Highlights
-
•
High-intensity exercise (HIE) causes an immediate increase in brain-derived neurotrophic factor (BDNF).
-
•
Compared to non-exercise or light-intensity exercise, HIE significantly increases BDNF.
-
•
The benefits of HIE remain similar despite differences in exercise length or baseline cardiorespiratory fitness.
-
•
HIE could be recommended to maximize circulating BDNF in healthy adults.
Keywords: Cognition, Exercise, Neurogenesis, Neuroplasticity
Abstract
Background
Although brain-derived neurotrophic factor (BDNF) has been identified as a molecular biomarker of the neurophysiological effects induced by exercise, the acute effects of high-intensity exercise (HIE) on BDNF levels are inconclusive. This study aims to estimate the immediate effects of HIE on BDNF levels in healthy young adults.
Methods
A systematic search was conducted in the MEDLINE, Scopus, Cochrane CENTRAL, and SPORTDiscuss databases up to December 2020. Randomized controlled trials (RCTs) and non-RCTs reporting pre–post changes in serum or plasma BDNF after an acute intervention of HIE compared to a control condition were included. Pooled effect sizes (p-ESs) and 95% confidence intervals (95%CIs) were calculated for RCTs using a random effects model with Stata/SE (Version 15.0; StataCorp., College Station, TX, USA). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed. PROPERO registration number: CRD42020221047.
Results
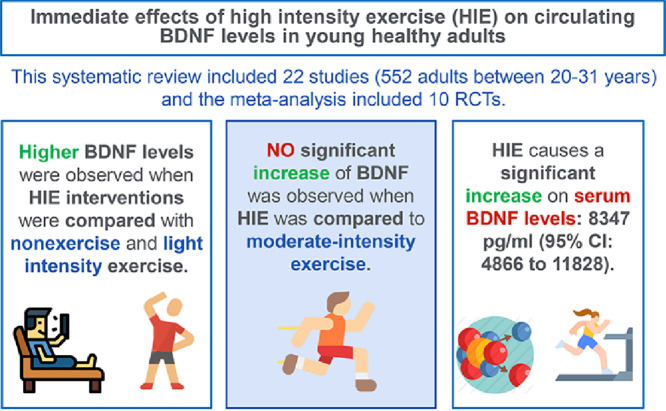
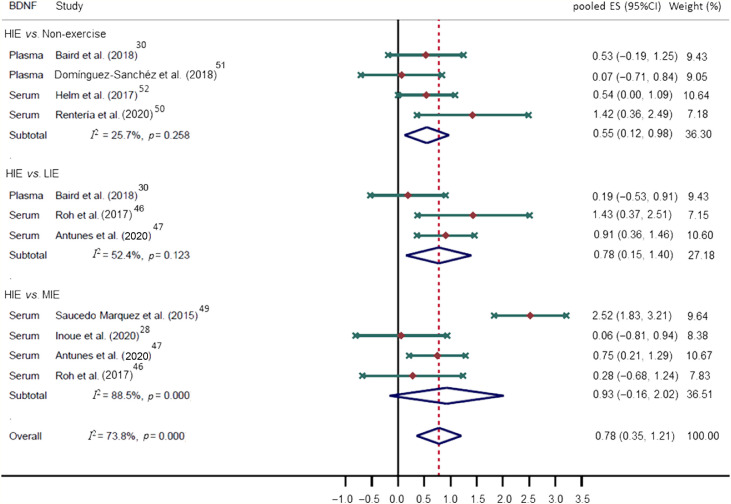
A total of 22 studies with 552 individuals (age range: 20–31 years; 59.1% male) were included. The meta-analysis included 10 RCTs that reported valid outcome data. Higher BDNF levels were observed when HIE interventions were compared with non-exercise (p-ES = 0.55, 95%CI: 0.12–0.98; I2 = 25.7%; n = 4 studies) and light-intensity exercise (p-ES = 0.78, 95%CI: 0.15–1.40; I2 = 52.4%; n = 3 studies) but not moderate-intensity exercise (p-ES = 0.93, 95%CI: –0.16 to 2.02; I2 = 88.5%; n = 4 studies) conditions.
Conclusion
In comparison to non-exercise or light-intensity exercises, an immediate increase in BDNF levels may occur when young adults perform HIE. Given the benefits obtained maximizing circulating BDNF when performing HIE and its potential effects on brain health, our findings suggest that HIE could be recommended by clinicians as a useful exercise strategy to healthy adults.
Graphical Abstract
1. Introduction
Brain-derived neurotrophic factor (BDNF), a protein member of the neurotrophin family of growth factors, has been associated with the modulation of cognition, neuroplasticity, angiogenesis, and neural connectivity. The role of BDNF in the improvement of metabolic and cardiovascular functions and in delaying the onset of neurodegenerative diseases has received increased attention.1,2 Current evidence has pointed out the influence of BDNF levels on structural and functional brain changes,3 including hippocampal neurogenesis,1 long-term potentiation,4 increased hippocampal volume, and survival of new born hippocampal neurons.5 Thus, higher BDNF levels have been associated with better cognitive performance, attention, and spatial memory.6 Indeed, reduced BDNF concentrations have been found in patients with dementia,7 mild cognitive impairment, Alzheimer's disease,8,9 and major depression.10
Consistent evidence supports that interventions based on physical exercise are effective in reducing physical and mental illness incidence and all-cause mortality.11,12 It has been suggested that exercise induces BDNF release,13 such that BDNF could be an intermediate variable in the relationship between exercise and cognition.4,14 Accordingly, BDNF seems to act as a potential mediator of exercise effects on the brain because it could enhance neuroplasticity via different pathways (i.e., neurogenesis, synaptogenesis, long-term potentiation, etc.). Therefore, the BDNF may be located in the causal path between exercise and cognition. Support for this idea comes from studies showing that higher BDNF levels induced by exercise were associated with improvements in cognition14 and memory.15,16 In addition, among the characteristics of the exercise prescription (i.e., type, duration, frequency, etc.), intensity has been identified as having the most relevant effect on acute release of BDNF.17 Higher serum BDNF concentrations have been found following high-intensity interval training (HIIT) as a consequence of the greater synthesis of BDNF in the brain.18
Some studies have suggested that high-intensity exercise (HIE), which includes HIIT and sprint interval training, may enhance serum BDNF levels even more than moderate- or low-intensity continuous training.17,19 A previous meta-analysis reported that aerobic exercise programs with moderate-to-vigorous intensity in adolescents did not result in a significant increase in BDNF levels.1 Another systematic review reported a dose response in terms of the frequency and intensity of the programmed sessions in samples including people with a variety of health conditions (i.e., healthy individuals, chronic patients).20 However, neither of these explored the immediate effect of exercise at different intensities on BDNF levels. Although it is known that exercise intensity is correlated with heart rate (which has been linked to BDNF levels21) and that the abovementioned reviews suggest the intensity of exercise is positively related to increases in BDNF levels, there is a lack of evidence about whether or not there is a threshold of exercise intensity from which a significant increase in BDNF concentrations can be observed.4,5,13 This information could be relevant to guide individual exercise prescriptions aimed at improving cognitive performance.
Therefore, the aim of the present study was to estimate the immediate changes in BDNF levels after exercise interventions at different intensities based on clinical trials in healthy young adults. Additionally, we explored whether acute changes in BDNF levels varied based on time spent on HIE (i.e., ≥30 min or <30 min) or with respect to baseline cardiorespiratory fitness.
2. Methods
2.1. Search strategy and study selection
The present systematic review and meta-analysis was conducted based on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions22 and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).23 This review was previously registered in the PROSPERO database (registration number: CRD42020221047).
We conducted a systematic search in the MEDLINE (via PubMed), Scopus, Cochrane CENTRAL, and SPORTDiscus databases from inception until December 2020 for studies reporting serum or plasma BDNF levels after an acute intervention of HIE. The search strategy was conducted by combining medical subject headings, free terms, and matching synonyms, and it included the following words: “brain derived neurotrophic factor”, “BDNF”, “high-intensity interval training”, “high-intensity exercise”, “HIIT”, or “SIT”. Additionally, we screened the references in the selected papers. The complete search strategy for MEDLINE is available in Supplementary Table 1.
2.2. Eligibility criteria
Two independent reviewers (RFR and CÁB) examined the titles and abstracts of retrieved articles to identify potentially eligible studies. The studies in which the titles and abstracts were related to the purpose of the present review were selected for full text screening. The inclusion criteria were as follows: (1) type of studies: randomized controlled trials (RCTs), non-RCTs, or pre–post studies; (2) type of participants: healthy young adults aged 18–35 years; (3) type of intervention: physical exercise described as HIE (i.e., HIIT, sprint interval training, vigorous continuous training that reached 75% maximal heart rate or maximal oxygen consumption (VO2max) in aerobic or strength modalities); (4) comparison: control condition as resting (non-exercise) or another physical exercise intervention with moderate or low intensity assessed through maximal heart rate or VO2max (moderate-intensity continuous training or low-intensity continuous training); and (5) outcome: BDNF levels (in plasma or in serum). No language restrictions were applied. Moreover, the studies were excluded when: (1) participants were diagnosed with any pathology or when animal models were used, and (2) if the laboratory assessment of BDNF was not detailed. A third reviewer (BNP) was consulted to resolve disagreements between reviewers.
2.3. Data extraction and risk of bias assessment
The following information was independently extracted from the included studies by 2 reviewers (RFR and CÁB): the first author's name and year of publication, study design, characteristics of the study population (age and sex), baseline VO2max, total sample size and sample size by groups, intervention characteristics (HIE modality and training protocol), comparison groups, outcome measure (BDNF levels in plasma or in serum), and main results. A third reviewer (BNP) was consulted to resolve disagreements between reviewers.
The Cochrane risk-of-bias tool was used for randomized trials (RoB 2.0)24 to assess the risk of bias of the included RCTs. The following domains were assessed: randomization process (allocation sequence and concealment), deviations from intended interventions (occurrence of non-protocol interventions and failures in implementing the protocol interventions that could affect the outcomes, or non-adherence to the assigned intervention by participants), missing outcome data (evidence regarding the result was not biased by missing outcome data), measurement of the outcome (measurement error for continuous outcomes differential or non-differential in relation to intervention assignment), and selection of the reported results. Each domain was assessed as “low risk of bias”, “some concerns”, or “high risk of bias”.25 Accordingly, the overall risk of bias for each study was classified as (1) “low risk of bias” when a low risk of bias was determined for all domains, (2) “some concerns” when at least 1 domain was assessed as raising some concerns, but no single domain was assessed as having a high risk of bias, or (3) “high risk of bias” when a high risk of bias was reached for at least 1 domain or when there were some concerns for multiple domains.24
Non-RCTs and quasi-experimental studies were assessed using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I)26 by which 7 domains were evaluated: (1) confounding (when: (a) at least 1 prognostic variable that predicts the outcome could also be implicated in the intervention received at baseline, (b) individuals switch between the interventions being compared, and (c) post-baseline prognostic variables affect the intervention received at baseline); (2) selection of participants into the study (when each trial excludes: (a) some eligible participants, (b) initial follow-up time of some participants, or (c) some outcomes events related to both intervention and outcome); (3) classification of the interventions (when the interventions are defined and categorized with the knowledge of subsequent outcomes: non-differential misclassification or differential misclassification); (4) biases due to deviations from intended interventions; (5) missing data; (6) measurement of outcomes; and (7) selection of the reported results. Each domain was rated as having a low, moderate, serious, or critical risk of bias. Studies were categorized as (1) “low risk of bias” when a low risk of bias was determined for all domains; (2) “moderate risk of bias” when a low or moderate risk of bias was determined for all domains; (3) “serious risk of bias” when there was at least 1 domain determined to have a serious risk of bias, but no domain was assessed as having a critical risk of bias; or (4) “critical risk of bias” when there was at least 1 domain determined to have a critical risk of bias.26
Two reviewers (RFR and CÁB) independently assessed the risk of bias of the included studies. A third reviewer (BNP) was consulted to resolve disagreements.
2.4. Quality of evidence
The Grades of Recommendations, Assessment, Development, and Evaluation (GRADE) tool was used to evaluate and summarize the quality of the evidence.27 Based on the design of the studies, the BDNF outcome measure was rated as high-, moderate-, low-, or very low-quality evidence considering the following domains: risk of bias, inconsistency, indirect evidence, imprecision, and publication bias.
2.5. Dealing with missing data
We contacted authors to receive missing outcome data to compute the analysis. Inoue et al.,28 Rodriguez et al.,29 and Baird et al.30 sent additional data.
2.6. Data analysis
Once the primary data from each study were extracted (including pre–post mean BDNF values, standard deviations, and sample sizes of intervention and control groups), the pooled effect sizes (p-ESs) and their 95% confidence intervals (95%CIs) were calculated for each study31 using the DerSimonian and Laird random effects method.32 The p-ESs for the effect of HIE vs. the control condition—that is, low-intensity exercise (LIE), moderate-intensity exercise (MIE), or non-exercise—were estimated for the RCTs. Likewise, to show the change in BDNF units of measurement (pg/mL), we computed the pooled raw mean difference after transforming all outcome data into the same unit (considering that 1 ng/mL = 1000 pg/mL). We evaluated heterogeneity using the I2 statistic, with I2 values of 0%–40% considered to be “not important” heterogeneity, 30%–60% representing “moderate” heterogeneity, 50%–90% representing “substantial” heterogeneity, and 75%–100% representing “considerable” heterogeneity,22 taking into account the corresponding p-values and 95%CIs.33
A sensitivity analysis was conducted to determine the robustness of the summary estimates by removing each included study from the analysis one by one. Furthermore, subgroup analyses based on comparison group (non-exercise, LIE, and MIE), training length (≥30 min or <30 min), sex, BDNF (serum/plasma), and high-intensity interval vs. non-interval exercise on serum BDNF response, as well as meta-regression models with duration in minutes and VO2max, were conducted to determine their potential influence on the p-ES estimates. Finally, publication bias was evaluated through visual inspection of funnel plots and Egger's regression asymmetry test for the assessment of small study effects.34 All statistical analyses were performed using Stata/SE (Version 15.0; StataCorp., College Station, TX, USA).
3. Results
3.1. Study selection
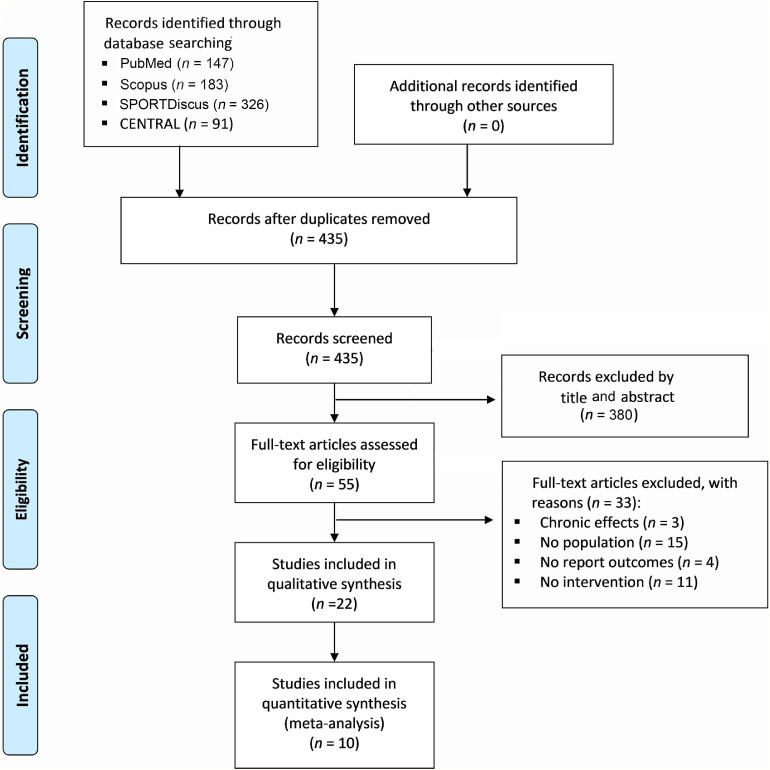
A total of 55 full-text articles were assessed for eligibility, of which 22 were included in the systematic review. Of those included, 10 RCTs were included for the meta-analysis because 2 RCTs35,36 did not contain the data needed for the meta-analysis (Fig. 1).
Fig. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram with the flow of studies through the review.
3.2. Characteristics of studies
Among the 22 studies included in the present systematic review, 10 studies were non-RCTs,6,29,37, 38, 39, 40, 41, 42, 43, 44 and 12 studies were RCTs,28,30,35,36,45, 46, 47, 48, 49, 50, 51, 52 of which 5 studies used a parallel design and 7 studies used a crossover design. The studies were conducted between 2012 and 2020. Further details are available in Supplementary Tables 2 and 3.
3.3. Participants
A total of 552 healthy young adults were included. Their mean age ranged between 20 and 31 years. Most studies (13 studies out of 22 studies; 59.1%) only included young male participants;28,29,36,37,39, 40, 41, 42,46, 47, 48, 49,51 6 studies (27.3%) were conducted in a mixed population;30,35,38,43,45,52 and only 3 studies (13.6%) were conducted in young females.6,44,50 The baseline VO2max values of the participants ranged from 33.0 mL/kg/min to 59.9 mL/kg/min (Supplementary Tables 2 and 3).
3.4. Interventions
Although the HIE protocols were different across the intervention groups, the intensity was constantly set at the individual's maximal or submaximal capacity. The training length was set between 7 min and 60 min, depending on the time spent in warm-up, main body of the training session, and calm down. Moreover, 3 studies performed HIE based on resistance training,6,41,42 and 19 studies were performed with an aerobic modality,28, 29, 30,35, 36, 37, 38, 39, 40,43, 44, 45, 46, 47, 48, 49, 50, 51, 52 mainly treadmill and cyclo-ergometer protocols (Supplementary Tables 2 and 3). It should be stated that because of the non-randomized design of resistance exercise trials, the meta-analyses were only based on aerobic modality.
The comparison groups were those with control conditions (non-exercise, sitting, or resting)36,38,39,42,45,49, 50, 51, 52 or MIE28,29,35,42,46,47,49 and LIE.30,43,46,47 BDNF was measured after the execution of exercise for the intervention groups or after the sitting and resting time.
3.5. Outcome
BDNF levels were measured in plasma in 5 studies,30,37,40,42,51 in serum in 16 studies,31, 32, 33, 34, 35,38,39,41,43, 44, 45, 46, 47, 48, 49, 50 and in both plasma and serum in 1 study.36 According to the unit of measure employed across the studies, 10 studies out of 22 used ng/mL,6,28,36,44, 45, 46,48,51,52 while 12 studies employed the pg/mL unit of measure30,35,37, 38, 39, 40, 41, 42, 43,47,49,50 (Supplementary Tables 2 and 3). The difference between serum and plasma levels (approximately 100–200 units) is because plasma measures represent free circulating BDNF, while blood serum measures represent total measurable blood-borne BDNF.53 Therefore, the contribution of plasma BDNF to total circulating levels is substantially lower than serum. It should be stated that measures in plasma cannot be generalized to serum. Moreover, the plasma measures of BDNF are unstable and have very low retest stability. Finally, in all studies, BDNF was analyzed according to the clinical standards of the laboratory or the manufacturer's guidelines with enzyme-linked immunosorbent assay; there was 1 exception, in which a study used a multiplexing assay.42
3.6. Risk of bias
According to the RoB 2.0 Cochrane tool,24 5 studies were rated as “low risk of bias”28,36,49, 50, 51 and 7 studies were rated as “some concerns”.30,35,45, 46, 47, 48,52 The risk of bias assessment is displayed in Supplementary Fig. 1. Based on the ROBINS-I tool, 3 studies were rated as “low risk of bias”,6,39,42 3 studies as “moderate risk of bias”,38,40,43 and 4 studies as “serious risk of bias”29,37,41,44 (Supplementary Fig. 2).
3.7. Quality of evidence
The quality of evidence was rated as low, since the certainty assessment showed serious concerns regarding risk of bias, inconsistency, and imprecision. A table with a summary of the findings is available in Supplementary Table 4.
3.8. Data synthesis
3.8.1. Meta-analysis
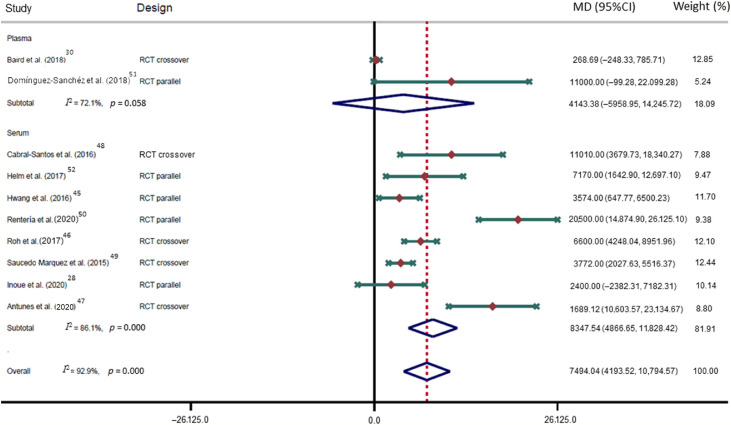
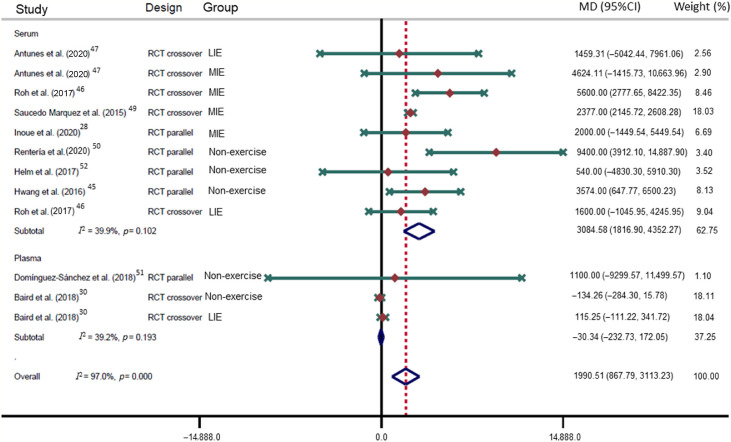
The p-ES for the BDNF effect of HIE vs. the comparison group (MIE, LIE, or non-exercise intervention) was 0.78 (95%CI: 0.35–1.21; I2 = 73.8%; Fig. 2); for HIE vs. non-exercise, it was 0.55 (95%CI: 0.12–0.98; I2 = 25.7%). The mean raw difference in BDNF levels following HIE in serum levels was 8347.54 pg/mL (95%CI: 4866.65–11,828.42; I2 = 86.1%), and in plasma, the difference was 4143.38 pg/mL (95%CI: –5958.95 to 14,245.72; I2 = 72.1%; Fig. 3). The mean raw difference in BDNF levels in the comparison group (MIE, LIE, or non-exercise intervention) in serum levels was 3084.58 pg/mL (95%CI: 1816.90–4352.27; I2 = 39.9%), and in plasma, the difference was –30.34 pg/ml (95%CI: –232.73 to 172.05; I2 = 39.2%; Fig. 4).
Fig. 2.
Forest plot of the pooled ES on BDNF for the effect of HIE vs. comparison group (MIE, LIE, or non-exercise intervention). Weights are from random effects analysis. 95%CI = 95% confidence interval; BDNF = brain-derived neurotrophic factor; ES = effect size; HIE = high-intensity exercise; LIE = low-intensity exercise; MIE = moderate-intensity exercise.
Fig. 3.
Forest plot of the mean raw differences in BNDF after high-intensity exercise. Weights are from random effects analysis. 95%CI = 95% confidence interval; BDNF = brain-derived neurotrophic factor; MD = mean difference; RCT = randomized controlled trial.
Fig. 4.
Forest plot of the mean raw differences in BNDF of the comparison groups (MIE, LIE, or non-exercise intervention). Weights are from random effects analysis. 95%CI = 95% confidence interval; BDNF = brain-derived neurotrophic factor; LIE = low-intensity exercise; MIE = moderate-intensity exercise; MD = mean difference; RCT = randomized controlled trial.
3.8.2. Sensitivity analysis
The p-ES was not modified when removing each included study one by one. Further details are displayed in Supplementary Table 5.
3.8.3. Subgroup analyses and meta-regression
We conducted subgroup analyses based on the comparison group (HIE vs. non-exercise, HIE vs. LIE, and HIE vs. MIE), showing that HIE vs. MIE was the only comparison that was not significant (p-ES = 0.93, 95%CI: –0.16 to 2.02; Fig. 2). Moreover, subgroup analyses based on training length (≥30 min or <30 min) and sex did not substantially modify the p-ES. Regarding the BDNF measures (plasma vs. serum), plasma levels did not show a significant increase after HIE (p-ES = 0.27, 95%CI: –0.15 to 0.70). These subgroup analyses are available in the Supplementary Table 6. Finally, we explored the serum BDNF response when comparing high-intensity interval to non-interval exercise (p-ES = 1.09, 95%CI: –0.16 to 2.34; Supplementary Fig. 3). The mean raw differences in serum BDNF were only significant for high-intensity interval exercise: 9813.91 pg/mL (95%CI: 4315.88–15,311.93; I2 = 91.0%; Supplementary Fig. 4).
Moreover, meta-regression models showed no significant influence of the duration in minutes: –0.01 (p = 0.63), or of baseline VO2max levels: 0.107 (p = 0.10). Further details are displayed in Supplementary Figs. 5 and 6.
3.8.4. Publication bias
We confirmed by funnel plot asymmetry and Egger's test that there was no significant publication bias regarding BDNF outcomes in the RCTs included (p = 0.726) (Supplementary Table 7).
4. Discussion
To our knowledge, this is the first systematic review and meta-analysis aimed at synthesizing the evidence examining acute HIE on BDNF levels in healthy young adults. Our study supports the notion that HIE moderately increases serum BDNF levels when compared with non-exercise and LIE, but there were no significant differences observed when HIE was compared with MIE. No significant associations between HIE and plasma BDNF were observed. Consistent results were observed for pooled mean differences in raw serum BDNF. Overall, these findings remained similar across different subgroups based on sex, training length, and baseline cardiorespiratory fitness.
The available evidence regarding exercise effects on BDNF has been inconsistent. While 2 previous reviews reported no changes in BDNF levels after LIE13 and MIE,20 another study reported an increase in circulating BDNF after exercise in healthy individuals.14 In accordance with the latter, our analyses showed that exercise intensity plays a key role in the association between exercise and BDNF increases in healthy young adults. These inconsistent results could be explained by the heterogeneity in the samples (e.g., individuals with risk factors, known cardiometabolic or neurological diseases, sedentary or trained individuals) or may be the result of animal models being used in the aforementioned reviews. Moreover, sex and exercise duration could also influence the effect of exercise on circulating BDNF levels.14 Otherwise, apart from sex and exercise duration, circulating serum levels are highly correlated with platelet count but also could be influenced by race, menstrual cycle, pathologies, or medication.53
The significant moderate effect of HIE on serum BDNF level shown in our study may be of interest for clinicians and professionals who prescribe physical exercise for young adults in the general population. Concretely, in addition to the cardiometabolic benefits of HIE, an acute improvement in cognitive functioning due to the increased serum BDNF levels following HIE could be considered an extra benefit, regardless of cardiorespiratory fitness and training length. Although the present results are based on findings in healthy young adults, the increase in BDNF levels, and presumably its related cognitive benefits,4 could be potentially extrapolated to people with aging-related cognitive disorders.2,54 Nevertheless, we should carefully interpret these results because of the high variability in BDNF responses.37,44
This variability may be related to the heterogeneity of organs, tissues, and mechanisms that have been proposed to account for the increased BDNF circulating after exercise (i.e., brain tissue, vascular endothelium, peripheral blood mononuclear cells, platelets, and skeletal muscle).55,56 Improvement in cerebral flow has been postulated as one of the major contributors, as neurons produce and release BDNF in a manner dependent on physical activity.57 Moreover, as a consequence of the activation of tropomyosin receptor kinase B by circulating BDNF during exercise, the vascular endothelial tissue increases the production of BDNF.58 In addition, the physiological stress caused by acute exercise increases peripheral blood mononuclear cells59 and, thus, their expression of BDNF. The exercise also stimulates platelets, which contribute to the bioavailable pool of BDNF by increasing platelet count and the amount of BDNF per platelet.53 Finally, although the skeletal muscle is not directly implicated by the increased BDNF during exercise, it may contribute to the maximized hippocampal BDNF expression, thereby causing long-term improvements in brain health.55
Among the mechanisms related to the intensity-dependent nature of the acute BDNF response,21 the following could be noted: first, the release of BDNF by platelets via thrombocytosis based on increased catecholamines and sympathetic nerve activity induced by exercise bouts;55 second, the association between the exercise intensity and the lactate pathway, which also plays a role in brain metabolism;4 third, HIE-related brain hypoxia and muscle damage may trigger an immediate response in circulating BDNF;18 and finally, the hyperthermia induced by exercise increases blood−brain barrier permeability, thereby facilitating the transport of the BDNF produced by neurons.55
Some limitations of our systematic review and meta-analysis should be acknowledged. First, the small sample size of the included studies as well as the scarcity of studies identified could have affected the precision of our estimations. In fact, only 6 studies calculated and reported the power analysis for the required sample size (Supplementary Table 8).28,29,40,45,48,51 Second, gender bias cannot be dismissed because only 3 studies included exclusively women,6,44,50 probably due to the difficulty of controlling for the variability of estrogen levels associated with the menstrual cycle. Estrogen levels are known to be associated with BDNF levels and, thus, are likely to influence their response to exercise.28 Third, the effects induced by exercise on BDNF might have been affected by blood collection methods and commercial enzyme-linked immunosorbent assay kits (Supplementary Table 8),60 as well as by age, health, smoking status, or alcohol consumption. Fourth, the presence of a single nucleotide polymorphism (in approximately 30% of the population) may alter BDNF secretion.5 Fifth, although our results were not influenced by the potential mediators or moderators analyzed, trained individuals with a higher capacity to use BDNF who may also have higher cardiorespiratory fitness could show lower levels of serum BDNF after exercise.17,61 Ultimately, high-quality studies with greater sample sizes are needed to clarify the role of potential moderators (e.g., training protocol, trained/untrained participants, sex, etc.) as well as to extrapolate these findings to a larger population.
5. Conclusion
Our results showed that HIE has a positive effect on increasing BDNF, which is not related to the exercise length or baseline cardiorespiratory fitness of healthy young adults. Therefore, given the potential benefits to mental and cardiometabolic health by increasing BDNF through the performance of HIE, our findings could support the recommendation of HIE by clinicians as promising time-efficient intervention that may maximize circulating BDNF.
Authors’ contributions
RFR conceived the study, participated in its design, carried out the literature search, data extraction, analysis, and interpretation, and was involved in the writing of the manuscript; CÁB and BNP participated in the data extraction and quality assessment; AEM and VMV conceived the study and participated in its design; IAMO carried out technical assessment. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2021.08.004.
Appendix. Supplementary materials
References
- 1.Azevedo KPM, de Oliveira VH, Medeiros GCBS, et al. The effects of exercise on BDNF levels in adolescents: A systematic review with meta-analysis. Int J Environ Res Public Health. 2020;17:1–14. doi: 10.3390/ijerph17176056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer's disease. Arch Med Res. 2012;43:615–621. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cefis M, Prigent-Tessier A, Quirié A, Pernet N, Marie C, Garnier P. The effect of exercise on memory and BDNF signaling is dependent on intensity. Brain Struct Funct. 2019;224:1975–1985. doi: 10.1007/s00429-019-01889-7. [DOI] [PubMed] [Google Scholar]
- 4.Müller P, Duderstadt Y, Lessmann V, Müller NG. Lactate and BDNF: Key mediators of exercise induced neuroplasticity? J Clin Med. 2020;9:1136. doi: 10.3390/jcm9041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinoff A, Herrmann N, Swardfager W, et al. The Effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): A meta-analysis. PLoS One. 2016;11:e0163037. doi: 10.1371/journal.pone.0163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gmiat A, Micielska K, Kozłowska M, et al. The impact of a single bout of high intensity circuit training on myokines’ concentrations and cognitive functions in women of different age. Physiol Behav. 2017;179:290–297. doi: 10.1016/j.physbeh.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein G, Beiser AS, Choi SH, et al. Serum brain-derived neurotrophic factor and the risk for dementia: The Framingham Heart Study. JAMA Neurol. 2014;71:55–61. doi: 10.1001/jamaneurol.2013.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laske C, Stransky E, Leyhe T, et al. BDNF serum and CSF concentrations in Alzheimer's disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Forlenza OV, Diniz BS, Teixeira AL, et al. Effect of brain-derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment. World J Biol Psychiatry. 2010;11:774–780. doi: 10.3109/15622971003797241. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, Ishikawa M, Niitsu T, et al. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One. 2012;7:e42676. doi: 10.1371/journal.pone.0042676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chekroud SR, Gueorguieva R, Zheutlin AB, et al. Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry. 2018;5:739–746. doi: 10.1016/S2215-0366(18)30227-X. [DOI] [PubMed] [Google Scholar]
- 12.Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Deng F, Wang J, et al. Intensity-dependent effects of consecutive treadmill exercise on spatial learning and memory through the p-CREB/BDNF/NMDAR signaling in hippocampus. Behav Brain Res. 2020;386 doi: 10.1016/j.bbr.2020.112599. [DOI] [PubMed] [Google Scholar]
- 14.Dinoff A, Herrmann N, Swardfager W, Lanctôt KL. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: A meta-analysis. Eur J Neurosci. 2017;46:1635–1646. doi: 10.1111/ejn.13603. [DOI] [PubMed] [Google Scholar]
- 15.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lourenco M V, Frozza RL, de Freitas GB, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat Med. 2019;25:165–175. doi: 10.1038/s41591-018-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez-Maldonado A, Rentería I, García-Suárez PC, Moncada-Jiménez J, Freire-Royes LF. The impact of high-intensity interval training on brain derived neurotrophic factor in brain: A mini-review. Front Neurosci. 2018;12:839. doi: 10.3389/fnins.2018.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Church DD, Hoffman JR, Mangine GT, et al. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J Appl Physiol. 2016;121:123–128. doi: 10.1152/japplphysiol.00233.2016. [DOI] [PubMed] [Google Scholar]
- 20.Feter N, Alt R, Dias MG, Rombaldi AJ. How do different physical exercise parameters modulate brain-derived neurotrophic factor in healthy and non-healthy adults? A systematic review, meta-analysis and meta-regression. Sci Sport. 2019;34:293–304. [Google Scholar]
- 21.Ross RE, Saladin ME, George MS, Gregory CM. High-intensity aerobic exercise acutely increases brain-derived neurotrophic factor. Med Sci Sports Exerc. 2019;51:1698–1709. doi: 10.1249/MSS.0000000000001969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (Updated March 2011). Available at: https://handbook-5-1.cochrane.org/. [accessed 18.01.2020 ].
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo M, Rita Banzi R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol. 2020;126:37–44. doi: 10.1016/j.jclinepi.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Inoue DS, Monteiro PA, Gerosa-Neto J, et al. Acute increases in brain-derived neurotrophic factor following high or moderate-intensity exercise is accompanied with better cognition performance in obese adults. Sci Rep. 2020;10:13493. doi: 10.1038/s41598-020-70326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez AL, Whitehurst M, Fico BG, et al. Acute high-intensity interval exercise induces greater levels of serum brain-derived neurotrophic factor in obese individuals. Exp Biol Med (Maywood) 2018;243:1153–1160. doi: 10.1177/1535370218812191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baird JF, Gaughan ME, Saffer HM, et al. The effect of energy-matched exercise intensity on brain-derived neurotrophic factor and motor learning. Neurobiol Learn Mem. 2018;156:33–44. doi: 10.1016/j.nlm.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thalheimer W, Cook S. How to calculate effect sizes from published research articles: A simplified methodology. Available at: http://coshima.davidrjfikis.com/EPRS8530/Effect_Sizes_pdf4.pdf. [accessed 28.01.2020 ].
- 32.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. John Wiley & Sons, Ltd.; Chichester: 2000. Methods for meta-analysis in medical research. [Google Scholar]
- 33.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 34.Sterne JAC, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etnier JL, Wideman L, Labban JD, et al. The effects of acute exercise on memory and brain-derived neurotrophic factor (BDNF) J Sport Exerc Psychol. 2016;38:331–340. doi: 10.1123/jsep.2015-0335. [DOI] [PubMed] [Google Scholar]
- 36.Slusher AL, Patterson VT, Schwartz CS, Acevedo EO. Impact of high intensity interval exercise on executive function and brain derived neurotrophic factor in healthy college aged males. Physiol Behav. 2018;191:116–122. doi: 10.1016/j.physbeh.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Di Battista AP, Moes KA, Shiu MY, et al. High-intensity interval training is associated with alterations in blood biomarkers related to brain injury. Front Physiol. 2018;9:1367. doi: 10.3389/fphys.2018.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mang CS, Snow NJ, Campbell KL, Ross CJD, Boyd LA. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J Appl Physiol. 2014;117:1325–1336. doi: 10.1152/japplphysiol.00498.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolini C, Michalski B, Toepp SL, et al. A single bout of high-intensity interval exercise increases corticospinal excitability, brain-derived neurotrophic factor, and uncarboxylated osteolcalcin in sedentary, healthy males. Neuroscience. 2020;437:242–255. doi: 10.1016/j.neuroscience.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 40.Martínez-Díaz IC, Escobar-Muñoz MC, Carrasco L. Acute effects of high-intensity interval training on brain-derived neurotrophic factor, cortisol and working memory in physical education college students. Int J Environ Res Public Health. 2020;17:8216. doi: 10.3390/ijerph17218216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira ES, Neto WK, Calefi AS, et al. Significant acute response of brain-derived neurotrophic factor following a session of extreme conditioning program is correlated with volume of specific exercise training in trained men. Front Physiol. 2018;9:823. doi: 10.3389/fphys.2018.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reycraft JT, Islam H, Townsend LK, Hayward GC, Hazell TJ, MacPherson REK. Exercise intensity and recovery on circulating brain-derived neurotrophic factor. Med Sci Sports Exerc. 2020;52:1210–1217. doi: 10.1249/MSS.0000000000002242. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt-Kassow M, Schädle S, Otterbein S, et al. Kinetics of serum brain-derived neurotrophic factor following low-intensity versus high-intensity exercise in men and women. Neuroreport. 2012;23:889–893. doi: 10.1097/WNR.0b013e32835946ca. [DOI] [PubMed] [Google Scholar]
- 44.García-Suárez PC, Rentería I, Moncada-Jiménez J, Fry AC, Jiménez-Maldonado A. Acute systemic response of BDNF, lactate and cortisol to strenuous exercise modalities in healthy untrained women. Dose Response. 2020;18 doi: 10.1177/1559325820970818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang J, Brothers RM, Castelli DM, et al. Acute high-intensity exercise-induced cognitive enhancement and brain-derived neurotrophic factor in young, healthy adults. Neurosci Lett. 2016;630:247–253. doi: 10.1016/j.neulet.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 46.Roh HT, Cho SY, Yoon HG, So WY. Effect of exercise intensity on neurotrophic factors and blood–brain barrier permeability induced by oxidative- nitrosative stress in male college students. Int J Sport Nutr Exerc Metab. 2017;27:239–246. doi: 10.1123/ijsnem.2016-0009. [DOI] [PubMed] [Google Scholar]
- 47.Antunes BM, Rossi FE, Teixeira AM, Lira FS. Short-time high-intensity exercise increases peripheral BDNF in a physical fitness-dependent way in healthy men. Eur J Sport Sci. 2020;20:43–50. doi: 10.1080/17461391.2019.1611929. [DOI] [PubMed] [Google Scholar]
- 48.Cabral-Santos C, Castrillón CIM, Miranda RAT, et al. Inflammatory cytokines and BDNF response to high-intensity intermittent exercise: Effect the exercise volume. Front Physiol. 2016;7:509. doi: 10.3389/fphys.2016.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saucedo Marque CM, Vanaudenaerde B, Troosters T, Wenderoth N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J Appl Physiol (1985) 2015;119:1363–1373. doi: 10.1152/japplphysiol.00126.2015. [DOI] [PubMed] [Google Scholar]
- 50.Rentería I, García-Suárez PC, Martínez-Corona DO, Moncada-Jiménez J, Plaisance EP, JiméNez-Maldonado A. Short-term high-Intensity interval training increases systemic brain-derived neurotrophic factor (BDNF) in healthy women. Eur J Sport Sci. 2020;20:516–524. doi: 10.1080/17461391.2019.1650120. [DOI] [PubMed] [Google Scholar]
- 51.Domínguez-Sanchéz MA, Bustos-Cruz RH, Velasco-Orjuela GP, et al. Acute effects of high intensity, resistance, or combined protocol on the increase of level of neurotrophic factors in physically inactive overweight adults: The BrainFit study. Front Physiol. 2018;9:741. doi: 10.3389/fphys.2018.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helm EE, Matt KS, Kirschner KF, Pohlig RT, Kohl D, Reisman DS. The influence of high intensity exercise and the Val66Met polymorphism on circulating BDNF and locomotor learning. Neurobiol Learn Mem. 2017;144:77–85. doi: 10.1016/j.nlm.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serra-Millàs M. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation? World J Psychiatry. 2016;6:84–101. doi: 10.5498/wjp.v6.i1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devenney KE, Guinan EM, Kelly ÁM, et al. Acute high-intensity aerobic exercise affects brain-derived neurotrophic factor in mild cognitive impairment: A randomised controlled study. BMJ Open Sport Exerc Med. 2019;5 doi: 10.1136/bmjsem-2018-000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh JJ, Tschakovsky ME. Exercise and circulating BDNF: Mechanisms of release and implications for the design of exercise interventions. Appl Physiol Nutr Metab. 2018;43:1095–1104. doi: 10.1139/apnm-2018-0192. [DOI] [PubMed] [Google Scholar]
- 56.Pareja-Galeano H, Alis R, Sanchis-Gomar F, et al. Methodological considerations to determine the effect of exercise on brain-derived neurotrophic factor levels. Clin Biochem. 2015;48:162–166. doi: 10.1016/j.clinbiochem.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Seifert T, Brassard P, Wissenberg M, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298:R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- 58.Meuchel LW, Thompson MA, Cassivi SD, Pabelick CM, Prakash YS. Neurotrophins induce nitric oxide generation in human pulmonary artery endothelial cells. Cardiovasc Res. 2011;91:668–676. doi: 10.1093/cvr/cvr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunelli A, Dimauro I, Sgrò P, et al. Acute exercise modulates BDNF and pro-BDNF protein content in immune cells. Med Sci Sports Exerc. 2012;44:1871–1880. doi: 10.1249/MSS.0b013e31825ab69b. [DOI] [PubMed] [Google Scholar]
- 60.Polacchini A, Metelli G, Francavilla R, et al. A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Sci Rep. 2015;5:17989. doi: 10.1038/srep17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hebisz P, Hebisz R, Murawska-Ciałowicz E, Zatoń M. Changes in exercise capacity and serum BDNF following long-term sprint interval training in well-trained cyclists. Appl Physiol Nutr Metab. 2019;44:499–506. doi: 10.1139/apnm-2018-0427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.