Abstract
Energy metabolism maintains the activation of intracellular and intercellular signal transduction, and plays a crucial role in immune response. Under environmental stimulation, immune cells change from resting to activation and trigger metabolic reprogramming. The immune system cells exhibit different metabolic characteristics when performing functions. The study of immune metabolism provides new insights into the function of immune cells, including how they differentiate, migrate and exert immune responses. Studies of immune cell energy metabolism are beginning to shed light on the metabolic mechanism of disease progression and reveal new ways to target inflammatory diseases such as autoimmune diseases, chronic viral infections, and cancer. Here, we discussed the relationship between immune cells and metabolism, and proposed the possibility of targeted metabolic process for disease treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11033-022-07474-2.
Keywords: Immune cells, Immune metabolism, Metabolic reprogramming, Targeted therapy
Introduction
Immune cells refer to all the cells and their precursors related to the immune response, which can be divided into innate immune cells and adaptive immune cells. The former include dendritic cells (DCs), macrophages, and natural killer (NK) cells; the latter refer to T lymphocytes and B lymphocytes that play a major role in the immune response. In most normal cases, immune cells are in a relatively static state [1]. When the body is confronted with abnormal interference such as infection, trauma and inflammation, it will rapidly activate to exert immune function, eliminate target substances, and maintain homeostasis [2]. In immune cells, metabolic changes may occur in response to indicative signals received from other cells or from environmental changes, such as the presence of danger signals or antigens [3]. The transformation of cells from resting to excited state involves a series of metabolic changes, especially the transformation of intracellular energy materials and metabolic pathways [4]. Previous studies have shown that the participation of immune receptors such as TLR and IL-2 receptors, metabolic transformation and immune cell function are highly correlated [5]. For example, the activation of immune receptors promotes glycolysis, which is the energy source of regulatory T cells (Tregs) migration and determines the polarization direction of macrophages [6].
With the development of immunology and metabolism, more and more studies have found that metabolism can affect the differentiation and function of immune cells [7]. Immune metabolism refers to the interaction between metabolism and immune response, which has been proved to be related to immune activation in many diseases [8]. The activation, differentiation and function of immune cells are dependent on energy supply and metabolic transformation (Fig. 1A). Assessing the response of immune cells to metabolic processes in health and disease states, may provide new therapeutic approaches for clinic.
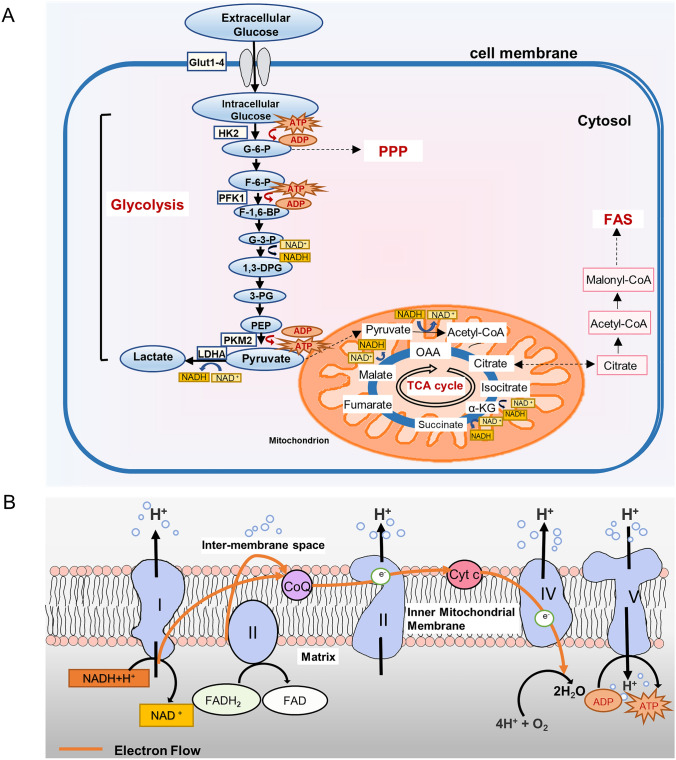
Fig. 1.
Simplified representation of glucose metabolic pathways. A Glycolysis occurs in the cytoplasm, converting glucose into pyruvate and then into lactic acid in the mitochondria. Under aerobic conditions, pyruvic acid is oxidized and decarboxylated to form acetyl CoA, which can be completely oxidized into TCA. Intermediates in glucose metabolism can flow to other metabolic pathways, such as PPP and FAS. B ETC refers to the structure of NADH or FADH2 transferring electrons to oxygen. OXPHOS causes the gain and loss of electrons on the electron carrier, which is transferred between redox substrates to form ETC
In this review, we summarize several common metabolic reprogramming of immune cells, focusing on the metabolic pathways (Figure S1 and reviewed in [9, 10]) of cell resting and activation. In addition, we will discuss metabolism in health and disease, as well as current treatment strategies for targeted metabolism, and aim to apply metabolic reprogramming to disease treatment.
Innate immune cells
Dendritic cells
Dendritic cells (DCs), as the most functional professional antigen-presenting cells, are the bridge between innate immunity and adaptive immunity [11]. DCs can produce antigen-carrying MHC molecules and rapidly deploy them to the cell surface, which is crucial for their ability to initiate naive T (Tn) cells [12].
DCs undergo metabolic reprogramming during activation, which is driven by PI3K/Akt pathway and antagonized by the AMPK pathway, similar to the Warburg effect in tumor cells [13]. This metabolic transformation is marked by the transition of mitochondrial OXPHOS from lipid oxidation to aerobic glycolysis [11] (Fig. 1B). Recent evidence suggests that the early glycolytic burst in BMDCs can partly bypass the requirement for glucose during activation [14]. The development of DCs from progenitor cells is associated with mitochondrial biogenesis, which is driven by PGC-1α and promoted by PPARγ, mTOR and MYC [15] (Table S1). Activated DCs provide energy through glycolysis and lactic acid fermentation and convert glycolysis intermediates into the PPP [16]. TLR signal is the main pathway for DCs activation. It leads to increased glycolysis metabolism and triggers the necessary metabolic reprogramming for DCs maturation [7, 11]. CCR7 stimulates the activation of HIF1α transcription factor pathway in DCs, leading to the conversion of metabolic reprogramming to glycolysis for DCs migration [17]. Regardless of the stimulation and activation phenotypes of PRRs, inducing the glycolysis of DCs in vivo is necessary for supporting the movement of DCs and CCR7 oligomerization [18].
In addition, it has recently been found that fatty acid metabolism is related to different functions of DCs [19]. When the synthesis of fatty acids was blocked, the number of DCs from PBMC precursors decreased [20]. Blocking of FAS also inhibits the DCs development of human PBMC and induces the apoptosis of DCs precursors [19].
Delivery antigen DCs play a pro-inflammatory role, enhance and prolong T cell response [21]. Thus, DCs vaccine work better than other anticancer vaccines because they integrate other immune-related signals to effectively induce antigen-directed T cell responses on a cellular platform [22]. The “second generation” DCs vaccine strategy is more effective in clinical practice, so it becomes the preferred choice of cancer immunologists [23].
Macrophage
Macrophages exist in all mammalian tissues. They can not only resist infection, but also are key members of homeostasis and tissue repair. Macrophages are considered to be the most plastic cells in innate immune system and the first line of defense against external infection [24].
In resting state, macrophages use TCA cycle to breathe normally. The expression of PRRs on cell surface allows resting macrophages to quickly recognize PAMPs, thereby inducing inflammation [25]. Macrophages activated by the bacterial product LPS are known as M1 or classically activated macrophages, and have very different metabolic characteristics compared with IL-4 activated macrophages, namely M2 or alternately activated macrophages [26]. The former has pro-inflammatory properties, while the latter has anti-inflammatory and parasitic effects [27]. In LPS-induced macrophages, TCA cycle is broken and results in elevated levels of intermediates such as succinate and malate [26, 28]. Glycolysis can play a role in M1 and M2, but the way is different. In the former, glycolysis is associated with the activation of the PPP for biosynthesis of biomolecules and ATP production, while in the latter, glycolysis is used to support OXPHOS [29, 30]. In M1, glucose uptake is enhanced and switches to glycolysis, while in M2, the main metabolic feature is enhanced FAO and OXPHOS [31].
Changes in metabolic processes are inseparable from enzymes involved in glycolysis. PKM is a rate-limiting enzyme in glycolysis, converting PEP to pyruvate. PKM2 exists in the proliferation of a few normal cells, while it is present at high levels in activated immune cells [32]. Palsson-McDermott indicated that PKM2 is a key determinant of macrophage glycolytic reprogramming, and that PKM2 is activated in macrophages to inhibit lipopolysaccharide-induced glycolysis and HIF1α expression related genes [33]. A previous study showed that PKM2 selectively promotes the activation of macrophages inflammasome by activating EIF2AK2 phosphorylation [32]. Glycolytic activator PFKFB3 promotes macrophages to clear infected cells and enhance their antiviral ability [34]. A recent study shows that macrophages mobilize glycogen metabolism, which governs macrophage-mediated inflammatory response [35].
The nuclear receptor PPARγ is known to regulate lipid metabolism in many tissues, including macrophages. PPARγ controls macrophage glutamine metabolism, providing a link between transcription and metabolism [36]. As reports, endogenous oxidized lipids promote simultaneous OXPHOS, aerobic glycolysis, and the hyperproduction of IL-1β in LPS-stimulated macrophages [37]. Fatty acid metabolism pathways can be categorized into FAS and FAO, which are highly activated in M1 and M2 [38]. It has been suggested that the activation of inflammatory macrophages is dependent on glycolysis, while IL-4-induced macrophages are driven by FAO [27]. Citrate will be withdrawn for fatty acid biosynthesis, one hallmark of the M1 [39]. However, M2 has a fully functional respiratory redox chain that allows FAO without producing ROS [29] (Fig. 2).
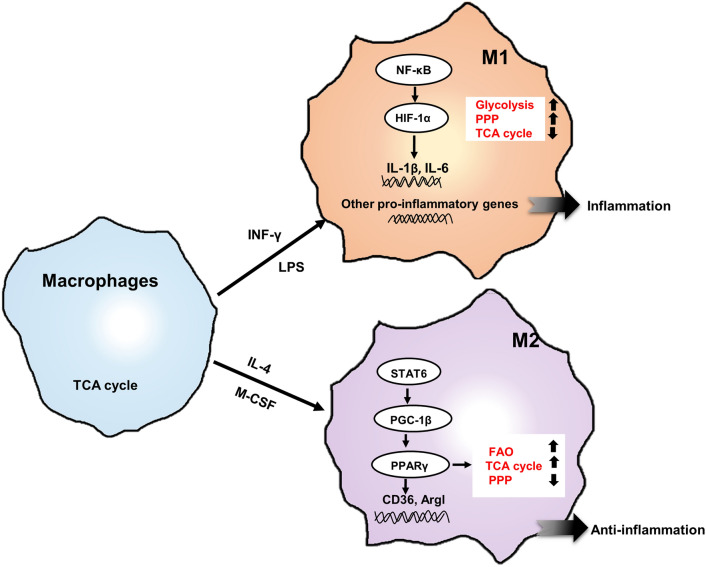
Fig. 2.
Major metabolic patterns in macrophages. The metabolic modes of M1 and M2 are different. In M1, the increase of glucose uptake is used for glycolysis and PPP. While in M2, the main metabolic characteristics are the increase of FAO and OXPHOS. M1 induced by LPS/INF-γ are regulated by HIF1α. In LPS (+ IFN-γ)-activated inflammatory macrophages, HIF1α not only promotes glycolysis, but also induces the expression of genes encoding inflammatory cytokines, especially IL-1β and IL-6. In IL-4-induced macrophages, PPARγ as the main regulator can use the transported glucose for TCA cycle. FAO can promote the development and activity of anti-inflammatory macrophages. Moreover, FAO has been proved to support mitochondrial oxidation and metabolism of M2, and ATP is continuously through OXPHOS. In M2, the anti-inflammatory effect was exerted by activating the expression of anti-inflammatory genes such as CD36 and Argl
Van den Bossche et al. show that the activation of inflammatory M1 inhibited mitochondrial function, thereby preventing the repolarization of anti-inflammatory M2 phenotype [26]. Blocking oxidative metabolism not only blocks M2 phenotype, but also makes macrophages enter M1 state [27]. Therefore, intervening in the metabolic pathway of macrophages and regulating the phenotypic transformation of M1/M2 may help to resist autoimmune diseases, tumors and other immune-related diseases [40].
Natural killer cell
Human NK cells are lymphocytes that connect innate immunity and adaptive immunity. They have excellent antiviral and anti-tumor properties, produce cytokines IFN, and directly kill target cells through cytotoxic mechanisms [41].
Glucose is the key fuel of NK cells, which directly affects the rate of glycolysis and OXPHOS [42]. At rest, NK cells preferentially use OXPHOS [43]. The levels of glycolysis and OXPHOS in stationary NK cells of mice were low but sufficient to sustain a rapid initial immune response [42]. Using cytokines to stimulate NK cells to activate, the incidence of glycolysis and OXPHOS is greatly increased. OXPHOS is required for the secretion of IFN-γ by receptor-stimulated NK cells [43]. 13C-glucose-tracing metabolomics have shown that NK cells use glucose to promote the biosynthesis of amino acids and fatty acids 18 h after cytokine stimulation [44]. Activating mouse NK cells with IL-2 and IL-12 breaks down glucose into pyruvate, which then produces lactic acid through aerobic glycolysis [42].
Transcription factors play an important role in the activation and metabolism of NK cells. The activity of transcription factor SREBP is necessary for the increase of glycolysis and OXPHOS. The glycolysis is increased by regulating the expression of citric acid and malic acid reverse transporter SLC25A1 and ACLY15 [44]. MYC controls the activation of metabolic pathways in NK cells, supporting glycolysis and mitosis by increasing the expression of glucose transporters and glycolytic enzymes to improve mitochondrial quality to support high levels of OXPHOS [45]. In NK cells stimulated by cytokines, elevated glycolysis requires mTORC1 signal transduction [46, 47].
In recent years, the research on the metabolic characteristics of NK cells in tumor microenvironment has become a new direction for cancer treatment. NK cell activity is negatively correlated with the incidence of cancer. New evidence suggests that NK cell infiltration into squamous cell lung is associated with better prognosis [48]. Brand et al. found that LDHA increased the production of lactic acid in cancer cells. The accumulation of lactic acid will destroy the production of IFN-γ in tumor-infiltrating T cells and NK cells, thereby inhibiting tumor immune surveillance and promoting tumor growth [49]. A high concentration of lactic acid in tumor microenvironment can damage the function of NK cells [48, 50]. Changes in NK cell metabolism may be an important factor in NK cell dysfunction (Table 1). Therefore, if we want to further explore the anti-tumor effect of NK cells, it will be a good choice from the perspective of metabolism.
Table 1.
Major metabolic pathways in innate cells
| Cell type | Energy sources in quiescent state | Energy sources in activition state | Related pathway changes | References |
|---|---|---|---|---|
| DCs | TCA cycle, OXPHOS |
Glycolysis, lactic fermentation |
TBK1-IKKε/AKT/HK-II pathway,PI3K/AKT/mTOR pathway,mTOR/HIF1α/iNOS pathway | [13, 15, 17, 18] |
| Macrophage | TCA cycle, OXPHOS |
M1: aerobic glycolysis, FAO M2: FAO, gluconeogenesis |
HIF-1a/PDK1 pathway, Akt/mTORC1 pathway, JAK/STAT6 Pathway, PPARγ/LXR/ABCA1 pathway | [32, 33, 36, 38] |
| NK cells | TCA cycle, OXPHOS, glutaminolysis |
OXPHOS, aerobic glycolysis |
PI3K/Akt/mTORC1 pathway, JAK/STAT pathways,NF-kB pathways |
[44, 46–48] |
Adaptive immune cells
T lymphocytes
T lymphocytes are a highly heterogeneous group, containing a large number of subsets, which play a central role in the immune response of foreign antigens [51, 52]. The latest research progress shows that metabolic changes have taken place in various biological processes of T cells, such as TCR-mediated activation and auxiliary T cell differentiation [53], suggesting that metabolic pathways can seriously affect T cell function. In the activation process of TCR-dependent T cells, glucose, glutamine, and other biological molecules as nutrients determine whether T cells can be activated and play a role [52]. T cell subsets perform different functions in the immune response and have different metabolic patterns.
NAIVE T cell
T lymphocytes develop and mature in the thymus, named Tn cells, which are recirculated through the paracortical regions of SLOs to recognize antigens [51].
The function of Tn cells maintained by TCR engagement by self-peptide-MHC molecules and the cytokine IL-7. Tn cells are mostly in static state, lack of mTOR expression, priority use OXPHOS as fuel [53, 54]. The absence of negative regulatory factors activated by TCR or the uninhibition of AKT activity will lead to the loss of resting state of Tn cells and increase the proliferation of homeostasis [55]. After T cells were activated, mTORC1 up-regulated C-MYC expression, indirectly accelerating glycolysis and glutamine metabolism [54, 55].
Memory T cell
Memory T(Tm) cells are named for making the immune system remember. Tm cells can persist in the body for decades. Tm cells circulate in the blood and exist in lymphatic organs, which is an important part of long-standing T cell immunity. When the antigen first stimulates the human body, it forms a Tm cells subset that enhances human immunity [56]. As the most important T cell subsets, memory CD8 + T cells and memory CD4 + T cells have attracted the attention of many studies.
Like Tn cells, memory CD8 + T cells are metabolically quiescent cells that use OXPHOS for energy conversion [54]. After infection, memory CD8 + T cells can remain in the tissue, rather than through the blood circulation of Tm cells in the tissue [56]. Owning to they are stationary in tissues, their glycolysis is at a low level, mainly using exogenous fatty acids as fuel. In the study of memory CD8 + T cells in the skin, it is found that the oxidation of fatty acids is necessary for cell growth. When the oxidation of free fatty acids in mitochondria is inhibited, memory persistence is reduced [57, 58]. CD4 + memory T cells preferentially adhere to mucosal tissues, and the number of CD4 + T cells is more than that of CD8 + T cells [59]. At present, there are few studies on CD4 + memory T cells, and no special metabolic pathways have been found.
Regulatory T cell
Tregs are a kind of T cell subsets that control the autoimmunity in the body. They inhibit the activation and proliferation of potential autoreactive T cells in the normal body through active regulation, thereby regulating the immunity of the body [60].
Tregs are more likely to use FAO and OXPHOS for energy, suggesting that T cells can function through lipid oxidation without glucose [61]. Valerie A et al. reported that Tregs not only oxidize lipids at high rates but also produce pyruvate by glycolysis [62]. The expression of Foxp3 leads to the up-regulation of mitochondrial protein-coding genes, thereby promoting the respiratory capacity of Tregs and enhancing their ability to utilize fatty acids to provide energy for OXPHOS. Foxp3 maintains regulatory function by inhibiting the expression of MYC and the glycolysis of Tregs, thereby giving Tregs metabolic advantages in low lactate environments [63, 64]. After inhibition of mTORC1 in mice, Tregs could not effectively synthesize lipids from glucose, and the cholesterol content was also reduced [65]. Deborah Cluxton et al. showed that Tregs oxidizes exogenous fatty acids as metabolic fuel instead of glucose [66].
Effector T cells
During the transition from Tn cells to effector T cells(Teffs), glucose uptake is upregulated and most glucose-derived pyruvate is excreted to lactic acid, which is similar to tumor cells’ Warburg physiology [53]. CD4 + T cells proliferate and differentiate into Teffs or Tregs that mediate or regulate immunity after activation [67]. Teffs use large amounts of glucose and high-speed glycolysis to meet energy needs [62]. mTOR signaling pathway has a profound impact on the fate of CD4 + T cells by regulating metabolic reprogramming [56]. A recent study showed that inhibition of mTORC1 activity attenuates glycolysis and reduces effector function, while absence of mTORC2 enhances glycolysis and increases effector function [68]. In addition, mTOR activation induced the expression of glucose transporter Glut1, which enhanced the proliferation of T cells and the production of cytokines [69]. Glut1 is the main transporter in glucose uptake and maintains a high level of expression in effector T cells. Glut1 mediates a sharp increase in glucose intake, which is necessary to promote cell growth, effector function and Teffs proliferation [70] (Fig. 3). In the absence of Glut1, CD4 + and CD8 + effector T cells can only maintain limited proliferation [67].
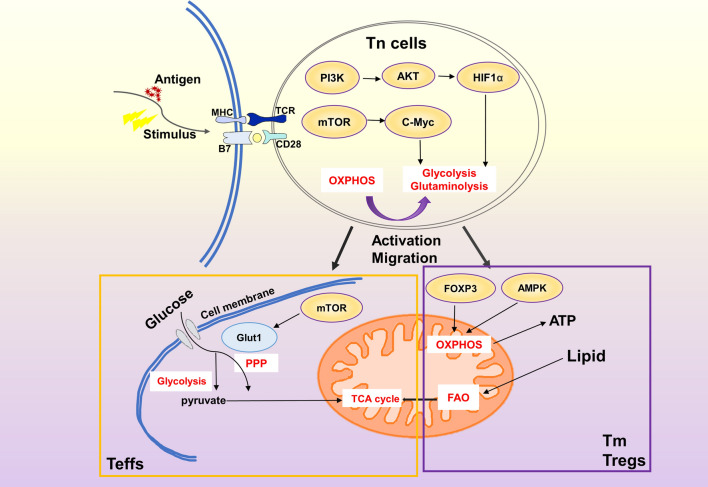
Fig. 3.
The metabolic differences of Teffs, Tm, Tregs. When Tn cells were stimulated to recognize antigens, the resting state of energy supply by OHPHOS was broken. With the assistance of HIF1α and C-Myc, glutamine decomposition was enhanced, and a large amount of energy was provided by glycolysis. It will undergo a development process characterized by rapid growth, proliferation and acquisition of special effects, and then differentiate into Teffs, Tregs and Tm cells. FAO plays an important role in regulating T cell response. So far, FAO has been observed to regulate the balance between inflammatory Teffs and inhibitory Tregs, and maintain the function of long-lived Tm cells. Compared with Teffs, Tregs and Tm cells showed more FAO and OXPHOS, and FAO promoted the generation of Tregs. Interestingly, the down-regulation of FAO by Teffs depends more on glycolysis and TCA cycle energy supply. FOXP3 and AMPK promote the OXPHOS and increase ATP production to meet the energy supply in Tm cells and Tregs. mTOR increases glucose uptake by targeting Glut1, making Teffs play an effective role in the body
T lymphocytes, as the effector cells of cellular immunity, maintain homeostasis and play an immunoregulatory role. T cells use different metabolic energy at different stages. Since metabolism controls the function and fate of T cells, it is necessary to study the changes made by different types of T cells in the state of disease.
B lymphocytes
B lymphocytes perform a variety of functions in adaptive immunity, the most critical of which are the secretion of disease-specific antibodies, antibody class switching, and affinity maturation [71]. During the immune response, B cells diversify their immunoglobulin genes in GCs, thus producing high-affinity, transformation-like antibodies that mediate humoral immunity [72].
Similar to other cells in the body, B cells mainly source of energy and carbon from glucose. Recently, it has been found that constitutively active GSK3 is a metabolic checkpoint regulator of resting B cells. Without antigen or growth factor stimulation, it assists cell survival by limiting protein synthesis and mitochondrial function [73]. Compared with the naïve B cells, the activated B cells absorb glucose, consume oxygen and secrete lactic acid more [74], suggesting that OXPHOS and glycolysis play a role simultaneously. Glucose is necessary to support cell activation, and to a certain extent supports the generation of new fats through ACLY activity, providing sufficient phospholipids for activated B cells to maintain morphological changes [75, 76]. Amino acids also contribute significantly to their metabolism. Amino acid consumption, alanine and glutamate production increased during B cell activation [76]. The increased absorption of amino acids such as leucine and lysine help to make the signal flow through in the downstream pathway of PI3K, and enhance the ability of solute transporters to absorb amino acid synthesis [77].
The metabolic process of B cells changes from static state to active state, and the role of molecular signals cannot be underestimated. Molecules such as TAPP [78], mTORC1 [79] and c-MYC [80] can control the activation of B cells. When TAPP expression is decreased, OXPHOS and glycolysis are increased, and the proliferation and autoimmunity of B cells in the germ center also increased [78]. Transcription factors c-MYC, HIF1α and STAT6 promote glycolysis gene expression, while Bcl6 inhibits transcription of certain target genes and glycolytic pathway [77].
GC B cells that react in GC will differentiate into long-lived memory B cells or plasma cells that produce antibodies [74]. GC B cells showed that mTORC1 activation and c-MYC accumulation were increased, and genes related to glycolysis were also up-regulated [81]. Due to the increased protein expression of glycolysis, TCA cycle and ETC in these cells, the number of mitochondria and the expression of HIF1α also increased [82]. Compared with the naive B cells, plasma cells have a higher protein synthesis rate, absorb more amino acids and glucose, and produce a large number of ROS [83]. In activated B cells, the decrease of oxygen concentration reduces mTORC1 signal transduction and inhibits the conversion of immunoglobulins isoforms [77]. However, hypoxia promotes plasma cell survival and supports regulatory B cell function. Hypoxia promotes the expression of HIF1α [77, 83], triggers steady transcription and regulates the expression of glucose transporters and glycolytic enzymes when oxygen concentration is limited [77]. With the participation of BCR or IL-4 stimulation, activated B cells became larger, total protein and CD138 expression increased [84]. In addition, BCR signaling pathway increases Glut1 expression and glucose uptake in PI3K-dependent mechanisms [85] (Table 2).
Table 2.
Major metabolic pathways in adopted cells
| Cell type | Energy sources in quiescent state | Energy sources in activition state | Related molecular changes | References |
|---|---|---|---|---|
| Tn cells | OXPHOS | Glycolysis | IL-7, mTORC1,MYC | [53, 54, 63] |
| Tm cell | FAO, mitochondrial metabolism | OXPHOS, TCA cycle |
Akt, mTORC2, aquaporin 9 |
[54, 56] |
| Tregs |
FAO, OXPHOS |
Glycolysis | AMPK, HIF1α Foxp3 | [62, 65, 98] |
| Teffs | FAO |
Glycolysis, glutaminolysis, TCA cycle |
mTOR, HIF1α | [68, 69] |
| B lymphocytes | OXPHOS |
Glycolysis, glutaminolysis, TCA cycle |
TAPP, mTORC1, C-MYC | [78–80] |
B cells transit from static state and recycling to activation, proliferate rapidly, and produce a large number of antibodies. Only when metabolism, extracellular stimulation and intracellular signal transduction work together, the humoral response dominated by B cells can be successfully carried out. Breaking this balance will lead to malignant transformation of B cells. Therefore, the discovery of metabolic differences between B cell activation and malignant tumors is very important for the treatment and prevention of B cell lymphoma.
Conclusion
Nowadays, studies on immune cells and metabolism have received extensive attention. Immune cells are involved in the progression of many human diseases, such as cancer [86–88], autoimmune diseases [89], and heart disease [90].
The metabolic pathways of sugar, fat and amino acids interact with each other, which are closely related to the survival and activation of immune cells. Studies have shown that immune cell subsets in disease state show different metabolic pathways to promote cell survival, lineage generation and function. Enhanced glycolysis enables immune cells to produce sufficient ATP and biosynthetic intermediates to perform their specific effector functions [89]. In acute lymphoblastic leukemia, carcinogenic signaling induces metabolic stress, increases glucose uptake and aerobic glycolysis of activated T cells [91]. After COVID-19 infection, monocytes and macrophages trigger mitochondrial ROS production, stabilize HIF1α expression, and promote glycolysis to maintain high viral replication level [92]. T cells in rheumatoid arthritis divert intracellular glucose to PPP and produce NADPH, which uses lactic acid from the external environment to meet their energy needs [93]. In normal kidney, the level of OXPHOS of Tregs and DCs was higher than that of glycolysis, and the two metabolic patterns were exchanged during acute kidney injury [94]. In addition, the accumulation of lactic acid in tumor promotes DCs OXPHOS and induces Tregs response [95]. Therefore, glycolysis enhancement is considered to be a marker metabolic change for the rapid activation of most immune cells.
It has therapeutic potential to change the state or function of immune cells by regulating immune cell metabolism in diseases. For example, In multiple sclerosis, targeted glycolysis enhances the inhibitory ability of Tregs and affects the differentiation of proinflammatory cells [96]. Glycolysis and TCA cycle have significant changes in autoimmune diseases (such as systemic lupus erythematosus). And there have been studies in animal models and preliminary human trials confirmed the efficacy of intervention in metabolic pathways. Besides, studies have shown that targeting key processes to reduce gluconeogenesis or increase glucose excretion can improve the prognosis of patients with type 1 diabetes [97].
In this review, we discuss the metabolic patterns of immune cells in different states. With the development of metabolomics, research in this field advances rapidly. However, due to the dynamic changes of the body and the complex and diverse metabolic pathways, there are still many problems to be solved in the treatment of diseases by reprogramming immune cell metabolism. Further elucidating the mechanism of metabolism mediated by immune cells, especially in the state of disease, is expected to realize the prospect of immunotherapy in this field.
Supplementary Information
Below is the link to the electronic supplementary material.
Figure S1. Major metabolic pathways in cells. Glycolysis is the decomposition of glucose into pyruvate and produces energy in a relatively hypoxic environment. Even under aerobic conditions, proliferating cells tend to convert glucose into lactic acid, a process known as aerobic glycolysis. TCA cycle consists of a series of enzymatic reactions. Pyruvate enters mitochondria and decarboxylates to form acetyl CoA under aerobic conditions. OXPHOS refers to the oxidation of metabolic substrate by electron respiratory chain to ATP in TCA cycle. PPP refers to the metabolic pathways that produce NADPH and 5-phosphate ribose using glycolysis intermediates(such as G-6-P), excluding ATP production and consumption. FAO is a process in which fatty acids are decomposed into acetyl CoA to produce large amounts of ATP to provide energy to the body. Most of the raw materials for FAS are derived from food, which is exogenous fatty acid and can be used by the body through modification in vivo. Supplementary file1 (TIF 1311 kb)
Abbreviations
- 1,3-DPG
1,3-Diphosphoglyceric acid
- 3-PG
3-Phosphoglycerate
- α-KG
α-Ketoglutarate
- Acetyl-CoA
Acetyl-coenzyme A
- ACLY
ATP-citrate lyase
- ADP
Adenosine diphosphate
- AMPK
AMP-activated protein kinase
- ATP
Adenosine-triphosphate
- BCR
B cell antigen receptor
- BMDCs
Bone marrow-derived dendritic cells
- CCR7
C–C motif chemokine receptor 7
- CD
Cluster of differentiation
- CoQ
Coenzyme Q
- Cyt c
Cytochrome c
- EIF2AK2
Eukaryotic translation initiation factor 2-alpha kinase 2
- ETC
Electron transport chain
- F-1,6-BP
Fructose 1,6-bisphosphate
- F-6-P
Fructose-6-phosphate
- FAD
Flavin adenine dinucleotide
- FAO
Fatty acid oxidation
- FAS
Fatty acid synthesis
- FOXP3
Forkhead box P3
- GCs
Germinal centers
- GLS
Glutaminase
- Glut-1
Glucose transportase-1
- GSK3
Glycogen synthase kinase 3
- G-3-P
Glyceraldehyde-3-phosphate
- G-6-P
Glucose-6-phosphate
- HIF1α
Hypoxia-inducible factor 1α
- HK
Hexokinase
- IFN
Interferon
- IL
Interleukin
- LDHA
Lactate dehydrogenase A
- LPS
Lipopolysaccharide
- M-CSF
Macrophage colony-stimulating factor
- MDSCs
Myeloid-derived suppressor cells
- MHC
Major histocompatibility complex
- mTOR
Mammalian targets of the rapamycin
- NAD
Nicotinamide adenine dinucleotide
- NADH
Nicotinamide adenine dinucleotide
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NF-κB
Nuclear factor-k-gene binding
- OAA
Oxaloacetate
- OXPHOS
Oxidative phosphorylation
- PAMPs
Pathogen-associated molecules
- PBMC
Peripheral blood mononuclear cells
- PEP
Phosphoenolpyruvate
- PFKFB3
Phosphofructokinase-2/fructose-2,6-bisphosphatase
- PGC
Peroxisome-proliferator-activated receptorγcoactivator
- PI3K
Phosphoinositol 3-kinase
- PKM
Pyruvate kinase
- PPARγ
Receptor peroxisome proliferator-activated receptor γ
- PPP
Pentose phosphate pathway
- PRRs
Pattern-recognition receptors
- ROS
Reactive oxygen species
- SLOs
Secondary lymphoid organs
- STAT
Signal transducers and activators of transcription
- T1D
Type I diabetes
- T-ALL
T cell acute lymphoblastic leukemia
- TAPP
Tandem PH domain containing protein
- TCA cycle
Tricarboxylic acid cycle
- TCR
T cell receptor
- TLR
Toll-like receptor
- TME
Tumor microenvironment
- TRAF3
TNF receptor-associated factor 3
Author contributions
CH drafted the manuscript. YX, XZ, and YL performed the literature search and revised the manuscript.KY and SY critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82073154) and the Natural Science Foundation of Shaanxi Province (No. 2020SF-200) and Scientific Research Fund (No. BWS19J011).
Data availability
This is a review, so there is no available data.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The review does not cover human participants and animal studies.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenchen Hu and Yuxin Xuan have contributed equally.
Contributor Information
Shuya Yang, Email: yangshuxiaoya@163.com.
Kun Yang, Email: yangkunkun@fmmu.edu.cn.
References
- 1.Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, Larbi A, Weinberger B, Cossarizza A. Aging of the immune system: focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–2301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 2017;169:570–586. doi: 10.1016/j.cell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loftus RM, Finlay DK. Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem. 2016;291:1–10. doi: 10.1074/jbc.R115.693903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou WC, Rampanelli E, Li X, Ting JP. Impact of intracellular innate immune receptors on immunometabolism. Cell Mol Immunol. 2021 doi: 10.1038/s41423-021-00780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce EJ, Pearce EL. Immunometabolism in 2017: driving immunity: all roads lead to metabolism. Nat Rev Immunol. 2018;18:81–82. doi: 10.1038/nri.2017.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Man K, Kutyavin VI, Chawla A. Tissue immunometabolism: development, physiology, and pathobiology. Cell Metab. 2017;25:11–26. doi: 10.1016/j.cmet.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira AV, Domiguez-Andres J, Netea MG. The role of cell metabolism in innate immune memory. J Innate Immun. 2022;14:42–50. doi: 10.1159/000512280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muri J, Kopf M. Redox regulation of immunometabolism. Nat Rev Immunol. 2021;21:363–381. doi: 10.1038/s41577-020-00478-8. [DOI] [PubMed] [Google Scholar]
- 11.Worbs T, Hammerschmidt SI, Forster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 12.Vyas JM. The dendritic cell: the general of the army. Virulence. 2012;3:601–602. doi: 10.4161/viru.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovanelli P, Sandoval TA, Cubillos-Ruiz JR. Dendritic cell metabolism and function in tumors. Trends Immunol. 2019;40:699–718. doi: 10.1016/j.it.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Pearce EJ, Everts B. Dendritic cell metabolism. Nat Rev Immunol. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wculek SK, Khouili SC, Priego E, Heras-Murillo I, Sancho D. Metabolic control of dendritic cell functions: digesting information. Front Immunol. 2019;10:775. doi: 10.3389/fimmu.2019.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Zhang X, Chen K, Cheng Y, Liu S, Xia M, Chen Y, Zhu H, Li Z, Cao X. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1alpha-mediated glycolysis. Immunity. 2019;50(600–615):e15. doi: 10.1016/j.immuni.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Guak H, Al Habyan S, Ma EH, Aldossary H, Al-Masri M, Won SY, Ying T, Fixman ED, Jones RG, McCaffrey LM, Krawczyk CM. Glycolytic metabolism is essential for CCR7 oligomerization and dendritic cell migration. Nat Commun. 2018;9:2463. doi: 10.1038/s41467-018-04804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lima TL, Peron G, Oliveira J, da Rosa LC, Thome R, Verinaud L. The impact of metabolic reprogramming on dendritic cell function. Int Immunopharmacol. 2018;63:84–93. doi: 10.1016/j.intimp.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 20.He Z, Zhu X, Shi Z, Wu T, Wu L. Metabolic regulation of dendritic cell differentiation. Front Immunol. 2019 doi: 10.3389/fimmu.2019.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawless SJ, Kedia-Mehta N, Walls JF, McGarrigle R, Convery O, Sinclair LV, Navarro MN, Murray J, Finlay DK. Glucose represses dendritic cell-induced T cell responses. Nat Commun. 2017;8:15620. doi: 10.1038/ncomms15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577–593. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Choi SY, Jung NC, Song JY, Seo HG, Lee HS, Lim DS. The effect of the tumor microenvironment and tumor-derived metabolites on dendritic cell function. J Cancer. 2020;11:769–775. doi: 10.7150/jca.38785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Prados J-C, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, Cascante M, Boscá L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 26.Van den Bossche J, O'Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Galvan-Pena S, O'Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan DG, O'Neill LAJ. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol. 2020;38:289–313. doi: 10.1146/annurev-immunol-081619-104850. [DOI] [PubMed] [Google Scholar]
- 29.Caputa G, Flachsmann LJ, Cameron AM. Macrophage metabolism: a wound-healing perspective. Immunol Cell Biol. 2019;97:268–278. doi: 10.1111/imcb.12237. [DOI] [PubMed] [Google Scholar]
- 30.Icard P, Fournel L, Wu Z, Alifano M, Lincet H. Interconnection between metabolism and cell cycle in cancer. Trends Biochem Sci. 2019;44:490–501. doi: 10.1016/j.tibs.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Zhang S, Vuckovic I, Jeon R, Lerman A, Folmes CD, Dzeja PP, Herrmann J. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab. 2018;28(463–475):e4. doi: 10.1016/j.cmet.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, Sun X, Yang M, Billiar TR, Wang H, Cao L, Jiang J, Tang D. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7:13280. doi: 10.1038/ncomms13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR, Domingo-Fernandez R, Johnston DG, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O'Neill LA. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21:65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Shi H, Sun M, Wang Y, Meng Q, Guo P, Cao Y, Chen J, Gao X, Li E, Liu J. PFKFB3-driven macrophage glycolytic metabolism is a crucial component of innate antiviral defense. J Immunol. 2016;197:2880–2890. doi: 10.4049/jimmunol.1600474. [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Wei K, Liu J, Tang K, Zhang H, Zhu L, Chen J, Li F, Xu P, Chen J, Liu J, Fang H, Tang L, Wang D, Zeng L, Sun W, Xie J, Liu Y, Huang B. Glycogen metabolism regulates macrophage-mediated acute inflammatory responses. Nat Commun. 2020;11:1769. doi: 10.1038/s41467-020-15636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson VL, Nguyen HCB, Garcia-Canaveras JC, Briggs ER, Ho WY, DiSpirito JR, Marinis JM, Hill DA, Lazar MA. PPARgamma is a nexus controlling alternative activation of macrophages via glutamine metabolism. Genes Dev. 2018;32:1035–1044. doi: 10.1101/gad.312355.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Gioia M, Spreafico R, Springstead JR, Mendelson MM, Joehanes R, Levy D, Zanoni I. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat Immunol. 2020;21:42–53. doi: 10.1038/s41590-019-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang S, Ji L, Kang L, Hu X. Metabolic regulation of innate immunity. Adv Immunol. 2020;145:129–157. doi: 10.1016/bs.ai.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 39.O'Neill LA. A broken krebs cycle in macrophages. Immunity. 2015;42:393–394. doi: 10.1016/j.immuni.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen HJ, Boshuizen MC, Ahmed M, Hoeksema MA, de Vos AF, de Winther MP. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur J Immunol. 2002;32:1205–1211. doi: 10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. 2019;19:282–290. doi: 10.1038/s41577-019-0139-2. [DOI] [PubMed] [Google Scholar]
- 43.Keppel MP, Saucier N, Mah AY, Vogel TP, Cooper MA. Activation-specific metabolic requirements for NK Cell IFN-gamma production. J Immunol. 2015;194:1954–1962. doi: 10.4049/jimmunol.1402099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Assmann N, O'Brien KL, Donnelly RP, Dyck L, Zaiatz-Bittencourt V, Loftus RM, Heinrich P, Oefner PJ, Lynch L, Gardiner CM, Dettmer K, Finlay DK. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat Immunol. 2017;18:1197–1206. doi: 10.1038/ni.3838. [DOI] [PubMed] [Google Scholar]
- 45.Loftus RM, Assmann N, Kedia-Mehta N, O'Brien KL, Garcia A, Gillespie C, Hukelmann JL, Oefner PJ, Lamond AI, Gardiner CM, Dettmer K, Cantrell DA, Sinclair LV, Finlay DK. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat Commun. 2018;9:2341. doi: 10.1038/s41467-018-04719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donnelly RP, Loftus RM, Keating SE, Liou KT, Biron CA, Gardiner CM, Finlay DK. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193:4477–4484. doi: 10.4049/jimmunol.1401558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mah AY, Rashidi A, Keppel MP, Saucier N, Moore EK, Alinger JB, Tripathy SK, Agarwal SK, Jeng EK, Wong HC, Miller JS, Fehniger TA, Mace EM, French AR, Cooper MA. Glycolytic requirement for NK cell cytotoxicity and cytomegalovirus control. JCI Insight. 2017 doi: 10.1172/jci.insight.95128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cong J, Wang X, Zheng X, Wang D, Fu B, Sun R, Tian Z, Wei H. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. 2018;28(243–255):e5. doi: 10.1016/j.cmet.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, Kastenberger M, Bogdan C, Schleicher U, Mackensen A, Ullrich E, Fichtner-Feigl S, Kesselring R, Mack M, Ritter U, Schmid M, Blank C, Dettmer K, Oefner PJ, Hoffmann P, Walenta S, Geissler EK, Pouyssegur J, Villunger A, Steven A, Seliger B, Schreml S, Haferkamp S, Kohl E, Karrer S, Berneburg M, Herr W, Mueller-Klieser W, Renner K, Kreutz M. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Souza-Fonseca-Guimaraes F, Cursons J, Huntington ND. The Emergence of Natural Killer Cells as a Major Target in Cancer Immunotherapy. Trends Immunol. 2019;40:142–158. doi: 10.1016/j.it.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Sharabi A, Tsokos GC. T cell metabolism: new insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol. 2020;16:100–112. doi: 10.1038/s41584-019-0356-x. [DOI] [PubMed] [Google Scholar]
- 52.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 53.Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. 2016;28:514–524. doi: 10.1016/j.smim.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Chapman NM, Boothby MR, Chi H. Metabolic coordination of T cell quiescence and activation. Nat Rev Immunol. 2020;20:55–70. doi: 10.1038/s41577-019-0203-y. [DOI] [PubMed] [Google Scholar]
- 55.Cekic C, Sag D, Day YJ, Linden J. Extracellular adenosine regulates naive T cell development and peripheral maintenance. J Exp Med. 2013;210:2693–2706. doi: 10.1084/jem.20130249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 57.Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O'Malley JT, Gehad A, Teague JE, Divito SJ, Fuhlbrigge R, Puigserver P, Krueger JG, Hotamisligil GS, Clark RA, Kupper TS. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543:252–256. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, Hess C. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 59.Schreiner D, King CG. CD4+ memory T cells at home in the tissue: mechanisms for health and disease. Front Immunol. 2018;9:2394. doi: 10.3389/fimmu.2018.02394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreno Ayala MA, Li Z, DuPage M. Treg programming and therapeutic reprogramming in cancer. Immunology. 2019;157:198–209. doi: 10.1111/imm.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beier UH, Angelin A, Akimova T, Wang L, Liu Y, Xiao H, Koike MA, Hancock SA, Bhatti TR, Han R, Jiao J, Veasey SC, Sims CA, Baur JA, Wallace DC, Hancock WW. Essential role of mitochondrial energy metabolism in Foxp3(+) T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, Haeberli L, Huck C, Turka LA, Wood KC, Hale LP, Smith PA, Schneider MA, MacIver NJ, Locasale JW, Newgard CB, Shinohara ML, Rathmell JC. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 64.Weinberg SE, Singer BD, Steinert EM, Martinez CA, Mehta MM, Martinez-Reyes I, Gao P, Helmin KA, Abdala-Valencia H, Sena LA, Schumacker PT, Turka LA, Chandel NS. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature. 2019;565:495–499. doi: 10.1038/s41586-018-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cluxton D, Petrasca A, Moran B, Fletcher JM. Differential regulation of human Treg and Th17 cells by fatty acid synthesis and glycolysis. Front Immunol. 2019;10:115. doi: 10.3389/fimmu.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen BJ, Hale LP, Rathmell JC. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, Wen J, Delgoffe GM, Powell JD. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J Clin Invest. 2015;125:2090–2108. doi: 10.1172/JCI77746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kishton RJ, Sukumar M, Restifo NP. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017;26:94–109. doi: 10.1016/j.cmet.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geltink RIK, Kyle RL, Pearce EL. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol. 2018;36:461–488. doi: 10.1146/annurev-immunol-042617-053019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gitlin AD, von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent roles of switching and hypermutation in the development and persistence of B lymphocyte memory. Immunity. 2016;44:769–781. doi: 10.1016/j.immuni.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jellusova J, Cato MH, Apgar JR, Ramezani-Rad P, Leung CR, Chen C, Richardson AD, Conner EM, Benschop RJ, Woodgett JR, Rickert RC. Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol. 2017;18:303–312. doi: 10.1038/ni.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green DR, Rathmell JC. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol. 2014;192:3626–3636. doi: 10.4049/jimmunol.1302062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dufort FJ, Gumina MR, Ta NL, Tao Y, Heyse SA, Scott DA, Richardson AD, Seyfried TN, Chiles TC. Glucose-dependent de novo lipogenesis in B lymphocytes: a requirement for atp-citrate lyase in lipopolysaccharide-induced differentiation. J Biol Chem. 2014;289:7011–7024. doi: 10.1074/jbc.M114.551051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jellusova J. Metabolic control of B cell immune responses. Curr Opin Immunol. 2020;63:21–28. doi: 10.1016/j.coi.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Boothby M, Rickert RC. Metabolic regulation of the immune humoral response. Immunity. 2017;46:743–755. doi: 10.1016/j.immuni.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jayachandran N, Mejia EM, Sheikholeslami K, Sher AA, Hou S, Hatch GM, Marshall AJ. TAPP adaptors control B cell metabolism by modulating the phosphatidylinositol 3-kinase signaling pathway: a novel regulatory circuit preventing autoimmunity. J Immunol. 2018;201:406–416. doi: 10.4049/jimmunol.1701440. [DOI] [PubMed] [Google Scholar]
- 79.Raybuck AL, Cho SH, Li J, Rogers MC, Lee K, Williams CL, Shlomchik M, Thomas JW, Chen J, Williams JV, Boothby MR. B Cell-Intrinsic mTORC1 promotes germinal center-defining transcription factor gene expression, somatic hypermutation, and memory B cell generation in humoral immunity. J Immunol. 2018;200:2627–2639. doi: 10.4049/jimmunol.1701321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kieffer-Kwon KR, Nimura K, Rao SSP, Xu J, Jung S, Pekowska A, Dose M, Stevens E, Mathe E, Dong P, Huang SC, Ricci MA, Baranello L, Zheng Y, Tomassoni Ardori F, Resch W, Stavreva D, Nelson S, McAndrew M, Casellas A, Finn E, Gregory C, St Hilaire BG, Johnson SM, Dubois W, Cosma MP, Batchelor E, Levens D, Phair RD, Misteli T, Tessarollo L, Hager G, Lakadamyali M, Liu Z, Floer M, Shroff H, Aiden EL, Casellas R. Myc regulates chromatin decompaction and nuclear architecture during B cell activation. Mol Cell. 2017;67(566–578):e10. doi: 10.1016/j.molcel.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ersching J, Efeyan A, Mesin L, Jacobsen JT, Pasqual G, Grabiner BC, Dominguez-Sola D, Sabatini DM, Victora GD. Germinal center selection and affinity maturation require dynamic regulation of mTORC1 kinase. Immunity. 2017;46(1045–1058):e6. doi: 10.1016/j.immuni.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho SH, Raybuck AL, Stengel K, Wei M, Beck TC, Volanakis E, Thomas JW, Hiebert S, Haase VH, Boothby MR. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature. 2016;537:234–238. doi: 10.1038/nature19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lam WY, Jash A, Yao CH, D'Souza L, Wong R, Nunley RM, Meares GP, Patti GJ, Bhattacharya D. Metabolic and transcriptional modules independently diversify plasma cell lifespan and function. Cell Rep. 2018;24(2479–2492):e6. doi: 10.1016/j.celrep.2018.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clarke AJ, Riffelmacher T, Braas D, Cornall RJ, Simon AK. B1a B cells require autophagy for metabolic homeostasis and self-renewal. J Exp Med. 2018;215:399–413. doi: 10.1084/jem.20170771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akkaya M, Traba J, Roesler AS, Miozzo P, Akkaya B, Theall BP, Sohn H, Pena M, Smelkinson M, Kabat J, Dahlstrom E, Dorward DW, Skinner J, Sack MN, Pierce SK. Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol. 2018;19:871–884. doi: 10.1038/s41590-018-0156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu Q, Qin Y, Ji S, Xu W, Liu W, Sun Q, Zhang Z, Liu M, Ni Q, Yu X, Xu X. UHRF1 promotes aerobic glycolysis and proliferation via suppression of SIRT4 in pancreatic cancer. Cancer Lett. 2019;452:226–236. doi: 10.1016/j.canlet.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 87.Wu JY, Huang TW, Hsieh YT, Wang YF, Yen CC, Lee GL, Yeh CC, Peng YJ, Kuo YY, Wen HT, Lin HC, Hsiao CW, Wu KK, Kung HJ, Hsu YJ, Kuo CC. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell. 2020;77(213–227):e5. doi: 10.1016/j.molcel.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 88.Sengupta D, Pratx G. Imaging metabolic heterogeneity in cancer. Mol Cancer. 2016;15:4. doi: 10.1186/s12943-015-0481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J, Duan Y, Sluijter JP, Xiao J. Lymphocytic subsets play distinct roles in heart diseases. Theranostics. 2019;9:4030–4046. doi: 10.7150/thno.33112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andrejeva G, Rathmell JC. Similarities and distinctions of cancer and immune metabolism in inflammation and tumors. Cell Metab. 2017;26:49–70. doi: 10.1016/j.cmet.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Codo AC, Davanzo GG, Monteiro LdB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F, Jimenez Restrepo JL, Vendramini PH, Reis-de-Oliveira G, Bispo dos Santos K, Toledo-Teixeira DA, Parise PL, Martini MC, Marques RE, Carmo HR, Borin A, Coimbra LD, Boldrini VO, Brunetti NS, Vieira AS, Mansour E, Ulaf RG, Bernardes AF, Nunes TA, Ribeiro LC, Palma AC, Agrela MV, Moretti ML, Sposito AC, Pereira FB, Velloso LA, Vinolo MAR, Damasio A, Proença-Módena JL, Carvalho RF, Mori MA, Martins-de-Souza D, Nakaya HI, Farias AS, Moraes-Vieira PM. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32:437–446.e5. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiu J, Wu B, Goodman SB, Berry GJ, Goronzy JJ, Weyand CM. Metabolic control of autoimmunity and tissue inflammation in rheumatoid arthritis. Front Immunol. 2021;12:652771. doi: 10.3389/fimmu.2021.652771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Basso PJ, Andrade-Oliveira V, Camara NOS. Targeting immune cell metabolism in kidney diseases. Nat Rev Nephrol. 2021;17:465–480. doi: 10.1038/s41581-021-00413-7. [DOI] [PubMed] [Google Scholar]
- 95.Biswas SK. Metabolic reprogramming of immune cells in cancer progression. Immunity. 2015;43:435–449. doi: 10.1016/j.immuni.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 96.Galgani M, Bruzzaniti S, Matarese G. Immunometabolism and autoimmunity. Curr Opin Immunol. 2020;67:10–17. doi: 10.1016/j.coi.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Warshauer JT, Bluestone JA, Anderson MS. New frontiers in the treatment of type 1 diabetes. Cell Metab. 2020;31:46–61. doi: 10.1016/j.cmet.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szwed A, Kim E, Jacinto E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol Rev. 2021;101:1371–1426. doi: 10.1152/physrev.00026.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol. 2021;18:809–823. doi: 10.1038/s41569-021-00569-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Major metabolic pathways in cells. Glycolysis is the decomposition of glucose into pyruvate and produces energy in a relatively hypoxic environment. Even under aerobic conditions, proliferating cells tend to convert glucose into lactic acid, a process known as aerobic glycolysis. TCA cycle consists of a series of enzymatic reactions. Pyruvate enters mitochondria and decarboxylates to form acetyl CoA under aerobic conditions. OXPHOS refers to the oxidation of metabolic substrate by electron respiratory chain to ATP in TCA cycle. PPP refers to the metabolic pathways that produce NADPH and 5-phosphate ribose using glycolysis intermediates(such as G-6-P), excluding ATP production and consumption. FAO is a process in which fatty acids are decomposed into acetyl CoA to produce large amounts of ATP to provide energy to the body. Most of the raw materials for FAS are derived from food, which is exogenous fatty acid and can be used by the body through modification in vivo. Supplementary file1 (TIF 1311 kb)
Data Availability Statement
This is a review, so there is no available data.