Abstract
Failure of weight-loss programs is high. We evaluated a whole-food plant-based (WFPB) lifestyle program. We investigated the obesity indices of 151 healthy adults who were on our ongoing, community-based program for the short (0.5 to ≤2 years), medium (2 to ≤5 years), or long term (5 to 10 years). Body composition indices were measured by medically approved bioimpedance. Body composition changes were favorable for all 3 groups and both genders. There were no differences in body composition between the males for all 3 groups, while there were lower body mass (BM), body mass index (BMI), and muscle mass in females on long-term versus short-term programs. All participants experienced a decrease in BMI (−2.5 kg/m2), BM (−7.1 kg), and body fat percentage (−6.4%; P < .001 for all). The reductions for those with a baseline BMI of obese, overweight, and normal were −5.6, −2.4, and −0.9 kg/m2 for BMI, −16.1, −7.1, and −2.5 kg for total BM, and −9.5%, −6.6%, and −4.8% for body fat percentage (baseline vs current; P < .001 for all). A total of 86% of parents of underage children introduced the WFPB lifestyle to children. Our WFPB lifestyle program provides a long-term reversal of obesity.
Keywords: plant-based diet, obesity, body composition, weight loss
O verweight and obesity is one of the major health challenges of the 21st century. 1 The failure rate of various weight-loss interventions is high, at up to 99.8%.2,3 In a meta-analysis of 29 long-term weight-loss studies in the United States, over 50% of the lost body mass (BM) was regained within 2 years, and 80% was regained within 5 years. 4 A healthy lifestyle includes healthy nutrition, maintaining a proper BM, avoiding smoking, limiting alcohol consumption, and being physically active, which might account for a 50% to 60% reduced risk of all-cause mortality. 5 In recent years, adopting a strict plant-based (vegan) diet (PBD) that is appropriately planned and supervised has become increasingly popular due to its well-documented health effects.6,7
We developed an ongoing, community-based whole-food plant-based (WFPB) lifestyle program (described under Materials and Methods) that enabled participants to maintain short-, medium- and long-term normal BM, body composition, and health.8-10
This study is part of a larger cross-sectional study of diet, lifestyle, and cardiovascular risk factors of healthy participants from Slovenia to our WFPB lifestyle program for 0.5 to 10 years. 10 In the present study, we evaluated body composition indices (body mass index [BMI] and body composition) of participants according to their stay in our ongoing, community-based program, that is, over the short, medium, and long term. We aimed to evaluate changes in obesity markers (BMI units, total BM, and body fat [BF] percentage) from the time of entering our program as practicing the Western-type diet (baseline) to the present, by gender. Our hypothesis was that there would be a significant difference in the body composition indices between participants that have been in the program short-term compared to those in the medium-term, but not between participants in the medium-term group compared to participants in the long-term group (H0). According to our clinical experiences and our previous interventional studies,8,9 we assumed that most of the benefits for a weight loss would be achieved within the first 2 years.
Materials and Methods
Study Design and Eligibility
We included free-living participants from 6 regions of Slovenia who had been in our ongoing, community-based WFPB lifestyle program for 0.5 to 10 years, 10 but also included some of their baseline anthropometric measures. Of note, to compare differences in body composition, investigators used participants’ baseline data (from the time when they started our program) and compared it with their recent body composition status. Participants were not remunerated financially to participate in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the national Medical Ethics Committee of the Republic of Slovenia (Approval Document 0120-380/2019/17) and the Slovenian Ethical Committee in the field of sports (No. 05:2019). This trial was registered on June 6, 2019, at https://clinicaltrials.gov (NCT03976479).
Subjects
We invited 2555 adults, aged 18 to 80 years, through closed plant-based social media support groups and by personal contact of PBD health coaches. All participants were in the same support system, from the less consistent PBD (ie, plant-rich diet) to more consistent PBD (ie, strict PBD, in our case supplemented WFPB diet). All participants were previously on a Western-type diet (ie, high intake of ultra-processed foods, low intake of fresh vegetables and fruits, resulting in high intake of saturated fats, salt, free sugars, while low in dietary fiber intake) and lifestyle, were not highly motivated, and did not have a preference toward PBD, which we assessed from a dietary and lifestyle questionnaire.
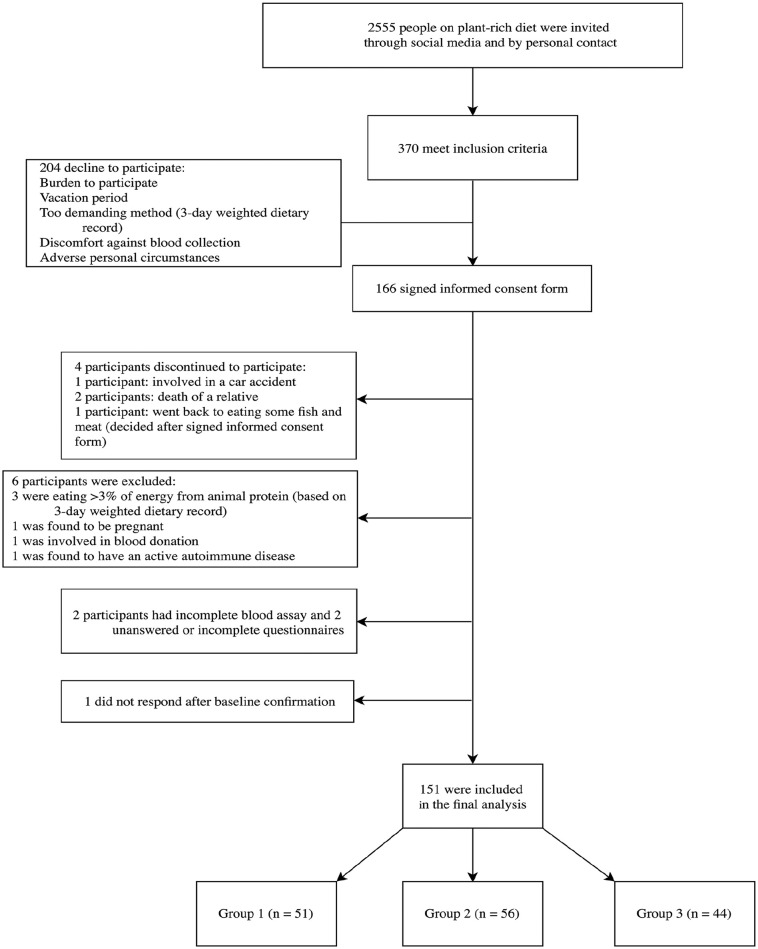
In this primary prevention setting, the exclusion criteria were pregnancy/lactation, competitive/top-level athletes, current use of drugs for medication affecting plasma lipids and glucose or blood pressure (recommended secondary prevention), major musculoskeletal restrictions, active malignant diseases (eg, cancer, cardiovascular disease, type 2 diabetes, autoimmune and neurodegenerative diseases), those acquiring >3% of energy from animal protein (based from 3-day weighted dietary protocol), incomplete blood assay, and unanswered questionnaires. Participants were included if they had some dietary restrictions (eg, gluten, peanuts). The majority of the invited participants were female. In total, 370 individuals met the inclusion criteria after 2-phase interviews, while 204 declined to participate (being stressed, vacation period, demanding methods [3-day weighted dietary record (3-DR)], discomfort against blood collection, and/or a challenging personal situation). A total of 166 individuals signed the informed-consent form, out of which 4 discontinued their participation; we excluded 6 participants as 3 were still eating a significant amount of animal-based products on a regular basis (3-DR evaluation), one was found to be pregnant, one was involved in blood donation, and one was found to have an autoimmune disease. Two participants had an incomplete blood assay, 2 had unanswered questionnaires, while 1 did not want to complete them (Figure 1). None of the participants were currently on lipids, blood pressure, or blood sugar control medications, reflecting the primary prevention settings of the program.
Figure 1.
Enrolment of the participants and completion of the study.
The study lasted from June until August 2019, while the clinical part of the study was completed within 14 days. In the final analysis, we included 151 participants (91% of initially included). For the inclusion criteria, we did not set limits concerning current BMI since we knew their baseline BM and BF percentage. Based on the duration of the WFPB lifestyle program, we divided participants into 3 groups: short term (0.5 to ≤2 years), medium term (2 to ≤5 years), and long term (5 to ≤10 years).
WFPB Lifestyle Program
The WFPB lifestyle program included a nutritional part, a physical activity component, and a support system.
Nutritional Part
The nutritional part included a 60-minute lecture every week for 10 weeks with an experienced health coach (PhD in sport science, university physical education teachers, sport trainers, and certified instructors of fitness, aerobics, and Pilates), with topics covering the WFPB diet, individual counselling and meal plans, shopping guidance, cooking workshops, and recipes. The dietary program consisted of ≥90% of energy from the WFPB diet. 10 The WFPB diet allows ad libitum intake of whole grains, fruits, vegetables, and legumes; and a moderate intake of nuts, seeds, avocados, and soy (eg, tofu) or wheat products with no added fat. It includes little or no added refined fat (eg, olive oil, coconut, and palm oil). Ultra-processed foods (defined by NOVA classification), 11 highly refined carbohydrates (eg, white rice, white flour), and foods containing added sugars and sweeteners were omitted. Moreover, it excludes all animal products. 12
Up to ≤10% of the energy intake was supplemented with nutrient-enriched plant-based meal replacement (MR; 36 g soy or pea protein/100 g) and dietary supplements (eg, 1000 µg B12/day; 2-3 times/week for the whole year, 3000 IU D3/day 7 times/week through October to April; optionally, 625 mg eicosapentaenoic acid [C20:5n-3, EPA] and docosahexaenoic acid [C22:6n-3, DHA]/day). This is known as the supplemented WFPB diet. The supplemented WFPB diet was individually optimized by an experienced health coach to meet nutritional needs, cultural preferences, and lifestyle. Despite the fact that weight management was a core part of our program, there was no need for calorie counting, and recommended WFPB diet foods were consumed ad libitum to full satiety at each meal. 10
In brief, all nutrients, with the exception of vitamin B12 and potentially vitamin D, and EPA and DHA, can be found in plant food sources. 13 However, MR was used to increase adherence of participants to a PBD8,9,14 with greater ease due to simplification,15-17 to help sustaining favorable body mass and body composition,8,9,15 and to ensure nutritional adequacy without excessive energy intake. 18 Furthermore, the supplemented dose of vitamin B12 was based on the absorption rate from dietary supplements, approximately 1%. 19 The decision on the intake dose of vitamin D3 was adopted based on our clinical experiences (monitored blood assays from most participants in our program and how they respond after intervention), the latitude of Slovenia (46 °N), and studies that considered individual response, 20 especially in relation to body mass. 21
Physical Activity Component
The physical activity component included habitual, organized, and other physical activity (PA). During the introduction phase, the participants were encouraged to engage in at least two 45-minute guided moderate-intensity exercise sessions/week. After the introduction phase, the participants performed the prescribed resistance-exercise activities by themselves. Participants were also encouraged to perform at least 30 min/day of low- to moderate-intensity aerobic activity (brisk walking or biking) and a longer low- to moderate-intensity activity during the weekend (45-120 minutes, 1-2 sessions; ie, brisk walking or hiking). 10
Support System
The support system consisted of frequent body composition measurements, meal plan evaluation, assistance in PA, individual and group support on challenges during behavioral changes, and social media support groups including (1) cooking recipes, (2) professional summaries of nutrition topics, (3) posted organized group workouts and testimonials, and (4) a discussion board. The goal was to motivate participants to comply with the prescribed dietary regiment, to receive accurate information, and to share new experiences to help face daily challenges, all with the aim of improving their well-being and long-term health. 10
Outcomes
Sociodemographic and Economic Status
We adopted and modified the questionnaire for adults 22 (Table 1).
Table 1.
Current Demographic and Other Characteristics of All Participants, Grouped According to Their Length of Engagement Time in Our Program (by Gender) a .
| Parameter | Group 1 (n = 51) | Group 2 (n = 56) | Group 3 (n = 44) | |||
|---|---|---|---|---|---|---|
| Gender | Female | Male | Female | Male | Female | Male |
| N (%) | 35 (69) | 16 (31) | 43 (77) | 13 (23) | 31 (70) | 13 (30) |
| Age (years) | 36.9 ± 11.5 | 37.3 ± 17.6 | 42.6 ± 10.9 | 36.1 ± 10.3 | 42.4 ± 13.5 | 35.8 ± 11.0 |
| Time on (WFPB) lifestyle program (years) | 1.3 (0.9-1.9) | 1.2 (0.8-1.8) | 3.9 (2.5-4.9) | 4.0 (2.5-4.9) | 6.9 (5.5-10.0) | 7.2 (5.5-10.0) |
| Smoking, n (%) | ||||||
| Never | 40 (78.4) | 44 (78.6) | 33 (75.0) | |||
| Former | 4 (7.8) | 2 (3.6) | 1 (2.3) | |||
| Current | 7 (13.7) | 9 (16.1) | 10 (22.7) | |||
| N/A (did not disclose) | 0 | 1 (1.8) | 0 | |||
| Alcohol, n (%) | 2 (3.9) | 1 (1.8) | 0 | |||
| Alcohol (mL/day) | 0.7 | 0 | 0 | |||
Abbreviation: WFPB, whole-food plant-based.
Data are mean ± standard deviation (SD) for normally distributed variables and median (minimum–maximum) for nonnormally distributed ones.
Lifestyle Factors
To assess (1) health-related PA, we used the long, self-administered International Long Physical Activity Questionnaire (L-IPAQ). 23 To evaluate (2) sleep quality, patterns, and disturbance, we used 19 self-rated questions of the Pittsburgh Sleep Quality Index (PSQI) questionnaire. 24 Finally, to measure (3) perceived stress status, we used a 30-question Perceived Stress Questionnaire (PSQ). 25 Lifestyle factors are presented in more detail in a separate article.
Anthropometric and Body Composition Measures
Participants were measured in light underwear. Baseline data (at entry to our program) were measured by 3 investigators and their teams, with the same bioelectrical impedance analysis technology (Tanita, Japan). Data were archived according to the General Data Protection Regulation (GDPR). The baseline original printout was given to all participants (and its duplicate was archived) at the start of their lifestyle change in our program. In the current study, all measurements were made by the same researcher, with the assistance of another. Heights (cm) were measured by the body height gauge (Kern, MPE 250K100HM, Kern and Sohn). Body composition was assessed by an 8-electrode medically approved and calibrated bioelectrical impedance body composition monitor (Tanita 780 S MA, Tanita Corporation), which provides an accurate tool to measure total BF percentage and fat free mass in healthy young males and females, regardless of their level of habitual PA. 26 BMI (kg/m2) and muscle mass index (MMI, kg/m2) were calculated from body height and BM, and body height and muscle mass were measured by body-height gauge and body composition monitor. Body composition indices included BM, BMI, BF percentage (BF %) relative to total BM, muscle mass, MMI, and total body water. Before the bioimpedance test, participants were asked not to eat or drink for at least 1 hour, not exercise for at least 24 hours, and not urinate for at least 30 minutes. Females were not measured 3 days before or 3 days after their menstrual cycle.
Dietary Intake and Cardiovascular Risk Factors
We evaluated dietary and supplements intakes by 3-DR as well as several cardiovascular diseases risk factors, which is presented in a separate article. 10
Statistical Analysis
We assumed a large Cohen effect size and test power of 80%. The calculation for the t test gave us a sample size of 126, and for ANOVA test there were 39 subjects per group. Quantitative variables were inspected for their distribution and potential outliers. As we used tests where variables were on the same scale, we did not need to use log or standardization/normalization procedures. Statistical analyses were completed with R 3.5.2 using packages “dplyr,” 27 “ggplot2,” 28 and “arsenal.” 29 For numerical variables, we used ANOVA to assess the differences between 3 different groups and the Tukey post hoc test when differences were statistically significant. Where the subsample was small, we referred to the Kruskal-Wallis test. When we analyzed dependent samples, we used t test for dependent samples. Similar for the categorical variables, we used the χ2 test, and Fisher’s exact test where the subsample was small. The threshold for statistical significance was P < .05. No missing data were present. We used a convenient sample. No sensitivity analysis was performed.
Results
Participant Characteristics
The whole sample included 151 active adults, 109 female (72%) and 42 male (28%). The age (mean ± SD) and current BMI (mean [min–max]) of the participants was 39.6 ± 12.5 years and 23.9 (17.7-41.4) kg/m2. None of the participants were currently on cholesterol, blood pressure, or blood sugar control medications.
Participants in our study had 196 children, 118 were underage (<18 years; 60% of all children), while 22 were adult (>18 years; 40% of all children). A total of 85.6% of underage children (n = 101 of 118) and 28.2% of adult children (n = 22 of 78) were on WFPB diet. A total of 8.2% children (n = 16) were born to parents while being on our WFPB diet lifestyle program (Supplemental Table 1S). During pregnancy, 7 females gained 7 to 10 kg (mean BM of their infants: 3683 g), 8 women gained 10.1 to <12 kg (mean BM of their infants: 3477 g), and 1 women gained 12.1 to <15 kg (mean BM of her infant: 3270 g).
Energy Intake, Physical Activity, and Lifestyle Factors
The mean (±SD) energy intake was 1841 ± 539 kcal/day for females (groups 1, 2 and 3 [mean]: 1963, 1734, and 1841 kcal/day, respectively, P > .05) and 2618 ± 726 kcal/day for males (groups 1, 2, and 3: 2621, 2570, and 2618 kcal/day, respectively, P > .05). Participants were physically very active (L-IPAQ score for females (groups 1, 2, and 3 [mean]: 5265, 4483, and 6438 METs min/week, P = .086) and for males (groups 1, 2, and 3: 6374, 6466, and 5696 METs min/week, P = .920), had good sleep quality (PSQI score: 2.7 ± 1.8) and perceived low stress (PSQ score: 0.3 ± 0.1).
Anthropometric and Body Composition Measures
There were no differences in maximal lifetime BM and BMI between the 3 groups of male participants (baseline vs current [after short-term, medium-term and long-term duration in program (groups 1, 2, and 3) and combined 0.5-10 years in our WFPB lifestyle program]), while there was a significant difference in maximal lifetime BM between the 3 female groups.
At baseline (at the entrance to WFPB lifestyle program), there were no differences in BM, BMI, and BF percentage and muscle mass between the 3 male groups. Female groups had statistically significantly different body height and BM, but not baseline BMI and BF percentage. Furthermore, female group 3 had significantly lower current BM, BMI, and absolute muscle mass (MM) compared to group 1. Therefore, MMI was a more appropriate tool for monitoring differences in muscle mass change and was not shown to be significantly different when we compared the female and male groups (Table 2).
Table 2.
Anthropometric and Body Composition Measures of All Participants According to Their Length of Engagement Time in Our Program (by Gender) a .
| Parameter | Group 1 (n = 51) | Group 2 (n = 56) | Group 3 (n = 44) | P | |||
|---|---|---|---|---|---|---|---|
| Gender | Female | Male | Female | Male | Female | Male | Female/male |
| Height (cm) | 167.7 ± 7.6 | 178.5 ± 5.3 | 164.2 ± 5.0 | 178.8 ± 4.0 | 163.5 ± 4.8 | 179.8 ± 6.9 | .009/.725 |
| Max lifetime b | |||||||
| Body mass (kg) | 79.1 ± 22.6 | 90.2 ± 11.5 | 73.7 ± 13.7 | 99.6 ± 27.4 | 67.9 ± 10.5 | 94.9 ± 15.5 | .026/.742 |
| BMI (kg/m2) | 28.1 (19.8-54.9) | 28.2 (24.1-32.8) | 27.4 (20.3-39.8) | 31.0 (23.6-50.6) | 25.4 (20.4-35.8) | 29.4 (21.6-37.9) | .169/.727 |
| Baseline | |||||||
| Body mass (kg) | 74.8 ± 20.6 | 87.2 ± 11.8 | 71.1 ± 14.2 | 94.4 ± 27.5 | 65.4 ± 9.4 | 86.3 ± 16.2 | .049/.866 |
| BMI (kg/m2) | 26.6 (19.1-46.3) | 27.3 (23.0-32.8) | 26.4 (17.7-38.3) | 29.3 (21.8-50.6) | 24.5 (19.2-32.5) | 26.7 (19.0-35.3) | .229/.621 |
| Body fat (%) | 30.3 ± 9.6 | 22.4 ± 6.0 | 33.3 ± 7.9 | 23.1 ± 9.4 | 29.4 ± 6.7 | 21.2 ± 10.0 | .094/.733 |
| Current | |||||||
| Body mass (kg) | 68.0 ± 13.1 | 77.9 ± 8.2 | 64.0 ± 9.6 | 85.5 ± 18.8 | 58.4 ± 6.6 | 82.6 ± 10.2 | .001/.341 |
| BMI (kg/m2) | 24.2 (18.7–37.3) | 24.5 (20.8–29.0) | 23.8 (17.7–30.3) | 26.6 (22.8–41.4) | 21.8 (18.3–29.5) | 25.5 (21.3–26.9) | .025/.351 |
| Body fat (%) | 24.4 ± 8.0 | 17.1 ± 6.3 | 25.8 ± 6.3 | 20.0 ± 5.8 | 21.9 ± 5.7 | 15.3 ± 5.3 | .061/.061 |
| Muscle mass (kg) | 48.1 ± 6.6 | 61.1 ± 4.8 | 44.7 ± 4.6 | 64.1 ± 8.2 | 43.0 ± 3.7 | 66.3 ± 6.6 | <.001/.099 |
| Muscle mass index (kg/m2) | 17.1 ± 2.1 | 19.2 ±1.3 | 16.6 ± 1.7 | 20.0 ± 1.9 | 17.4 ± 2.4 | 20.5 ± 1.5 | .061/.078 |
Abbreviations: BMI, body mass index.
Data are mean ± standard deviation (SD) for normally distributed variables, and median (minimum–maximum) for nonnormally distributed ones.
Maximal reported body mass (BM) that a participant reached at any time during their life. One-way ANOVA was used for comparing females across groups. Kruskal-Wallis test was used for comparing males across groups.
Body composition changes were favorable for all 3 groups, for both genders and for the whole sample. There were no differences in BF percentage, MMI, and total body water between the 3 groups of participants in both genders.
The results of the baseline versus current comparison of BMI, BM, and BF % according to BMI classification (Supplemental Table 2S) showed that all participants (N = 151) significantly improved their obesity indices. They experienced a mean BMI reduction of −2.5 kg/m2 (from baseline mean overweight BMI range [26.4 kg/m2] to normal BMI [23.9 kg/m2]; P < .001). They also achieved a BM reduction of 7.1 kg (P < .001) and BF percentage reduction of 6.4% (P < .001). Participants who had the highest BMI at baseline (BMI ≥30 to >40; obesity classes 1-3) lost the most BMI units, total BM, and BF percentage. BMI units (−5.6, −2.4, and −0.9 kg/m2), total BM (−16.1, −7.2, and −2.5 kg), and BF percentage points (−9.5%, −6.6%, and −4.8%) all decreased statistically significantly for participants who had baseline BMI in obese, overweight, and normal range, respectively (baseline vs current, P < .001 for all).
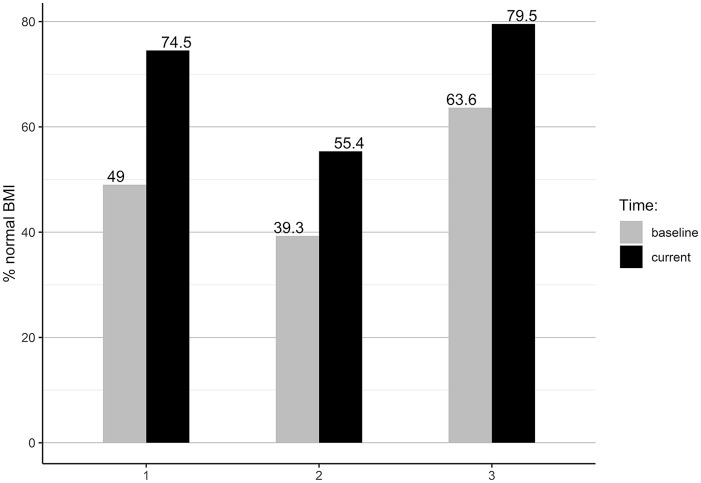
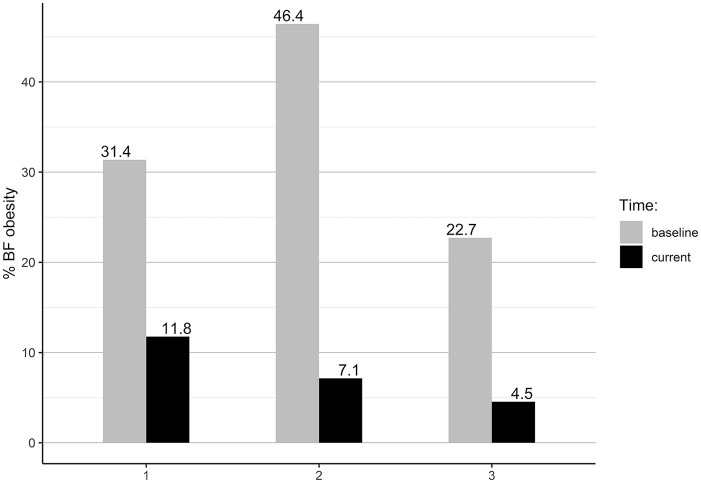
According to the World Health Organization (WHO) BMI obesity classification, 30 57.8% females and 28.6% males were within a normal BMI range at the baseline. The current BMI results showed that the proportion of normal BMI increased by 16.5% for females and by 26.2% for males. The improvement toward normal BMI was seen in all groups’ comparisons (Figure 2). According to the WHO BF % obesity classification, 31 at the baseline, 34.9% females and 33.3% males were obese (Figure 3). The current BF % status showed improvements to a normal BF % range by 27.6% of females and 23.8% of males. At the end of the study, 92.7% of females and 90.5% of males were within the normal BF % range.
Figure 2.
Group comparison of participants with normal BMI (18.5-24.9 kg/m2).
Figure 3.
Group comparison of obesity by body fat percentage.
The group comparison showed that group 1 improved the proportion of normal BMI by 25.5%, group 2 by 16.1%, and Group 3 by 15.9%. Using BF % classification, group 1 improved the proportion of participants with normal BF % by 19.6%, group 2 by 39.3%, and group 3 by 18.2%. One female was underweight at entry to our WFPB lifestyle program, while tw2 females were underweight at entry to the lifestyle program and at time of final measurement (BMI < 18.5 kg/m2), but had proper BF % (24.6% and 16.9%).
Discussion
An evaluation of the long-term (0.5-10 years) effect of a WFPB lifestyle program on lifestyle and BM management of healthy, active adults demonstrated a statistically significant BM loss, and a decrease in BMI as well as BF percentage in participants to our program that took part in the short, medium, and long term (ie, all 3 groups). There were no significant differences in relative body composition changes (BM, BMI, BF %, and MMI) in males participating in short-, medium-, and long-term programs, but there were significant differences in BM and BMI between the females participating in short-, medium-, and long-term programs; therefore, our hypothesis was only partly rejected. The most significant and long-lasting changes occurred within the first 2 years in a WFPB lifestyle program. The new WFPB lifestyle and dietary pattern was transferred to 85.6% of underage children of participants in the program. All children were regularly medically supervised by pediatricians.
Anthropometric and Body Composition Measures
At the baseline, when entering into our WFPB lifestyle program, participants were not highly motivated and did not have a preference toward a WFPB dietary pattern—this is evident from their average maximal BM, baseline anthropometric, body composition status, and assessed dietary and lifestyle questionnaire (at the baseline). Due to the BMI limitations, we combined in our study the BMI with body composition profile measurements, which is especially important for smaller-scale observational studies and for people with sarcopenic obesity. 32 Our results of body composition status of the whole sample and of the 3 compared groups (by gender) showed an improvement in adopting the WFPB lifestyle. The possible reasons for the favorable outcome may be due to the quality and quantity of supplemented WFPB diet with the combination of being physically active and other lifestyle-related factors (presented in another manuscript).
Studies on plant-based dieters have found large variety in BMI status. We could not directly compare our study results with other cross-sectional studies since they did not follow the sample in the transition from the end of a Western-type diet through the transitional period to a PBD lifestyle, and many of them did not divide the samples by gender. However, the average BMI of vegans in the Epic Oxford study was 22.49 and 21.98 kg/m2 for females and males, 33 respectively, while in the Adventist Health Study 2, 5548 vegans were found to have BMI of 24.1 kg/m2. 34 Similarly, researchers measured the BMI of British Indian vegans and found that their BMI was just below overweight level (24.8 kg/m2), but with a high BF percentage (32.7%). 35 The average BMI was also within normal BMI range for 75 Danish (21 kg/m2) 36 and 43 Swiss vegans (21.6 kg/m2), but we do not know the gender differences, 37 which is a limitation. The average BMI of 26 Italian vegans (9 males, 17 females) was 23.7 kg/m2 with a BF of 25.6% (measured with the BIA), 38 but the researchers did not divide results by gender. Another European cohort study (Epic Norfolk) that included only a small sample of vegans (12 males and 16 females) found that vegans had BMI in overweight range, 28 and 27 kg/m2 for males and females, 39 respectively. A meta-analysis of 37 observational studies on 12 241 vegans showed that BMI was on average within the healthy-weight range, but there was no difference in BMI for vegans compared to controls in Asia (23.3 kg/m2); however, for non-Asian studies, the difference was −1.92 kg/m2. 40 A recent randomized controlled trial that used a WFPB diet on overweight and obese adults showed impressive weight loss results at 3 (−8.6 kg), 6 (−12.1 kg), and 12 month (−11.5 kg) follow-ups. 41
Participants’ Children
We found that the new, healthy WFPB lifestyle was transferred by participants to 85.6% of children aged <18 years and to 28.2% of children aged >18 years, showing that it was more than 3 times more likely to be transferred to underage than to adult children. Such a high transfer rate of the WFPB dietary pattern in Slovenia is of special significance, as in Slovenia families and children are not encouraged to practice a WFPB diet, as has been the case in the United States for 10 years already,6,42 as well as Australia, 43 Canada, 44 Portugal, 45 Great Britain, 46 and Italy. 47
Strengths and Limitations
The strength of our study is its long-term nature, since the participants were on our WFPB lifestyle program from 0.5 to 10 years. We followed their entire transformation from a Western-type, more sedentary lifestyle to the present WFPB lifestyle, which gives the study generalizability (external validity). A unique feature of our study is describing the sample in detail for both genders. The study also has a good geographical representation, since our participants lived dispersed across the country in urban, suburban, and rural areas.
All measurements were performed within a short period of 14 days. Participants were measured in the same manner by the same researchers, which decreased sources of potential bias. For the inclusion criteria, we did not set limits concerning current BMI, which significantly limits potential selection bias of participants. Furthermore, the entire study protocol and analyzed data were under the supervision of 2 independent experts in the field, while analyzed data were performed by an independent professor of statistics. This study confirmed the previously shown health benefits of the WFPB lifestyle program to have a short-, medium-, and long-term effect.8-10 Our results were not limited to diet only, but to healthy, active lifestyle and the support system. Regular PA is associated with lower BMI and BF percentage, especially in combination with a weight-management dietary program. 48 An additional factor for the success of our program was the enhanced and extensive support system, 10 since behavior changes 49 and motives related to personal well-being and health 50 may be a crucial reason to remain a plant-based dieter in the long term. In the future, it would be valuable to investigate the reasons why most of our screened people that adopted plant-rich diets did not adopt a strict supplemented WFPB diet or exclusively WFPB diet. It is also the first cross-sectional study on supplemented WFPB dieters that assessed how many children (<18 years and >18 years) that were born to participants began practicing a WFPB lifestyle. We discovered an extremely high successful transfer of healthy lifestyles to children (up to 85.6% of children aged <18 years).
The main limitation of the study is that we did not have a control group, but only a comparison of the same participants at baseline versus present. In addition, we could not exclude the possible unknown impact of people who were within the set criteria but did not respond or were not willing to participate in the study. Additionally, at the time of the study, there were more than 2500 participants to our program. However, only a few of them adhere to a strict supplemented WFPB lifestyle (about 15% according to inclusion/exclusion criteria, which is consistent with the flowchart in Figure 1). Another limitation, which could be a potential bias, is the use of a medically approved bio-impedance body composition monitor instead of DXA (dual X-ray absorptometry). However, the bioelectrical impedance analyzer that we used has been shown to be an accurate tool to measure the total BF percentage and fat free mass measures in healthy females and males, regardless of the PA level compared to DXA scans. 26 Nevertheless, concordance between BIA and DXA methods at the individual level is lacking, particularly in participants with BMI below 18 kg/m2. 51
Conclusions
Our study assessed the effects of a short-, medium-, and long-term (0.5-10 years) WFPB lifestyle program on the lifestyle and body composition indices compared to baseline characteristics. We discovered no differences in body composition indices between the male participants of our program over the short-, medium-, and long-term, while there were lower BM, BMI, and MM in females on long-term versus short-term programs. Body composition changes were favorable for all 3 groups, both genders, and all participants. The novelty of our results is that, through an extensive support system, the majority of beneficial effects were achieved within the first 2 years, and successfully maintained in the long term (5-10 years). Our results contradict the majority of previous weight-loss studies, which have a high failure rate within the first 2 years and in the long term. Our data support the benefits of a WFPB lifestyle program, including very effective and sustainable BM loss and a reduction in BF percentage. We also discovered that participants were passing on the healthy and active lifestyle to the next generation (to 85.6% of underage children of participants).
Supplemental Material
Supplemental material, Sj-pdf-1-ajl-10.1177_1559827620949205 for Whole-Food Plant-Based Lifestyle Program and Decreased Obesity by Boštjan Jakše, Barbara Jakše, Stanislav Pinter, Jernej Pajek and Nataša Fidler Mis in American Journal of Lifestyle Medicine
Acknowledgments
The authors wish to thank all health coaches for their collaboration. We would like to thank Uroš Godnov (Faculty of Management, University of Primorska) for his assistance with the statistical analysis and Alenka Polajnar Gantar for the language review. Last, but not least, we would like to show our gratitude to all participants in the study; this work would not have been possible without them.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JP and NFM do not declare any conflict of interest and collaborated as independent researchers. Barbara Jakše and SP are receiving royalty compensation at Herbalife Nutrition, which did not have any role in the design of the study, collection, analysis, and interpretation of data, nor in writing the manuscript. Boštjan Jakše is Barbara Jakše’s spouse and therefore derives income from the same sources.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was partly financially supported by the Slovenian Research Agency (Research Program P3-0395: Nutrition and Public Health) and partly by resource of Barbara Jakše, sole proprietor.
Ethical Approval: The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the national Medical Ethics Committee of the Republic of Slovenia (Approval Document 0120-380/2019/17) and the Slovenian Ethical Committee in the field of sports (No. 05:2019). This trial was registered on 06 June 2019 at https://clinicaltrials.gov with number NCT03976479.
Informed Consent: All participants in this study signed the informed consent form.
Trial Registration: This trial was registered on June 6, 2019, at https://clinicaltrials.gov with number NCT03976479.
ORCID iD: Boštjan Jakše  https://orcid.org/0000-0001-7424-7258
https://orcid.org/0000-0001-7424-7258
Supplemental Material: Supplemental materials for this article is available online.
Contributor Information
Boštjan Jakše, Department of Food science, Biotechnical Faculty, University of Ljubljana, Ljubljana, Slovenia.
Barbara Jakše, Barbara Jakše sole proprietor, Domžale, Slovenia.
Stanislav Pinter, Basics of Movements in Sport, Faculty of Sport, University of Ljubljana, Ljubljana, Slovenia.
Jernej Pajek, Department of Nephrology, University Medical Centre Ljubljana, Ljubljana, Slovenia and Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia.
Nataša Fidler Mis, Department of Gastroenterology, Hepatology and Nutrition, University Children’s Hospital, University Medical Centre Ljubljana, Ljubljana, Slovenia.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 suppl):222S-225S. doi: 10.1093/ajcn/82.1.222S [DOI] [PubMed] [Google Scholar]
- 3.Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health. 2015;105:e54-e59. doi: 10.2105/AJPH.2015.302773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579-584. doi: 10.1093/ajcn/74.5.579 [DOI] [PubMed] [Google Scholar]
- 5.Veronese N, Li Y, Manson JE, Willett WC, Fontana L, Hu FB. Combined associations of body weight and lifestyle factors with all cause and cause specific mortality in men and women: prospective cohort study. BMJ. 2016;355:i5855. doi: 10.1136/bmj.i5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melina V, Craig W, Levin S. Position of the Academy of Nutrition and Dietetics: vegetarian diets. J Acad Nutr Diet. 2016;116:1970-1980. doi: 10.1016/j.jand.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 7.Bodai BI, Nakata TE, Wong WT, et al. Lifestyle medicine: a brief review of its dramatic impact on health and survival. Perm J. 2017;22:17-25. doi: 10.7812/TPP/17-025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakše B, Pinter S, Jakše B, Bucˇar Pajek M, Pajek J. Effects of an ad libitum consumed low-fat plant-based diet supplemented with plant-based meal replacements on body composition indices. Biomed Res Int. 2017;2017:9626390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakše B, Jakše B, Pajek J, Pajek M. Effects of ad libitum consumed, low-fat, high-fiber plant-based diet supplemented with plant-based meal replacements on cardiovascular risk factors. Food Nutr Res. 2019;63. doi: 10.29219/fnr.v63.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakše B, Jakše B, Pinter S, et al. Dietary intakes and cardiovascular health of healthy adults in short-, medium-, and long-term whole-food plant-based lifestyle program. Nutrients. 2019;12:55. doi: 10.3390/nu12010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67-77.e3. doi: 10.1016/j.cmet.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell TC, Campbell TM. The China Study: The Most Comprehensive Study of Nutrition Ever Conducted and the Startling Implications for Diet, Weight Loss and Long-Term Health. BenBella Books; 2005. [Google Scholar]
- 13.Hever J. Plant-based diets: a physician’s guide. Perm J. 2016;20:15-82. doi: 10.7812/TPP/15-082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao A. Role of meal replacements on weight management, health and nutrition. In: Waisundara VY, ed. Superfood and Functional Food—An Overview of Their Processing and Utilization. InTech; 2017. doi: 10.5772/66331 [DOI] [Google Scholar]
- 15.EFSA. Scientific opinion on the substantiation of health claims related to meal replacements for weight control (as defined in Directive 96/8/EC on energy restricted diets for weight loss) and reduction in body weight (ID 1417), and maintenance of body weight. EFSA J. 2010;8:1466. doi: 10.2903/j.efsa.2010.1466 [DOI] [Google Scholar]
- 16.Raynor HA, Champagne CM. Position of the Academy of Nutrition and Dietetics: interventions for the treatment of overweight and obesity in adults. J Acad Nutr Diet. 2016;116:129-147. doi: 10.1016/j.jand.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 17.Heymsfield SB, van Mierlo CAJ, van der Knaap HCM, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27:537-549. doi: 10.1038/sj.ijo.0802258 [DOI] [PubMed] [Google Scholar]
- 18.Ashley JM, Herzog H, Clodfelter S, Bovee V, Schrage J, Pritsos C. Nutrient adequacy during weight loss interventions: a randomized study in women comparing the dietary intake in a meal replacement group with a traditional food group. Nutr J. 2007;6:12. doi: 10.1186/1475-2891-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul C, Brady DM. Comparative Bioavailability and utilization of particular forms of B12 supplements with potential to mitigate B12-related genetic polymorphisms. Integr Med (Encinitas). 2017;16:42-49. [PMC free article] [PubMed] [Google Scholar]
- 20.Heaney RP. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13-19. doi: 10.1016/j.jsbmb.2005.06.020 [DOI] [PubMed] [Google Scholar]
- 21.Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014;9:e111265. doi: 10.1371/journal.pone.0111265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NIJZ and Partners. Priloga Specifikacije Zahtev Za Izvedbo Ankete v Raziskavi :EU Menu Slovenija. EU Menu Slovenija: POTEK ANKETE MLADOSTNIK/ODRASLI LOT 2. Published 2019. Accessed August 10, 2019. https://www.nijz.si/sites/www.nijz.si/files/uploaded/p-5_priloga_k_specifikaciji_2_a.pdf
- 23.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755-762. [DOI] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 25.Levenstein S, Prantera C, Varvo V, et al. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res. 1993;37:19-32. [DOI] [PubMed] [Google Scholar]
- 26.Verney J, Schwartz C, Amiche S, Pereira B, Thivel D. Comparisons of a multi-frequency bioelectrical impedance analysis to the dual-energy X-ray absorptiometry scan in healthy young adults depending on their physical activity level. J Hum Kinet. 2015;47:73-80. doi: 10.1515/hukin-2015-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickham H, François R, Henry L, Müller K. A grammar of data manipulation. R package version 0.8.1; 2019. https://cran.r-project.org/web/packages/dplyr/index.html
- 28.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. 2nd ed. Springer Nature; 2016. [Google Scholar]
- 29.Heinzen E, Sinnwell J, Atkinson E, et al. An arsenal of “R” functions for large-scale statistical summaries [R package arsenal version 3.3.0]. Published 2019. Accessed May 10, 2020. https://cran.r-project.org/web/packages/arsenal/index.html
- 30.World Health Organization. Body mass index—BMI. Published September 29, 2019. Accessed September 30, 2019. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi
- 31.World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. World Health Organization; 1995. Accessed September 30, 2019. https://apps.who.int/iris/bitstream/handle/10665/37003/WHO_TRS_854.pdf?sequence=1&isAllowed=y [PubMed] [Google Scholar]
- 32.Sangachin MG, Cavuoto LA, Wang Y. Use of various obesity measurement and classification methods in occupational safety and health research: a systematic review of the literature. BMC Obes. 2018;5:28. doi: 10.1186/s40608-018-0205-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer EA, Appleby PN, Davey GK, Key TJ. Diet and body mass index in 38 000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int J Obes Relat Metab Disord. 2003;27:728-734. doi: 10.1038/sj.ijo.0802300 [DOI] [PubMed] [Google Scholar]
- 34.Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173:1230-1238. doi: 10.1001/jamainternmed.2013.6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong TY, Key TJ, Sobiecki JG, Bradbury KE. Anthropometric and physiologic characteristics in white and British Indian vegetarians and nonvegetarians in the UK Biobank. Am J Clin Nutr. 2018;107:909-920. doi: 10.1093/ajcn/nqy042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kristensen NB, Madsen ML, Hansen TH, et al. Intake of macro- and micronutrients in Danish vegans. Nutr J. 2015;14:115. doi: 10.1186/s12937-015-0103-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schüpbach R, Wegmüller R, Berguerand C, Bui M, Herter-Aeberli I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur J Nutr. 2017;56:283-293. doi: 10.1007/s00394-015-1079-7 [DOI] [PubMed] [Google Scholar]
- 38.Losasso C, Eckert EM, Mastrorilli E, et al. Assessing the influence of vegan, vegetarian and omnivore oriented westernized dietary styles on human gut microbiota: a cross sectional study. Front Microbiol. 2018;9:317. doi: 10.3389/fmicb.2018.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welch AA, Shakya-Shrestha S, Lentjes MA, Wareham NJ, Khaw KT. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the precursor-product ratio of α-linolenic acid to long-chain n-3 polyunsaturated fatty acids: results from the EPIC-Norfolk cohort. Am J Clin Nutr. 2010;92:1040-1051. doi: 10.3945/ajcn.2010.29457 [DOI] [PubMed] [Google Scholar]
- 40.Benatar JR, Stewart RAH. Cardiometabolic risk factors in vegans: a meta-analysis of observational studies. PLoS One. 2018;13:e0209086. doi: 10.1371/journal.pone.0209086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:e256. doi: 10.1038/nutd.2017.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Craig WJ, Mangels AR. Position of the American Dietetic Association: vegetarian diets. J Am Diet Assoc. 2009;109:1266-1282. doi: 10.1016/s0002-8223(96)00305-7 [DOI] [PubMed] [Google Scholar]
- 43.Amit M. Vegetarian diets in children and adolescents [in French]. Paediatr Child Health (Oxford). 2010;15:303-314. doi: 10.1093/pch/15.5.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietitians of Canada. What you need to know about following a vegan eating plan—unlock food. Published 2018. Accessed October 4, 2019. https://www.unlockfood.ca/en/Articles/Vegetarian-and-Vegan-Diets/What-You-Need-to-Know-About-Following-a-Vegan-Eati.aspx
- 45.Gomes SC, João S, Pinho P, et al. National Programme for the Promotion of Healthy Eating Guidelines for a Healthy Vegetarian Diet. Published 2015. Accessed September 2, 2019. https://www.alimentacaosaudavel.dgs.pt/activeapp/wp-content/files_mf/1451330068Guidelinesforahealthyvegetariandiet.pdf
- 46.The Association of UK Dietitians. British Dietetic Association confirms well-planned vegan diets can support healthy living in people of all ages. Published 2017. Accessed June 3, 2019. https://www.bda.uk.com/news/view?id=179
- 47.Agnoli C, Baroni L, Bertini I, et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr Metab Cardiovasc Dis. 2017;27:1037-1052. doi: 10.1016/j.numecd.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 48.Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P. Behavioural Weight Management Review Group. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114:1557-1568. doi: 10.1016/j.jand.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118-126. doi: 10.1016/j.amepre.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffman SR, Stallings SF, Bessinger RC, Brooks GT. Differences between health and ethical vegetarians. Strength of conviction, nutrition knowledge, dietary restriction, and duration of adherence. Appetite. 2013;65:139-144. doi: 10.1016/j.appet.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 51.Achamrah N, Colange G, Delay J, et al. Comparison of body composition assessment by DXA and BIA according to the body mass index: a retrospective study on 3655 measures. PLoS One. 2018;13:e0200465. doi: 10.1371/journal.pone.0200465 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Sj-pdf-1-ajl-10.1177_1559827620949205 for Whole-Food Plant-Based Lifestyle Program and Decreased Obesity by Boštjan Jakše, Barbara Jakše, Stanislav Pinter, Jernej Pajek and Nataša Fidler Mis in American Journal of Lifestyle Medicine