Abstract
To investigate the relationship between human immunodeficiency virus (HIV) infection and the risk of mortality among coronavirus disease 2019 (COVID-19) patients based on adjusted effect estimate by a quantitative meta-analysis. A random-effects model was used to estimate the pooled effect size (ES) with corresponding 95% confidence interval (CI). I2 statistic, sensitivity analysis, Begg’s test, meta-regression and subgroup analyses were also conducted. This meta-analysis presented that HIV infection was associated with a significantly higher risk of COVID-19 mortality based on 40 studies reporting risk factors-adjusted effects with 131,907,981 cases (pooled ES 1.43, 95% CI 1.25–1.63). Subgroup analyses by male proportion and setting yielded consistent results on the significant association between HIV infection and the increased risk of COVID-19 mortality. Allowing for the existence of heterogeneity, further meta-regression and subgroup analyses were conducted to seek the possible source of heterogeneity. None of factors might be possible reasons for heterogeneity in the further analyses. Sensitivity analysis indicated the robustness of this meta-analysis. The Begg’s test manifested that there was no publication bias (P = 0.2734). Our findings demonstrated that HIV infection was independently associated with a significantly increased risk of mortality in COVID-19 patients. Further well-designed studies based on prospective study estimates are warranted to confirm our findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-022-00840-1.
Keywords: COVID-19, HIV, Mortality, Meta-analysis
Introduction
Evidence has suggested that increasing age and comorbidities were interrelated with worse outcomes in coronavirus disease 2019 (COVID-19) [1–5]. Due to the immunocompromised status, people living with human immunodeficiency virus (HIV) were assumed more likely to suffer from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related mortality [6–8]. Several previous meta-analyses have addressed the association between HIV infection and the risk of COVID-19 mortality with conflicting conclusions, moreover they were stuck in failing to consider the influence of certain confounding factors on the pooled effects owing to that un-adjusted effect estimates extracted from individual original literature were used to calculate the pooled effects in the previous meta-analyses [9–14]. In fact, the influence of certain confounding factors on the association between HIV infection and the risk of mortality in COVID-19 patients may bear on the output of results. For instance, Zimmermann et al. reported HIV infection status was associated with a significantly lower risk of mortality of COVID-19 patients (hazard ratio (HR): 0.757, 95% confidence interval (CI): 0.632–0.906) in the univariate model, while the significant association reversed in the multiple regression analysis (HR: 1.360, 95% CI (1.13–1.63) [15]. It is not the only study that dramatic change came up after adjusting age and sex (un-adjusted HR: 0.77. 95% CI 0.54–1.11; adjusted HR: 1.45, 95% CI 1.00–2.12) during assessing the association between HIV status and mortality in COVID-19 patients [16]. With the emergence of researches focusing on the confounding factors-adjusted association between HIV infection and COVID-19 mortality, an update meta-analysis based on adjusted effect estimates is necessary to assess whether there exists a relationship between HIV infection and COVID-19 mortality or not.
Methods
Search strategy
A comprehensive search of the literature was performed in line with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17] based on the central databases of PubMed, Springer Link, Web of Science, Wiley Library, Elsevier ScienceDirect, Scopus and Cochrane Library, which retrieved all potential articles published from inception to November 25, 2021. Pertinent keywords and terms were used to magnify the output from the literature search containing three parts: (1) “COVID-19,” “coronavirus disease 2019,” “2019-nCoV,” “2019 novel coronavirus,” “SARS-CoV-2,” “severe acute respiratory syndrome coronavirus 2”; (2) “HIV,” “AIDS,” “human immunodeficiency virus,” “acquired immunodeficiency syndrome”; and (3) “mortality,” “fatality,” “death,” “non-survivor,” “deceased.” (Detailed search strategy is shown in Table S1.) Additional articles, originating from references of the included studies and relevant reviews, were also included in the campaign of research.
Eligibility criteria and data extraction
Only in these ways were studies included in this meta-analysis: (1) studies reporting adult confirmed cases of COVID-19 regardless of country or antiretroviral therapy (ART); (2) peer-reviewed articles in English; (3) the endpoint contained death of the participants in COVID-19; and (4) studies with available data on the adjusted effect size with 95% CI regarding the association between HIV infection and COVID-19 mortality. Case reports, review papers, preprints, duplications, and errata must cast away accordingly. Searching for literature, study selection and data extraction were performed by two investigators independently. In case of disagreement, the third person would be brought in to resolve the disputes on a negotiating basis. The extracted information is at list: first author, study design, country, number of participants, the mean (standard deviation) or median (interquartile range) age, proportion of males, frequency of HIV infection (percentage of patients), the adjusted effect size (HR, OR (odds ratio) and RR (risk ratio)) with 95% CI and setting.
Statistical analysis
The pooled ES with corresponding 95% CI evaluating the association between HIV infection and COVID-19 mortality was conducted by a random-effects meta-analysis model [2] providing a visual depiction of the meta-analysis findings through forest plots. I2 statistic was used to test for heterogeneity across the included studies. Sensitivity analysis by removing single study at a time was carried out to assure the robustness of the findings. Begg’s test was applied to evaluate the potential publication bias. The analysis was performed using the package “meta” on R software (version 4.1.1). Statistical significance of testing standard was set as P < 0.05.
Results
Characteristics of the included studies
There existed 16,575 records from electronic databases and 30 records by cross-referencing from the identified studies. Forty and three records were identified after screening abstracts/titles (16,605) and full texts (4,222) with discarding duplicated articles, reviews, case reports, expert consensus, corrections, protocols, comments or data not related to HIV infection and data deficiencies. There were 40 eligible articles [6–8, 15, 16, 18–52] that included more than 13 million individuals after three studies were excluded allowing for less sample sizes from the same sources. The detail of selection process is shown by a flow chart (Fig. 1). A total of 131,907,981 sample sizes ranging from 270 to 66,050,590 were ultimately included in this meta-analysis. The included studies originated from 17 countries spread on four regions—Americas (n = 19 studies), Europe (n = 11 studies), Asia (n = 5 studies) and Africa (n = 5 studies). Across most studies (28/40), the number of HIV infection and percentage in all COVID-19 infection was relatively low (< 3%). The summary information of included studies is presented in Table 1.
Fig. 1.
Flow chart of the process of study selection of PRISMA
Table 1.
The general information of the eligible studies in the meta-analysis
| Author | Location | Study design | Sample size | Age | Male (%) | HIV (n/%) | Adjusted effect (95% CI) | Outcome | Setting |
|---|---|---|---|---|---|---|---|---|---|
| Adrish M | USA | Retrospective study | 469 | 54.49 ± 16.19 | 59.5 | 37 (7.89) | HR: 3.53 (1.57–4.03) | In-hospital mortality | Hospitalized |
| Bennett KE | Ireland | Retrospective study | 19,789 | NR | NR | 364 (1.84) | OR: 1.24 (0.77–2.00) | In-hospital mortality | Hospitalized |
| Bergman J | Swedish | Retrospective study | 68,575 | 46 ± 21 | 39.1 | 84 (0.12) | HR: 1.24 (0.56–2.77) | Death | Hospitalized |
| Bhaskaran K | UK | Retrospective study | 17,282,905 | 50.0 ± 22.2 | 50.0 | 27,840 (0.16) | HR: 2.90 (1.96–4.30) | Mortality | All patients |
| Brown AE | UK | Retrospective study | 45,657,664 | NR | 68.63 | 92,643 (0.20) | RR: 2.18 (1.76–2.70) | Death | All patients |
| Bushman D | USA | Case–control study | 1029 | 56 (23–64) | 65.5 | 28 (2.72) | OR: 2.42 (1.05–5.59) | Death | Hospitalized |
| Cai M | USA | Retrospective study | 49,238 | 63.3 (49.8–73.1) | 88.50 | 504 (1.02) | OR: 1.05 (0.67–1.58) | 30-day mortality | All patients |
| Chanda D | Zambia | Prospective study | 443 | NR | 57.3 | 122 (27.54) | HR: 0.88 (0.49–1.56) | Death | Hospitalized |
| Choi YJ | South Korea | Retrospective study | 7590 | 47.3 | 40.8 | 7 (0.09) | OR: 7.080 (0.462–108.575) | Mortality | All patients |
| Deiana G | Italy | Matched case–control study | 1223 | 58.47 (45.3–78.3) | 40.8 | NR | OR: 1.8 (0.2–13.5) | Deceased | All patients |
| Durstenfeld MS | USA | Retrospective study | 21,528 | 62.24 ± 17.87 | 54.09 | 220 (1.02) | OR: 1.14 (0.78–1.68) | In-hospital mortality | Hospitalized |
| Emami A | Iran | Retrospective study | 1239 | 51.48 ± 19.54 | 55.9 | 5 (0.40) | HR: 10.46 (3.27–33.45) | Death | Hospitalized |
| Filardo TD | USA | Retrospective study | 270 | 58 (50–67) | 67.4 | 5 (1.85) | RR: 1.46 (0.49–4.31) | Mortality | Hospitalized |
| Ge E | Canada | Retrospective study | 167,500 | 42.7 (21.9) | 48.00 | 332 (0.20) | HR: 1.60(0.85–3.00) | 30-day all-cause mortality | All patients |
| Geretti AM | UK | Prospective study | 47,592 | 72.7 ± 17.8 | 56.9 | 122 (0.26) | HR: 1.69 (1.15–2.48) | 28-day mortality | Hospitalized |
| Janssen NAF | Netherland, Belgium | Retrospective study | 519 | 64 (55–72) | 72.80 | 6 (1.16) | OR: 18.96 (1.84–195.11) | Death | Hospitalized |
| Jassat W | South Africa | Retrospective study | 151,779 | 53.4 | 44.9 | 13,793 (9.09) | OR: 1.23 (1.15–1.33) | In-hospital mortality | Hospitalized |
| Kabarriti R | USA | Retrospective study | 5902 | 57.5 (mean) | 46.9 | 92 (1.56) | HR: 0.88 (0.51–1.51) | Death | Hospitalized |
| Kaplan-Lewis E | USA | Retrospective study | 304 | 55.33 ± 13.4 | 70.07 | 110 (36.18) | OR: 0.41 (0.19–0.86) | Mortality | All patients |
| Kelly JD | USA | Prospective study | 27,640 | 56.03 | 88.6 | 332 (1.20) | OR: 1.03 (0.58–1.83) | 30-day Mortality | All patients |
| Laracy J | USA | Retrospective study | 340 | 58.4 ± 12.7 | 71 | 68 (20) | HR: 0.9 (0.3–2.3) | In-hospital mortality | Hospitalized |
| Lee SG | Korea | Retrospective study | 7339 | 47.1 ± 19.0 | 40.1 | 4 (0.05) | OR: 106.93 (6.38- > 999) | Death | All patients |
| Li S | USA | Retrospective study | 6218 | 59.0 ± 19.1 | 47.60 | 35 (0.56) | OR: 1.077 (0.172–6.753) | In-hospital mortality | Hospitalized |
| Lundon DJ | USA | Cross-sectional analysis | 8928 | 58.0 ± 18.8 | 46.2 | 140 (1.57) | OR: 1.37 (0.91–2.05) | Mortality | Hospitalized |
| Makker J | USA | Retrospective study | 1206 | 62.0 ± 12.0 | 60.70 | 79 (6.55) | OR: 0.54 (0.24–1.23) | In-hospital mortality | Hospitalized |
| Mascarello KC | Brazil | Cross-sectional analysis | 104,359 | 42.2 | 46.9 | NR | RR: 2.40 (1.26–4.57) | Death | All patients |
| Mollalo Aa | USA | Retrospective study | NR | NR | NR | NR | OR: 1.23 ( 0.52–2.91) | Mortality | All patients |
| Oh TK | South Korea | Retrospective study | 8070 | 49.34 | 39.5 | 9 (0.11) | HR: 0.96 (0.68–1.35) | In-hospital mortality | Hospitalized |
| Orlando V | Italy | Retrospective study | 3497 | NR | 55.6 | 68 (1.94) | OR: 1.04 (0.58–1.86) | Death | All patients |
| Osibogun A | Nigeria | Retrospective study | 2184 | 43 (33–55) | 65.8 | 7 (0.32) | OR: 16.13 (2.74–95.07) | Death | Hospitalized |
| Paul Ra | USA | Retrospective study | 3104 | NR | NR | 301 (9.70) | RR: 1.002 (1.000–1.004) | Mortality | All patients |
| Perez-Guzman PN | UK | Retrospective study | 614 | 69 (56.5–81.5) | 62.21 | 9 (1.47) | OR: 1.32 (0.24–7.36) | In-hospital mortality | Hospitalized |
| Robles-Pérez E | USA | Retrospective study | 70,531 | NR | 43.2 | 70 (0.10) | OR: 6.97 (1.92–25.28) | Death | All patients |
| Rosenthal N | USA | Prospective study | 64,781 | 56.1 ± 19.9 | 49.3 | 252 (0.39) | OR: 0.68 (0.44–1.04) | In-hospital mortality | Hospitalized |
| Semenzato L | France | Retrospective study | 66,050,590 | 43 ± 24 | 48 | 146,204 (0.22) | HR: 1.93 (1.51- 2.47) | In-hospital mortality | Hospitalized |
| Sohrabi MR | Iran | Prospective study | 205,645 | 52.8 ± 21.1 | 54.5 | 154 (0.07) | OR: 1.77 (1.053–2.973) | Mortality | Hospitalized |
| Venturas J | South Africa | Prospective study | 384 | 50 (39–60) | 53 | 108 (28.13) | OR: 0.50 (0.20–1.40) | In-hospital mortality | Hospitalized |
| WCPHDCb | the Western Cape | Cohort study | 22,308 | NR | 31.6 | 3978 (17.83) | HR: 2.14 (1.70–2.70) | Death | All patients |
| Yang X | USA | Cohort study | 1,436,622 | 47 (32–61) | 44.96 | 13,170 (0.92) | OR: 1.43 (1.29–1.59) | Death | All patients |
| Zimmermann IR | Brazil | Retrospective study | 398,063 | 61.5 | 55.5 | 467 (0.12) | HR: 1.360 (1.13–1.63) | In-hospital mortality | Hospitalized |
The age (years) was presented as mean ± standard deviation or median (interquartile range, IQR); CI, confidence interval; NR, not clearly reported; USA, The United states of America; UK, The United Kingdom; a indicates combined effects based on subgroups; b indicates Western Cape Department of Health in collaboration with the National Institute
Estimated risk of HIV infection on COVID-19 mortality
This meta-analysis based on risk factors-adjusted effects presented that HIV infection was associated with a significantly higher risk of COVID-19 mortality (pooled ES 1.43, 95% CI 1.25–1.63) (Fig. 2), although between-study variation was high (I2 = 89%, P < 0.01). There is nearly a 45% excess risk of death among HIV patients as compared to the individuals without HIV infection. Subsequently stratified analyses based on the type of effects showed that HIV infection was a risk factor for death in COVID-19 patients compared with those without HIV infection for the subgroups with OR and HR (pooled OR 1.20, 95% CI 1.01–1.44 and pooled HR 1.67, 95% CI 1.30–2.15, separately), but not for RR (pooled RR 1.64, 95% CI 0.91–1.44) (Table 2 and Figure S1). Subgroup analyses by male proportion and setting of participants yielded consistent results on significantly positive association between HIV infection and COVID-19 mortality (pooled ES 1.50, 95% CI 1.16–1.94 for male proportion ≥ 50% and pooled ES 1.45, 95% CI 1.20–1.74 for male proportion < 50%; and pooled ES 1.58, 95% CI 1.23–2.03 for all patients and pooled ES 1.34, 95% CI 1.12–1.61 for hospitalization, Table 2, Fig. S2 and Fig. S3). Following subgroup analyses with age, sample size and study design demonstrated that this significant association between HIV infection and the increased risk of COVID-19 mortality did exist among studies with separated subgroups: age < 60 (pooled ES 1.50, 95% CI 1.25–1.81); sample sizes ≥ 8000 (pooled ES 1.47, 95% CI 1.28–1.70); sample sizes < 8000 (pooled ES 1.52, 95% CI 1.04–2.22); retrospective study (pooled ES 1.45, 95% CI 1.23–1.71); except with separated subgroup: prospective study (pooled ES 1.05, 95% CI 0.71–1.55) (Table 2, Fig. S4, Fig. S5 and Fig. S6). As for subgroup analysis for region manifested that HIV infection could increase the risk of COVID-19 mortality in Asia (pooled ES 3.99, 95% CI 1.37–11.60), Europe (pooled ES 1.74, 95% CI 1.37–2.20) and Americas (pooled ES 1.23, 95% CI 1.03–1.47), but did not exist in Africa (pooled ES 1.38, 95% CI 0.87–2.20) (Table 2 and Fig. S7). Coming through further meta-regression to pursue the source of heterogeneity, none of factors mentioned above might be possible reasons of heterogeneity (age: P value = 0.6579; male proportion: P value = 0.2859; sample size: P value = 0.7892; setting: P value = 0.3483; study design: P value = 0.0826; region: P value = 0.1033) (Table 2).
Fig. 2.
Forest plot presents the relationship between HIV infection and COVID-19 mortality with pooled effect and its 95% confidence intervals (CI). * indicates combined effects based on subgroups; ** indicates Western Cape Department of Health in collaboration with the National Institute
Table 2.
Subgroup analysis and meta-regression
| Variables | No. of studies | Meta-regression | Subgroup analysis | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| Tau2 | Z-Value | P value | Pooled Effect (95% CI) | I2 | τ2 | P value | ||
| Age (years) | 0.1392 | − | 0.6579 | |||||
| ≥ 60 | 7 | − | − 0.9049 | 0.3655 | 1.25 (0.95 − 1.65) | 54% | 0.0584 | 0.04 |
| < 60 | 25 | − | − | − | 1.50 (1.25 − 1.81) | 79% | 0.1058 | < 0.01 |
| NR | 8 | − | − 0.3686 | 0.7124 | 1.42 (0.97 − 2.10) | 93% | 0.2360 | < 0.01 |
| Male (%) | 0.1044 | − | 0.2859 | |||||
| ≥ 50 | 21 | − | 0.1127 | 0.9103 | 1.50 (1.16 − 1.94) | 79% | 0.2186 | < 0.01 |
| < 50 | 16 | − | − | − | 1.45 (1.20 − 1.74) | 78% | 0.0650 | < 0.01 |
| NR | 3 | − | − 1.4583 | 0.8802 | 1.002 (1.000 − 1.004) | 0% | 0 | 0.84 |
| Sample size | 0.1026 | − | 0.7892 | |||||
| ≥ 8000 | 20 | − | 0.6288 | 0.5295 | 1.47 (1.28 − 1.70) | 80% | 0.0606 | < 0.01 |
| < 8000 | 19 | − | − | − | 1.52 (1.04 − 2.22) | 80% | 0.3879 | < 0.01 |
| NR | 1 | − 0.1521 | 0.8791 | 1.23 (0.52 − 2.91) | − | − | − | |
| Setting | 0.1341 | − 0.9378 | 0.3483 | |||||
| All patients | 16 | − | − | − | 1.58 (1.23 − 2.03) | 93% | 0.1543 | < 0.01 |
| Hospitalized | 24 | − | − | − | 1.34 (1.12 − 1.61) | 73% | 0.0964 | < 0.01 |
| Study design | 0.0880 | − | 0.0826 | |||||
| Retrospective study | 28 | − | − 2.2147 | 0.0268 | 1.45 (1.23 − 1.71) | 89% | 0.0894 | < 0.01 |
| Prospective study | 6 | − | − 1.1487 | 0.2507 | 1.05 (0.71 − 1.55) | 69% | 0.1516 | < 0.01 |
| Other | 6 | − | − | − | 1.75 (1.36 − 2.24) | 62% | 0.0438 | 0.02 |
| Region | 0.1007 | − | 0.1033 | |||||
| Asia | 5 | − | 1.2369 | 0.2161 | 3.99 (1.37 − 11.60) | 87% | 0.9965 | < 0.01 |
| Europe | 11 | − | 0.9406 | 0.3469 | 1.74 (1.37 − 2.20) | 57% | 0.0704 | < 0.01 |
| Americas | 19 | − | − 0.5381 | 0.5905 | 1.23 (1.03 − 1.47) | 85% | 0.0771 | < 0.01 |
| Africa | 5 | − | − | − | 1.38 (0.87 − 2.20) | 88% | 0.1811 | < 0.01 |
| Effect | 0.1202 | − | 0.1361 | |||||
| OR | 23 | − | − 1.9172 | 0.0552 | 1.20 (1.01 − 1.44) | 69% | 0.0667 | < 0.01 |
| RR | 4 | − | − 0.1810 | 0.8564 | 1.64 (0.91 − 2.96) | 95% | 0.2864 | < 0.01 |
| HR | 13 | − | − | − | 1.67 (1.30 − 2.15) | 80% | 0.1423 | < 0.01 |
NR, not clearly reported; CI, confidence interval; OR, odds ratio; RR, risk ratio; HR, hazard ratio
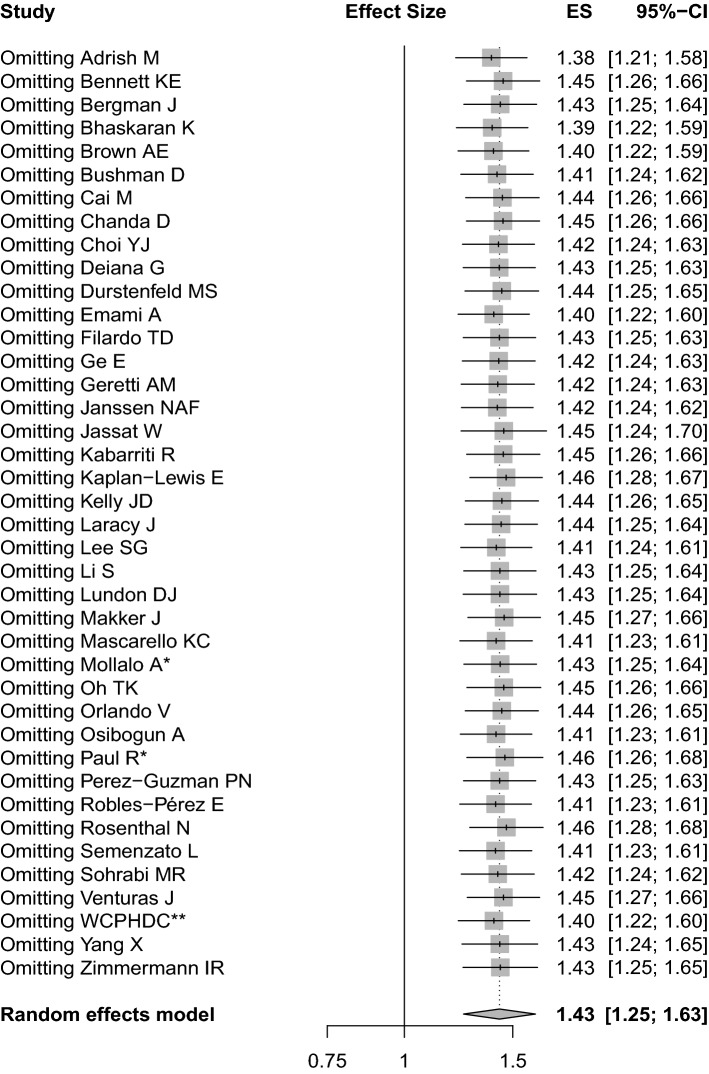
Sensitivity analysis and publication bias
After removing single study at a time, the forest plot still indicated that the pooled effect did not drastically alter, which indicated the robustness of this meta-analysis (Fig. 3). Graphically, the funnel plot analysis intuitively presented symmetry among the included studies (Fig. 4). Statistically, the Begg’s test manifested that there was no publication bias for the publications (P = 0.2734).
Fig. 3.
Sensitivity analysis for pooled effect and 95% CI by deleting single study at a time. * indicates combined effects based on subgroups; ** indicates Western Cape Department of Health in collaboration with the National Institute
Fig. 4.

Publication bias in Begg’s test based on funnel plot
Discussion
Although several meta-analyses have investigated the association between HIV infection and COVID-19 mortality, they obtained contradictory findings and the influence of certain confounding factors on the pooled effect was not taken into account [9–14]. This meta-analysis investigated the relationship between HIV infection and COVID-19 mortality on the basis of risk factors-adjusted effects. Our findings demonstrated that HIV infection was significantly associated with an increased risk of COVID-19 mortality based on 40 eligible articles with 131,907,981 COVID-19 patients reporting risk factors-adjusted effects, which suggests that HIV infection might be an independent risk factor for predicting fatal COVID-19. Subgroup analyses by setting and male proportion yielded consistent results on the significant association between HIV infection and the increased risk of COVID-19 mortality. Allowing for the existence of heterogeneity, further meta-regression and subgroup analyses were conducted to seek the possible source of heterogeneity. None of factors for subgroups might be possible reasons for heterogeneity in the further analyses that can be utilized to interpret the source of heterogeneity.
Clinical features of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection varied from asymptomatic outcome to the dead endpoint. Evidence exists that elevated levels of infectious biomarkers and inflammatory cytokines may result in the significant decrease of the number of T cells in COVID-19 patients and both helper T cells and suppressor T cells in patients with COVID-19 were below normal levels [53]. This indicated the novel coronavirus might impact T lymphocytes. Moreover, the distinctive characteristic of HIV infection was persistent immune dysfunction manifested as low counts of CD4+ T cells, which was subject to death than the HIV-negative patients [48]. This can be attributed to that immune activation, inflammation and coagulopathy are more common in HIV infection despite mitigation with drug-induced control of HIV virus replication [54–56]. Then, in regard to the individual co-infected with HIV and SARS-CoV-2, it is prone to interfering with immune homeostasis, promoting tissue damage and resulting in severe outcome of patients in a double whammy to consume CD4+ T cells. Mentioned above statements, to some extent, explained the relationship between HIV infection and COVID-19 death. It is worth pursuing the mechanisms in detail under the further investigations on which pathway might have increased lethality for the HIV-positive compared with the rest among people with a diagnosis of COVID-19.
After an in-depth subgroup analysis with age, we found a higher estimate for mortality in the < 60 years of age group compared with the ≥ 60 years of age group (1.50 vs 1.25). Younger persons living with diagnosed HIV may also be at higher risk of mortality due to COVID-19 complications [57]. This is an interesting phenomenon. Persons living with diagnosed HIV hospitalized and dying from COVID-19 were younger than persons living without diagnosed HIV [58–60]. This finding may lend support to the hypothesis that HIV infection can accelerate biological aging [61, 62]. Subgroup with male proportion ≥ 50% in HIV and the novel coronavirus co-infection had a slightly higher risk of death than those < 50% (1.50 vs 1.45). Meanwhile, as reported in Yang et al.’s study, male patients had higher risk of COVID-19 death compared with females whether or not with HIV infection [49]. With a large burden of other comorbidities may also influence the association between HIV infection and death of the novel coronavirus. As mentioned in the background, comorbidities were interrelated with worse outcomes in COVID-19. When stratifying for existence of comorbidities, the association between HIV and COVID-19 mortality was significantly higher in those with comorbidities [21, 51]. Region is also an influencing factor. When performing subgroup by continents, we found different risk effects among regions, which may be related to the prevalence of HIV infection and different measures for COVID-19. CD4+ T cells counts, viral load, and type of antiretroviral therapy that associated HIV status can influence the progression of the physical condition in turn to affect the severity of COVID-19 [51, 63].
We acknowledged several limitations in the review, which were mainly distributed in the following areas. First, prospective studies are more capable of proving the cause declared in this study and are more likely to delivery conservative results and conclusions, but most of the included studies were retrospective, only six prospective studies were included and failed to deliver to the significant association between HIV infection and COVID-19 mortality on our pooled effect, which might result from the relatively small number of included prospective studies. Therefore, further meta-analyses based on a large number of prospective studies are warranted to verify our findings in future, while the more relevant articles by study design in prospective study are available. Second, across most studies (28/40), the numbers of HIV infection and percentage in all COVID-19 patients were relatively low (< 3%). In another aspect, there were also several articles with high prevalence rates in HIV infection, which may result from selection bias despite the robust results of the sensitivity analysis. Third, most of the included studies originated from Americas and Europe. Finally, HIV/acquired immunodeficiency syndrome (AIDS) as a long-lasting disease, dosage use of drugs in convention was not reported clearly so that the meta-analysis did not address the influence of AIDS therapy on the association between HIV infection and COVID-19 mortality presently.
This study suggested that effective measures to mitigate COVID-19 risk among the HIV-positive should be included in HIV programs in order to reduce the mortality in COVID-19. Under the management of HIV patients without COVID-19, beyond the routine disease treatment, prevention and control for HIV patients, precautionary measures for COVID-19 should be more targeted and conventional. Patients with HIV infection need priority consideration for the SARS-CoV-2 vaccine through a combination of prevention and treatment, especially in countries with severe HIV burdens and higher numbers of vulnerable populations. In addition, patients in COVID-19 with HIV infection need to pay attention to deal with possibly poor progression and death in view of physicians.
In conclusion, our findings demonstrated that HIV infection was independently associated with a significantly increased risk of mortality in COVID-19 patients, which suggests that HIV infection might be a predictor for fatal COVID-19. Further well-designed studies based on prospective study estimates are warranted to confirm our findings. This study suggested that routine interventions and treatment for HIV patients with COVID-19 should be given rise to wider attention.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Yang Li, Peihua Zhang, Jian Wu, Xuan Liang, Wenwei Xiao, Li Shi, Ruiying Zhang and Mengke Hu (all are from Department of Epidemiology, School of Public Health, Zhengzhou University) for their kind help in searching articles and collecting data, and valuable suggestions for analyzing data.
Author contributions
Haiyan Yang and Yadong Wang conceptualized the study. Xueya Han, Jie Xu, Hongjie Hou and Ying Wang performed literature search and data extraction. Xueya Han, Hongjie Hou, Jiahao Ran and Shuwen Li analyzed the data. Xueya Han wrote the manuscript. All the authors approved the final manuscript.
Data availability statement
The data that support the findings of this study are included in this article and available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Declarations
Conflicts of interest
All authors report that they have no potential conflicts of interest.
Human and animal rights
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou H, Xu J, Li Y, Wang Y, Yang H. The association of asthma with COVID-19 mortality: an updated meta-analysis based on adjusted effect estimates. J Allergy Clin Immunol Pract. 2021;9(11):3944–68.e5. doi: 10.1016/j.jaip.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Xu J, Liang X, Shi L, Wang Y. Autoimmune diseases are independently associated with COVID-19 severity: Evidence based on adjusted effect estimates. J Infect. 2021;82(4):e23–e26. doi: 10.1016/j.jinf.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Xiao W, Liang X, et al. The association of cerebrovascular disease with adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. J Cerebrovas Diseases: Official J Nat Stroke Assoc. 2020;29(11):105283. doi: 10.1016/j.jstrokecerebrovasdis.2020.105283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang X, Shi L, Wang Y, et al. The association of hypertension with the severity and mortality of COVID-19 patients: Evidence based on adjusted effect estimates. J Infect. 2020;81(3):e44–e47. doi: 10.1016/j.jinf.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deiana G, Azara A, Dettori M, et al. Deaths in SARS-Cov-2 positive patients in Italy: the influence of underlying health conditions on lethality. Int J Environ Res Public Health. 2020 doi: 10.3390/ijerph17124450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emami A, Javanmardi F, Akbari A, et al. Survival rate in hypertensive patients with COVID-19. Clin Exp Hypertens. 2021;43(1):77–80. doi: 10.1080/10641963.2020.1812624. [DOI] [PubMed] [Google Scholar]
- 8.Filardo TD, Khan MR, Krawczyk N, et al. Comorbidity and clinical factors associated with COVID-19 critical illness and mortality at a large public hospital in New York City in the early phase of the pandemic (March-April 2020) PLoS ONE. 2020;15(11):e0242760. doi: 10.1371/journal.pone.0242760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Y, Li Z, Ding S, et al. HIV infection and risk of COVID-19 mortality: A meta-analysis. Medicine. 2021;100(26):e26573. doi: 10.1097/md.0000000000026573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. 2021;11(1):6283. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Feng R, Xu J, Shi L, Feng H, Yang H. An updated meta-analysis on the association between HIV infection and COVID-19 mortality. AIDS. 2021;35(11):1875–1878. doi: 10.1097/qad.0000000000002968. [DOI] [PubMed] [Google Scholar]
- 12.Liang M, Luo N, Chen M, et al. Prevalence and Mortality due to COVID-19 in HIV Co-Infected Population: A Systematic Review and Meta-Analysis. Infectious diseases and therapy. 2021;10(3):1267–1285. doi: 10.1007/s40121-021-00447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE. 2020;15(8):e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kouhpayeh H, Ansari H. HIV infection and increased risk of COVID-19 mortality: A Meta-Analysis. Eur J Trans Myol. 2021 doi: 10.4081/ejtm.2021.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann IR, Sanchez MN, Frio GS, et al. Trends in COVID-19 case-fatality rates in Brazilian public hospitals: a longitudinal cohort of 398,063 hospital admissions from 1st March to 3rd October 2020. PLoS ONE. 2021;16(7):e0254633. doi: 10.1371/journal.pone.0254633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geretti AM, Stockdale AJ, Kelly SH, et al. Outcomes of coronavirus disease 2019 (COVID-19) Related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) Clinical characterization protocol (UK): a prospective observational study. Clin Infect Dis. 2021;73(7):e2095–e2106. doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adrish M, Chilimuri S, Sun H, Mantri N, Yugay A, Zahid M. The association of renin-angiotensin-aldosterone system inhibitors with outcomes among a predominantly ethnic minority patient population hospitalized with covid-19: the bronx experience. Cureus. 2020;12(9):e10217. doi: 10.7759/cureus.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett KE, Mullooly M, O'Loughlin M, et al. Underlying conditions and risk of hospitalisation, ICU admission and mortality among those with COVID-19 in Ireland: A national surveillance study. Lancet Reg Health Eur. 2021;5:100097. doi: 10.1016/j.lanepe.2021.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergman J, Ballin M, Nordström A, Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021;36(3):287–298. doi: 10.1007/s10654-021-00732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. The Lancet HIV. 2021;8(1):e24–e32. doi: 10.1016/s2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown AE, Croxford SE, Nash S, et al. COVID-19 mortality among people with diagnosed HIV compared to those without during the first wave of the COVID-19 pandemic in England. HIV Med. 2021 doi: 10.1111/hiv.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bushman D, Davidson A, Pathela P, et al. Risk factors for death among hospitalized patients aged 21–64 years diagnosed with COVID-19-New York City, March 13-April 9, 2020. J Racial Ethn Health Disparities. 2021 doi: 10.1007/s40615-021-01098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai M, Bowe B, Xie Y, Al-Aly Z. Temporal trends of COVID-19 mortality and hospitalisation ratES an observational cohort study from the US Department of Veterans Affairs. BMJ Open. 2021;11(8):e047369. doi: 10.1136/bmjopen-2020-047369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chanda D, Minchella PA, Kampamba D, et al. COVID-19 Severity and COVID-19-Associated Deaths Among Hospitalized Patients with HIV Infection - Zambia, March-December 2020. MMWR Morbidity and mortality weekly report. 2021;70(22):807–10. 10.15585/mmwr.mm7022a2 [DOI] [PMC free article] [PubMed]
- 26.Choi YJ, Park JY, Lee HS, et al. Variable effects of underlying diseases on the prognosis of patients with COVID-19. PLoS ONE. 2021;16(7):e0254258. doi: 10.1371/journal.pone.0254258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durstenfeld MS, Sun K, Ma Y, et al. Association of human immunodeficiency virus infection with outcomes among adults hospitalized with COVID-19. AIDS. 2021 doi: 10.1097/QAD.0000000000003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen NAF, Nyga R, Vanderbeke L, et al. Multinational observational cohort study of COVID-19-associated pulmonary Aspergillosis(1) Emerg Infect Dis. 2021;27(11):2892–2898. doi: 10.3201/eid2711.211174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabarriti R, Brodin NP, Maron MI, et al. Association of race and ethnicity with comorbidities and survival among patients with covid-19 at an urban medical center in New York. JAMA Netw Open. 2020;3(9):e2019795. doi: 10.1001/jamanetworkopen.2020.19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan-Lewis E, Banga J, Khan M, et al. HIV diagnosis and the clinical course of COVID-19 among patients seeking care within the New York city public hospital system during the initial pandemic peak. AIDS Patient Care STDS. 2021 doi: 10.1089/apc.2021.0124. [DOI] [PubMed] [Google Scholar]
- 31.Kelly JD, Bravata DM, Bent S, et al. Association of social and behavioral risk factors with mortality among US veterans with COVID-19. JAMA Netw Open. 2021;4(6):e2113031. doi: 10.1001/jamanetworkopen.2021.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laracy J, Zucker J, Castor D, et al. HIV-1 infection does not change disease course or inflammatory pattern of SARS-CoV-2-infected patients presenting at a large urban medical center in New York City. Open Forum Infect Dis. 2021 doi: 10.1093/ofid/ofab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S-G, Park GU, Moon YR, Sung K. Clinical characteristics and risk factors for fatality and severity in patients with coronavirus disease in Korea: a nationwide population-based retrospective study using the Korean health insurance review and assessment service (HIRA) database. Int J Environ Res Public Health. 2020 doi: 10.3390/ijerph17228559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Sarangarajan R, Jun T, et al. In-hospital use of ACE inhibitors/angiotensin receptor blockers associates with COVID-19 outcomes in African American patients. J Clin Invest. 2021 doi: 10.1172/JCI151418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundon DJ, Mohamed N, Lantz A, Goltz HH, Kelly BD, Tewari AK. Social Determinants Predict Outcomes in Data From a Multi-Ethnic Cohort of 20,899 Patients Investigated for COVID-19. Frontiers in Public Health. 2020 doi: 10.3389/fpubh.2020.571364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makker J, Mantri N, Patel HK, et al. The incidence and mortality impact of gastrointestinal bleeding in hospitalized COVID-19 Patients. Clin Exp Gastroenterol. 2021;14:405–411. doi: 10.2147/CEG.S318149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascarello KC, Vieira A, Souza ASS, Marcarini WD, Barauna VG, Maciel ELN. COVID-19 hospitalization and death and relationship with social determinants of health and morbidities in Espirito Santo State, Brazil: a cross-sectional study. Epidemiol Serv Saude. 2021;30(3):e2020919. doi: 10.1590/S1679-49742021000300004. [DOI] [PubMed] [Google Scholar]
- 38.Mollalo A, Rivera KM, Vahabi N. Spatial statistical analysis of pre-existing mortalities of 20 diseases with COVID-19 mortalities in the continental United States. Sustain Cities Soc. 2021;67:102738. doi: 10.1016/j.scs.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh TK, Song IA, Song KH, Jeon YT. Comparison of all-cause mortality between individuals with COVID-19 and propensity score-matched individuals without COVID-19 in South Korea. Open Forum Infect Dis. 2021 doi: 10.1093/ofid/ofab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlando V, Rea F, Savare L, et al. Development and validation of a clinical risk score to predict the risk of SARS-CoV-2 infection from administrative data: A population-based cohort study from Italy. PLoS ONE. 2021;16(1):e0237202. doi: 10.1371/journal.pone.0237202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osibogun A, Balogun M, Abayomi A, et al. Outcomes of COVID-19 patients with comorbidities in southwest Nigeria. PLoS ONE. 2021;16(3):e0248281. doi: 10.1371/journal.pone.0248281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul R, Arif A, Pokhrel K, Ghosh S. The association of social determinants of health with COVID-19 mortality in rural and urban counties. J Rural Health. 2021;37(2):278–286. doi: 10.1111/jrh.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Guzman PN, Daunt A, Mukherjee S, et al. Clinical characteristics and predictors of outcomes of hospitalized patients with COVID-19 in a multi-ethnic London NHS Trust: a retrospective cohort study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robles-Perez E, Gonzalez-Diaz B, Miranda-Garcia M, Borja-Aburto VH. Infection and death by COVID-19 in a cohort of healthcare workers in Mexico. Scand J Work Environ Health. 2021;47(5):349–355. doi: 10.5271/sjweh.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients With COVID-19. JAMA Netw Open. 2020;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semenzato L, Botton J, Drouin J, et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur. 2021;8:100158. doi: 10.1016/j.lanepe.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohrabi M-R, Amin R, Maher A, et al. Sociodemographic determinants and clinical risk factors associated with COVID-19 severity: a cross-sectional analysis of over 200,000 patients in Tehran. Iran. BMC Infectious Diseases. 2021 doi: 10.1186/s12879-021-06179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venturas J, Zamparini J, Shaddock E, et al. Comparison of outcomes in HIV-positive and HIV-negative patients with COVID-19. J Infect. 2021;83(2):217–227. doi: 10.1016/j.jinf.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Sun J, Patel RC, et al. Associations between HIV infection and clinical spectrum of COVID-19: a population level analysis based on US National COVID Cohort Collaborative (N3C) data. The Lancet HIV. 2021;8(11):e690–e700. doi: 10.1016/s2352-3018(21)00239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge E, Li Y, Wu S, Candido E, Wei X. Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study. PLoS ONE. 2021;16(10):e0258154. doi: 10.1371/journal.pone.0258154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jassat W, Cohen C, Tempia S, et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. The Lancet HIV. 2021;8(9):e554–e567. doi: 10.1016/s2352-3018(21)00151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA. Risk Factors for Coronavirus Disease (COVID-19) death in a population cohort study from the Western Cape Province. South Africa. Clin Infect Dis. 2019 doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin C, Zhou L, Hu Z, et al. Dysregulation of Immune response in patients with coronavirus 2019 (COVID-19) in Wuhan. China Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013;119:51–83. doi: 10.1016/b978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiau S, Krause KD, Valera P, Swaminathan S, Halkitis PN. The Burden of COVID-19 in People Living with HIV: A Syndemic Perspective. AIDS Behav. 2020;24(8):2244–2249. doi: 10.1007/s10461-020-02871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gervasoni C, Meraviglia P, Riva A, et al. Clinical Features and Outcomes of Patients With Human Immunodeficiency Virus With COVID-19. Clin Infect Dis. 2020;71(16):2276–2278. doi: 10.1093/cid/ciaa579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blanco JL, Ambrosioni J, Garcia F, et al. COVID-19 in patients with HIV: clinical case series. The lancet HIV. 2020;7(5):e314–e316. doi: 10.1016/s2352-3018(20)30111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tesoriero JM, Swain CE, Pierce JL, et al. Elevated COVID-19 outcomes among persons living with diagnosed HIV infection in New York State: Results from a population-level match of HIV, COVID-19, and hospitalization databases. 2020; 10.1101/2020.11.04.20226118
- 61.De Francesco D, Wit FW, Bürkle A, et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS. 2019;33(2):259–268. doi: 10.1097/qad.0000000000002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lagathu C, Cossarizza A, Béréziat V, Nasi M, Capeau J, Pinti M. Basic science and pathogenesis of ageing with HIV: potential mechanisms and biomarkers. AIDS. 2017;31(Suppl 2):S105–S119. doi: 10.1097/qad.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 63.Tesoriero JM, Swain CE, Pierce JL, et al. COVID-19 Outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open. 2021;4(2):e2037069. doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are included in this article and available from the corresponding author upon reasonable request.
Not applicable.