Abstract
Background
Health utility values (HUVs) are important inputs to the cost-utility analysis of breast cancer interventions.
Purpose
Provide a catalog of breast cancer–related published HUVs across different stages of breast cancer and treatment interventions.
Data Sources
Systematic searches of MEDLINE, MEDLINE In-Process, EMBASE, Web of Science, CINAHL, PsycINFO, EconLit, and Cochrane databases (2005–2017).
Study Selection
Studies published in English that reported mean or median HUVs using direct or indirect methods of utility elicitation for breast cancer.
Data Extraction
Independent reviewers extracted data on a preestablished and piloted form; disagreements were resolved through discussion.
Data Analysis
Mixed-effects meta-regression using restricted maximum likelihood modeling was conducted for intervention type, stage of breast cancer, and typical clinical and treatment trajectory of breast cancer patients to assess the effect of study characteristics (i.e., sample size, utility elicitation method, and respondent type) on HUVs.
Data Synthesis
Seventy-nine studies were included in the review. Most articles (n = 52, 66%) derived HUVs using the EQ-5D. Patients with advanced-stage breast cancer (range, 0.08 to 0.82) reported lower HUVs as compared with patients with early-stage breast cancer (range, 0.58 to 0.99). The meta-regression analysis found that undergoing chemotherapy and surgery and radiation, being diagnosed with an advanced stage of breast cancer, and recurrent cancer were associated with lower HUVs. The members of the general public reported lower HUVs as compared with patients.
Limitations
There was considerable heterogeneity in the study population, health states assessed, and utility elicitation methods.
Conclusion
This review provides a catalog of published HUVs related to breast cancer. The substantial heterogeneity in the health utility studies makes it challenging for researchers to choose which HUVs to use in cost-utility analyses for breast cancer interventions.
Keywords: breast cancer, economic evaluation, meta-regression, health status, health states, health-related quality of life, health state utility values, health utilities, PRISMA, review, utility score
Introduction
Patient-centered decisions about breast cancer treatments often involve tradeoffs between the possible benefits and harms. Such tradeoffs are personal judgments that may differ among individuals; some women may judge that the survival benefits of cancer treatment outweigh the potential toxicity, while others may place greater value on health-related quality of life (HRQOL) over survival. The measure of quality-adjusted life-years (QALYs) combines both survival and impact on HRQOL. The HRQOL impact (the “Q”) in a QALY is measured by health utilities. 1 Health utilities are cardinal values that represent the strength of an individual’s preferences for the health outcome or health state under consideration.2,3 Hence, a more desirable health outcome will have higher health utility value and vice versa. Health utilities are anchored at 0 for death and 1 for full health or the best possible outcome. Health states that are considered worse than death are indicated by negative values. 3 In breast cancer, health utilities have been measured using direct utility elicitation methods such as standard gamble (SG), time tradeoff (TTO), or rating scales or using indirect methods with self-reported, generic preference-based instruments such as the EQ-5D, 4 the Short Form–6D (SF-6D),5,6 and the Health Utilities Index Mark 3 (HUI3). 7
The health utility values for the same health outcome or health state can vary substantially depending on the method of health utility estimation, the population used to derive utility scores (patients, caregivers, health professionals, or the general public), and the context (setting, method or mode of administration, or description of health state). This heterogeneity in the health utility values makes it challenging for researchers to choose which values to use for the calculation of QALY in cost-utility analyses. A previous systematic review by Peasgood et al. 8 summarized published utilities in breast cancer and pooled utilities for some breast cancer–related health states for peer-reviewed studies published up to 2007. Peasgood et al. 8 concluded that because of substantial variation in the method of utility estimation and the source of utility values in breast cancer, the pooling of most utility values was problematic. To the best of our knowledge, no comprehensive systematic review of the breast cancer literature has been conducted since then. Hence, the objective of this systematic review of the literature was to identify and descriptively summarize the published health utility values related to breast cancer. The scope of this review covers the full spectrum of cancer care, ranging from screening to palliative care, whenever reported.
Methods
Search Strategy
A review of the literature published between January 1, 2005 and August 2017 was conducted. This timeline was chosen to ensure that the included health utilities are relevant to the current diagnostic and treatment guidelines. The electronic databases of MEDLINE, MEDLINE In-Process, EMBASE, Web of Science, CINAHL, PsycINFO, EconLit, and Cochrane databases (Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness [DARE], Cochrane Central Register of Clinical Trials, Health Technology Assessment [HTA], and NHS Economic Evaluation Database [NHS EED]) were searched. The electronic search strategy, designed with the help of a medical librarian, used health utility and utility elicitation method-specific terms, combined with breast cancer. The database search was complemented with a bibliographic hand search of citations included in the articles that met the study inclusion criteria. The search strategy is provided in the supplementary material.
Study Eligibility
The studies were screened in 2 phases. In phase 1, the titles and abstracts retrieved from the electronic databases search were reviewed by 1 author (M.N.K.). Studies in which the title or the abstract clearly indicated that the health utility values were elicited for adult patients with breast cancer were included for the phase 2 screening. We excluded literature reviews, meta-analyses, psychometric evaluations, editorials, comment letters, animal studies, conference abstracts, studies published in languages other than English, and studies in which health utility values were obtained from the literature.
In the phase 2 screening, full texts of the studies that met the inclusion criteria in phase 1 were reviewed by 2 independent reviewers (M.N.K. and P.D., J.P.D., or M.S.) using a predetermined screening form that was piloted using 5 studies. Studies were included if they 1) reported health utility values for adult breast cancer patients, including treatment-related and adverse events, and 2) described methods of utility assessment. Interreviewer disagreements were resolved through discussion, and a senior author (F.X.) was consulted if the disagreement persisted.
Data Extraction and Management
The data from the included studies were extracted onto a predesigned data extraction form, which was piloted with 5 articles. The data extraction was completed by 2 reviewers independently (M.K. and J.P.D., D.P., or M.S.). The following variables were recorded: 1) first listed author, publication year, country, journal, and funding source; 2) study design; 3) number and type of respondents from whom utilities were elicited (i.e., patients or nonpatients); 4) method of utility elicitation (direct or indirect); for direct studies, data on whether pilot testing was completed, whether interviewer was trained, and whether inconsistencies were assessed and recorded; for indirect studies, data on the country of scoring algorithm (where provided) were recorded; 5) administration method; and 6) reported mean or median utility, with variance (where provided). Disutilities were converted to utilities for the purposes of consistency in reporting and analyses.
A thematic approach was adopted for data management whereby the health utilities extracted from articles were classified into 2 main categories: 1) intervention-specific utilities and 2) breast cancer stage–specific utilities. Intervention-specific health utility values were further organized into 1) screening, 2) noninvasive and invasive diagnostic procedures, 3) local therapy (i.e., radiation or/and surgery), 4) systemic therapy (i.e., chemotherapy, endocrine therapy, targeted therapy), 5) allied health and complementary medicine, and 6) adverse events and their treatments. Breast cancer stage–specific health utility values were organized into 1) early breast cancer, 2) advanced or metastatic breast cancer, and 3) nonspecific breast cancer for when the stage of breast cancer was not specified or could not be ascertained from the article.
Descriptive analyses were completed to summarize the results. To assess the effect of study characteristics (i.e., sample size, valuation method, and type of respondent) on utility values, a meta-regression analysis was performed with utility value as the dependent variable and the study characteristics as independent variables. The heterogeneity of variance was estimated by fitting 2 separate mixed-effects models for type of intervention and stage of breast cancer using restricted maximum likelihood. The 2 models were in alignment with the thematic approach of the paper (i.e., describing health utilities by intervention and stage of breast cancer). Combining these 2 models into 1 model was deemed inappropriate, as it would have resulted in substantial heterogeneity, potentially rendering the model estimates unusable. For the purposes of meta-regression analysis, the type of intervention was categorized into screening, noninvasive diagnostic procedure, invasive diagnostic procedure, surgery, radiation, surgery and radiation, chemotherapy, and endocrine therapy. The stage of breast cancer was categorized into early, advanced or metastatic, and nonspecific breast cancer. The valuation methods were organized into EQ-5D, SG, TTO, visual analog scale (VAS), and others. Respondents were organized into patients and public. For studies that reported multiple utility values for the same defined health states by intervention or stage, the utility values were averaged and treated as one record for the analysis. The meta-regression analysis was completed using R version 4.0.3 and the lme4 package.
Results
Review Process
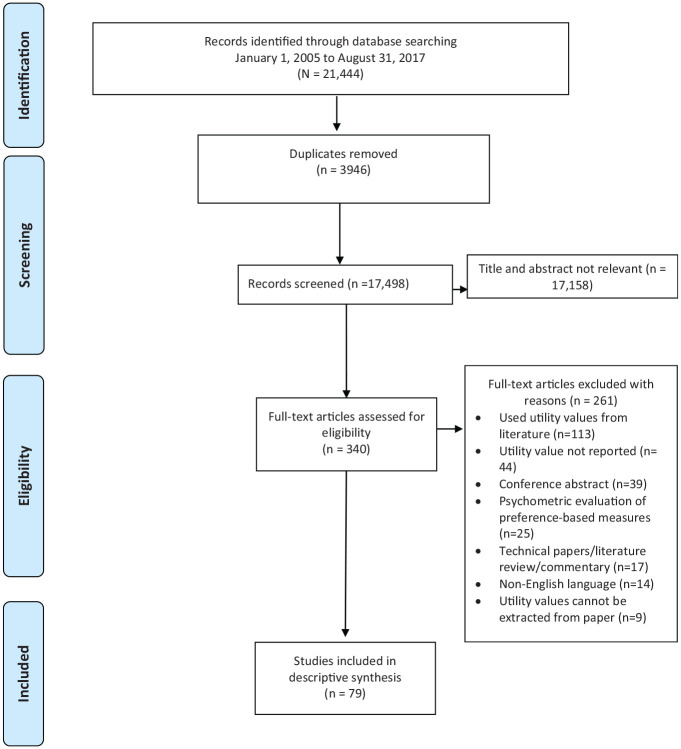
As shown in the PRISMA diagram (Figure 1), the electronic literature search yielded 21,444 records. Of these, 3,946 records were published in duplicate and were removed. After phase 1 screening, 17,158 records were excluded based on title and abstract screening. The remaining 340 full-text articles were retrieved and reviewed for inclusion. Of these, 79 were included in the review.
Figure 1.
PRISMA flow diagram for the systematic literature review of published health utility values in breast cancer.
Study Characteristics
A summary of the study characteristics are shown in Table 1 (for a detailed description of study characteristics, please see the supplementary material). Thirty-nine studies (49.4%) were published in oncology journals, followed by health economics and outcomes research journals (n = 22, 27.8%), and the remaining articles were published in other medical or public health journals (n = 18, 22.8%). A total of 37 articles (46.8%) received funding from not-for-profit or academic sources, 18 from for-profit sources (22.8%), and 3 from a combination of the two (3.8%). The remaining 21 articles (26.6%) did not receive funding or disclose a funding source. The number of articles published per year gradually increased since 2005, with the highest number of articles (n = 12, 15.2%) published in 2017. The corresponding author(s) for most of the publications were based in the United States (n = 23, 29.1%), the Netherlands (n = 8, 10.1%), Australia (n = 6, 7.6%), and Canada (n = 6, 7.6%).
Table 1.
Summary of Study Characteristics
| Study Characteristic | N |
|---|---|
| Country of corresponding author | |
| United States | 23 |
| The Netherlands | 9 |
| United Kingdom | 7 |
| Canada | 6 |
| Australia | 6 |
| Japan | 4 |
| Sweden | 4 |
| Germany, Greece, Iran, Korea, Singapore, Spain | 2 each |
| China, Finland, Lebanon, Malaysia, South Korea, Switzerland, Taiwan, Thailand | 1 each |
| Study design for preference elicitation study | |
| Cross-sectional | 46 |
| Randomized controlled trial | 16 |
| Cohort, prospective | 14 |
| Cohort, retrospective | 3 |
| Respondents | |
| Patients | 56 |
| Public, women | 11 |
| Public | 5 |
| Public, women and patients | 3 |
| Public and patients | 2 |
| Health care professionals | 2 |
| Health utility elicitation method | |
| EQ-5D | 51 |
| SG | 13 |
| TTO | 12 |
| VAS | 10 |
| SF-6D | 4 |
| SHE | 2 |
| TMI | 2 |
| AQOL-4D, 15D, QOL VAS, VR-6D, HALex | 1 each |
AQOL, Australian Quality of Life; SF-6D, HALex, Health and Activities Limitation Index, Short Form-6D; SG, standard gamble; SHE, Subjective Health Estimation; TMI, Testing Morbidities Index; TTO, time tradeoff; QOL, quality of life; VAS, visual analog scale; VR-6D, Veterans RAND-6D.
Indirect methods using multiattribute utility instruments were more common than direct methods of utility estimation. Direct methods of utility estimation were used in 18 studies (22.8%), where SG was the most common approach, followed by TTO and VAS. Indirect methods were used in 55 studies (69.6%) and a combination of direct and indirect methods in 6 studies (7.6%). Of 18 articles reporting on direct studies, 7 (38.9%) studies piloted the methods prior to administration, 9 (50%) reported on using trained interviewers, and 7 (38.9%) assessed inconsistencies in responses and adjusted their analyses accordingly. The health states to be assessed were identified and defined using literature review (n = 10, 55.6%), consultation with health care professionals experienced in treating women with breast cancer (n = 8, 44.4%), interviews with women diagnosed with breast cancer (n = 4, 22.2%), published guidelines or medical labeling information (n = 3, 16.7%), epidemiological data (n = 1, 5.6%), a previously developed questionnaire (n = 1, 5.6%), and breast cancer web forums (n = 1, 5.6%). Six (33.3%) studies did not specify how health states were developed.
Of 55 studies that used the indirect methods, the EQ-5D-3L was the most common preference-based measure (n = 48, 87.3%), followed by the SF-6D (n = 4, 7.3%). The remaining studies used the Finnish 15D, 9 HUI3, 10 or Assessment of Quality of Life 4–dimension (AQOL-4D). 11 Five studies mapped the data from EORTC-QLQ-C 30 to the EQ-5D-3L,12–16 and 1 study mapped the SF-12 to VR-6D 17 using published algorithms. Three studies used the 5L version of the EQ-5D.18–20 Four studies (5.1%) compared direct and indirect methods,21–24 6 studies (7.6%) compared 1 or more types of direct utility estimation methods,20,21,25–28 and 3 studies (3.8%) compared 1 or more types of indirect utility elicitation methods.9,13,29 Three studies (5.4%) compared country-specific algorithms.19,21,30
In terms of the respondents who completed the utility estimation exercise, most studies used women diagnosed with breast cancer (n = 57, 72.2%), followed by members of the general public (n = 14, 17.7%), a combination of the general public and women diagnosed with breast cancer (n = 6, 7.6%), and health care professionals (n = 2, 2.5%). The full list of breast cancer–relevant health states and the utilities are provided in the supplementary material.
Intervention-Specific Health Utility Values
Screening or diagnostic interventions
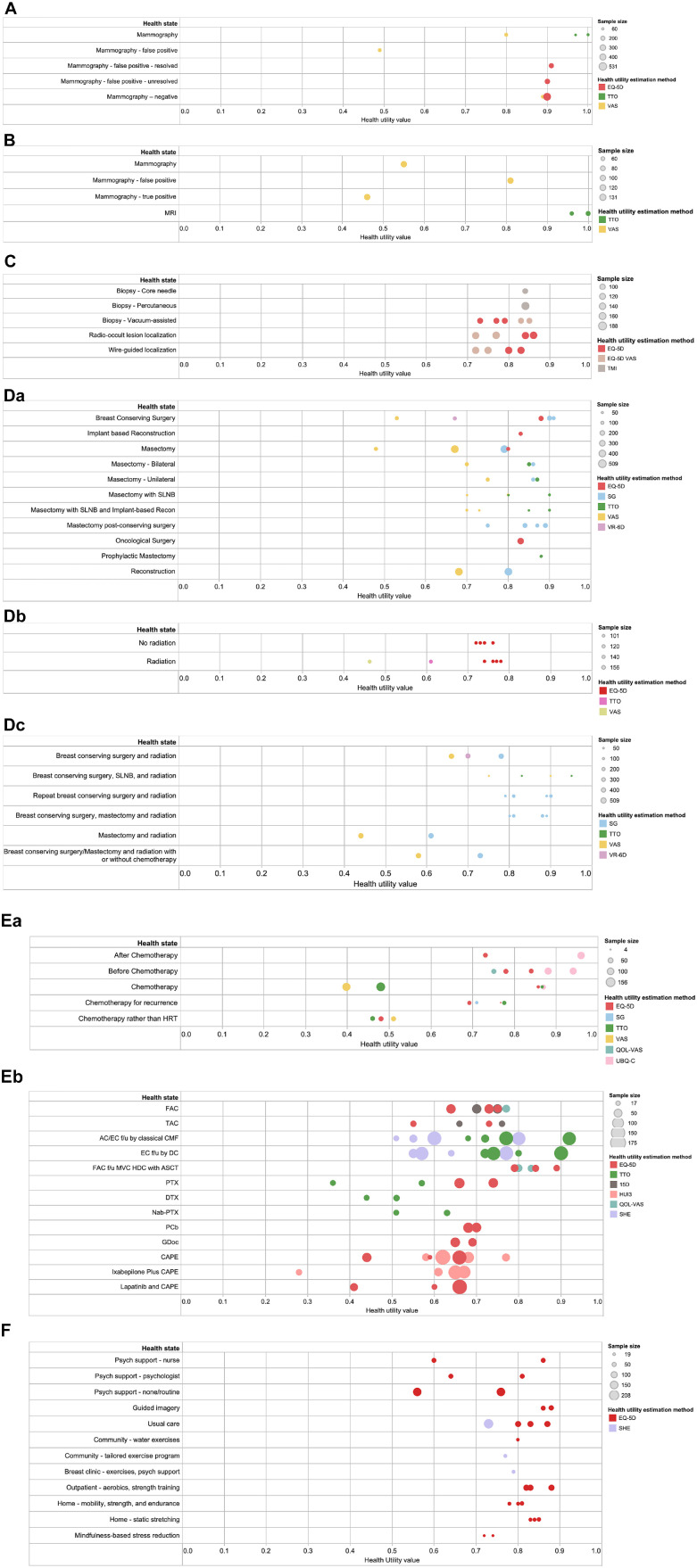
Eight studies55,62,64,78,79,83,84,89 measured utility values for health states related to breast cancer screening or diagnostic interventions and are shown in Figure 2a-c. The mean values ranged from 0.46 to 1.00. Three mammography-related health states of false-positive results on screening mammography and receiving diagnostic mammography and a true-positive result on diagnostic mammography had lower mean utilities (range, 0.46 to 0.55) compared with the other health states (range, 0.72 to 1.00). Most of the health states were measured using either the EQ-5D or VAS, and others used the Testing Morbidities Index and TTO. The values obtained from the VAS were placed on the lower end of the 0–1 scale, the EQ-5D values were in the middle, and the TTO values fell on the higher end.
Figure 2.
Health utility values in breast cancer, by treatment intervention. (a) Screening interventions. (b) Noninvasive diagnostic interventions. (c) Invasive diagnostic interventions. (d) Local interventions: a, surgery; b, radiation; c, radiation and surgery. (e) Systematic interventions: a, chemotherapy, drug not specified; b, chemotherapy, drugs specified. (f) Allied health or complementary medicine interventions.
Local therapy
Thirteen studies17,20,26,27,46,55,64,73,77,81,82,87,97 used direct (SG, TTO, VAS) and indirect (EQ-5D, VR-6D) methods to obtain health utilities for breast cancer surgery (Figure 2b). Breast cancer surgery–related mean utilities were found to have large variation; utilities for breast-conserving surgery (range, 0.53 to 0.91) and mastectomy (range, 0.48 to 0.87) were found to be lower compared with utilities for mastectomy followed by breast-conserving surgery (range, 0.75 to 0.89) and bilateral mastectomy (range, 0.70 to 0.86). Breast reconstruction–related utilities were found to be between 0.68 and 0.90. The mean utilities derived from VAS tended to be lower (range, 0.48 to 0.80) than those from other methods, which clustered between 0.67 and 0.91.
Four studies48,52,55,87 measured utilities for radiation as compared with no radiation. The sample size of the studies was similar, and the median utilities for no radiation were slightly higher as compared with radiation. The utilities derived using the VAS were found to be on the lower end, TTO fell in the middle, and the EQ-5D values were on the higher end.
Five studies17,20,46,63,97 measured utilities related to breast cancer surgery and radiation combined. The values for mastectomy and radiation were lowest (range, 0.44 to 0.61), whereas the utilities for breast-conserving surgery (with or without mastectomy) and radiation or repeat breast-conserving surgery and radiation fell between 0.66 and 0.95. The utilities derived from VAS were at the lower end (range, 0.44 to 0.90) as compared with SG, TTO, and VR-6D. Most of the utilities in this category were derived from SG (range, 0.61 to 0.90).
Systemic therapy
The utilities associated with chemotherapy were categorized into when no drug was specified (n = 9)21,23,24,48,51,55,80,87,90 or drugs were specified (n = 11)10,13,23,54,56,58-60,70,85,92. When no drug was specified, the utilities for chemotherapy health states (for primary and recurrence) were found to be lower and had much larger variability (range, 0.40 to 0.92) as compared with those before and after chemotherapy (range, 0.73 to 0.84). The utilities derived from the VAS were on the lower end. When the drug or drug combination for chemotherapy-related utilities was specified, substantial variation in the values was observed (Figure 2c). Utilities ranged from 0.28 to 0.92 and were measured using primarily self-reported instruments, the EQ-5D, Finnish 15D, HUI3, QOL-VAS, and Subjective Health Estimation.
Ten studies21,24,48,51,55,57,64,71,87,94 measured endocrine therapy–related utilities. The utilities for nonspecific hormone replacement therapy ranged from 0.52 to 0.93, whereas for tamoxifen, the utilities were on the higher end and ranged from 0.75 to 0.95. One study with a sample size of 152 patients 71 assessed the utility for goserelin therapy using SG and reported a mean utility value of 0.81. None of the identified studies reported utilities for targeted therapies for breast cancer.
Allied health and complementary medicine
Ten studies14,45,61,65,67,74,86,95,96,98 assessed utilities associated with allied health and complementary medicine–related health states (Figure 2d). Most of the studies used the EQ-5D, and the mean utility values ranged from 0.56 to 0.88.
Adverse events and their treatments
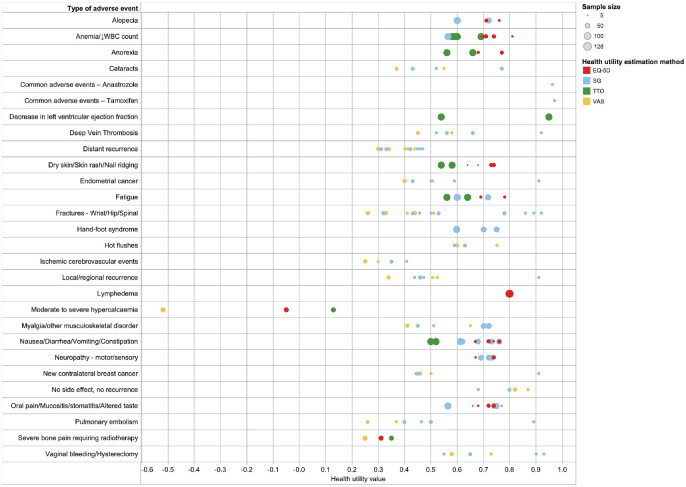
A total of 10 studies21,22,25,28,47,51,57,75,76,90 reported on a range of breast cancer treatment–related adverse events (Figure 3). Lower utilities (<0.5) were found to be associated with fractures, severe bone pain, local or distant recurrence that may or may not require treatment(s), lymphedema, pulmonary embolism, deep vein thrombosis, ischemic cerebrovascular events, endometrial or contralateral breast cancer, and cataracts. The utilities for adverse event–related health states were predominantly estimated using the VAS or SG. VAS values were found to be lower as compared with SG. For health states in which the EQ-5D and TTO were used, utilities estimated using TTO tended to be lower compared with utilities estimated using EQ-5D.
Figure 3.
Health utility values for adverse effects of breast cancer treatment interventions.
Breast Cancer Stage-Specific Utilities
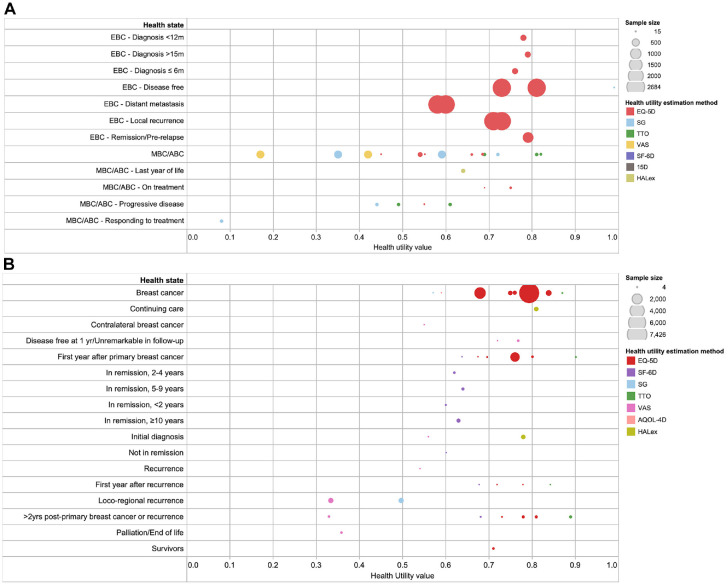
A total of 512,48,51,87,99 and 139,18,24,29,47,48,53,72,75,82,91,97,101 studies assessed the utilities for early breast cancer and advanced/metastatic breast cancer–related health states, respectively. As seen in Figure 4a, most of the studies for early breast cancer derived utilities using the EQ-5D. One study with large sample size 99 (>1000) consistently found the early breast cancer health states to be between 0.58 and 0.81. The health utilities for advanced breast cancer states were mainly measured using direct methods (SG, TTO, and VAS). The utilities for local recurrence were found to be lower than for early breast cancer without recurrence but higher than for advanced or metastatic disease.
Figure 4.
Health utility values in breast cancer, by stage of breast cancer. (a) Early and advanced-stage breast cancer. (b) Nonspecific breast cancer.
Figure 4b shows the utilities for when the stage of breast cancer was not specified (n = 20)11,15,16,19,24,29,30,49,50,53,55,57,64,66,68,69,88,93,97,100. Several studies with small sample sizes found that the utilities for participants in mid- to long-term remission were lower as compared with locoregional recurrence. The reported utilities from the initial diagnosis of breast cancer to 2 years following primary or recurrent breast cancer were lower as compared with longer term (2 years or more) follow-up.
Meta-regression
The results of the meta-regression analysis are shown Table 2. For the regression model concerning health utilities by intervention, we found that compared with screening, invasive diagnostic procedures, local therapies, and systemic breast cancer therapies had lower utilities, with the lowest value being in patients undergoing chemotherapy followed by surgery and radiation (R2 = 0.57). For breast cancer stage, health utilities for advanced or metastatic breast cancer states were lower compared with early breast cancer and when breast cancer stage was not specified (R2 = 0.37); however, the differences were not statistically significant. For both models, utilities elicited using the VAS were found to be lower compared with the EQ-5D, whereas SG- and TTO-derived utilities were found to be higher than EQ-5D. Lastly, utilities elicited from the general population were found to be lower than from patients.
Table 2.
Results from Meta-regression Analyses
| Variable | Model 1 a | Model 2 b | ||
|---|---|---|---|---|
| Coefficient | P Value | Coefficient | P Value | |
| Intervention | ||||
| Reference: screening | ||||
| Noninvasive diagnostic | 0.00 (0.00) | 0.78 | — | — |
| Invasive diagnostic | –0.06 (0.06) | 0.30 | — | — |
| Surgery | –0.15 (0.03) | <0.001* | — | — |
| Radiation | –0.17 (0.04) | <0.001* | — | — |
| Surgery and radiation | –0.24 (0.04) | <0.001* | — | — |
| Chemotherapy | –0.19 (0.04) | <0.001* | — | — |
| Endocrine therapy | –0.12 (0.03) | <0.001* | — | — |
| Breast cancer stage | ||||
| Reference: early | ||||
| Advanced/metastatic | — | — | –0.11 (0.08) | 0.18 |
| Non-specific | — | — | –0.02 (0.08) | 0.76 |
| Study sample size | 0.00 (0.00) | 0.53 | 0.00 (0.00) | 0.82 |
| Valuation method | ||||
| Reference: EQ-5D | ||||
| SG | 0.09 (0.03) | <0.01* | 0.14 (0.08) | 0.09 |
| TTO | 0.06 (0.03) | 0.08 | 0.17 (0.06) | <0.05* |
| VAS | –0.04 (0.02) | <0.05* | –0.03 (0.08) | 0.69 |
| Other | –0.01 (0.06) | 0.84 | –0.01 (0.05) | 0.86 |
| Respondents | ||||
| Reference: Patients | ||||
| Public | –0.04 (0.03) | 0.13 | –0.26 (0.07) | < 0.01* |
| Constant | 0.92 (0.04) | < 0.001* | 0.80 (0.08) | < 0.001* |
By the intervention in model 1: observations: N of health utilities in the model = 88; R2 = 0.57. Studies included in model 1: reference number (number of utilities contributed by the study): 17(2), 21(6), 23(4), 24(14), 26(3), 27(3), 46(4), 48(3), 51(2), 52(1), 55(6), 56(3), 57(1), 60(2), 62(2), 63(1), 64(6), 70(4), 78(4), 79(1), 80(1), 84(3), 87(3), 89(1), 92(2), 94(1), 97(4).
By breast cancer stage in model 2: observations: N of health utilities in the model = 52; R2 = 0.37. Studies included in model 2: reference number (number of utilities contributed by the study): 9(2), 16(1),18(1),19(1), 24(8), 29(8), 30(3), 48(2), 49(1), 51(1), 53(3), 55(3), 57(1), 64(2), 68(1), 72(1),75(2), 82(1), 88(1), 91(1), 97(4), 99(1), 100(1), 101(2).
Significant P value.
Discussion
There has been a continued interest in the measurement of health utilities for breast cancer since 2005. This systematic literature review identified the full range of published health utility values relevant to breast cancer from diagnostic or screening, local and systemic therapies, allied health or complementary medicine–related interventions, and treatment-related adverse events. Only 1 of the 79 identified studies in the review explicitly estimated the utility values for Indigenous women with breast cancer, 11 which is concerning because of the higher incidence and mortality rates of cancer in this population. 31
We found that the utility values for women undergoing screening and noninvasive diagnostic interventions were equivalent to being in full health. In addition, women with false-positive results on screening mammography and women with confirmed positive results on diagnostic mammography were similar in terms of their health utility values. This observation is supported by a recently published systematic review of 27 studies reporting on the disutilities associated with cancer-screening programs, 32 which concluded that cancer-screening programs resulted in low disutilities and that diagnostic and treatment programs are associated with low to moderate disutilities. This finding suggests that women experience low health utilities even with suspected breast cancer diagnosis. Subsequently, women should be promptly evaluated for psychological well-being and offered appropriate support and resources to cope with the diagnosis from the outset.
For local interventions, we found that the utility values for breast cancer surgery (i.e., breast-conserving surgery or mastectomy) were lower compared with women undergoing breast reconstruction after cancer surgery. This finding is corroborated by the evidence to date that suggests that undergoing breast reconstructive surgery significantly improves HRQOL and satisfaction with breasts.33–35 Health utility values were most assessed for chemotherapy-related health states. The high number of studies reporting on chemotherapy was conceivably due to the rapid turnover of research on new chemotherapeutic agents and possible drug combinations requiring health utility–relevant evidence prior to market entry. This may also have influenced the dominance of generic preference-based measures in this category.
Substantial variation was noted between the different chemotherapy regimens; however, 2 trends were noted. First, patients who were on chemotherapy reported lower values as they progressed in their treatment, such that the values for the last treatment session were lower as compared with the first session. Second, utilities for patients who were on chemotherapy were lower compared with patients who had completed chemotherapy, and utilities after chemotherapy continued to increase. This has important implications when designing cost-effectiveness analyses, as the time frame during which the costs and the benefits accrued may have been different. We did not identify studies estimating utility values for patients on targeted therapies and identified few studies reporting on the utility values for specific HRT drugs. This lack of utility values in the HRT or targeted therapy literature was unforeseen because of the higher uptake of these interventions in breast cancer in recent years.
In terms of breast cancer stage, predictable patterns were observed. We found that the utilities for early-stage breast cancer states were higher compared with advanced or metastatic stages. This finding is similar to Peasgood et al., 8 who reported higher values for early-stage breast cancer without recurrence and a sharp decline in progressive advanced or metastatic stage breast cancer health states. An interesting finding was that patients who were in remission (<2 y to ≥10 y) had lower utility values, which indicates the long-term impacts of breast cancer and its associate treatments on HRQOL. It also demonstrates the need to look beyond the treatment phase and assess the costs and HRQOL in patients in the survivor phase.
With respect to the methods used to assess utilities in the breast cancer population, we found that compared with EQ-5D–derived values, utilities elicited using the VAS were lower and SG and TTO were higher. Choice-based methods such as SG and TTO take into account the risk attitude or the tradeoffs in eliciting health state preferences, but only SG incorporates risk preferences. The VAS method is relatively easier but may be prone to response spreading bias 36 and end-state aversion bias. 37 Alternatively, indirect methods are inexpensive, easy to implement, and less cognitively burdensome compared with direct application of elicitation tasks and therefore are recommended by some health technology assessment (HTA) agencies. We also found that patients reported higher utility values compared with members of the general public, a finding that is corroborated in the literature. This discrepancy in utility values by population may be due to the hypothetical scenario presented to the general public, adaption to the health state by patients, or potential response shift. 38 Notably, in the meta-regression analyses, the differential between the patient and public in utility values was found to be much smaller by intervention as compared with stage of breast cancer. This difference may be due to the direct and indirect experiences of the general population for the intervention and breast cancer stage–related health states. The general population may be more familiar with the experience of undergoing oncology treatments. For example, nausea and hair loss are well known side effects of chemotherapy and are often used to typify the image of someone with cancer in the media and other cancer-related reports. In contrast, the general population may not be as aware of the diagnostic, treatment experience, and quality-of-life implications of different stages of cancer. This may cause the general population to report lower utilities compared with patients. Altogether, the context, method, and population used to elicit utility values are important considerations when selecting health utilities for cost-effectiveness analyses.
Study Limitations
Our study included publications between 2005 and August 2017 in the English language. While our search was comprehensive and designed with the help of a medical librarian, we identified many irrelevant articles, indicating that the search was highly sensitive but lacked precision. More recently, Arber et al.39,40 published search filters for Ovid MEDLINE for studies reporting health utility values that maximize sensitivity, balance sensitivity and precision, and maximize precision. The latter 2 strategies may be considered in the future to reduce the number of irrelevant articles. The authors of the publications were not contacted for further clarification or missing data, which might have resulted in the exclusion of relevant studies. Further, we found an increasing trend in the number of studies reporting health utility values from 2012 onward. Our review is limited to studies published until 2017 and does not include recent literature. Reviews of this nature are time-consuming, especially in breast oncology because of the number of publications annually on this topic. Hence, it will be important to update the review findings on an ongoing basis. An important limitation of this review is that we were unable to conduct quality assessment of the included studies. This was due to the lack of such an appraisal tool. To that effect, the senior author is leading a Health Utility Book (HUB) project. Once concluded, the HUB will consist of a registry of published health utility values and a quality assessment tool for health utility studies.41,42 We believe that the HUB project will fill an important gap in the literature and improve the credibility of health utility studies in the HTA submission process. Finally, for the meta-regression analysis by breast cancer stage, the results should be interpreted with caution, as the model included only a handful of studies for early-stage breast cancer (n = 3) as compared with 18 and 33 studies in advanced and nonspecific breast cancer, respectively. In addition, the purpose of the meta-regression analysis was to evaluate the influence of study characteristics on the utility values and not to generate a utility prediction model. Subsequently, the regression parameters should not be used to generate predicted utility weights.
Implications: Selecting Health Utility Values for Use in Cost-Effectiveness Models
Given the methodological variation among health utility studies, it is a challenging task to choose from multiple published health utilities for cost-effectiveness models. Recent ISPOR Good Research Practices Task Force Reports43,44 recommend evaluating the “fit for purpose” of the health utilities for a cost-effectiveness model by taking into account 1) the population used to elicit utility values (general public versus patient and or caregivers), 2) the population’s sociodemographic (e.g., age, educational level) characteristics, 3) clinical (e.g., stage of breast cancer, HER2 status, menopausal status, comorbidities) and treatment (e.g., active treatment versus survivorship, treatment-related adverse events, or acute clinical events) characteristics, 4) description of the health state (individual health state versus aggregate as a function of the clinical status), and 5) utility study design and setting (e.g. country, sample size, mode of administration). We believe the catalog of the breast cancer–related health utilities provided in our study could be useful for future cost-effectiveness models, whereby the authors will be able to weigh the aforementioned characteristics and ascertain the value(s) to use for their analysis.
Conclusion
This study provides a catalog of the published health utility values related to breast cancer. We found that even though a variety of utility estimation methods have been used in the breast cancer literature, the EQ-5D is most commonly used. A higher proportion of studies identified in the review measured health utilities for interventions, more specifically for chemotherapy and treatment-related adverse events. Further, substantial variation was noted in the utility values among studies reporting on the same health state. This variation was larger for systemic interventions and treatment-related adverse events than for screening-, diagnostic-, local-, or allied health and complementary medicine–related interventions. The utilities for early breast cancer health states were higher compared with the advanced or metastatic breast cancer health states.
Another important contribution of this systematic literature review is understanding the source of variation in utility values by method, population, and type of health state (i.e., intervention or breast cancer stage). The catalog may be useful for future economic evaluations; however, a word of caution is in order due to the heterogeneity of published utilities.
Supplemental Material
Supplemental material, sj-docx-1-mdm-10.1177_0272989X211065471 for A Systematic Literature Review of Health Utility Values in Breast Cancer by Manraj N. Kaur, Jiajun Yan, Anne F. Klassen, Justin P. David, Dilshan Pieris, Manraj Sharma, Louise Bordeleau and Feng Xie in Medical Decision Making
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: No financial support was received for this study. Manraj N. Kaur was supported by the Canadian Institute of Health Research Canada’s Best Graduate Scholarship Doctoral Award (2015-19). Dr. Kaur’s funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Authors’ Note: Preliminary results of this review were presented as a poster (Lee B. Lusted Finalist Student Competition–Poster) at the 39th Annual North American Meeting of the Society of Medical Decision Making held in Pittsburgh, Pennsylvania, United States (October 22–25, 2017).
Research Data: All data from the review are presented in the article or in the electronic supplementary material.
ORCID iDs: Manraj N. Kaur  https://orcid.org/0000-0002-1911-0395
https://orcid.org/0000-0002-1911-0395
Dilshan Pieris  https://orcid.org/0000-0003-3906-3263
https://orcid.org/0000-0003-3906-3263
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making website at http://journals.sagepub.com/home/mdm.
Contributor Information
Manraj N. Kaur, School of Rehabilitation Sciences, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada.
Jiajun Yan, Department of Health Research Methods, Evidence and Impact, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada.
Anne F. Klassen, Department of Pediatrics, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada
Justin P. David, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
Dilshan Pieris, Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Manraj Sharma, Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Louise Bordeleau, Department of Oncology, Division of Medical Oncology, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada.
Feng Xie, Department of Health Research Methods, Evidence and Impact, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada.
References
- 1. Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(1):5–21. [DOI] [PubMed] [Google Scholar]
- 2. Tolley K. What Are Health Utilities? London: Hayward Medical Communications; 2009. [Google Scholar]
- 3. Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care. 1989;5(4):559–75. [DOI] [PubMed] [Google Scholar]
- 4. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108. [DOI] [PubMed] [Google Scholar]
- 5. Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 Health Survey. J Clin Epidemiol. 1998;51(11):1115–28. [DOI] [PubMed] [Google Scholar]
- 6. Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–92. [DOI] [PubMed] [Google Scholar]
- 7. Feeny D, Furlong W, Torrance GW, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40(2):113–28. [DOI] [PubMed] [Google Scholar]
- 8. Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2010;10(5):553–66. [DOI] [PubMed] [Google Scholar]
- 9. Farkkila N, Torvinen S, Roine RP, et al. Health-related quality of life among breast, prostate, and colorectal cancer patients with end-stage disease. Qual Life Res. 2014;23(4):1387–94. [DOI] [PubMed] [Google Scholar]
- 10. Reed SD, Li Y, Anstrom KJ, Schulman KA. Cost effectiveness of ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2009;27(13):2185–91. [DOI] [PubMed] [Google Scholar]
- 11. Garvey G, Cunningham J, He VY, et al. Health-related quality of life among Indigenous Australians diagnosed with cancer. Qual Life Res. 2016;25(8):1999–2008. [DOI] [PubMed] [Google Scholar]
- 12. Wolowacz SE, Cameron DA, Tate HC, Bagust A. Docetaxel in combination with doxorubicin and cyclophosphamide as adjuvant treatment for early node-positive breast cancer: a cost-effectiveness and cost-utility analysis. J Clin Oncol. 2008;26(6):925–33. [DOI] [PubMed] [Google Scholar]
- 13. Bastani P, Kiadaliri AA. Cost-utility analysis of adjuvant therapies for breast cancer in Iran. Int J Technol Assess Health Care. 2012;28(2):110–4. [DOI] [PubMed] [Google Scholar]
- 14. Arving C, Brandberg Y, Feldman I, Johansson B, Glimelius B. Cost–utility analysis of individual psychosocial support interventions for breast cancer patients in a randomized controlled study. Psychooncology. 2014;23(3):251–8. [DOI] [PubMed] [Google Scholar]
- 15. Brown DS, Trogdon JG, Ekwueme DU, et al. Health state utility impact of breast cancer in US women aged 18–44 years. Am J Prev Med. 2016;50(2):255–61. [DOI] [PubMed] [Google Scholar]
- 16. Trogdon JG, Ekwueme DU, Chamiec-Case L, Guy GP. Breast cancer in young women. Am J Prev Med. 2016;50(2):262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ali AA, Xiao H, Tawk R, et al. Comparison of health utility weights among elderly patients receiving breast-conserving surgery plus hormonal therapy with or without radiotherapy. Curr Med Res Opin. 2017;33(2):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallwiener M, Heindl F, Brucker SY, et al. Implementation and feasibility of electronic Patient-Reported Outcome (ePRO) data entry in the PRAEGNANT real-time advanced and metastatic breast cancer registry. Geburtshilfe Frauenheilkd. 2017;77(8):870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu L, Li S, Wang M, Chen G. Comparison of eQ-5D-5l health state utilities using four country-specific tariffs on a breast cancer patient sample in mainland china. Patient Prefer Adher. 2017;11:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knuttel FM, van den Bosch MA, Young-Afat DA, et al. Patient preferences for minimally invasive and open locoregional treatment for early-stage breast cancer. Value Health. 2017;20(3):474–80. [DOI] [PubMed] [Google Scholar]
- 21. Milne RJ, Heaton-Brown KH, Hansen P, Thomas D, Harvey V, Cubitt A. Quality-of-life valuations of advanced breast cancer by New Zealand women. Pharmacoeconomics. 2006;24(3):281–92. [DOI] [PubMed] [Google Scholar]
- 22. Cheville AL, Almoza M, Courmier JN, Basford JR. A prospective cohort study defining utilities using time trade-offs and the Euroqol-5D to assess the impact of cancer-related lymphedema. Cancer. 2010;116(15):3722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conner-Spady B, Cumming C, Nabholtz J, Jacobs P, Stewart D. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant. 2005;36(3):251. [DOI] [PubMed] [Google Scholar]
- 24. Lidgren M, Wilking N, Jönsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16(6):1073–81. [DOI] [PubMed] [Google Scholar]
- 25. Tan X-Y, Aung M-M, Ngai M-I, Xie F, Ko Y. Assessment of preference for hormonal treatment–related health states among patients with breast cancer. Value Health Reg Issues. 2014;3:27–32. [DOI] [PubMed] [Google Scholar]
- 26. Sinno H, Izadpanah A, Thibaudeau S, Christodoulou G, Lin SJ, Dionisopoulos T. An objective assessment of the perceived quality of life of living with bilateral mastectomy defect. Breast. 2013;22(2):168–72. [DOI] [PubMed] [Google Scholar]
- 27. Sinno H, Izadpanah A, Vorstenbosch J, et al. Living with a unilateral mastectomy defect: a utility assessment and outcomes study. J Reconstr Microsurg. 2014;30(5):313–8. [DOI] [PubMed] [Google Scholar]
- 28. Shih V, Chan A, Xie F, Ko Y. Health state utility assessment for breast cancer. Value Health Reg Issues. 2012;1(1):93–7. [DOI] [PubMed] [Google Scholar]
- 29. Yousefi M, Najafi S, Ghaffari S, Mahboub-Ahari A, Ghaderi H. Comparison of SF-6D and EQ-5D scores in patients with breast cancer. Iran Red Crescent Med J. 2016;18(5):e23556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naik H, Howell D, Su S, et al. EQ-5D health utility scores: data from a comprehensive Canadian cancer centre. Patient. 2017;10(1):105–15. [DOI] [PubMed] [Google Scholar]
- 31. Moore SP, Antoni S, Colquhoun A, et al. Cancer incidence in indigenous people in Australia, New Zealand, Canada, and the USA: a comparative population-based study. Lancet Oncol. 2015;16(15):1483–92. [DOI] [PubMed] [Google Scholar]
- 32. Li L, Severens JL, Mandrik O. Disutility associated with cancer screening programs: a systematic review. PloS One. 2019;14(7):e0220148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCarthy CM, Hamill JB, Kim HM, Qi J, Wilkins E, Pusic AL. Impact of bilateral prophylactic mastectomy and immediate reconstruction on health-related quality of life in women at high risk for breast carcinoma: results of the Mastectomy Reconstruction Outcomes Consortium Study. Ann Surg Oncol. 2017;24(9):2502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg. 2013;132(2):201e–9e. [DOI] [PubMed] [Google Scholar]
- 35. Sait M, Srinivasaiah N. Quality of life issues in breast cancer surgery—a review. Indian J Surg. 2019;81(1):57–64. [Google Scholar]
- 36. Brazier J, Green C, McCabe C, Stevens K. Use of visual analog scales in economic evaluation. Exp Rev Pharmacoecon Outcomes Res. 2003;3(3):293–302. [DOI] [PubMed] [Google Scholar]
- 37. Torrance GW, Feeny D, Furlong W. Visual analog scales: do they have a role in the measurement of preferences for health states? Med Decis Making. 2001;21(4):329–34. [DOI] [PubMed] [Google Scholar]
- 38. Gandhi M, San Tan R, Ng R, et al. Comparison of health state values derived from patients and individuals from the general population. Qual Life Res. 2017;26(12):3353–63. [DOI] [PubMed] [Google Scholar]
- 39. Arber M, Garcia S, Veale T, et al., eds. Sensitivity of a search filter designed to identify studies reporting health state utility values. Presented at: HTAi 12th Annual Conference; Oslo, Norway; June 2015. [Google Scholar]
- 40. Arber M, Garcia S, Veale T, Edwards M, Shaw A, Glanville JM. Performance of Ovid MEDLINE search filters to identify health state utility studies. Int J Technol Assess Health Care. 2017;33(4):472–80. [DOI] [PubMed] [Google Scholar]
- 41. Xie F, Zoratti M, Chan K, et al. Toward a centralized, systematic approach to the identification, appraisal, and use of health state utility values for reimbursement decision making: introducing the Health Utility Book (HUB). Med Decis Making. 2019;39(4):371–9. [DOI] [PubMed] [Google Scholar]
- 42. Zoratti MJ, Zhou T, Chan K, et al. Health Utility Book (HUB)–cancer: protocol for a systematic literature review of health state utility values in cancer. MDM Policy Pract. 2019;4(2):2381468319852594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolowacz SE, Briggs A, Belozeroff V, et al. Estimating health-state utility for economic models in clinical studies: an ISPOR good research practices task force report. Value Health. 2016;19(6):704–19. [DOI] [PubMed] [Google Scholar]
- 44. Brazier J, Ara R, Azzabi I, et al. Identification, review, and use of health state utilities in cost-effectiveness models: an ISPOR good practices for outcomes research task force report. Value Health. 2019;22(3):267–75. [DOI] [PubMed] [Google Scholar]
- 45. Gordon LG, Scuffham P, Battistutta D, Graves N, Tweeddale M, Newman B. A cost-effectiveness analysis of two rehabilitation support services for women with breast cancer. Breast Cancer Res Treat. 2005;94(2):123–33. [DOI] [PubMed] [Google Scholar]
- 46. Hayman JA, Kabeto MU, Schipper MJ, Bennett JE, Vicini FA, Pierce LJ. Assessing the benefit of radiation therapy after breast-conserving surgery for ductal carcinoma-in-situ. J Clin Oncol. 2005;23(22):5171–7. [DOI] [PubMed] [Google Scholar]
- 47. Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schleinitz MD, DePalo D, Blume J, Stein M. Can differences in breast cancer utilities explain disparities in breast cancer care? J Gen Intern Med. 2006;21(12):1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shih Y-CT, Wang XS, Cantor SB, Cleeland CS. The association between symptom burdens and utility in Chinese cancer patients. Qual Life Res. 2006;15(8):1427–38. [DOI] [PubMed] [Google Scholar]
- 50. Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mansel R, Locker G, Fallowfield L, Benedict A, Jones D. Cost-effectiveness analysis of anastrozole vs tamoxifen in adjuvant therapy for early stage breast cancer in the United Kingdom: the 5-year completed treatment analysis of the ATAC (‘Arimidex’, Tamoxifen alone or in combination) trial. Br J Cancer. 2007;97(2):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prescott R, Kunkler I, Williams L, et al. A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial. Health Technol Assess. 2007;11(31):1–170. [DOI] [PubMed] [Google Scholar]
- 53. Yabroff KR, McNeel TS, Waldron WR, et al. Health limitations and quality of life associated with cancer and other chronic diseases by phase of care. Med Care. 2007:629–37. [DOI] [PubMed] [Google Scholar]
- 54. Bernhard J, Zahrieh D, Zhang J, et al. Quality of life and quality-adjusted survival (Q-TWiST) in patients receiving dose-intensive or standard dose chemotherapy for high-risk primary breast cancer. Br J Cancer. 2008;98(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bonomi AE, Boudreau DM, Fishman PA, et al. Quality of life valuations of mammography screening. Qual Life Res. 2008;17(5):801–14. [DOI] [PubMed] [Google Scholar]
- 56. Fountzilas G, Dafni U, Dimopoulos M, et al. A randomized phase III study comparing three anthracycline-free taxane-based regimens, as first line chemotherapy, in metastatic breast cancer. Breast Cancer Res Treat. 2009;115(1):87. [DOI] [PubMed] [Google Scholar]
- 57. Melnikow J, Birch S, Slee C, McCarthy TJ, Helms LJ, Kuppermann M. Tamoxifen for breast cancer risk reduction: impact of alternative approaches to quality-of-life adjustment on cost-effectiveness analysis. Med Care. 2008;46(9):946–53. [DOI] [PubMed] [Google Scholar]
- 58. Sherrill B, Amonkar M, Stein S, Walker M, Geyer C, Cameron D. Q-TWiST analysis of lapatinib combined with capecitabine for the treatment of metastatic breast cancer. Br J Cancer. 2008;99(5):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dranitsaris G, Cottrell W, Spirovski B, Hopkins S. Economic analysis of albumin-bound paclitaxel for the treatment of metastatic breast cancer. J Oncol Pharm Pract. 2009;15(2):67–78. [DOI] [PubMed] [Google Scholar]
- 60. Zhou X, Cella D, Cameron D, et al. Lapatinib plus capecitabine versus capecitabine alone for HER2+ (ErbB2+) metastatic breast cancer: quality-of-life assessment. Breast Cancer Res Treat. 2009;117(3):577–89. [DOI] [PubMed] [Google Scholar]
- 61. de Kok M, Dirksen CD, Kessels AG, et al. Cost-effectiveness of a short stay admission programme for breast cancer surgery. Acta Oncol. 2010;49(3):338–46. [DOI] [PubMed] [Google Scholar]
- 62. Domeyer PJ, Sergentanis TN, Zagouri F, Zografos GC. Health-related quality of life in vacuum-assisted breast biopsy: short-term effects, long-term effects and predictors. Health Qual Life Outcomes. 2010;8(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Freedman GM, Li T, Anderson PR, Nicolaou N, Konski A. Health states of women after conservative surgery and radiation for breast cancer. Breast Cancer Res Treat. 2010;121(2):519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grann VR, Patel P, Bharthuar A, et al. Breast cancer-related preferences among women with and without BRCA mutations. Breast Cancer Res Treat. 2010;119(1):177. [DOI] [PubMed] [Google Scholar]
- 65. Haines TP, Sinnamon P, Wetzig NG, et al. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res Treat. 2010;124(1):163–75. [DOI] [PubMed] [Google Scholar]
- 66. Lux MP, Reichelt C, Wallwiener D, et al. Results of the Zometa® cost-utility model for the german healthcare system based on the results of the ABCSG-12 study. Oncol Res Treat. 2010;33(7):360–8. [DOI] [PubMed] [Google Scholar]
- 67. Kimman M, Dirksen C, Voogd A, et al. Economic evaluation of four follow-up strategies after curative treatment for breast cancer: results of an RCT. Eur J Cancer. 2011;47(8):1175–85. [DOI] [PubMed] [Google Scholar]
- 68. Matalqah LM, Radaideh KM, Yusoff ZM, Awaisu A. Health-related quality of life using EQ-5D among breast cancer survivors in comparison with age-matched peers from the general population in the state of Penang, Malaysia. J Public Health. 2011;19(5):475. [Google Scholar]
- 69. Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–4. [DOI] [PubMed] [Google Scholar]
- 70. Shiroiwa T, Fukuda T, Shimozuma K, et al. Comparison of EQ-5D scores among anthracycline-containing regimens followed by taxane and taxane-only regimens for node-positive breast cancer patients after surgery: the N-SAS BC 02 trial. Value Health. 2011;14(5):746–51. [DOI] [PubMed] [Google Scholar]
- 71. Cheng TF, Der Wang J, Uen WC. Cost-utility analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy in premenopausal women with breast cancer. BMC Cancer. 2012;12(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Oh DY, Crawford B, Kim SB, et al. Evaluation of the willingness-to-pay for cancer treatment in Korean metastatic breast cancer patients: a multicenter, cross-sectional study. Asia-Pacific J Clin Oncol. 2012;8(3):282–91. [DOI] [PubMed] [Google Scholar]
- 73. Robertson S, Wengström Y, Eriksen C, Sandelin K. Breast surgeons performing immediate breast reconstruction with implants—assessment of resource-use and patient-reported outcome measures. Breast. 2012;21(4):590–6. [DOI] [PubMed] [Google Scholar]
- 74. Diane Serra R, Parris CR, Carper E, et al. Outcomes of guided imagery in patients receiving radiation therapy for breast cancer. Clin J Oncol Nurs. 2012;16(6):617–23. [DOI] [PubMed] [Google Scholar]
- 75. Frederix GW, Quadri N, Hövels AM, et al. Utility and work productivity data for economic evaluation of breast cancer therapies in the Netherlands and Sweden. Clin Ther. 2013;35(4):e1–e7. [DOI] [PubMed] [Google Scholar]
- 76. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101–7. [DOI] [PubMed] [Google Scholar]
- 77. Moro-Valdezate D, Peiró S, Buch-Villa E, et al. Evolution of health-related quality of life in breast cancer patients during the first year of follow-up. J Breast Cancer. 2013;16(1):104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Postma EL, Koffijberg H, Verkooijen H, Witkamp A, van den Bosch M, van Hillegersberg R. Cost-effectiveness of radioguided occult lesion localization (ROLL) versus wire-guided localization (WGL) in breast conserving surgery for nonpalpable breast cancer: results from a randomized controlled multicenter trial. Ann Surg Oncol. 2013;20(7):2219–26. [DOI] [PubMed] [Google Scholar]
- 79. Humphrey KL, Lee JM, Donelan K, et al. Percutaneous breast biopsy: effect on short-term quality of life. Radiology. 2014;270(2):362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Min YH, Lee JW, Shin Y-W, et al. Daily collection of self-reporting sleep disturbance data via a smartphone app in breast cancer patients receiving chemotherapy: a feasibility study. J Med Internet Res. 2014;16(5):e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Moro-Valdezate D, Buch-Villa E, Peiró S, et al. Factors associated with health-related quality of life in a cohort of Spanish breast cancer patients. Breast Cancer. 2014;21(4):442–52. [DOI] [PubMed] [Google Scholar]
- 82. Songtish D, Praditsitthikorn N, Teerawattananon Y. A cost-utility analysis comparing standard axillary lymph node dissection with sentinel lymph node biopsy in patients with early stage breast cancer in Thailand. Value Health Reg Issues. 2014;3:59–66. [DOI] [PubMed] [Google Scholar]
- 83. Timmers JM, Damen JA, Pijnappel RM, et al. Cost-effectiveness of non-invasive assessment in the Dutch breast cancer screening program versus usual care: a randomized controlled trial. Can J Public Health. 2014;105(5):e342e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tosteson AN, Fryback DG, Hammond CS, et al. Consequences of false-positive screening mammograms. JAMA Intern Med. 2014;174(6):954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dranitsaris G, Yu B, King J, Kaura S, Zhang A. Nab-paclitaxel, docetaxel, or solvent-based paclitaxel in metastatic breast cancer: a cost-utility analysis from a Chinese health care perspective. Clinicoecon Outcomes Res. 2015;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Eyles C, Leydon GM, Hoffman CJ, et al. Mindfulness for the self-management of fatigue, anxiety, and depression in women with metastatic breast cancer: a mixed methods feasibility study. Integr Cancer Ther. 2015;14(1):42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hall P, Hamilton P, Hulme C, et al. Costs of cancer care for use in economic evaluation: a UK analysis of patient-level routine health system data. Br J Cancer. 2015;112(5):948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kimman M, Jan S, Monaghan H, Woodward M. The relationship between economic characteristics and health-related quality of life in newly diagnosed cancer patients in Southeast Asia: results from an observational study. Qual Life Res. 2015;24(4):937–49. [DOI] [PubMed] [Google Scholar]
- 89. Swan JS, Kong CY, Hur C, et al. Comparing morbidities of testing with a new index: screening colonoscopy versus core-needle breast biopsy. J Am Coll Radiol. 2015;12(3):295–301. [DOI] [PubMed] [Google Scholar]
- 90. Tachi T, Teramachi H, Tanaka K, et al. The impact of outpatient chemotherapy-related adverse events on the quality of life of breast cancer patients. PloS One. 2015;10(4):e0124169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pickard AS, Jiang R, Lin H-W, Rosenbloom S, Cella D. Using patient-reported outcomes to compare relative burden of cancer: EQ-5D and functional assessment of cancer therapy-general in eleven types of cancer. Clin Ther. 2016;38(4):769–77. [DOI] [PubMed] [Google Scholar]
- 92. Shiroiwa T, Fukuda T, Shimozuma K, et al. Long-term health status as measured by EQ-5D among patients with metastatic breast cancer: comparison of first-line oral S-1 and taxane therapies in the randomized phase III SELECT BC trial. Qual Life Res. 2017;26(2):445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang S-Y, Hsu SH, Gross CP, et al. Association between time since cancer diagnosis and health-related quality of life: a population-level analysis. Value Health. 2016;19(5):631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yagata H, Ohtsu H, Komoike Y, et al. Joint symptoms and health-related quality of life in postmenopausal women with breast cancer who completed 5 years of anastrozole. Support Care Cancer. 2016;24(2):683–9. [DOI] [PubMed] [Google Scholar]
- 95. Enblom A, Lindquist H, Bergmark K. Participation in water-exercising long-term after breast cancer surgery: experiences of significant factors for continuing exercising as a part of cancer rehabilitation. Eur J Cancer Care. 2017;27(1):e12736.. [DOI] [PubMed] [Google Scholar]
- 96. Gordon LG, DiSipio T, Battistutta D, et al. Cost-effectiveness of a pragmatic exercise intervention for women with breast cancer: results from a randomized controlled trial. Psychooncology. 2017;26(5):649–55. [DOI] [PubMed] [Google Scholar]
- 97. Kim S-H, Jo M-W, Ock M, Lee H-J, Lee J-W. Estimation of health state utilities in breast cancer. Patient Prefer Adher. 2017;11:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. May AM, Bosch MJ, Velthuis MJ, et al. Cost-effectiveness analysis of an 18-week exercise programme for patients with breast and colon cancer undergoing adjuvant chemotherapy: the randomised PACT study. BMJ Open. 2017;7(3):e012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Seferina SC, Ramaekers BL, Maaike de, Boer M, et al. Cost and cost-effectiveness of adjuvant trastuzumab in the real world setting: a study of the Southeast Netherlands Breast Cancer Consortium. Oncotarget. 2017;8(45):79223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. The Action Study Group. Health-related quality of life and psychological distress among cancer survivors in Southeast Asia: results from a longitudinal study in eight low-and middle-income countries. BMC Med. 2017;15(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. van Kampen R, Ramaekers B, Lobbezoo D, et al. Real-world and trial-based cost-effectiveness analysis of bevacizumab in HER2-negative metastatic breast cancer patients: a study of the Southeast Netherlands Breast Cancer Consortium. Eur J Cancer. 2017;79:238–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mdm-10.1177_0272989X211065471 for A Systematic Literature Review of Health Utility Values in Breast Cancer by Manraj N. Kaur, Jiajun Yan, Anne F. Klassen, Justin P. David, Dilshan Pieris, Manraj Sharma, Louise Bordeleau and Feng Xie in Medical Decision Making