Abstract
Background
Poor adherence to clinical practice guidelines for eosinophilic esophagitis (EoE) has been described and the diagnostic delay of the disease continues to be unacceptable in many settings.
Objective
To analyze the impact of improved knowledge provided by the successive international clinical practice guidelines on reducing diagnostic delay and improving the diagnostic process for European patients with EoE.
Methods
Cross‐sectional analysis of the EoE CONNECT registry based on clinical practice. Time periods defined by the publication dates of four major sets of guidelines over 10 years were considered. Patients were grouped per time period according to date of symptom onset.
Results
Data from 1,132 patients was analyzed and median (IQR) diagnostic delay in the whole series was 2.1 (0.7‐6.2) years. This gradually decreased over time with subsequent release of new guidelines (p < 0.001), from 12.7 years up to 2007 to 0.7 years after 2017. The proportion of patients with stricturing of mixed phenotypes at the point of EoE diagnosis also decreased over time (41.3% vs. 16%; p < 0.001), as did EREFS scores. The fibrotic sub‐score decreased from a median (IQR) of 2 (1‐2) to 0 (0‐1) when patients whose symptoms started up to 2007 and after 2017 were compared (p < 0.001). In parallel, symptoms measured with the Dysphagia Symptoms Score reduced significantly when patients with symptoms starting before 2007 and after 2012 were compared. A reduction in the number of endoscopies patients underwent before the one that achieved an EoE diagnosis, and the use of allergy testing as part of the diagnostic workout of EoE, also reduced significantly over time (p = 0.010 and p < 0.001, respectively).
Conclusion
The diagnostic work‐up of EoE patients improved substantially over time at the European sites contributing to EoE CONNECT, with a dramatic reduction in diagnostic delay.
Keywords: Diagnostic delay, Endoscopy, Gastrointestinal, Eosinophilic esophagitis, Guideline Adherence, Practice Guidelines as Topic, Registries
Key summary.
Established knowledge
The incidence of eosinophilic esophagitis (EoE) has increased rapidly over the last decade, but its diagnosis is still challenging.
The wide and variable symptomatic spectrum of EoE patients contributes to its frequent under and misdiagnosis, and has produced an unacceptably long diagnostic delay.
Fibrous remodeling resulting from untreated, long‐lasting eosinophilic inflammation determines the appearance of fibrotic sequelae of EoE in the esophagus and impairs symptoms and health‐related quality of life.
The impact that improved knowledge on reducing diagnostic delay in EoE, provided by the release of successive clinical practice guidelines, has not yet been assessed.
New or significant findings
Among the 1,132 EoE cases registered in EoE CONNECT, the median diagnosis delay reduced from 12.7 to 0.7 years when patients, whose symptoms began before the first EoE guidelines were published in 2007, were compared with those with onset of symptoms after the publication of the latest guidelines in 2017.
Stricturing and mixed EoE phenotypes, endoscopic features and scores for symptoms at diagnosis showed significant successive reduction when patients were distributed between periods of time defined by the release of four sets of international clinical practice guidelines.
The mean number of previous upper endoscopies before the one that allowed diagnosis of EoE were significantly higher among patients with symptoms onset up to 2007, compared to any other time thereafter.
Although not recommended, a proportion of patients underwent allergy testing as part of the diagnostic work out of EoE. The proportion, however, significantly reduced over time, as did the number of allergy tests performed per patient.
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic immune‐mediated inflammatory condition affecting the esophagus, 1 largely independent of immunoglobulin (Ig)E. 2 It is triggered and maintained by ongoing exposure to dietary antigens, 3 , 4 with a minor role also being attributed to environmental antigens. 5 From its first characterization as a distinct clinico‐pathological syndrome 3 decades ago, 6 , 7 the prevalence of EoE has increased sharply in recent years, 8 to currently represent the main cause of dysphagia in children and young adults in developed countries and the second cause of chronic esophagitis after gastroesophageal reflux disease (GERD). 9 An increased awareness, together with a true rise in incidence, explain the expanding epidemiology of EoE. 10 , 11 , 12
The diagnosis of EoE relies on the finding of distinctive histopathological features 13 in a patient with symptoms of esophageal dysfunction. 4 , 11 Despite this apparent simplicity, achieving a rapid diagnosis of EoE is still challenging. This is largely due to its wide symptomatic spectrum, which ranges from very mild food avoidance, occasional vomiting, non‐cardiac chest or abdominal pain, to severe dysphagia with food impaction. Many EoE patients present GERD‐related symptoms (e.g., heartburn, regurgitation, belching), that may improve under acid secretion blocker therapy, 15 , 16 thus leading to EoE being under or misdiagnosed with other conditions, and with GERD in particular. In addition, patients often develop adaptive eating behaviours 17 , 18 and restrictive strategies 19 to cope with chronic symptoms making it easier for them to go unnoticed unless questioned carefully. 20 , 21 As endoscopic findings in EoE can be subtle and unspecific, 22 and almost absent in up to 25% of patients, 23 they may be overlooked and esophageal biopsies not routinely performed. 24 , 25 As a result, the diagnostic delay reported for EoE is unacceptably long 26 , 27 , 28 , 29 and increases the risk of finding fibrotic complications of the disease. 26 , 30 , 31
Since 2007, several international clinical practice guidelines, 1 , 32 , 33 , 34 consensus documents 14 , 35 and technical reviews 36 have been released to provide a scientific framework from which to approach the management of patients with EoE. 37 At the same time, various studies have revealed a wide heterogeneity 27 , 38 , 39 , 40 and variable degree of adherence in clinical practice 41 , 42 , 43 , 44 to the recommendations provided. Therefore, whether a better knowledge of the disease has resulted in real improvements in clinical practice remains unknown.
Given such ambiguity, this study, using data from the largest known clinical practice registry, aims to analyze whether a better knowledge of EoE has resulted in a reduction of diagnostic delay and an improvement in the diagnostic process of EoE patients in Europe.
MATERIAL AND METHODS
Study design and database
This is a cross‐sectional analysis of EoE CONNECT, a large, collaborative, prospectively maintained European database promoted by United European Gastroenterology, as a part of the Link Award program “Harmonizing diagnosis and therapy of Eosinophilic Oesophagitis across Europe (HaEoE‐EU)”. EoE CONNECT is managed by EUREOS, the European Consortium for Eosinophilic Diseases of the GI Tract (www.eureos.online).
All patients recruited are diagnosed with EoE based on the criteria of the evidence‐based guidelines 1 and the AGREE conference. 14 EoE CONNECT records, both prospectively and retrospectively, demographic and clinical characteristics, diagnostic work out and therapy outcomes in patients recruited at various sites across Europe. The definitions, detailed study protocol and operational procedures of EoE CONNECT have been published elsewhere. 45
Patients and variables
Data collected for this analysis included patient sex and birth date; date of onset of symptoms, and of EoE diagnosis; endoscopic features at diagnosis according to EREFS (edema, rings, exudates, furrows and strictures) score; 46 and EoE phenotype (inflammatory, structuring or mixed). Symptoms were evaluated with the Straumann’s Dysphagia Symptoms Score (DSS). 47 We also retrieved type and number of allergic tests performed after diagnosing EoE, to inform the therapeutic approach.
For the primary outcomes, planned subgroup analyses were performed based on study periods defined by the date of publication of the major international clinical practice guidelines (ie, up to 2007, 32 2008‐2011, 33 2012‐2013, 34 2014‐2017 1 , and after 2017), and patients were distributed to these periods according to the point of symptom onset.
Statistical analysis
Mean and standard deviation (SD) were used for continuous variables with a normal distribution and median and interquartile ranges (IQR) for those with a non‐normal distribution. Normality was evaluated using the Kolgomorov‐Smirnov test. Comparisons were performed with Chi‐square (χ2) or Fisher’s exact tests for categorical variables and Kruskal‐Wallis test for continuous variables. Post‐hoc comparisons between groups were performed with Dunn and Mann‐Whitney tests.
Analyses were carried out using PASW 18.0 statistical analysis software (SPSS Inc, Chicago, IL, USA) and GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). Statistical significance was considered when p<0.05.
Ethics
The study protocol was approved by the Ethics Research Committee of Hospital Universitario de La Princesa, Madrid, Spain (central Ethics Committee for EoE CONNECT) as well as ethics committees at each individual participating study site. All patients or legal guardians provided informed written consent to participate.
RESULTS
Demographic data and diagnostic delay
At the point of data extraction on January 11 2022, 1,511 patients were registered in EoE CONNECT, with 1,132 (865 males; 76.4%) having the date of symptom onset and EoE diagnosis recorded (Table 1). Mean (SD) age at diagnosis was 33.3 (15.1) years old. No differences were detected for gender and age among groups. Overall, median (IQR) diagnostic delay (i.e. time from first symptom onset to definitive EoE diagnosis) in the whole series was 2.1 (0.7‐6.2) years. This gradually decreased as new guidelines were released (Figure 1A). Thus, a significant reduction, from a median of 12.7 years for symptom onset before the publication of first guidelines in 2007 to only 8 months after last guidelines were published in 2017, was seen.
TABLE 1.
Demographic and clinical characteristics of patients with eosinophilic esophagitis (EoE) classified into groups according to the year of symptom onset, in line with the year of publication of major international EoE clinical practice guidelines.
| Overall | Up to 2007 | 2008‐2011 | 2012‐2013 | 2014‐2017 | 2018 of after | p‐value | |
|---|---|---|---|---|---|---|---|
| Number of patients | 1132 | 214 | 214 | 122 | 394 | 188 | ‐ |
| Male patients, n (%) | 865 (76.4) | 174 (81.3) | 166 (77.6) | 92 (75.4) | 294 (74.6) | 139 (73.9) | 0.353 a |
| Age at diagnosis, years, mean (SD) | 33.3 (15.1) | 33.4 (13.5) | 32.7 (13.9) | 33.8 (15.5) | 34.4 (15.5) | 31.3 (17.0) | 0.169 b |
| Diagnostic delay, years, median (IQR) | 2.1 (0.7‐6.2) | 12.7 (8.0‐19.1) | 5.0 (1.9‐8.3) | 2.8 (0.7‐5.2) | 1.2 (0.5‐2.9) | 0.7 (0.2‐1.3) | <0.001 b |
| EoE phenotype, n | 1062 | 206 | 208 | 111 | 368 | 169 | <0.001 a |
| Inflammatory, n (%) | 782 (73.6) | 121 (58.7) | 146 (70.2) | 80 (72.1) | 293 (79.6) | 142 (84.0) | |
| Mixed, n (%) | 147 (13.9) | 41(19.9) | 33 (15.9) | 20 (18.0) | 38 (10.3) | 15 (8.9) | |
| Stricturing, n (%) | 133 (12.5) | 44 (21.4) | 29 (13.9) | 11 (9.9) | 37 (10.1) | 12 (7.1) | |
| EREFS score, median (IQR) | 2 (1‐4) | 3 (2‐4) | 2 (1‐4) | 2 (2‐4) | 2 (2‐4) | 2 (2‐3) | <0.001 b |
| EREFS fibrotic sub‐score, median ( IQR) | 1 (0‐2) | 2 (1‐2) | 1 (0‐2) | 1 (0‐2) | 1 (0‐2) | 0 (0‐1) | <0.001 b |
| DSS, median (IQR) | 8 (6‐10) | 9 (7‐10) | 8 (6‐10) | 8 (5‐9) | 8 (6‐10) | 8 (5‐10) | 0.024 b |
Abbreviations: DSS, dysphagia symptom score; EREFS; edema, rings, exudates, furrows and strictures; EREFS, fibrosis sub‐score: furrows and strictures; IQR, interquartile range; rings, SD, standard deviation; sum of scores for rings and strictures.
ap‐value for chi‐square test.
bp‐value for Kruskal‐Wallis test.
FIGURE 1.

Box plot for diagnostic delay (time span from symptom onset to reaching an EoE diagnosis) (a) and proportion of patients exhibiting different EoE phenotypes (b), in patient groups defined according to the year of symptom onset. Differences in diagnostic delay according to patient phenotypes were also found (c). *p < 0.05, **p < 0.01, ***p < 0.001.
Impact of reducing diagnostic delay on esophageal fibrosis
We next investigated the potential impact that an early diagnosis of EoE could have on a shorter progression of esophageal fibrosis and EoE phenotype at diagnosis on the 1,062 patients with this data registered. Overall, a stricturing EoE phenotype at diagnosis was present in 133 (12.5%) patients, 147 (13.9%) presented a mixed phenotype and the remaining 782 (73.6%) patients presented an inflammatory EoE phenotype at disease onset. When they were grouped according to the time period their symptoms appeared, a significant decrease over time in the proportion of patients with stricturing and mixed phenotypes was demonstrated (p < 0.001) (Figure 1B). Thus, 41.3% of patients whose symptoms appeared before 2007 had stricturing or mixed phenotypes, in comparison to only 16% of those in whom EoE debuted after 2017. In addition, the median (IQR) diagnostic delay among the 133 patients with a stricturing phenotype at the moment of EoE diagnosis was 4.9 (1.9‐11.3) years, significantly longer than the 3.2 (1.0‐8.4) years in the 147 patients with a mixed phenotype or than the 2.0 (0.7‐5.3) years found among the 782 patient with an inflammatory phenotype (p < 0.001) (Figure 1C).
To check this finding, we then analyzed fibrotic and inflammatory features captured by EREFS. 46 The score was higher among patients with symptom onset before 2007, displaying significant differences among groups (p < 0.001) (Figure 2A). When the fibrotic EREFS sub‐score was considered, a significant difference among groups was also detected (p < 0.001), decreasing from a median (IQR) of 2 (1‐2) to 0 (0‐1) over time.
FIGURE 2.

Box plots for EREFS (edema, rings, exudates, furrows and strictures) score calculated at diagnostic endoscopy (a) and for DSS (dysphagia symptom score) at EoE diagnosis (b), in patients classified according to the year of symptom onset. *p < 0.05, **p < 0.01, ***p < 0.001.
Effect of diagnostic delay on symptoms severity
Finally, the severity of EoE symptoms at diagnosis measured by using the DSS was also different among groups (p = 0.024), with DSS again being higher in patients with symptom onset before 2007 than in patient groups diagnosed in 2012 or later (2012‐2013, 2014‐2017 and after 2017) (Figure 2B).
Endoscopies performed before diagnosis of eosinophilic esophagitis reduced over time
We next hypothesized that patients presenting with EoE symptoms before the first EoE guidelines were published might have undergone further upper endoscopic examinations, due to unrecognized EoE and/or food impactions, without a consistent EoE diagnosis. Therefore, the number of previous upper endoscopies performed before the one that led to the diagnosis of EoE was evaluated. We found 100 patients in EoE CONNECT who had undergone at least one upper endoscopy prior to the examination that led to a diagnosis of EoE. The date of symptom onset was available for 92 out of these 100 patients. To verify the impact of increased awareness of EoE, we compared patients who presented with esophageal symptoms in or before 2007 ( n = 27) with those who presented with them after 2008 (n = 65): The mean (SD) number of upper endoscopies was higher among patients in the first group (1.6 [0.8] vs. 1.3 [0.7]; p = 0.010). In addition, the proportion of patients requiring more than one upper endoscopy before the one that allowed the EoE diagnosis was higher in the first group (44.4% vs. 18.5%, p = 0.018) (Figure 3A). Consequently, a patient with esophageal symptoms that began before the first EoE guidelines were published had significantly higher chances (odds ratio: 3.5; 95% confidence interval: 1.3‐9.5) of having undergone additional endoscopies before the one that achieved a diagnosis of EoE.
FIGURE 3.

Histogram showing the percentage of patients with between 1 to 5 endoscopies prior to the one which lead to an EoE diagnosis, and classified according to the year of symptom onset (in 2007 or before and in 2008 or later) (a). Box plot for number of allergy tests per patient after EoE diagnosis, for patients classified in four groups according to the year of EoE diagnosis (b). ***p < 0.001.
Allergy testing performed to manage eosinophilic esophagitis
As EoE is associated with various atopic diseases, 1 skin or serum allergy tests in these patients are frequently positive. However, research has indicated that the results of skin prick tests or serum IgE against food are not able to improve EoE in most patients, and their use for this purpose is discouraged in the most recent guidelines. 1 , 33 , 34 Therefore, we analyzed the evolving use of skin and/or serum IgE tests to direct dietary therapy after EoE diagnosis.
At least one allergic test was performed in 297 patients after being diagnosed with EoE; these included skin prick tests in 79.1%, food‐specific serum IgE in 65.3% or atopy patch testing in 3% of patients. When patients were classified in time groups according to the date of EoE diagnosis (Table 2), a significant decrease in the use of allergy tests to manage EoE was demonstrated over time (p < 0.001). A post‐hoc analysis also demonstrated a significant reduction in the mean number of allergy tests performed on patients between those diagnosed up to 2011 and after the release of the subsequent guidelines (Figure 3B). In addition, the proportion of patients who underwent three or more allergy tests reduced significantly over time (Table 2). Comparing patients diagnosed in 2011 or earlier with those diagnosed from the beginning of 2012, the former presented a higher proportion of patients with three or more allergic tests after diagnosis of EoE (37.9% vs. 6.5%, p < 0.001). These differences were also significant for each individual allergic testing modality: skin prick tests (87.9% vs. 76.6%, p = 0.047), food‐specific serum IgE (78.8% vs. 61.5%, p = 0.009) and atopy patch testing (10.6% vs. 0.9%, p < 0.001).
TABLE 2.
Allergic tests, including skin prick test, food‐specific serum immunoglobulin (Ig)E and/or atopy patch testing, performed on patients after a diagnosis of eosinophilic esophagitis (EoE) was provided. The last column shows p‐values for the difference between patients diagnosed up to 2011 and those diagnosed in 2012 or later.
| Overall | Period of EoE diagnosis | <2011 vs. >2012 | ||||
|---|---|---|---|---|---|---|
| Up to 2011 | 2012‐2013 | 2014‐2017 | 2018 or after | p‐value | ||
| Number of patients, n | 297 | 66 | 48 | 119 | 64 | ‐ |
| Skin prick test, n (%) | 235 (79.1) | 58 (87.9) | 41 (85.4) | 95 (79.8) | 41 (64.1) | 0.047 a |
| Serum IgE test, n (%) | 194 (65.3) | 52 (78.8) | 23 (47.9) | 78 (65.5) | 41 (64.1) | 0.009 a |
| Atopy patch test, n (%) | 9 (3.0) | 7 (10.6) | 0 (0) | 1 (0.8) | 1 (1.6) | <0.001 b |
| Number of tests/patient, mean (SD) | 1.7 (1.1) | 2.5 (1.7) | 1.6 (0.9) | 1.6 (0.6) | 1.4 (0.6) | ‐ |
| Number of tests/patient, median (IQR) | 2 (1‐2) | 2 (1‐3) | 1 (1‐2) | 2 (1‐2) | 1 (1‐2) | <0.001 c |
| Patients with ≥3 tests, n (%) | 40 (13.5) | 25 (37.9) | 5 (10.4) | 7 (5.9) | 3 (4.7) | <0.001 a |
Abbreviations: IQR, interquartile range SD, standard deviation.
ap‐value for chi‐square test.
bp‐value for Fisher’s exact test.
cp‐value for Mann‐Whitney test.
Subgroup analyses
To check whether the findings of this study were homogeneous across the different recruitment centers, we analyzed the differences between patients with EoE recruited in Spain (n = 932), compared to those diagnosed in Italy (n = 163), Denmark (n = 33) and France (n = 4). Diagnostic delays and reductions along time were similarly demonstrated among Spanish and non‐Spanish patients (Figure 4). The proportion of patients with stricturing or mixed phenotypes reduced along time in Spanish and non‐Spanish participants. However, significant differences were exclusively demonstrated for patients recruited at Spain (p < 0.001) while the limited sample size of the remaining only allow to document a trend (p = 0.21) (Supplementary Table 1).
FIGURE 4.
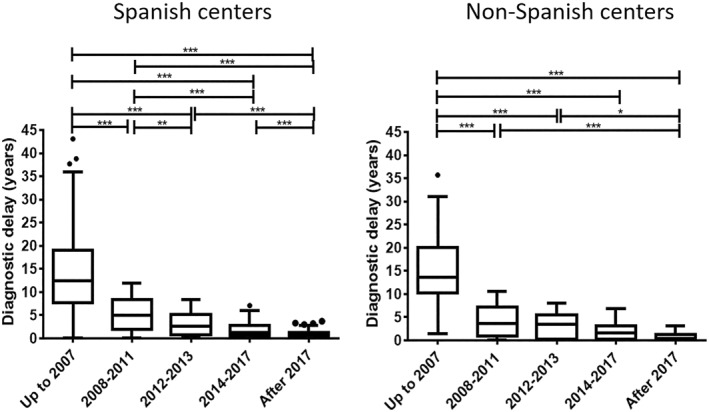
Box plot for diagnostic delay (time span from symptom onset to reaching an EoE diagnosis) among patients recruited in Spain compared to non‐Spanish patients. *p < 0.05, **p < 0.01, ***p < 0.001.
Regarding previous endoscopies performed before the one that allowed diagnosing EoE and allergy tests after EoE diagnosis, the subgroup of patients recruited from Spanish sites reproduced the same findings as the entire patient cohort. Only 16 non‐Spanish patients underwent any prior endoscopy before being diagnosed with EoE. At least two examinations were performed in 2 of the 3 patients with previous endoscopies who were diagnosed before 2007 (66.7%) but only in 4 of the 13 diagnosed after 2007 (30.8%). Allergy testing was performed to manage EoE in 26,3% and 26% patients recruited in Spain and other countries, respectively. A similar mean [SD] number of tests per patient (1.8 [1.2] vs. 1.6 [0.7]) and proportion of patients with 3 or more tests performed (13.1% vs. 15.4%) was found among Spanish and not Spanish patients, respectively. Finally, the use of allergy testing reduced along time in all patient subgroups (Supplementary Table 1).
DISCUSSION
To our knowledge, this is the first study that systematically evaluates the impact that a better understanding of EoE (defined through the publication of successive clinical practice guidelines) has had on improving care for a large cohort of European patients. Throughout the study period, which is defined by the publication of four sets of international clinical guidelines over a decade, we have documented a significant reduction in the diagnostic delay of EoE, in the impact of the disease (defined by lower symptom and endoscopic scores) and in the fibrotic sequelae that EoE causes on the esophagus.
Despite some discrepancies related to the position of PPIs in the management of EoE, guidelines have nonetheless provided a structured and evidence‐based framework for the management of patients with EoE. However, the literature has shown poor adherence to guideline recommendations in different settings. 41 , 44 This is especially true in relation to the use of PPI therapy (guidelines changes have probably been the cause of considerable confusion among providers, particularly gastroenterologists with smaller volumes of EoE patients) 44 and monitoring of response to treatment, which dispenses with endoscopy in favor of symptoms. 48 These aspects have been addressed in a previous analysis of EoE CONNECT. 49 Considering that the centers that contribute to this registry are experts in EoE, they do not necessarily reflect the reality of the EoE diagnosis problem. However, the shortening in diagnostic delay times and its consequences in the reduction of fibrosis demonstrated in our work transcends the expertise of the participating sites. Recently, Lenti et al 29 showed that two variables contributed to diagnostic delay in EoE: patient‐dependent diagnostic delay (the time span occurring between the onset of symptoms and the first medical assessment) and physician‐dependent diagnostic delay (the time span occurring between the first medical assessment and the definitive diagnosis). Unfortunately, EoE CONNECT does not register these differences, but indirect data suggests diagnostic delay for EoE reduced dramatically over the last decade. 27
We also found the number of endoscopies required to reach a diagnosis of EoE has reduced since the publication of the first EoE guideline, probably because esophageal biopsies are now taken more frequently in patients with suspected EoE, regardless of the endoscopic appearance of the esophagus.
However, there is still room for further improvement. According to the results of a recent international web‐based survey documenting practice patterns in patients with esophageal food impaction 50 (the most common situation leading to an EoE diagnosis) significant differences in the proportion of endoscopists who recommended obtaining esophageal biopsies in EoE‐suspicious food impaction during the initial endoscopy were documented based on level of EoE‐experience; differences were also noted when pediatric and adult endoscopists were compared.
Allergy tests are useful in the evaluation of concomitant atopies in EoE patients, but their utility in addressing the causes of the disease and directing its therapy, mostly from restrictive diets, has been very limited. Therefore, current disease management guidelines do not recommend these tests. 1 , 34 However, it is still common for many patients with EoE to be referred to the allergy clinic after diagnosis, to carry out batteries of tests whose results confound the effective management of patients. 51 The reduction in the use of these tests since the publication of Liacouras et al guideline in 2011, in addition to better adherence to the most recent guideline recommendations, could also mean less use of dietary in favor of drug treatment strategies for EoE. The latter are increasingly positioned as the recommended first line treatment in certain environments. 44 , 52
One of the strengths of our study is the inclusion of a large series of 1,132 patients, recruited in various hospitals throughout Spain, Italy, Denmark and France. Patients were accurately characterized following a previously defined protocol; the study covered a sufficiently long period of time; and the results from the different analyses performed all concur. Diagnostic delay and trends along time were similar among patients of different countries, and no differences were found in the management of patients across the study sites.
Some study weaknesses must also be recognized. Due to the retrospective registration of part of the clinical information, inaccuracies might have been generated. However, this was minimized as much as possible by quality monitoring of the data registered in EoE CONNECT. Furthermore, our study considers the years of publication of the main clinical practice guidelines as time milestones, all of an international nature, and does not consider the merit that other activities might have in the improvement of EoE care. Among these, the publication of local versions of the guidelines, consensus documents, educational activities developed by scientific organizations, and the role of patient organizations and advocacy groups, 53 must also be recognized as being directly responsible for the changes documented in our study, although their impact is more difficult to measure. Also, we did not assess changes in therapy choice or monitoring of response. Finally, the results of this study are applicable to the centers that participate in EoE CONNECT, and may not reflect the reality of care in settings less aware of this disease.
In conclusion, substantial improvement over time in EoE management, in European centers, has been demonstrated in this EoE CONNECT analysis. This is reflected in a reduction in diagnostic delay, endoscopic severity and symptom scores at diagnosis; less usage of endoscopies to obtain the diagnosis; and a decrease in allergy testing after diagnosis. Our findings provide a rationale that supports the creation of evidence‐based practice guidelines and further study into best practice.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. The Ethic Committees at all sites contributing to EoE CONNECT evaluated the protocol and approved participation.
Supporting information
Table S1
ACKNOWLEDGEMENTS
P Navarro is recipient of a Sara Borrell grant (CD19/00102) and EJ Laserna‐Mendieta of a Juan Rodes grant (JR19/00005), and both from the Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Health – Social Services and Equality, which is partly funded by the European Social Fund (period 2014‐2020).
Navarro P, Laserna‐Mendieta EJ, Casabona S, Savarino E, Pérez‐Fernández MT, Ghisa M, et al. Accurate and timely diagnosis of Eosinophilic Esophagitis improves over time in Europe. An analysis of the EoE CONNECT Registry. United European Gastroenterol J. 2022;10(5):507–17. 10.1002/ueg2.12240
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lucendo AJ, Molina‐Infante J, Arias A, Arnim U, Bredenoord AJ, Bussmann C, et al. Guidelines on eosinophilic esophagitis: evidence‐based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5(3):335–58. 10.1177/2050640616689525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C, et al. Eosinophilic esophagitis is characterized by a non‐IgE‐mediated food hypersensitivity. Allergy. 2016;71(5):611–20. 10.1111/all.12846 [DOI] [PubMed] [Google Scholar]
- 3. Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid‐based formula. Gastroenterology. 1995;109(5):1503–12. 10.1016/0016-5085(95)90637-1 [DOI] [PubMed] [Google Scholar]
- 4. Molina‐Infante J, Gonzalez‐Cordero PL, Arias A, Lucendo AJ. Update on dietary therapy for eosinophilic esophagitis in children and adults. Expet Rev Gastroenterol Hepatol. 2017;11(2):115–23. 10.1080/17474124.2017.1271324 [DOI] [PubMed] [Google Scholar]
- 5. Cianferoni A, Jensen E, Davis CM. The role of the environment in eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2021;9:3268–74. 10.1016/j.jaip.2021.07.032 [DOI] [PubMed] [Google Scholar]
- 6. Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38(1):109–16. 10.1007/bf01296781 [DOI] [PubMed] [Google Scholar]
- 7. Straumann A, Spichtin HP, Bernoulli R, Loosly J, Vögtlin J. [Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings]. Schweiz Med Wochenschr. 1994;124:1419–29. [PubMed] [Google Scholar]
- 8. Navarro P, Arias Á, Arias‐González L, Laserna‐Mendieta EJ, Ruiz‐Ponce M, Lucendo AJ. Systematic review with meta‐analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population‐based studies. Aliment Pharmacol Ther. 2019;49(9):1116–25. 10.1111/apt.15231 [DOI] [PubMed] [Google Scholar]
- 9. Arias Á, Lucendo AJ. Epidemiology and risk factors for eosinophilic esophagitis: lessons for clinicians. Expet Rev Gastroenterol Hepatol. 2020;14(11):1069–82. 10.1080/17474124.2020.1806054 [DOI] [PubMed] [Google Scholar]
- 10. Dellon ES, Erichsen R, Baron JA, Shaheen NJ, Vyberg M, Sorensen HT, et al. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: national population‐based estimates from Denmark. Aliment Pharmacol Ther. 2015;41(7):662–70. 10.1111/apt.13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molina‐Infante J, Gonzalez‐Cordero PL, Ferreira‐Nossa HC, Mata‐Romero P, Lucendo AJ, Arias A. Rising incidence and prevalence of adult eosinophilic esophagitis in midwestern Spain (2007‐2016). United Eur Gastroenterol J. 2018;6(1):29–37. 10.1177/2050640617705913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arias Á, Lucendo AJ. Incidence and prevalence of eosinophilic oesophagitis increase continiously in adults and children in Central Spain: a 12‐year population‐based study. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2019;51(1):55–62. 10.1016/j.dld.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 13. Collins MH. Histopathology of eosinophilic esophagitis. Dig Dis Basel Switz. 2014;32(1‐2):68–73. 10.1159/000357012 [DOI] [PubMed] [Google Scholar]
- 14. Dellon ES, Liacouras CA, Molina‐Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. 2018;55(4):1022–33.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krarup AL, Villadsen GE, Mejlgaard E, Olesen SS, Drewes AM, Funch‐Jensen P. Acid hypersensitivity in patients with eosinophilic oesophagitis. Scand J Gastroenterol. 2010;45(3):273–81. 10.3109/00365520903469931 [DOI] [PubMed] [Google Scholar]
- 16. Frandsen LT, Westmark S, Melgaard D, Krarup AL. Effectiveness of PPI treatment and guideline adherence in 236 patients with eosinophilic oesophagitis‐Results from the population‐based DanEoE cohort shows a low complication rate. United Eur Gastroenterol J. 2021;9(8):910–18. 10.1002/ueg2.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alexander R, Alexander JA, Ravi K, Geno D, Tholen C, Mara K, et al. Measurement of observed eating behaviors in patients with active and inactive eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019;17(11):2371–3. 10.1016/j.cgh.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 18. Hiremath G, Rogers E, Kennedy E, Hemler J, Acra S. A comparative analysis of eating behavior of school‐aged children with eosinophilic esophagitis and their caregivers’ quality of life: perspectives of caregivers. Dysphagia. 2019;34(4):567–74. 10.1007/s00455-019-09984-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robson J, Laborda T, Fitzgerald S, Andersen J, Peterson K, O’Gorman M, et al. Avoidant/restrictive food intake disorder in diet‐treated children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2019;69(1):57–60. 10.1097/mpg.0000000000002323 [DOI] [PubMed] [Google Scholar]
- 20. Kanakala V, Lamb CA, Haigh C, Stirling RW, Attwood SE. The diagnosis of primary eosinophilic oesophagitis in adults: missed or misinterpreted? Eur J Gastroenterol Hepatol. 2010;22(7):848–55. 10.1097/meg.0b013e32832c7709 [DOI] [PubMed] [Google Scholar]
- 21. Lin SK, Zhang S, Kalra N, Ghaffari G. Challenges in managing patients referred for eosinophilic esophagitis: a telephone survey and retrospective review. Allergy Asthma Proc. 2018;39(6):449–55. 10.2500/aap.2018.39.4163 [DOI] [PubMed] [Google Scholar]
- 22. Lucendo AJ, Arias A, Molina‐Infante J, Arias‐González L. The role of endoscopy in eosinophilic esophagitis: from diagnosis to therapy. Expet Rev Gastroenterol Hepatol. 2017;11(12):1135–49. 10.1080/17474124.2017.1367664 [DOI] [PubMed] [Google Scholar]
- 23. Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63(1):3–12. 10.1016/j.gie.2005.07.049 [DOI] [PubMed] [Google Scholar]
- 24. Hiremath G, Vaezi MF, Gupta SK, Acra S, Dellon ES. Management of esophageal food impaction varies among gastroenterologists and affects identification of eosinophilic esophagitis. Dig Dis Sci. 2018;63(6):1428–37. 10.1007/s10620-018-4972-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenwald K, Pan Z, Andrews R, Menard‐Katcher C. Follow‐up and symptom persistence after esophageal food impaction. Dis Esophagus Off J Int Soc Dis Esophagus. 2021;34(10):doab029. 10.1093/dote/doab029 [DOI] [PubMed] [Google Scholar]
- 26. Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon H, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time‐dependent manner. Gastroenterology. 2013;145(6):1230–6.e1–2. 10.1053/j.gastro.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 27. Lucendo AJ, Arias T, Molina‐Infante J, Rodriguez‐Sanchez J, Rodrigo L, Nantes O, et al. Diagnostic and therapeutic management of eosinophilic oesophagitis in children and adults: results from a Spanish registry of clinical practice. Dig Liver Dis. 2013;45(7):562–8. 10.1016/j.dld.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 28. Melgaard D, Westmark S, Laurberg PT, Krarup AL. A diagnostic delay of 10 years in the DanEoE cohort calls for focus on education ‐ a population‐based cross‐sectional study of incidence, diagnostic process and complications of eosinophilic oesophagitis in the North Denmark Region. United Eur Gastroenterol J. 2021;9(6):688–98. 10.1002/ueg2.12092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lenti MV, Savarino E, Mauro A, Penagini R, Racca F, Ghisa M, et al. Diagnostic delay and misdiagnosis in eosinophilic oesophagitis. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2021;53(12):1632–9. S1590‐8658(21)00268–1. 10.1016/j.dld.2021.05.017 [DOI] [PubMed] [Google Scholar]
- 30. Dellon ES, Kim HP, Sperry SLW, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79(4):577–85.e4. 10.1016/j.gie.2013.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lipka S, Kumar A, Richter JE. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol. 2016;50(2):134–40. 10.1097/mcg.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 32. Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–63. 10.1053/j.gastro.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 33. Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.e6. quiz 21–22. [DOI] [PubMed] [Google Scholar]
- 34. Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679–92. quiz 693. 10.1038/ajg.2013.71 [DOI] [PubMed] [Google Scholar]
- 35. Molina‐Infante J, Bredenoord AJ, Cheng E, Dellon ES, Furuta GT, Gupta SK, et al. Proton pump inhibitor‐responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut. 2016;65(3):521–31. 10.1136/gutjnl-2015-310991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rank MA, Sharaf RN, Furuta GT, Aceves SS, Greenhawt M, Spergel JM, et al. Technical review on the management of eosinophilic esophagitis: a report from the AGA institute and the joint task force on allergy‐immunology practice parameters. Gastroenterology. 2020;158(5):1789–810.e15. 10.1016/j.anai.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lucendo AJ, Arias A, Redondo‐Gonzalez O, Molina‐Infante J. Quality assessment of clinical practice guidelines for eosinophilic esophagitis using the AGREE II instrument. Expet Rev Gastroenterol Hepatol. 2017;11(4):383–90. 10.1080/17474124.2017.1285696 [DOI] [PubMed] [Google Scholar]
- 38. Dellon ES, Collins MH, Bonis PA, Leung J, Capocelli KE, Dohil R, et al. Substantial variability in biopsy practice patterns among gastroenterologists for suspected eosinophilic gastrointestinal disorders. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2016;14(12):1842–4. 10.1016/j.cgh.2016.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharma A, Eluri S, Philpott H, Lemberg DA, Dellon ES. EoE down under is still EoE: variability in provider practice patterns in Australia and New Zealand among pediatric gastroenterologists. Dig Dis Sci. 2021;66(7):2301–10. 10.1007/s10620-020-06534-6 [DOI] [PubMed] [Google Scholar]
- 40. Miller TL, Desai AD, Garrison MM, Lee D, Muir A, Lion KC. Drivers of variation in diagnosis and management of eosinophilic esophagitis: a survey of pediatric gastroenterologists. Dig Dis Sci. 2021. 10.1007/s10620-021-07039-6 [DOI] [PubMed] [Google Scholar]
- 41. Zifman E, Banai H, Shamir R, Ringel‐Kulka T, Zevit N. Practice differences in the diagnosis and management of eosinophilic esophagitis among adult and pediatric gastroenterologists in Israel. J Pediatr Gastroenterol Nutr. 2018;67(1):34–9. 10.1097/mpg.0000000000001909 [DOI] [PubMed] [Google Scholar]
- 42. Huang KZ, Jensen ET, Chen HX, Landes LE, McConnell KA, Almond MA, et al. Practice pattern variation in pediatric eosinophilic esophagitis in the carolinas EoE collaborative: a research model in community and academic practices. South Med J. 2018;111(6):328–32. 10.14423/smj.0000000000000817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miehlke S, von Arnim U, Schlag C, Frieling T, Madisch A, Loibl R, et al. Clinical management of eosinophilic esophagitis ‐ a nationwide survey among gastroenterologists in Germany. Z Gastroenterol. 2019;57(06):745–52. 10.1055/a-0885-1963 [DOI] [PubMed] [Google Scholar]
- 44. Chang JW, Saini SD, Mellinger JL, Chen JW, Zikmund‐Fisher BJ, Rubenstein JH. Management of eosinophilic esophagitis is often discordant with guidelines and not patient‐centered: results of a survey of gastroenterologists. Dis Esophagus Off J Int Soc Dis Esophagus. 2019;32(6). 10.1093/dote/doy133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lucendo A, Santander C, Savarino E, Guagnozzi D, Perez‐Martinez I, Perello A, et al. EoE CONNECT, the European registry of clinical, environmental and genetic determinants in eosinophilic esophagitis: rationale, design and study protocol of a large‐scale epidemiological study in Europe. Ther Adv Gastroenterol. 2022;15:175628482210742. 10.1177/17562848221074204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–95. 10.1136/gutjnl-2011-301817 [DOI] [PubMed] [Google Scholar]
- 47. Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, et al. Long‐term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2011;9(5):400–9. 10.1016/j.cgh.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 48. Schoepfer AM, Panczak R, Zwahlen M, Kuehni CE, Coslovsky M, Maurer E, et al. How do gastroenterologists assess overall activity of eosinophilic esophagitis in adult patients? Am J Gastroenterol. 2015;110(3):402–14. 10.1038/ajg.2015.32 [DOI] [PubMed] [Google Scholar]
- 49. Laserna‐Mendieta EJ, Casabona S, Savarino E, Perello A, Perez‐Martinez I, Guagnozzi D, et al. Efficacy of therapy for eosinophilic esophagitis in real‐world practice. Clin Gastroenterol Hepatol. 2020;18(13):2903–11. 10.1016/j.cgh.2020.01.024 [DOI] [PubMed] [Google Scholar]
- 50. Schreiner P, Safroneeva E, Schoepfer A, Greuter T, Biedermann L, Schlag C, et al. Management of eosinophilic esophagitis associated food impaction in Europe and the United States. Dis Esophagus Off J Int Soc Dis Esophagus. 2022. 10.1093/dote/doac003 [DOI] [PubMed] [Google Scholar]
- 51. Terrados S, Villafana L, Antolín‐Amérigo D, Camarero C, Martinez‐Botas J, Sanchez‐Ruano L, et al. Effectiveness of allergy testing in milk induced eosinophilic esophagitis. Description and follow‐up of patients. Allergol Immunopathol. 2020;48(6):576–81. 10.1016/j.aller.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 52. Tourlamain G, Garcia‐Puig R, Gutiérrez‐Junquera C, Papadopoulou A, Roma E, Kalach N, et al. Differences in management of eosinophilic esophagitis in Europe: an assessment of current practice. J Pediatr Gastroenterol Nutr. 2020;71(1):83–90. 10.1097/mpg.0000000000002672 [DOI] [PubMed] [Google Scholar]
- 53. James C, Assa’ad A. The global face of eosinophilic esophagitis: advocacy and research groups. Clin Rev Allergy Immunol. 2018;55(1):99–105. 10.1007/s12016-018-8683-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.


