Abstract
Background
Lateral pelvic lymph node dissection (LPLND) is an option in the treatment of rectal cancer and may reduce local recurrence/improve disease-free survival. Advancements in minimally invasive technology have improved the ability to identify anatomy and neurovascular structures that may help in LPLND. The aim of this retrospective study was to evaluate the technical feasibility and oncological safety of laparoscopic LPLND compared with the open LPLND.
Method
Between July 2010 and July 2019, patients from three tertiary referral hospitals who underwent LPLND with total mesorectal excision for primary rectal cancer were included. Baseline patient characteristics, perioperative outcomes, pathologic results, recurrence, and survival were compared between the laparoscopic and open groups.
Results
There were 126 and 70 patients in the laparoscopic and open groups respectively. The laparoscopic group had less estimated blood loss (100 ml versus 300 ml, P < 0.001) and lower transfusion rate (0.8 per cent versus 10.0 per cent; P = 0.003) but longer operating times (318 min versus 270 min, P = 0.004). The laparoscopic group had fewer wound infections (1.6 per cent versus 10.0 per cent, P = 0.011) and neuropathy (0 per cent versus 4.3 per cent, P = 0.044). Lateral pelvic recurrence rate was 7.6 per cent in the laparoscopic group and 19.6 per cent in the open group (P = 0.053). Recurrence-free survival (72.2 per cent versus 63.5 per cent; P = 0.190) and overall survival (93.3 per cent versus 85.0 per cent; P = 0.118) were not significantly different.
Conclusion
Laparoscopic LPLND was associated with improved perioperative outcomes and non-inferior oncological outcomes.
This is a multicentre retrospective cohort study that aimed to evaluate the surgical and oncological outcomes of laparoscopic lateral pelvic lymph node dissection (LPLND) compared with those of open LPLND using statistical analyses. In addition, we report the technical feasibility and oncological safety of laparoscopic LPLND.
Introduction
Locoregional recurrence after curative resection of locally advanced rectal cancer reduces patient survival and impairs quality of life1,2. Over the last 30 years, improvements in technique, including total mesorectal excision (TME), have decreased local recurrence in patients with rectal cancer3–5. In addition to the adoption of the TME technique, neoadjuvant chemoradiotherapy further reduces local recurrence6,7. However, chemoradiotherapy followed by TME still has a significant risk of local recurrence of 5–9 per cent5–7.
Recent studies have reported that local recurrences that occur after preoperative chemoradiotherapy and TME mainly recur in the lateral pelvic lymph nodes (LPLNs), with more than 50 per cent of all local recurrences occurring only in the lateral compartment8–11. LPLN dissection (LPLND) was developed with the aim of reducing local recurrence. The oncological benefits of LPLND performed for patients with suspicious metastatic LPLNs based on pre-treatment radiology have been reported in patients who received preoperative chemoradiotherapy12–16. A large international pooled analysis demonstrated that 5-year lateral local recurrence was reduced from 19.5 to 5.7 per cent with LPLND in patients with LPLNs of more than or equal to 7 mm in the short axis14. Other studies reported that local recurrence was 3–5.39 per cent (with LPLND) versus 11–20.13 per cent (without LPLND) in patients with LPLNs of more than or equal to 5 mm in the short axis and preoperative chemoradiotherapy15,16. LPLND with TME after preoperative chemoradiotherapy is an option for managing local recurrence in advanced rectal cancer with enlarged LPLNs; however, LPLND is considered a challenging procedure due to the complex neurovascular anatomy of the lateral pelvis, which leads to longer operative times, greater blood loss and increased urinary/sexual dysfunction17–20. To date, several studies have evaluated the outcomes of the laparoscopic LPLND technique and reported this technique to have better short-term results, such as reduced blood loss and shorter duration of hospital stay, than the open approach21–27. However, in most studies, LPLND was performed in all patients according to Japanese guidelines, and only a small number of patients who received preoperative chemoradiotherapy were included. Therefore, it has been difficult to directly apply these results to institutions that implement Western radiotherapy-based neoadjuvant therapy. Presently, studies reporting short-term results and long-term oncological safety of laparoscopic LPLND with preoperative chemoradiotherapy for locally advanced rectal cancer are lacking.
Since 2010, the institutions of Seoul Colorectal Research Group (SECOG) has been using radiation therapy-based treatment for advanced rectal cancer, and LPLND was selectively performed based on pre-treatment MRI. Based on this treatment strategy, the present study aimed to evaluate the technical feasibility and oncological safety of laparoscopic LPLND performed in patients with locally advanced rectal cancer by comparing its short-term and long-term outcomes with those undergoing an open approach.
Methods
Study design
This was a retrospective study based on prospectively collected databases of three tertiary referral hospitals. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (institutional review board number 2107-195-1237) and conformed with the Declaration of Helsinki. The institutional review board waived the need for informed consent due to the study’s retrospective nature.
Patients
Consecutive patients who had undergone LPLND with TME for primary rectal cancer with curative intent between July 2010 and July 2019 at three different tertiary referral hospitals, performing more than 700 laparoscopic surgeries and more than 200 rectal cancer surgeries annually, were eligible for this study. Patients with a histologically proven primary rectal adenocarcinoma located within 15 cm of the anal verge, radiologically suspected LPLN metastasis, and without M1 disease were included. Patients who had undergone palliative surgery with a history of other malignancies or synchronous multiple cancer were excluded.
The collected variables were the baseline patient characteristics, perioperative outcomes, pathological examination results, all types of recurrence, including local and metastatic, and survival.
Preoperative investigations
All patients underwent digital rectal examination (DRE), colonoscopy, chest X-ray, CT of the abdomen and pelvis, and rectal MRI before surgery to evaluate the preoperative cancer stage. The tumour height from the anal verge was determined based on DRE and colonoscopy findings by surgeons. Patients with LPLNs with a short-axis diameter more than or equal to 5 mm when initially assessed by way of rectal MRI were radiologically suspected to have LPLN metastasis8.
Neoadjuvant treatment
Preoperative chemoradiotherapy was performed in patients with clinical T3/T4 or node-positive rectal cancer. Radiation was delivered to the entire pelvis at a dose of 45 Gy in 25 fractions, followed by a 5.4 Gy boost in three fractions to the primary tumour. The radiation field encompassed the volume, including the gross tumour, mesorectum, presacral space, the whole sacral hollow, and the regional lymphatics, including perirectal, presacral, internal iliac, and distal common iliac nodes, and did not change with the LPLNs status. The fluoropyrimidine-based preoperative chemotherapy was concurrently initiated on the first day of pelvic radiotherapy and administered on the days of radiotherapy: two cycles of an intravenous bolus of fluorouracil (400 mg/m2 per day) and leucovorin (20 mg/m2 per day) for 3 days in the first and fifth weeks of radiotherapy; or continuous oral administration of capecitabine (825 mg/m2 twice daily) during radiotherapy28.
Surgical procedure
Surgical resection was performed 6–9 weeks after completion of preoperative chemoradiotherapy. Radical proctectomy with TME, inferior mesenteric vessel ligation, and autonomic nerve preservation were performed in all patients. Proctectomy was divided into three operation types based on the tumour height from the anal verge and whether the anal sphincter complex or pelvic floor structures were invaded: low anterior resection with double-stapling anastomosis, intersphincteric resection with hand-sewn coloanal anastomosis, and abdominoperineal resection. As reported in the previous study of Seoul Colorectal Research Group (SECOG), LPLND was performed in patients with a LPLN more than or equal to 5 mm in the short axis on preoperative MRI, regardless of the chemoradiotherapy response15. The procedure was performed by complete LPLNs removal in the adipose tissue located in the pelvic cavity, lateral to the pelvic plexus. All internal and external iliac and obturator nodes on the side of radiologically suspected LPLN metastasis were cleared, and the autonomic nerves and pelvic vessels were preserved unless they were invaded by the metastatic LPLN. The anatomical landmarks of LPLND were the external iliac artery and obturator muscle on the lateral side, pelvic plexus on the medial side, sciatic nerves on the dorsal side, and levator ani muscle on the caudal side29. One patient group underwent both proctectomy and LPLND laparoscopically (laparoscopic group), while the others underwent both proctectomy and LPLND by laparotomy (open group).
Pathological examination findings
Surgical specimens were evaluated by a board-certified pathologist who determined the pathological stage of all specimens based on the eighth edition of the American Joint Committee on Cancer Staging System30. Pathological outcomes that could affect the quality of the surgical procedure and oncological results, including the number of collected lymph nodes and resection margin status, were evaluated. Circumferential and distal resection margins were considered positive if the distance from the tumour to the surgical resection margin was microscopically less than 1 mm31,32.
Postoperative and oncological outcomes
Postoperative outcomes, including morbidity and mortality within 30 days after surgery, were evaluated. The severity of complications was evaluated according to the modified Clavien–Dindo classification33. Patient follow-up was performed every 3 months for the first 2 years after surgery, then every 6 months for up to 5 years, and once every year thereafter. Recurrence was demonstrated by pathological results obtained by surgical resection, biopsy, or cytology of the recurrent tumour and/or radiological findings of an increase in the size of the tumour over time. Lateral pelvic recurrence was defined as the detection of tumour recurrence within the pelvic cavity, except for anastomotic and mesorectal recurrences. Finally, systemic recurrence was defined as any recurrence outside the pelvic cavity.
Statistical analysis
The characteristics of patients in the laparoscopic and open groups were compared with a Student’s t test or the Mann–Whitney U test for continuous variables, and the chi-squared test or Fisher's exact test for categorical variables. Survival curves were estimated with the Kaplan–Meier method and comparisons between curves were performed with a log rank test.
For the time to the lateral pelvic recurrence, the first lateral pelvic recurrence was defined as an event and death due to any cause after surgery for overall survival (OS). In the determination of recurrence-free survival (RFS), any local, metastatic recurrence, or death, due to any cause after surgery was defined as an event. The impact of potential risk factors for lateral pelvic recurrence, RFS, and OS were analysed by way of univariable and multivariable Cox proportional hazard regression models. Variables remaining in the multivariable model were selected with a backward selection method. A P value ≤0.1 was used for inclusion of variable in the multivariable analysis.
Statistical significance was defined as a P value <0.050. All statistical analyses were performed with SPSS® version 25 (IBM, Armonk, New York, USA).
Results
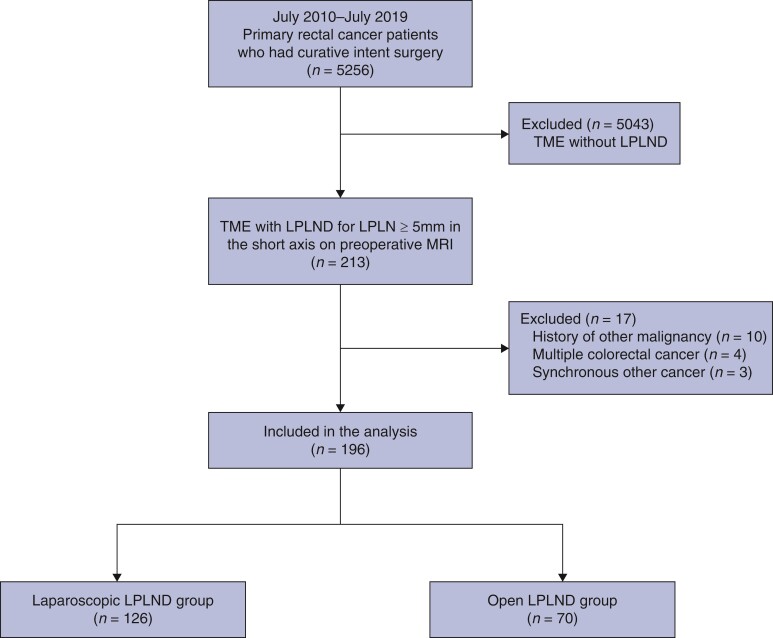
A total of 196 patients were enrolled and analysed with a median age of 58.0 (30–82) years; 84 (42.9 per cent) were women. There were 126 patients in the laparoscopic group and 70 in the open group (Fig. 1). The patient baseline and operative characteristics are listed in Table 1 and were similar between the two groups. Preoperative chemoradiotherapy was administered to 108 of 126 patients (85.7 per cent) in the laparoscopic group and 53 (75.7 per cent) patients in the open group (P = 0.080). Operation type, diverting stoma rate, and extent of LPLND were not statistically different between the two groups. The conversion from laparoscopic to open surgery occurred in 3 out of 126 patients (2.4 per cent) in the laparoscopic group. The reasons for conversion to open surgery were difficulty in securing adequate distal resection margins for rectal cancer (n = 2) and T4 disease (n = 1).
Fig. 1.
Flow chart of the study population
TME, total mesorectal excision; LPLND, lateral pelvic lymph node dissection; LPLN, lateral pelvic lymph node.
Table 1.
Baseline and surgical characteristics
| Variables | Lap (n = 126) | Open (n = 70) | P* |
|---|---|---|---|
| Age, (years) median (range) | 58 (30−81) | 59 (31−82) | 0.950 |
| Sex | 0.763 | ||
| Male | 73 (57.9) | 39 (55.7) | |
| Female | 53 (42.1) | 31 (44.3) | |
| BMI, median (range) | 24.1 (17.9–34.8) | 22.9 (17.8–35.0) | 0.070 |
| ASA PS | 0.351 | ||
| I–II | 124 (98.4) | 67 (95.7) | |
| III–IV | 2 (1.6) | 3 (4.3) | |
| Previous abdominal surgery | 38 (30.2) | 21 (30.0) | 0.981 |
| Tumour distance from AV, median (range) | 5.0 (0–15.0) | 5.0 (0–15.0) | 0.410 |
| Preoperative CRT | 0.080 | ||
| No | 18 (14.3) | 17 (24.3) | |
| Yes | 108 (85.7) | 53 (75.7) | |
| Pretreatment CEA level, median (range) | 3.3 (0–703.7) | 3.7 (0.5–250.7) | 0.285 |
| Operation type | 0.095 | ||
| Low anterior resection | 93 (73.8) | 43 (61.4) | |
| Intersphincteric resection | 22 (17.5) | 14 (20.0) | |
| Abdominoperineal resection | 11 (8.7) | 13 (18.6) | |
| Diverting stoma | 119 (94.4) | 63 (90.0) | 0.247 |
| Extent of LPLND | 0.050 | ||
| Unilateral | 103 (81.7) | 48 (68.6) | |
| Bilateral | 23 (18.3) | 22 (31.4) | |
| Conversion rate | 3 (2.4) | NA | NA |
Lap, laparoscopic; PS, physical status classification; AV, anal verge; CRT, chemoradiotherapy; CEA, carcinoembryonic antigen; LPLND, lateral pelvic lymph node dissection; NA, not applicable.
P values were calculated using the Mann–Whitney U test for continuous variables and the chi-squared test for categorical variables. Values are n (%) unless otherwise indicated.
The laparoscopic group had longer operating times, less estimated blood loss, and lower transfusion rates than the open group (Table 2). In the subgroup analysis of estimated blood loss according to the extent of LPLND, the laparoscopic group had less blood loss than the open group in both unilateral LPLND (median 100 (range 10–1000) ml versus median 255 (range 20–2000) ml; P < 0.001) and bilateral LPLND (median 150 (range 30–500) ml versus median 325 (range 30–2000) ml; P = 0.008). Intraoperative adverse events occurred in 6 out of 126 patients (4.8 per cent) in the laparoscopic group and in 3 out of 70 patients (4.3 per cent) in the open group (P > 0.999). Most of the adverse events were intraoperative bleeding during lymph node dissection in 5 of 126 patients (4.0 per cent) (laparoscopic group) versus 2 of 70 patients (2.9 per cent) (open group). The laparoscopic group had similar overall morbidity rates (40 of 126 patients (31.7 per cent) versus 25 of 70 (35.7 per cent); P = 0.572) and Clavien–Dindo classification (grade I and II, 31 of 126 patients (24.6 per cent) versus 19 of 70 (27.1 per cent); grade III and IV, 9 of 126 patients (7.1 per cent) versus 6 of 70 (8.6 per cent); P = 0.889) compared with the open group (Table 2). Wound infection (2 of 126 patients (1.6 per cent) versus 7 of 70 (10.0 per cent); P = 0.011) and neuropathy (0 of 126 patients (0 per cent) versus 3 of 70 (4.3 per cent); P = 0.044) were significantly lower in the laparoscopic group. Three patients had neuropathy in the open group, of whom two had sciatic neuropathy and one had obturator neuropathy. All three patients with postoperative bleeding in the laparoscopic group were found to have intraluminal anastomotic bleeding, which was not related to the LPLND site. Postoperative duration of hospital stay was similar between the two groups (9 (3–46) days versus 9 (5–64) days, P = 0.454), and no postoperative mortality within postoperative 30 days occurred in either group.
Table 2.
Perioperative outcomes of laparoscopic versus open lateral pelvic lymph node dissection
| Variables | Lap (n = 126) | Open (n = 70) | P * |
|---|---|---|---|
| Operative time, (mins) median (range) | 318 (145–650) | 270 (150–675) | 0.004 |
| Estimated blood loss, (ml) median (range) | 100 (10–1000) | 300 (20–2000) | <0.001 |
| Transfusion | 1 (0.8) | 7 (10.0) | 0.003 |
| Intraoperative adverse event | 6 (4.8) | 3 (4.3) | >0.999 |
| Bleeding | 5 (4) | 2 (2.9) | >0.999 |
| Ureter injury | 1 (0.8) | 1 (1.4) | >0.999 |
| Postoperative complication | 40 (31.7) | 25 (35.7) | 0.572 |
| Urinary retention | 12 (9.5) | 4 (5.7) | 0.351 |
| Ileus | 12 (9.5) | 3 (4.3) | 0.186 |
| Wound infection | 2 (1.6) | 7 (10.0) | 0.011 |
| Pelvic abscess | 4 (3.2) | 5 (7.1) | 0.286 |
| lymphocele | 5 (4.0) | 4 (5.7) | 0.724 |
| Anastomotic leakage | 4 (3.2) | 1 (1.4) | 0.657 |
| Bleeding | 3 (2.4) | 0 (0) | 0.554 |
| Neuropathy | 0 (0) | 3 (4.3) | 0.044 |
| Pulmonary-related complication | 3 (2.4) | 2 (2.9) | >0.999 |
| Stoma-related complication | 3 (2.4) | 0 (0) | 0.554 |
| Clavien–Dindo classification | 0.889 | ||
| Grade <3 | 31 (24.6) | 19 (27.1) | |
| Grade ≥3 | 9 (7.1) | 6 (8.6) | |
| Postoperative duration of hospital stay, median (range) | 9 (3–46) | 9 (5–64) | 0.454 |
| Mortality | 0 (0) | 0 (0) | NA |
Lap, laparoscopic; NA, not applicable.
P values were calculated using the Mann–Whitney U test for continuous variables and the chi-squared test for categorical variables. Values are n (%) unless otherwise indicated.
The p/ypT category, p/ypN category, tumour differentiation, resection margin status, and pathological complete response rate of the laparoscopic group were comparable to the open group (Table 3). The overall pathological LPLN metastasis rate of the study population was 29.1 per cent (57 of 196 patients), of which 27.6 per cent (54 of 196) had unilateral metastasis, and 1.5 per cent (3 of 196) had bilateral metastases. The pathological LPLN metastasis rate (32 of 126 patients (25.4 per cent) versus 25/70 (35.7 per cent); P = 0.128) and the median number of metastatic LPLNs (0 (0–7) versus 0 (0–7); P = 0.098) were comparable between the groups. The median number of collected LPLNs was significantly higher in the open group than in the laparoscopic group (overall 7 (0–23) versus 10 (0–46); P = 0.027; unilateral 6 (0–16) versus 9 (0–29); P = 0.021). The mesorectal lymph node metastasis rate, number of metastatic, and collected mesorectal lymph nodes were also similar between the two groups.
Table 3.
Pathological outcomes of laparoscopic versus open lateral pelvic lymph node dissection
| Variables | Lap (n = 126) | Open (n = 70) | P* |
|---|---|---|---|
| p/ypT category | 0.121 | ||
| T0–2 | 48 (38.1) | 19 (27.1) | |
| T3–4 | 78 (61.9) | 51 (72.9) | |
| p/ypN category | 0.443 | ||
| N0 | 63 (50.0) | 31 (44.3) | |
| N1–2 | 63 (50.0) | 39 (55.7) | |
| Differentiation | 0.307 | ||
| WD/MD | 117 (92.9) | 61 (88.6) | |
| PD/mucinous/SRC | 9 (7.1) | 8 (11.4) | |
| Positive resection margin | 12 (9.5) | 8 (11.4) | 0.673 |
| CRM involvement (<1 mm) | 10 (8.1) | 7 (10.3) | 0.603 |
| DRM involvement (<1 mm) | 2 (1.6) | 1 (1.5) | 0.552 |
| Pathologic complete response | 7 (5.6) | 8 (11.4) | 0.138 |
| Pathological LPLN metastasis | 32 (25.4) | 25 (35.7) | 0.128 |
| Unilateral | 30 (23.8) | 24 (34.3) | 0.116 |
| Bilateral | 2 (1.6) | 1 (1.4) | >0.999 |
| Number of metastatic LPLNs, median (range) | 0 (0–7) | 0 (0–7) | 0.098 |
| Unilateral | 0 (0–4) | 0 (0–3) | 0.238 |
| Bilateral | 0 (0–7) | 0 (0–7) | 0.482 |
| Number of collected LPLNs, median (range) | 7 (0–23) | 10 (0–46) | 0.027 |
| Unilateral | 6 (0–16) | 9 (0–29) | 0.021 |
| Bilateral | 14 (2–23) | 13 (3–46) | 0.459 |
| Mesorectal LN metastasis | 58 (46.0) | 26 (37.7) | 0.260 |
| Number of metastatic mesorectal LNs, median (range) | 0 (0–9) | 0 (0–8) | 0.370 |
| Number of collected mesorectal LNs, median (range) | 26 (6–97) | 20 (3–76) | 0.095 |
Lap, laparoscopic; pCR, pathologic complete response; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; SRC, signet ring cell; CRM, circumferential resection margin; DRM, distal resection margin; LPLN, lateral pelvic lymph node; LN, lymph node.
P values were calculated using the Mann–Whitney U test for continuous variables and the chi-squared test for categorical variables. Values are n (%) unless otherwise indicated.
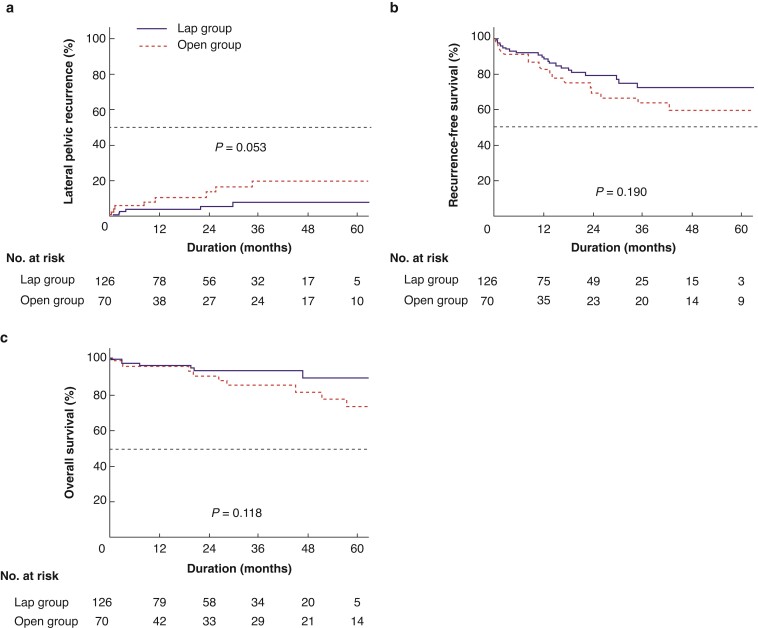
Kaplan–Meier curves of the lateral pelvic recurrence, RFS, and OS according to the surgical procedure are shown in Fig. 2. The median follow-up time in the entire study population was 21.0 (range 0.2–79.0) months. The median duration of follow-up was 21.0 (range 0.2–73.4) and 21.8 (range 0.3–79.0) months in the laparoscopic and open groups respectively (P = 0.104). Oncological outcomes, including the lateral pelvic recurrence rate (3-years, 7.6 per cent versus 19.6 per cent; P = 0.053; Fig. 2a), RFS (3-years, 72.2 per cent versus 63.5 per cent; P = 0.190; Fig. 2b), and OS (3-years, 93.3 per cent versus 85.0 per cent; P = 0.118; Fig. 2c), were comparable between the two groups. Subgroup analysis of oncological outcomes in patients with pathological LPLN metastasis was performed and the lateral pelvic recurrence rate (3-years, 7.2 per cent versus 26.5 per cent; P = 0.114), RFS (3-years, 65.1 per cent versus 50.0 per cent; P = 0.141), and OS (3-years, 91.6 per cent versus 73.1 per cent; P = 0.063) were also comparable between the two groups.
Fig. 2.
Kaplan–Meier curves of lateral pelvic recurrence rate, recurrence-free survival, and overall survival according to surgical approach
a, Lateral pelvic recurrence rate. b, Recurrence-free survival rate. c, Overall survival rate. Rates were similar between the laparoscopic group and the open group. Lap, laparoscopic.
Cox regression analysis for variables associated with lateral pelvic recurrence, RFS, and OS is shown in Table 4 and Table S1. Multivariable analysis revealed that preoperative CEA (HR 1.003, 95 per cent c.i. 1.001 to 1.006; P = 0.018), transfusion rate (HR 11.886, 95 per cent c.i. 2.376 to 59.463; P = 0.003), and p/ypN category (HR 7.513, 95 per cent c.i. 1.588 to 35.555; P = 0.011) were independent prognostic factors for lateral pelvic recurrence, whereas estimated blood loss (HR 1.001, 95 per cent c.i. 1.000 to 1.002; P = 0.025) and p/ypN category (HR 5.072, 95 per cent c.i. 2.219 to 11.596; P < 0.001) were prognostic factors for RFS. Estimated blood loss (HR 1.001, 95 per cent c.i. 1.000 to 1.003; P = 0.025), postoperative hospital stay (HR 1.042, 95 per cent c.i. 1.008 to 1.078; P = 0.015), and mesorectal lymph node metastasis (HR 3.408, 95 per cent c.i. 1.062 to 10.937; P = 0.039) were predictors of OS. Surgical approaches, laparoscopy, or laparotomy were not prognostic factors for lateral pelvic recurrence, RFS, and OS.
Table 4.
Multivariable Cox proportional hazard regression model analysis for lateral pelvic recurrence, recurrence-free survival, and overall survival
| Lateral pelvic recurrence | Recurrence-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR (95% c.i.) | P | HR (95% c.i.) | P | HR (95% c.i.) | P | |
| Surgical approach | ||||||
| Open | Reference | Reference | Reference | |||
| Lap | 0.547 (0.166–1.805) | 0.322 | 0.980 (0.451–2.129) | 0.959 | 0.880 (0.283–2.730) | 0.824 |
| Pretreatment CEA level | 1.003 (1.001–1.006) | 0.018 | ||||
| Estimated blood loss | 1.001 (1.000–1.002) | 0.025 | 1.001 (1.000–1.003) | 0.025 | ||
| Transfusion | ||||||
| No | Reference | |||||
| Yes | 11.886 (2.376–59.463) | 0.003 | ||||
| Postoperative hospital stay | 1.042 (1.008–1.078) | 0.015 | ||||
| p/ypN catergory | ||||||
| N0 | Reference | Reference | ||||
| N1–2 | 7.513 (1.588–35.555) | 0.011 | 5.072 (2.219–11.596) | <0.001 | ||
| Mesorectal LN metastasis | ||||||
| No | Reference | |||||
| Yes | 3.408 (1.062–10.937) | 0.039 | ||||
Lap, laparoscopic; CEA, carcinoembryonic antigen; LN, lymph node.
Discussion
Laparoscopic LPLND enabled better perioperative results by preserving the neurovascular structures of the lateral pelvis more meticulously and resulted in adequate oncological outcomes in rectal cancer patients with LPLN size larger or equal to 5 mm before treatment. Laparoscopic LPLND resulted in better outcomes in terms of intraoperative bleeding and neuropathy compared with those observed with the open approach. The pathological and oncological outcomes of laparoscopic LPLND were similar to those of the open approach.
In 2011, three technical notes retrospectively reviewed the feasibility of laparoscopic LPLND in 11–34 patients and reported morbidity rates of 20.6–35.7 per cent and local recurrence rates of 6.1–11.2 per cent21–23. Three retrospective studies demonstrated that patients in the laparoscopic LPLND group had significantly less haemorrhage and similar recurrence and survival rates to those in the open group24–26. Although laparoscopic LPLND of locally advanced rectal cancer is not yet widely performed because of its technical difficulty and the anatomical complexity of the lateral pelvic compartment, previous studies suggest that laparoscopic LPLND might be superior to open LPLND.
Laparoscopic LPLND can be more technically challenging in patients after chemoradiotherapy with tissue fibrosis, oedema, and neural degeneration34,35. Tissue fibrosis interferes with dissection in the correct anatomical plane and tissue oedema can cause misting, further reducing visibility of structures27. Therefore, without considerable caution during surgery, damage to blood vessels, nerves, or ureters may increase, leading to postoperative complications. Despite these concerns, the findings of this study confirm the safety and oncological adequacy of laparoscopic LPLND with preoperative chemoradiotherapy. In the present study, intraoperative adverse events occurred at similar rates in the laparoscopic and open groups. Moreover, only three cases (2.4 per cent) required conversion to open surgery in the laparoscopic group, all of which occurred during the proctectomy procedure. Furthermore, the short-term outcomes in the laparoscopic group, overall morbidity rate (31.7 per cent) were comparable to those of the open group. Among the short-term outcomes, wound infection, blood loss, and neuropathy, were significantly better, and among the long-term outcomes, lateral pelvic recurrence rate, although not statistically significant, tended to be better in the laparoscopic group in the present study. Considering that the extent of LPLND may affect the amount of blood loss, unilateral LPLND and bilateral LPLND were further analysed separately in the subgroup analysis and the laparoscopic group still demonstrated less blood loss than the open group.
These favourable results of laparoscopic LPLND may have been facilitated with advanced laparoscopic devices that allow for more meticulous node dissection with a magnified surgical view. Advanced laparoscopes provide better visualization of the obturator foramen and Alcock’s canal, wherein 85 per cent of LPLN metastases occur, and are located in the deepest pelvis when approached from the abdomen36. Advanced energy devices that utilize ultrasonic and bipolar energy may have reduced lymphatic spillage and cancer cell contamination. Other possible explanations include recent increased understanding of lateral pelvic anatomy with the advent of laparoscopic approaches and possibly less tumour growth stimulation due to a decrease in perioperative surgical stress37. Consequently, this may have led to better operative outcomes, including reduced blood loss and neuropathy.
In the present study, the median number of collected LPLNs, which can measure adequate lymph node dissection, was significantly lower in the laparoscopic group (7 versus 10; P = 0.027). This may have resulted from the greater number of patients who underwent preoperative chemoradiotherapy in the laparoscopic group, although the difference was not statistically significant (108 of 126 patients (85.7 per cent) versus 53 of 70 (75.7 per cent); P = 0.080). Previous studies have reported that the number of lymph nodes retrieved after preoperative chemoradiotherapy for rectal cancer significantly decrease by 28.9–32.6 per cent due to apoptosis and degeneration38,39. Meanwhile, the pathological LPLN metastasis rate reported in a previous study as 13.9–40.0 per cent24,36,40–43 was similar between two groups (32 of 126 patients (25.4 per cent) versus 25 of 70 (35.7 per cent); P = 0.128). Therefore, it can be concluded that LPLND to retrieve the metastatic lymph nodes was performed appropriately.
This study had limitations. Firstly, because of its retrospective design, the possibility of selection bias cannot be excluded. Although this study was based on a prospectively maintained cohort from three institutions, this study was not a randomized clinical trial, and the inherent limitations were inevitable because surgeons determined whether the operation would be performed laparoscopically or not. Therefore, multivariable analysis was used to assess the individual effect of the surgical approach on the oncological outcomes and confirmed that the surgical approach was not an independent prognostic factor for all oncological outcomes. Further prospective randomized studies are needed to overcome this limitation. Secondly, due to the variety of regimens, doses, and completion statuses of individuals, postoperative chemotherapy, which can influence the oncological outcomes, could not be stratified and analysed. Finally, sexual dysfunction caused by nerve injury could not be assessed due to a lack of data on postoperative functional outcomes. Despite these limitations, considering the limited number of institutions performing laparoscopic LPLND, this study reports clinically important messages regarding the possible benefits of laparoscopic LPLND in intraoperative and postoperative outcomes and the long-term safety of LPLND after preoperative chemoradiotherapy.
Supplementary Material
Acknowledgements
The authors thank M. H. Kim (Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Gyeonggi, Republic of Korea) and S. S. Park (Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Gyeonggi, Republic of Korea) for their help with data collection.
Disclosure. The authors declare no conflict of interest
Contributor Information
Han-Ki Lim, Department of Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea; Colorectal Cancer Center, Seoul National University Cancer Hospital, Seoul, Republic of Korea.
Minjung Kim, Department of Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea; Colorectal Cancer Center, Seoul National University Cancer Hospital, Seoul, Republic of Korea; Cancer Research Institute, Seoul National University, Seoul, Republic of Korea.
Ji Won Park, Department of Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea; Colorectal Cancer Center, Seoul National University Cancer Hospital, Seoul, Republic of Korea; Cancer Research Institute, Seoul National University, Seoul, Republic of Korea.
Seung-Bum Ryoo, Department of Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea; Colorectal Cancer Center, Seoul National University Cancer Hospital, Seoul, Republic of Korea.
Kyu Joo Park, Department of Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea; Colorectal Cancer Center, Seoul National University Cancer Hospital, Seoul, Republic of Korea.
Heung-Kwon Oh, Department of Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea; Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Gyeonggi, Republic of Korea.
Duck-Woo Kim, Department of Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea; Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Gyeonggi, Republic of Korea.
Sung-Bum Kang, Department of Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea; Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Gyeonggi, Republic of Korea.
Dong Woon Lee, Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Gyeonggi, Republic of Korea.
Sung Chan Park, Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Gyeonggi, Republic of Korea.
Jae Hwan Oh, Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Gyeonggi, Republic of Korea.
Seung-Yong Jeong, Department of Surgery, Seoul National University College of Medicine, Seoul, Republic of Korea; Colorectal Cancer Center, Seoul National University Cancer Hospital, Seoul, Republic of Korea; Cancer Research Institute, Seoul National University, Seoul, Republic of Korea.
Seoul Colorectal Research Group (SECOG):
H.-K. Lim, M. J. Kim, J. W. Park, S.-B. Ryoo, K. J. Park, M. H. Kim, H.-K. Oh, D.-W. Kim, S.-B. Kang, S. S. Park, D. W. Lee, S. C. Park, J. H. Oh, R. M. Shin, S. C. Heo, and S.-Y. Jeong
Collaborators
Seoul Colorectal Research Group (SECOG). H.-K. Lim (Seoul National University Hospital, Seoul, Republic of Korea), M. J. Kim (Seoul National University Hospital, Seoul, Republic of Korea), J. W. Park (Seoul National University Hospital, Seoul, Republic of Korea), S.-B. Ryoo (Seoul National University Hospital, Seoul, Republic of Korea), K. J. Park (Seoul National University Hospital, Seoul, Republic of Korea), M. H. Kim (Seoul National University Bundang Hospital, Seongnam, Republic of Korea), H.-K. Oh (Seoul National University Bundang Hospital, Seongnam, Republic of Korea), D.-W. Kim (Seoul National University Bundang Hospital, Seongnam, Republic of Korea), S.-B. Kang (Seoul National University Bundang Hospital, Seongnam, Republic of Korea), S. S. Park (National Cancer Center, Goyang, Republic of Korea), D. W. Lee (National Cancer Center, Goyang, Republic of Korea), S. C. Park (National Cancer Center, Goyang, Republic of Korea), J. H. Oh (National Cancer Center, Goyang, Republic of Korea), R. M. Shin (Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Republic of Korea), S. C. Heo (Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Republic of Korea), S.-Y. Jeong (Seoul National University Hospital, Seoul, Republic of Korea).
Funding
This study was supported by the Seoul National University Hospital (grant no. 2920190030).
Supplementary material
Supplementary material is available at BJS online.
Data availability
The data described in the manuscript are not provided due to privacy and ethical restrictions; however, anonymous data necessary to reproduce the results will be available from the corresponding author on reasonable request.
References
- 1. Temple WJ, Saettler EB. Locally recurrent rectal cancer: role of composite resection of extensive pelvic tumors with strategies for minimizing risk of recurrence. J Surg Oncol 2000;73:47–58 [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Aguilar J, Cromwell JW, Marra C, Lee SH, Madoff RD, Rothenberger DA. Treatment of locally recurrent rectal cancer. Dis Colon Rectum 2001;44:1743–1748 [DOI] [PubMed] [Google Scholar]
- 3. Heald RJ, Ryall RDH. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;327:1479–1482. [DOI] [PubMed] [Google Scholar]
- 4. Havenga K, Enker WE, Norstein J, Moriya Y, Heald RJ, Van Houwelingen HCet al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol 1999;25:368–374 [DOI] [PubMed] [Google Scholar]
- 5. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish rectal cancer trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644–5650 [DOI] [PubMed] [Google Scholar]
- 6. Van Gijn W, Marijnen CAM, Nagtegaal ID, Kranenbarg EMK, Putter H, Wiggers Tet al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575–582 [DOI] [PubMed] [Google Scholar]
- 7. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess Cet al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926–1933 [DOI] [PubMed] [Google Scholar]
- 8. Kim TH, Jeong SY, Choi DH, Kim DY, Jung KH, Moon SHet al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol 2008;15:729–737 [DOI] [PubMed] [Google Scholar]
- 9. Kim TG, Park W, Choi DH, Park HC, Kim SH, Cho YBet al. Factors associated with lateral pelvic recurrence after curative resection following neoadjuvant chemoradiotherapy in rectal cancer patients. Int J Colorectal Dis 2014;29:193–200 [DOI] [PubMed] [Google Scholar]
- 10. Kim MJ, Kim TH, Kim DY, Kim SY, Baek JY, Chang HJet al. Can chemoradiation allow for omission of lateral pelvic node dissection for locally advanced rectal cancer? J Surg Oncol 2015;111:459–464 [DOI] [PubMed] [Google Scholar]
- 11. Kusters M, Slater A, Muirhead R, Hompes R, Guy RJ, Jones OMet al. what to do with lateral nodal disease in low locally advanced rectal cancer? A call for further reflection and research. Dis Colon Rectum 2017;60:577–585 [DOI] [PubMed] [Google Scholar]
- 12. Akiyoshi T, Ueno M, Matsueda K, Konishi T, Fujimoto Y, Nagayama Set al. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol 2014;21:189–196 [DOI] [PubMed] [Google Scholar]
- 13. Matsuda T, Sumi Y, Yamashita K, Hasegawa H, Yamamoto M, Matsuda Yet al. Outcomes and prognostic factors of selective lateral pelvic lymph node dissection with preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 2018;33:367–374 [DOI] [PubMed] [Google Scholar]
- 14. Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda Set al. Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low ct3/4 rectal cancer. J Clin Oncol 2019;37:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim MJ, Chang GJ, Lim HK, Song MK, Park SC, Sohn DKet al. Oncological impact of lateral lymph node dissection after preoperative chemoradiotherapy in patients with rectal cancer. Ann Surg Oncol 2020;27:3525–3533 [DOI] [PubMed] [Google Scholar]
- 16. Kroon HM, Malakorn S, Dudi-Venkata NN, Bedrikovetski S, Liu J, Kenyon-Smith Tet al. Local recurrences in western low rectal cancer patients treated with or without lateral lymph node dissection after neoadjuvant (chemo)radiotherapy: an international multi-centre comparative study. Eur J Surg Oncol 2021;47:2441–2449. [DOI] [PubMed] [Google Scholar]
- 17. Nagawa H, Muto T, Sunouchi K, Higuchi Y, Tsurita G, Watanabe Tet al. Randomized, controlled trial of lateral node dissection vs. nerve-preserving resection in patients with rectal cancer after preoperative radiotherapy. Dis Colon Rectum 2001;44:1274–1280 [DOI] [PubMed] [Google Scholar]
- 18. Yano H, Moran BJ. The incidence of lateral pelvic side-wall nodal involvement in low rectal cancer may be similar in Japan and the West. Br J Surg 2008;95:33–49 [DOI] [PubMed] [Google Scholar]
- 19. Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJet al. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol 2009;10:1053–1062 [DOI] [PubMed] [Google Scholar]
- 20. Fujita S, Akasu T, Mizusawa J, Saito N, Kinugasa Y, Kanemitsu Yet al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol 2012;13:616–621 [DOI] [PubMed] [Google Scholar]
- 21. Park JS, Choi GS, Lim KH, Jang YS, Kim HJ, Park SYet al. Laparoscopic extended lateral pelvic node dissection following total mesorectal excision for advanced rectal cancer: initial clinical experience. Surg Endosc 2011;25:3322–3329 [DOI] [PubMed] [Google Scholar]
- 22. Liang JT. Technical feasibility of laparoscopic lateral pelvic lymph node dissection for patients with low rectal cancer after concurrent chemoradiation therapy. Ann Surg Oncol 2011;18:153–159 [DOI] [PubMed] [Google Scholar]
- 23. Konishi T, Kuroyanagi H, Oya M, Ueno M, Fujimoto Y, Akiyoshi Tet al. Lateral lymph node dissection with preoperative chemoradiation for locally advanced lower rectal cancer through a laparoscopic approach. Surg Endosc 2011;25:2358–2359 [DOI] [PubMed] [Google Scholar]
- 24. Nagayoshi K, Ueki T, Manabe T, Moriyama T, Yanai K, Oda Yet al. Laparoscopic lateral pelvic lymph node dissection is achievable and offers advantages as a minimally invasive surgery over the open approach. Surg Endosc 2016;30:1938–1947 [DOI] [PubMed] [Google Scholar]
- 25. Yamaguchi T, Konishi T, Kinugasa Y, Yamamoto S, Akiyoshi T, Okamura Ret al. Laparoscopic versus open lateral lymph node dissection for locally advanced low rectal cancer: a subgroup analysis of a large multicenter cohort study in Japan. Dis Colon Rectum 2017;60:954–964 [DOI] [PubMed] [Google Scholar]
- 26. Watanabe J, Ishibe A, Suwa Y, Ozawa M, Nakagawa K, Suwa Het al. Short- and long-term outcomes of laparoscopic versus open lateral lymph node dissection for locally advanced middle/lower rectal cancer using a propensity score-matched analysis. Surg Endosc 2021;35:4427–4435 [DOI] [PubMed] [Google Scholar]
- 27. Matsuda T, Hasegawa H, Yamashita K, Tanaka T, Yamamoto M, Kanaji Set al. Laparoscopic lateral pelvic lymph node dissection for lower rectal cancer treated with preoperative chemoradiotherapy. Surg Endosc 2020;34:1425–1431 [DOI] [PubMed] [Google Scholar]
- 28. Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DWet al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637–645 [DOI] [PubMed] [Google Scholar]
- 29. Malakorn S, Ouchi A, Sammour T, Bednarski BK, Chang GJ. Robotic lateral pelvic lymph node dissection after neoadjuvant chemoradiation: view from the west. Dis Colon Rectum 2018;61:1119–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amin MB. AJCC Cancer Staging System (8th edn). New York: Springer, 2017 [Google Scholar]
- 31. Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas Met al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes the ACOSOG Z6051 randomized clinical trial. JAMA 2015;314:1346–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stevenson ARL, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJet al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 2015;314:1356–1363 [DOI] [PubMed] [Google Scholar]
- 33. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishizawa Y, Fujii S, Saito N, Ito M, Ochiai A, Sugito Met al. The association between anal function and neural degeneration after preoperative chemoradiotherapy followed by intersphincteric resection. Dis Colon Rectum 2011;54:1423–1429 [DOI] [PubMed] [Google Scholar]
- 35. Koushi K, Nishizawa Y, Kojima M, Fujii S, Saito N, Hayashi Ret al. Association between pathologic features of peripheral nerves and postoperative anal function after neoadjuvant therapy for low rectal cancer. Int J Colorectal Dis 2016;31:1845–1852 [DOI] [PubMed] [Google Scholar]
- 36. Kobayashi H, Mochizuki H, Kato T, Mori T, Kameoka S, Shirouzu Ket al. Outcomes of surgery alone for lower rectal cancer with and without pelvic sidewall dissection. Dis Colon Rectum 2009;52:567–576 [DOI] [PubMed] [Google Scholar]
- 37. Agalar F, Daphan C, Hayran M, Sayek I. Laparoscopic surgery is associated with less tumour growth stimulation than conventional surgery: an experimental study. Br J Surg 1997;84:1480. [DOI] [PubMed] [Google Scholar]
- 38. Wichmann MW, Muller C, Meyer G, Strauss T, Hornung HM, Lau-Werner Uet al. Effect of preoperative radiochemotherapy on lymph node retrieval after resection of rectal cancer. Tech Coloproctol 2002;6:199–200 [PubMed] [Google Scholar]
- 39. Ha YH, Jeong SY, Lim SB, Choi HS, Hong YS, Chang HJet al. Influence of preoperative chemoradiotherapy on the number of lymph nodes retrieved in rectal cancer. Ann Surg 2010;252:336–340 [DOI] [PubMed] [Google Scholar]
- 40. Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka Set al. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum 2006;49:1663–1672 [DOI] [PubMed] [Google Scholar]
- 41. Ueno H, Mochizuki H, Hashiguchi Y, Hase K. Prognostic determinants of patients with lateral nodal involvement by rectal cancer. Ann Surg 2001;234:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oh HK, Kang SB, Lee SM, Lee SY, Ihn MH, Kim DWet al. Neoadjuvant chemoradiotherapy affects the indications for lateral pelvic node dissection in mid/low rectal cancer with clinically suspected lateral node involvement: a multicenter retrospective cohort study. Ann Surg Oncol 2014;21:2280–2287 [DOI] [PubMed] [Google Scholar]
- 43. Lim SB, Yu CS, Kim CW, Yoon YS, Park SH, Kim TWet al. Clinical implication of additional selective lateral lymph node excision in patients with locally advanced rectal cancer who underwent preoperative chemoradiotherapy. Int J Colorectal Dis 2013;28:1667–1674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the manuscript are not provided due to privacy and ethical restrictions; however, anonymous data necessary to reproduce the results will be available from the corresponding author on reasonable request.