Abstract
Background
The probability of undergoing treatment with curative intent according to the hospital of diagnosis varies for esophagogastric cancer in the Netherlands. Little is known about the factors contributing to this variation. This study aimed to improve the understanding of the differences between the multidisciplinary team meeting treatment proposal and the treatment that was actually carried out and to qualitatively investigate the differences in treatment decision‐making after the multidisciplinary team meeting treatment proposal between hospitals.
Methods
To gain an in‐depth understanding of treatment decision‐making, quantitative data (i.e., multidisciplinary team meeting proposal and treatment that was carried out) were collected from the Netherlands Cancer Registry. Changes in the multidisciplinary team meeting proposal and applied treatment comprised changes in the type of treatment option (i.e., curative or palliative, or no change) and were calculated according to the multivariable multilevel probability of undergoing treatment with curative intent (low, middle, and high). Qualitative data were collected from eight hospitals, including observations of 26 outpatient clinic consultations, 30 in‐depth interviews with clinicians, seven focus groups with clinicians, and three focus groups with patients. Clinicians and patients' perspectives were assessed using thematic content analysis.
Results
The multidisciplinary team meeting proposal and applied treatment were concordant in 97% of the cases. Clinicians' implementation of treatment decision‐making in clinical practice varied, which was mentioned by the clinicians to be due to the clinician's personality and values. Differences between clinicians consisted of discussing all treatment options versus only the best fitting treatment option and the extent of discussing the benefits and harms. Most patients aimed to undergo curative treatment regardless of the consequences, since they believed this could prolong their life.
Conclusion
Since changes in the multidisciplinary team meeting‐proposed treatment and actual treatment were rarely observed, this study emphasizes the importance of an adequately formulated multidisciplinary team meeting proposal.
Keywords: clinicians' perspectives, esophageal and gastric cancer, multidisciplinary team meeting, patients' perspectives, treatment decision‐making
The probability of undergoing treatment with curative intent according to the hospital of diagnosis has been shown to vary considerably for esophagogastric cancer in the Netherlands. Little is known about the factors that contribute to this variation in practice. In this study, we aimed to improve our understanding of the differences between the multidisciplinary team meeting (MDTM) treatment proposal and the treatment that was actually carried out and to qualitatively investigate the differences in treatment decision‐making (TDM) after the MDTM treatment proposal between hospitals.

INTRODUCTION
In the Netherlands, almost 4200 patients are annually diagnosed with esophageal and gastric (esophagogastric) cancer, with over 3000 cancer‐related deaths per year. 1 Guidelines state that treatment with curative intent should be considered in patients without distant metastasis, depending on the patients' general health status and local extent of disease. The cornerstones of treatment with curative intent for nonmetastatic esophagogastric cancer and early‐stage tumors are resection with or without (neo)adjuvant chemo(radiation) therapy and endoscopic resection, respectively. 2 , 3 , 4 , 5 For patients with esophageal cancer, definitive chemoradiation is a reasonable alternative with curative intent for frail patients, patients with unresectable locally advanced cancer, or patients who refrain from surgery. 3 Nevertheless, the probability of undergoing treatment with curative intent has been shown to vary considerably depending on the hospital of diagnosis, with a significant effect on survival in 2010–2013 (hazard ratios of hospitals with a high probability of undergoing treatment with curative intent in esophageal cancer 1.15, 95% confidence interval [CI] 1.07–1.24 and gastric cancer 1.21, 95% CI 1.04–1.41). 6 , 7 , 8
Practice variation can only partially be explained by differences in patient‐ and tumor‐related factors. 6 , 7 , 8 Hence, other factors might explain variation in treatment decisions, such as organization of clinical pathways, the emphasis and subsequent advice of multidisciplinary team meetings, and treatment decision‐making during the outpatient clinic visit after the multidisciplinary team meeting, which encompasses a treatment decision‐making process between physicians and patients. 9 , 10 , 11 , 12 Thus, the best available evidence of possible outcomes should be shared with the patient, to guide the patient's considerations on treatment preferences. 13 Hence, one of the aspects that could attribute to practice variation may be a change in the multidisciplinary team meeting proposal caused by this process. Knowledge about these changes could shed light on whether variation in practice between hospitals occurs at the multidisciplinary team meeting level or more during the treatment decision‐making process thereafter in the outpatient clinic.
This study aimed to quantitatively investigate the compliance to the multidisciplinary team meeting proposal when compared to the treatment carried out and to gain insight into factors explaining these changes based on qualitative observations during outpatient clinic visits, interviews with clinicians, and focus groups with clinicians and patients.
METHODS
This study is part of a mixed methods multiple case study investigating causes of hospital practice variation in the curative treatment of esophagogastric cancer: the VARIATE‐project (Box 1 describes the study design of the VARIATE‐project). The current sub‐study focuses on identifying compliance with the multidisciplinary team meeting treatment proposal, as a result of the treatment decision‐making process during the outpatient clinic visit after the multidisciplinary team meeting combined with quantitative and qualitative data. Quantitative data were used to assess the differences between the multidisciplinary team meeting proposal and actual treatment carried out. Outpatient clinic observations, semi‐structured interviews, and focus groups with clinicians were used to gain insight into the clinicians' perspective on treatment decision‐making, and patient focus groups were organized to gain insight into the patients' perspectives on treatment decision‐making. Clinicians' perspectives included the physicians' convictions and values regarding treatment options, their beliefs and attitudes regarding treatment decision‐making, and the input of a patient's proxy. Patients' perspectives included the patients' experiences regarding treatment decision‐making during the outpatient clinic visit, reasons for selecting a specific treatment modality, their experiences with the discussions with their doctor, and their treatment experiences.
BOX 1.
All patients diagnosed with esophageal and gastric cancer in the Netherlands are registered in the Netherlands Cancer Registry (NCR). Previous multivariable multilevel analyses of potentially curable patients diagnosed in the period 2015–2017 have shown that the probability of receiving treatment with curative intent differed according to the hospital of diagnosis. 8 Hospitals were divided into three tertiles: low, middle, or high probability of undergoing treatment with curative intent. Patients diagnosed in a hospital with a high probability of receiving treatment with curative intent had a significant better long‐term survival. 8 In order to obtain in‐depth information and knowledge of the underlying mechanisms of hospital practice variation in proposing treatment with curative intent the VARIATE‐ study (VariAtion in the cuRatIve treatment of esophAgeal and gasTric cancEr) was developed, which was financed by the Dutch Cancer Society.
Received treatment with curative intent was defined as endoscopic or surgical resection, initiation of surgery (without resection), definitive chemoradiation (external beam radiotherapy and concurrent chemotherapy; including initiation of chemoradiation). Palliative treatment was defined as: palliative systemic therapy, palliative radiotherapy, and best supportive care.
Design:
The VARIATE study is a mixed methods multiple case study, which combines qualitative and quantitative research. A purposive sample 43 of eight cases (i.e., hospitals) participated. These hospitals were a representative sample of Dutch hospitals regarding the probability of offering treatment with curative intent (low, low, or middle for gastric or esophageal cancer, and high), hospital type, size, and geographical location.
Recruitment: Surgeons or medical oncologists from 11 different hospitals were invited by email. After interest was voiced, JL presented the study during the multidisciplinary team meeting (MDTM) of the eight interested hospital to assess the interest of the multidisciplinary team. All hospitals and team members who saw the presentation wished to participate in this study.
This study used an iterative approach for qualitative data collection and analyses, data collection consisted of:
Observations of (Upper‐GI specific) MDTMs (2–4 MDTMs per hospital) and outpatient clinic visits (minimum of two outpatient clinic visits per hospital)
Semi‐structured interviews (n = 30) with clinicians involved in the multidisciplinary care for esophageal and gastric cancer (i.e., surgeons (S, n = 8), medical oncologist (MO, n = 6), radiation oncologist (RO, n = 5), gastroenterologists (GE, n = 6), and case managers (CM, n = 5))
Focus groups with clinicians in order to validate and further enrich the results of their own hospital (n = 7).
Focus groups with patients diagnosed with potentially curable esophageal‐ or gastric cancer were organized to explore factors related to their treatment choices (n = 3: low, middle, and high probability hospital).
Based on the analysis of the first three hospitals the following decisions regarding the quantitative and qualitative data collection in the further hospitals were made:
Depending on the emerging topics from previous interviews the topic list was altered (more focus on: MDTMs, cases of doubt, shared decision‐making).
Clinicians in the other five hospitals were selected for interviewing through emergent sampling (i.e., gastroenterologist that did not treat early carcinomas were not invited for participation, recent new members in multidisciplinary teams were not invited for participation).
Additional quantitative data for potentially curable patients diagnosed in 2015–2017 was gathered in XX hospitals in the Netherlands (i.e., data were gathered by the NCR regarding diagnostics, the MDTM treatment proposal and outpatient clinic visits) in order to gain insight in clinical pathways and alterations in MDTM treatment proposal.
The VARIATE‐study focusses on the organization of clinical pathways and MDTMs and the outpatient clinic visit.
Analyses:
Qualitative analyses: Interviews were audio recorded, transcribed per verbatim and summarized (all by JL) and shared with the interviewed clinicians serving as member check. Next, the interviews were reviewed and coded, using open coding as described by Strauss and Corbin. 44 To minimize subjectivity the first 11 transcripts were independently coded by two researchers (JL, PV) and discussed until consensus was reached. 45 The remaining 19 transcripts were coded by JL. A summary was written for each interview and each hospital. Using thematic content analyses emerging themes were found. 18 Simultaneously, through a constant comparison across and within cases, relations were searched for and themes were identied. 19 The core study group (JL, PV, RV, GN) met weekly to discuss analyses, refine the codebook and identify emerging themes. The coding process was facilitated by Atlas ti 8 software.
Quantitative analyses: Quantitative data was analyzed according to the probability of receiving treatment with curative intent using SAS® version 9.4 (SAS Institute). A p‐value below 0.05 was considered statistically significant.
Data collection procedures
Quantitative research
Patients with potentially curable (cT1‐4a or cTx, any cN, cM0) esophagogastric cancer diagnosed between 2015 and 2017 were selected from the nationwide population‐based Netherlands Cancer Registry (NCR). Patients diagnosed with a gastroesophageal junction tumor were included in the esophageal cancer group. Information on patient characteristics, such as sex, age, modified Charlson Comorbidity Index score, and Eastern Cooperative Oncology Group (ECOG) performance status, and tumor characteristics, such as tumor location, histology, stage, and treatment, were collected from medical records by specially trained data managers of the NCR.
Additional data regarding the final multidisciplinary proposed treatment plan prior to the start of treatment were collected from 38 hospitals for patients with esophageal cancer and 68 hospitals for patients with gastric cancer. As the incidence rate of gastric cancer is lower than that of esophageal cancer in the Netherlands, more hospitals (n = 68) were included as a representative sample.
Differences between the proposed and actual treatments were assessed, and treatments with curative intent were defined as endoscopic resection (endoscopic mucosal resection or endoscopic submucosal dissection), surgical resection of the primary tumor (with or without [neo]adjuvant chemo[radio]therapy), or definitive chemoradiation (for esophageal cancer). Palliative treatment was defined as systemic therapy only, radiotherapy only, and best supportive care (e.g., stent placement).
Qualitative research: Observations, interviews, and focus groups
Eight hospitals were selected based on hospital type (academic resection hospital [n = 3], regional resection hospital [n = 4], and referring non‐resecting hospital [n = 1]), probability of offering treatment with curative intent (low [L] n = 2, low/middle [L/M] n = 2, and high probability [H], n = 4), 8 and size and geographical location in the Netherlands (see methods regarding hospital selection in Box 1), to achieve a deviant case sampling. 14 A detailed description regarding the calculation of probability of treatment with curative intent classification has been described previously.. 8
From January 2019 to November 2020, observations during multidisciplinary team meetings and outpatient clinic visits, interviews with clinicians, and focus groups (with clinicians and patients) were conducted. Data were collected and analyzed iteratively. All data were collected by a medical doctor (JL), who was trained to interview, organize focus groups, and analyze the data together with two experienced researchers in the field of qualitative research (LB, MW). Medical oncologists, surgeons, radiation oncologists, gastroenterologists, and case managers (e.g., nurse practitioners, physician assistants) involved in esophagogastric care were all observed and interviewed in the first three hospitals. After the iterative analyses of these three hospitals, emergent themes were discussed within the research team. Thereafter, clinicians in the other five hospitals were selected for interviewing through emergent sampling (see method section Box 1), which implies that sampling decisions were made during data collection as the study progressed. 15
Observations
Twenty‐six outpatient clinic visits in eight hospitals were observed in half‐day sessions (approximately 4 h), focusing on how clinicians and patients dealt with the multidisciplinary team meeting proposal, discussed treatment options, discussed benefits and harms, and clinician‐patient interaction. Field notes were kept during the observations and summarized at the end of each observation. Observations and informal conversations were helpful in building a relationship of trust (rapport) with the clinicians and were used as inputs for the interviews and focus groups.
Interviews
Semi‐structured interviews with clinicians were conducted using a topic list (Supplementary 1), based on the expertise of the research team and literature, such as the organization of healthcare and multidisciplinary team meetings 10 , 11 , 16 and physician attitudes toward treatment options. 17 During all interviews, the opportunity was provided to discuss topics not part of the topic list, exploring new themes that evolved during the interview. Broad topics (Supplementary 1) were discussed during the interviews conducted in the first three hospitals. During the course of this study and through iterative analyses, the topic list evolved, encompassing factors contributing to treatment decision‐making during the outpatient clinic visit, such as the physicians' convictions and values regarding treatment options, their beliefs and attitudes regarding shared decision‐making, and the input of a patient's proxy. These factors led to more focused interviews in the last five hospitals. Semi‐structured interviews were performed by one researcher (JL) with a mean duration of 39 (range 25–54) min. Interviews were audio‐recorded and transcribed ad verbatim (JL). All interviews were summarized. Summaries were sent to each interviewed clinician to assess for accuracy, serving as a member check. All clinicians agreed to the summaries' accuracy.
Clinician focus groups
In seven of the eight hospitals, focus groups were conducted after the observations and interviews with 3–4 clinicians. In the included referring hospital, only observations and interviews were conducted, and no focus groups were performed, since only two clinicians were involved in the upper gastrointestinal (GI) clinical pathway. Each focus group started with a presentation of the most important results of the observations and interviews, followed by a discussion in which the clinicians were encouraged to further discuss, complement, or contradict the results of the findings in their hospital. Focus groups were physically organized within the hospital (n = 3) or videoconference (n = 4) due to the severe acute respiratory syndrome‐coronavirus disease 2019 pandemic and lasted for an average of 1 h and 30 min. The focus groups were observed by one of the two members of the research group (RV, PV) and were all moderated by JL. The focus group moderator and observer discussed all focus groups directly after the focus groups; thereafter, the focus group audio recordings were summarized.
Patient focus groups
In three resection hospitals, patients who were diagnosed with a curable tumor stage were recruited for the patient focus group. Thus, patients with a curable tumor stage undergoing palliative treatment were excluded in the focus groups. Patients (n = 18) were informed by their treating physician about the VARIATE‐project. After consent was provided, the patients were invited to participate in a focus group in the hospital in which they underwent treatment. All but one invited patient agreed to participate. One patient did not participate due to logistical reasons (see Supplementary 2 for patient demographics).
The focus group guide (Supplementary 3) was used to structure patient's discussion. The focus groups addressed the patients' experiences regarding treatment decision‐making during the outpatient clinic visit, reasons for selecting a specific treatment modality, their experiences with patient‐doctor discussions, and their treatment experiences. Focus groups were organized in a conference room of the hospital and lasted for an average of 1 h and 29 min (range 1 h 15 min–1 h 43 min). Three focus groups were conducted with five to six patients per focus group, moderated by LB, and observed by JL.
Data analyses
Quantitative data analyses
The primary quantitative outcome parameter was the proportion of patients in whom the multidisciplinary team meeting proposal differed from the actual treatment carried out. Secondary outcomes were the proportion of patients discussed during a multidisciplinary team meeting, the type of multidisciplinary team meeting proposal, and received treatment according to the hospitals of diagnosis' probability of undergoing treatment with curative intent.
Patients' baseline characteristics and outcome parameters were reported as frequencies with percentages. To evaluate baseline characteristics and outcome parameters, the chi‐squared test and Fisher exact test were used, when appropriate.
Qualitative data analyses
The data used for analyses comprised outpatient clinic field notes, transcripts of the interviews focusing on factors influencing the treatment decision‐making during the outpatient clinic visit, and summaries of the clinician focus groups and patient focus groups. A thematic content analysis was used to identify individual and hospital experiences, 18 focusing on the clinicians' perceptions and beliefs regarding treatment decision‐making during the outpatient clinic (e.g., aims, beliefs, personal characteristics, discussed treatment options) (see Box 1 for a complete overview of the coding process and the identification of emerging themes and subthemes) and was based on the treatment decision‐making model created by Elwyn et al. 13
For each hospital, a similar thematic map summarizing each theme and subtheme per clinician was created. Through constant comparison within and across cases, associations and deviant cases were identified. 19 Preliminary conclusions from the thematic map were thoroughly discussed by the core research team (JL, PV, RV, GN, MW). The thematic map comprised the themes and interrelations between the codes and themes, such as discussed treatment options, influence of patient characteristics, and influence of clinician's personality traits. Thereafter, the themes were discussed with two experts in the field of shared decision‐making (LB and LT).
Ethics
Ethics approval was not required according to the Medical Research Ethics Committees United of the Netherlands (number: W.18.166). All participating hospitals approved this study. All quantitative data collected by the NCR were de‐identified and coded. Written informed consent was obtained from all participants prior to the interview or start of the patient focus groups. Privacy and confidentiality were protected through pseudonymization. The transcripts and summaries will be stored pseudonymized for a minimum of 10 years, on the secured network of IKNL, to which only the core research team members have access. This study was funded by the Dutch Cancer Society (project number: 10895).
RESULTS
Quantitative results: Changes in the multidisciplinary team meeting proposal
Table 1 shows patient characteristics according to the hospital's diagnosis probability of undergoing treatment with curative intent. For esophageal cancer, no significant differences were observed between the probability groups; however, for gastric cancer, significant differences were observed in the number of comorbidities, ECOG performance status, and cT tumor stage. Surgery with curative intent was proposed during multidisciplinary team meeting in 63%, 65%, and 72% of patients diagnosed with esophageal cancer in a hospital with a low, middle, or high probability of undergoing treatment with curative intent, respectively. For gastric cancer, the rates were 75%, 82%, and 82%, respectively. Definitive chemoradiation was proposed during the multidisciplinary team meeting in 13%, 12%, and 15% of patients diagnosed with esophageal cancer in a hospital with a low, middle, or high probability of undergoing treatment with curative intent, respectively. In hospitals with a low, middle, or high probability of proposing treatment with curative intent, the proposed treatment plan for patients with esophageal cancer changed by 5%, 4%, and 3% (p = 0.24), respectively, and for gastric cancer, these were 1%, 0%, and 0% (p = 0.18), respectively (Table 2). Changes in the multidisciplinary team meeting proposal and actual treatment carried out comprised changes in the type of curative treatment option, such as a surgical proposal changing to definitive chemoradiation (n = 42). Nonetheless, no changes from curative intent multidisciplinary team meeting proposals to palliative treatment proposals or vice versa were observed (Table 2). Of the patients diagnosed with esophageal cancer in which the treatment plan was known (n = 1293), neoadjuvant treatment followed by resection was proposed in 65% of the patients. Of them, 18% did not undergo the proposed treatment plan: 12% underwent primary surgery, 5% underwent definitive chemoradiation, and 1% underwent endoscopic resection. Of the patients diagnosed with gastric cancer in which the treatment plan was known (n = 935), neoadjuvant treatment followed by surgery was proposed in 51% of the patients. Of them, 17% did not receive chemotherapy but directly underwent surgery. Of the patients in which perioperative treatment followed by resection was proposed, 83% underwent neoadjuvant treatment followed by resection of whom 39% did not undergo the adjuvant component. Direct surgery was proposed in 28% of the patients diagnosed with gastric cancer of which 98% underwent surgery (Table 3).
TABLE 1.
Patient characteristics esophageal and gastric cancer patients according to the hospital of diagnosis low, middle, or high probability of receiving treatment with curative intent
| Esophageal cancer | Gastric cancer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probability | ||||||||||||||
| Low | Middle | High | p value | Low | Middle | High | p value | |||||||
| ALL | 477 | 100% | 548 | 100% | 625 | 100% | 507 | 100% | 327 | 100% | 545 | 100% | ||
| Sex | 0.75 | 0.53 | ||||||||||||
| Female | 136 | 29% | 147 | 27% | 179 | 29% | 199 | 39% | 122 | 37% | 224 | 41% | ||
| Male | 341 | 71% | 401 | 73% | 446 | 71% | 308 | 61% | 205 | 63% | 321 | 59% | ||
| Age | 0.09 | 0.72 | ||||||||||||
| 60–74 | 239 | 50% | 287 | 52% | 340 | 54% | 169 | 33% | 122 | 37% | 195 | 36% | ||
| <60 | 100 | 21% | 83 | 15% | 111 | 18% | 76 | 15% | 46 | 14% | 71 | 13% | ||
| GE 75 | 138 | 29% | 178 | 32% | 174 | 28% | 262 | 52% | 159 | 49% | 279 | 51% | ||
| cT Classification | 0.59 | 0.03 | ||||||||||||
| cT1 | 28 | 6% | 35 | 6% | 34 | 5% | 21 | 4% | 12 | 4% | 29 | 5% | ||
| cT2 | 133 | 28% | 143 | 26% | 197 | 32% | 202 | 40% | 117 | 36% | 190 | 35% | ||
| cT3 | 239 | 50% | 273 | 50% | 305 | 49% | 130 | 26% | 66 | 20% | 117 | 21% | ||
| cT4 | 6 | 1% | 10 | 2% | 10 | 2% | 16 | 3% | 20 | 6% | 19 | 3% | ||
| cTX | 71 | 15% | 87 | 16% | 79 | 13% | 138 | 27% | 112 | 34% | 190 | 35% | ||
| cN Classification | 0.22 | 0.7 | ||||||||||||
| cN0 | 176 | 37% | 211 | 39% | 272 | 44% | 275 | 54% | 182 | 56% | 317 | 58% | ||
| cN+ | 260 | 55% | 288 | 53% | 303 | 48% | 161 | 32% | 99 | 30% | 151 | 28% | ||
| cNX | 41 | 9% | 49 | 9% | 50 | 8% | 71 | 14% | 46 | 14% | 77 | 14% | ||
| Histology | 0.07 | 0.475 | ||||||||||||
| Adenocarcinoma | 348 | 73% | 386 | 70% | 477 | 76% | 491 | 97% | 321 | 98% | 532 | 98% | ||
| Squamous cell carcinoma | 123 | 26% | 145 | 26% | 134 | 21% | NA | NA | NA | |||||
| Not otherwise specified | 6 | 1% | 17 | 3% | 14 | 2% | 16 | 3% | 6 | 2% | 13 | 2% | ||
| Number of Comorbidities | 0.83 | 0.01 | ||||||||||||
| 0 comorbidities | 192 | 40% | 213 | 39% | 259 | 41% | 166 | 33% | 127 | 39% | 219 | 40% | ||
| 1 comorbidity | 151 | 32% | 184 | 34% | 204 | 33% | 187 | 37% | 111 | 34% | 153 | 28% | ||
| 2 or more | 116 | 24% | 117 | 21% | 141 | 23% | 135 | 27% | 74 | 23% | 150 | 28% | ||
| Unknown | 18 | 4% | 34 | 6% | 21 | 3% | 19 | 4% | 15 | 5% | 23 | 4% | ||
| ECOG performance status | 0.53 | 0.001 | ||||||||||||
| ECOG 0 and 1 | 319 | 67% | 368 | 67% | 415 | 66% | 260 | 51% | 141 | 43% | 267 | 49% | ||
| ECOG 2 | 39 | 8% | 53 | 10% | 43 | 7% | 37 | 7% | 24 | 7% | 51 | 9% | ||
| ECOG 3 and 4 | 16 | 3% | 16 | 3% | 17 | 3% | 31 | 6% | 10 | 3% | 12 | 2% | ||
| Unknown ECOG | 103 | 22% | 111 | 20% | 150 | 24% | 179 | 35% | 152 | 46% | 215 | 39% | ||
TABLE 2.
Treatment and treatment plan in patients diagnosed with esophageal‐ and gastric cancer according to the probability of receiving treatment with curative intent
| Esophageal cancer | Gastric cancer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low probability | Middle probability | High probability | p value | Low probability | Middle probability | High probability | p value | |||||||
| Total | 477 | 100% | 548 | 100% | 625 | 100% | 507 | 100% | 327 | 100% | 545 | 100% | ||
| Discussed in multidisciplinary team meeting | 414 | 87% | 486 | 89% | 547 | 88% | 0.645 | 411 | 81% | 269 | 82% | 457 | 84% | 0.434 |
| Involvement expert center | 407 | 85% | 429 | 78% | 535 | 86% | <0.0001 | 362 | 71% | 213 | 65% | 389 | 71% | 0.02 |
| Treatment plan known | 383 | 80% | 420 | 77% | 490 | 78% | 343 | 67% | 220 | 67% | 372 | 68% | ||
| Treatment plan | 0.001 | 0.217 a | ||||||||||||
| Endoscopic resection | 21 | 5% | 13 | 3% | 8 | 2% | 7 | 2% | 3 | 1% | 10 | 2% | ||
| Resection | 240 | 63% | 274 | 65% | 351 | 72% | 258 | 75% | 180 | 82% | 304 | 82% | ||
| Definitive chemoradiation | 51 | 13% | 50 | 12% | 72 | 15% | ||||||||
| Palliative | 58 | 15% | 67 | 16% | 53 | 11% | 37 | 11% | 18 | 8% | 24 | 6% | ||
| Best supportive care | 13 | 4% | 16 | 4% | 6 | 1% | 41 | 12% | 19 | 9% | 34 | 9% | ||
| Treatment | <0.0001 a | <0.0001 a | ||||||||||||
| Endoscopic resection | 17 | 4% | 10 | 2% | 9 | 2% | 7 | 2% | 3 | 1% | 7 | 2% | ||
| Neoadjuvant and/or resection | 229 | 60% | 263 | 63% | 341 | 70% | 258 | 75% | 180 | 82% | 307 | 83% | ||
| Definitive chemoradiation | 66 | 17% | 64 | 15% | 81 | 17% | ||||||||
| Palliative systemic therapy | 6 | 2% | 2 | 1% | 5 | 1% | 5 | 1% | 4 | 2% | 4 | 1% | ||
| Palliative radiation therapy | 34 | 9% | 46 | 11% | 29 | 6% | 21 | 6% | 4 | 2% | 8 | 2% | ||
| Best supportive care | 31 | 8% | 35 | 8% | 25 | 5% | 52 | 15% | 29 | 13% | 46 | 12% | ||
| Alteration in treatment plan | 18 | 5% | 18 | 4% | 13 | 3% | 0.24 | 2 | 1% | 0 | 0% | 0 | 0% | 0.18 a |
| Endoscopic resection → surgical resection¥ | 1 | 5% | ||||||||||||
| Surgical resection → endoscopic resection | 0 | 0 | 2 | 15% | 2 | 100% | ||||||||
| Surgical resection → definitive chemoradiation | 16 | 89% | 16 | 89% | 10 | 77% | ||||||||
| Definitive chemoradiation → surgery | 1 | 5% | 2 | 11% | 1 | 8% | ||||||||
Note: The treatment plan is solely assessed of patients discussed in a multidisciplinary team meeting prior to treatment. Treatment plan, treatment, and alteration in treatment plan were solely assessed if the treatment plan was known.
For groups with numbers <5 Fishers exact test was used.
TABLE 3.
Multidisciplinary team meeting (MDTM) proposed treatment plan versus actual received treatment
| Esophageal cancer | Gastric cancer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDTM proposal (n = 1293) | Received treatment | MDTM proposal (n = 935) | Received treatment | ||||||||
| Endoscopic resection | 42 | 3% | Endoscopic resection | 34 | 81% | Endoscopic resection | 20 | 2% | Endoscopic resection | 15 | 75% |
| Neoadjuvant treatment followed by resection | 7 | 17% | Neoadjuvant treatment followed by resection | 3 | 15% | ||||||
| Primary resection | 1 | 2% | Primary resection | 2 | 10% | ||||||
| Neoadjuvant treatment followed by resection | 841 | 65% | Neoadjuvant treatment followed by resection | 697 | 82% | Neoadjuvant treatment followed by resection | 474 | 51% | Neoadjuvant treatment followed by resection | 393 | 83% |
| Primary resection | 100 | 12% | Primary resection | 81 | 17% | ||||||
| Endoscopic resection | 2 | 1% | |||||||||
| Definitive chemoradiation | 42 | 5% | |||||||||
| Primary resection | 24 | 2% | Primary resection | 24 | 100% | Primary resection | 266 | 28% | Primary resection | 261 | 98% |
| Neoadjuvant treatment followed by resection | 3 | 1% | |||||||||
| Definitive chemoradiation | 173 | 14% | Definitive chemoradiation | 169 | 97% | Endoscopic resection | 2 | 1% | |||
| Neoadjuvant treatment followed by resection | 2 | 1.5% | |||||||||
| Primary resection | 2 | 1.5% | |||||||||
| Palliative treatment | 213 | 16% | Palliative treatment | 213 | 100% | Palliative treatment | 175 | 19% | Palliative treatment | 173 | 99% |
| Primary resection | 2 | 1% | |||||||||
| Alterations in treatment plan | 156 | 12% | 93 | 10% | |||||||
Qualitative results: From treatment proposal to the actual treatment carried out
Figure 1 shows a visualization of the treatment decision‐making process during the outpatient clinic visit, in which the multidisciplinary team meeting proposal was discussed with the patient. In this process, the patient should become aware of having a choice, understand the treatment options (option communication) with the possible benefits and harms (risk communication), and feel that there is sufficient time and support to consider which treatment option fits most to the patients' preferences (shared decision‐support), resulting in decision communication (treatment decision‐making). A comprehensive description of the factors that play a role in treatment decision‐making during the clinician‐patient interaction and deliberation is shown in Table 4, such as clinician's aims, personality, and conversation style. These themes and subthemes are described in more detail below and complemented by the patient's perspectives on treatment decision‐making, which were expressed during the focus groups (Table 5 displays quotes of the patient focus groups).
FIGURE 1.
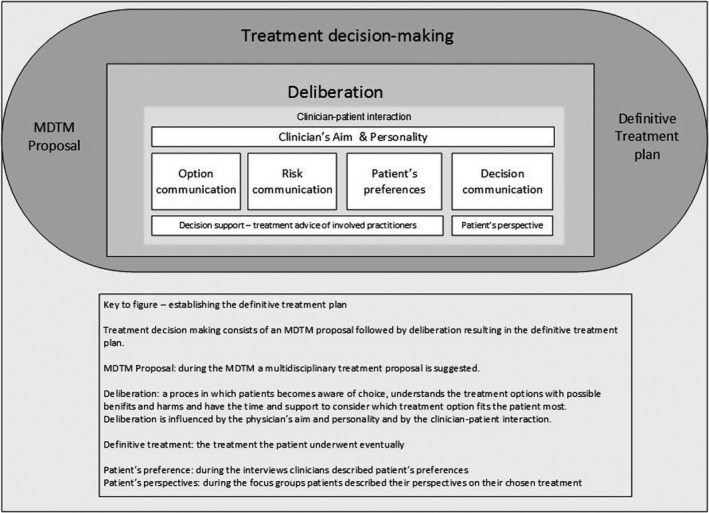
Treatment decision‐making
TABLE 4.
Treatment decision‐making during the outpatient clinic visit: a clinician's perspective
| Theme | Subtheme | Category |
|---|---|---|
| Discussing the multidisciplinary team meeting proposal with the patient | Context | Setting and participants (e.g., multidisciplinary outpatient clinic visit vs. monodisciplinary outpatient clinic visit, referring clinician vs. treating clinician discusses treatment proposal with patient) |
| Clinician's aims |
Accomplishing informed consent (e.g., following the Medical Treatment Contracts Act [WGBO], taking into account the patient's ability to reason, considered and broadly carried decision) Providing tailored information (e.g., providing patient with realistic perspective, outlining context and uncertainties, adjusting perspective of patients who are misinformed by the referral center) |
|
| Conversation style |
Physician's attitude (e.g., providing sufficient time and allowing the patients to tell their stories, providing the feeling that the patients and their families are heard; decision should be made together with the patient and their family) Relationship of trust (e.g., trust between physician and patient, trust that best decision has been made) Communication style (e.g., jargon‐free communication; conversation flows better in patients diagnosed with multimorbidity since their perspective is more realistic) |
|
| Deliberation | Personal practice style |
Personal communication style (e.g., decisive vs. elaborative, physician showing his/her vulnerability in cases of doubt, empathetic, humor) Personality (e.g., providing hope, prepared to give the treatment a chance, daring to propose experimental treatment options, daring to let the patient make the treatment decision) Personal treatment believes (e.g., the feasibility of a certain treatment, primum non nocere – first do no harm) |
| Discussion about treatment options |
Option communication
Risk communication
Patient's preferences
Decision‐communication
Decision‐support
|
Abbreviation: SES, socioeconomic status.
TABLE 5.
Treatment decision‐making during the outpatient clinic visit: a patient's perspective
| Theme | Subtheme | Category | Patient quotes |
|---|---|---|---|
| Conversation about treatment decision‐making | Patient's experience regarding option communication | Physician input regarding discussed treatment options | “In my case, definitive chemoradiation was not discussed” FG2 |
| “For a little while, I was in doubt, since the radiation oncologist explained the option of chemoradiation without surgery; however, we had already chosen for the treatment including surgery” FG3 | |||
| “There was the possibility to ask questions, but there was no room in the treatment protocol, so once I had the appointment with the surgeon, it was we are going to do this and that and operate” FG2 | |||
| “They said you have got one of the most aggressive forms of cancer; in our opinion, surgery is your best option, so I just went along with that” FG3 | |||
| “There was no attention for my spouse; nevertheless, they also have to deal with the disease” FG2 | |||
| Various media input regarding treatment options and consequences | “Prior to the outpatient clinic visit, I have read about the treatment options and complications on the internet” FG3 | ||
| Information load | “A lot of information is discussed in a short time, so you have to filter; I did not remember everything they told me” FG3 | ||
| “You are in a kind of whirlwind, but at the end of the day, you know where you stand” FG3 | |||
| “It is better if you bring family members to the outpatient clinic consult, so you can discuss the options afterward” FG2 | |||
| Patient's experiences regarding risk communication | Not concerned about the effect of harms | “You know that problems can occur, they told you several, but at that time, you are not concerned about them” FG2 | |
| “Let happen what needs to be done” FG1 | |||
| Treatment decision‐making | Patient's preferences influencing treatment decision‐making | Patient's desire for quality of life | “I have said, from the beginning, no surgery. All the doctors wanted to know why I did not wanted surgery; I believe that quality of life is more important than the duration of my life; then I have to add; I am single and I do not have a safety net” FG3 |
| Patients hoping for cure | “I do not have a choice, I just want to be able to walk the earth for a very long time” FG1 | ||
| “You trust the physician completely, they propose a treatment plan, you want to be cured, so you go for it” FG3 | |||
| Patient's trust in physician | “You just give your life in the hands of the other, the one, that will try to fix it” FG1 | ||
| “At that time you are not fully accountable; all you can think about is how can I get rid of this […]; then you have to trust the physician” FG3 |
Abbreviation: FG, focus group.
Discussing the multidisciplinary team meeting proposal with the patient
Context
The setting and participants during the outpatient clinic visit differed between hospitals; for example, some hospitals organized an outpatient clinic visit directly after the multidisciplinary team meeting involving the whole multidisciplinary team, as opposed to other hospitals where patients were seen separately by different clinicians on different occasions after the multidisciplinary team meeting. Additionally, in some hospitals, the referring clinician discussed the multidisciplinary team meeting proposal with the patient, whereas in other hospitals, an upper‐GI involved clinician from the expert center discussed the multidisciplinary team meeting proposal with the patient.
Clinician‐patient interaction
Clinician aims and conversation style
Most frequently, a clinician's intention was to accomplish an informed treatment decision by explaining how the multidisciplinary team meeting decision was substantiated, facilitating the patient to make a balanced decision during treatment decision‐making as explained by a surgeon: “If he understands and comprehends what is happening, and is treatable, also in the postoperative phase (i.e., no mental impairment leading to declining nasogastric feeding), then we would perform surgery” (surgeon 3, low/middle probability). Additionally, most clinicians believed that the treatment decision should be made together with the patient and family members, in which they experience respect and trust that the best treatment decision has been made: “I would like the patient and the family to have the feeling that they are heard and that they understand that we are on the same page regarding the treatment aims” (surgeon 1, low probability). Hence, clinicians believed that it is important that the patient feels that sufficient time is available in which the patients is allowed to discuss their arguments. Clinicians believed that the trust between them and the patient was important. Patients mentioned that they trusted to put their lives into the clinician's hands, and since their will to survive prevailed, they agreed with the proposed (curative) treatment proposal.
The observations showed that communication styles varied among clinicians, such as elaborating and discussing treatment and complications in more detail, which was also explained by a case manager during an interview: “They definitely differ; one of them is more short but decisive, and the other elaborates more; however, their aims are similar” (case manager 8, low/middle probability). Empathy or humor was helpful in putting the patient at ease, as explained by other clinicians.
Deliberation
Clinician's personality and practice style
The clinician's practice style, communication style, and treatment belief are all part of a clinician's personality, which differed between clinicians and was mentioned to affect discussion. For instance, some clinicians were more inclined to guide the patient toward the multidisciplinary team meeting‐proposed treatment plan, whereas others provided more room to let the patient make a treatment decision. According to a gastroenterologist, clinicians should express their vulnerability when discussing uncertainties, as this would benefit the patient's decision‐making: “Naturally, at times, patients do not know which choice to make, and at these times perhaps, we should take more responsibility; a lot of doctors are unsure to discuss their uncertainties with patients about choice A or choice B, since they hold the belief that the doctor should provide the patient with a superior choice” (gastroenterologist 3, low/middle probability).
Option communication
Most clinicians explained that all treatment options, including the multidisciplinary team meeting proposal, should be discussed during treatment option communication, as opposed to others who mentioned that they only explain other treatment options if the patient asked for them: “Only if patients ask for it, then we discuss definitive chemoradiation, but we do tell them that it does not provide the best odds” (case manager 2, high probability). Moreover, in the patient focus groups, patients mentioned that not all treatment options were discussed. Clinicians differed in their discussion of the multidisciplinary team meeting proposal during the outpatient clinic. Based on the observations and interviews, some clinicians only discussed the multidisciplinary team meeting proposal, as opposed to others who discussed their personal treatment beliefs in addition to the multidisciplinary team meeting proposal: “Well, that's the question, ultimately, the multidisciplinary team meeting proposal is only an advice; you can deviate from this advice as treating physician, but you have to motivate your decision […], so if the multidisciplinary team meeting's advice is on the conservative spectrum, and I personally belief a more invasive treatment option is feasible, ‘I would like to discuss this with you and then we can make a shared decision’” (surgeon 6, high probability). During treatment decision‐making in the outpatient clinic visit, the clinician's personal treatment beliefs and values played a role according to a surgeon: “The person's ethics, the beliefs of the clinician, are important in how that person comes to a decision; perhaps, life experiences play a role as well” (surgeon 2, high probability).
Most clinicians mentioned that providing tailored information is important, such as providing the patient with a realistic perspective: “Occasionally, patients imagine total alopecia due to chemotherapy; that is however no longer the case in patients treated with chemotherapy for esophageal cancer.” (case manager 1, low probability). Most clinicians aimed to outline the context, such as the effect of patient age on treatment outcome and the uncertainties associated with certain (experimental) treatment options.
Risk communication
Most clinicians mentioned that they explain the benefits and harms of the treatment options during risk communication: “In all openness, the benefits and harms are discussed with the patient, weighing what the best treatment option is for this individual patient” (medical oncologist 3, low/middle probability). However, it was observed that the extent of discussing the benefits and harms of the proposed treatment varied among clinicians. Some clinicians only mentioned the probability of postoperative complications, whereas others discussed complications in more detail. Most patients mentioned that they were not concerned about the impact or harms, since they had a strong will to be cured.
Patient's preferences
During the patient focus groups, most patients mentioned that they did not experience that there was a choice, since their will to be cured prevailed. Some clinicians mentioned exceptions, for instance, patients who felt that they had a complete life or did not want to compromise their quality of life standards by surgery. This was also explained by one of the patients, stressing that preserving quality of life was more important than prolonging life, which made her choose for definitive chemoradiation. Patients' motivation, preferences, home situation, and family opinions were taken into account during treatment decision‐making according to most clinicians: “I think that we take the interest of the patient into account, or the patient's opinion, what does the patient want, and I can imagine that there are hospitals that are more guiding” (surgeon 8, low/middle probability). Some clinicians explained that they always stress that the patient always has a choice: “I always try to tell them, “yes, you do have a choice” […] I always discuss that, and that we can also refrain from treatment” [medical oncologist 5, high probability). However, most clinicians explained that refraining from treatment was rarely an option for patients.
Decision‐communication
Most clinicians recognized their ability to influence the patient's treatment decision‐making during decision‐communication: “It is not like I tell the patient to make a choice, as choosing your food at the Chinese restaurant […], yet I realize that we are able to guide the patient's treatment decision‐making […] it's not only explaining the options, but you can also influence the patient's decision” (surgeon 1, low probability). Based on the observations, clinicians used different techniques, ranging from describing all treatment options without the treating physician's personal preference to guiding the patient to the definitive treatment plan, which was also mentioned by a surgeon: “I try to get an impression how the patient responds to all the treatment options, how they consider the treatment options, or whether they did their own search on the internet” (surgeon 8, low/middle probability). Some clinicians felt that doctors need a certain maturity to guide the patient properly during treatment decision‐making: “It is important that doctors have a certain maturity, a certain confidence, that during uncertainties, they can guide the patient” (gastroenterologist 3, low/middle probability).
Decision‐support
During the focus groups, patients mentioned that they needed time to process the discussed treatment options and their own preferences and were not able to decide during the outpatient clinic visit, which was also mentioned by some clinicians. One patient mentioned that he received a significant amount of information during the visit and needed time to think about what was explained during the consultation. In these situations, the clinician organized a second appointment to support the patient in the treatment decision‐making or involved the patient's general practitioner. Most clinicians felt that the patient should discuss the treatment proposal with all involved clinicians prior to a definitive treatment choice is made: “I insist that the patient has also a consultation with the surgeon prior to the start of treatment; this was however not always the case in the beginning” (radiation oncologist 6, high probability). Based on these observations, patients rarely seek contact with peers or provide pamphlets to explain treatment options.
DISCUSSION
In this mixed method multiple case study, the quantitative results demonstrated that differences between the multidisciplinary team meeting proposal and applied treatment rarely occurred. Despite the qualitative finding that most clinicians held similar opinions regarding the decisional process, the implementation of treatment decision‐making in practice varied. Based on the observations, differences comprised discussing all treatment options versus only the best fitting treatment option and the thoroughness of discussing the benefits and harms of treatment options. Most patients aimed to undergo treatment with curative intent and were less concerned about the possible harms.
Based on the principle that multidisciplinary case discussions lead to improved treatment recommendations, multidisciplinary team meetings have been widely implemented in cancer care. The multidisciplinary team meeting structure enhances communication, decision‐making, and coordination, based on evidence‐based and updated knowledge or expert opinions. 20 The quantitative results of the present study indicate that changes in the multidisciplinary team meeting proposal and the actual treatment decision during the outpatient clinic visit only rarely occurred. Hence, variation in the probability of undergoing treatment with curative intent between hospitals mainly originates during clinical decision‐making during the multidisciplinary team meeting. Furthermore, it can be hypothesized that the multidisciplinary tumor board generally proposes an adequate treatment plan; thus, sufficient information is available during the multidisciplinary team meeting, including patient characteristics, such as the patient's opinion, resulting in a fitting treatment proposal that matches the individual needs of a specific patient. Therefore, based on the results of the current study, adequate organization of a multidisciplinary team meeting and clinical decision‐making during a multidisciplinary team meeting is invaluable, since most clinicians mentioned that they value the upper‐GI multidisciplinary team meeting treatment proposal and communicate this during the outpatient clinic visit as a treatment advice. Due to the development of multidisciplinary team meetings, the process of individual physician decision‐making has shifted to multidisciplinary decision‐making, yet the definitive treatment decision is a shared decision between the clinician and patient made during the outpatient clinic visit.
In 1956, three basic models of the physician‐patient relationship were described (i.e., activity‐passivity, guidance‐cooperation, and mutual participation). 21 Recently, treatment decision‐making should consist of mutual participation, in which the physician and patient work together as partners toward a mutually agreed treatment plan. First, awareness of equipoise should be created (explaining that there is no best choice), followed by discussing the benefits and harms. Subsequently, the patient's concerns and preferences should be elicited, and the patient should be supported in the process of deliberation. 22 The process of treatment decision‐making during the outpatient clinic visit is pivotal, and regardless of the multidisciplinary team meeting proposal, the discussion toward a definitive treatment plan should be personalized to accompany the patient in the treatment decision‐making. During the patient focus groups, it became clear that most patients trusted their clinician in the treatment advice and their strong intention to be cured prevailed, which resulted in following the treatment proposal. In line with a previous study, 23 the current study demonstrated that clinicians expressed that during treatment decision‐making, a partnership between the clinician and patient in which the patient feels supported and encouraged is important. Furthermore, clinicians explained the importance of the patient having the feeling that they were heard, and that the best treatment decision was made as a joined venture. Importantly, previous studies have demonstrated that greater patient participation during decision‐making has a positive effect on patient satisfaction, coping, and treatment adherence. 24 , 25 , 26 Hence, although the multidisciplinary team meeting treatment proposal only rarely changed during the outpatient clinic visit, treatment decision‐making is an important process in treatment decision‐making that enhances patient empowerment and possibly results in increased patient satisfaction and treatment adherence.
During the interviews, it became apparent that most clinicians recognized their ability to influence patients' decision‐making during decision‐communication. In addition, differences were observed, such as describing all treatment options, including personal preferences, and guiding the patient to the multidisciplinary team meeting proposal or offering treatment options that differed from the multidisciplinary team meeting proposal. In line with previous studies, 27 , 28 the qualitative results of the present study demonstrate that the clinician's personality, personal beliefs, and values on treatment (subjective considerations) influence treatment decision‐making during the process of clinical decision‐making during outpatient clinic visits. In 1984, Wennberg reported that subjective physician considerations play a decisive role during clinical treatment decision‐making (i.e., the practice style factor). 29 He found that physicians held different opinions regarding the best therapy, and that this was unrelated to scientific controversies. 29 More recent literature has described that the practice style factor reflects a deeply rooted behavioral mechanism regarding convictions of the individual clinician with respect to how to practice medicine, 30 possibly attributed to the observed differences in offering guidance or withholding a personal opinion in the current study, which is in concordance with a previous study. 31 Furthermore, variation in practice was observed regarding the discussed treatment options and the extent of discussing benefits and harms during the outpatient clinic visit. The frequent absence of addressing alternative options or refraining from treatment and differences in the extensiveness of discussing potential benefits and harms have also been described in previous studies. 23 , 32 , 33 A potential explanation for this phenomenon may be that clinicians might feel that fostering hope may contribute to the patient's well‐being, 33 which might explain the finding that clinicians refrained from discussing alternative treatment options with a lower likelihood of survival. Another suggested explanation is that the therapeutic relationship might be damaged by a clinician sharing information on gaps in scientific knowledge, uncertainties, and controversies. 34 This means that the process of treatment decision‐making might be influenced by the individual practice style factor but yet rarely leads to changes in the multidisciplinary team meeting treatment proposal.
Strengths and limitations
The main strength of this study is the combination of quantitative data and the clinicians' and patients' perspectives, providing a complete understanding of differences between clinicians toward treatment decision‐making during outpatient clinic visits. Furthermore, the reliability and validity of the data increased due to data triangulation and member checking. 35 , 36 Nevertheless, this study has some limitations when interpreting the results. For instance, our findings based on the patient focus groups should be interpreted with caution, since only patients who underwent treatment with curative intent were included. It would have been of added value when patients diagnosed with a curable stage, but who refrained from treatment with curative intent, would have been included. However, we were unable to recruit the patient group. Nevertheless, the outcomes of this study provide insight into the perspectives of patients who underwent treatment with curative intent and their reasoning. Additionally, all observations and interviews were performed by one researcher; nonetheless, potential researcher bias was tried to be prevented during data collection and analyses by peer debriefing. 37 Furthermore, the last multidisciplinary team meeting prior to treatment was defined as the multidisciplinary team meeting treatment proposal in the quantitative analyses. Since multiple multidisciplinary team meetings were held in some patients, the patient's feedback on previously discussed treatment options might be used as input during the multidisciplinary team meeting in which the multidisciplinary team meeting proposal is finalized. This means that during multidisciplinary team meeting, information regarding the patient's preference might be known and taken into consideration. In addition, at times, an “if then” treatment decision was made during the multidisciplinary team meeting, and since the data managers were only able to formulate one multidisciplinary team meeting proposal, these cases might have affected the quantitative outcomes of our results.
Future directions
To overcome the gap in treatment information that is provided to patients, personalized pamphlets (infographics) describing treatment options (i.e., curative and palliative options), and their outcomes (i.e., survival, functional, and quality of life outcomes) could be provided directly after diagnosis, informing patients about all treatment options prior to the outpatient clinic consultation for finally deciding on the treatment. Additionally, decision aids have been helpful in facilitating patient engagement in treatment decision‐making and could enhance the patient's question asking behavior. 38 , 39 , 40 , 41 An ongoing trial (the SOURCE trial: NCT04232735) investigates the effects of personalized predictions of treatment outcomes, 42 used by caregivers who are trained in using this specific tool on tailored information provision to patients diagnosed with esophagogastric cancer. The outcomes of such studies may improve personalized and tailored information provisions in clinical consultations.
Conclusion
Since changes in the multidisciplinary team meeting proposal and actual treatment carried out rarely occurred, the multidisciplinary team meeting proposal is pivotal. Differences in the beliefs and personalities of the attending clinicians might have contributed to the variation during the outpatient clinic consultation. Patients who aimed to be cured of their cancer trusted their treating clinician and were less concerned with the harms of a certain treatment.
CONFLICT OF INTEREST
PS: Research support or funding: EndoStim, Pentax, Norgine, Motus GI and The Enose company Advisory Board: Motus GIE; HvL: Consultant or advisory role: BMS, Lilly, MSD, Nordic Pharma, Servier, Research funding and/or medication supply: Bayer, BMS, Celgene, Janssen, Lilly, Nordic Pharma, Philips, Roche, Servier; RV: received research grants from Roche and Bristol‐Myers Squibb; For the remaining authors no conflict of interest was declared.
AUTHOR CONTRIBUTIONS
JL: conception, design, analysis, acquisition of data, interpretation of data, drafting manuscript, revising manuscript, final approval, accuracy, and integrity; LB: analysis, interpretation of data, acquisition of data, revising manuscript, final approval, accuracy, and integrity; PV: conception, design, analysis, interpretation of data, revising manuscript, final approval, accuracy, and integrity; AW: interpretation of data, revising manuscript, final approval, accuracy, and integrity; FW: interpretation of data, revising manuscript, final approval, accuracy, and integrity; JH: interpretation of data, revising manuscript, final approval, accuracy, and integrity; SM: interpretation of data, revising manuscript, final approval, accuracy, and integrity; JOH: interpretation of data, revising manuscript, final approval, accuracy, and integrity; MvD: interpretation of data, revising manuscript, final approval, accuracy, and integrity; LT: interpretation of data, revising manuscript, final approval, accuracy, and integrity; MH: interpretation of data, revising manuscript, final approval, accuracy, and integrity; HvL: conception, design, interpretation of data, acquisition of finances, revising manuscript, final approval, accuracy, and integrity; CR: conception, design, interpretation of data, acquisition of finances, revising manuscript, final approval, accuracy, and integrity; PS: conception, design, interpretation of data, acquisition of finances, revising manuscript, final approval, accuracy, and integrity; MW: conception, design, analyses, interpretation of data, acquisition of finances, revising manuscript, final approval, accuracy, and integrity; RV: conception, design, analyses, interpretation of data, acquisition of finances, revising manuscript, final approval, accuracy, and integrity; GN: conception, design, analyses, interpretation of data, acquisition of finances, revising manuscript, final approval, accuracy, and integrity.
ETHICS APPROVAL
The Medical Research Ethics Committees of the Netherlands confirmed that ethical approval was not required for this study (W.18.166). The participating hospitals approved this study. Written informed consent was obtained from all the participants prior to the interviews and focus groups
Supporting information
supinfo.
ACKNOWLEDGMENTS
The authors thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the NCR. This research was not preregistered. Data and methods can be requested at the Netherlands Comprehensive Cancer Organization (IKNL). The authors would like to thank the clinicians and patients for their time and openness during the interviews and focus groups. This study was funded by a grant from the Dutch Cancer Society (project number 10895). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Luijten JC, Brom L, Vissers PA, et al. Treatment decision‐making during outpatient clinic visit of patients with esophagogastric cancer. The perspectives of clinicians and patients, a mixed method, multiple case study. Cancer Med. 2022;11:2427–2444. doi: 10.1002/cam4.4596
Pauline A. J. Vissers, M. J. Westerman, Rob H. A. Verhoeven and Grard A. P. Nieuwenhuijzen shared senior authorship.
Contributor Information
Josianne C. H. B. M. Luijten, Email: j.luijten@iknl.nl.
Rob H. A. Verhoeven, Email: r.verhoeven@iknl.nl.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to for the privacy of individuals that participated in the study. Data are however available from the authors upon reasonable request and with permission of Netherlands Comprehensive Cancer Organization (IKNL).
REFERENCES
- 1.Accessed October 19, 2020. Available at: https://www.iknl.nl/nkr‐cijfers
- 2. Nederlandse Vereniging van Maag‐Darm‐Leverartsen, Type: Landelijke richtlijn Oesophaguscarcinoom. May, 1 2015. Accessed November 25, 2019. Available at: www.oncoline.nl/oesofaguscarcinoom
- 3. Lordick F, Mariette C, Haustermans K, Obermannova R, Arnold D, Committee EG. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27(suppl 5):v50‐v57. [DOI] [PubMed] [Google Scholar]
- 4. Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27(suppl 5):v38‐v49. [DOI] [PubMed] [Google Scholar]
- 5. Landelijke werkgroep Gastro‐intestinale Tumoren, Type: Landelijke richtlijn. Landelijke richtlijn Maagcarcinoom. March 1, 2017. Accessed November 25, 2019. Available at: https://www.oncoline.nl/maagcarcinoom
- 6. van Putten M, Koëter M, van Laarhoven HWM, et al. Hospital of diagnosis influences the probability of receiving curative treatment for esophageal cancer. Ann Surg. 2018;267(2):303‐310. [DOI] [PubMed] [Google Scholar]
- 7. van Putten M, Verhoeven RH, van Sandick JW, et al. Hospital of diagnosis and probability of having surgical treatment for resectable gastric cancer. Br J Surg. 2016;103(3):233‐241. [DOI] [PubMed] [Google Scholar]
- 8. Luijten JCHBM, Vissers PAJ, Lingsma H, et al. Changes in hospital variation in the probability of receiving treatment with curative intent for esophageal and gastric cancer. Cancer Epidemiol. 2021;71(Pt A):101897. [DOI] [PubMed] [Google Scholar]
- 9. van Hagen P, Spaander MC, van der Gaast A, et al. Impact of a multidisciplinary tumour board meeting for upper‐GI malignancies on clinical decision making: a prospective cohort study. Int J Clin Oncol. 2013;18(2):214‐219. [DOI] [PubMed] [Google Scholar]
- 10. Davies AR, Deans DA, Penman I, et al. The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro‐esophageal cancer. Dis Esophagus. 2006;19(6):496‐503. [DOI] [PubMed] [Google Scholar]
- 11. Du CZ, Li J, Cai Y, Sun YS, Xue WC, Gu J. Effect of multidisciplinary team treatment on outcomes of patients with gastrointestinal malignancy. World J Gastroenterol. 2011;17(15):2013‐2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Légaré F, Ratté S, Stacey D, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2010;(5):CD006732. [DOI] [PubMed] [Google Scholar]
- 13. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green T. Qualitative Methods for Health Research. Sage; 2014:121‐122. [Google Scholar]
- 15. Patton MQ. Qualitative Research and Evaluation Methods. Sage Publications; 2002. [Google Scholar]
- 16. Siemerink EJ, Schaapveld M, Plukker JT, Mulder NH, Hospers GA. Effect of hospital characteristics on outcome of patients with gastric cancer: a population based study in north‐East Netherlands. Eur J Surg Oncol. 2010;36(5):449‐455. [DOI] [PubMed] [Google Scholar]
- 17. Thornblade LW, Truitt AR, Davidson GH, Flum DR, Lavallee DC. Surgeon attitudes and practice patterns in managing small bowel obstruction: a qualitative analysis. J Surg Res. 2017;219:347‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Green J, Thorogood N. Analysing qualitative data. In: Silverman D, ed. Qualitative Methods for Health Research. 1st ed. Sage Publications; 2004:173‐200. [Google Scholar]
- 19. Yin RK. Case Study Research: Design and Methods. Sage; 1994. [Google Scholar]
- 20. El Saghir NS, Keating NL, Carlson RW, Khoury KE, Fallowfield L. Tumor boards: optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. Am Soc Clin Oncol Educ Book. 2014;34:e461‐e466. [DOI] [PubMed] [Google Scholar]
- 21. Szasz TS, Hollender MH. A contribution to the philosophy of medicine; the basic models of the doctor‐patient relationship. AMA Arch Intern Med. 1956;97(5):585‐592. [DOI] [PubMed] [Google Scholar]
- 22. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the Centre of healthcare. BMJ. 2012;344:e256. [DOI] [PubMed] [Google Scholar]
- 23. Brom L, De Snoo‐Trimp JC, Onwuteaka‐Philipsen BD, Widdershoven GA, Stiggelbout AM, Pasman HR. Challenges in shared decision making in advanced cancer care: a qualitative longitudinal observational and interview study. Health Expect. 2017;20(1):69‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golin C, DiMatteo MR, Duan N, Leake B, Gelberg L. Impoverished diabetic patients whose doctors facilitate their participation in medical decision making are more satisfied with their care. J Gen Intern Med. 2002;17(11):857‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Street RL Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician‐patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295‐301. [DOI] [PubMed] [Google Scholar]
- 26. Rood JA, Van Zuuren FJ, Stam F, van der Ploeg T, Huijgens PC, Verdonck‐de Leeuw IM. Cognitive coping style (monitoring and blunting) and the need for information, information satisfaction and shared decision making among patients with haematological malignancies. Psycho‐Oncol. 2015;24(5):564‐571. [DOI] [PubMed] [Google Scholar]
- 27. Logan RL, Scott PJ. Uncertainty in clinical practice: implications for quality and costs of health care. Lancet. 1996;347(9001):595‐598. [DOI] [PubMed] [Google Scholar]
- 28. Andersen TF, Hammond ME. The Challenges of Medical Practice Variations. MacMillan Press; 1990. [Google Scholar]
- 29. Wennberg JE. Dealing with medical practice variations: a proposal for action. Health Aff (Millwood). 1984;3(2):6‐32. [DOI] [PubMed] [Google Scholar]
- 30. Grytten J, Sørensen R. Practice variation and physician‐specific effects. J Health Econ. 2003;22(3):403‐418. [DOI] [PubMed] [Google Scholar]
- 31. Thorne S, Oliffe JL, Stajduhar KI. Communicating shared decision‐making: cancer patient perspectives. Patient Educ Couns. 2013;90(3):291‐296. [DOI] [PubMed] [Google Scholar]
- 32. de Haes H, Koedoot N. Patient centered decision making in palliative cancer treatment: a world of paradoxes. Patient Educ Couns. 2003;50(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 33. Buiting HM, Rurup ML, Wijsbek H, van Zuylen L, den Hartogh G. Understanding provision of chemotherapy to patients with end stage cancer: qualitative interview study. BMJ. 2011;342:d1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coulter A. Partnerships with patients: the pros and cons of shared clinical decision‐making. J Health Serv Res Policy. 1997;2(2):112‐121. [DOI] [PubMed] [Google Scholar]
- 35. Britten N. Qualitative interviews in medical research. BMJ. 1995;311(6999):251‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sim J, Sharp K. A critical appraisal of the role of triangulation in nursing research. Int J Nurs Stud. 1998;35(1–2):23‐31. [DOI] [PubMed] [Google Scholar]
- 37. Lincoln YS. CENiBH. Sage Publications; 1985. [Google Scholar]
- 38. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;2017(4):CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brandes K, Linn AJ, Butow PN, van Weert JC. The characteristics and effectiveness of question prompt list interventions in oncology: a systematic review of the literature. Psychooncology. 2015;24(3):245‐252. [DOI] [PubMed] [Google Scholar]
- 40. Lindig A, Hahlweg P, Frerichs W, Topf C, Reemts M, Scholl I. Adaptation and qualitative evaluation of ask 3 questions – a simple and generic intervention to foster patient empowerment. Health Expect. 2020;23(5):1310‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van der Hout A, Jansen F, van Uden‐Kraan CF, et al. Cost‐utility of an eHealth application 'Oncokompas' that supports cancer survivors in self‐management: results of a randomised controlled trial. J Cancer Surviv. 2021;15(1):77‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van den Boorn HG, Abu‐Hanna A, Ter Veer E, et al. SOURCE: a registry‐based prediction model for overall survival in patients with metastatic oesophageal or gastric cancer. Cancer. 2019;11(2):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patton MQ. Qualitative Evaluation and Research Methods. Sage; 1990. [Google Scholar]
- 44. Strauss AL, Corbin JM. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Sage Publications; 1990. [Google Scholar]
- 45. Korstjens I, Moser A. Series: practical guidance to qualitative research. Part 4: trustworthiness and publishing. Eur J Gen Pract. 2018;24(1):120‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supinfo.
Data Availability Statement
The data underlying this article cannot be shared publicly due to for the privacy of individuals that participated in the study. Data are however available from the authors upon reasonable request and with permission of Netherlands Comprehensive Cancer Organization (IKNL).


