Abstract
The generation of vesicles is a constitutive attribute of mitochondria inherited from bacterial ancestors. The physiological conditions and mild oxidative stress promote oxidation and dysfunction of certain proteins and lipids within the mitochondrial membranes; these constituents are subsequently packed as small mitochondrial‐derived vesicles (MDVs) (70–150 nm in diameter) and are transported intracellularly to lysosomes and peroxisomes to be degraded. In this way, MDVs remove the damaged mitochondrial components, preserve mitochondrial structural and functional integrity and restore homeostasis. An outline of the current knowledge on MDVs seems to be necessary for understanding the potential impact of this research area in cellular (patho)physiology. The present synopsis is an attempt towards the accomplishment of this demand, highlighting also the still unclear issues related to MDVs. Here, we discuss (i) MDVs budding and generation (molecules and mechanisms), (ii) the distinct cargoes packed and transported by MDVs, (iii) the MDVs trafficking pathways and (iv) the biological role of MDVs, from quality controllers to the involvement in organellar crosstalk, mitochondrial antigen presentation and peroxisome de novo biogenesis. These complex roles uncover also mitochondria integration into the cellular environment. As the therapeutic exploitation of MDVs is currently limited, future insights into MDVs cell biology are expected to direct to novel diagnostic tools and treatments.
Keywords: extracellular vesicles, lysosomes, Parkin, peroxisome, PINK1, Quality control
1. INTRODUCTION
Mitochondria are essential intracellular organelles well known for their function in generating the energy required to maintain normal cellular processes and safeguard cell homeostasis. Mitochondria execute and coordinate a wide range of metabolic pathways (phospholipid transfer, inflammation, calcium balance, ion homeostasis, aldehyde metabolism, etc.) and contribute to the cell's survival or death. Dysregulated homeostasis implies the damage and dysfunction of mitochondria triggered by various stressors, mainly the reactive oxygen species (ROS), the side‐products of adenosine triphosphate (ATP) biosynthesis. Therefore, the maintenance of a healthy mitochondrial population imposes the clearance of dysfunctional/damaged organelles. In eukaryotic cells, this task is accomplished by the ‘quality control’ (QC) system that engages specific regulatory pathways, depending on the nature and severity of mitochondrial dysfunction. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Examples are (i) the antioxidant enzymes (that cope against ROS‐mediated toxicity), (ii) the activities of the ubiquitin‐proteasome system (that execute the QC of outer mitochondrial membrane, OMM, proteins), and of the mitochondrial proteases, and chaperones (that refold or degrade the damaged/mislocalized mitochondrial proteins), (iii) the packaging of oxidized proteins or lipids (resulting from exposure to subtoxic doses of stressors such as glucose oxidase and xanthine oxidase/xanthine) into mitochondrial‐derived vesicles (MDVs); these vesicles are subsequently delivered to the late endosomes/lysosomes or to the peroxisome for degradation 1 , 8 , 10 and (iv) the autophagic elimination (mitophagy) of extensively damaged mitochondria, in response to stressors such as the strong oxidants. 4 Compared with the latter pathway, MDVs allow degradation of not yet depolarized mitochondria, are a kinetically faster QC route, and help the clearance of defective mitochondrial proteins and lipids, before executing the degradation of the entire organelle by mitophagy. 1 , 10 , 11 The failure of the QC systems above intervenes in human neurodegenerative diseases and cardiomyopathy. 8 , 12 , 13 For reviews, see. 14 , 15 , 16
The ability of mitochondria to load their contents into vesicles is a constitutive and evolutionary conserved process, inherited from the bacterial ancestors. 16 , 17 The bacterial vesicles are generated by the local expansion of the envelopes, the budding of the vesicles and their release extracellularly. Although a large diversity of bacterial envelopes exist, two main types of extracellular vesicles (EV) are prevalent: the outer membrane vesicles (OMVs) derived from the outer layer, and the outer‐inner membrane vesicles (O‐IMVs) containing both outer and inner constituents. 18 The composition of OMVs includes the outer layer lipopolysaccharides (LPS), peptidoglycans of the periplasmic space, and cytosol. 19 The O‐IMVs include DNA, cytoplasmic and inner membrane proteins, and ATP. 20 , 21 The biological role of bacterial EV consists in signalling within the colony via quorum sensing, 22 intercellular communication by transport of proteins, modulation of immunogenic host invasion, execution of bacterial competitors and biofilms formation. 23 The vesicular transport has been conserved during evolution, and MDVs are an example of this inheritance.
The bacterial EV and MDVs share several elements in common: (i) the single‐/double‐membrane, (ii) the propensity for transport tasks, (iii) the involvement in immune response and (iv) the possibility of EV formation under specific stress conditions. 11 , 24 , 25 Among the differences between the bacterial EV and MDVs one can quote: (i) the peculiar composition of their membrane (see above for bacterial EV, and of mitochondrial origin for MDVs), (ii) the size of OMVs (diameter is up to 500 nm) is larger than that of MDVs, (iii) the particular transport pathways, intercellular, for the bacterial vesicles and intracellular, for the MDVs engaged in inter‐organellar communication. 1 , 16
At present, MDVs define an emerging research area, validated by the recent discoveries on their mechanistic and roles. However, numerous open questions still deserve deeper insights. Therefore, an overview of the literature is necessary to bring the unsolved issues to attention and focus on the MDVs potential exploitation in therapy. The following subjects are discussed here: (i) MDVs budding and generation (molecules and mechanisms), (ii) the identity of cargoes packed and transported by MDVs, (iii) the MDVs trafficking pathways and (iv) the biological role of MDVs. The review is concluded by (v) the future perspectives of this dynamic research area.
2. MDVs BUDDING AND FORMATION (MOLECULES AND MECHANISMS)
The MDVs have a small size (diameter between 70 and 150 nm), as observed by electron microscopy; these vesicles are generated by mitochondria in both basal and stress‐related conditions, independent of the fission protein, dynamin‐related protein 1 (Drp1). 1 , 3 , 8 , 11 , 26 Several molecules intervene in the budding of damaged segments of mitochondria into MDVs; vital are the Parkinson's disease‐associated proteins PINK1, Parkin and the Vacuolar sorting protein 35 (Vps35). 10 , 27 , 28 , 29 PINK1 (PTEN‐induced kinase 1) is a mitochondrial serine/threonine‐protein kinase (encoded by the PINK1 gene), and Parkin is an E3 ubiquitin protein ligase; in terms of structure, Parkin contains at its N terminus a ubiquitin‐like domain (Ubl) and four zinc‐coordinating RING‐like domains. The Parkin‐dependent ubiquitination of mitochondrial proteins is the central mechanism involved in the elimination of the damaged segments of mitochondria. 27
In mildly oxidative stress conditions, the proteins of the mitochondrial membranes become oxidized; moreover, ROS and oxidative stress initiate the local activation of PINK1 and Parkin, leading to the budding of oxidized membrane proteins into vesicles. 10 , 30 As observed in Parkinson's disease, the loss of PINK1/Parkin‐mediated MDVs formation is due to the inability of mitochondria to remove the oxidized/ damaged proteins, leading to mitochondrial dysfunction. 10 The role of Vps35 in MDV generation is acknowledged by the fact that its mutation impairs MDVs formation. 28
The restoration of mitochondrial homeostasis by the removal of oxidized proteins via MDVs is upregulated in stress conditions, 8 under remote ischaemic preconditioning, 31 and in connection with cannabidiol treatment. 32 This compound (C21H30O2) is the major non‐psychoactive phytocannabinoid, used in nutraceutical and medical treatment. Recently, Ramirez et al. 32 showed that cannabidiol generates MDVs via the PINK1‐Parkin pathway, and heals dysfunctional mitochondria by opening the mitochondrial permeability transition pore. According to the working hypothesis of PINK1/Parkin‐mediated MDVs formation, 27 the mechanism implies four steps: (i) ROS and/or defects in protein assembly direct the aggregation of oxidized or unfolded proteins within the matrix; in this stage, cardiolipin oxidation generates phosphatidic acid, a contributor to the alteration of membrane curvature, (ii) the protein aggregates saturate chaperones, affecting the import of ‘an individual’ channel in a process affected by cardiolipin oxidation; PINK1 is imported fast and accumulates at the site of the failed import channels, (iii) next, PINK1 phosphorylates ubiquitin and the ubiquitin‐like domain of Parkin, an event with two consequences: it stabilizes activated Parkin and facilitates MDVs generation; (iv) MDVs are formed and released. Matheoud et al. 30 showed that MDVs biogenesis requires the recruitment of Rab9 (a small GTPase associated with pathways towards the endo‐lysosomal compartments) and of Sorting nexin 9 (SNX9), although the regulatory process is still incompletely understood. 26
Among the techniques currently used for MDVs evaluation, one can quote the transmission electron microscopy, flow cytometry, electron tomography analysis, immunofluorescence and confocal microscopy. 1 , 31 , 33 , 34
3. THE IDENTITY OF CARGOES PACKED AND TRANSPORTED BY MDVs
The mild oxidative stress induces the packaging of mitochondrial oxidized proteins into single or double‐membrane vesicles; these will be ultimately targeted to lysosomes for degradation. 11 , 14 , 32 Specific mitochondrial compartments are employed for vesicles generation. Thus, of the OMM, the single‐membrane MDVs recruit all ß‐Barrel proteins, 12 the mitochondrial‐anchored protein ligase (MAPL or Mul1), 28 and the translocase of the outer membrane 20 (TOM20). 35 The TOM complex is evaluated to be ‘the entry gate’ for the precursor proteins biosynthesized on cytosolic ribosomes. 36 , 37 The double‐membrane MDVs are generated from both OMM and inner mitochondrial membrane (IMM) carrying sometimes matrix proteins. 3 , 8 , 10 , 11 , 15 , 27 Of the IMM, MDVs incorporate specifically the OXPHOS complexes, complexes III and V, and the Fe‐S cluster; the latter functions in preventing mitochondrial Fe overload and in the removal of the irreversibly damaged proteins. 17 Of the mitochondrial matrix, MDVs might include pyruvate dehydrogenase, 38 the TCA cycle, fatty acids ß‐oxidation 17 and SOD2. 11 , 26 Moreover, mtDNA can be transferred by MDVs, a process associated with systemic inflammation in Parkinson's disease. 39 In this disorder, the defective complex I activity is due to the mutations in (NADH): ubiquinone oxidoreductase subunit S3 (NDUFS3). 40 Another group of MDVs transfers both OMM‐related TOM20 and the matrix SOD2. 11 , 26
Besides oxidative stress, hypoxia induces MDVs formation. Thus, when loaded with Bcl‐2, MDVs inhibit mitochondrial apoptosis, and help alleviate myocardial ischemia. 34 In addition, the stress induced by starvation generates also MDVs which are transported to lysosomes for the subsequent degradation. 10 , 11
Recently, proteomic analysis acknowledged the presence of 107 high‐confidence cargoes in TOM20 positive MDVs. 12 In brain MDVs, have been identified 72 proteins (31% from the OXPHOS) along with the small TIM chaperones. 13 , 41 In cardiac MDVs, Vasam et al. 17 reported the occurrence of proteins containing hyper‐reactive cysteine residues, redox enzymes and enzymes that mediate iron metabolism. These examples may imply MDVs tissue specificity.
In living organisms, MDVs are a heterogeneous population of vesicles. 23 , 42 Their selectivity in cargo incorporation is commanded by the nature of the mitochondrial stress. 11 , 27 According to Ryan and Tumbarello, 42 the cargo and ‘potentially’ the membrane constituents outline three attributes of MDVs: the trafficking mechanism employed, the intracellular route and their ultimate destination.
4. THE MDVs TRAFFICKING PATHWAYS
The specific destinations of MDVs trafficking are the lysosomes, the peroxisomes, the bacterial phagosomes and the extracellular vesicles (EV). The lysosomal route is mainly taken by MDVs containing oxidized proteins and engages the PINK1/Parkin pathway 10 , 11 , 27 , 41 (Figure 1). The loss of this pathway limits the mitochondria's capability to degrade the damaged proteins, followed by mitochondrial dysfunction. Such a defective process occurs in Parkinson's disease 10 and is due to the mutations of the corresponding genes. Recent reports demonstrate that the recessive early‐onset Parkinson's disease is associated with biallelic mutations in PINK1 43 and the loss‐of‐function mutations in the PARK2 gene, resulting in Parkin depletion. 44 In Parkin‐deficient mice, mutations in the mouse Parkin gene (Park2) are accomplished by the targeted deletion of Parkin exon 2. 45
FIGURE 1.
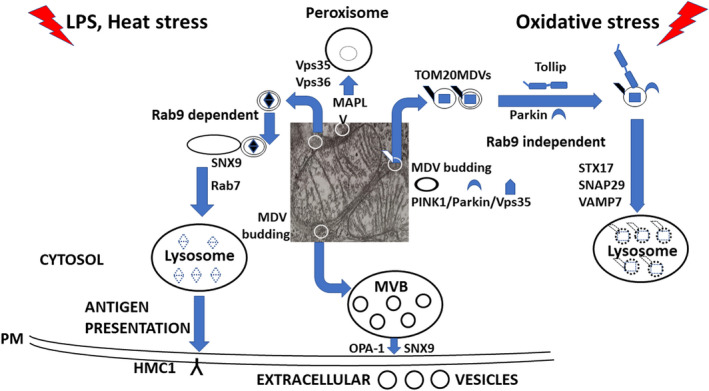
Outline of the MDVs generation and trafficking routes
Recent studies uncovered both the proteins beyond the MDVs flux to lysosomes/endo‐lysosomal compartment and those required for the fusion between the MDVs (containing oxidized proteins and lipids) and the degradative organelle. Thus, in response to mitochondrial stress, the endosomal adaptor Toll interacting protein (Tollip), in coordination with Parkin enables the transport of single‐membrane TOM20 MDVs to the endo‐lysosomal compartment, 35 , 42 following a Rab9 GTPase independent pathway. 10 Subsequently, MDVs fusion with the lysosomes is facilitated by the SNARE protein Syntaxin‐17 (STX17) that forms a complex with SNAP29 and VAMP7; this process is Rab9‐independent. 10 Next, a retromer‐dependent pathway could allow the ‘recycling’ of Tollip back from lysosomes to the early endosomes, either directly or via Rab7. 35
Another group of MDVs targets the peroxisome 6 (Figure 1). MDVs fusion with peroxisome, followed by the delivery into the peroxisome lumen is still unclear. However, a small number of contributing proteins have been identified so far. 14 Thus, MAPL‐containing MDVs are transported to the peroxisome, 27 , 28 and the recruitment involves two components of the retromer complex: Vps35 and the Vacuolar sorting protein 36 (Vps36). This complex is known for regulating the vesicle's retrograde transport from the endosomes to the trans‐Golgi network. 28
The MDVs could also converge to the bacteria‐containing phagosomes. Abuaita et al. 26 demonstrated that the infection of macrophages with methicillin‐resistant Staphylococcus aureus stimulated the generation of MDVs packed with SOD2. The loaded MDVs are delivered to the bacteria‐containing phagosomes, where SOD2 converts superoxide anions () into hydrogen peroxide (H2O2), which is used to kill the invading bacteria; this is an example of MDVs operation in antimicrobial defense. 34 One can conclude that MDVs trafficking pathways (described above) prove the interaction of MDVs with certain intracellular organelles.
Under stress conditions, the lysosomal degradation may be exceeded, and the MDVs containing dysfunctional parts could operate as pro‐inflammatory damage‐associated molecular patterns (DAMPs); cells prevent such an event by packaging the MDVs inside EV that will be extracellularly discharged 17 , 34 , 46 (Figure 1). Recently, Todkar et al. 46 reported that the EV route is followed by MDVs containing two proteins: the optic atrophy 1 (OPA1) and the SNX9. Moreover, the presence of Parkin inhibits this route, and MDVs are targeted for lysosomal degradation.
5. BIOLOGICAL ROLES OF MDVs
There are several generally acknowledged roles of MDVs:
a player in mitochondrial QC, ensuring the preservation of mitochondrial proteome (containing >1,000 proteins) and the functional integration of mitochondria according to the cellular demands. 8 , 15 , 17 , 26 , 34 , 38 Recently, Lv et al. 31 affirmed that MDVs act as ‘the first line of defence’ for the removal of the harmed mitochondrial components, before the degradation of the entire organelle by mitophagy. In cells where mitophagy is inactive or defective (such as the cancer cells), the MDVs pathway operates as a compensatory, adaptive mechanism supporting mitochondrial health. 33 , 47 , 48 The potential therapeutic targeting of mitochondrial QC pathways is particularly important for the alleviation of mitochondrial dysfunction in pathophysiology 13 , 49 , 50 , 51 ;
MDVs maintain the mitochondrial turnover, as the degraded damaged cargo could be replaced by novel proteins and lipids via biogenesis 52 ;
MDVs participate in inter‐organellar communication, an additional evolutionary conserved trait of mitochondria. 3 , 16 , 17 , 26 , 34 While transported within the cytosol, MDVs routes converge to the endo‐lysosomal compartment, to the lysosomes, and to the peroxisome and exchange proteins and lipids at the contact sites; moreover, in hypoxic conditions, mitochondria enriched in Bcl‐2 could transfer it to less healthy mitochondria 34 ;
MDVs mediate mitochondrial antigen presentation (MitAP), a process important in immune tolerance and immune responses. 30 These vesicles are generated in response to LPS exposure or heat stress. Their formation requires the presence of Rab7 (a small GTPase that monitors vesicular transport to late endosomes and lysosomes), Rab9, and SNX9, and is inhibited by PINK1 and Parkin. 17 , 30 , 34 This set of MDVs is transported also to the lysosomes where the mitochondrial antigens are processed, and MHC class I molecules are presented at the cell surface 30 (Figure 1);
a distinct group of MDVs are implied in de novo peroxisome biogenesis 2 , 53 and are not subjected to lysosomal degradation. 53 Previously, it was demonstrated that MDVs containing the E3 ubiquitin ligase MAPL target peroxisome. 27 This small organelle is born either by the growth and division of the existing cellular population or by de novo biogenesis. 54 Although the latter issue was extensively documented in yeasts, fewer studies deciphered it in mammaliancells. 55 According to the recent results, immature pre‐peroxisomes are formed by the fusion of vesicles containing peroxisome biogenesis‐initiating proteins known as ‘peroxins’ (Pex). 2 , 54 Interestingly, the vesicles involved in the fusion originate from two different organelles: the mitochondria, which bud MDVs enriched in Pex3 (descended from OMM)/Pex14 (an integral membrane protein implicated in peroxisomal matrix import), and the endoplasmic reticulum, that generates Pex16 holdng vesicles. The resulting fused structure imports peroxisomal membrane proteins into the lipid bilayer (with the contribution of Pex3 and Pex16), and recruits the matrix (lumen) proteins from the cytosol. 54 The fully competent mature peroxisome continues these imports, grows, elongates and divides into two to five ‘daughter’ organelles, adjusting their abundance, according to the cellular demands. 2 , 54 However, numerous questions are still open and deserve further insights. Thus, Schrader and Pellegrini 55 mention several gaps in the mechanism of OMM Pex3 transport and engagement in MDVs fusion, the details of Pex16 and Pex3/Pex14 vesicles fusion, and the understanding of the maturation process. The peroxisome is a ubiquitous organelle, with numerous cellular functions, including the involvement in ROS and lipid metabolism. The dysregulated activity of peroxisome in certain diseases is another issue worth to be unveiled by further research.
MDVs could operate in antimicrobial defence 26 (discussed in section #4).
MDVs have a broad biomedical significance. This extends from myocardial ischemia 34 and neurodegenerative diseases 12 , 27 to skeletal myocytes, 56 liver, 50 , 57 brown adipose tissue 58 and cancer cell metabolism, 47 to give a few examples only. Thus, in skeletal myocytes MDVs contribute to the maintenance of mitochondrial homeostasis, and to the immune signalling associated with muscle remodelling. 56 Previous reports demonstrated that MDVs have a protective role against alcohol‐induced liver injury. 50 However, these vesicles are absent or decreased in the liver of Parkin knock‐out mice. 57 A recent report acknowledges that the release of EV can be considered a biomarker in liver diseases. 59 In the brown adipose tissue, mitochondrial‐derived EV reduced the PPARγ signalling and the levels of uncoupling protein 1 (UCP1). 58 In human renal cell carcinomas, vesicles of mitochondrial and endoplasmic reticulum origin have been observed by electron microscopy. 60 In addition, the rare autophagy‐deficient clones are characterized by MDVs increased levels 47 ; this is an adaptation that compensates for autophagy loss and targets mitochondrial homeostasis maintenance. 61
6. FUTURE PERSPECTIVES
The urgent issues to be clarified include the uncovering of regulatory events behind MDVs generation in the cardiovascular diseases pathophysiology, 15 , 32 deciphering the relationships between the QC pathways, 10 , 62 disclosure of the potential mechanisms beyond coordination of mitochondrial‐lysosomal axis and EV trafficking, 14 characterization of mitochondrial EV in various pathologies, 8 , 14 , 15 stimulation of cardiomyocytes to generate Bcl‐2 containing MDVs, potentially useful for the therapy of myocardial ischemia 34 and finding those mitochondrial pathways that could delay inception of neurodegenerative diseases. 12 , 27 Besides the above objectives, one should be aware that the present knowledge on MDVs is based on in vitro studies; therefore, in vivo identification of MDVs metabolism (generation, wrapping and transport to the correct intra(extra)cellular organelle) using animal models is crucial for their potential use in clinical settings. 34
AUTHOR CONTRIBUTION
LUCIA DOINA POPOV: Conceptualization (lead); Formal analysis (lead); Visualization (lead); Writing – original draft (lead); Writing – review & editing (lead).
CONFLICT OF INTEREST
The author confirms that there is no conflict of interest.
Popov L‐D. Mitochondrial‐derived vesicles: Recent insights. J Cell Mol Med. 2022;26:3323–3328. doi: 10.1111/jcmm.17391
REFERENCES
- 1. Soubannier V, McLelland G‐L, Zunino R, et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012;22:135‐141. [DOI] [PubMed] [Google Scholar]
- 2. Sugiura A, Mattie S, Prudent J, McBride HM. Newly born peroxisomes are a hybrid of mitochondrial and ER‐derived pre‐peroxisomes. Nature. 2017;542:251‐254. [DOI] [PubMed] [Google Scholar]
- 3. Neuspiel M, Schauss AC, Braschi E, et al. Cargo‐selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol. 2008;18:102‐108. [DOI] [PubMed] [Google Scholar]
- 4. Hui L, Wu H, Wang T‐W, et al. Hydrogen peroxide‐induced mitophagy contributes to laryngeal cancer cells survival via the upregulation of FUNDC1. Clin Transl Oncol. 2019;21:596‐606. [DOI] [PubMed] [Google Scholar]
- 5. Voos W. Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Res Microbiol. 2009;160:718‐725. [DOI] [PubMed] [Google Scholar]
- 6. Juhász G. A mitochondrial‐derived vesicle HOPS to endolysosomes using Syntaxin‐17. J Cell Biol. 2016;214:241‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3‐5. [DOI] [PubMed] [Google Scholar]
- 8. Cadete VJJ, Deschênes S, Cuillerier A, et al. Formation of mitochondrial‐derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system. J Physiol. 2016;594(18):5343‐5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song J, Herrmann JM, Becker T. Quality control of the mitochondrial proteome. Nat Rev Mol Cell Biol. 2021;22(1):54‐70. [DOI] [PubMed] [Google Scholar]
- 10. McLelland G‐L, Soubannier V, Chen CX, et al. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. The EMBO J. 2014;33:282‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soubannier V, Rippstein P, Kaufman BA, et al. Reconstitution of mitochondria derived vesicle formation demonstr ates selective enrichment of oxidized cargo. PLoS One. 2012;7:e52830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. König T, Nolte H, Aaltonen MJ, et al. MIROs and DRP1 drive mitochondrial‐derived vesicle biogenesis and promote quality control. Nat Cell Biol. 2021;23:1271‐1286. [DOI] [PubMed] [Google Scholar]
- 13. Roberts RF, Bayne AN, Goiran T, et al. Proteomic profiling of mitochondrial‐derived vesicles in the brain reveals enrichment of respiratory complex sub‐assemblies and small TIM chaperones. J Proteome Res. 2021;20:506‐517. [DOI] [PubMed] [Google Scholar]
- 14. Picca A, Guerra F, Calvani R, et al. Mitochondrial dysfunction and aging: insights from the analysis of extracellular vesicles. Int J Mol Sci. 2019;20:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Picca A, Guerra F, Calvani R, et al. Generation and release of mitochondrial‐derived vesicles in health, aging, and disease. J Clin Med. 2020;9:1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andrade‐Navarro MA, Sanchez‐Pulido L, McBride HM. Mitochondrial vesicles: an ancient process providing new links to peroxisomes. Curr Opin Cell Biol. 2009;21:560‐567. [DOI] [PubMed] [Google Scholar]
- 17. Vasam G, Nadeau R, Cadete VJJ, et al. Proteomics characterization of mitochondrial‐derived vesicles under oxidative stress. The FASEB J. 2021;35:e21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beveridge TJ. Structures of gram‐negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725‐4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaparakis‐Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375‐387. [DOI] [PubMed] [Google Scholar]
- 20. Perez‐Cruz C, Carriὀn O, Delgado L, et al. New type of outer membrane vesicle produced by the Gram‐negative bacterium Shewanella vesiculosa M7T: implications for DNA content. Appl Environ Microb. 2013;79:1874‐1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pérez‐Cruz C, Delgado L, López‐Iglesias C, et al. Outer‐inner membrane vesicles naturally secreted by gram‐negative pathogenic bacteria. PLoS One. 2015;12:e0116896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437(7057):422‐425. [DOI] [PubMed] [Google Scholar]
- 23. Deatherage BL, Cookson BT. Membrane vesicle release in Bacteria, Eukaryotes, and Archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80(6):1948‐1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221‐225. [DOI] [PubMed] [Google Scholar]
- 26. Abuaita BH, Schultz TL, O'Riordan MX. Mitochondria‐derived vesicles deliver antimicrobial reactive oxygen species to control phagosome‐localized Staphylococcus aureus. Cell Host Microbe. 2018;24(5):625‐636.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sugiura A, McLelland G‐L, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial‐derived vesicles. The EMBO J. 2014;33:2142‐2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braschi E, Goyon V, Zunino R, et al. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol. 2010;20(14):1310‐1315. [DOI] [PubMed] [Google Scholar]
- 29. Roberts RF, Fon EA. Presenting mitochondrial antigens: PINK1, Parkin and MDVs steal the show. Cell Res. 2016;26:1180‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matheoud D, Sugiura A, Bellemare‐Pelletier A, et al. Parkinson's disease‐related proteins PINK1 and Parkin repress mitochondrial antigen presentation. Cell. 2016;166:314‐327. [DOI] [PubMed] [Google Scholar]
- 31. Lv J, Guan W, You Q, et al. RIPC provides neuroprotection against ischemic stroke by suppressing apoptosis via the mitochondrial pathway. Sci Rep. 2020;10:5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramirez A, Old W, Selwood DL, Liu X. Cannabidiol activates PINK1‐Parkin‐dependent mitophagy and mitochondrial‐derived vesicles. Eur J Cell Biol. 2022;101:151185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Towers CG, Wodetzki DK, Thorburn J, et al. Mitochondrial‐derived vesicles compensate for the loss of LC3‐mediated mitophagy. Dev Cell. 2021;56:2029‐2042.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li B, Zhao H, Wu Y, et al. Mitochondrial‐derived vesicles protect cardiomyocytes against hypoxic damage. Front Cell Dev Biol. 2020;8:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ryan TA, Phillips EO, Collier CL, et al. Tollip coordinates Parkin‐dependent trafficking of mitochondrial‐derived vesicles. The EMBO J. 2020;39:e102539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abe Y, Shodai T, Muto T, et al. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell. 2000;100:551‐560. [DOI] [PubMed] [Google Scholar]
- 37. Yamano K, Yatsukawa Y‐I, Esaki M, et al. Tom20 and Tom22 share the common signal recognition pathway in mitochondrial protein import. J Biol Chem. 2008;283:3799‐3807. [DOI] [PubMed] [Google Scholar]
- 38. Ng F, Tang BL. Pyruvate dehydrogenase complex (PDC) export from the mitochondrial matrix. Mol Membr Biol. 2014;31:207‐210. [DOI] [PubMed] [Google Scholar]
- 39. Picca A, Beli R, Calvani R, et al. Older adults with physical frailty and sarcopenia show increased levels of circulating small extracellular vesicles with a specific mitochondrial signature. Cells. 2020;9:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Picca A, Guerra F, Calvani R, et al. Mitochondrial signatures in circulating extracellular vesicles of older adults with Parkinson's disease: results from the EXosomes in PArkiNson's disease (EXPAND) study. J Clin Med. 2020;9(2):504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roberts RF, Bayne AN, Goiran T, et al. Proteomic profiling of mitochondrial‐derived vesicles in brain reveals enrichment of respiratory complex sub‐assemblies and small TIM chaperones. J Proteome Res. 2021;20:506‐517. [DOI] [PubMed] [Google Scholar]
- 42. Ryan TA, Tumbarello DA. A central role for mitochondrial‐derived vesicles in the innate immune response: implications for Parkinson’s disease. Neural Regen Res. 2021;16:1779‐1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nybø CJ, Gustavsson EK, Farrer MJ, Aasly JO. Neuropathological findings in PINK1‐associated Parkinson's disease. Parkinsonism Relat Disord. 2020;78:105‐108. [DOI] [PubMed] [Google Scholar]
- 44. Gaweda‐Walerych K, Sitek EJ, Narożańska E, Buratti E. Parkin beyond Parkinson's disease‐A functional meaning of Parkin downregulation in TDP‐43 proteinopathies. Cells. 2021;10:3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perez FA, Palmiter RD. Parkin‐deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci USA. 2005;102:2174‐2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Todkar K, Chikhi L, Desjardins V, et al. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nature Comm. 2021;12:1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poillet‐Perez L, White E. MDVs to the rescue: How autophagy‐deficient cancer cells adapt to defective mitophagy. Dev Cell. 2021;56:2010‐2012. [DOI] [PubMed] [Google Scholar]
- 48. Mondal P, Towers C. Beyond mitophagy: mitochondrial‐derived vesicles can get the job done! Autophagy. 2022;18:449‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ikeda G, Santoso MR, Tada Y, et al. Mitochondria‐rich extracellular vesicles from autologous stem cell‐derived cardiomyocytes restore energetics of ischemic myocardium. J Am Coll Cardiol. 2021;77:1073‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eid N, Ito Y, Otsuki Y. Mitophagy in steatotic hepatocytes of ethanol‐treated wild‐type and Parkin knockout mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G513‐G514. [DOI] [PubMed] [Google Scholar]
- 51. Paley EL, Perry G, Sokolova O. Tryptamine induces axonopathy and mitochondriopathy mimicking neurodegenerative diseases via tryptophanyl‐tRNA deficiency. Curr Alzheimer Res. 2013;10:987‐1004. [DOI] [PubMed] [Google Scholar]
- 52. Pickles S, Vigié P, Youle RJ. The art of mitochondrial maintenance. Curr Biol. 2018;28):R170‐R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim P. Peroxisome biogenesis: a union between two organelles. Curr Biol. 2017;27:R271‐R274. [DOI] [PubMed] [Google Scholar]
- 54. Smith JJ, Aitchison JD. Peroxisomes take shape. Nat Rev Mol Cell Biol. 2013;14:803‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schrader M, Pellegrini L. The making of a mammalian peroxisome, version 2.0: mitochondria get into the mix. Cell Death Differ. 2017;24:1148‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Picca A, Guerra F, Calvani R, et al. Mitochondrial‐derived vesicles in skeletal muscle remodeling and adaptation. Semin Cell Dev Biol. 2022;S1084–9521(22):00095‐97. [DOI] [PubMed] [Google Scholar]
- 57. Williams JA, Ding WX. Mitophagy, mitochondrial spheroids, and mitochondrial‐derived vesicles in alcohol‐induced liver injury. Am J Physiol Gastrointest Liver Physiol. 2015;309:G515. [DOI] [PubMed] [Google Scholar]
- 58. Rosina M, Ceci V, Turchi R, et al. In brown adiopose tissue, the ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022;S1550‐4131(22)00088‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thietart S, Rautou PE. Extracellular vesicles as biomarkers in liver diseases: a clinician's point of view. J Hepatol. 2020;73:1507‐1525. [DOI] [PubMed] [Google Scholar]
- 60. Thoenes W, Störkel S, Rumpelt HJ. Human chromophobe cell renal carcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;48:207‐217. [DOI] [PubMed] [Google Scholar]
- 61. Towers CG, Wodetzki DK, Thorburn J, et al. Mitochondrial‐derived vesicles compensate for loss of LC3‐mediated mitophagy. Dev Cell. 2021;56:2029‐2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McLelland GL, Lee SA, Mcbride HM, Fon EA. Syntaxin‐17 delivers PINK1/Parkin‐dependent mitochondrial vesicles to the endolysosomal system. J Cell Biol. 2016;214:275‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]


