Abstract
Objective
To estimate the change in odds of covid-19 over time following primary series completion of the inactivated whole virus vaccine CoronaVac (Sinovac Biotech) in São Paulo State, Brazil.
Design
Test negative case-control study.
Setting
Community testing for covid-19 in São Paulo State, Brazil.
Participants
Adults aged ≥18 years who were residents of São Paulo state, had received two doses of CoronaVac, did not have a laboratory confirmed SARS-CoV-2 infection before vaccination, and underwent reverse transcription polymerase chain reaction (RT-PCR) testing for SARS-CoV-2 from 17 January to 14 December 2021. Cases were matched to test negative controls by age (in 5 year bands), municipality of residence, healthcare worker status, and epidemiological week of RT-PCR test.
Main outcome measures
RT-PCR confirmed symptomatic covid-19 and associated hospital admissions and deaths. Conditional logistic regression was adjusted for sex, number of covid-19 associated comorbidities, race, and previous acute respiratory illness.
Results
From 202 741 eligible people, 52 170 cases with symptomatic covid-19 and 69 115 test negative controls with covid-19 symptoms were formed into 43 257 matched sets. Adjusted odds ratios of symptomatic covid-19 increased with time since completion of the vaccination series. The increase in odds was greater in younger people and among healthcare workers, although sensitivity analyses suggested that this was in part due to bias. In addition, the adjusted odds ratios of covid-19 related hospital admission or death significantly increased with time compared with the odds 14-41 days after series completion: from 1.25 (95% confidence interval 1.04 to 1.51) at 70-97 days up to 1.94 (1.41 to 2.67) from 182 days onwards.
Conclusions
Significant increases in the risk of moderate and severe covid-19 outcomes occurred three months after primary vaccination with CoronaVac among people aged 65 and older. These findings provide supportive evidence for the implementation of vaccine boosters in these populations who received this inactivated vaccine. Studies of waning should include analyses designed to uncover common biases.
Introduction
Since the authorization and licensing of the first covid-19 vaccines in late 2020, more than eight billion doses have been distributed worldwide,1 of which nearly two billion have been CoronaVac, an inactivated whole virus vaccine produced by the Chinese pharmaceutical company Sinovac.2 A key driver of future dynamics of covid-19, as well as public health policy, will be the durability of vaccine protection against infection and severe disease.
Following large scale vaccination campaigns in higher income countries, observational studies have found evidence for waning effectiveness of several covid-19 vaccines against infection, symptomatic covid-19, and severe covid-19 associated outcomes.3 4 5 6 7 8 9 10 However, the smaller evidence base on the effectiveness over time of inactivated vaccines, such as CoronaVac,11 12 presents an urgent need given the reliance on these vaccines in dozens of lower and middle income countries in South and Central America, Africa, and Asia. Inactivated vaccines elicit lower neutralizing antibody responses than do other vaccine platforms in the short and long term, reaching almost undetectable levels six months after completion of a two dose series.13 Observational studies of waning effectiveness may be subject to multiple sources of bias, including the differential build-up of immunity among unvaccinated people and association between time of vaccination and risk of covid-19 in risk prioritized vaccination campaigns.14 These biases may result in overestimation of waning.
In this study, we used a well characterized, linked surveillance database in São Paulo State, Brazil,15 16 17 to conduct a matched test negative case-control study among vaccinated people, with the aim of estimating a change in vaccine effectiveness over time following completion of the two dose schedule of CoronaVac.
Methods
Study setting
The study setting and design have been described in detail elsewhere.15 16 17 We assembled individual level data on demographic and clinical characteristics, SARS-CoV-2 testing, and covid-19 vaccination from four databases: the São Paulo State laboratory testing registry (GAL), national surveillance databases covering acute respiratory illness (e-SUS) and severe acute respiratory illness (SIVEP-Gripe), and the São Paulo State vaccine registry (Vacina Já) covering all people vaccinated in São Paulo State. The surveillance databases include hospital admissions and all other health visits conducted through public and private health systems, and notification of SARS-CoV-2 test results and suspected covid-19 cases, hospital admissions, and deaths is compulsory. We retrieved information from these databases on 10 February 2022. The STROBE checklist is shown in supplementary table A.
Study population and design
We conducted a test negative case-control study, including people with respiratory illness who received a reverse transcription polymerase chain reaction (RT-PCR) test for SARS-CoV-2.18 19 20 The study population consisted of adults ≥18 years old who had a residential address in São Paulo State and had complete and consistent information between data sources on age, sex, residence, and vaccination and testing status and dates. Eligible cases (or controls) had a symptomatic illness, received a positive (or negative) RT-PCR test for SARS-CoV-2 during the study period of 17 January 2021 to 14 December 2021 with a sample collection date within 10 days after symptom onset, received two doses of CoronaVac before sample collection, and did not have a positive RT-PCR test for SARS-CoV-2 before vaccination. Each person could contribute up to four different negative tests as controls and could contribute as both controls and cases.
To control for predictors of the timing of vaccination and changes in infection risk and variant circulation over time and space, we matched cases to controls by week of RT-PCR testing, age (in 5 year bands), municipality of residence, and vaccination priority group (healthcare worker versus non-healthcare worker, which included older people, teachers, general population, etc). Details of the matching process are given in the supplementary methods.
Outcomes and covariates
We estimated the association between time from receipt of the second dose of CoronaVac vaccine to date of sample collection for RT-PCR and the odds of testing positive for SARS-CoV-2. We categorized this time in the following intervals: 0-13, 14-41 (selected as the reference group, when IgG seropositivity peaks21), 42-69, 70-97, 98-125, 126-153, 154-181, and ≥182 days. In this design, the odds ratio can estimate the relative risk for later compared with earlier vaccine recipients if transmission between the earliest and latest vaccination dates is limited, meaning that an odds ratio greater than one can be interpreted as evidence of lower effectiveness than during the reference period.14 22 In this context, when vaccination was conducted during a large epidemic, odds ratios will likely overestimate waning owing to accrual of immunity before vaccination in later vaccinees.
In addition, we estimated the association between days from second dose of vaccine until RT-PCR sample collection and the odds of severe covid-19 (covid-19 associated hospital admission or covid-19 associated death and covid-19 associated death only). To do these analysis, we fitted the primary model to cases who had the outcome of interest and their matched controls.16 Controls represented people with negative RT-PCR tests with a range of severity but were representative of the population with access to RT-PCR testing.23 We did analyses within subgroups defined by age (18-39, 40-64, 65-79, and ≥80 years) and by priority status (healthcare worker versus non-healthcare worker). As misclassification of healthcare workers over the age of 65 was possible, we restricted analysis of healthcare workers to people aged under 65 years.
We accounted for the following covariates as potential confounders: age as a linear term, sex, self-reported skin color (pardo, preta, branca, amarela, and indigena24), previous acute respiratory illness (defined as at least one previous symptomatic event that was reported to surveillance systems between 1 February 2020 and 16 January 2021), and number of covid-19 associated comorbidities documented at the time of the RT-PCR test (cardiovascular, renal, neurologic, hematologic, or hepatic comorbidities, diabetes, chronic respiratory disorder, obesity, or immunosuppression; categorized as none, one to two, and three or more).
Statistical analysis
We used conditional logistic regression to estimate the association between days since vaccination and each outcome, accounting for the matched design and including potential confounders as additional covariates. We included a missing indicator for self-reported race, as it was likely missing at random. To determine whether evidence existed for interactions between age, healthcare worker status, and time since vaccination, we fitted an interaction between time since vaccination and age categories and then added an interaction with healthcare worker status, assessing the significance of each interaction with a likelihood ratio test (P<0.05). We used R, version 4.1.2, for all data processing and analyses.
Sensitivity analyses
The test negative case-control design can have several biases, which could lead to spurious conclusions of waning effectiveness. Therefore, we did two sensitivity analyses to detect bias.
Even after adjustment for age and priority status, people vaccinated earlier may be at higher risk of RT-PCR confirmed covid-19. This would lead to apparent waning as “early adopters,” at increased risk of SARS-CoV-2 infection, would be over-represented in later time periods since vaccination. To identify differences in risk by calendar time of vaccination, we did a sensitivity analysis restricting the study population to people who received an RT-PCR test within 14-90 days of receipt of their second dose, a period in which less waning is expected. We did the primary analysis with month of vaccination as the exposure variable. If changes in effectiveness were driven by “early adopters,” we would expect to observe elevated odds ratios in earlier months relative to later months. On the other hand, if changes in effectiveness were due to waning, we should observe little difference in odds of covid-19 by month of vaccination. We did the analyses by healthcare worker status subgroup.
Although we excluded people with a positive RT-PCR test before vaccination, many infections will have been acquired without receipt of such a test. People vaccinated later may therefore have acquired infection and be less susceptible to reinfection, which could induce apparent waning. To reduce this depletion of susceptible people during the time period of vaccination among healthcare workers, we did the primary analysis on a population with an additional eligibility criterion that they should be healthcare workers who received the second dose in February 2021. See supplementary methods for details of other sensitivity analyses.
Public and patient involvement
Members of the public or patients were not directly involved in setting the design of the study as this study used routine surveillance data sources. Nevertheless, the decision about outcomes and the interpretation of the results received inputs from the public community. No members of the public and patients were involved in writing up the results.
Results
Study setting
The second wave of covid-19 in São Paulo State peaked in March 2021, with substantial declines in transmission from June 2021. This decline is reflected in the decreased proportion of RT-PCR tests that were positive over time in the study population (supplementary figure A).
Study population
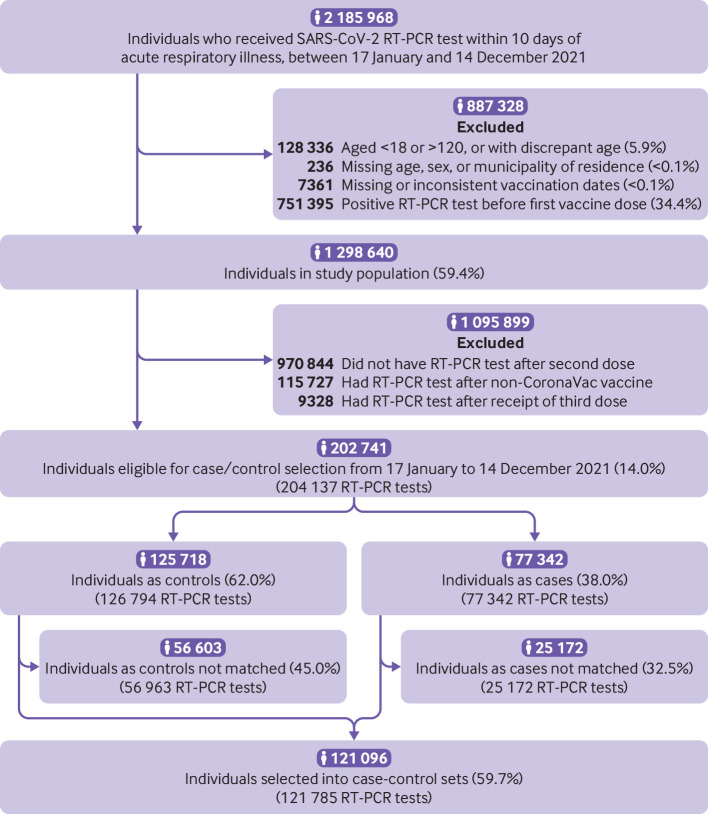
Among 202 741 people with 204 137 RT-PCR tests eligible for selection as a case or control, 121 785 RT-PCR tests from 121 096 people were matched into 43 257 case-control sets for the primary analysis. In the study population, 1367 people were tested multiple times, with no individual receiving more than four RT-PCR tests. A total of 487 people contributed multiple negative tests, and 187 people contributed a negative test and a positive test to the analysis population. Figure 1 shows the flowchart of enrollment, and figure 2 shows the timing of vaccination by age group and healthcare worker status. Table 1 shows demographic and clinical characteristics for matched cases and controls. Most matched sets with discordant cases and controls (that is, with different time category since completion of primary series) were vaccinated close in time (supplementary table B).
Fig 1.
Flowchart of study population and case and control selection. RT-PCR=reverse transcription polymerase chain reaction
Fig 2.
Distribution of second dose timing by age group in matched cases and controls, stacked by healthcare worker (HCW) status
Table 1.
Demographic and clinical characteristics of matched cases and controls. Values are numbers (percentages) unless stated otherwise
| Characteristics | Controls (n=69 615) | Cases (n=52 170) |
|---|---|---|
| Mean (SD) age, years | 52.01 (20.68) | 56.24 (20.23) |
| Age group, years: | ||
| 18-39 | 26 463 (38.0) | 15 074 (28.9) |
| 40-64 | 15 946 (22.9) | 12 391 (23.8) |
| 65-79 | 22 579 (32.4) | 20 007 (38.3) |
| ≥80 | 4627 (6.6) | 4698 (9.0) |
| Male sex | 25 275 (36.3) | 20 656 (39.6) |
| Race: | ||
| Branca | 36 047 (51.8) | 28 768 (55.1) |
| Pardo | 14 791 (21.2) | 8628 (16.5) |
| Preta | 3221 (4.6) | 1904 (3.6) |
| Yellow | 662 (1.0) | 524 (1.0) |
| Indigena | 12 (0.0) | 14 (0.0) |
| Missing | 14 882 (21.4) | 12 332 (23.6) |
| Healthcare worker | 17 569 (25.2) | 15 639 (30.0) |
| No of comorbidities: | ||
| 0 | 55 125 (79.2) | 37 555 (72.0) |
| 1-2 | 13 571 (19.5) | 13 396 (25.7) |
| ≥3 | 919 (1.3) | 1219 (2.3) |
| ≥1 previous acute respiratory infection | 1852 (2.7) | 718 (1.4) |
| Days since second dose: | ||
| 0-13 | 6470 (9.3) | 5614 (10.8) |
| 14-41 | 14 797 (21.2) | 9628 (18.5) |
| 42-69 | 12 640 (18.1) | 9342 (17.9) |
| 70-97 | 11 142 (16.0) | 8159 (15.6) |
| 98-125 | 9405 (13.5) | 7049 (13.5) |
| 126-153 | 6418 (9.2) | 4969 (9.5) |
| 154-181 | 4762 (6.8) | 3877 (7.4) |
| ≥182 | 3981 (5.7) | 3532 (6.8) |
| Mean (SD) interdose interval, days | 25.88 (7.37) | 24.83 (6.02) |
| Mean (SD) interval from second dose to PCR test, days | 80.76 (56.10) | 83.23 (57.85) |
| Mean (SD) interval from symptom onset to PCR test, days | 3.12 (2.20) | 3.78 (2.44) |
| Admitted to hospital | 6862 (9.9) | 10 345 (19.8) |
| Died | 2106 (3.0) | 4327 (8.3) |
PCR=polymerase chain reaction; SD=standard deviation.
Change in odds of covid-19 over time since vaccination
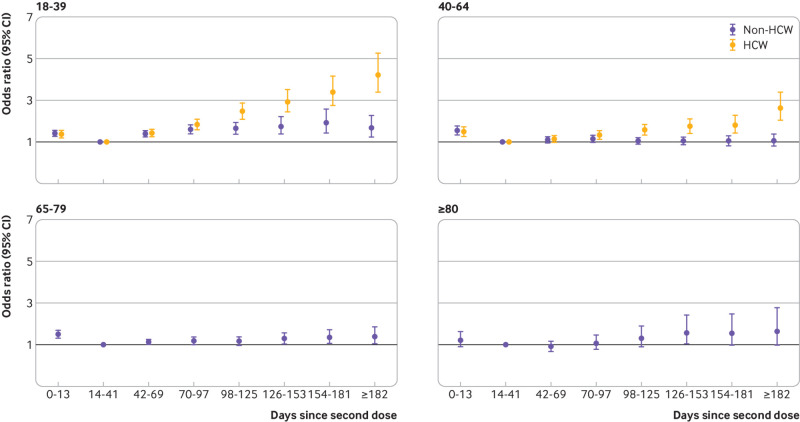
The odds ratio of symptomatic covid-19 increased with time from vaccination, the pattern of which varied by age group (P<0.001) and healthcare worker status (P<0.001) (fig 3; supplementary table C). Among people aged ≥80 years, those vaccinated 126-153 days before their test date had increased odds of covid-19 relative to those vaccinated 14-41 days before (odds ratio 1.58, 95% confidence interval 1.04 to 2.42), and the odds ratio was similar at later times. Overall, the odds ratio of covid-19 associated with time since vaccination was of lower magnitude among 65-79 year olds, with a maximum odds ratio of 1.40 (1.05 to 1.86), and we saw no increase in odds over time among 40-64 year old non-healthcare workers. Among 18-39 year old non-healthcare workers, significantly increased odds of covid-19 infection began at 42-69 days (odds ratio 1.38, 1.24 to 1.54), increasing to 1.92 (1.43 to 2.58) at 154-181 days.
Fig 3.
Odds ratio of symptomatic polymerase chain reaction confirmed covid-19 disease against days since vaccination, relative to 14-41 days from vaccination, by age group and healthcare worker (HCW) status
Among healthcare workers, increased odds of covid-19 infection began at 42-69 days among those aged 18-39 years (odds ratio 1.42, 1.24 to 1.61) and at 70-97 days among 40-64 year olds (1.31, 1.12 to 1.53), and the odds ratio increased with time since vaccination (odds ratio from 182 days: 4.22 (3.39 to 5.26) among 18-39 year old healthcare workers; 2.62 (2.03 to 3.38) among 40-64 year old healthcare workers). The pattern was similar for 18-39 year old and 40-64 year old healthcare workers, with greater increases among the younger age group. For both age groups, the increase in odds of covid-19 over time was greater among healthcare workers than non-healthcare workers (supplementary table C).
In all groups except those aged ≥80 years, people receiving their second dose between zero and 13 days before the RT-PCR test had significantly increased odds of covid-19, indicating that vaccine effectiveness within 13 days of the second dose was reduced. For those aged ≥80 years, the odds were not significantly increased (odds ratio 1.22, 0.91 to 1.63), possibly owing to lack of power in this age group.
Change in odds of severe covid-19 outcomes over time since vaccination
Longer time from second dose was associated with increased odds of covid-19 related hospital admission or death, with a significant increase observed starting at 70-97 days (odds ratio 1.25, 1.04 to 1.51) (fig 4; supplementary table D), increasing to 1.94 (1.41 to 2.67) after 182 days.
Fig 4.
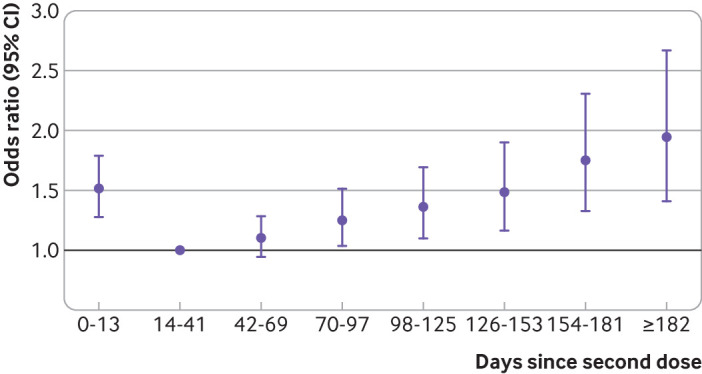
Odds ratio of polymerase chain reaction confirmed covid-19 related hospital admission or death for days since vaccination, relative to 14-41 days from vaccination
Exploring sources of bias
We estimated the association between month of vaccination and covid-19 among recent vaccinees (sensitivity analysis 1, supplementary figure B). Among non-healthcare workers, the odds of covid-19 was significantly lower among people vaccinated in May and from September to November, compared with April (odds ratios ranging from 0.31 to 0.83), but those vaccinated in February and March were at otherwise similar risk. On the other hand, early adopters among healthcare workers seemed to be at significantly higher risk of covid-19 than later adopters (for example, odds ratio comparing healthcare workers vaccinated in February with those vaccinated in April: 1.65, 1.36 to 2.01).
Restricting the analysis to healthcare workers vaccinated in February (sensitivity analysis 2) led to a pattern of waning that was later in onset among 40-64 year olds and lower in magnitude among 18-39 year olds (supplementary figure C), albeit with lower precision. These analyses suggest that the waning observed in the primary analysis may be overestimated among healthcare workers in particular owing to depletion of susceptible healthcare workers between February and April, when most of them were vaccinated (fig 2).
Vaccine effectiveness
We observed similar patterns of waning in the estimates of vaccine effectiveness against symptomatic covid-19 (supplementary table E) and against covid-19 related hospital admission or death (supplementary table F). We note in particular that vaccine effectiveness after 182 days was negative among 18-39 year olds and 40-64 year olds, likely owing to healthcare workers being over-represented in these later time periods. Consequently, we observed a greater decrease in vaccine effectiveness over time in these age groups, compared with 65-79 and ≥80 year olds, consistent with our primary analysis. Finally, we saw a smaller decrease in vaccine effectiveness against severe disease, which was more similar across age groups. Other sensitivity analyses results returned similar results (supplementary figures D-H).
Discussion
In this study, we found evidence of an increase in covid-19 disease and covid-19 related hospital admission over time since vaccination in people receiving two doses of CoronaVac in São Paulo State, Brazil, between February and December 2021. We observed a greater increase in the risk of covid-19 over time since vaccination in younger people and among healthcare workers, which is likely because of bias due to early vaccine recipients becoming infected and gaining immunity.
Given the results of our sensitivity analyses, our results could be interpreted as an upper bound on the degree of waning. For example, an odds ratio of 1.40 at ≥182 days, as observed among 65-79 year olds, would translate to a maximum drop in vaccine effectiveness from 50% to 30% or from 40% to 16%. Similarly, an odds ratio of 1.94 at ≥182 days, as observed for covid-19 related hospital admission or death, would translate to a maximum drop in vaccine effectiveness from 70% to 42% or from 60% to 22%.
Possible explanations for findings
The difference in magnitude of apparent waning by age (fig 3), with waning in all groups except those aged 40-64 years, has several potential explanations. Immune response to vaccination is lower in older people,25 26 particularly those above 80, which could lead to earlier waning when a protective threshold is crossed. Differences in the apparent magnitude could also be explained by differences in initial effectiveness, with greater waning among younger people for whom initial vaccine effectiveness was likely higher.16 Behavioral changes following vaccination, in particular relaxing of other risk mitigation practices or likelihood of getting tested on symptomatic illness, may occur in younger people. Finally, if exposures experienced by healthcare workers are generally associated with higher viral loads, such exposures could cause breakthrough infections earlier than low viral load exposures, by overcoming a higher concentration of circulating antibodies.27
The stark difference in patterns according to age and healthcare worker status should raise some concern about validity, and our sensitivity analyses uncovered potential overestimation of waning among the healthcare workers. We found suggestive evidence that healthcare workers vaccinated earlier were at higher risk of covid-19 and that infection of healthcare workers before vaccination may have led to overestimation of waning in this group. Our estimates in the older population, who also contributed most to the estimates of waning against severe disease, are likely less affected by bias owing to lower incidence in these age groups, as suggested by serologic data,28 and better adherence to risk mitigation, as observed in other contexts.29 30
Implications of findings
With these caveats in mind, these observed increases in covid-19 over time indicate a waning of vaccine effectiveness against moderate and severe outcomes in older age groups. Although the vaccine effectiveness in later time periods is uncertain, such a finding has important implications globally. Most doses of CoronaVac have been given to people in lower and middle income countries, and several countries, including Indonesia,31 Pakistan,32 and Hong Kong,33 continue to recommend a primary series of CoronaVac. Current World Health Organization recommendations are for an extended primary series of CoronaVac for older and immunocompromised people only,37 and our analysis suggests that a booster dose, if effective, would also benefit people aged ≥65 years who have received a two dose series. Additional analyses are needed to determine the effectiveness of heterologous and homologous booster doses by time of administration. For individual patients, our results can provide doctors with evidence to encourage maintenance of other risk mitigation practices among vaccinated people.
These findings will likely remain relevant in the context of the omicron variant, as waning of effectiveness independent of changing variant prevalence will continue to dictate epidemic dynamics and guide vaccination policy. An assumption that inactivated vaccines will remain a key tool even as new variants emerge is reasonable, so gathering evidence for the effectiveness of inactivated vaccines in these contexts is critical.
Other observational studies, which can all be affected by depletion of susceptibles bias, have found evidence of waning effectiveness across age groups.5 7 9 Mizrahi and colleagues found an increased incidence rate of breakthrough infections among early vaccinees compared with late vaccinees, concluding that this provided evidence of waning.4 A similar case-control study conducted in Israel among people who received two doses of BNT162b2 (Pfizer-BioNTech) found a greater than twofold increase in odds of covid-19 starting at 90 days following BNT162b2 series completion, with the largest increases in the 40-59 year age group.8
Strengths and limitations of study
A major strength of this study is our ability to distinguish people vaccinated as healthcare workers from those vaccinated under some other priority group. Given the strong associations between healthcare worker status, age, vaccine timing, and risk of SARS-CoV-2 infection, studies of waning effectiveness must account for the presence of healthcare workers. Previous studies have done so by restricting to healthcare workers,6 adjusting for healthcare worker status,7 or using frequency of previous PCR testing as a proxy for occupational exposure,34 whereas other observational studies of vaccine effectiveness have excluded healthcare workers.35 36 Current and future studies of vaccine effectiveness must be designed to overcome bias introduced by healthcare workers and other people at high risk who are prioritized for vaccination, and preferably to examine patterns within risk groups.
Our study has several limitations. We did not have data on the SARS-CoV-2 variant for each case included in the study. Therefore, some of the patterns observed may result from changes in the dominant variant from gamma to delta. Our sensitivity analysis including only the period dominated by the delta variant was underpowered but also found evidence of waning. The analysis of hospital admission or death used test negative controls from all settings, who may have differential access to healthcare compared with cases needing hospital admission; thus, changes in the relation between healthcare access and vaccination over time may have led to bias. We evaluated healthcare workers separately as we suspected that they might have different exposure risks compared with non-healthcare workers; we could not account for potential changes in use of personal protective equipment or other risk mitigation factors over time, or attribute risk to healthcare versus other settings. Our sensitivity analyses suggested bias but were unable to fully correct for it. For example, we found evidence suggesting that healthcare workers vaccinated earlier were at higher risk of covid-19. However, waning of humoral immune response has been observed on this timescale,21 so the extent to which increased risk of SARS-CoV-2 infection among early vaccine recipients contributes to apparent waning remains unclear. Finally, even within non-healthcare workers, CoronaVac was available only to certain high risk groups before May, leading to over-representation of people at higher risk and increased odds of covid-19 in later time periods.
Conclusion
We have provided evidence that moderate and severe covid-19 outcomes increased over time following completion of a primary series of CoronaVac in older people, and we have suggested sensitivity analyses that could be conducted to understand bias in observational studies such as this.
What is already known on this topic
The effectiveness of the inactivated whole virus vaccine CoronaVac (Sinovac Biotech) against moderate and severe covid-19 has been shown in clinical trials and observational studies
The effectiveness of other covid-19 vaccines decreases over time, prompting many countries to deploy additional doses for people who have completed their primary series
Evidence for change in the rate of breakthrough infection in people who have received a primary series of CoronaVac is limited
What this study adds
In people receiving two doses of CoronaVac, the odds of symptomatic covid-19 increased over time since completion of the primary vaccination series
Odds of covid-19 related hospital admission or death increased over time since series completion, but to a lesser extent
Vaccine effectiveness of CoronaVac seems to decline starting at three months following vaccination in people aged 65 and older
Acknowledgments
We are grateful for the support of the Pan American Health Organization and the São Paulo State in making the databases available for analysis.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: All authors conceived the study. MDTH completed analyses with guidance from OTR, NED, JRA, DATC, AIK, and JC. MSST, OFPP, OTR, and MDTH curated and validated the data. MDTH wrote the first draft of the manuscript. TLD, RCP, OFPP, EFMV, MA, RS, JCG, and WNA provided supervision. MLL and MD edited and reviewed the manuscript. All authors contributed to and approved the final manuscript. JC is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by grant R01-AI139761 from the National Institute for Allergy and Infectious Diseases to NED and the Sendas Family and Beatrice Kleinberg Neuwirth Funds to AIK. JC is supported by the Oswaldo Cruz Foundation (Edital Covid-19 – resposta rápida: 48111668950485). OTR is funded by a Sara Borrell fellowship (CD19/00110) from the Instituto de Salud Carlos III. OTR acknowledges support from the Spanish Ministry of Science and Innovation through the Centro de Excelencia Severo Ochoa 2019-2023 Program and from the Generalitat de Catalunya through the CERCA Program. All funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The Health Secretary of State of São Paulo and PRODESP reviewed the data and findings of the study, but the academic authors retained editorial control. OTR, MDTH, and JC had full access to de-identified data in the study, and OTR and MDTH verified the data; all authors approved the final version of the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Institute for Allergy and Infectious Diseases and from the Sendas Family and Beatrice Kleinberg Neuwirth Funds; MDTH and DATC report a contract from Merck (to the University of Florida) for research unrelated to this manuscript; no other relationships or activities that could appear to have influenced the submitted work.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Results will be disseminated to the public in São Paulo and across Brazil. Dissemination of results to people who were selected into the study is not possible owing to anonymisation of the data.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study was approved by the Ethical Committee for Research of Federal University of Mato Grosso do Sul (CAAE: 43289221.5.0000.0021). The free and informed consent form was waived because the study involved de-identified surveillance datasets.
Data availability statement
Deidentified databases as well as the R codes will be deposited in the repository https://github.com/juliocroda/VebraCOVID-19.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/.
- 2.Mallapaty S. China’s COVID vaccines have been crucial — now immunity is waning. 2021. https://www.nature.com/articles/d41586-021-02796-w. [DOI] [PubMed]
- 3. Goldberg Y, Mandel M, Bar-On YM, et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med 2021;385:e85. 10.1056/NEJMoa2114228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 2021;12:6379. 10.1038/s41467-021-26672-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021;398:1407-16. 10.1016/S0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pilishvili T, Gierke R, Fleming-Dutra KE, et al. Vaccine Effectiveness among Healthcare Personnel Study Team . Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. N Engl J Med 2021;385:e90. 10.1056/NEJMoa2106599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med 2021;385:e83. 10.1056/NEJMoa2114114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ 2021;375:e067873. 10.1136/bmj-2021-067873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2022;375:331-6. 10.1126/science.abm0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katikireddi SV, Cerqueira-Silva T, Vasileiou E, et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet 2022;399:25-35. 10.1016/S0140-6736(21)02754-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cerqueira-Silva T, Katikireddi SV, de Araujo Oliveira V, et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med 2022;28:838-43. 10.1038/s41591-022-01701-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suah JL, Husin M, Tok PSK, et al. Waning COVID-19 Vaccine Effectiveness for BNT162b2 and CoronaVac in Malaysia: An Observational Study. Int J Infect Dis 2022;119:69-76. 10.1016/j.ijid.2022.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan H, Wu Q, Zeng G, et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18-59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. medRxiv [Preprint] 2021. 10.1101/2021.07.23.21261026 [DOI]
- 14. Lipsitch M, Goldstein E, Ray GT, Fireman B. Depletion-of-susceptibles bias in influenza vaccine waning studies: how to ensure robust results. Epidemiol Infect 2019;147:e306. 10.1017/S0950268819001961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hitchings MDT, Ranzani OT, Torres MSS, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: A test-negative case-control study. Lancet Reg Health Am 2021;1:100025. 10.1016/j.lana.2021.100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ 2021;374:n2015. 10.1136/bmj.n2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hitchings MDT, Ranzani OT, Dorion M, et al. Effectiveness of ChAdOx1 vaccine in older adults during SARS-CoV-2 Gamma variant circulation in São Paulo. Nat Commun 2021;12:6220. 10.1038/s41467-021-26459-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness. Am J Epidemiol 2016;184:345-53. 10.1093/aje/kww064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewnard JA, Tedijanto C, Cowling BJ, Lipsitch M. Measurement of vaccine direct effects under the test-negative design. Am J Epidemiol 2018;187:2686-97. 10.1093/aje/kwy163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lewnard JA, Patel MM, Jewell NP, et al. Theoretical Framework for Retrospective Studies of the Effectiveness of SARS-CoV-2 Vaccines. Epidemiology 2021;32:508-17. 10.1097/EDE.0000000000001366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sauré D, O’Ryan M, Torres JP, Zuniga M, Santelices E, Basso LJ. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis 2022;22:56-63. 10.1016/S1473-3099(21)00479-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ray GT, Lewis N, Klein NP, Daley MF, Lipsitch M, Fireman B. Depletion-of-susceptibles Bias in Analyses of Intra-season Waning of Influenza Vaccine Effectiveness. Clin Infect Dis 2020;70:1484-6. 10.1093/cid/ciz706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grimes DA, Schulz KF. Compared to what? Finding controls for case-control studies. Lancet 2005;365:1429-33. 10.1016/S0140-6736(05)66379-9 [DOI] [PubMed] [Google Scholar]
- 24.Brazilian Institute of Geography and Statistics - IBGE. Ethnic and racial characteristics of the population: classifications and identities. 2013. https://biblioteca.ibge.gov.br/visualizacao/livros/liv63405.pdf.
- 25. Levin EG, Lustig Y, Cohen C, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med 2021;385:e84. 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bichara CDA, Queiroz MAF, da Silva Graça Amoras E, et al. Assessment of Anti-SARS-CoV-2 Antibodies Post-Coronavac Vaccination in the Amazon Region of Brazil. Vaccines (Basel) 2021;9:1169. 10.3390/vaccines9101169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marc A, Kerioui M, Blanquart F, et al. Quantifying the relationship between SARS-CoV-2 viral load and infectiousness. Elife 2021;10:e69302. 10.7554/eLife.69302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moya Rios do Vale N, Roche Moreira Latini F, Prisco Arnoni C, et al. Increasing rate of anti-SARS-CoV-2 antibodies between the first and second waves of COVID-19 in São Paulo, Brazil: A cross-sectional blood donors-based study. Clinics (Sao Paulo) 2022;77:100016. 10.1016/j.clinsp.2022.100016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hutchins HJ, Wolff B, Leeb R, et al. COVID-19 Mitigation Behaviors by Age Group - United States, April-June 2020. MMWR Morb Mortal Wkly Rep 2020;69:1584-90. 10.15585/mmwr.mm6943e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Myerson J, Strube MJ, Green L, Hale S. Individual differences in COVID-19 mitigation behaviors: The roles of age, gender, psychological state, and financial status. PLoS One 2021;16:e0257658. 10.1371/journal.pone.0257658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakarta COVID-19 Response Team. Covid-19. https://corona.jakarta.go.id/en/kuota-vaksinasi-jaki.
- 32.Government of Pakistan. Guidelines and Standard Operating Procedures (SOPs): Sinovac Vaccine (CoronaVac). 2021. https://storage.covid.gov.pk/new_guidelines/14November2021_20211112_Guidelines_for_Sinovac_Vaccine_6305.pdf.
- 33.Government of the Hong Kong Special Administrative Region. Recommendations on use of COVID-19 vaccines. 2022. https://www.covidvaccine.gov.hk/en/faq.
- 34. Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet 2021;397:1646-57. 10.1016/S0140-6736(21)00677-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med 2021;384:1412-23. 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021;398:2093-100. 10.1016/S0140-6736(21)02249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac. 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-2021.1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
Deidentified databases as well as the R codes will be deposited in the repository https://github.com/juliocroda/VebraCOVID-19.