Abstract
Background:
Discussing advance care planning (ACP) with care partners may be a steppingstone to the completion of advance directives (ADs) for persons with cognitive impairment (PwCIs).
Objectives:
To examine whether PwCI-reported occurrence of and PwCI-care partner agreement about ACP discussions are associated with completion of ADs.
Design and Subjects:
We conducted a secondary, cross-sectional analysis of data from 1672 PwCI-care partner dyads in the BLINDED study. PwCIs were Medicare beneficiaries in the US, aged >65 years, and diagnosed with mild cognitive impairment or dementia. Care partners were identified by PwCIs as being most involved in their health care.
Measurements:
PwCIs’ completion of ADs was determined by 1 or more affirmative responses to dichotomous indicators for formalizing a living will, medical directive, or durable power of attorney for health care. Discussion occurrence was based on PwCI reports and agreement between PwCI and care partner reports of prior conversations about PwCIs’ ACP preferences between PwCIs and care partners.
Results:
In logistic regression models adjusted for PwCI and care partner characteristics, PwCIs who had (vs. had not) discussed ACP were 10% more likely to complete ADs. PwCIs from dyads agreeing (vs. disagreeing) a discussion occurred were 7% more likely to complete ADs. PwCIs from care dyads in agreement (vs. disagreement) about non-discussion were 11% less likely to formalize ADs.
Conclusions:
Discussing ACP with care partners plays a direct, positive role in completing ADs among PwCIs. Health care providers who approach ACP as a dyadic, communicative decision-making process from the outset may facilitate PwCIs’ uptake of ADs.
Keywords: advance care planning, advance directives, formal preparation, informal discussion, end-of-life care, mild cognitive impairment, dementia, care dyads
Introduction
Persons with cognitive impairment (PwCIs), which includes mild cognitive impairment (MCI) and dementia, become less able to independently make decisions about their own health care as cognitive impairment progresses and other significant life changes occur.1 Amid the unpredictable clinical trajectory of cognitive decline, it is paramount that PwCIs engage in advance care planning (ACP) while still cognitively intact to ensure their values, goals, and preferences for future health care are known and respected in the event of incapacitation.1–3 ACP comprises formal preparation and informal discussion.4,5 Formal preparation entails the completion of advance directives (ADs). ADs are legal documents or tools that enable individuals to predetermine the treatment they do or do not wish to receive should a future health crisis or other events leave them unable to make decisions about their medical care at that time.6,7 Among PwCIs, ADs have been associated with improved quality of care, reduced Medicare costs, increased receipt of care consistent with their advance wishes, and decreased hospitalization, hospital death, and intensive care unit utilization.8,9
Relative to ADs, an informal discussion has received less attention but is nonetheless critical in its own right.10,11 Informal discussion refers to, in part, meaningful conversations with care partners, practitioners, and others regarding one’s values, goals, and preferences for future care.5,12 Some health care providers consider informal discussion between PwCIs and care partners, or care dyads, to be 1 of the most important aspects of preparing for future care.5,12–15 As cognitive decline progresses, both PwCIs and health care providers increasingly depend on care partners to make decisions that adequately represent the PwCIs’ care wishes.16,17 Without informal discussion, care partners must make assumptions or use their own judgment about these wishes, which may be no more accurate than chance and can jeopardize the PwCIs’ receipt of desired care.4,10
Despite widespread recognition of the importance and benefits of ADs, completion remains low among PwCIs.3,18,19 Identifying modifiable factors that contribute to the completion of ADs may increase their uptake. It has been suggested, but not shown, that informal discussion maybe 1 such potential factor and serve as a steppingstone to ADs.3,5,20 ADs are considered most complete or effective when informal discussion precedes their completion, as discussion occurrence may signify that care partners know, understand, and are prepared to represent PwCIs’ choices.5,20 Informal discussion has been linked to decreased decisional conflict and improved psychosocial outcomes for PwCIs and care partners alike.21,22 Further, the extent to which PwCIs and care partners agree about their participation in informal discussion may also be influential for ADs. Informal discussion is a subjective experience, and disagreement about its occurrence is likely.23,24 Agreement about informal discussion may reflect consensus or understanding of PwCIs’ wishes that provide the impetus for more binding decisions about ACP.25
Thus, the present study examined the relationship between informal discussion about and formal preparation for ACP among PwCIs. We hypothesized that engagement in the informal discussion would be positively associated with completion of ADs based on (1) PwCIs’ self-reported discussion of ACP with care partners and (2) PwCI-care partner agreement about informal discussion occurrence.
Methods
Participants and Setting
Data derive from Caregivers’ Reactions and Experience (CARE), an add-on study of the Imaging Dementia – Evidence for Amyloid Scanning (IDEAS) Study. Methodological details about both studies have been previously reported.26 Briefly, the CARE IDEAS study examined how amyloid-β positron emission tomography scans influence clinical management among 18 295 Medicare beneficiaries in the US who are aged ≥65 years with MCI or dementia. A diagnosis of MCI or dementia was determined with a diagnostic and statistical manual of mental disorders, fourth edition and/or National Institutes of Aging-Alzheimer’s Association, and by verification from a dementia specialist within 2 years. Of the 3717 individuals who agreed to be contacted in the CARE IDEAS study, 2228 PwCIs and 1872 care partners completed the baseline telephone interview. Care partners were identified by study participants who reported having a family member or friend who was involved in their health care decisions and were willing to share that person’s contact information with the study team. All participants provided oral consent. The current study includes baseline survey responses for 1672 care dyads with complete responses to ACP questions. We excluded 81 dyads who either did not provide information about informal discussion (ie, refused or responded “I don’t know”), or did not provide information about completing ADs (ie, living will, medical directive, or durable power of attorney for health care [DPAHC]). We also excluded 119 dyads in which PwCIs reported that the recruited care partner was not the person most involved in their health care decisions. The The Brown University and Duke University Institutional Review Boards Institutional Review Boards approved the CARE IDEAS study (#1606001534 and #00076890). Codebooks and model syntax are available at https://doi.org/10.26300/1esq-ge69.
Measures
Advance Directives.
Formal preparation for ACP was determined by self-reported completion (yes/no) of 3 types of ADs: (1) living will (a written document describing wishes for future medical care if the patient is no longer able to convey them), (2) medical directive (a written document describing future preferences regarding consent or refusal to resuscitation and other life-saving procedures, including cardiopulmonary resuscitation and do not resuscitate orders), and (3) DPAHC appointment (designation of another person to make health care decisions on one’s behalf in the event of incapacitation).27–31 Care partners answered the same 3 yes/no questions regarding PwCIs’ completion of ADs. At least 1 affirmative response was considered to reflect completion.
Informal Discussion.
PwCIs and care partners reported whether they had discussed PwCIs’ end-of-life care preferences in the event of their incapacitation (yes/no).
PwCI-Care Partner Agreement.
Dyadic agreement regarding informal discussion about ACP was determined by categorizing answers from both members of the care dyad into 1 of 3 responses, such that the dyad: (1) agreed a discussion had occurred, (2) agreed a discussion had not occurred (ie, non-discussion), or (3) disagreed about discussion occurrence (ie, 1 member reported the discussion occurred and the other reported non-discussion). The first 2 categories reflect the dyadic agreement, whereas the third reflects the dyadic disagreement.
Covariates.
Covariates were chosen based on their potential to confound the association between informal discussion and patient-reported completion of ADs. Covariates included PwCI and care partner self-reported sociodemographic characteristics including age, gender, race, and level of education. Using 5-point Likert scales, each member of the care dyad self-rated their own general health status (1=excellent, 5=poor) and health literacy (1=always require help with medical forms, 5=never require help with medical forms).
PwCI-Only Covariates.
PwCI-only covariates included self-reported satisfaction with current financial matters on a 5-point Likert scale (1=not at all satisfied, 5=completely satisfied). To account for negative skew, responses were dichotomized into not at all/not very/somewhat satisfied versus very/completely satisfied. Cognitive status was assessed using an abbreviated version of the telephone interview cognitive status (TICS-M),32 in which possible scores range from 0 to 41.33 The instrument includes items of immediate and delayed 10-noun-free recall, serial seven subtraction, counting backward, recall of the date, naming the president and the vice-president, and naming 2 common items. PwCIs reported their relationship to the care partner as spouse/significant other, child, other family, friend, or other. With the majority reporting a spouse/significant other relation, all other relations were grouped into other.
Care Partner-Only Covariates.
Care partner-only covariates included 4 measures to account for involvement in PwCIs’ care. To assess care hours, care partners estimated total weekly hours spent caring for PwCIs, categorized as 5 h or fewer, 6 to 19 h, 20 to 39 h, and 40 h or more; the 2 latter categories were combined. Care partners reported how often they accompanied PwCIs to (1) primary and (2) specialty care appointments on a 5-point Likert scale (0=never, 4=always). We combined responses by taking the greater of the 2 and then dichotomizing them into never/sometimes versus always/most of the time. Subjective caregiver burden was assessed with the 12-item Zarit Burden Interview (ZBI).34 Item responses range from never (0) to nearly always (4). The ZBI total score is summed and can range from 0 to 48. A score >16 suggests a clinically significant caregiver burden.34,35 Care partners also answered questions from the caregiver perceptions about communication with clinical team members (CAPACITY) instrument.36,37 The 12-item instrument measures perceptions of health care interactions in 2 domains, quality of communication and consideration of care partners’ capacity and preferences for caregiving. Responses are scored on a 5-point Likert scale. Domain scores were averaged, with higher scores reflecting more positive health care interactions.
Statistical Analysis
Baseline characteristics of PwCIs and care partners were described overall and by PwCI-reported occurrence of informal discussion about ACP (yes/no). Group differences were tested using chi-square tests/Kruskal–Wallis tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Multicollinearity was assessed using the SAS VARCLUS procedure; variables included in the final analysis were not considered collinear.
We imputed missing responses for covariates using the mode of non-missing values for categorical variables, and the mean of non-missing values for continuous variables. We report missing values for covariates in Table 1. Due to a large number of missing values for care partners’ CAPACITY domain scores (n=101), a sensitivity analysis was conducted on a subsample of care dyads without missing CAPACITY scores. We ran additional sensitivity analyses to examine moderation by cognitive impairment status (MCI vs. dementia), patient education, and patient marital status because we hypothesized that the outcome effects might differ by these 3 factors. Specifically, we assessed the interaction coefficient between informal discussion and the 3 aforementioned covariates.
Table 1.
PwCI and Care Partner Baseline Characteristics, Overall and Grouped by PwCI-Reported Occurrence of Informally Discussing ACP with Care Partner.
| Variable | PwCI-reported occurrence of informal discussing ACP with care partner |
|||
|---|---|---|---|---|
| Overall | Yes | No | P-value | |
|
| ||||
| N | 1672 | 1058 | 614 | |
| PwCI characteristics | ||||
| Age, mean (SD) | 75 (6) | 74.(6) | 75 (6) | 0.07 |
| Male | 1051 (63%) | 664 (63%) | 387 (63%) | 0.91 |
| Non-Hispanic White | 1553 (93%) | 986 (93%) | 567 (92%) | 0.51 |
| Bachelor‘s degree or higher | 1003 (60%) | 643 (61%) | 360 (59%) | 0.39 |
| Self-rated general health status, mean (SD) | 3 (1) | 3 (1) | 3 (1) | 0.15 |
| Health literacy, mean (SD) | 4 (2) | 3 (2) | 4 (2) | 0.28 |
| Cognitive functioning (TICS-M), mean (SD) | 21 (6) | 21 (6) | 20 (6) | <0.001 |
| Very or completely satisfied with present financial situation | 1148 (69%) | 747 (71%) | 401 (65%) | 0.02 |
| Care partner is spouse or significant other | 1504 (90%) | 947 (90%) | 557 (91%) | 0.43 |
| Care partner characteristics | ||||
| Age, mean (SD) | 70 (9) | 70 (9) | 70 (10) | 0.39 |
| Male | 518 (31%) | 321 (30%) | 197 (32%) | 0.46 |
| Non-Hispanic White | 1564 (94%) | 997 (94%) | 567 (92%) | 0.13 |
| Bachelor‘s degree or higher | 971 (58%) | 626 (59%) | 345 (56%) | 0.23 |
| Self-rated general health status, mean (SD) | 2 (1) | 2 (1) | 2(1) | 0.51 |
| Health literacy, mean (SD) | 5 (1) | 5 (1) | 5 (1) | 0.87 |
| Cognitive functioning (TICS-M), mean (SD) | 28 (5) | 28 (5) | 28 (5) | 0.41 |
| Caregiver burden, mean (SD) | 11 (8) | 11 (8) | 11 (8) | 0.53 |
| Time providing care for the PwCI | 0.33 | |||
| 5 h or fewer a week | 505 (30%) | 317 (30%) | 188 (31%) | |
| 6 to 19 h a week | 222 (13%) | 134 (13%) | 88 (14%) | |
| 20 or more hours a week | 160 (10%) | 111 (11%) | 49 (8%) | |
| Don‘t know/refused/missing | 785 (47%) | 496 (47%) | 289 (47%) | |
| Accompanies PwCI to primary care or specialty care appointments most of the time or always | 1461 (87%) | 913 (86%) | 548 (89%) | 0.08 |
| CAPACITY: communication domain score, mean (SD) | 3 (1) | 3 (1) | 3 (1) | 0.006 |
| CAPACITY: capacity domain score, mean (SD) | 2 (1) | 2 (1) | 2 (1) | 0.048 |
| Informal discussion about ACP | ||||
| Care partner reports discussion has occurred | 1256 (75%) | 904 (85%) | 352 (57%) | <0.001 |
| PwCI and care partner responses agree about discussion occurrence | 1166 (70%) | 904 (85%) | 262 (43%) | <0.001 |
| ADs (PwCI-report) | ||||
| Any type of advance directive | 1508 (90%) | 1002 (95%) | 506 (82%) | <0.001 |
| Living will | 1346 (82%) | 923 (88%) | 423 (71%) | <0.001 |
| Medical directive | 1196 (74%) | 850 (82%) | 346 (60%) | <0.001 |
| Health care proxy | 1355 (83%) | 932 (89%) | 423 (71%) | <0.001 |
| ADs (care partner report) | ||||
| Any type of advance directive | 1532 (92%) | 990 (94%) | 542 (88%) | <0.001 |
| Living will | 1374 (83%) | 907 (87%) | 467 (77%) | <0.001 |
| Medical directive | 1270 (79%) | 847 (83%) | 423 (72%) | <0.001 |
| Health care proxy | 1424 (87%) | 938 (90%) | 486 (82%) | <0.001 |
Abbreviations: PwCI, persons with mild cognitive impairment or dementia; ACP, advance care planning; CAPACITY, caregiver perceptions about communication with clinical team members; TICS-M, an abbreviated version of the telephone interview cognitive status.
Note. Statistics in the table comprise imputed values for missing observations; there were no meaningful differences when statistics from the complete case sample was compared to statistics presented herein. Between-group differences were estimated chi-square tests/Kruskal-Wallis tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Higher scores for cognitive functioning and caregiver burden indicate high degrees of functional impairment and subjective burden. Higher scores for general health status, health literacy, and CAPACITY indicate better health and a higher degree of health literacy and perceived communication/support from the PwCI’s health care team. The number of imputed values per variable were: PwCIs’ financial satisfaction (n =26), education (n = 18), health literacy (n = 16), general health status (n = 10), and TICS-M score (n = 4), as well as care partners’ CAPACITY scores (both domains, n = 101), burden (n = 34), education (n = 11), age (n = 10), health literacy (n = 4), and general health status (n = 2).
To test how (a) PwCI-reported and (b) care dyad agreement about informal discussion occurrence related to PwCI-reported completion of ADs, we estimated separate logistic regression models and calculated both odds ratios and marginal effects adjusted for PwCI-only and care partner-only covariates. To investigate PwCI-care partner agreement about discussion occurrence and PwCIs’ completion of ADs, we calculated 2×2 concordance matrices and kappa statistics. The Kappa statistic quantifies the degree of agreement, with 1.0 indicating perfect agreement and 0 indicating agreement due to chance alone.
Results
Descriptive Results
Table 1 displays sample characteristics overall and by PwCI-reported informal discussion occurrence. PwCIs had a mean age of 75 years. More than half were male (63%), college graduates (60%), and non-Hispanic White (93%). Care partners had a mean age of 70 years. The majority were female (69%), non-Hispanic White (94%), and college graduates (58%). Ninety percent of dyads were spouses/significant others. When applying the threshold for cognitive impairment from the original TICS-M to the 41-point version used in the present study,38,39 mean TICS-M scores indicate performance in the impaired range for patients (M=21, SD=6) and perform well within the range for normal cognition for care partners (M=28, SD=5). Sixty-three percent of PwCIs reported that they had discussed ACP with their care partner. PwCIs who had discussions (vs. not) demonstrated higher cognitive functioning (21 vs. 20, p < 0.001), more financial satisfaction (71% vs. 65%, p < 0.05), and had a care partner who reported more communication with health care providers (3.1 vs. 3.0, p < 0.001).
Relationship Between PwCI-Reported Occurrence of Informally Discussing ACP and Completion of ADs
Descriptive.
Over 90% of PwCIs and care partners reported that the PwCI had completed ADs. PwCIs who reported informally discussing ACP with care partners had higher rates of ADs (95% vs. 82%, p < 0.001).
Primary.
Table 2 presents results from examining whether PwCI-reported discussion occurrence was associated with PwCI-reported completion of ADs in logistic regression models adjusting for PwCI and care partner characteristics. PwCIs who informally discussed ACP were 10% (95% CI: 8%, 13%) more likely to have completed ADs.
Table 2.
Marginal Effects and Odds Ratios Testing the Association Between PwCI-Reported Occurrence of Informally Discussing ACP with Care Partner and PwCI-Reported Completion of ADs.
| Predictors | Marginal effects (95% CI) | Odds ratios (95% CI) |
|---|---|---|
|
| ||
| PwCI-reported occurrence of informally discussing ACP with care partner (yes) | 0.10 (0.08, 0.13) | 3.77 (2.64, 5.39) |
| PwCI covariates | ||
| Age | 0.01 (0.00, 0.01) | 1.07 (1.03, 1.12) |
| Male | 0.04 (0.01, 0.07) | 1.58 (1.07, 2.34) |
| Non-Hispanic White | 0.00 (−0.05, 0.05) | 1.03 (0.54, 1.96) |
| Bachelor‘s degree or higher | 0.03 (−0.00, 0.06) | 1.45 (0.99, 2.13) |
| Self-rated health status | 0.00 (−0.01, 0.02) | 1.06 (0.89, 1.28) |
| Health literacy | −0.00 (−0.01, 0.01) | 0.98 (0.86, 1.11) |
| Cognitive status, TICS-M | 0.00 (0.00, 0.00) | 1.03 (1.00, 1.06) |
| Very or completely satisfied with present financial situation | 0.06 (0.03, 0.09) | 2.07 (1.44, 2.98) |
| Care partner characteristics | ||
| Age | 0.00 (−0.00, 0.00) | 1.02 (0.99, 1.05) |
| Non-Hispanic White | 0.07 (0.02, 0.12) | 2.50 (1.35, 4.63) |
| Bachelor‘s degree or higher | −0.02 (−0.05, 0.01) | 0.81 (0.55, 1.18) |
| Self-rated health status | −0.00 (−0.02, 0.01) | 0.95 (0.79, 1.14) |
| Health literacy | 0.01 (−0.01, 0.02) | 1.07 (0.88, 1.31) |
| Caregiver burden | 0.00 (−0.00, 0.00) | 1.01 (0.99, 1.04) |
| Hours per week providing care for the PwCI (ref = missing) | ||
| ≤5 h | 0.01 (−0.02, 0.05) | 1.18 (0.79, 1.77) |
| 6 to 19 h | 0.04 (0.00, 0.08) | 1.76 (0.98, 3.17) |
| ≥20 h | 0.06 (0.02, 0.10) | 2.43 (1.15, 5.15) |
| Accompanies PwCI to appointments most of the time or always | 0.00 (−0.04, 0.05) | 1.01 (0.58, 1.77) |
| CAPACITY, capacity domain | 0.02 (−0.01, 0.04) | 1.23 (0.93, 1.62) |
| CAPACITY, communication domain | −0.01 (−0.04, 0.01) | 0.85 (0.62, 1.16) |
| Married or living with a partner | −0.00 (−0.07, 0.07) | 0.99 (0.43, 2.29) |
Abbreviations: PwCI, persons with mild cognitive impairment or dementia; ACP, advance care planning; AD, advance directive.
Note. Both odds ratios and marginal effects (the estimated % change in the dependent variable for every one unit change in the predictor) were estimated from a logistic regression model in which PwCI-reported completion of ADs was the dependent variable.
Dyadic Agreement
Descriptive.
Table 1 shows that 75% of care partners reported informally discussing ACP with PwCIs. This proportion significantly differed by PwCI-reported discussion occurrence (p < 0.001); care partner-reported discussion occurrence was proportionately higher when in agreement with PwCI-reported discussion occurrence (85%) than when in disagreement (57%). Figure 1 provides further information about dyadic agreement regarding informal discussion and ADs. Discussion agreement was quantified by Kappa=0.3 (0.25, 0.35), which indicated a fair amount of agreement not due to chance.40 Dyadic agreement about PwCIs’ completion of ADs was higher (Kappa=0.50).
Figure 1.
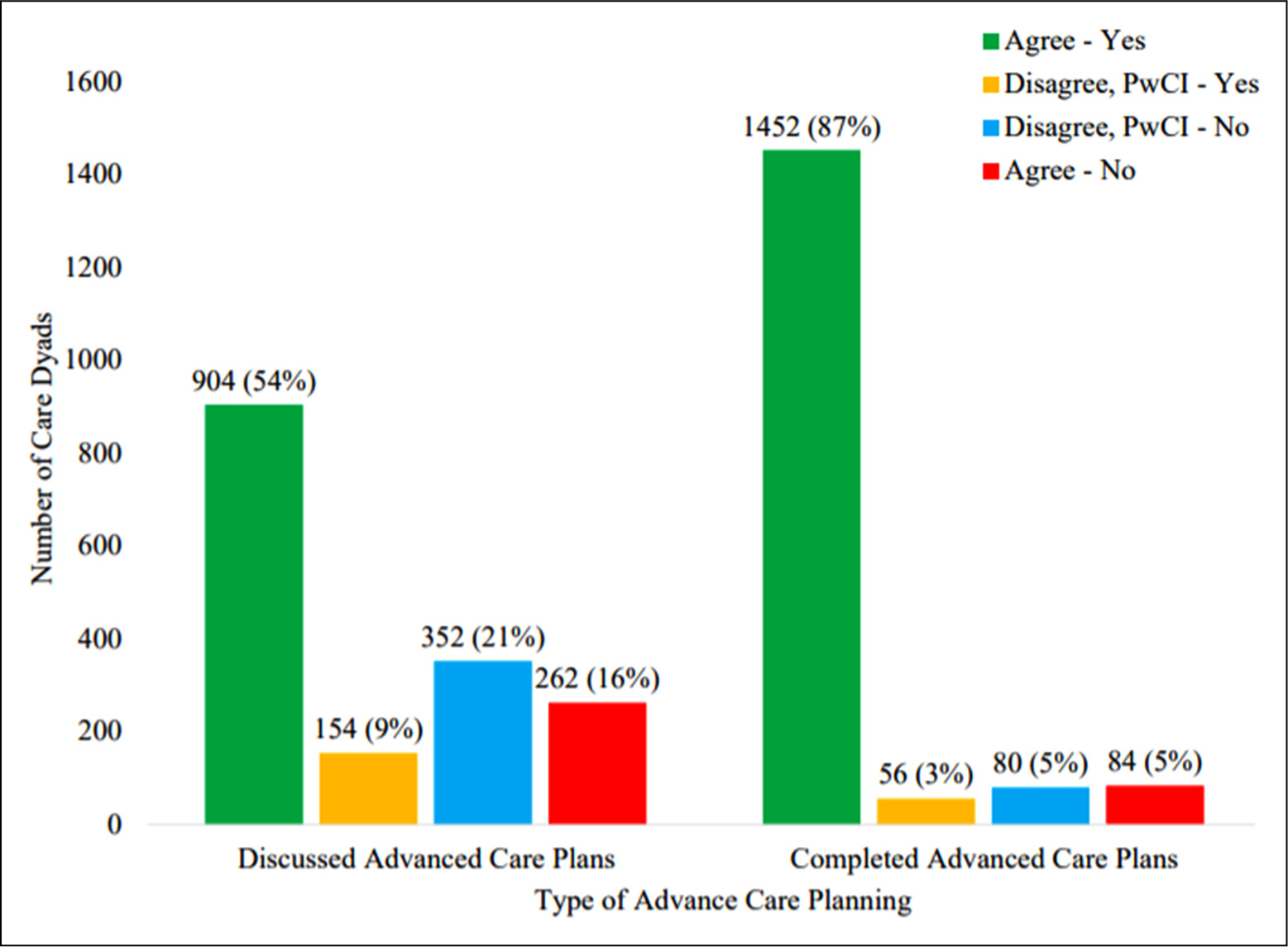
Care dyad agreement about informally discussing ACP and the person with cognitive impairment’s completion of ADs.
Abbreviations: ACP, advance care planning; AD, advance directive.
Primary.
Table 3 shows adjusted associations between dyadic agreement about discussion occurrence and PwCI-reported completion of ADs. For care dyads agreeing a discussion occurred, PwCIs were 7% (95% CI: 4%, 10%) more likely to report completing ADs than PwCIs from dyads disagreeing about discussion occurrence. The PwCI was 11% (−16%, −5%) less likely to report completing ADs when dyads agreed about non-discussion than when dyads disagreed about non-discussion.
Table 3.
Marginal Effects and Odds Ratios Testing the Association Between Care Dyad Agreement About Informally Discussing ACP and PwCI-Reported Completion of ADs.
| Predictors | Marginal effects (95% CI) | Odds ratios (95% CI) |
|---|---|---|
|
| ||
| Care dyad agreement about informally discussing ACP (ref = disagreed) | ||
| Agreed, yes | 0.07 (0.04, 0.10) | 2.94 (1.91, 4.53) |
| Agreed, no | −0.11 (−0.16, −0.05) | 0.44 (0.29, 0.66) |
| PwCI covariates | ||
| Age | 0.01 (0.00, 0.01) | 1.07 (1.03, 1.11) |
| Male | 0.04 (0.01, 0.07) | 1.63 (1.10, 2.43) |
| Non-Hispanic White | 0.00 (−0.05, 0.05) | 1.02 (0.53, 1.96) |
| Bachelor‘s degree or higher | 0.03 (−0.00, 0.06) | 1.39 (0.94, 2.04) |
| Self-rated health status | 0.00 (−0.01, 0.02) | 1.04 (0.86, 1.26) |
| Health Literacy | −0.00 (−0.01, 0.01) | 0.99 (0.87, 1.12) |
| Cognitive Status, TICS-M | 0.00 (−0.00, 0.00) | 1.02 (0.99, 1.06) |
| Very or completely satisfied with present financial situation | 0.05 (0.03, 0.08) | 2.01 (1.39, 2.91) |
| Care partner covariates | ||
| Age | 0.00 (−0.00, 0.00) | 1.02 (0.99, 1.05) |
| Non-Hispanic White | 0.07 (0.02, 0.12) | 2.49 (1.34, 4.62) |
| Bachelor‘s degree or higher | −0.02 (−0.05, 0.01) | 0.81 (0.55, 1.18) |
| Self-rated health status | −0.00 (−0.02, 0.01) | 0.96 (0.79, 1.15) |
| Health literacy | 0.00 (−0.01, 0.02) | 1.03 (0.84, 1.27) |
| Caregiver burden | 0.00 (−0.00, 0.00) | 1.01 (0.99, 1.04) |
| Hours per week providing care for the patient (ref = missing) | ||
| ≤5 h | 0.01 (−0.02, 0.05) | 1.17 (0.78, 1.77) |
| 6 to 19 h | 0.04 (−0.00, 0.08) | 1.71 (0.95, 3.09) |
| ≥20 h | 0.06 (0.02, 0.10) | 2.36 (1.10, 5.06) |
| Accompanies PwCI to appointments most of the time or always | −0.00 (−0.04, 0.04) | 0.99 (0.57, 1.74) |
| CAPACITY, capacity domain | 0.01 (−0.01, 0.03) | 1.14 (0.86, 1.52) |
| CAPACITY, communication domain | −0.02 (−0.04, 0.01) | 0.82 (0.60, 1.13) |
| Married or living with a partner | 0.01 (−0.06, 0.07) | 1.11 (0.48, 2.60) |
Abbreviations: PwCI, person with mild cognitive impairment or dementia; ACP, advance care planning; AD, advance directive; CAPACITY, caregiver perceptions about communication with clinical team members; TICS-M, an abbreviated version of the telephone interview cognitive status.
Note. Both odds ratios and marginal effects (the estimated % change in the dependent variable, for every one unit change in the predictor), were estimated from a logistic regression model in which PwCI-reported completion of ADs was the dependent variable.
p < 0.05
p < 0.01
p < 0.001.
Sensitivity Analyses
The aforementioned sensitivity analysis on a subsample of care dyads without missing CAPACITY scores revealed that results were unchanged (Tables 1S and 2S). We also observed no difference in effects by cognitive impairment status, patient education, or patient marital status.
Discussion
In this study of PwCI-care partner dyads, PwCIs who informally discussed ACP with care partners were more likely to formalize their plans through a living will, medical directive, and/or DPAHC. Although these discussions are presumed to be a steppingstone to completion of ADs,41 we believe ours is the first study to show that they are directly and positively associated with the completion of ADs for PwCIs. Our finding upholds recommendations to approach ACP as a dyadic and communicative decision-making process from the outset and prioritize discussions within care dyads.11,13,23 For care dyads prepared to engage in ACP, simply involving the care partner in health care provider-led discussions may serve as an intervention in and of itself.23 For others, health care providers’ use or distribution of decision aids may provide needed support for PwCIs’ and care partners’ engagement in discussions.42
Additionally, 70% of dyads agreed about discussion occurrence, such that 54% and 16% of dyads agreed discussions had and had not occurred, respectively. To our knowledge, no studies have examined agreement between PwCIs and care partners regarding discussion occurrence. However, the overall rate and distribution of agreement observed in this study are consistent with the broader literature on older adults’ informal discussions.24,43 Prior research has identified dyadic agreement about discussion occurrence as a correlate of care partners’ knowledge of PwCIs’ treatment goals.23 Our study adds to this body of evidence by showing that PwCIs in agreeing dyads were more likely and PwCIs in disagreeing dyads were less likely to have ADs. These findings have implications for how health care providers can help PwCIs and care partners navigate ACP. For example, a brief assessment of dyadic agreement about discussion occurrence may be insightful for determining the next steps. When dyads agree on discussion occurrence, health care providers should capitalize on the opportunity to learn about the breadth and depth of past communication, identify topics that still need to be discussed, and assess readiness to progress to completing ADs. When dyads agree on non-discussion, health care providers should employ evidence-based strategies to assist PwCIs and care partners in overcoming discussion barriers such as encouraging clear, open communication and collaborative decision-making.44,45
The level of dyadic agreement in our study was moderate, as a substantial proportion of dyads disagreed about having discussed ACP on behalf of the PwCI. Such a disagreement is a reminder of the subjective nature of discussions and additional challenges that may arise after care partners are engaged in ACP, namely achieving a shared interpretation about discussion occurrence, especially in cases where PwCIs are unable to accurately recall discussions or communicate their preferences. Given that we can only speculate on reasons for disagreement here, we recommend future research examine sources of disagreement in PwCI-care partner dyads to shed light on how the quality of their ACP-related communication can be improved. Interestingly, PwCIs’ completion of ADs was more likely in disagreeing dyads than in dyads agreeing about non-discussion. This finding implies dyads’ disagreement may have more positive effects for formalizing preparations than a shared understanding of non-discussion. More research is warranted to understand the implications of this finding, such as potential consequences for PwCIs’ receipt of desired care.
This study has several limitations. First, we used a cross-sectional design, which does not allow us to infer causality in the interpretation of results or the direction of effects. Future longitudinal research is required to more thoroughly elucidate the relationships observed in this study. Second, generalizability may be limited because the majority of care dyads were White, married couples with a college education. As such, additional studies are needed with more diverse care dyads that reflect the broader population of PwCIs and their care partners. Also, without state-level identifiers, we were unable to confirm where participants resided in the US. Third, half of our sample comprised older adults with a verified diagnosis of MCI or dementia, which may raise questions about the reliability of ACP reporting. However, recent studies have called for more ACP research focused on this particular patient population and have also suggested that PwCIs can meaningfully engage in informal discussions about ACP.22,46–48 Relatedly, our sensitivity analysis showed that findings did not differ between PwCIs with more (dementia) or less (MCI) cognitive impairment. Fourth, we determined the completion of ADs with self-reported responses to single-item indicators. Although completion of ADs has typically been ascertained in this way, we recommend that future research confirm or supplement self-reported responses with ACP documents from electronic medical records. Relatedly, our assessment of informal discussions was restricted to any previous conversations about PwCIs’ end-of-life care preferences. Consequently, we were unable to account for characteristics regarding engagement in discussions, such as frequency or quality, which may confound the relationship between informal discussion and formal preparation. Understanding the role of discussion characteristics in this relationship through further studies could be informative for future ACP efforts.
Conclusion
This study of PwCI-care partner dyads highlights the importance of informal discussion about ACP for PwCIs’ formal preparation. Completion of ADs was more likely among PwCIs who had informally discussed ACP with care partners. Similarly, PwCIs’ completion of ADs was more likely when both the PwCI and care partner agreed a discussion had occurred. PwCIs were less likely to complete ADs when dyads agreed on non-discussion than when dyads disagreed about discussion occurrence. Findings suggest health care providers should approach ACP as a dyadic, communicative decision-making process between PwCIs and care partners to facilitate PwCIs’ completion of ADs.
Supplementary Material
Acknowledgments
We would like to acknowledge the input of Dr. Terrie Wetle, Dr. Vince Mor, Dr. Brenda Plassman, and Dr. James Burke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder had no role in the design, methods, data collection, analysis, or preparation of the paper.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Institute Of Aging of the National Institutes of Health under Award Number R01AG053934 and by the American College of Radiology Imaging Network and the Alzheimer’s Association. Support was provided by the National Institute on Aging (NIA,K01AG070284) to N.D.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Cheong K, Fisher P, Goh J, et al. Advance care planning in people with early cognitive impairment. BMJ Support Palliat Care. 2015;5:63–69. [DOI] [PubMed] [Google Scholar]
- 2.Guzmán A, Gillanders D, Stevenson A, Ross K. Psychosocial adjustment to mild cognitive impairment: the role of illness perceptions, cognitive fusion and cognitive impairment. Dementia 2020;20(1):464–484. doi: 10.1177/1471301219893862 [DOI] [PubMed] [Google Scholar]
- 3.Bryant J, Sellars M, Sinclair C, et al. Inadequate completion of advance care directives by individuals with dementia: national audit of health and aged care facilities. BMJ Support Palliat Care. 2021. doi: 10.1136/bmjspcare-2020-002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr D, Luth EA. Advance care planning: contemporary issues and future directions. Innovat Aging. 2017;1(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boerner K, Moorman SM, Carr D, Ornstein KA. Insufficient advance care planning? Correlates of planning without personal conversations. J Gerontol B. 2021;76(1):104–108. [DOI] [PubMed] [Google Scholar]
- 6.Casey DA, Walker DM. The clinical realities of advance directives. Widener Law Rev. 2011;17:429. [Google Scholar]
- 7.Hague SB, Moody LE. A study of the public’s knowledge regarding advance directives. Nurs Econ. 1993;11(5):303–307. [PubMed] [Google Scholar]
- 8.Wendrich-van Dael A, Bunn F, Lynch J, et al. Advance care planning for people living with dementia: an umbrella review of effectiveness and experiences. Int J Nurs Stud. 2020;107. doi: 10.1016/j.ijnurstu.2020.103576 [DOI] [PubMed] [Google Scholar]
- 9.Dixon J, Karagiannidou M, Knapp M. The effectiveness of advance care planning in improving end-of-life outcomes for people with dementia and their carers: a systematic review and critical discussion. J Pain Symptom Manage. 2018;55(1):132–150. [DOI] [PubMed] [Google Scholar]
- 10.Siconolfi D, Bandini J, Chen E. Individual, interpersonal, and health care factors associated with informal and formal advance care planning in a nationally-representative sample of midlife and older adults. Patient Educ Couns.. 2020;104(7):1806–1813. doi: 10.1016/j.pec.2020.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace CL. Family communication and decision making at the end of life: a literature review. Palliat Support Care. 2015;13(3):815. [DOI] [PubMed] [Google Scholar]
- 12.Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage. 2017;53(5):821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doukas DJ, Hardwig J. Using the family covenant in planning end-of-life care: obligations and promises of patients, families, and physicians. J Am Geriatr Soc. 2003;51(8):1155–1158. [DOI] [PubMed] [Google Scholar]
- 14.Peterson LJ, Hyer K, Meng H, et al. Discussing end-of-life care preferences with family: role of race and ethnicity. Res Aging. 2019;41(9):823–844. [DOI] [PubMed] [Google Scholar]
- 15.Kale MS, Ornstein KA, Smith CB, Kelley AS. End-of-life discussions with older adults. J Am Geriatr Soc. 2016;64(10):1962–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fetherstonhaugh D, McAuliffe L, Shanley C, et al. Did I make the right decision?”: the difficult and unpredictable journey of being a surrogate decision maker for a person living with dementia. Dementia. 2019;18(5):1601–1614. [DOI] [PubMed] [Google Scholar]
- 17.Harrison Dening K, King M, Jones L, et al. Advance care planning in dementia: do family carers know the treatment preferences of people with early dementia? PLoS One. 2016;11(7):e0159056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piers R, Albers G, Gilissen J, et al. Advance care planning in dementia: recommendations for healthcare professionals. BMC Palliat Care. 2018;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moorman SM, Carr D. Spouses’ effectiveness as end-of-life health care surrogates: accuracy, uncertainty, and errors of overtreatment or undertreatment. Gerontologist. 2008;48(6):811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bomba P Supporting the patient voice: building the foundation of shared decision-making. Generations. 2017;41(1):21–30. [Google Scholar]
- 21.Chiarchiaro J, Buddadhumaruk P, Arnold RM, White DB. Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc. 2015;12(10):1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geshell L, Kwak J, Radhakrishnan K. Perspectives and experiences of persons with dementia with advance care planning: an integrative literature review. J Geriatr Psychiatry Neurol. 2019;32(5):231–245. [DOI] [PubMed] [Google Scholar]
- 23.Fried TR, Zenoni M, Iannone L, et al. Engagement in advance care planning and surrogates’ knowledge of patients’ treatment goals. J Am Geriatr Soc. 2017;65(8):1712–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried T, Zenoni M, Iannone L. A dyadic perspective on engagement in advance care planning. J Am Geriatr Soc. 2017;65(1):172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott IA, Mitchell GK, Reymond EJ, Daly MP. Difficult but necessary conversations—the case for advance care planning. Med J Aust. 2013;199(10):662–666. [DOI] [PubMed] [Google Scholar]
- 26.Jutkowitz E, Van Houtven CH, Plassman BL, Mor V. Willingness to undergo a risky treatment to improve cognition among persons with cognitive impairment who received an amyloid PET scan. Alzheimer Dis Assoc Disord. 2020;34(1):1–9. doi: 10.1097/WAD.0000000000000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker EF, Marco CA. Advance directives in the emergency department. J Am Coll Emerg Phys Open. 2020;1(3):270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doukas DJ, Doukas MA. Considering advance directives for oncology patients. Prim Care: Clin Off Pract. 1998;25(2):423–431. [DOI] [PubMed] [Google Scholar]
- 29.Obernberger S When love and abuse are not mutually exclusive: the need for government intervention. Issues L Med. 1996;12(4):355. [PubMed] [Google Scholar]
- 30.Rauscher J, Nacinovich MR. Concerns and wishes–communication of advance care planning. J Commun Healthc. 2012;5(1):1–2. [Google Scholar]
- 31.Emanuel LL, von Gunten CF, Ferris FD. Advance care planning. Arch Fam Med. 2000;9(10):1181. [DOI] [PubMed] [Google Scholar]
- 32.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Cogn Behav Neurol. 1993;6(2):103–110. [Google Scholar]
- 33.Ofstedal MB, Fisher GG, Herzog AR. Documentation of Cognitive Functioning Measures in the Health and Retirement Study; 2005. http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf. Accessed September 9, 2020.
- 34.Bedard M, Molloy DW, Squire L, et al. The Zarit burden interview: a new short version and screening version. Gerontologist. 2001;41(5):652–657. [DOI] [PubMed] [Google Scholar]
- 35.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649–655. [DOI] [PubMed] [Google Scholar]
- 36.Van Houtven CH, Miller KEM, O’Brien EC, et al. Development and initial validation of the caregiver perceptions about communication with clinical team members (CAPACITY) measure. Med Care Res Rev. 2019;76(6):784–806. [DOI] [PubMed] [Google Scholar]
- 37.Van Houtven C, Lippmann S, Belanger E, et al. Measurement properties of the CAPACITY instrument to assess perceived communication with the health care team among care partners of patients with cognitive impairment. Med Care. 2020;58(9):842–849. doi: 10.1097/MLR.0000000000001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallo JJ, Breitner JC. Alzheimer’s disease in the NAS-NRC registry of aging twin veterans, IV. Performance characteristics of a two-stage telephone screening procedure for Alzheimer’s dementia. Psychol Med. 1995;25(6):1211–1219. [DOI] [PubMed] [Google Scholar]
- 39.Brandt J, Welsh KA, Breitner JC, Folstein MF, Helms M, Christian JC. Hereditary influences on cognitive functioning in older men. A study of 4000 twin pairs. Arch Neurol. 1993;50-(6):599–603. [DOI] [PubMed] [Google Scholar]
- 40.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue M, Tompkins C, Scruggs K, Robles J. Advance directives information delivery in medicare/Medicaid-funded agencies: an exploratory study. J Soc Work End Life Palliat Care. 2018;14(2–3):177–193. [DOI] [PubMed] [Google Scholar]
- 42.Davies N, Schiowitz B, Rait G, Vickerstaff V, Sampson EL. Decision aids to support decision-making in dementia care: a systematic review. Int Psychogeriatr. 2019;31(10):1403–1419. [DOI] [PubMed] [Google Scholar]
- 43.Fried TR, Redding CA, Robbins ML, O’Leary JR, Iannone L. Agreement between older persons and their surrogate decision-makers regarding participation in advance care planning. J Am Geriatr Soc. 2011;59(6):1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung MY, Matthews AK. A systematic review of clinical interventions facilitating end-of-life communication between patients and family caregivers. Am J Hosp Palliat Med 2020;38(2):180–190. doi: 10.1177/1049909120929323 [DOI] [PubMed] [Google Scholar]
- 45.Anderson RJ, Bloch S, Armstrong M, et al. Communication between healthcare professionals and relatives of patients approaching the end-of-life: a systematic review of qualitative evidence. Palliat Med. 2019;33(8):926–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song MK, Ward SE, Hepburn K, et al. Can persons with dementia meaningfully participate in advance care planning discussions? A mixed-methods study of spirit. J Palliat Med. 2019. Nov 1;22-(11):1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van den Block L Advancing research on advance care planning in dementia. Palliat Med. 2019;33(3):259–261. [DOI] [PubMed] [Google Scholar]
- 48.Read ST, Toye C, Wynaden D. The participation of people with dementia in the planning of their care and support: an integrative literature review. Dementia. 2020;19(3):691–707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


