Abstract
Introduction
Cancer is a major public health problem and a global leading cause of death where the screening, diagnosis, prediction, survival estimation, and treatment of cancer and control measures are still a major challenge. The rise of Artificial Intelligence (AI) and Machine Learning (ML) techniques and their applications in various fields have brought immense value in providing insights into advancement in support of cancer control.
Methods
A systematic and thematic analysis was performed on the Scopus database to identify the top 100 cited articles in cancer research. Data were analyzed using RStudio and VOSviewer.Var1.6.6.
Results
The top 100 articles in AI and ML in cancer received a 33 920 citation score with a range of 108 to 5758 times. Doi Kunio from the USA was the most cited author with total number of citations (TNC = 663). Out of 43 contributed countries, 30% of the top 100 cited articles originated from the USA, and 10% originated from China. Among the 57 peer-reviewed journals, the “Expert Systems with Application” published 8% of the total articles. The results were presented in highlight technological advancement through AI and ML via the widespread use of Artificial Neural Network (ANNs), Deep Learning or machine learning techniques, Mammography-based Model, Convolutional Neural Networks (SC-CNN), and text mining techniques in the prediction, diagnosis, and prevention of various types of cancers towards cancer control.
Conclusions
This bibliometric study provides detailed overview of the most cited empirical evidence in AI and ML adoption in cancer research that could efficiently help in designing future research. The innovations guarantee greater speed by using AI and ML in the detection and control of cancer to improve patient experience.
Keywords: artificial intelligence, machine learning, cancer, diagnosis, prevention, control, Scopus database
Introduction
Cancer is a significant public health problem worldwide, characterized by an increasing prevalence and mortality rate. 1 According to an update on global cancer burden using the GLOBOCAN 2020 database, about 19.3 million new cases and almost 10 million deaths were estimated. 2 Breast cancer remains the most prevalent, whereas lung, colorectal, prostate, and stomach cancers are the most commonly reported cases. Lung cancer remains the leading cause of cancer death, with an estimated 1.8 million deaths, followed by colorectal, liver, stomach, and breast cancers. 2 Treatment and prevention measures of cancer are still challenging 3,4, but the emergence of artificial intelligence (AI) and machine learning (ML) positively supports treatment and control of cancer.5,6
Recently, the increasing knowledge of AI techniques caused significant positive waves in healthcare by gradually altering the global landscape of healthcare and biomedical research. 7 Machine learning (ML) is a subfield of AI that can be regarded as an umbrella term encompassing various algorithms that can automatically learn and improve with experience. 8 Whereas Deep Learning (DL) is the subset of ML that uses neural network-based models to mimic the human brain’s ability for processing massive amounts of complex data, such as image recognition, languages processing, drug discovery, to name a few, all of which acts as a decision support system for humans. 9
Since the early 1970s, AI successfully made revolutions in medicine. 10 Oncology research focused on decoding the molecular onset of cancer by understanding the complex biological architecture of cancer cell proliferation. 11 Moreover, AI in clinical decision-making process was believed to increase the chances of early disease diagnosis and prediction by using next-generation sequencing (NGS) and high-resolution imaging techniques. 12 There was a recent successful application of AI in cancer classification through gene selection to determine whether those genes were active, hyperactive, or silent in normal or cancerous tissue. 12 On the other hand, the use of support vector machines in skin cancer diagnostic can potentially provide low-cost universal access to vital diagnostic care, 13 cancer gene expression signatures using artificial neural networks, 14 and in breast cancer research prediction and prognosis.15,16
Remarkable progress has been made in adopting innovative technology in cancer research to fast-track diagnosis, prediction, and possible treatment modes. Consequently, research reported the variety of AI and ML techniques as templates for global practices,7,9,16 which enabled big data and cognition capable computers to support cancer research specialists to revolutionize medicine by performing complex tasks to improve diagnostic accuracy, increase the efficiency of throughputs, improve clinical workflow, decrease human resource costs, and improve treatment choices. 17
Cancer research in lungs, breast, ovary, and pancreas has innovatively explored AI and ML to provide an evidence-based approach in the field. While some studies investigated the use of AI for breast screening based on ethical and social issues of the adoption 18 and radiologist performance, 19 there are evidences of new findings like DL supporting the discovery of lymph node metastases from breast cancer. 20 Similarly, by adopting CADe ML models in colorectal screening, endoscopic images and videos were processed in record time and gave allowance for real-time recognition of polyps with an excellent and high accuracy.21,22 Other ML learning approaches have been used to detect mismatch repair deficiency (dMMR) in colorectal screening. 23 In liver cancers, AI approaches using CS-SVM on liver cancer rehabilitation groups found that the methods can explicitly project the time and location of cancer reoccurrence. 24 While the adoption of AI and ML for cancer prediction, diagnosis, and rehabilitation is increasingly discussed among researchers, accessing the scope, progress, and achievement may serve as a reference for future research and applications of innovative technologies.
Notably, there is an increasing number of articles reporting the advancements in the AI and ML applications that enabled fast-tracked and ease in cancer screening, diagnostic, test precision, cancer classification, and cancer prediction and prognosis.7-19 As new approaches, AI and ML are practical tools to ensure improvements in patient care and control of cancer cases to reduce mortality rates globally.
Therefore, a bibliometrics analytical method was used to assess the research field’s critical areas to extend the discourse in this domain. Bibliometrics approach are widely adopted among researchers to identify various research themes and quality of the published work, thus providing information on research performance, publication patterns, and characteristics, determine research gaps, and predict the direction for future studies.25-31 Moreover, with global limited healthcare resources, bibliometric studies can be seen as a guiding tool for scientists and research funding agencies to map areas where restriction or increase in research may need to be considered. 32 The study adopted a bibliometric and systematic approach in exploring the top 100 cited articles to extend the empirical evidence on the adoption of ML and AI in cancer prediction, diagnosis, and other related research. Therefore, our study was timely conducted to provide a current and broad understanding of AI and ML in cancer research. The findings of this study can support the scientific community with a perspective on the evolution of cancer research adopting AI and ML over the past years and highlight possible future directions. This study aimed to;
i. Identify and characterize the top 100 cited articles published on AI and ML application in cancer research and provide useful information on the citation analysis, manuscript impact, research performance, and author productivity in the scientific community.
ii. Systematically determine and deliberate the status and current evidence and scopes of the top 100 cited articles.
iii. Provide an overview of the conceptual framework and the thematic change and evolution of the mostly influenced topics regarding AI and ML application in cancer.
Methods and Material
Study Design
The study adopted a systematic search and thematic analysis to examine the top-cited research on AI and ML to accentuate their impacts and innovations. The study approach has previously adopted multiple research domains to monitor and evaluate research performance for direction and necessary future action.33,34
Data Source
The data used in this study was extracted from the Scopus database, a large repository for scientific publications (www.scopus.com/). The data was downloaded from public source databases, and no ethical approval is needed for this study.
Search Strategy
Documents relating to artificial intelligence (AI), machine learning (ML) applications in cancer research published between 1978 and July 10, 2021, were searched in Scopus (https://www.scopus.com/) database.
The search query was uniquely developed for the current study for all published documents using the following terms: Title (Benign neoplasms* or malignancy* or malignant neoplasms* or neoplasia neoplasm* or neoplasms* or benign* or tumors*), and (artificial intelligence* or machine learning* or deep learning* or convolution neural network* or neural network* or random forest* or support vector machine* or fuzzy logic* or computer vision* or automatic programming* or speech understanding* or autonomous robots* or intelligent tutoring* or intelligent agents* or neural network* or voice recognition* or text mining*).
Inclusion and Exclusion Criteria
The search results were subsequently refined to include only original articles and reviews published in English. Documents published in “(“Chinese” or “Japanese” or “Persian” or “German” or “Russian” or “Spanish” or “Polish” or “Korean” or “Portuguese” or “Turkish” or “Arabic” or “Danish” or “French” language were excluded from this study. We further restricted the search to the final publication stage, and other articles in the press were also excluded from the analysis.
In total, 3263 documents within the Timespan: 1978 to July 10, 2021 were identified and then the top 100 cited articles were selected based on the citation times. The Metadata was exported into comma-separated value (csv) excel and BiB TeX format files for further in-depth analysis. The retrieved data included citation information, bibliographical information, abstract and keywords, countries, institutions, funding agencies, funding details, and other information such as references.
Top-cited articles were sorted by the “Times cited” and two researchers independently screened the document’s based on the research title. All bibliographic information such as Title, authors, affiliation, keywords analysis (author keyword, abstract, and title keyword), and countries of origin were included with the aims of identifying the countries with the most significant number of publications based on the corresponding author, research categories, funding agencies, and journals sources.
Ranking of Top-performing Authors, Journals and Articles
The journal impact factor and journal quartile range were obtained from the Journal Citation Report (JCR), which offers an updated list of journals ranked by Journal Impact Factor (JIF) to assess the quality of contributed journals in AI, ML, and cancer research. We also investigated the association between citation number in journals, authors, and the number of publications per year with the number of citations. The frequency distribution analysis for scientific productivity for an author’s contributions in AI, ML, and cancer is evaluated in according to Lotka’s law. 35
Data Analysis
Data analysis was conducted using Bibliometrix, an R package applied in R version 4.0.4. The VOSviewer.Var1.6.6 36 was used to analyze the co-authorship regarding two standard weight attributes “Links attribute” (L) and “Total link strength attribute” (TLS). 37 The Spearman correlation coefficient ® was used to calculate the association between the number of publications and the number of times cited. GraphPad Prism 6.03 for Windows (GraphPad Software Inc., San Diego, CA, USA) 38 was used for statistical analysis and P-values less than .05 was considered statistically significant.
Results
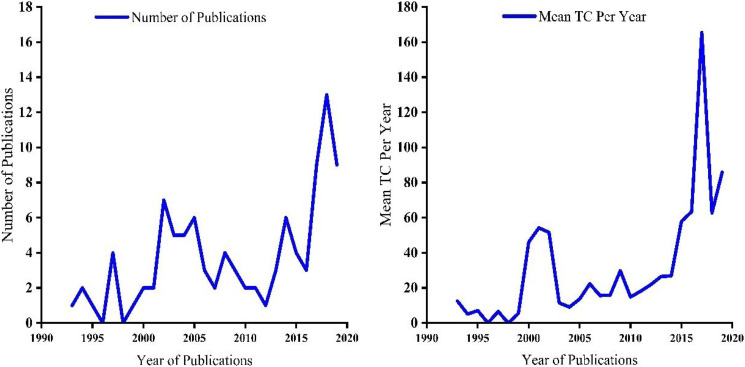
The top 100 cited articles on artificial intelligence and machine learning in cancer research were published from 1993 to 2019. Overall, the retrieved documents received a total of 33 920 citations, with 339,2 average citations per year per document. The studies involved 628 authors, with single authors per document and 6.28 co-authors per document. The top 100 cited articles were published in 57 journals with 43 contributing countries. The retrieved documents have a collaboration index of 6.5, as reported in Table 1. Figure 1 shows an increasing trend in the annual number of publications and the Mean of Total Citation Per year after 2015 during the study period. The significant correlation between the number of publications and Mean TC per Year was (r=.5746, P<.0001).
Table 1.
Main Information on Artificial intelligence and machine learning in cancer.
| Description | Results | Description | Results |
|---|---|---|---|
| Timespan | 1993:2019 | Author’s keywords (DE) b | 223 |
| Sources (journals, books, etc) | 57 | Authors | |
| Country | 43 | Authors | 628 |
| Funding agencies | Author appearances | 692 | |
| Total number of publications funded | 13 | Authors of single-authored documents | 4 |
| Total number of publications not funded | 87 | Authors of multi-authored documents | 624 |
| Documents | 100 | Author productivity through Lotka’s Law (N. Of authors) | 6 |
| Average years from publication | 12.2 | Document written by one author | 570 |
| Average citations per documents | 339.2 | Document written by two authors | 52 |
| Average citations per year per doc | 33.89 | Document written by three authors | 6 |
| References | 4262 | Author’s collaboration | |
| Document types | Single-authored documents | 4 | |
| Article | 93 | Documents per author | .16 |
| Review | 7 | Authors per document | 6.28 |
| Document contents | Co-authors per documents | 6.92 | |
| Keywords plus (ID) a | 1065 | Collaboration index (CI) c | 6.5 |
aFrequency distribution of keywords associated with the document by Scopus.
bFrequency distribution of the authors’ keywords’.
cThe scientific collaboration on the social process by which two or more researchers are work together sharing their intellectual and material resources to produce new scientific knowledge.
Figure 1.
Annual growth of publications and mean of Total Citation Per Year (Mean TC Per Year) on Artificial Intelligence and Machine learning in cancer.
The top 10 most productive authors in AI and ML in cancer research with at least three studies are presented in Table 2. Aerts Hugo JWL was ranked first with total citations (TNC=566), followed by Collins William P with (TNC=565), and Doi Kunio with (TNC=663). There is a significant correlation between the number of publications and Total Citations (r=.2871, P<.0001). The top 100 cited articles on AI and ML in cancer research received a total of 33 923 citations, with an average of total citations ranging from (TNC=108 to 5758) as shown in Supplemental Table S1.
Table 2.
Author with at least 3 articles and more in Artificial intelligence and machine learning in cancer.
| SCR | Author (n=628) | Affiliation | h_index | TNC | TNP |
|---|---|---|---|---|---|
| 1 | Aerts Hugo J. W. L | Harvard Medical School, Boston, MA | 3 | 566 | 3 |
| 2 | Collins William P | King’s College Hospital, London, UK | 3 | 565 | 3 |
| 3 | Doi Kunio | The University of Chicago South Maryland Avenue, Chicago, USA | 3 | 663 | 3 |
| 4 | Timmerman Dirk | University Hospitals KU Leuven, Leuven, Belgium | 3 | 581 | 3 |
| 5 | Valentin Lil | Malmö University Hospital, Lund University, Malmö, Sweden | 3 | 619 | 3 |
SCR: Standard Competition Ranking; TNC: Total Number of Citation, TNP: Total Number of Publications.
Analysis of the top 100 cited articles based on the geographical origin of publication shows that 43 countries contributed to AI and ML in cancer research (Table 3). The USA was the most productive country with (TNP=30) articles, followed by China with (TNP=11) articles, and the United Kingdom with (TNP=7) articles. The multiple-country publication analysis also shows a high collaboration rate between Germany (MCP_Ratio; 0.800) and Belgium (MCP_Ratio; .500).
Table 3.
Countries contributing at least 3 articles and more in Artificial intelligence and machine learning in cancer.
| SCR | Country (n=43) | TNP | TNC | AAC | SCP | MCP | MCP_Ratio |
|---|---|---|---|---|---|---|---|
| 1 | USA | 30 | 15 084 | 503 | 18 | 12 | .400 a |
| 2 | China | 11 | 1911 | 174 | 7 | 4 | .364 a |
| 3 | United Kingdom | 7 | 1717 | 245 | 5 | 2 | .286 a |
| 4 | Germany | 5 | 969 | 194 | 1 | 4 | .800 b |
| 5 | Belgium | 4 | 712 | 178 | 2 | 2 | .500 b |
| 6 | Netherlands | 4 | 1154 | 288 | 0 | 4 | 1.000 a |
| 7 | Canada | 3 | 989 | 330 | 3 | 0 | .000 a |
| 8 | Japan | 3 | 455 | 152 | 3 | 0 | .000 a |
| 9 | Spain | 3 | 466 | 155 | 3 | 0 | .000 a |
| 10 | Turkey | 3 | 1032 | 344 | 3 | 0 | .000 a |
SCR: Standard Competition Ranking; TNP: Number of publications; TNC: Total Number of Citations; AAC: Average Article Citations; SCP: Single Country Publication (Intra-Country Collaboration). MCP: Multiple Country Publications (Inter-Country Collaboration).
aLower International Collaboration (Value: Less 0,50).
bHigh International Collaboration (Value: More than 0,50).
According to our results, the top 100 cited articles were published across 57 journals (Table 4). The journal of “Expert Systems with Applications” was ranked first based on the number of publications with (TNP=8) articles and total citations of (TNC=1826), followed by “Scientific Reports” with (TNP=6) having a total citations number of (TNC=958), and “Radiology” (TNP=5) articles with a total citations number of (TNC=893) times. The significant association between the number of articles and the total citation number reported in the journal was (r=.646, P<.0001).
Table 4.
Journal with at least 2 articles and more in Artificial intelligence and machine learning in cancer.
| SCR | Journal (n=57) | h_index | TNC | TNP | IF (2020) |
|---|---|---|---|---|---|
| 1 | Expert Systems with Applications | 8 | 1826 | 8 | 6.954 |
| 2 | Scientific Reports | 6 | 958 | 6 | 4.379 |
| 3 | Radiology | 5 | 892 | 5 | 11.105 |
| 4 | Artificial Intelligence in Medicine | 4 | 893 | 4 | 5.326 |
| 5 | Bioinformatics | 4 | 2520 | 4 | 6.937 |
| 6 | IEEE Transactions on Medical Imaging | 4 | 1243 | 4 | 10.048 |
| 7 | Nature Medicine | 4 | 3078 | 4 | 53.44 |
| 8 | Computer Methods and Programs in Biomedicine | 3 | 495 | 3 | 5.428 |
| 9 | Ultrasound in Obstetrics and Gynecology | 3 | 414 | 3 | 7.299 |
| 10 | Cancer | 2 | 400 | 2 | 6.86 |
| 11 | Cancer Research | 2 | 318 | 2 | 12.701 |
| 12 | Clinical Cancer Research | 2 | 347 | 2 | 12.531 |
| 13 | Gastrointestinal Endoscopy | 2 | 303 | 2 | 9.427 |
| 14 | IEEE/ACM Transactions on Computational Biology and Bioinformatics | 2 | 316 | 2 | 3.71 |
| 15 | Journal of Biomedical Informatics | 2 | 334 | 2 | 6.317 |
| 16 | Journal of Clinical Oncology | 2 | 481 | 2 | 44.544 |
| 17 | Journal of Investigative Dermatology | 2 | 431 | 2 | 8.551 |
| 18 | Pattern Recognition | 2 | 395 | 2 | 7.74 |
| 19 | Plos Medicine | 2 | 239 | 2 | 11.069 |
| 20 | Plos One | 2 | 562 | 2 | 3.24 |
SCR: Standard Competition Ranking; TNP: Total Number of publications; TNC: Total Number of Citations; IF: Impact factor.
The top 100 Keyword Plus were visualized and summarized using WorldCloud analysis for top 100 cited articles on AI, ML, and cancer research documents. The most frequently occurring keywords were: “Human” (85), “female” (76), “humans” (69), “aged” (57),’ adult (47), “sensitivity and specificity” (43), “artificial neural network” (38), “middle aged” (38), “artificial intelligence” (37), “computer-assisted” (31), “mammography” (30), “algorithms” (29), “breast cancer” (29), “machine learning” (29), and “deep learning” (26), among others keyword (Figure 2).
Figure 2.
WordCloud analysis of the top 100 Keywords based on the frequency occurrence of the keyword.
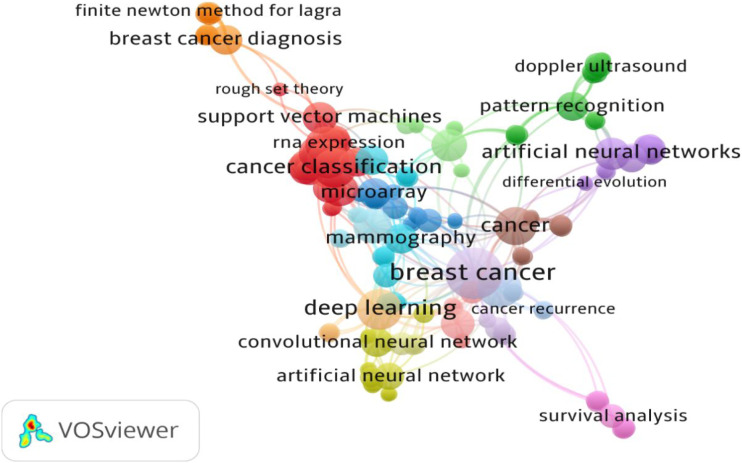
As a result of the co-occurrence analysis of Keywords Plus, a total of 179 keywords grouped in 16 clusters were uncovered with links (L=656) and a Total link Strength (TLS=688). Research involving breast cancer emerged as the hot researched area (L=48, TLS=56), followed by machine learning (L=27, TLS=33), and cancer classification (L=24, TLS=30), among others (Figure 3).
Figure 3.
Co-occurrence analysis of Keywords Plus based on the Total Links Strength (TLS).
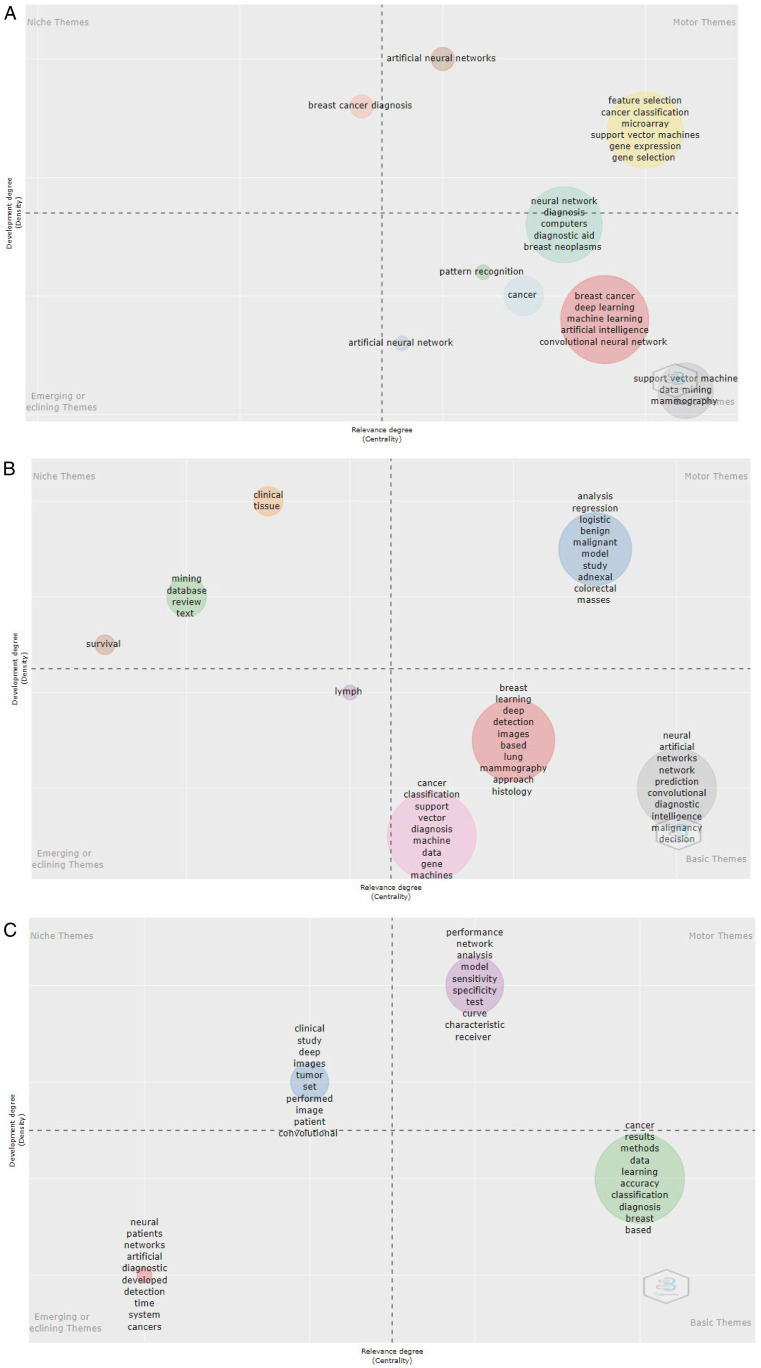
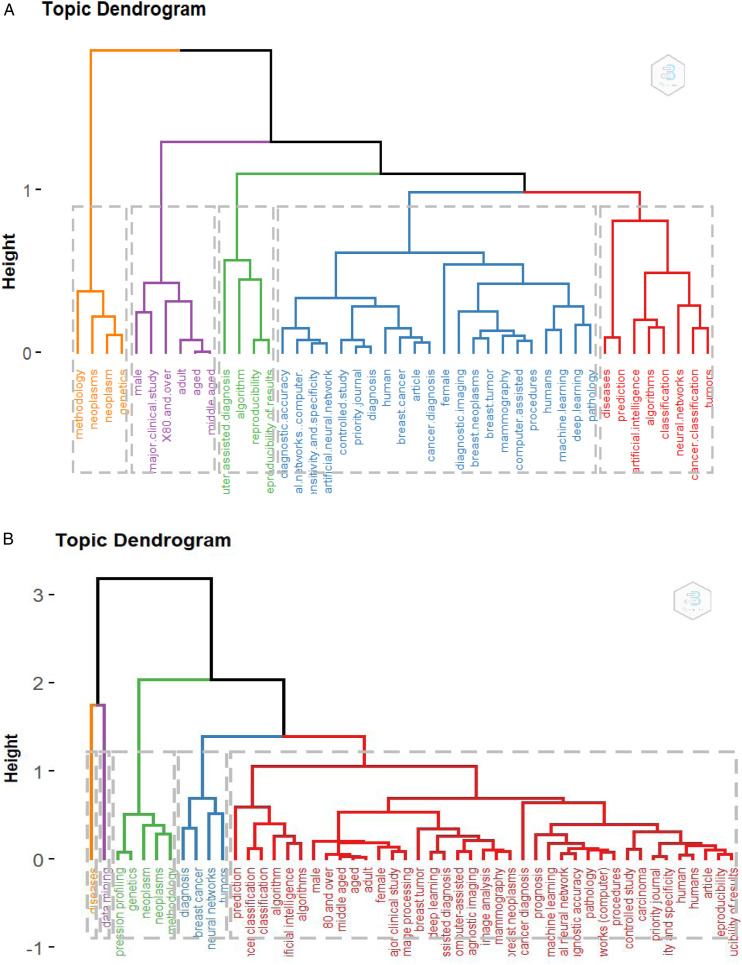
Figure 4 shows the conceptual structure analysis using a thematic map, the horizontal axis denotes the Relevance Degree (Centrality) and the vertical axis represents Development Degree (Density), and four quadrants comprise as: first quadrant (top right): motor-themes, both important and well-developed; second Quadrant (top left): Niche themes or Highly Development and Isolated Themes, which have developed well but are not important for the current field; third quadrant (bottom left): emerging topic or disappearing themes for AI and ML in Cancer topics; fourth quadrant (bottom right): basic themes or basic and Transversal Themes, it is an important to the field AI and ML in Cancer research, but not well-developed. The basic concept of topic themes published in the file is presented in (Figures 4A–4C).
Figure 4.
Conceptual structure analysis using thematic map analysis for (A) KeyWord Plus, (B) Authors Keywords, and Title (C) for Topics reported in Artificial Intelligence and Machine learning in cancer literature.
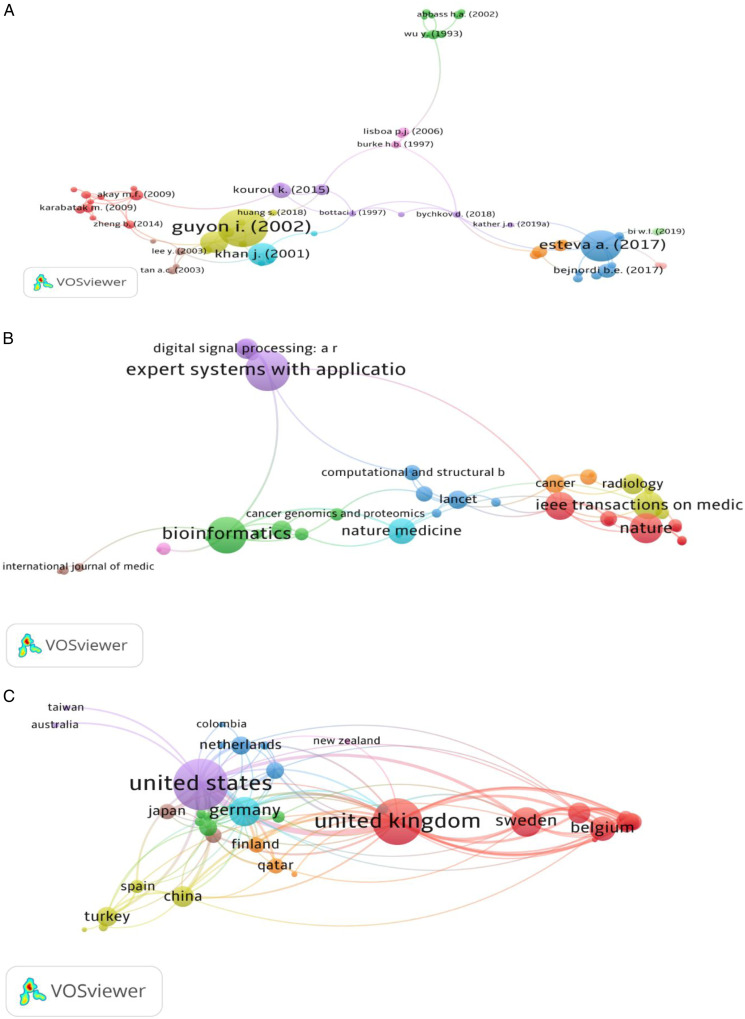
Analysis of the top 100 cited articles based on the number of received citations is depicted by Figure 5. A total of 59 top-cited articles formed 11 clusters with Links 85. The top-cited document was published by Guyon I (2002) and received (L=5758), followed by Esteve A. (2017), which received (L=3815) times (Figure 5A).
Figure 5.
Co-citation analysis between the top 100 cited documents by authors (A), Journal Sources (B), and country (C).
As previously mentioned, the initial analysis comprised a total of 57 journals. Only 41 journals met the threshold and formed 9 clusters with (TLS=79) times. Expert systems with the application (TLS=15), followed by Bioinformatics (TLS=13), Nature (TLS=10) and Nature medicine (TLS=9) times (Figure 5 B).
Only 39 countries met the threshold and are shown in the co-citations analysis across 9 clusters among the total contributed countries. The USA was the top-ranked country with (TLS=115), followed by the United Kingdom (TLS=95), Germany (TLS=40), Belgium (TLS=29), and the Netherlands (TLS=17) among other reported countries (Figure 5C).
The common conceptual structure for research in a dataset provides a clearer understanding for published research direction in the field. The common research, based on keywords associated with AI and ML in cancer research within the retrieved documents, was investigated using multiple correspondence analysis (MCA). The 50 keywords analyzed were clustered into five clusters. The first cluster included eight keywords (diseases, prediction, artificial intelligence, Algorithms, Classification, Neural networks, Cancer classification, and Tumor). The second cluster included had 22 keywords (Diagnostic accuracy, Networks computer, Sensitivity and Spasticity, controlled study, Diagnostic imaging, machine learning, deep learning, and pathology) as seen in Figure 6A.
Figure 6.
Multiple Correspondence Analysis (MCA) (A) and Correspondence Analysis (CA) (B) associated with Artificial Intelligence and Machine Learning in cancer literature analysis based on Keyword Plus.
Correspondence Analysis (CA) shows that the keywords most associated with AI and ML in cancer research studies are mainly grouped in two clusters 2 keywords (disease and data mining), 4 keywords (diagnosis, breast cancer, neural network, and tumors), 39 keywords (prediction, algorithms, and image processing computer-assisted), respectively (Figure 6B).
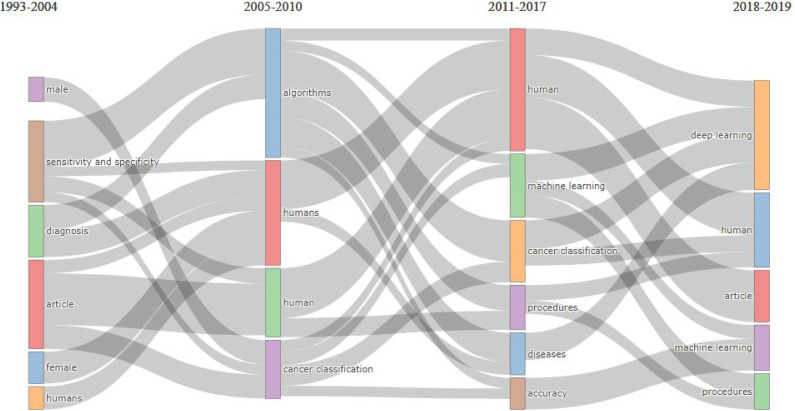
The identified themes and visualization of the topic evolution in AI and ML in cancer literature from 1993 to 2019 were explored. A thematic growth of the themes of research in AI and ML in cancer was detected in each period considering their keywords and evolution across four temporal periods, that is, (1993–2004), (2005–2010), (2011–2017), and (2018–2019) as shown as Figure 7. The identified themes with topic reported in each cluster, Time slice, Thematic Evolution themes, and Callon Centrality measures for a given cluster the intensity of its links with other clusters, and Callon Density to characterizes the strength of the links that tie the words making up the cluster of AI and ML in Cancer to be together are reported Supplemental Table S2.
Figure 7.
Thematic evolution of the research in Artificial Intelligence and Machine learning in cancer literature using: (A) KeyWords Plus, (1993–2019).
Top-Cited Articles by Cancer Types, Diagnosis, Prevention, and Treatment
The top-cited articles were systematically classified into specific cancer types Artificial Intelligence and Machine Learning was adopted in treatment, diagnosis, and possible prevention (Table 5). Most of the top-cited (37) innovative research focuses on breast cancer ML and AI adoption characteristics were focused on convolutional/artificial neural networks (ANN/CNN), deep learning (DL), and vector machines.15,39-74 Similarly, seven top-cited articles adopted image-based machine learning, DL and ANN in colorectal cancer diagnosis,75-81 (2) articles on Esophageal cancer research (two articles) used ANN and DL for detection,82,83 (2) articles on Gastric,84,85 (28) articles on Gene Expression, Multiple Cancer Prediction and diagnosis,5,12,14,16,86-108 (1) article on Head and Neck, 109 (1) article on Liver cancer, 110 (10) articles on Lungs cancer,111-120 (4) articles on Ovary,121-124 Pancreatic, 125 (4) research article on Prostate cancer,126-129 (1) article on Renal, 130 and (3) articles on Skin13,131,132 adopted Multiple monitoring and parametric MRI techniques along with ANN, Support Monitoring Machine and DL in profiling the cancer prevalence. Lastly, we classified generalized researches on tissues and gene expressions and reviews (27) articles that explored an all-inclusive approach in cancer prognostics such as gene expressions, therapy, and predictions.
Table 5.
Classification of Cancer types, Innovative Approach of artificial intelligence/Machine Learning in Predicting, Diagnosis, and Prevention.
| s/n | Cancer types | References | Innovative Approach of AI and ML | Applications |
|---|---|---|---|---|
| 1 | Breast cancer | 15,39-74 | Artificial neural network (ANNs) Deep learning or machine learning techniques Deep learning (DL) mammography-based model Convolutional neural networks (CNN) Support vector machine (SVM), genetically optimized neural network model (GONN), supporting vector machine classifier (RS_SVM) Computer-aided diagnosis (CAD) Particle swarm optimized wavelet neural network (PSOWNN) Least square support vector machine (LS-SVM) | ML and AI approach in detecting breast cancer types. A combined neural network and decision trees model for prognosis of breast cancer relapse. It is recommended that radiologists use an artificial intelligence support system for mammography in breast cancer detection without lengthening their reading time to improve their cancer detection at mammography Similarly, convolutional neural networks (CNN) outperformed the handcrafted feature-based classifier that was used to classify BCs based on histology images into benign and malignant, as well as benign and malignant sub-classes The SVM classifier for automatic classification of normal and malignant breast conditions was used to evaluate the feasibility of using thermal imaging as a potential tool Using particle swarm optimized wavelet neural network (PSOWNN) classifier improves classification accuracy for detecting breast abnormalities in digital mammograms Deep learning algorithms were applied for the detection of lymph node metastases The hybrid 4 algorithm of K-means and support vector machine algorithms shows promise in breast cancer diagnosis and time savings during the training phase |
| 2 | Colorectal cancer | 75-81 | Image-based machine learning, deep learning, artificial neutral network | Findings in the application of deep/machine learning and AI show that they extract more prognostic information from colorectal tissue morphology of colorectal cancer than human observers. Others proposed a new method of SC-CNN, and NEP produces the highest average F1 score relative to other approaches that benefit pathology practice in terms of quantitative analysis of tissue constituents. Similarly, combining SELDI-TOF mass spectrometry and artificial neural networks in the analysis of serum protein yields significantly increased sensitivity and specificity values for detecting and diagnosing colorectal cancer |
| 3 | Esophageal cancer | 82,83 | Artificial neural networks and deep learning | Deep learning was developed to detect esophageal cancer using convolutional neural networks (CNNs), which facilitated analyzing stored endoscopic images in record time with high sensitivity, which can also aid in early detection and prognosis |
| 4 | Gastric cancer | 84,85 | Convolutional neural network, deep learning | Deep learning determined microsatellite instability directly that gastrointestinal cancer responds exceptionally well to immunotherapy. By adopting, deep residual learning can predict MSI directly from H&E histology, which can immunize some patients with gastrointestinal cancer. Similarly, AI constructed CNN system processed numerous stored endoscopic images in a short time with a clinically relevant diagnostic ability to detect gastric cancer |
| 5 | Gene expression, multiple cancer prediction and diagnosis | 5,12,14,16,86-108 | Support vector machines, extreme learning machine, bayesian regularization, text mining, support vector, machine, genetic algorithms, artificial neural networks, multimodal deep learning | ANN supported tumor diagnosis and identification of candidates for therapy. In predicting gene sets of potential cancer cases, the genetic algorithm and support vector machine were used. Meanwhile, ML was used to select genes for cancer classifications. Text mining was used to sift through microRNA-cancer literature. The use of ANN improved the accuracy of predicting cancer survival |
| 6 | Head and neck cancer | 109 | Radiomic machine learning classifiers | Through radiomics-based prognostic analysis, it was possible to predict the survival of patients with head and neck cancer using machine learning |
| 7 | Liver cancer | 110 | Deep learning based multi-omics integration | A novel approach using deep learning to identify multi-omics features was linked to the differential survival of HCC patients and adaptable for future prediction of HCC prognosis prediction |
| 8 | Lungs cancer | 111-120 | Multi-crop convolutional neural networks, neural networks, deep learning, artificial neutral network | Significant advances in lung cancer research have been made by using convolutional network architecture through deep learning systems to achieve performance at classifying nodule type that outperforms one of the classical machine learning approaches. Some studies used a deep convolutional neural network (inception v3) trained on cancer genome atlas whole-slide images to accurately and automatically classify them as LUAD, LUSC, or normal lung tissue. Deeping learning methods generated contours to reduce the contouring time of OARs for lung radiotherapy while conforming to local clinical standards. ANN network was also used to assist radiologists in differentiating benign and malignant pulmonary nodes |
| 9 | Ovary cancer | 121-124 | Transvaginal B mode and color Doppler imaging | The transvaginal B mode and color Doppler imaging were applied to derive analytical logistics regression in scoring and discriminating between malignant and benign adnexal masses before operation. Particularly in ovarian cancer prediction, new models were derived to improve on the predictions of benign and malignant masses |
| 10 | Pancreatic cancer | 125 | Neural network analysis | Artificial neutral network analysis of EUS sequences was used to diagnose and distinguish between normal pancreas, chronic pancreatitis, and pancreatic cancer. The methods support artificial neural network processing of EUS elastography digitalized movies, enabling an optimal prediction of the types of pancreatic lesions |
| 11 | Prostate cancer | 126-129 | Artificial neural network, multi-parametric MRI, multiple monitoring approaches | Artificial neural networks (ANN) methods were used to detect prostate cancer in men with total prostate-specific antigen, which is superior in accurately predicting the ANN compared to conventional PSA parameters |
| 12 | Renal cancer | 130 | Neural network analysis | The study expanded evidence on the proteomic profiling of urinary proteins in renal cancer by using surface-enhanced laser desorption ionization and neural-network analysis |
| 13 | Skin cancer | 13,131,132 | Deep learning algorithm, support vector machine, deep neural network | A deep learning algorithm was used to classify clinical images of skin diseases such as basal cell carcinoma, squamous cell carcinoma, intraepithelial carcinoma, actinic keratosis, seborrheic keratosis, malignant melanoma, melanocytic nevus, lentigo, pyogenic granuloma, hemangioma, dermatofibroma, and wart. In other studies, artificial intelligence was shown to be capable of classifying skin cancer at a level of competence comparable to dermatologists. A novel approach for rapid, automated skin cancer diagnosis is supported by neural network analysis of near-infrared fourier transforms Raman spectra |
Discussion
The presented bibliometric study highlighted the characteristics of the 100 cited articles on AI, ML, and cancer research from the Scopus database. Our analysis shows the publication’s growth on AI and ML in cancer research. The cumulative amount of published documents showed increased interest in ML and AI techniques and applications that have improved the predictive ability and diagnosis in cancer research.5,96
AL and ML techniques were introduced in the medical diagnosis of cancer imaging starting from the late 1990s, 92 cancer immunotherapy, 133 and cancer diagnosis and prognosis. 134 Recently AI and ML have been increasingly implemented across healthcare and medical research.135-138 However, AI and ML-based approaches will have a more dominant influence in digital healthcare for diagnosis and treatment in the coming years.96,137 Among the articles published in 2015 is a review paper titled “Machine Learning Applications in Cancer Prognosis and Prediction,” which highlighted the use of AI in healthcare to identify the risk factors in patients with cancer.96,137 Another top-cited article focused on using AI to detect lymph node metastases in women with breast cancer in a healthcare unit. 139 Those published documents were premised on AI, ML to predict, diagnose, and control, or classify cancer diseases.
Breast cancer research was prominent in adopting AI and ML as evidence has shown it is a significant cause of death among women. 140 Meanwhile, it is confirmed that the early detection and accurate diagnosis of this disease might offer a long survival rate for the patients by using advanced tools such as artificial neural networks to predict breast cancer from mammographic findings.39,54,141 Using DL broader family of machine learning methods based on artificial neural networks techniques can be used to diagnose the disease of breast cancer with high classification accuracies.39,41,142 In addition, its application to the medical field can accurately detect breast cancer on screening mammograms using an “end-to-end” training approach. 40 ML techniques currently used in this field were to screen breast cancer with mammography as an effective method in reducing breast cancer–related death, or Neural network approach for early detection and identification of women at high risk of developing breast cancer 143 and prognosis of breast cancer. 40
In research on colorectal cancers, deep learning advances the approach of proposing a new approach using a new method of spatially Constrained Convolutional Neural Network (SC-CNN), and Neutral endopeptidase (NEP). Other researchers combine Surface-enhanced laser desorption ionization-time of flight mass spectrometry (SELDI-TOF) mass spectrometry and artificial neural networks to analyze serum protein with high production sensitivity and specificity values for detecting and diagnosing colorectal cancer.75,80 The esophageal and gastric cancer prognosis were conducted through CNNs constructed through AI to support early detection and speedy prediction.83,84 The application of AI and ML supported these outcomes and was evidence-based on the approaches’ speed and accuracy. Like in gene expression research applications, ANN supported tumor diagnosis, survival of patients, classification and identification of candidates for therapy, and genetic algorithm and support vector machine supported in predicting gene sets of potential cancer cases.82,88,144
AI and ML innovative approach cut across all cancer research to advance general healthcare practices. Significant achievements in lung cancer research have adopted convolutional network architecture through DL system achieves performance at classifying nodule type that surpasses one of classical machine learning approaches. Some studies used trained a deep convolutional neural network (inception v3) on whole-slide images of Cancer Genome Atlas to accurately and automatically classify them into LUAD, LUSC, or normal lung tissue.114,115 In ovarian prognosis, Transvaginal B mode and Color Doppler Imaging were applied to derive discriminating between malignant and benign adnexal masses before operation.97,124 While in liver cancer research, a novel approach using DL was used to identify multi-omics attributes of survival of HCC patients and adaptable for future HCC prognosis prediction. 110
Remarkable progress was made in adapting AI and ML to diagnose, predict, and control pancreatic, renal, skin, and prostate cancer research.86,92,125,130,145 A deep learning algorithm was tested to classify clinical images of skin diseases-basal cell carcinoma, squamous cell carcinoma, intraepithelial carcinoma, actinic keratosis, seborrheic keratosis, malignant melanoma, melanocytic nevus, lentigo, pyogenic granuloma, hemangioma, dermatofibroma, and wart.146,147 The study extended evidence on the Proteomic Profiling of Urinary Proteins in Renal Cancer by adopting Surface-Enhanced Laser Desorption Ionization and Neural Network Analysis. 130 Applied artificial neutral network analysis of sequences of EUS to diagnose and differentiate normal pancreas, chronic pancreatitis, and pancreatic cancer. The methods support artificial neural network processing of EUS elastography digitalized movies, enabling an optimal prediction of the types of pancreatic lesions. 125
Generally, the analysis shows that global interest among the scientific community is based on the number of times an article is cited, and we notice some articles received more than 1000 classical citations with the current study period.12-14,102 These studies can be considered baseline and fundamental building blocks for further studies. The articles describe, for example, the use of Support Vector Machines (SVM) in cancer classification, 12 Skin cancer, 13 and classification diagnostic and prediction of cancer using gene expression, Artificial Neural Network applications, 14 and use of automatic techniques to classify and validate cancer tissue in human. 102 A potential aiding factor to this rising interest can be attributed to the exponential growth of computing power and data storage capacity over the past few years.148,149 Aerts Hugo J. W. L, Collins William P and Doi Kunio were the most productive authors for using AI and ML technology in cancer research. The study also highlighted the multidisciplinary links among the authors and the interdisciplinary nature of AI and ML in cancer research.
Regarding the geographical distribution of the articles across countries, the USA published 30 articles and was the leading country in productivity based on a single country and multiple country publication, followed by China. Moreover, based on the analysis of Multiple Countries Publication (MCP), we notice that Germany and Belgium had a high ratio of collaboration in AI, ML, and cancer research productivity, respectively, which surpassed those of the China, USA, and the United Kingdom. This evidence leads us to believe that future collaboration for research in the field is highly required among the scientific community. Our findings show that the impact of articles published by the USA is higher. Nevertheless, and due to the massive technological advancements and rising interest, it is believed that the quality and quantity of studies coming from China and several other countries are expected to improve in the future.
In terms of journals, it was observed that the journal “Expert Systems with Applications” is the favorite publication destination, followed by the journal of “Scientific Reports”, the journal of “Radiology,” and the Journal of “Artificial Intelligence in Medicine”. We believe that further support and interest from publishing houses and journals such as launching special issues for works investigating the use of AI, ML in cancer research will help promote this field of research further. Through keyword analysis, it was observed that the focus on AI, ML, and cancer research within the distributed clusters offered an indicator that there is noticeable growth. There is a gradual shifting from “artificial neural network application using neural network (computer application)” to “use of computer application” in image processing, different diagnosis type of cancer such as (breast tumor, breast neoplasms, breast cancer, and mammography) over the past 26 years. Therefore, we believe that scientific breakthroughs might be related to these hotspots in recent years. As for future applications, we suggest that authors select research topics from the map and demonstrate their importance as a frontier hotspot. This approach might also support the funding organization in enhancing the research and directing the funds to a specific area yet to be covered by the scientific community.
Although this analysis is considered as the first comprehensive study of publications for AI and ML in cancer research, we also believe that there are some limitations regarding the survey techniques adopted in this study. Even though Scopus represents a comprehensive, abstract, and citation database with enriched data and linked scholarly content, including other similar databases such as Google Scholar, Web of Science, and PubMed can provide more insights and outlook into the field. Our study included only certain documents, such as original research articles and reviews published in English. Extending the analysis to include other document types and articles can also prospect future studies.
Despite the limitations mentioned above, and the best of our knowledge, this study is the first bibliometric analysis of publications involving AI and ML techniques for cancer research. Through this effort, we sought to provide researchers in the field and the general academic community with an overview of the evolution of publications over the past 26 years, current research status, and future research trends concerning the use of AI and ML techniques in cancer research. This study confirms our initial hypothesis that the use and implementation of recent computing paradigms in healthcare are enormously increasing.
Implications for Cancer Research and Control
AI and ML have the innovative potential and technological toolkit to diagnose, predict, and control cancer for global clinical and medical practices. This study demonstrated remarkable progress in clinical practices for cancer research and offered new methodological window for reviewing cancer research. Our findings showed that AI and ML have gained global interest and adoption, promoting accurate diagnosis of various cancer types. Nevertheless, significant challenges must be sorted to encourage robust global adoption. Ethical and private concerns should be resolved, especially those concerning patients and their privacy, which calls for global AI and ML control to ensure transparency, data protection, and best practices. Patient voice and opinion become relevant in the global adoption of AI and ML. Future studies should explore perception, attitude, and willingness to allow private information to go through the analytical process. The approach also supports disseminating AI and ML awareness in health systems to increase global support.
The study provides a comprehensive theoretical contribution of the top 100 cited articles in AI and ML in cancer research published from 1993 to 2019. AI and ML have ushered the theoretical approaches in understanding human behavior and potential behavioral changes. 150 These theories can develop insights by predicting behavioral changes of people with high-risk of cancer or undergoing cancer treatments. Similarly, AI and ML have achieved cutting-edge technical performance in precise cancer control and prevention. 151 While traditional screening methods have advanced research in the field, AI and ML can accurately monitor the health statuses of cancer patients, which can support the management of patients. 152 In particular, early detection of cancer through the adoption of AI and ML stands as one of the innovations of the 21st century that can significantly help control the prevalence of cancer globally. 153
The mapping and visualizing analysis of the keywords related to AI and ML in cancer research provides in-depth WordCloud analysis of the top 100 keywords based on the frequency, thematic analysis, structure, and development status of the topics found on similarities into five distinguish clusters, systematically and intuitively reveal the subject structure and development status and make predictions subject in the fields. From the systematic and bibliometric analysis findings, we revealed that AI and ML have helped push the limits of cancer control and reshape the future for better diagnosis, prevention, management, and control of cancer. Therefore, our research contributes to the literature on AI and ML in cancer control by helping researchers optimize research topic choices, seek collaboration with appropriate partners and scholars globally to understand better the current status of AI and ML in cancer research. Furthermore, it will assist researchers in staying up-to-date with the recent development and future research in the field of AI and ML application in cancer diagnosis, prevention, management, and control.
Furthermore, since the top-cited articles have shown that the global adoption is not parallel across different types of cancers, it becomes necessary to encourage the adoption of AI and ML in other unique prevailing cancers. While it is acknowledged that AI and ML advances the controlling, diagnosing and predicting various cancers types, clinical and medical practitioners need to be included in the adoption and learning to balance traditional cancer screening and treatment approval with technological advancement.
Conclusion
The progress of AI and ML techniques for cancer research is acknowledged, given the top 100 cited articles indexed in Scopus databases. The observed improvement towards cancer diagnoses and prevention gives insight into the rising global attention from the scientific community towards implementing advanced technology in this area. The progress in cancer research and control has enabled the early detection and accurate diagnosis of cancer through artificial neural networks to predict malignancies. Many cancer types have adopted deep learning in proposing a new approach using a new method of spatially SC-CNNs and NEP. AI and ML have achieved advanced precision in cancer control and prevention through accurately monitor the health statuses of cancer patients, which can support the management of patients.
One keynote for future consideration is the ethical and private concerns which call for global AI and ML control to ensure transparency, data protection, and best practices. Patient voice and opinion must be considered in adopting ML and AI in cancer research and control. Future studies should explore perception, attitude, and willingness to allow private information to go through analytical processes. The approach also supports disseminating AI and ML awareness in health systems to increase global awareness.
Supplemental Material
Supplemental Material for Artificial Intelligence and Machine Learning in Cancer Research: A Systematic and Thematic Analysis of the Top 100 Cited Articles Indexed in Scopus Database by Ibrahim H. Musa, Ibrahim Zamit, Taha H. Musa, Hassan H. Musa, Andrew Tassang, and Tosin Y. Akintunde in Cancer Control.
Author Contributions: Ibrahim Hussein Musa: Substantial contributions to the study, which include (Conceptualization/design, Methodology, Formal analysis, and Data curation). Lukman O. Afolabi: Writing, review of analysis, editing of the revised manuscript and providing final approval of the version to be published. Ibrahim Zamit: Methodology, Investigation, and Formal analysis, Writing – review or editing of the manuscript and provides final approval of the version to be published. Taha Hussein Musa: Conceptualization/design, Methodology, Formal analysis, Resources, Writing – drafting the initial manuscript, and approved the version to be published. Hassan Hussein Musa: Writing – review or editing of the manuscript and provide the final approval of the version to be published. Andrew Tassang: Writing – review or editing of the manuscript and provide final approval for all aspects of the work of the version to be published. Tosin Yinka Akintunde: Writing – review or editing of the manuscript, drafted the article, revised it critically for important intellectual content, and provided final approval of the version to be published. Wei Li: Gave final approval of the version to be published, Funding acquisition, and review of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Jiangsu Provincial Health Development Center Open Project in 2021 (JSHD2021018).
Data Availability: All data analyzed during this study are included in this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Ibrahim H. Musa https://orcid.org/0000-0001-8060-6341
Lukman O. Afolabi https://orcid.org/0000-0001-7659-128X
Ibrahim Zamit https://orcid.org/0000-0002-5517-5102
Taha H. Musa https://orcid.org/0000-0003-4452-1943
Hassan H. Musa https://orcid.org/0000-0003-0227-6484
Andrew Tassang https://orcid.org/0000-0001-7274-5042
Tosin Y. Akintunde https://orcid.org/0000-0002-9392-8726
References
- 1.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134-1150. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71:209-249. [DOI] [PubMed] [Google Scholar]
- 3.Afolabi LO, Afolabi MO, Sani MM, et al. Exploiting the CRISPR-Cas9 gene-editing system for human cancers and immunotherapy. Clinical & translational immunology 2021;10(6). 10.1002/cti2.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afolabi LO, Bi J, Li X, et al. Synergistic Tumor Cytolysis by NK Cells in Combination With a Pan-HDAC Inhibitor, Panobinostat. Frontiers in immunology 2021;12(701671). 10.3389/fimmu.2021.701671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman PH, Rosen DB, et al. Artificial Neural Networks Improve the Accuracy of Cancer Survival Prediction; 1996:857-862. [DOI] [PubMed] [Google Scholar]
- 6.Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Therapeut. 2016;38:1551–1566. [DOI] [PubMed] [Google Scholar]
- 7.Kamal VK, Kumari D. Use of artificial intelligence/machine learning in cancer research during the COVID-19 Pandemic. Asian Pacific J Cancer Care. 2020; 5:S1 doi: 10.31557/apjcc.2020.5.s1.251-253 [DOI] [Google Scholar]
- 8.Dananjayan S, Raj GM. Artificial Intelligence during a pandemic: The COVID-19 example. Int J Health Plann Manag. 2020;35:1260-1262. doi: 10.1002/hpm.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal MJ, Javed Z, Sadia H, et al. Clinical applications of artificial intelligence and machine learning in cancer diagnosis: looking into the future. Cancer Cell Int. 2021;21:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ognjanovic I. Artificial intelligence in healthcare. Stud Health Technol Inf. 2020;274:189-205. doi: 10.3233/SHTI200677 [DOI] [PubMed] [Google Scholar]
- 11.Dlamini Z, Francies FZ, Hull R, Marima R. Artificial intelligence (AI) and big data in cancer and precision oncology. Comput Struct Biotechnol J. 2020;18:2300-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46:389-422. doi: 10.1023/A:1012487302797 [DOI] [Google Scholar]
- 13.Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi: 10.1038/nature21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan J, Wei JS, Ringnér M, et al. Classification and diagnostic prediction of cancers using gene expression profiling and artificial neural networks. Nat Med. 2001;7:673-679. doi: 10.1038/89044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bejnordi BE, Veta M, Van Diest PJ, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA, J Am Med Assoc. 2017;318:2199-2210. doi: 10.1001/jama.2017.14585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz JA, Wishart DS. Applications of machine learning in cancer prediction and prognosis. Cancer Inf. 2006;2:59-77. doi: 10.1177/117693510600200030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldenberg SL, Nir G, Salcudean SE. A new era: artificial intelligence and machine learning in prostate cancer. Nat Rev Urol. 2019;16:391-403. [DOI] [PubMed] [Google Scholar]
- 18.Houssami N, Lee CI, Buist DSM, Tao D. Artificial intelligence for breast cancer screening: Opportunity or hype? Breast. 2017;36:31-33. [DOI] [PubMed] [Google Scholar]
- 19.Le EPV, Wang Y, Huang Y, Hickman S, Gilbert FJ. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366. [DOI] [PubMed] [Google Scholar]
- 20.Golden JA. Deep learning algorithms for detection of lymph node metastases from breast cancer. JAMA. 2017;318:2184. [DOI] [PubMed] [Google Scholar]
- 21.Mori Y, Kudo S-e. Detecting colorectal polyps via machine learning. Nature Biomedical Engineering. 2018;2:713-714. [DOI] [PubMed] [Google Scholar]
- 22.Wang K-W, Dong M. Potential applications of artificial intelligence in colorectal polyps and cancer: Recent advances and prospects. World J Gastroenterol. 2020;26:5090-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrand LA, Pierce CJ, Dennis M, Paracha M, Maoz A. Artificial intelligence for histology‐based detection of microsatellite instability and prediction of response to immunotherapy in colorectal cancer. Cancers. 2021;13:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jianzhu B, Shuang L, Pengfei M, Yi Z, Yanshu Z. Research on early warning mechanism and model of liver cancer rehabilitation based on CS-SVM. J Healthc Eng. 2021. doi: 10.1155/2021/6658776 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Kawuki J, Ghimire U, Papabathini SS, Obore N, Musa TH. A Bibliometric Analysis of Childhood Obesity Research from China Indexed in Web of Science. https://doi.org/10.21037/jphe-20-95. Journal of Public Health and Emergency; 2021. [Google Scholar]
- 26.Musa TH, Ahmad T, Li W, et al. A bibliometric analysis of global scientific research on scrub typhus. BioMed Res Int. 2020;16:5737893. doi: 10.1155/2020/5737893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musa TH, Akintunde TY, Musa HH, Ghimire U, Gatasi G. Malnutrition Research Output : A Bibliometric Analysis for Articles Index in Web of Science between 1900 and 2020, 18; 2021. [Google Scholar]
- 28.Akintunde TY, Musa TH, Musa HH, et al. Bibliometric analysis of global scientific literature on effects of COVID-19 Pandemic on Mental Health. Asian journal of psychiatry. 2021;63:102753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akintunde TY, Musa TH, Musa HH, Ibrahim E, Muhideen S, Kawuki J. Mapping the global research output on Ebola vaccine from research indexed in web of science and scopus : a comprehensive bibliometric analysis. Hum Vaccines Immunother. 2021;00:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musa HH, El-Sharief M, Musa IH, Musa TH, Akintunde TY, Akintunde TY. Global scientific research output on sickle cell disease: a comprehensive bibliometric analysis of web of science publication. Scientific African. 2021;12:e00774. [Google Scholar]
- 31.Yu Y, Li Y, Zhang Z, et al. A bibliometric analysis using VOSviewer of publications on COVID-19. Ann Transl Med. 2020;8:816. doi: 10.21037/atm-20-4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan MS, Ullah W, Riaz IB, et al. Top 100 cited articles in cardiovascular magnetic resonance: a bibliometric analysis. J Cardiovasc Magn Reson. 2016;18:87. doi: 10.1186/s12968-016-0303-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akintunde TY, Chen S, Musa TH, et al. Tracking the Progress in COVID-19 and Vaccine Safety Research – a Comprehensive Bibliometric Analysis of Publications Indexed in Scopus Database; 2021. doi: 10.1080/21645515.2021.1969851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musa IH, Musa TH, Zamit I, Okeke M. Artificial Intelligence and Machine Learning in Oncology: Historical Overview of Documents Indexed in the Web of Science Database. Eurasian J Med Oncol. 2021;5:239-248. [Google Scholar]
- 35.Coile RC. Lotka’s frequency distribution of scientific productivity. J Am Soc Inf Sci. 1977;28:366-370. doi: 10.1002/asi.4630280610 [DOI] [Google Scholar]
- 36.Dervis H. Bibliometric analysis using bibliometrix an R package. J Scientometr Res. 2019;3:156-160. doi: 10.5530/JSCIRES.8.3.32 [DOI] [Google Scholar]
- 37.Van Eck NJ, Waltman L. VOSviwer Manual Version 1.6.10. CWTS Meaningful metrics; 2019. [Google Scholar]
- 38.GraphPad Software . GraphPad Prism Version 6.03 for Windows. La Jolla Calif; 2014. [Google Scholar]
- 39.Andina D. (2011) Expert Systems with Applications WBCD breast cancer database classification applying artificial metaplasticity neural network. 38:9573–9579. [Google Scholar]
- 40.Ramos-jime G, Alba-conejo E. (2003) A combined neural network and decision trees model for prognosis of breast cancer relapse. 27:45–63. [DOI] [PubMed] [Google Scholar]
- 41.Shen L, Margolies LR, Rothstein JH, Fluder E, Mcbride R. Deep Learning to Improve Breast Cancer Detection on Screening Mammography, 1–12; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez-ruiz A, Krupinski E, Mordang J, Schilling K. Detection of Breast Cancer with Mammography : Effect of an Artificial Intelligence Support System; 2019. [DOI] [PubMed] [Google Scholar]
- 43.Yala A, Lehman C, Schuster T, Portnoi T, Barzilay R. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology. 2019;292:60-66. [DOI] [PubMed] [Google Scholar]
- 44.Bardou D, Zhang K, Ahmad SM. Classification of breast cancer based on histology images using convolutional neural networks. IEEE Access. 2018;6:24680-24693. [Google Scholar]
- 45.Abbass HA. An evolutionary artificial neural networks approach for breast cancer diagnosis. Artif Intell Med. 2002;25:265-281. [DOI] [PubMed] [Google Scholar]
- 46.Acharya UR, Ng EYK, Tan J-H, Sree SV. Thermography based breast cancer detection using texture features and support vector machine. J Med Syst. 2012;36:1503-1510. [DOI] [PubMed] [Google Scholar]
- 47.Akay MF. Support vector machines combined with feature selection for breast cancer diagnosis. Expert Syst Appl. 2009;36:3240-3247. [Google Scholar]
- 48.Albarqouni S, Baur C, Achilles F, Belagiannis V, Demirci S, Navab N. Aggnet: deep learning from crowds for mitosis detection in breast cancer histology images. IEEE Trans Med Imag. 2016;35:1313-1321. [DOI] [PubMed] [Google Scholar]
- 49.Azar AT, El-Said SA. Performance analysis of support vector machines classifiers in breast cancer mammography recognition. Neural Comput Appl. 2014;24:1163-1177. [Google Scholar]
- 50.Baker JA, Kornguth PJ, Lo JY, Williford ME, Floyd CE. Breast cancer: Prediction with artificial neural network based on BI-RADS standardized lexicon. Radiology. 1995;196:817-822. [DOI] [PubMed] [Google Scholar]
- 51.Becker AS, Marcon M, Ghafoor S, Wurnig MC, Frauenfelder T, Boss A. Deep Learning in Mammography. Invest Radiol. 2017;52:434-440. [DOI] [PubMed] [Google Scholar]
- 52.Bhardwaj A, Tiwari A. Breast cancer diagnosis using genetically optimized neural network model. Expert Syst Appl. 2015;42:4611-4620. [Google Scholar]
- 53.Chen D-R, Chang R-F, Kuo W-J, Chen M-C, Huang Yu.-L. Diagnosis of breast tumors with sonographic texture analysis using wavelet transform and neural networks. Ultrasound Med Biol. 2002;28:1301-1310. [DOI] [PubMed] [Google Scholar]
- 54.Chen HL, Yang B, Liu J, Liu DY. A support vector machine classifier with rough set-based feature selection for breast cancer diagnosis. Expert Syst Appl. 2011;38:9014–9022. [Google Scholar]
- 55.Chou S-M, Lee T-S, Shao YE, Chen I-F. Mining the breast cancer pattern using artificial neural networks and multivariate adaptive regression splines. Expert Syst Appl. 2004;27:133-142. [Google Scholar]
- 56.Chougrad H, Zouaki H, Alheyane O. Deep convolutional neural networks for breast cancer screening. Comput Methods Progr Biomed. 2018;157:19-30. [DOI] [PubMed] [Google Scholar]
- 57.Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: A Deep Learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450-46514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dheeba J, Albert Singh N, Tamil Selvi S. Computer-aided detection of breast cancer on mammograms: A swarm intelligence optimized wavelet neural network approach. J Biomed Inf. 2014;49:45-52. [DOI] [PubMed] [Google Scholar]
- 59.Floyd CE, Lo JY, Yun AJ, Sullivan DC, Kornguth PJ. Prediction of breast cancer malignancy using an artificial neural network. Cancer. 1994;74:2944-2948. [DOI] [PubMed] [Google Scholar]
- 60.Han Z, Wei B, Zheng Y, Yin Y, Li K, Li S. Breast Cancer Multi-classification from Histopathological Images with Structured Deep Learning Model. Sci Rep. 2017;7:4172-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C-L, Liao H-C, Chen M-C. Prediction model building and feature selection with support vector machines in breast cancer diagnosis. Expert Syst Appl. 2008;34:578-587. [Google Scholar]
- 62.Jerez JM, Molina I, García-Laencina PJ, et al. Missing data imputation using statistical and machine learning methods in a real breast cancer problem. Artif Intell Med. 2010;50:105-115. [DOI] [PubMed] [Google Scholar]
- 63.Karabatak M, Ince MC. An expert system for detection of breast cancer based on association rules and neural network. Expert Syst Appl. 2009;36:3465-3469. [Google Scholar]
- 64.Khan S, Islam N, Jan Z, Ud Din I, Rodrigues JJPC. A novel deep learning based framework for the detection and classification of breast cancer using transfer learning. Pattern Recogn Lett. 2019;125:1-6. [Google Scholar]
- 65.Liyang Wei L, Yongyi Yang Y, Nishikawa RM, Yulei Jiang Y. A study on several machine-learning methods for classification of malignant and benign clustered microcalcifications. IEEE Trans Med Imag. 2005;24:371-380. [DOI] [PubMed] [Google Scholar]
- 66.Polat K, Güneş S. Breast cancer diagnosis using least square support vector machine. Digit Signal Process. 2007;17:694-701. [Google Scholar]
- 67.Steiner DF, Macdonald R, Liu Y, et al. Impact of deep learning assistance on the histopathologic review of lymph nodes for metastatic breast cancer. Am J Surg Pathol. 2018;42:1636-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun W, Tseng T-L, Zhang J, Qian W. Enhancing deep convolutional neural network scheme for breast cancer diagnosis with unlabeled data. Comput Med Imag Graph. 2017;57:4-9. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Cruz-Roa A, Basavanhally A, et al. Mitosis detection in breast cancer pathology images by combining handcrafted and convolutional neural network features. J Med Imag. 2014;1:034003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Yang X, Cai H, Tan W, Jin C, Li L. Discrimination of breast cancer with microcalcifications on mammography by deep learning. Sci Rep. 2016;6:27327-27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H, Zheng B, Yoon SW, Ko HS. A support vector machine-based ensemble algorithm for breast cancer diagnosis. Eur J Oper Res. 2018;267:687-699. [Google Scholar]
- 72.Wolberg WH, Street WN, Mangasarian OL. Machine learning techniques to diagnose breast cancer from image-processed nuclear features of fine needle aspirates. Cancer Lett. 1994;77:163-171. [DOI] [PubMed] [Google Scholar]
- 73.Wu Y, Giger ML, Doi K, Vyborny CJ, Schmidt RA, Metz CE. Artificial neural networks in mammography: Application to decision making in the diagnosis of breast cancer. Radiology. 1993;187:81-87. [DOI] [PubMed] [Google Scholar]
- 74.Zheng B, Yoon SW, Lam SS. Breast cancer diagnosis based on feature extraction using a hybrid of K-means and support vector machine algorithms. Expert Syst Appl. 2014;41:1476-1482. [Google Scholar]
- 75.Bychkov D, Linder N, Turkki R, et al. Deep learning based tissue analysis predicts outcome in colorectal cancer. Sci Rep. 2018;8:3395-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bottaci L, Drew PJ, Hartley JE, et al. Artificial neural networks applied to outcome prediction for colorectal cancer patients in separate institutions. Lancet. 1997;350:469-472. [DOI] [PubMed] [Google Scholar]
- 77.Chen Y-d., Zheng S, Yu J-k., Hu X. Artificial neural networks analysis of surface-enhanced laser desorption/ionization mass spectra of serum protein pattern distinguishes colorectal cancer from healthy population. Clin Cancer Res. 2004;10:8380-8385. [DOI] [PubMed] [Google Scholar]
- 78.Ahmed FE. Artificial neural networks for diagnosis and survival prediction in colon cancer. Mol Cancer. 2005;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kather JN, Pearson AT, Halama N, et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat Med. 2019;25:1054-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sirinukunwattana K, Raza SEA, Tsang Y-W, Snead DRJ, Cree IA, Rajpoot NM. Locality sensitive deep learning for detection and classification of nuclei in routine colon cancer histology images. IEEE Trans Med Imag. 2016;35:1196-1206. [DOI] [PubMed] [Google Scholar]
- 81.Kather JN, Krisam J, Charoentong P, et al. Predicting survival from colorectal cancer histology slides using deep learning: a retrospective multicenter study. PLoS Medicine. 2019;16:e1002730-e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Y, Selaru FM, Yin J, et al. Artificial neural networks and gene filtering distinguish between global gene expression profiles of Barrett's esophagus and esophageal cancer. Cancer Research. 2002;62:3493-3497. [PubMed] [Google Scholar]
- 83.Horie Y, Yoshio T, Aoyama K, et al. Diagnostic outcomes of esophageal cancer by artificial intelligence using convolutional neural networks. Gastrointest Endosc. 2019;89:25-32. [DOI] [PubMed] [Google Scholar]
- 84.Hirasawa T, Aoyama K, Tanimoto T, et al. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer. 2018;21:653-660. [DOI] [PubMed] [Google Scholar]
- 85.Yokota T, Ishiyama S, Saito T, et al. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380-384. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y. Active learning with support vector machine applied to gene expression data for cancer classification. J Chem Inf Comput Sci. 2004;44:1936-1941. [DOI] [PubMed] [Google Scholar]
- 87.Peng S, Xu Q, Ling XB, Peng X, Du W, Chen L. Molecular classification of cancer types from microarray data using the combination of genetic algorithms and support vector machines. FEBS (Fed Eur Biochem Soc) Lett. 2003;555:358-362. [DOI] [PubMed] [Google Scholar]
- 88.Huang S, Cai N, Pacheco PP, Narrandes S, Wang Y, Xu W. Applications of support vector machine (SVM) learning in cancer genomics. CANCER GENOMICS PROTEOMICS. 2018;15:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Menden MP, Iorio F, Garnett M, et al. Machine learning prediction of cancer cell sensitivity to drugs based on genomic and chemical properties. PLoS One. 2013;8:e61318. doi: 10.1371/journal.pone.0061318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Statnikov A, Wang L, Aliferis CF. A comprehensive comparison of random forests and support vector machines for microarray-based cancer classification. BMC Bioinf. 2008;9:319-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spasić I, Livsey J, Keane JA, Nenadić G. Text mining of cancer-related information: review of current status and future directions. Int J Med Inf. 2014;83:605-623. [DOI] [PubMed] [Google Scholar]
- 92.Bi WL, Hosny A, Schabath MB, et al. Artificial Intelligence in Cancer Imaging: Clinical Challenges and Applications. CA Cancer J Clin; 2019. doi: 10.3322/caac.21552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiao Y, Wu J, Lin Z, Zhao X. A deep learning-based multi-model ensemble method for cancer prediction. Comput Methods Progr Biomed. 2018;153:1-9. [DOI] [PubMed] [Google Scholar]
- 94.Chan H-P, Sahiner B, Petrick N, et al. Computerized classification of malignant and benign microcalcifications on mammograms: texture analysis using an artificial neural network. Phys Med Biol. 1997;42:549-567. [DOI] [PubMed] [Google Scholar]
- 95.Hu Z, Tang J, Wang Z, Zhang K, Zhang L, Sun Q. Deep learning for image-based cancer detection and diagnosis − A survey. Pattern Recogn. 2018;83:134-149. [Google Scholar]
- 96.Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2015;13:8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang M, Li Z, Chen T, Zeng J. Integrative Data Analysis of Multi-Platform Cancer Data with a Multimodal Deep Learning Approach. IEEE ACM Trans Comput Biol Bioinf. 2015;12:928-937. [DOI] [PubMed] [Google Scholar]
- 98.Lisboa PJ, Taktak AFG. The use of artificial neural networks in decision support in cancer: A systematic review. Neural Network. 2006;19:408-415. [DOI] [PubMed] [Google Scholar]
- 99.Ongenaert M, Van Neste L, De Meyer T, Menschaert G, Bekaert S, Van Criekinge W. PubMeth: A cancer methylation database combining text-mining and expert annotation. Nucleic Acids Res. 2008;36:D842-D846. doi: 10.1093/nar/gkm788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Timmerman D, Bourne TH, Tailor A, et al. A comparison of methods for preoperative discrimination between malignant and benign adnexal masses: The development of a new logistic regression model. Am J Obstet Gynecol. 1999;181:57-65. [DOI] [PubMed] [Google Scholar]
- 101.Zhu F, Patumcharoenpol P, Zhang C, et al. Biomedical text mining and its applications in cancer research. J Biomed Inf. 2013;46:200-211. [DOI] [PubMed] [Google Scholar]
- 102.Furey TS, Cristianini N, Duffy N, Bednarski DW, Schummer M, Haussler D. Support vector machine classification and validation of cancer tissue samples using microarray expression data. Bioinformatics. 2000;16:906-914. [DOI] [PubMed] [Google Scholar]
- 103.Chu F, Wang L. Applications of support vector machines to cancer classification with microarray data. Int J Neural Syst. 2005;15:475-484. [DOI] [PubMed] [Google Scholar]
- 104.Runxuan Zhang R, Huang G-B, Sundararajan N, Saratchandran P. Multicategory classification using an extreme learning machine for microarray gene expression cancer diagnosis. IEEE ACM Trans Comput Biol Bioinf. 2007;4:485-495. [DOI] [PubMed] [Google Scholar]
- 105.Cawley GC, Talbot NLC. Gene selection in cancer classification using sparse logistic regression with Bayesian regularization. Bioinformatics. 2006;22:2348-2355. [DOI] [PubMed] [Google Scholar]
- 106.Xie B, Ding Q, Han H, Wu D. MiRCancer: A microRNA-cancer association database constructed by text mining on literature. Bioinformatics. 2013;29:638-644. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Tetko IV, Hall MA, et al. Gene selection from microarray data for cancer classification-a machine learning approach. Comput Biol Chem. 2005;29:37-46. [DOI] [PubMed] [Google Scholar]
- 108.Tan AC, Gilbert D. Ensemble machine learning on gene expression data for cancer classification. Appl Bioinf. 2003;2:S75-S83. [PubMed] [Google Scholar]
- 109.Parmar C, Grossmann P, Rietveld D, Rietbergen MM, Lambin P, Aerts HJWL. Radiomic machine-learning classifiers for prognostic biomarkers of head and neck cancer. Front Oncol. 2015. doi: 10.3389/fonc.2015.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chaudhary K, Poirion OB, Lu L, Garmire LX. Deep Learning-Based Multi-Omics Integration Robustly Predicts Survival in Liver Cancer. Clin Cancer Res. 2018;24:1248-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shen W, Zhou M, Yang F, et al. Multi-crop Convolutional Neural Networks for lung nodule malignancy suspiciousness classification. Pattern Recogn. 2017;61:663-673. [Google Scholar]
- 112.Kuruvilla J, Gunavathi K. Lung cancer classification using neural networks for CT images. Comput Methods Progr Biomed. 2014;113:202-209. [DOI] [PubMed] [Google Scholar]
- 113.Lustberg T, van Soest J, Gooding M, et al. Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer. Radiother Oncol. 2018;126:312-317. [DOI] [PubMed] [Google Scholar]
- 114.Hosny A, Parmar C, Coroller TP, et al. Deep learning for lung cancer prognostication: A retrospective multi-cohort radiomics study. PLoS Medicine. 2018;15:e1002711-e1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24:1559-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou Z-H, Jiang Y, Yang Y-B, Chen S-F. Lung cancer cell identification based on artificial neural network ensembles. Artif Intell Med. 2002;24:25-36. [DOI] [PubMed] [Google Scholar]
- 117.Nakamura K, Yoshida H, Engelmann R, et al. Computerized analysis of the likelihood of malignancy in solitary pulmonary nodules with use of artificial neural networks. Radiology. 2000;214:823-830. [DOI] [PubMed] [Google Scholar]
- 118.Ciompi F, Chung K, Van Riel SJ, et al. Towards automatic pulmonary nodule management in lung cancer screening with deep learning. Sci Rep. 2017;7:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suzuki K, Feng Li F, Sone S, Doi K. Computer-aided diagnostic scheme for distinction between benign and malignant nodules in thoracic low-dose CT by use of massive training artificial neural network. IEEE Trans Med Imag. 2005;24:1138-1150. [DOI] [PubMed] [Google Scholar]
- 120.Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961. [DOI] [PubMed] [Google Scholar]
- 121.Tailor A, Jurkovic D, Bourne TH, Collins WP, Campbell S. (1997) Soonography Prediction of Malignancy in Adnexal Masses Using Multivariate Logistic Regression Analysis. [DOI] [PubMed] [Google Scholar]
- 122.Timmerman D, Van Calster B, Testa AC, et al. Ovarian cancer prediction in adnexal masses using ultrasound-based logistic regression models: A temporal and external validation study by the IOTA group. Ultrasound Obstet Gynecol. 2010;36:226-234. [DOI] [PubMed] [Google Scholar]
- 123.Timmerman D, Testa AC, Bourne T, et al. Logistic regression model to distinguish between the benign and malignant adnexal mass before surgery: a multicenter study by the International Ovarian Tumor Analysis Group. J Clin Oncol. 2005;23:8794-8801. [DOI] [PubMed] [Google Scholar]
- 124.Valentin L, Hagen B, Tingulstad S, Eik-Nes S. Comparison of ‘pattern recognition' and logistic regression models for discrimination between benign and malignant pelvic masses: a prospective cross validation. Ultrasound Obstet Gynecol. 2001;18:357-365. [DOI] [PubMed] [Google Scholar]
- 125.Săftoiu A, Vilmann P, Gorunescu F, et al. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. [DOI] [PubMed] [Google Scholar]
- 126.Djavan B, Remzi M, Zlotta A, Seitz C, Snow P, Marberger M. Novel artificial neural network for early detection of prostate cancer. J Clin Oncol. 2002;20:921-929. [DOI] [PubMed] [Google Scholar]
- 127.Stephan C, Cammann H, Semjonow A, et al. Multicenter evaluation of an artificial neural network to increase the prostate cancer detection rate and reduce unnecessary biopsies. Clin Chem. 2002;48:1279-1287. [PubMed] [Google Scholar]
- 128.Langer DL, Van Der Kwast TH, Evans AJ, Trachtenberg J, Wilson BC, Haider MA. Prostate cancer detection with multi-parametric MRI: Logistic regression analysis of quantitative T2, diffusion-weighted imaging, and dynamic contrast-enhanced MRI. J Magn Reson Imag. 2009;30:327-334. [DOI] [PubMed] [Google Scholar]
- 129.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424. [DOI] [PubMed] [Google Scholar]
- 130.Rogers MA, Clarke P, Noble J, et al. Proteomic profiling of urinary proteins in renal cancer by surface enhanced laser desorption ionization and neural-network analysis: identification of key issues affecting potential clinical utility. Cancer Research. 2003;63:6971-6983. [PubMed] [Google Scholar]
- 131.Han SS, Kim MS, Lim W, Park GH, Park I, Chang SE. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. [DOI] [PubMed] [Google Scholar]
- 132.Gniadecka M, Philipsen PA, Wessel S, et al. Melanoma diagnosis by raman spectroscopy and neural networks: structure alterations in proteins and lipids in intact cancer tissue. J Invest Dermatol. 2004;122:443-449. [DOI] [PubMed] [Google Scholar]
- 133.Xu Z, Wang X, Zeng S, Ren X, Yan Y, Gong Z. Applying artificial intelligence for cancer immunotherapy. Acta Pharm Sin B. 2021. doi: 10.1016/j.apsb.2021.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang S, Yang J, Fong S, Zhao Q. Artificial Intelligence in Cancer Diagnosis and Prognosis: Opportunities and Challenges. Cancer Lett; 2020. doi: 10.1016/j.canlet.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 135.Khanna S. Artificial intelligence and machine learning techniques optimising patient management. Int. J. Rheum. Dis. 2019;4:rkaa005. [Google Scholar]
- 136.Tekkeşin Aİ. Artificial Intelligence in Healthcare: Past, Present and Future. Anatol J Cardiol. 2019;22:8-9. doi: 10.14744/AnatolJCardiol.2019.28661 [DOI] [PubMed] [Google Scholar]
- 137.Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc Neurol. 2017;2:230-243. doi: 10.1136/svn-2017-000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Badnjevic A, Avdihodzic H, Pokvic LG. Artificial intelligence in medical devices: Past, present and future. Psychiatr Danub. 2021;33:S336-S341. [PubMed] [Google Scholar]
- 139.Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. JAMA. 2017;318:2199-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Suri JS, Chang R-F, et al. Non-Extensive Entropy for CAD Systems of Breast Cancer Images. In: 2006 19th Brazilian Symp. Comput. Graph. Image Process; 2006:121-128. [Google Scholar]
- 141.Floyd CE, Lo JY, Yun AJ, Sullivan DC, Kornguth PJ. Prediction of breast cancer malignancy using an artificial neural network. Cancer. 1994;74:2944-2948. [DOI] [PubMed] [Google Scholar]
- 142.Liu HX, Zhang RS, Luan F, et al. Diagnosing breast cancer based on support vector machines. J Chem Inf Comput Sci. 2003;43:900-907. [DOI] [PubMed] [Google Scholar]
- 143.Furundzic D, Djordjevic M, Jovicevic Bekic A. Neural networks approach to early breast cancer detection. J Syst Architect. 1998;44:617-633. [Google Scholar]
- 144.Bouloy M, Janzen C, Vialat P, et al. Genetic Evidence for an Interferon-Antagonistic Function of Rift Valley Fever Virus Nonstructural Protein NSs. J Virol. 2001;75:1371. doi: 10.1128/jvi.75.3.1371-1377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patel SK, George B, Rai V. Artificial intelligence to decode cancer mechanism: beyond patient stratification for precision oncology. Front Pharmacol. 2020;11:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978-986. doi: 10.1111/j.1365-4632.2010.04474.x [DOI] [PubMed] [Google Scholar]
- 147.Okuboyejo DA, Olugbara OO, Odunaike SA. Automating skin disease diagnosis using image classification. Lect Notes Eng Comput Sci. 2013;2:850-854. [Google Scholar]
- 148.Tran B, Vu G, Ha G, et al. Global evolution of research in artificial intelligence in health and medicine: a bibliometric study. J Clin Med. 2019;8:360. doi: 10.3390/jcm8030360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fleming N. How artificial intelligence is changing drug discovery. Nature. 2018;557:S55-S57. doi: 10.1038/d41586-018-05267-x [DOI] [PubMed] [Google Scholar]
- 150.Misawa D, Fukuyoshi J, Sengoku S. Cancer prevention using machine learning, nudge theory and social impact bond. Int J Environ Res Publ Health. 2020;17(3):790. doi: 10.3390/ijerph17030790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chua IS, Gaziel‐Yablowitz M, Korach ZT, et al. Artificial intelligence in oncology: Path to implementation. Cancer Med. 2021;10:4138-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parimbelli E, Wilk S, Cornet R, et al. A review of AI and Data Science support for cancer management. Artif Intell Med. 2021;117:102111. [DOI] [PubMed] [Google Scholar]
- 153.Hunter B, Hindocha S, Lee RW. The Role of Artificial Intelligence in Early Cancer Diagnosis Cancers (Basel); 2022. doi: 10.3390/cancers14061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Artificial Intelligence and Machine Learning in Cancer Research: A Systematic and Thematic Analysis of the Top 100 Cited Articles Indexed in Scopus Database by Ibrahim H. Musa, Ibrahim Zamit, Taha H. Musa, Hassan H. Musa, Andrew Tassang, and Tosin Y. Akintunde in Cancer Control.