group I: healthy control rats, group II: melatonin administrated control rats, group III: non-treated STZ diabetic rats and group IV: melatonin treated STZ diabetic rats.
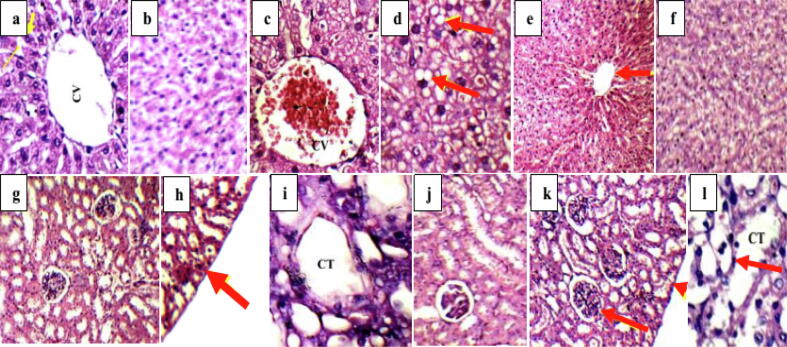
Liver sections of normal control group I and melatonin administrated group II showing average central veins with average hepatocytes that were arranged in single cell cords. On the other hand, mildly dilated congested central veins with marked micro-vesicular steatosis of hepatocytes were observed in liver sections of diabetic untreated group III. Melatonin administration, in group IV, decreased inflammation in liver sections where average central veins with average hepatocytes were obvious in Fig. 7.
Fig. 7.
a] and b] liver sections of normal control group I and melatonin administrated group II showing average central veins with average hepatocytes arranged in single cell cords and average intervening blood sinusoids (H&E; x400), c] and d] liver sections of diabetic untreated group III showing mildly dilated congested central veins with marked micro-vesicular steatosis of hepatocytes (arrows) (H&E; x400), e] and f] liver sections of melatonin treated diabetic group IV showing average central veins (arrow) with average hepatocytes (H&E; x200& 400, respectively), g] and h] Kidney sections of normal control group I and melatonin administrated group II showing average renal capsule (arrow), average glomeruli with average proximal tubules and preserved brush borders (H&E, x200), i] and j] kidney sections of diabetic untreated group III showing small-sized glomeruli with widened Bowman’s spaces, proximal tubules with mildly vacuolated epithelial lining and partial loss of brush borders (H&E, x400), k] and l] kidney sections of melatonin treated diabetic group IV showing average renal capsule (head arrow), average glomeruli with average proximal tubules, normal epithelial lining and preserved brush borders (arrow) (H&E, x400). CV: central vein and CT: collecting tubules.
Average renal capsule, average glomeruli with average proximal tubules and preserved brush borders were observed in kidney sections of normal control group I and melatonin administrated group II showing (Fig. 7). Diabetes induction in group III distorted the rats’ kidney, where small-sized glomeruli with widened Bowman’s spaces, proximal tubules with mildly vacuolated epithelial lining and partial loss of brush borders were demonstrated in Fig. 7. kidney sections of melatonin treated diabetic group IV showed average renal capsule, average glomeruli with average proximal tubules, normal epithelial lining and preserved brush borders.
4. Discussion
Melatonin regulates sleep, circadian and seasonal rhythms, and immune response; it is recommended in treatment of blind circadian rhythm sleep disorders, delayed sleep phase and insomnia (Bravo et al., 2013). It is a potent antioxidant (Tan et al., 2000), anti-inflammtory (Nabavi et al., 2019), free radical scavenger, useful in fighting infectious diseases (Maestroni, 2001). A reduction in melatonin secretion and increased insulin resistance has been reported with age; however, melatonin supplementation can relief these symptoms (Singh and Jadhav, 2014). Also, She et al. (2014) reported that melatonin administration reduced insulin resistance and elevated glucose intake in 3 T3-L1 adipocytes. Hardeland (2019) found that decreased level of circulating melatonin was associated with losing the anti-oxidant protection and anti-inflammatory effects.
In Goto Kakizaki diabetic rats, diabetes was linked to reduced melatonin level in blood (Frese et al., 2009). Balduini et al. (2019) reported that melatonin should be used with caution by people on hypertension or hypoglycemic medications due to its effect on lowering blood glucose level and hypertension. The expression of glucose transporter type IV gene was down regulated, in pinealectomized animals, leading to insulin resistance and glucose intolerance; exogenous melatonin administration has alleviated these symptoms (Rodrigues et al., 2013, Sun et al., 2018). Milosavljevi et al. (2018) reported the reduced melatonin level in human dental pulp tissues of diabetic patients; and added that exogenous melatonin administration increased the antioxidant enzymes level and protect the dental pulp tissue. In rats with STZ-induced diabetes, insulin and melatonin co-treatment for two months (1.5 U/100 g/day for insulin and 0.2 mg/kg/day in drinking water for melatonin) improved glucose homeostasis and insulin sensitivity in white adipose tissue compared to using only one of these treatments (Oliveira et al., 2018). Obese mice with insulin resistance have lower melatonin production, according to Sartori et al. (2009); who also found that melatonin therapy (100 mg/kg/day in drinking water for two months) improved glucose tolerance. Behram et al. (2018) showed that type II diabetic patients, at night, release lower melatonin than healthy individuals. Recent studies found that a single dose of melatonin (1–5 mg) decreases the tolerance of glucose tests at night and day in normal post- and premenopausal females (Heo et al., 2018, Xu et al., 2019). Melatonin decreased HbA1c levels when administered for five months according to Garfinkel et al. (2011); and enhanced the glycemic control in diabetic patients according to Rezvanfar et al. (2017). Kadhim et al. (2006) showed that administration of ten mg of melatonin with metformin, in poorly controlled type II diabetic patients, generated a higher cellular response. Sun et al. (2018) found that treatment with three mg of melatonin per day, for twelve weeks, improved insulin sensitivity and reduced inflammation in obese individuals with Acanthosis Nigricans. According to the review of Doosti-Irani et al. (2018), melatonin has a role in regulating glycemic homeostasis by increasing insulin sensitivity and decreasing the level of fasting glucose.
In this study, daily administration of 10 mg/kg melatonin in STZ diabetic rats has led to the reduction in FBG and FTA levels which were similar to the healthy control levels. STZ induced diabetes lead to insulin deficiency in untreated group to record an insulin level of 0.91 ng/ml; however, melatonin administration elevated serum insulin level (2.8 ng/ml) in treated group. After 8 weeks of melatonin administration, group IV showed a significantly lower AUC compared to non-treated group III. Thus, melatonin succeeded in the elevation of insulin level, glucose sensitivity and delayed diabetic progression. The same results were observed in diabetic model treated with melatonin in the study of Agil et al. (2012). Our results showed an improvement in kidney and liver function enzymes after melatonin administration. This was proofed by the histopathological examination of both liver and kidney. Where, liver sections of treated group showed normal liver architecture when compared to untreated diabetic group. Also, kidney sections of melatonin treated diabetic group showed average renal capsules, average proximal tubules, normal epithelial lining and preserved brush borders. Usually, high blood glucose level leads to high levels of triglycerides and cholesterol; according to He et al. (2015), STZ induced hyperglycemia led to hypertriglyceridemia and hypercholesterolemia. Our results were in agreement with that, where non-treated diabetic group had elevated hepatic cholesterol; and serum cholesterol, triglycerides and LDL-C levels.
Diabetes refers to a group of metabolic disorders that is characterized by hyperglycemia. Hypoglycemia inside body's cells provokes glucogenesis and gluconeogenesis, which causes breakdown of fat (known as diabetic ketoacidosis) and a reduction in synthesis of protein (causes weight and muscle loss, increased hunger, and poor wound healing), whereas extracellular hyperglycemia causes glycemic coma and increased urination (Ozougwu et al., 2013). The progression of vascular problems in diabetes is thought to be influenced by the oxidative stress (Pham-Huy, 2008). Increased production of free radicles, in diabetes, was linked to the reduction in the antioxidant enzymes like CAT, SOD, and GPx; which in turn led to tissues damage (Lipinski, 2001). Mortalities among diabetic patients, according to the epidemiological research of Pham-Huy (2008), were primarily due to an increase in vascular disorders rather than elevated blood glucose level. In diabetes, oxidative stress caused enzymes damage and elevated insulin resistance; where accumulation of free radicles was due to protein glycation, oxidation of glucose, and enhanced lipid peroxidation (Maritim et al., 2003). The main causes of oxidative stress are mitochondria; where a part of oxygen is reduced to water, and the leftover oxygen is converted to oxygen free radical (O-), a major reactive oxygen species that is transformed to other reactive species such as ONOO–, OH–, and H2O2 (Moussa, 2008) that in turn modulates insulin signalling (Erejuwa, 2012). The pathogenesis of diabetes mellitus has been linked to chronic oxidative stress and inflammation. According to Ambade and Mandrekar (2012), in pathological states, inflammation and oxidative stress are related closely; moreover, chronic inflammation promotes cellular damage primarily through the generation of free radicals in excess and the depletion of antioxidants enzymes (Fialkow et al., 2007). Free radicles accumulation drives pro-inflammatory cytokine expression by activating transcription factors like NF-kB and activator protein-1 (Oguntibeju, 2019). Due to natural negative feedback mechanisms, like increased synthesis of anti-oxidant chemicals or anti-inflammatory cytokines; oxidative stress and immune response stimulation were generally short-lived under healthy conditions. Both oxidative stress and inflammation become activated in certain chronic disease states, like diabetes, and can have a positive or negative impact on diabetes control depending on a variety of parameters (Hold and El-Omar, 2008).
As a result, inhibiting oxidative stress and inflammatory cytokines signaling mechanisms is a promising technique for diabetes treatment. In this study, melatonin administration decreased serum and hepatic cholesterol, serum triglycerides and serum LDL-C; thus melatonin acted as hypocholesterolemic agents and improved lipid profile in STZ diabetic rats. Other studies demonstrated the melatonin effect on lowering cholesterol absorption in high cholesterol fed rats, which in turn led to a significant reduction in the levels of cholesterol and triglyceride in plasma and liver (Hussain, 2007). Several studies reported the reduction effect of melatonin, independence of blood glucose level, on elevated MDA level in diabetic models (Salmanoglu et al., 2016). These studies were in agreement with our study, where melatonin administration lowered MDA; and elevated both SOD and GPx in treated rats. Melatonin was reported as an anti-oxidant agent; however, its effect on SOD and GPx was controversial. Where, Salmanoglu et al. (2016) reported that melatonin did not increase the activity of both SOD and GPx. Other studies reported that melatonin elevated SOD and GPx levels; Kahya and Nazıroğlu (2016) showed that melatonin up-regulate GSH and GPx levels and down-regulate oxidative stress in diabetic rats. Salmanoglu et al. (2016) found that melatonin, as an anti-oxidant, decreased MDA level in type II diabetic rats.
Diabetes is characterized by oxidative stress and chronic inflammation in pancreas that results from β cell death or injury of the islets of Langerhans; and the subsequent infiltration of macrophages and dendritic cells to the site of injury. The infiltrated mononuclear cells secrete pro-inflammatory cytokines as IL-1β and TNF-α that recruit T cells (Nakayama et al., 2005). Many studies proofed the role of IL-12 in the development of diabetes, where its administration induced premature diabetes onset with high level of INF-γ and high numbers of pancreas-infiltrating dendritic cells as well as Th1/Tc1 cells (Trembleau et al., 1995). IL-12 antagonists' administration reduced T1DM development in 3 weeks age mice (Adorini et al., 2001). High levels of different immunoglobulin were reported in patients with developing diabetes (Isomaa et al., 1999). However, our STZ diabetic animal model exhibited all the previous immunological abnormalities; where high level of IL-1β, IL-12 and immunoglobulins were observed in diabetic non-treated group. Furthermore, melatonin administration reduced the levels of cytokines and immunoglobulins that was subsequently reflected on reducing pancreatic inflammation and allowing regeneration of β cells. This was very obvious in pancreatic tissue section of melatonin treated group, where the islets size was improved to some extend in comparison to non-treated group. This improvement can be confirmed by the elevation in insulin level after melatonin administration. Also, this improvement was obvious in pancreatic sections stained by immunohistochemical methods, where melatonin administrated group showed brown coloured islets area larger than that of untreated diabetic group indicating an elevation in insulin secretion. Furthermore, expression of NF-κB was significantly reduced in melatonin treated diabetic group more than untreated diabetic group. This reduced expression was reflected on NF-κB target genes that in turn reduced pro-inflammatory cytokines (IL-1β and IL-12) production to give a chance for β cells regeneration. Melatonin administration in many animal models suppress NF-κB expression (Di Stefano and Paulesu, 1993, Habtemariam et al., 2017, Vriend and Reiter, 2014); as the work of Colombo et al., 2018 who reported the reduction of NF-κB gene expression in cancerous cells. In addition to Chuang et al. (1996) who reported that 10 mg/kg of exogenous melatonin decreased the binding activity of NF-κB to DNA in spleen cells after an hour of its administration. Moreover the work of Li et al., 2005 and Ahmed et al. (2022) showed that melatonin has an anti-colitis effect due to the suppression of NF-κB.
Previous studies reported the protective role of melatonin in pancreatitis, where it reduced the inflammatory markers such as edema, neutrophil recruitment and cells vacuolization (Sadek et al., 2017). Experimental models of acute pancreatitis treated with melatonin showed improvement of blood flow and reduced tissue damage (Baykal et al., 2000). Jaworek et al., 2012 reported pancreatic regeneration by melatonin administration after the recovery from acute pancreatitis. It was suggested that melatonin had a therapeutic effect on STZ diabetic rats by reducing oxidative markers and protection of β cells.
In conclusion, melatonin administration in STZ diabetic rats reduced hyperglycemia, hyperlipidemia and oxidative stress; moreover, improvement in liver and kidney functions was noticed. This can be explained by the antioxidant and free radicles scavenging capabilities of melatonin, which, in turn reduced the production of pro-inflammatory cytokines. Therefore, melatonin was helpful in restoring the islets of Langerhans allowing insulin production and diabetes management. It can be concluded that melatonin effectively protected β cells under severe inflammatory situations as a result of its antioxidant effect. However, further studies are required for monitoring blood glucose level and inflammatory cytokines in non-diabetic healthy control individuals that received melatonin for insomnia and improving sleep in different conditions. This can be followed by clinical trials in diabetic patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Monaem G., Farid A., Rabia I., El-Amir A. Evaluation of Echinostoma liei worm, metacercaria and redia antigens for schistosomiasis control. Experimental Parasitology. 2015;157:23–29. doi: 10.1016/j.exppara.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Adorini L. Interleukin 12 and autoimmune diabetes. Nature Genet. 2001;27(2):131–132. doi: 10.1038/84732. [DOI] [PubMed] [Google Scholar]
- Agil A., Rosado I., Ruiz R., Figueroa A., Zen N., Fernández-Vázquez G. Melatonin improves glucose homeostasis in young zucker diabetic fatty rats. J. Pineal Res. 2012;52(2):203–210. doi: 10.1111/j.1600-079X.2011.00928.x. [DOI] [PubMed] [Google Scholar]
- Ahmad Haja Y., Rai S., Basheer M., Ghosh H., Singh S. Protective role of melatonin in streptozotocin induced pancreatic damages in diabetic wistar rat. Pak J. Biol. Sci. 2018;21(9):423–431. doi: 10.3923/pjbs.2018.423.431. [DOI] [PubMed] [Google Scholar]
- Ahmed O., Abdel-Halim M., Farid A., Elamir A. Taurine loaded chitosan-pectin nanoparticle shows curative effect against acetic acid-induced colitis in rats. Chemico-Biological Interactions. 2022;351:109715. doi: 10.1016/j.cbi.2021.109715. [DOI] [PubMed] [Google Scholar]
- Ahmed O., Farid A., Elamir A. Dual role of melatonin as an anti-colitis and anti-extra intestinal alterations against acetic acid-induced colitis model in rats. Sci. Rep. 2022;12:6344. doi: 10.1038/s41598-022-10400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral F.G., Turati A.O., Barone M., Scialfa J.H., do Carmo Buonfiglio D., Peres R., Peliciari-Garcia R.A., Afeche S.C., Lima L., Scavone C., Bordin S., Reiter R.J., Menna-Barreto L., Cipolla-Neto J. Melatonin synthesis impairment as a new deleterious outcome of diabetes-derived hyperglycemia. J. Pineal Res. 2014;57(1):67–79. doi: 10.1111/jpi.12144. [DOI] [PubMed] [Google Scholar]
- Ambade A., Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int. J. Hepatol. 2012;2012:1–9. doi: 10.1155/2012/853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M.A., Eisenbarth G.S., Michels A.W. type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduini W., Weiss M.D., Carloni S., Rocchi M., Sura L., Rossignol C., Longini M., Bazzini F., Perrone S., Ott D., Wadhawan R., Buonocore G. Melatonin pharmacokinetics and dose extrapolation after enteral infusion in neonates subjected to hypothermia. J. Pineal Res. 2019;66(4):e12565. doi: 10.1111/jpi.12565. [DOI] [PubMed] [Google Scholar]
- Baykal A., Iskit A.B., Hamaloglou E., Oguz M., Hascelik G., Sayek Y. Melatonin modulates mesenteric blood flow and TNF alpha concentrations after lipopolysaccharide challenge. Eur. J. Surg. 2000;166:722–727. doi: 10.1080/110241500750008484. [DOI] [PubMed] [Google Scholar]
- Behram K.Y., Guntekin U., Tosun V., Korucuk N., Bozdemir M.N. Melatonin protects against streptozotocin-induced diabetic cardiomyopathy by the phosphorylation of vascular endothelial growth factor-A (VEGF-A) Cell Mol. Biol. 2018;64(14):47–52. [PubMed] [Google Scholar]
- Bouwens L., Rooman I. Regulation of pancreatic beta-cell mass. Physiol. Rev. 2005;85(4):1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- Bravo R., Matito S., Cubero J., Paredes S.D., Franco L., Rivero M., Rodríguez A.B., Barriga C. Tryptophan-enriched cereal intake improves nocturnal sleep melatonin serotonin and total antioxidant capacity levels and mood in elderly humans. Age (Dordr) 2013;35(4):1277–1285. doi: 10.1007/s11357-012-9419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J.I., Mohan N., Meltz M.L., Reiter R.J. Effect of melatonin on NF-kappa-B DNA-binding activity in the rat spleen. Cell Biol. Int. 1996;20(10):687–692. doi: 10.1006/cbir.1996.0091. [DOI] [PubMed] [Google Scholar]
- Colombo J., Jardim-Perassi B.V., Ferreira J.P.S., Braga C.Z., Sonehara N.M., Júnior R.P., Moschetta M.G., Girol A.P., Zuccari D.A.P.C. Melatonin differentially modulates NF-кB expression in breast and liver cancer cells. Anticancer Agents Med. Chem. 2018;18(12):1688–1694. doi: 10.2174/1871520618666180131112304. [DOI] [PubMed] [Google Scholar]
- Di Stefano A., Paulesu L. Inhibitory effect of melatonin on production of IFN gamma or TNF alpha in peripheral blood mononuclear cells of some blood donors. J. Pineal Res. 1993;17(4):164–169. doi: 10.1111/j.1600-079x.1994.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Dong Y., Fan C., Hu W., Jiang S., Ma Z., Yan X., Deng C., Di S., Xin Z., Wu G., Yang Y., Reiter R.J., Liang G. Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J. Pineal Res. 2016;60(3):253–262. doi: 10.1111/jpi.12300. [DOI] [PubMed] [Google Scholar]
- Donga E., van Dijk M., van Dijk J.G., Biermasz N.R., Lammers G.-J., van Kralingen K., Hoogma R.P., Corssmit E.P., Romijn J.A. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care. 2010;33:1573–1577. doi: 10.2337/dc09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doosti-Irani, A., Ostadmohammadi, V., Mirhosseini, N., Mansournia, M.A., Reiter, R.J., Kashanian, M., Rahimi, M., Razavi, M., Asemi, Z., 2018. Correction: the effects of melatonin supplementation on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Hormone Metabol Res 50(11), e6. [DOI] [PubMed]
- Eleazu C.O., Eleazu K.C., Chukwuma S., Essien U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013;12(1):60. doi: 10.1186/2251-6581-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erejuwa O.O. Oxidative stress in diabetes mellitus: is there a role for hypoglycemic drugs and/or antioxidants. Oxid. Stress Dis. 2012:217–246. [Google Scholar]
- Farid A., Abdel Malek A., Rabie I., Helmy A., El Amir A.M. Overview on cysteine protease inhibitors as chemotherapy for schistosomiasis mansoni in mice and also its effect on the parasitological and immunological profile. Pakistan Journal of Biological Sciences. 2013;16(24):1849–1861. doi: 10.3923/pjbs.2013.1849.1861. [DOI] [PubMed] [Google Scholar]
- Farid A., El-Dewak M., Safwat G., Diab A. Anti-apoptotic and antioxidant effects of melatonin protect spleen of whole body γ-irradiated male Sprague-dawley rats. International Journal of Radiation Research. 2021;19(4):861–872. [Google Scholar]
- Farid A., Hany A., Khaled A., Safwat G. Cytokines and autoantibodies profile during systemic lupus erythematosus and psoriasis diseases in Egypt. Journal of King Saud University-Science. 2022;34(4):102007. [Google Scholar]
- Farid A., Hesham M., El-Dewak M., Amin A. The hidden hazardous effects of stevia and sucralose consumption in male and female albino mice in comparison to sucrose. SPJ. 2020;28(10):1290–1300. doi: 10.1016/j.jsps.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid A., Yousry M., Safwat G. Garlic (Allium sativum Linnaeus) improved inflammation and reduced cryptosporidiosis burden in immunocompromised mice. Journal of Ethnopharmacology. 2022;292:115174. doi: 10.1016/j.jep.2022.115174. [DOI] [PubMed] [Google Scholar]
- Fialkow L., Wang Y., Downey G.P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007;42(2):153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Frese T., Bach A.G., Mühlbauer E., Pönicke K., Brömme H.-J., Welp A., Peschke E. Pineal melatonin synthesis is decreased in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2009;85(13-14):526–533. doi: 10.1016/j.lfs.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Furman B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015;70:5.47.1–5.47.20. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- Garfinkel, D., Zorin, M., Wainstein, J., Matas, Z., Laudon, M., Zisapel, N., 2011. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes, metabolic syndrome and obesity: targets and therapy 4, 307-313 [DOI] [PMC free article] [PubMed]
- Habtemariam S., Daglia M., Sureda A., Selamoglu Z., Fuat Gulhan M., Mohammad Nabavi S. Melatonin and respiratory diseases: a review. Curr. Topics Med. Chem. 2016;17(4):467–488. doi: 10.2174/1568026616666160824120338. [DOI] [PubMed] [Google Scholar]
- Hajam Y.A., Rai S. Melatonin supplementation revives diabetic induced biochemical, histological and hematological impairments in rats. Heliyon. 2020;6(4):e03770. doi: 10.1016/j.heliyon.2020.e03770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajam Y.A., Rai S., Pandi-Perumal S.R., Brown G.M., Reiter R.J., Cardinali D.P. Coadministration of melatonin and insulin improves diabetes-induced impairment of rat kidney function. Neuroendocrinology. 2021 doi: 10.1159/000520280. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Aging melatonin and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 2019;20(5):1223. doi: 10.3390/ijms20051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Hao L., Fu X., Huang M., Li R. Severe hypertriglyceridemia and hypercholesterolemia accelerating renal injury: a novel model of type 1 diabetic hamsters induced by short-term high-fat / high-cholesterol diet and low-dose streptozotocin. BMC Nephrol. 2015;16:51. doi: 10.1186/s12882-015-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy S., Farid A., Rabae I., El-Amir A. Novel IMB-ELISA assay for rapid diagnosis of human toxoplasmosis using SAG1 antigen. Japanese Journal of Infectious Diseases. 2015;68(6):474–480. doi: 10.7883/yoken.JJID.2014.444. [DOI] [PubMed] [Google Scholar]
- Heo J.-I., Yoon D.W., Yu J.H., Kim N.H., Yoo H.J., Seo J.A., Kim S.G., Choi K.M., Baik S.H., Choi D.S., Kim N.H. Melatonin improves insulin resistance and hepatic steatosis through attenuation of alpha-2-HS-glycoprotein. J. Pineal Res. 2018;65(2):e12493. doi: 10.1111/jpi.12493. [DOI] [PubMed] [Google Scholar]
- Hold G.L., El-Omar E.M. Genetic aspects of inflammation and cancer. Biochem. J. 2008;410:225–235. doi: 10.1042/BJ20071341. [DOI] [PubMed] [Google Scholar]
- Hussain S.-R. Effect of melatonin on cholesterol absorption in rats. J. Pineal Res. 2007;42(3):267–271. doi: 10.1111/j.1600-079X.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- Isomaa B., Almgren P., Henricsson M., Taskinen M.R., Tuomi T., Groop L., Sarelin L. Chronic complications in patients with slowly progressing autoimmune type I diabetes (LADA) Diabetic Care. 1999;8:1347–1353. doi: 10.2337/diacare.22.8.1347. [DOI] [PubMed] [Google Scholar]
- Jaworek J., Szklarczyk J., Jaworek A.K., Nawrot-Porąbka K., Leja-Szpak A., Bonior J., Kot M. Protective effect of melatonin on acute pancreatitis. Int. J. Inflam. 2012;2012:1–8. doi: 10.1155/2012/173675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhim H.M., Ismail S.H., Hussein K.I., Bakir I.H., Sahib A.S., Khalaf B.H., Hussain S.-R. Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with metformin. J. Pineal Res. 2006;41(2):189–193. doi: 10.1111/j.1600-079X.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- Kahya M.C., Nazıroğlu M. Melatonin reduces lens oxidative stress level in STZ-induced diabetic rats through supporting glutathione peroxidase and reduced glutathione values. J. Cell Neurosci. Oxid. Stress. 2016;8(2):588–594. [Google Scholar]
- Kamel D., Farid A., Ali E., ...Hendawy M., El Amir A.M. Diagnostic potential of target Giardia lamblia specific antigen for detection of human giardiasis using coproantigen sandwich ELISA. World Journal of Medical Sciences. 2013;9(2):113–122. [Google Scholar]
- Kanter M., Uysal H., Karaca T., Sagmanligil H.O. Depression of glucose levels and partial restoration of pancreatic beta-cell damage by melatonin in streptozotocin-induced diabetic rats. Arch Toxicol. 2006;80(6):362–369. doi: 10.1007/s00204-005-0055-z. [DOI] [PubMed] [Google Scholar]
- Klepac N., Rudes Z., Klepac R. Effects of melatonin on plasma oxidative stress in rats with streptozotocin induced diabetes. Biomed. Pharmacother. 2006;60(1):32–35. doi: 10.1016/j.biopha.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- Li J.H., Yu J.P., Yu H.G., Xu X.M., Yu L.L., Liu J., Luo H.S. Melatonin reduces inflammatory injury through inhibiting NF-kappa B activation in rats with colitis. Mediators Inflamm. 2005;2005(4):185–193. doi: 10.1155/MI.2005.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski B. Pathophysiology of oxidative stress in diabetes mellitus. J. Diabetes Complications. 2001;15(4):203–210. doi: 10.1016/s1056-8727(01)00143-x. [DOI] [PubMed] [Google Scholar]
- Maestroni G.JM. The immunotherapeutic potential of melatonin. Expert Opin. Investig. Drugs. 2001;10(3):467–476. doi: 10.1517/13543784.10.3.467. [DOI] [PubMed] [Google Scholar]
- Maritim A.C., Sanders R.A., Watkins J.B. Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Milosavljević A., DJukić L.j., Toljić B., Milašin J., DŽeletović B., Brković B., Roganović J. Melatonin levels in human diabetic dental pulp tissue and its effects on dental pulp cells under hyperglycaemic conditions. Int. Endodon. J. 2018;51(10):1149–1158. doi: 10.1111/iej.12934. [DOI] [PubMed] [Google Scholar]
- Mohie El-Dinn M.M., Abdel-Aal E.S., Eid L.N., Farid A.A., Aly I.R., El-Deeb S.O. Effect of Bacilli as feed additive on immune response of pregnant she-camel and its newborn calf. Archivos de zootecnia. 2018;67(258):270–277. [Google Scholar]
- Moussa S.A. Oxidative stress in diabetes mellitus. Romanian J. Biophys. 2008;18(3):225–236. [Google Scholar]
- Nabavi S.M., Nabavi S.F., Sureda A., Xiao J., Dehpour A.R., Shirooie S., Silva A.S., Baldi A., Khan H., Daglia M. Anti-inflammatory effects of Melatonin: a mechanistic review. Crit. Rev. Food Sci. Nutr. 2019;59(sup1):S4–S16. doi: 10.1080/10408398.2018.1487927. [DOI] [PubMed] [Google Scholar]
- Nakayama M., Abiru N., Moriyama H., Babaya N.L., Iu E., Miao D., Yu L., Wegmann D.R., Hutton J.C., Elliott J.F., Eisenbarth G.S. Prime role for an insulin epitope in the development of type 1 diabetes in nod mice. Nature. 2005;435(7039):220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguntibeju O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019;11(3):45–63. [PMC free article] [PubMed] [Google Scholar]
- Oliveira A.C.d., Andreotti S., Sertie R.A.L., Campana A.B., de Proença A.R.G., Vasconcelos R.P., Oliveira K.A.d., Coelho-de-Souza A.N., Donato-Junior J., Lima F.B. Combined treatment with melatonin and insulin improves glycemic control, white adipose tissue metabolism and reproductive axis of diabetic male rats. Life Sci. 2018;199:158–166. doi: 10.1016/j.lfs.2018.02.040. [DOI] [PubMed] [Google Scholar]
- Ozougwu J.C., Obimba K.C., Belonwu C.D., Unakalamba C.B. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013;4(4):46–57. [Google Scholar]
- Parasuraman S., Raveendran R., Kesavan R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010;1(2):87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedro P.F., Tsakmaki A., Bewick G.A. The glucose tolerance test in mice. Methods Mol. Biol. 2020;2128:207–216. doi: 10.1007/978-1-0716-0385-7_14. [DOI] [PubMed] [Google Scholar]
- Peschke E., Stumpf I., Bazwinsky I., Litvak L., Dralle H., Mühlbauer E. Melatonin and type 2 diabetes - a possible link? J. Pineal Res. 2007;42(4):350–358. doi: 10.1111/j.1600-079X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. IJBS. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- Rezvanfar M.R., Heshmati G., Chehrei A., Haghverdi F., Rafiee F., Rezvanfar F. Effect of bedtime melatonin consumption on diabetes control and lipid profile. Int. J. Diabetes Dev. Count. 2017;37(1):74–77. [Google Scholar]
- Rodrigues S.C., Pantaleão L., Lellis‐Santos C., Veras K., Amaral F., Anhê G., Bordin S. Increased corticosterone levels contribute to glucose intolerance induced by the absence of melatonin. Feder Am Soc Exp Biol. 2013;27(S1):1161. [Google Scholar]
- Saberzadeh-Ardestani B., Karamzadeh R., Basiri M., Hajizadeh-Saffar E., Farhadi A., Shapiro A.M.J., Tahamtani Y., Baharvand H. Type 1 diabetes mellitus, cellular and molecular pathophysiology at A glance. Autumn. 2018;20(3):294–301. doi: 10.22074/cellj.2018.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek A.S., Khattab R.S. The protective role of melatonin on l-arginine-induced acute pancreatitis. Folia Morphol. 2017;76:66–73. doi: 10.5603/FM.a2016.0029. [DOI] [PubMed] [Google Scholar]
- Salmanoglu D.S., Gurpinar T., Vural K., Ekerbicer N., Darıverenli E., Var A. Melatonin and l-carnitin improves endothelial disfunction and oxidative stress in type 2 diabetic rats. Redox Biol. 2016;8:199–204. doi: 10.1016/j.redox.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori C., Dessen P., Mathieu C., Monney A., Bloch J., Nicod P., Scherrer U., Duplain H. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology. 2009;150(12):5311–5317. doi: 10.1210/en.2009-0425. [DOI] [PubMed] [Google Scholar]
- Shater H., Fawzy M., Farid A., El-Amir A., Fouad S., Madbouly N. B-cell activating factor and a proliferation-inducing ligand in relation to intima-media thickness as biomarkers of premature atherosclerosis in systemic lupus erythematosus patients. The American Journal of the Medical Sciences. 2022 doi: 10.1016/j.amjms.2022.05.008. [DOI] [PubMed] [Google Scholar]
- She M., Hou H., Wang Z., Zhang C., Laudon M., Yin W. Melatonin rescues 3T3-L1 adipocytes from FFA-induced insulin resistance by inhibiting phosphorylation of IRS-1 on ser307. Biochimie. 2014;103:126–130. doi: 10.1016/j.biochi.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Singh M., Jadhav H.R. Melatonin: Functions and ligands. Drug Discov. Today. 2014;19(9):1410–1418. doi: 10.1016/j.drudis.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Sun H., Wang X., Chen J., Gusdon A.M., Song K., Li L., Qu S. Melatonin treatment improves insulin resistance and pigmentation in obese patients with acanthosis nigricans. Int. J. Endocrinol. 2018;2018:1–7. doi: 10.1155/2018/2304746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D.X., Manchester L.C., Reiter R.J., Qi W.B., Karbownik M., Calvo J.R. Significance of melatonin in antioxidative defense system: reactions and products. Biol. Signals Receptors. 2000;9(3–4):137–159. doi: 10.1159/000014635. [DOI] [PubMed] [Google Scholar]
- Tesch G.H., Allen T.J. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology. 2007;12(3):261–266. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- Trembleau S., Penna G., Bosi E., Mortara A., Gately M.K., Adorini L. IL-12 administration induces Th1 cells and accelerates autoimmune diabetes in NOD mice. J. Exp. Med. 1995;181:817. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend J., Reiter R.J. Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 2014;115(1-2):8–14. doi: 10.1016/j.lfs.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Wu J., Yan L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. 2015;8:181–188. doi: 10.2147/DMSO.S82272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Chen F., Li W.A., Geng X., Li C., Meng X., Feng Y., Liu W., Yu F. A review of sleep disorders and melatonin. Neurol. Res. 2017;39(6):559–565. doi: 10.1080/01616412.2017.1315864. [DOI] [PubMed] [Google Scholar]
- Xu J., Gao H., Zhang L.i., Rong S., Yang W., Ma C., Chen M., Huang Q., Deng Q., Huang F. Melatonin alleviates cognition impairment by antagonizing brain insulin resistance in aged rats fed a high-fat diet. J. Pineal Res. 2019;67(2) doi: 10.1111/jpi.v67.210.1111/jpi.12584. [DOI] [PubMed] [Google Scholar]
- Yavuz O., Cam M., Bukan N., Guven A., Silan F. Protective effect of melatonin on beta-cell damage in streptozotocin-induced diabetes in rats. Acta Histochem. 2003;105(3):261–266. doi: 10.1078/0065-1281-00711. [DOI] [PubMed] [Google Scholar]
- Yin D., Tao J., Lee D.D., Shen J., Hara M., Lopez J., Kuznetsov A., Philipson L.H., Chong A.S. Recovery of islet beta-cell function in streptozotocin-induced diabetic mice: an indirect role for the spleen. Diabetes. 2006;55(12):3256–3263. doi: 10.2337/db05-1275. [DOI] [PubMed] [Google Scholar]
- Zhang X., Xie L., Zhong M., Yang B., Yang Q., Yang H., Xie C. The association between melatonin receptor 1B gene polymorphisms and type 2 diabetes mellitus (T2DM) in Chinese populations: a meta-analysis. Ann. Palliat Med. 2020;9(3):957–966. doi: 10.21037/apm-20-691. [DOI] [PubMed] [Google Scholar]