Abstract
Preeclampsia (PE) is the most severe complication of pregnancy with substantial burden of morbidity and mortality for mother and neonate. The increased placental oxidative stress (OS) has been involved as central pathomechanism, yet the sources of reactive oxygen species (ROS) are partially elucidated. Monoamine oxidase (MAO) with 2 isoforms, A and B, at the outer mitochondrial membrane has emerged as a constant source of ROS in cardiometabolic pathologies. The present pilot study was purported to assess as follows: (i) the magnitude of placental OS in relation to the site of sampling and (ii) the expression of placental MAO in the setting of PE. To this aim, central and placental samples were harvested during cesarean section from mild and severe PE versus healthy pregnancies. ROS generation (dihydroethidium staining) and MAO expression were assessed (confocal microscopy). MAO gene transcript was evaluated by RT-PCR. The main findings are as follows: (i) a significant increase in placental OS was found in severe (but not in mild) PE with no regional differences between central and peripheral areas and (ii) placental MAO-A and B (gene and protein) were significantly increased in severe preeclampsia. The signal transduction of the latter finding, particularly in relation with mitochondrial dysfunction, is worth further studying.
Keywords: Preeclampsia, Human placental oxidative stress, Peripheral and central samples, Monoamine oxidase
Introduction
Preeclampsia (PE) is the most severe complication of pregnancy that requires both prompt diagnosis and management in order to prevent potential deleterious short- and long-term consequences for both mother and neonate. PE is classically defined according to the American College of Obstetricians and Gynecologists (ACOG) [1] and the International Society for the Study of Hypertension in Pregnancy (ISSHP) [2] as new-onset hypertension identified after 20 weeks of gestation either as a systolic blood pressure of ≥ 140 mmHg or a diastolic blood pressure of ≥ 90 mmHg on two readings or, in its severe form, as a systolic pressure of ≥ 160 mmHg or a diastolic pressure of ≥ 110 mmHg on two separate occasions. This multi-organ syndrome is typically associated with one or more of the following abnormalities: proteinuria (≥ 300 mg/24 h), hematological complications (thrombocytopenia—platelet count < 100,000/mm3, hemolysis, disseminated intravascular coagulation), impaired liver function (elevated transaminases twice as the upper normal limit or severe persistent right upper quadrant or epigastric pain), acute kidney injury (serum creatinine level of > 1.1 mg/dL or doubling of its serum level), pulmonary edema, and neurologic complications (severe headache, impaired mental status, hyperreflexia, stroke, visual disturbances, blindness); fetal growth restriction (FGR) may also occur in PE with severe features [1, 2].
The syndrome has a multifactorial, partially elucidated pathophysiology and the only accepted treatment so far is the preterm delivery of both fetus and placenta. The past decades have witnessed major advances in understanding its complexity and various pathomechanisms have been implicated, including (but not limited to) impaired placental oxygenation in the presence of increased generation of reactive oxygen species (ROS) and persistent inflammation, spiral artery remodeling, altered immune tolerance at the maternal–fetal interface, and dysregulated balance of angiogenic and antiangiogenic factors [3, 4]. While a mild oxidative stress is considered physiological during normal pregnancy, the repetitive sequence of intermittent hypoxia and reoxygenation elicited by PE has been reported to be at the root of the increased local oxidative stress [3, 5]. Indeed, in the first weeks of pregnancy, a transient hypoxic environment is needed for the embryonic implantation and several lines of evidence emphasize that an increased expression and activity of nuclear factor kappa B (NFkB) has a crucial role in this respect. At variance, in preeclampsia, the persistence of increased placental level of NFkB via partially elucidate mechanisms is responsible for the endothelial dysfunction and perpetuation of inflammation and oxidative stress (recently reviewed in ref. [6]).
Literature regarding the placental sources of ROS is somewhat inconclusive. The main source of placental ROS are the stressed mitochondria via the impairment of oxidative phosphorylation [7]. NADPH oxidase has also been reported as an important superoxide (O2−) generator in PE at the human fetal–maternal interface by some [8] but not all [9] authors. A dysfunctional placental endothelial nitric oxide synthase (eNOS) either due to uncoupling or to posttranslational modifications also contributes to the aggravation/persistence of local oxidative stress (recently reviewed in ref. [10]).
Monoamine oxidase (MAO), a flavoenzyme with two isoforms, MAO-A and MAO-B, located at the outer mitochondrial membrane, is responsible for the catabolism of dietary amines and neurotransmitters: catecholamines (norepinephrine, epinephrine), serotonin, and melatonin are preferentially metabolized by MAO-A, while phenylethylamine and benzylamine are largely oxidized by MAO-B. Both isoenzymes catalyze the deamination of tyramine, dopamine, octopamine, and tryptamine. MAOs have been systematically reported to be a constant source of oxidative stress in various tissues (heart, vessels, adipose tissue) and pathologies [11]. With respect to PE, high levels of neurotransmitters (serotonin and norepinephrine) in plasma, urine, and placenta [12] as well as a defective gene expression and/or function of the placental monoamines transporters have been reported [13].
Few studies have addressed to date whether differences occur in the magnitude of ROS production in central versus peripheral placental areas in PE. In healthy pregnancies, Burton reported an early burst of oxidative stress (at the beginning of the first trimester) in the periphery of placenta; however, at approximately 11 weeks the differences disappeared in normal pregnancies [14]. Whether the same pattern occurs in PE it is not known.
The present pilot study performed in was double aimed (i) to determine the magnitude of placental oxidative stress in relation to the site of sampling (peripheral vs central) and (ii) to assess gene and protein expression of placental monoamine oxidase MAOs in PE as compared to healthy pregnancies.
Materials and methods
Chemicals
All chemicals were purchased from Biorad, Abcam, and Biotech.
Study population
The study was conducted in accordance with the statements of the Declaration of Helsinki and the protocol was approved by the Committee of Research Ethics of “Victor Babeș” University of Medicine and Pharmacy, Timișoara, Romania (no. 7 /30.01.2019 and no. 28/25.06.2020).
Nineteen patients were included in the study of local oxidative stress assessment after obtaining the written informed consent. The PE group comprised 11 patients that were further divided according the syndrome severity into 2 subgroups: mild PE (n = 4, gestational age/GA = 38 ± 0.7 weeks) and severe PE (n = 7, GA = 34.43 ± 1.77 weeks), respectively. In the control group 8 healthy, normotensive parturients with singleton-term pregnancies scheduled for elective cesarean section (GA = 38.88 ± 0.29 weeks) were included. In the second study, MAO expression (gene and protein) was evaluated in placental samples harvested from severe PE (n = 3) and control (n = 3) groups.
The diagnosis of preeclampsia was based on the criteria proposed by the American College of Obstetricians and Gynecologists (ACOG) [1] and the International Society for the Study of Hypertension in Pregnancy (ISSHP), respectively [2]. The severe forms of PE presented one or more of the following: fetal growth restriction, hepatocytolysis, blood pressure > 160/110 mmHg, refractory hypertension, low platelet count, and neurologic complications (headache, photophobia, visual disturbances). The exclusion criteria were diagnosis of chronic hypertension, coexisting diabetes mellitus, chronic kidney disease, twin pregnancy, inflammatory disorders, cancer, cardiovascular disease and coagulation disorders, and ruptured membranes/labor. On the day of cesarean section, biometrical data and informed consent were collected and patients were observed by a senior specialist who also reviewed their medical records.
Placental samples were collected immediately after delivery following non-labored cesarean section. Small fragments of villous tissue from the maternal surface (excluding areas of calcifications, microinfarctions) were randomly harvested from central and peripheral regions and placed in ice cold 0.9% KCl solution; fragments were stored at − 80 °C until further analysis.
Assessment of local oxidative stress by means of DHE staining (confocal microscopy)
Placental samples were cut in 20-μm-thick sections with the cryomicrotome and placed on microscope slides. The cryosections were subjected to three successive washes with PBS (5 min each) and afterward incubated with dihydroethidium (DHE) for 30 min at room temperature, in the dark. After the incubation period, the excess of DHE was washed away by three additional washes with PBS. Sections were mounted with Vectashield (Vector Laboratories) and immediately analyzed with the confocal microscope (Olympus Fluoview FV1000). Images were obtained using laser excitation at 488 nm with a × 20 UPLSAPO objective (NA = 0.75), and for each section three randomly chosen regions were analyzed. Image analysis was performed using the Icy bioimage analysis software [15].
DHE fluorescence intensity was measured as integrated fluorescence intensity (IFI) as described in Vaduva et al. ROS production was evaluated using a dihydroethidium (DHE)-based fluorescence method. DHE is used in fluorimetric detection assays because upon reaction with superoxide anion is converted to 2-hydroxyethidium, following DNA intercalation and emits red fluorescence [16].
Assessment of MAO gene transcript (RT-PCR)
Total RNA was isolated (Aurum Total RNA Mini Kit, Biorad) and used for reverse transcription (iScript Advanced cDNA Synthesis Kit, Biorad). Quantitative RT-PCR was performed in placenta samples. In order to determine gene transcript, primers against MAO isoforms were designed using sequence information from the NCBI database (5ʹ → 3ʹ): human MAO-A forward, 5 = –CTG ATC GAC TTG CTA AGC TAC–3 and human MAO-A reverse, 5 = –ATG CAC TGG ATG TAA AGC TTC-3. The housekeeping gene (18 s) and its primers were as follows (5ʹ → 3ʹ): 18 s forward GTA ACC CGT TGA ACC CCA TT and 18 s reverse: CAT CCA ATC GGT AGT AGC G.
Assessment of MAO protein expression (immunohistochemistry)
Monoamine oxidase protein expression was quantified in frozen sections of human placenta using the MAO-A (Abcam, ab126751), MAO-B (Abcam, ab175136) primary antibody, and Alexa Fluor-labeled secondary goat anti-rabbit antibody (Invitrogen, A32731), respectively, as previously described [17]. Nuclear staining was obtained with DAPI (Santa Cruz, SC3598). The slides were examined using the Olympus Fluoview FV1000 confocal microscope. Quantification of MAO was performed by measurement of fluorescence intensity (expressed as arbitrary units) using ImageJ software.
Statistics
Statistical data processing was performed with the GraphPad Prism software Version 9.3.1 (GraphPad, USA). Data are presented as mean ± SEM. To compare two groups, the unpaired t test was applied; to compare three groups, the one-way Anova with Tukey’s multiple comparisons test was applied. Statistical significance was set at a value of p < 0.05.
Results
The characteristics of the study groups are depicted in Table 1. The following demographic and laboratory characteristics of pregnant women (PE and healthy pregnancies) were included: maternal age, gestational age, maternal blood pressure, MAP (mean arterial pressure), and fetal weight. MAP is calculated by the following formula, (Syst BP + 2 X Diast BP)/3.
Table 1.
Characteristics of the study groups
| Parameter | Healthy pregnancies (n = 8) | PE mild (n = 4) | PE severe (n = 7) |
|---|---|---|---|
| Maternal age (years) | 29.63 ± 1.7 | 28.5 ± 2.02 | 36.86 ± 1.37** |
| Gestational age (years) | 38.88 ± 0.29 | 38 ± 0.7 | 34.43 ± 1.77* |
| Systolic BP (mmHg) | 106.8 ± 5.81 | 147.8 ± 3.42*** | 160.1 ± 4.71**** |
| Diastolic BP (mmHg) | 69.13 ± 2.9 | 90.25 ± 1.9*** | 93 ± 2.77**** |
| MAP (mmHg) | 82.67 ± 1.45 | 110.3 ± 1.25** | 116 ± 3.45**** |
| Fetal weight (g) | 3423 ± 132.8 | 3145 ± 98.8 | 2086 ± 234.6* |
| Proteinuria (24 h) | – | 0.97 ± 0.435 | 2.3 ± 1.07 |
Data are presented as mean ± SEM. MAP—Mean arterial pressure, PE—preeclampsia
*p < 0.05, ** p < 0.01, *** p < 0.001, ****p < 0.0001 versus healthy pregnancies
As previously mentioned, PE pregnancies were further divided in two subgroups: mild preeclampsia (n = 4) and severe preeclampsia (n = 7). The mild forms of preeclampsia were associated with a good responsiveness to treatment (absence of refractory hypertension or fetal distress), only proteinuria was associated to hypertension, and all pregnancies reached term and the newborns were normal for gestational age, as showed by ultrasound investigation and anthropometric measurements at childbirth. The severe forms of preeclampsia were associated with intrauterine growth restriction (85.71%), hepatocytolysis (28.57%), preterm delivery (57.14%), low platelet count (14.28%), blood pressure > 160/110 mmHg (57.14%), hypertension refractory to treatment (28.57%), and neurologic complications (14.28%).
Oxidative stress is increased in both central and peripheral placental samples harvested from severe (but not mild) preeclampsia
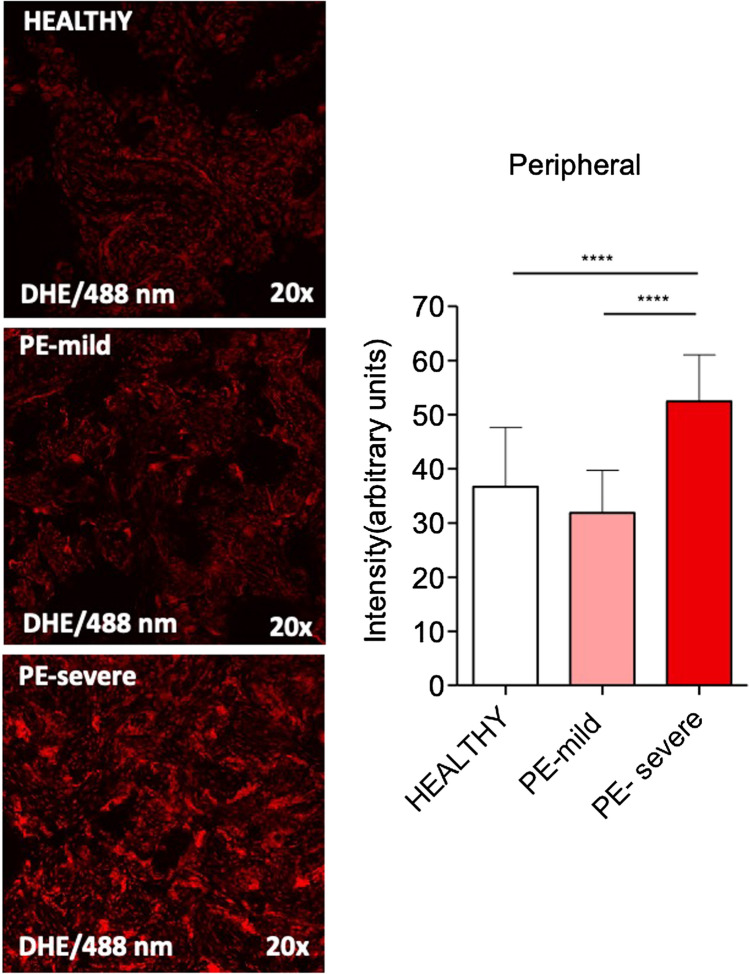
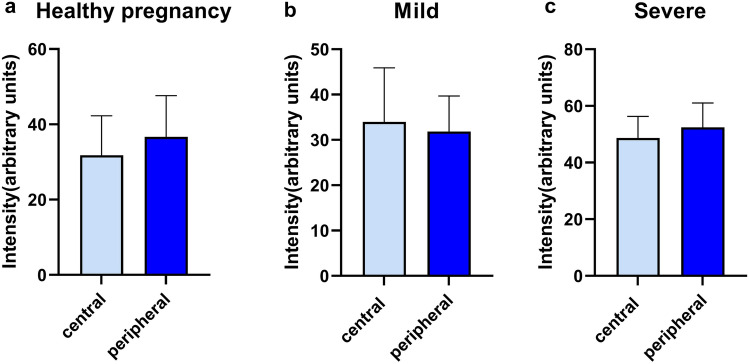
As shown in Figs. 1 and 2, the severe forms of PE presented significantly higher increased oxidative stress in both central and peripheral regions as compared to healthy pregnancies (p < 0.0001) or mild PE (p < 0.001). The cases of mild preeclampsia displayed a minor (6.53%) increase in ROS levels in the central placental areas; at variance, a mild reduction (13.08%) of oxidative stress in the peripheral areas was observed. No significant differences in ROS level were found between the central and peripheral placental regions in either normal (Fig. 3a) or pathological conditions (Fig. 3b, c). Interestingly, the pattern of placental oxidative stress was comparable in both normal and severe PE pregnancies with a lower increase in ROS production in the peripheral areas vs the central ones.
Fig. 1.
Assessment of placental oxidative stress (DHE staining) in central samples harvested from healthy (n = 8) versus mild (n = 4) and severe (n = 7) PE pregnancies. Data are presented as mean ± SEM. One-way ANOVA with Tukey’s post hoc multiple comparisons test was applied. ****p < 0.0001, ***p < 0.001
Fig. 2.
Assessment of placental oxidative stress (DHE staining) in peripheral samples harvested from healthy (n = 8) versus mild (n = 4) and severe PE (n = 7) pregnancies. Data are presented as mean ± SEM. One-way ANOVA with Tukey’s post hoc multiple comparisons test was applied. ****p < 0.0001
Fig. 3.
Comparison of placental oxidative stress (DHE staining) in central versus peripheral samples of healthy (a, n = 8), mild PE (b, n = 4), and severe (c, n = 7) PE pregnancies. Data is presented as mean ± SEM. Unpaired t test was applied
Preeclampsia is associated with increased gene transcript and protein expression of both MAO-A and MAO-B isoforms in placental tissue
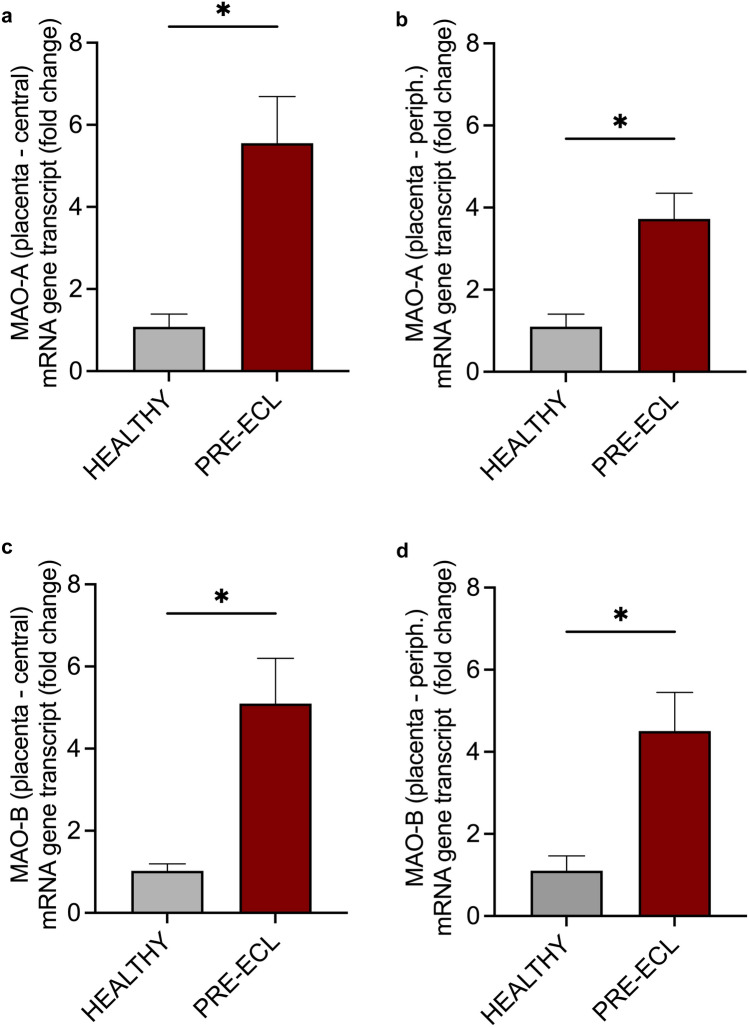
Both MAO isoforms were identified in placental tissue, being significantly (p < 0.05) upregulated in samples isolated from severe PE pregnancies versus controls, as revealed by the mRNA gene transcripts. No significant differences in gene expression of MAO appeared between the central (Fig. 4a, c) and peripheral (Fig. 4b, d) placental regions in either normal or pathological conditions. Accordingly, there was a fivefold increase in the central areas and around fourfold increase in the peripheral areas for both MAO isoforms in the PE placentas. Interesting, as for the ROS generation, a slight increase in gene transcripts of both MAO isoforms was observed in central as compared to peripheral placental areas.
Fig. 4.
Assessment of central and peripheral placental MAO-A (a, b) and MAO-B isoform (c, d) gene transcript in samples from healthy vs severe PE pregnancies (RT-PCR). Data are presented as mean ± SEM. Unpaired t test was applied. *p < 0.05
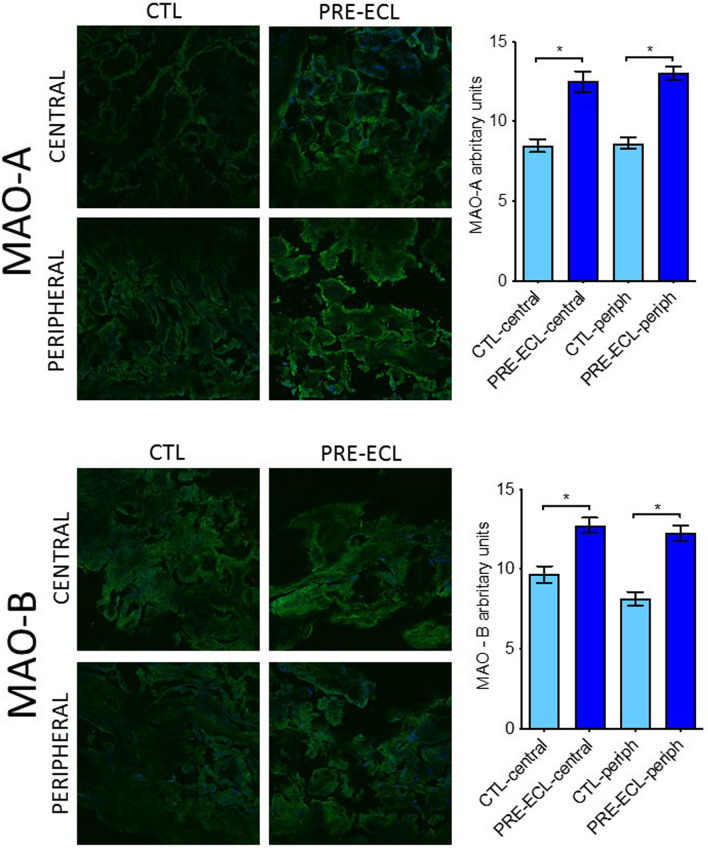
Additionally, MAOs protein expression was evaluated by means of immunohistochemistry in healthy placental tissue vs severe preeclampsia using specific antiMAO-A and antiMAO-B antibodies. As shown in Fig. 5, both isoforms were increased in PE placentas as compared to controls and, similarly to the gene expression, no differences were found between central and peripheral regions.
Fig. 5.
Assessment of central and peripheral placental MAO-A and B protein expression in samples harvested from healthy vs severe PE pregnancies (immunohistochemistry: green anti MAO-A/B antibody; blue DAPI)
Discussion
In the present pilot study, we assessed the level of oxidative stress in central and peripheral placental samples determined (in a certain set of experiments) both MAO mRNA and protein expression in preeclamptic versus healthy pregnancies. The major findings are as follows: (i) severe (but not mild) PE is associated with increased placental oxidative stress, (ii) no significant regional differences in the magnitude of oxidative stress are evident in either normal or pathological conditions (yet the central areas appear to be primarily involved), (iii) MAO gene transcripts of MAO-A and B are increased in preeclamptic placentas, particularly in the central areas, and (iv) MAO protein expression of both isoforms is also present in PE placental tissue, regardless the place of harvesting.
Normal pregnancy is a state of oxidative stress characterized by a balance between ROS production and detoxification. Continuous local production of small amounts of ROS exert a beneficial role in the setting of normal pregnancies being essential in the establishment of a normal placental bed by regulating local angiogenesis [8]. The imbalance in the oxidant/antioxidant activities is at the root of placental malfunction and has been reported to occur in all complicated pregnancies, particularly in preeclampsia with or without fetal growth restriction.
Over the past decades several placental sources of oxidative stress have been systematically studied in relation to PE, such as the mitochondrial electron transport system, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, and the uncoupled nitric oxide synthase [18]. Superoxide anion (O2−⋅), the precursor of most ROS, is a key mediator in several oxidative reactions being produced in both cytoplasm and organelles. The non-enzymatic production of O2−⋅ occurs mainly when a single electron is directly transferred to the oxygen due to the leakage of the mitochondrial respiratory chain complexes that occurs on both sides of the inner mitochondrial membrane. The principal enzymatic sources are the NADPH oxidases and the proteolytic conversion of xanthine dehydrogenase to xanthine oxidase. In order to maintain the steady-state concentration of superoxide low, it is further converted to hydrogen peroxide (H2O2) by a family of metalloenzymes with multicompartment distribution called superoxide dismutases. The main H2O2 detoxifying enzymes are glutathione peroxidase and catalase with variable tissue-dependent distribution [19]. The fact that no significant differences in ROS level were found between the central and peripheral placental regions in either normal or pathological conditions is in line with the pioneering work by Jauniaux et al. who reported almost two decades ago that starting with the second trimester of pregnancy no differences occur in the central and peripheral regions once the placental blood flow is completely settled [20]. Superoxide has been firstly reported to be increased in placental trophoblast cells isolated from PE placentas two decades ago [21]. More recently, H2O2 production in preeclamptic placentas has been reported to be influenced by gestational age, with higher values being reported for term pregnancies as compared to the preterm ones. Interestingly, healthy-term placentas subjected to hypoxia reoxygenation behave exactly like term PE placentas by increasing H2O2 production [22]. At variance, a recent study showed reduced mitochondrial ROS in placentas isolated from preeclamptic (both term and preterm) versus healthy pregnancies; interestingly, ROS production was significantly lower in preterm placentas (< 34 weeks) as compared to placentas harvested > 34 weeks of pregnancy [23]. Of note, we have also recently reported a dichotomous behavior in placental mitochondria respiration in relation to the fetal birth weight. PE pregnancies with fetal growth restriction were associated with decreased active respiration (OXPHOS capacity) and maximal uncoupled respiration, while an increase in both respiratory parameters was found in PE pregnancies with normal weight fetuses [24].
The rate of ROS production increases linearly as oxygen concentration increases and, in theory, should decrease with hypoxia. However, a paradoxical increase in oxidative stress under moderately hypoxic in vitro conditions has been reported in cells with functioning mitochondria (but not in mutant cells lacking functional mitochondria) when incubated with dichlorofluorescein, another ROS probe; the increase in fluorescence was eliminated in the presence of severe hypoxia [25]. Dihydroethidium (DHE) or hydroethidine is the most widely used redox-sensitive probe originally designed to detect intracellular superoxide. The reaction between superoxide and DHE generates 2-hydroxyethidium (2-OH-E+), a specific red fluorescent product. The ethidium cation binds to DNA and remains trapped inside the cells because of its charge. Since it has been proven that DHE-based confocal and fluorescence microscopy do not provide trustworthy information on intracellular superoxide formation, the general term ROS was used in the present paper [26, 27].
The deleterious effects resulting from the ROS formation are largely prevented in physiological conditions by various antioxidant systems. The impairment of placental antioxidant mechanisms has been reported to occur in women with preeclampsia, as shown by the decreased expression of superoxide dismutase and glutathione peroxidase when compared with normal pregnancies [28]. In line with our findings showing a significantly higher ROS generation in severe (but not in mild) PE, it is tempting to speculate that the increase in local oxidative stress elicited in the setting of mild PE can be counteracted by a comparable increase in the placental antioxidant defense; however, this cannot be recapitulated in the severe forms of disease leading to the permanent oxidative stress.
There is an unmet need to characterize the placental sources of ROS in humans and their contribution to the oxidative stress in both normal and preeclamptic pregnancies in order to identify placental-targeted therapies able to mitigate it in the latter case. MAO-related oxidative stress has been widely acknowledged as an important pathomechanism in the cardiovascular pathology, in both experimental models [29–31] and in humans [32, 33]. In this respect, it has to be mentioned that MAO activity in placental tissue was reported to play an important role in normal fetal development [34]. However, the literature regarding MAO contribution to oxidative stress in preeclamptic placentas is scarce: one study reported significantly reduced MAO-A activity due to impaired catalytic turnover [35], while another pioneering one showed more than three decades ago, using an radioenzymatic assay, a reduced MAO activity in high-risk pregnancies (chronic hypertension, preeclampsia, and diabetes mellitus) [36].
In the present study, we detected comparable levels of MAO-A and B isoforms at both gene and protein levels in healthy placental samples. Regarding MAO activity in placental tissue, the literature is controversial, MAO-A is expressed predominantly in the syncytiotrophoblastic layer with no detection in the vascular endothelium or smooth muscles, while MAO-B is present at low levels in the syncytiotrophoblasts, cytotrophoblasts, and the vascular endothelium [37–39] or was even absent [35]. Our results showed a significant upregulation of MAO mRNA transcript and protein expression in severe preeclampsia, with no significant differences between the central and peripheral placental regions, a finding in line with the results regarding the placental ROS generation, as detected in confocal microscopy.
Among the deleterious effects of increased ROS levels is the degradation NFkB inhibitors, preeclampsia being associated with increased NFkB activity which favors the oxidative stress persistence [6]. We have previously demonstrated that induction of endogenous MAOs in murine vascular rings occurred after acute incubation with angiotensin II and lipopolysaccharide via NFkB [40]. Whether this observation can be also recapitulated in PE placentas is worth further investigation.
A couple of limitations to this pilot study need to be acknowledged. Firstly, the reduced sample size due to the ups and downs of the COVID-19 pandemic, which prevented us to recruit the patients as initially designed. A second limitation is that we did not assess the PE-related changes in the local antioxidant systems, since these techniques are not available so far in our research center. Last but not least, the signal transduction of the increased MAO expression has to be elucidated.
Conclusion
Placental oxidative stress is the hallmark of severe (but not mild) forms of preeclampsia with no regional differences being evident between the central vs peripheral areas at delivery. An increased gene transcripts and protein expression of both MAO isoforms was found in severe preeclampsia. A thorough characterization of the sources of placental oxidative stress assessed by a larger randomized controlled trial is worth in order to provide the background for the development of innovative therapeutic approaches to treat preeclampsia.
Acknowledgements
We thank Andreea Anechitei for the expert technical assistance. We also thank all the pregnant women and volunteers for participating in this study.
Author contributions
AMB contributed to writing and preparation of the original draft, investigation, and data curation; AS contributed to investigation, methodology, and data curation; OMA contributed to methodology and visualization; II contributed to visualization; AGM contributed to sample collection and investigation; EB contributed to sample collection and investigation; CB contributed to visualization and supervision; ZLP contributed to sample collection, writing, reviewing, & editing of the manuscript, and formal analysis; MLC contributed to sample collection, visualization, and supervision; DMM contributed to conceptualization, writing, reviewing, & editing of the manuscript, and formal analysis; OMC contributed to project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the university internal funds allocated to the research centers. There was no additional external funding received for this study.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Institutional review board statement
The study protocol and informed consent were approved by the Committee of Research Ethics of “Victor Babeș” University of Medicine and Pharmacy, Timișoara, Romania (no. 7 /30.01.2019 and 28/25.06.2020).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anca M. Bînă and Adrian Sturza have contributed equally to this work.
Contributor Information
Zoran L. Popa, Email: popa.zoran@umft.ro
Danina M. Muntean, Email: popa.zoran@umft.ro
References
- 1.American College of Obstetricians and Gynecologists ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:1. doi: 10.1097/aog.0000000000003018. [DOI] [PubMed] [Google Scholar]
- 2.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/hypertensionaha.117.10803. [DOI] [PubMed] [Google Scholar]
- 3.Rani N, Dhingra R, Arya DS, Kalaivani M, Bhatla N, Kumar R. Role of oxidative stress markers and antioxidants in the placenta of preeclamptic patients. J Obstet Gynaecol Res. 2010;36:1189–1194. doi: 10.1111/j.1447-0756.2010.01303.x. [DOI] [PubMed] [Google Scholar]
- 4.Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15:275–289. doi: 10.1038/s41581-019-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25:287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakowicz A. The targeting of nuclear factor kappa B by drugs adopted for the prevention and treatment of preeclampsia. Int J Mol Sci. 2022 doi: 10.3390/ijms23052881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo DD, Zarco-Zavala M, Olvera-Sanchez S, Pardo JP, Juarez O, Martinez F, Mendoza-Hernandez G, García-Trejo JJ, Flores-Herrera O. Atypical cristae morphology of human syncytiotrophoblast mitochondria: role for complex V. J Biol Chem. 2011;286:23911–23919. doi: 10.1074/jbc.M111.252056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guedes-Martins L, Silva E, Gaio AR, Saraiva J, Soares AI, Afonso J, Macedo F, Almeida H. Fetal-maternal interface impedance parallels local NADPH oxidase related superoxide production. Redox Biol. 2015;5:114–123. doi: 10.1016/j.redox.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raijmakers MT, Peters WH, Steegers EA, Poston L. NAD(P)H oxidase associated superoxide production in human placenta from normotensive and pre-eclamptic women. Placenta. 2004;25(Suppl A):S85–S89. doi: 10.1016/j.placenta.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Guerby P, Tasta O, Swiader A, Pont F, Bujold E, Parant O, Vayssiere C, Salvayre R, Negre-Salvayre A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021;40:101861. doi: 10.1016/j.redox.2021.101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturza A, Muntean DM, Crețu OM. Monoamine oxidase, obesity and related comorbidities: discovering bonds. In: Tappia PS, Ramjiawan B, Dhalla NS, editors. Cellular and biochemical mechanisms of obesity. Cham: Springer International Publishing; 2021. pp. 199–213. [Google Scholar]
- 12.Phoswa WN. Dopamine in the pathophysiology of preeclampsia and gestational hypertension: monoamine oxidase (MAO) and catechol-O-methyl Transferase (COMT) as possible mechanisms. Oxid Med Cell Longev. 2019;2019:3546294. doi: 10.1155/2019/3546294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslén B, Marsál K, Hansson SR. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004;25:518–529. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140066. doi: 10.1098/rstb.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Chaumont F, Dallongeville S, Chenouard N, Hervé N, Pop S, Provoost T, Meas-Yedid V, Pankajakshan P, Lecomte T, Le Montagner Y, Lagache T, Dufour A, Olivo-Marin JC. Icy: an open bioimage informatics platform for extended reproducible research. Nat Methods. 2012;9:690–696. doi: 10.1038/nmeth.2075. [DOI] [PubMed] [Google Scholar]
- 16.Văduva AO, Glămeanu C, Negrea R, Muntean MD, Dema ALC. In vivo confocal microscopy quantification of reactive oxygen species: a working model in rat kidney. Rom J Morphol Embryol. 2017;58:953–960. [PubMed] [Google Scholar]
- 17.Sturza A, Olariu S, Ionică M, Duicu OM, Văduva AO, Boia E, Muntean DM, Popoiu CM. Monoamine oxidase is a source of oxidative stress in obese patients with chronic inflammation (1) Can J Physiol Pharmacol. 2019;97:844–849. doi: 10.1139/cjpp-2019-0028. [DOI] [PubMed] [Google Scholar]
- 18.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003;162:115–125. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta. 2001;22:206–212. doi: 10.1053/plac.2000.0608. [DOI] [PubMed] [Google Scholar]
- 22.Holland OJ, Cuffe JSM, Dekker Nitert M, Callaway L, Kwan Cheung KA, Radenkovic F, Perkins AV. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis. 2018;9:1150. doi: 10.1038/s41419-018-1190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaka R, Deer E, Cunningham M, McMaster KM, Wallace K, Cornelius DC, Amaral LM, LaMarca B. Characterization of mitochondrial bioenergetics in preeclampsia. J Clin Med. 2021 doi: 10.3390/jcm10215063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bînă AM, Aburel OM, Avram VF, Lelcu T, Lința AV, Chiriac DV, Mocanu AG, Bernad E, Borza C, Craina ML, Popa ZL, Muntean DM, Crețu OM. Impairment of mitochondrial respiration in platelets and placentas: a pilot study in preeclamptic pregnancies. Mol Cell Biochem. 2022 doi: 10.1007/s11010-022-04415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumacker PT. Hypoxia, anoxia, and O2 sensing: the search continues. Am J Physiol Lung Cell Mol Physiol. 2002;283:L918–L921. doi: 10.1152/ajplung.00205.2002. [DOI] [PubMed] [Google Scholar]
- 26.Kalyanaraman B. Pitfalls of reactive oxygen species (ROS) measurements by fluorescent probes and mitochondrial superoxide determination using MitoSOX. In: Berliner LJ, Parinandi NL, editors. Measuring oxidants and oxidative stress in biological systems. Cham CH: Springer Nature Switzerland AG; 2020. pp. 7–9. [PubMed] [Google Scholar]
- 27.Xiao Y, Meierhofer D. Are hydroethidine-based probes reliable for reactive oxygen species detection? Antioxid Redox Signal. 2019;31:359–367. doi: 10.1089/ars.2018.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregnancy. 2002;21:205–223. doi: 10.1081/prg-120015848. [DOI] [PubMed] [Google Scholar]
- 29.Kaludercic N, Mialet-Perez J, Paolocci N, Parini A, Di Lisa F. Monoamine oxidases as sources of oxidants in the heart. J Mol Cell Cardiol. 2014;73:34–42. doi: 10.1016/j.yjmcc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas M-H, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 31.Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, Bedja D, De Mario A, Chen K, Gabrielson KL, Lindsey ML, Pacak K, Takimoto E, Shih JC, Kass DA, Di Lisa F, Paolocci N. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid Redox Signal. 2014;20:267–280. doi: 10.1089/ars.2012.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturza A, Popoiu CM, Ionică M, Duicu OM, Olariu S, Muntean DM, Boia ES. Monoamine oxidase-related vascular oxidative stress in diseases associated with inflammatory burden. Oxid Med Cell Longev. 2019;2019:8954201. doi: 10.1155/2019/8954201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manni ME, Rigacci S, Borchi E, Bargelli V, Miceli C, Giordano C, Raimondi L, Nediani C. Monoamine oxidase is overactivated in left and right ventricles from ischemic hearts: an intriguing therapeutic target. Oxid Med Cell Longev. 2016;2016:4375418. doi: 10.1155/2016/4375418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus S, Barry K, Flynn H, Tandon R, Greden J. Treatment guidelines for depression in pregnancy. Int J Gynaecol Obstet. 2001;72:61–70. doi: 10.1016/S0020-7292(00)00318-0. [DOI] [PubMed] [Google Scholar]
- 35.Sivasubramaniam SD, Finch CC, Billett MA, Baker PN, Billett EE. Monoamine oxidase expression and activity in human placentae from pre-eclamptic and normotensive pregnancies. Placenta. 2002;23:163–171. doi: 10.1053/plac.2001.0770. [DOI] [PubMed] [Google Scholar]
- 36.Barnea ER, MacLusky NJ, DeCherney AH, Naftolin F. Monoamine oxidase activity in the term human placenta. Am J Perinatol. 1986;3:219–224. doi: 10.1055/s-2007-999871. [DOI] [PubMed] [Google Scholar]
- 37.Riley LA, Waguespack MA, Denney RM. Characterization and quantitation of monoamine oxidases A and B in mitochondria from human placenta. Mol Pharmacol. 1989;36:54. [PubMed] [Google Scholar]
- 38.Sekizawa A, Ishikawa H, Morimoto T, Hirose K, Suzuki A, Saito H, Yanaihara T, Arai Y, Oguchi K. A study of monoamine oxidase activity in fetal membranes. Acta Obstet Gynecol Scand. 1996;75:423–427. doi: 10.3109/00016349609033347. [DOI] [PubMed] [Google Scholar]
- 39.Auda GR, Kirk SH, Billett MA, Billett EE. Localization of monoamine oxidase mRNA in human placenta. J Histochem Cytochem. 1998;46:1393–1400. doi: 10.1177/002215549804601208. [DOI] [PubMed] [Google Scholar]
- 40.Sturza A, Leisegang MS, Babelova A, Schröder K, Benkhoff S, Loot AE, Fleming I, Schulz R, Muntean DM, Brandes RP. Monoamine oxidases are mediators of endothelial dysfunction in the mouse aorta. Hypertension. 2013;62:140–146. doi: 10.1161/hypertensionaha.113.01314. [DOI] [PubMed] [Google Scholar]