Abstract
This paper examines the synergistic action of carbon dioxide and nisin on Listeria monocytogenes Scott A wild-type and nisin-resistant (Nisr) cells grown in broth at 4°C. Carbon dioxide extended the lag phase and decreased the specific growth rate of both strains, but to a greater degree in the Nisr cells. Wild-type cells grown in 100% CO2 were two to five times longer than cells grown in air. Nisin (2.5 μg/ml) did not decrease the viability of Nisr cells but for wild-type cells caused an immediate 2-log reduction of viability when they were grown in air and a 4-log reduction when they were grown in 100% CO2. There was a quantifiable synergistic action between nisin and CO2 in the wild-type strain. The MIC of nisin for the wild-type strain grown in the presence of 2.5 μg of nisin per ml increased from 3.1 to 12.5 μg/ml over 35 days, but this increase was markedly delayed for cultures in CO2. This synergism between nisin and CO2 was examined mechanistically by following the leakage of carboxyfluorescein (CF) from listerial liposomes. Carbon dioxide enhanced nisin-induced CF leakage, indicating that the synergistic action of CO2 and nisin occurs at the cytoplasmic membrane. Liposomes made from cells grown in a CO2 atmosphere were even more sensitive to nisin action. Liposomes made from cells grown at 4°C were dramatically more nisin sensitive than were liposomes derived from cells grown at 30°C. Cells grown in the presence of 100% CO2 and those grown at 4°C had a greater proportion of short-chain fatty acids. The synergistic action of nisin and CO2 is consistent with a model where membrane fluidity plays a role in the efficiency of nisin action.
The ability of Listeria monocytogenes to resist environmental stresses has made this food-borne pathogen a major concern to the food industry. This pathogen is found on various foods and has been implicated in several large food-borne outbreaks worldwide (C. B. Dalton, C. Austin, J. Sobel, P. Hayes, B. Bibb, J. Mellen, and P. Griffin, Proc. 44th Annu. Epidemic Intelligence Serv. Conf., abstr. 19, 1995; 11). New preservation strategies have been developed to control the growth of L. monocytogenes in foods, including application of nisin (10, 15). Nisin is an antimicrobial peptide which kills L. monocytogenes as well as many other gram-positive bacteria. It acts on the cytoplasmic membrane of sensitive cells by forming transient pores which allow efflux of small hydrophilic compounds like ATP, ADP, monovalent cations, and amino acids (1, 25). This pore formation leads to dissipation of the membrane potential and ionic gradient across the membrane and subsequently results in the destruction of energy metabolism and cell death (3, 18).
The practical application of nisin as a food preservative can be compromised by the existence of L. monocytogenes strains which are naturally resistant to nisin (13, 17) and selection of nisin-resistant bacteria during exposure to progressively higher nisin concentrations (16, 17). However, the use of a multiple-hurdle system may reduce development of nisin resistance. Carbon dioxide inhibits growth of both gram-positive and gram-negative bacteria (6, 8). Our model studies and trials with cold-smoked salmon (20) have shown that a CO2 atmosphere improves the antilisterial activity of nisin. Despite several publications on the effect of CO2 on bacterial growth, the mechanism of its inhibitory activity still remains unclear.
The objective of this study was to gain mechanistic insight into the combined antilisterial effect of nisin and CO2. Our data indicate that nisin and CO2 atmosphere act synergistically on the cytoplasmic membrane of wild-type L. monocytogenes Scott A cells by enhancing membrane permeabilization. We also examined whether nisin and CO2 have a synergistic antibacterial effect against a nisin-resistant (Nisr) derivative of the Scott A strain. Alternate mechanisms of CO2 action against wild-type Scott A cells, including inhibition of cell division, were investigated and excluded.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Wild-type L. monocytogenes Scott A and its Nisr mutant L. monocytogenes ATCC 700302 (16) were grown in brain heart infusion (BHI) broth (Difco, Detroit, Mich.). The Nisr strain was maintained on BHI agar (Difco) containing 25 μg of nisin per ml at 4°C. Lactococcus lactis strain NZ 9000 was transformed with recombinant plasmid pNZ8008 carrying the gusA gene fused to the nisA promoter (7) as described previously (14). The recombinant strain NZ 9000(pNZ8008) was grown at 30°C in M17 broth (Difco) supplemented with 0.5% (wt/vol) glucose (GM17) and chloramphenicol (10 μg/ml). This strain was used to determine nisin concentration (see below).
Chemicals.
Nisin stock solutions were prepared from Nisaplin (a gift from Aplin and Barrett, Trowbridge, United Kingdom) for in vitro studies or pure nisin (Ambicin; a gift of AMBI Inc., Tarrytown, N.Y.) for in vivo studies. The stock solution was dissolved in nisin diluent (0.02 N HCl–0.75% NaCl, pH 5.3) and filter sterilized through a 0.45-μm-pore-size membrane filter (Acrodisc; Gelman Sciences, Ann Arbor, Mich.). When required, BHI was buffered (B-BHI) with 0.1 N phosphate buffer (K2HPO4-KH2PO4) to a pH of 6.2.
Growth of wild-type and Nisr L. monocytogenes Scott A in the presence of nisin and in a CO2 atmosphere.
Wild-type and Nisr cells of L. monocytogenes were grown at 30°C to an optical density at 600 nm (OD600) of 0.5 to 0.6 (Shimadzu UV 160U; Tokyo, Japan). Wild-type cells were inoculated in B-BHI broth to give an initial cell concentration of approximately 5 × 106 CFU/ml. Nisin was added at time zero at a concentration of 2.5 μg/ml. Cultures were incubated at 4°C with agitation (120 rpm) in air or in an atmosphere of 100% CO2. To create a 100% CO2 atmosphere, flasks were placed in anaerobic jars which had an external valve system (GasPak Jar System; BBL). Air was evacuated from the jars (−0.9 bar), and the atmosphere composition was adjusted to 100% CO2 by filling the anaerobic jars with CO2 (gas purity was >99.8%; JWS Technologies). At appropriate intervals, CFU, MICs, and residual nisin concentrations were determined. CFU were determined by spiral plating (Model D; Spiral Biotech, Inc., Bethesda, Md.) of appropriate dilutions on BHI agar. After 24 to 30 h of incubation at 30°C, plates were counted with a laser bacterial colony counter (Model 500A; Spiral Biotech, Inc.).
Determination of nisin resistance.
Nisin resistance in L. monocytogenes was measured by determining the MIC of nisin. L. monocytogenes cells were grown for 18 h at 30°C in BHI broth. Fresh BHI broth was inoculated with 106 cells. The culture broth was inoculated into the wells of a microtiter plate (Corning Costar Corporation, Cambridge, Mass.), and nisin (12.5 μg/ml) was added in the first row of wells. Twofold dilutions of nisin in the culture were made, and the MIC was determined as the lowest nisin concentration which prevented L. monocytogenes growth after 24 h at 30°C.
Determination of nisin concentration by the β-glucuronidase assay.
The concentration of residual nisin in B-BHI broth was measured with the quantitative β-glucuronidase assay. Lactococcus lactis NZ 9000(pNZ8008) cells were grown in M17 broth containing 0.5% (wt/vol) glucose (GM17) and 10 μg of chloramphenicol per ml at 30°C to an OD600 of 0.5. Nisin was added as described by de Ruyter et al. (7). The specific β-glucuronidase activity was measured in 96-well black plates with flat bottoms (Dynex Technologies), using an LS50B spectrometer (Perkin-Elmer) with excitation at 365 nm and emission at 455 nm. Nisin concentrations were derived from a dose-response standard curve (7).
Lipid extraction and fatty acid composition.
L. monocytogenes Scott A cells were grown with aeration in B-BHI at 30 and 4°C, in the absence or presence of nisin (2.5 μg/ml), and in air or a 100% CO2 atmosphere. Cells were harvested at mid-log phase (OD600 of 0.6 to 0.8) and washed once with 0.1% peptone water (Difco), and lipids were extracted using the Bligh and Dyer (2) method, with modifications (25). Lipids were resuspended in chloroform-methanol (9:1) and stored at −20°C for up to 2 weeks before analysis for fatty acid composition (16).
Preparation of CF-loaded liposomes.
Liposomes were made and loaded with carboxyfluorescein (CF) as previously described (25) and stored on ice for up to 4 h until use. The phospholipid concentration of liposomes was determined using the Bartlett assay as described by New (19).
CF release assay.
The ability of nisin to cause CF efflux from liposomes derived from wild-type L. monocytogenes Scott A cells was studied in the presence of air and 100% CO2 at 4 and 22°C. The effect of CO2 at 22°C was not investigated since the solubility of the gas and consequently the antimicrobial effect are decreased at increasing temperatures (6). The release of CF from liposomes was determined as an increase in fluorescence intensity at 516 nm with excitation at 490 nm (F1T11 spectrofluorometer; Spex Industries, Metuchen, N.J.) as previously described (25). The assay buffer was changed to 0.1 N KHPO4 due to its superior ability to stabilize pH in the presence of CO2. The buffer's temperature was adjusted to the assay's temperature (4 or 22°C), and pH was adjusted to 6.2 with either 0.1 N K2HPO4 or 0.1 N KH2PO4 prior to each experiment. Precise temperature control was maintained by connecting a circulating temperature control bath (PolyScience, Niles, Ill.) to the cuvette holder in the spectrofluorometer. For the experiment in a 100% CO2 atmosphere, the assay buffer (0.1 N KHPO4, 4°C) was saturated with CO2 by flushing 100% CO2 directly into the buffer for approximately 5 min. The pH was then adjusted to 6.2 with either 0.1 N K2HPO4 or 0.1 N KH2PO4. During the assay, CO2 was flushed into the cuvette chamber to saturate the space above the assay buffer with CO2.
The release of CF from the liposomes was expressed as the percentage of CF release relative to the maximal CF release from liposomes. This was calculated from the equation % efflux = [(Ft − F0)/(Fm − F0)] × 100, where Ft was the fluorescence intensity at time t, F0 was the fluorescence intensity for the control (addition of nisin diluent in the presence of air) at time t, and Fm was maximum release of CF (4). Maximal CF release was determined by addition of 10% Triton X-100 solution to a final concentration of 0.2% (vol/vol). The rate of CF efflux (percent per minute) was calculated from the slope of the tangent to the efflux curve after 200 s after the addition of nisin or diluent.
RESULTS
Influence of nisin and CO2 on growth of Nisr and wild-type L. monocytogenes Scott A.
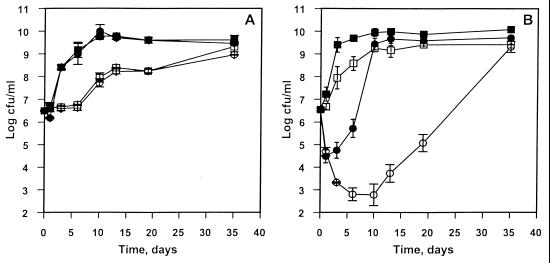
When grown in air at 4°C, Nisr cells reached 8 × 109 CFU/ml after 10 days (Fig. 1A). The growth patterns of the Nisr strain in the presence and the absence of nisin were identical. Nisin (2.5 μg/ml) had no effect on Nisr cells cultured in air or CO2 atmospheres. A carbon dioxide atmosphere, on the other hand, extended the lag phase of the Nisr culture for 6 days and decreased the final cell density by 2 log10. Furthermore, carbon dioxide was more inhibitory to the growth of the Nisr strain than to the growth of the wild-type strain (Fig. 1B).
FIG. 1.
Growth of L. monocytogenes Scott A Nisr (A) and wild-type (B) strains in B-BHI broth at 4°C. Closed symbols indicate results in air; open symbols indicate results in 100% CO2. Squares represent cultures in the absence of nisin, and circles represent cultures in the presence of nisin (2.5 μg/ml). Vertical bars represent the means and standard deviations from two independent experiments.
Wild-type cultures reached a maximum of approximately 1010 CFU/ml after 6 days of incubation (Fig. 1B). The CO2 atmosphere prolonged the lag phase slightly and reduced the maximum cell number by 1 loga. In air, nisin addition (2.5 μg/ml) to B-BHI broth caused an immediate 2-log reduction of viable cell count, and the wild-type cells reached maximum cell density after 10 days of incubation. In the presence of CO2, exposure of wild-type cells to nisin caused a similar immediate viability reduction and further extended the die-off such that a 4-log decline in viable cell count was reached after 6 days (Fig. 1B). The time required to reach maximum cell density (109 CFU/ml) was extended to more than 20 days. Therefore, for wild-type L. monocytogenes cells, the combined effect of CO2 and nisin is greater than the sum effects of CO2 and nisin alone, with respect to both lethality and growth retardation (time required to reach maximum cell density).
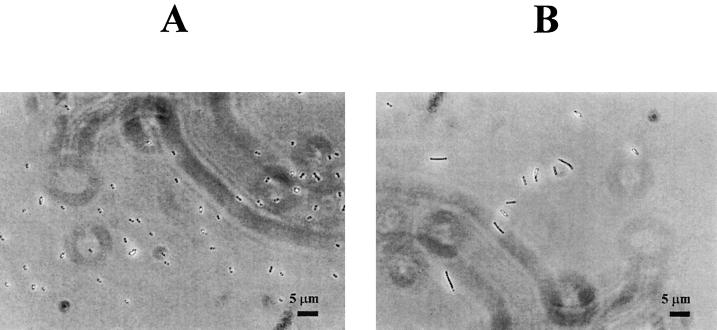
Effect of carbon dioxide on cell morphology of wild-type L. monocytogenes Scott A.
The morphology of wild-type cells grown for 20 days in air and in the presence of 100% CO2 atmosphere at 4°C is illustrated in Fig. 2. In the presence of 100% CO2, cells elongated to two to five times their normal length.
FIG. 2.
Phase-contrast photomicrographs of L. monocytogenes Scott A (wild type) in the presence of air (A) and 100% CO2 (B).
Development of nisin resistance in wild-type cells.
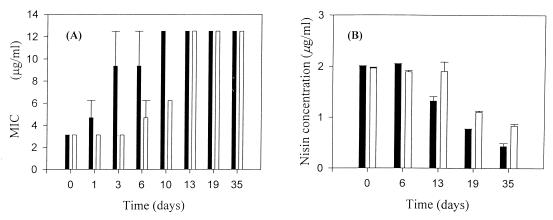
When the wild-type cells were grown in the presence of an initial concentration of 2.5 μg of nisin per ml, the MICs increased over time, both in air and in CO2. For wild-type cells grown in air, the initial MIC was 3.1 μg/ml. This increased rapidly to 9.3 μg/ml after 3 days of incubation and to a maximum MIC of 12.5 μg/ml by day 10 (Fig. 3A). When grown in the presence of 100% CO2, the MIC for the cells was stable at 3.1 μg/ml for 3 days, which was then increased gradually to 12.5 μg/ml by day 13.
FIG. 3.
MICs of nisin (A) and residual concentrations of nisin (B) for L. monocytogenes wild-type cells grown at 4°C in the presence of air (black bars) or 100% CO2 atmosphere (open bars) when nisin (2.5 μg/ml) was added to the broth at time zero. Vertical bars represent the means and standard deviations from two independent experiments.
The residual nisin concentration was measured during the course of these experiments. At time zero, 80% of the initial nisin concentration was detected in the culture broth (Fig. 3B). Over the course of the experiment, the residual nisin concentration decreased to approximately 25 to 30% of the initial concentration.
Influence of temperature and culture growth conditions on nisin-mediated CF efflux from liposomes.
Temperature had a dramatic influence on the sensitivity of Listeria liposomes to nisin. CF-loaded liposomes were prepared using lipids extracted from cells cultured under three different conditions, 30°C in air, 4°C in air, and 4°C in a CO2 atmosphere, in order to examine the effect of lipid composition. CF efflux was compared at two different temperatures to determine the role of membrane fluidity. For all three culture conditions examined, decreasing the assay temperature (and, thus, fluidity) markedly decreased the initial rates and overall extent of CF efflux and increased the time required for CF efflux to reach a plateau (Table 1). Liposomes derived from cells grown at 30°C in air were completely insensitive to nisin's action at 4°C. The negative CF efflux from liposomes of cells grown at 30°C in air when assayed at 4°C indicated that control liposomes (to which nisin diluent had been added) were slightly more leaky for CF than were liposomes treated with 2.5 μg of nisin per ml. The intrinsic (i.e., caused by diluent without nisin) leakiness of liposomes from cells grown in air at 30°C was 0.19%/min, 0.64% for liposomes from cells grown in air at 4°C, and 0.90%/min for cells grown at 4°C with a CO2 atmosphere (data not shown).
TABLE 1.
Effect of the assay temperature on nisin-induced CF efflux from liposomes derived from L. monocytogenes Scott A grown under various conditionsa
| Cell growth conditionsb | CF effluxc at given temp
|
|||||
|---|---|---|---|---|---|---|
| Initial efflux rate (%/min)
|
Overall efflux (%)
|
Time to plateau (s)
|
||||
| 4°C | 22°C | 4°C | 22°C | 4°C | 22°C | |
| 30°C, air | −0.1 | 5.5 | −0.3 | 30.9 | >1,200 | 600 |
| 4°C, air | 0.3 | 20.5 | 5.1 | 82.6 | >1,200 | 380 |
| 4°C, CO2 | 2.1 | 24.8 | 18.1 | 92.5 | >1,200 | 300 |
Nisin concentration was 2.5 μg/ml. Values represent the averages from two independent experiments.
Cells were grown in B-BHI broth in the absence of nisin under conditions indicated.
The initial efflux rate of CF was calculated from the slope of a tangent to the efflux curve at 200 s after addition of nisin. Overall percentage of CF efflux was calculated at 600 s. Time for plateau of CF leakage was calculated after addition of nisin (seconds). The lipid concentration for cells preadapted to 30°C in air, 4°C in air, and 4°C in 100% CO2 was 2.5, 2.45, and 3.0 μM, respectively.
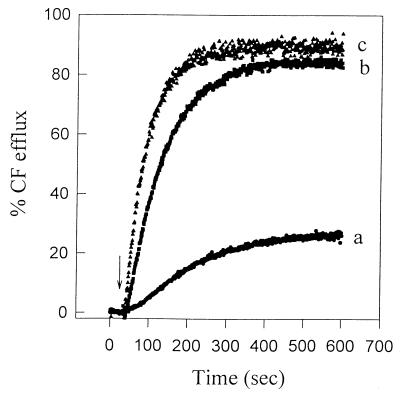
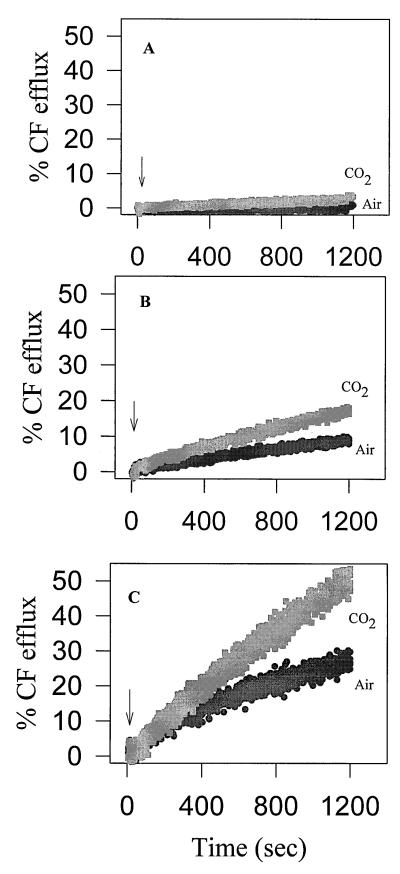
Liposomes derived from cells grown at 4°C were more sensitive to nisin than were liposomes derived from cells grown at 30°C (Table 1), at both assay temperatures. In fact, liposomes derived from cells grown at 4°C in the presence of CO2 were the most sensitive, having the highest CF efflux rate and overall efflux. Figure 4 illustrates the CF release kinetics for these three types of liposomes. Nisin-induced CF efflux from liposomes derived from cells grown at 4°C in air was comparable to that from liposomes derived from cells grown at 4°C in the CO2 atmosphere. Liposomes derived from cells grown at 30°C in air were the least sensitive to nisin (Fig. 4 and Table 1).
FIG. 4.
Nisin-induced CF efflux from liposomes derived from wild-type L. monocytogenes Scott A grown in B-BHI broth at 30°C in air (a), 4°C in air (b), and 4°C in 100% CO2 (c). Nisin (2.5 μg/ml) was added at the time indicated by the arrow. The lipid concentration for cells preadapted at 30°C in air, 4°C in air, and 4°C in 100% CO2 was 2.5, 2.45, and 3.0 μM, respectively. The assay temperature was 22°C.
Influence of CO2 atmosphere and growth conditions on nisin-mediated CF efflux at 4°C.
When liposomes were exposed to a CO2 atmosphere in the absence of nisin, there was a weak CF release over time, as previously noted. The overall CF efflux values (which had been corrected for baseline fluorescence) were only 0.77 to 1.9% after 600 S. Thus, the CO2 atmosphere had some influence on the permeability of the liposomes. Interestingly, nisin-induced CF efflux was greatly enhanced in the presence of CO2 atmosphere for all three types of liposomes examined (Fig. 5). Liposomes derived from cells grown at 30°C in air exhibited the greatest synergistic action between CO2 and nisin. This was demonstrated by a 430% increase of the overall CF efflux at 1,200 s (Fig. 5A), albeit that the CF efflux levels were low. When assayed under air or a CO2 atmosphere, liposomes derived from cells which had been grown at refrigeration temperature had higher CF efflux levels than did liposomes derived from cells grown at 30°C (Fig. 5B and C). Carbon dioxide atmosphere nonetheless increased CF efflux to higher levels. The overall CF efflux at 1,200 s increased by 186% (from 9.8 to 18.3%) and by 176% (from 30.1 to 53.2%) for liposomes derived from cells grown at 4°C in air (Fig. 5B) and at 4°C in 100% CO2 (Fig. 5C), respectively. For all three types of liposomes, the combined effect of nisin and CO2 on CF efflux was greater than the sum effects of CO2 and nisin alone.
FIG. 5.
Influence of atmosphere composition on nisin-induced CF efflux from L. monocytogenes-derived liposomes. Liposomes were made from wild-type L. monocytogenes Scott A grown at 30°C in air (A), 4°C in air (B), and 4°C in 100% CO2 (C). Liposomes were exposed to air and 100% CO2, as listed next to the efflux curves. Nisin (2.5 μg/ml) was added at the time indicated by an arrow. The lipid concentration for cells preadapted to 30 and 4°C in air and 4°C in 100% CO2 was 2.5, 2.45, and 3.0 μM, respectively.
Membrane fatty acid composition of wild-type cells grown at 4°C.
L. monocytogenes Scott A cells grown in the presence of 100% CO2 had a greater proportion of short-chain fatty acids than did cells grown in air (Table 2). This was found both in the absence and in the presence of nisin. The primary response to a decrease in growth temperature from 30 to 4°C was a marked increase of monounsaturated fatty acids in the cell membrane composition (data not shown).
TABLE 2.
Fatty acid composition of wild-type L. monocytogenes Scott A
| Growth condition(s) | % of totala
|
|||||||
|---|---|---|---|---|---|---|---|---|
| C10:0 | C12:0 | C15:0 | C15:1 | C17:0 | C17:1 | C18:0 | Other | |
| Air | 0 | 0 | 0 | 43.9 | 0 | 19.4 | 14.1 | 22.6 |
| CO2 | 0 | 31.4 | 28.1 | 40.5 | 0 | 0 | 0 | 0 |
| Air, nisinb | 4.7 | 36.6 | 0 | 10.3 | 0 | 43.9 | 4.4 | 0.1 |
| CO2, nisin | 19.8 | 40.7 | 0 | 39.5 | 0 | 0 | 0 | 0 |
Lipids were extracted from culture grown in B-BHI broth (pH 6.2) at 4°C until late-exponential growth phase.
Nisin (2.5 μg/ml) was added to the growth broth at time zero.
DISCUSSION
These results demonstrated a quantifiable synergistic action of nisin and CO2 on the cells (growth inhibition) and liposomes (membrane permeability) of wild-type L. monocytogenes Scott A. Carbon dioxide extended the lag phase and decreased the specific growth rate of both L. monocytogenes Scott A wild-type and Nisr cells. CO2 was more bacteriostatic against the Nisr strain than was the wild-type strain. High CO2 concentrations often extend the lag phase and inhibit growth (9). Results from this study verify that CO2 acts similarly in L. monocytogenes cells. Wild-type cells grown in a 100% CO2 atmosphere were two to five times longer than cells grown in air. Inhibition of cell division due to CO2 has not, to our knowledge, previously been reported. However, CO2 changes the cell morphology of Streptococcus mutans; at a high bicarbonate/K+ ratio, spherical cells are produced, and at a low bicarbonate/K+ ratio, the cells remain bacillary (23).
Lethality of nisin to L. monocytogenes cells and pore formation in liposomes were both enhanced in the presence of CO2. Carbon dioxide reportedly acts like anaesthetic gases which expand hydrophobic regions of the membrane lipids. This perturbs membrane fluidity and could be expected to alter the function of a biological membrane (8). The increased CF efflux from liposomes derived from wild-type L. monocytogenes grown in the presence of CO2 demonstrates that it destabilized the membrane. Moreover, adding CO2 to the assay atmosphere enhanced the nisin-induced CF leakage. The correlation between our in vivo and in vitro data suggests that the synergistic action of CO2 and nisin occurs at the cytoplasmic membrane.
In addition to increasing membrane permeability, carbon dioxide also caused the cells to modify their membrane fatty acid composition. The presence of CO2 during growth of L. monocytogenes Scott A increased the proportion of short-chain fatty acids. The increase in short-chain fatty acids at the expense of long-chain fatty acids is suggestive of increased membrane fluidity (16). This in turn may enhance pore formation by nisin. Indeed, higher nisin-induced CF leakage was confirmed in the liposomes derived from cells grown in CO2 than was confirmed in liposomes derived from cells grown in air.
The enhanced lethal action of nisin on cells grown in a CO2 atmosphere could be attributed to a similar change in membrane fluidity. Carbon dioxide significantly reduces the MIC of nisin against L. monocytogenes cells grown at 10°C (20). The distinctive shift towards greater proportions of short-chain fatty acids, which is consistent with a more fluid membrane, would make cells adapted to a CO2 atmosphere more susceptible to nisin. Resistance to nisin in L. monocytogenes is also accompanied by changes in lipid composition which are suggestive of a more rigid membrane (5, 16). The alterations in fatty acid profile may underlie the ability of CO2 to delay the development of nisin resistance in wild-type cells.
Liposomes derived from cells grown at 4°C were dramatically more sensitive to nisin than were liposomes derived from cells grown at 30°C when CF efflux was measured at the same temperature. Extension of these in vitro findings to in vivo conditions would suggest that cells at low temperatures could be more sensitive to nisin, reducing the concern about high nisin tolerance developing in L. monocytogenes cells from refrigerated foods. Abee et al. (1) reported that nisin Z-induced K+ leakage from L. monocytogenes Scott A cells was greater in cells grown at 4°C than in cells grown at 30°C. This was explained by the increased proportion of unsaturated fatty acyl chains of the membrane lipids at 4°C, which helped to maintain an optimum membrane fluidity (1). Previously, we reported that the content of fatty acids with lower melting points is greater in L. monocytogenes cells grown at 10°C than in cells grown at 30°C (16). In agreement with these results, the present study found an increased proportion of monounsaturated fatty acids in the cell membrane at 4°C, compared to the proportion at 30°C. The alteration in fatty acid profile for cells cultured at the lower temperature, again suggestive of increased membrane fluidity, correlates with dramatically increased liposomal sensitivity to nisin.
Clearly, when the fatty acid composition is altered in response to culture conditions (i.e., CO2 atmosphere and temperature) there have to have been changes in the nisin-induced CF efflux, presumably through modulation of membrane fluidity. The influence of assay temperature on the sensitivity of the liposomes to nisin further supports this membrane fluidity model. Temperature had a dramatic influence on pore formation of nisin. Decreasing the assay temperature of liposomes with the same lipid composition (i.e., derived from cells grown at a given condition) dramatically reduced nisin-mediated CF efflux. When the liposomes were switched to the lower temperature, a more-ordered fatty acid alignment may have resulted, making the membrane bilayer more rigid and thus more sensitive to nisin.
CO2 atmosphere and nisin might be used in combination to control L. monocytogenes in refrigerated foods. The action of CO2 on growing L. monocytogenes cells altered fatty acid composition, inhibited cell division, and delayed the development of nisin resistance. The synergistic action of nisin and CO2 provides further support for a membrane fluidity model of interaction between nisin and the cytoplasmic membrane of a target cell. Several lines of evidence obtained from the present study suggest that membrane fluidity modulation in L. monocytogenes may underlie membrane poration by nisin. There is now a strong correlation between membrane fluidity and nisin sensitivity. The hypothesis that the observed changes in CF efflux (functional differences) are actually caused by changes in fluidity (difference in the physical state of membrane) is under investigation.
ACKNOWLEDGMENTS
We gratefully acknowledge Richard Ludescher for helpful discussion and comments, Karen Schaich for use of the spectrophotometer, and Oscar Kuipers for providing us with L. lactis NZ 9000 and plasmid pNZ8008.
This work was supported by the Danish Food Technology (FØTEK) program, the U.S. Department of Agriculture CSRS NRI Food Safety Program (grant no. 94-37201-0994), and other state and federal support provided by the New Jersey Agricultural Experiment Station.
REFERENCES
- 1.Abee T, Rombouts F M, Hugenholtz J, Guihard G, Letellier L. Mode of action of nisin Z against Listeria monocytogenes Scott A grown at high and low temperatures. Appl Environ Microbiol. 1994;60:1962–1968. doi: 10.1128/aem.60.6.1962-1968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 3.Bruno M E C, Kaiser A, Montville T J. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992;58:2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Shapira R, Eisenstein M, Montville T J. Functional characterization of pediocin PA-1 binding to liposomes in the absence of a protein receptor and its relationship to a predicted tertiary structure. Appl Environ Microbiol. 1997;63:524–531. doi: 10.1128/aem.63.2.524-531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crandall A D, Montville T J. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl Environ Microbiol. 1998;64:231–237. doi: 10.1128/aem.64.1.231-237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels J A, Krishnamurthi R, Rizvi S S H. A review of effects of carbon dioxide on microbial growth and food quality. J Food Prot. 1985;48:532–537. doi: 10.4315/0362-028X-48.6.532. [DOI] [PubMed] [Google Scholar]
- 7.de Ruyter P G G A, Kuipers O P, Beerthuyzen M M, van Alen-Boerrigter A, de Vos W M. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol. 1996;178:3434–3439. doi: 10.1128/jb.178.12.3434-3439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon N M, Kell D B. The inhibition by CO2 of the growth and metabolism of micro-organisms. J Appl Bacteriol. 1989;67:109–136. doi: 10.1111/j.1365-2672.1989.tb03387.x. [DOI] [PubMed] [Google Scholar]
- 9.Farber J M. Microbiological aspects of modified-atmosphere packaging technology—a review. J Food Prot. 1991;54:58–70. doi: 10.4315/0362-028X-54.1.58. [DOI] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. Nisin preparation: affirmation of GRAS status as a direct human food ingredient. Fed Regist. 1988;53:11247–11251. [Google Scholar]
- 11.Goulet V, Jacquet C, Vaillant V, Rebiere I, Mouret E, Lorente C, Maillot E, Stäiner F, Rocourt J. Listeriosis from consumption of raw-milk cheese. Lancet. 1995;345:1581–1582. doi: 10.1016/s0140-6736(95)91135-9. [DOI] [PubMed] [Google Scholar]
- 12.Guihard G, Benedetti H, Besnard M, Letellier L. Phosphate efflux through the channels formed by colicins and phage T5 in Escherichia coli cells is responsible for the fall in cytoplasmic ATP. J Biol Chem. 1993;268:17775–17780. [PubMed] [Google Scholar]
- 13.Harris L J, Flemming H P, Klaenhammer T R. Sensitivity and resistance of Listeria monocytogenes ATCC 19115, Scott A, and UAL500 to nisin. J Food Prot. 1991;54:836–840. doi: 10.4315/0362-028X-54.11.836. [DOI] [PubMed] [Google Scholar]
- 14.Holo H, Nes J F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzapfel W H, Geisen R, Schillinger U. Review paper. Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes. Int J Food Microbiol. 1995;24:343–362. doi: 10.1016/0168-1605(94)00036-6. [DOI] [PubMed] [Google Scholar]
- 16.Mazzotta A S, Montville T J. Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10°C and 30°C. J Appl Microbiol. 1997;82:32–38. doi: 10.1111/j.1365-2672.1997.tb03294.x. [DOI] [PubMed] [Google Scholar]
- 17.Ming X, Daeschel M A. Nisin resistance of foodborne bacteria and the specific resistance responses of Listeria monocytogenes Scott A. J Food Prot. 1993;56:944–948. doi: 10.4315/0362-028X-56.11.944. [DOI] [PubMed] [Google Scholar]
- 18.Moll G N, Clark J, Chan W C, Bycroft B W, Roberts G C K, Konings W N, Driessen A J M. Role of transmembrane pH gradient and membrane binding in nisin pore formation. J Bacteriol. 1997;179:135–140. doi: 10.1128/jb.179.1.135-140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.New R R C. Characterization of liposomes. In: New R R C, editor. Liposomes: a practical approach. Oxford, England: IRL Press; 1992. pp. 105–162. [Google Scholar]
- 20.Nilsson L, Huss H H, Gram L. Inhibition of Listeria monocytogenes on cold-smoked salmon by nisin and carbon dioxide atmosphere. Int J Food Microbiol. 1997;38:217–227. doi: 10.1016/s0168-1605(97)00111-6. [DOI] [PubMed] [Google Scholar]
- 21.Razavilar V, Genigeorgis C. Interactive effect of temperature, atmosphere and storage time on the probability of colony formation on blood agar by four Listeria species. J Food Prot. 1992;55:88–92. doi: 10.4315/0362-028X-55.2.88. [DOI] [PubMed] [Google Scholar]
- 22.Tan K H, Gill C O. Physiological basis of CO2 inhibition of a meat spoilage bacterium, Pseudomonas fluorescens. Meat Sci. 1982;7:9–17. doi: 10.1016/0309-1740(82)90093-6. [DOI] [PubMed] [Google Scholar]
- 23.Tao L, Tanzer J M, MacAlister T J. Bicarbonate and potassium regulation of the shape of Streptococcus mutans NCTC 10449S. J Bacteriol. 1987;169:2543–2547. doi: 10.1128/jb.169.6.2543-2547.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teixeira De Mattos M J, Plomp P J A M, Neijssel O M, Tempest D W. Influence of metabolic end-products on the growth efficiency of Klebsiella aerogenes in anaerobic chemostat culture. Antonie Leeuwenhoek. 1984;50:461–472. doi: 10.1007/BF02386220. [DOI] [PubMed] [Google Scholar]
- 25.Winkowski K, Ludescher R D, Montville T J. Physiochemical characterization of the nisin-membrane interaction with liposomes derived from Listeria monocytogenes. Appl Environ Microbiol. 1996;62:323–327. doi: 10.1128/aem.62.2.323-327.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]