Abstract
Background:
Cell-based therapies for multiple sclerosis (MS), including those employing autologous bone marrow-derived mesenchymal stromal cells (MSC) are being examined in clinical trials. However, recent studies have identified abnormalities in the MS bone marrow microenvironment.
Objective:
We aimed to compare the secretome of MSC isolated from control subjects (C-MSC) and people with MS (MS-MSC) and explore the functional relevance of findings.
Methods:
We employed high throughput proteomic analysis, enzyme-linked immunosorbent assays and immunoblotting, as well as in vitro assays of enzyme activity and neuroprotection.
Results:
We demonstrated that, in progressive MS, the MSC secretome has lower levels of mitochondrial fumarate hydratase (mFH). Exogenous mFH restores the in vitro neuroprotective potential of MS-MSC. Furthermore, MS-MSC expresses reduced levels of fumarate hydratase (FH) with downstream reduction in expression of master regulators of oxidative stress.
Conclusions:
Our findings are further evidence of dysregulation of the bone marrow microenvironment in progressive MS with respect to anti-oxidative capacity and immunoregulatory potential. Given the clinical utility of the fumaric acid ester dimethyl fumarate in relapsing–remitting MS, our findings have potential implication for understanding MS pathophysiology and personalised therapeutic intervention.
Keywords: Multiple sclerosis, fumarate hydratase, mesenchymal stromal cells, neuroprotection, oxidative stress
Introduction
Cell-based therapy for the treatment of multiple sclerosis (MS) has undergone rapid translation from in vitro and in vivo studies to clinical trials. In neurological disease, including MS, the potential of autologous cells isolated from a systemic source and expanded ex vivo is particularly attractive given the limited capacity of the central nervous system (CNS) for repair. 1 The protective properties of multipotent mesenchymal stromal cells (MSC) and their secretome, in both in vitro and in vivo models of neurodegenerative disease, mean they are widely regarded as one of the most promising cell types for use in cell-based therapies.2–5
If autologous cells are to be employed in cell-based therapies, it is important to demonstrate that their therapeutic properties have not been compromised by exposure to disease. 6 In MS, there is increasing concern that MSC isolated from people with MS have altered functional properties. We have previously demonstrated that MSC isolated from people with progressive with MS (MS-MSC) can be expanded in vitro and have the expected cell surface phenotype and mesenchymal differentiation potential. 7 However, in subsequent, larger studies which take the effect of age into consideration, we demonstrated that MS-MSC have reduced ex vivo expansion potential, 8 and failure or in adequate ex vivo expansion of autologous MSC was also reported in approximately 5% participants in the MEsenchymal StEm cells for MS (MESEMS) study (NCT01854957; A. Uccelli, ECTRIMS 2018). 9 Our previous investigations have also demonstrated that MS-MSC have an in vitro phenotype consistent with premature ageing, with increased expression of markers of senescence and accelerated telomere shortening. 8 Furthermore, we have also shown that the MS-MSC secretome offers reduced neuroprotection in vitro, 10 and MS-MSC have increased susceptibility to nitrosative stress and display dysregulated anti-oxidant responses including reduced secretion of a range of trophic factors and anti-oxidants. 11 Others have reported reduced immunosuppressive function and altered cytokine expression in MS-MSC12,13 as well as reduced therapeutic efficacy of MS-MSC in a murine model of MS (experimental autoimmune encephalomyelitis, EAE). 14
Here, we compared the composition of the MSC secretome when MSC were isolated from people with MS or control subjects with the aim of identifying differences which may contribute to the reduced neuroprotective potential of the MSC secretome and dysregulated anti-oxidant responses previously reported.
Material and methods
MSC isolation and culture
Bone marrow aspirates were obtained from people with progressive MS (MS-MSC) participating in the clinical trials ‘Repeat Infusion of Autologous bone Marrow Cells in MS (SIAMMS-II)’ (NCT01932593; United Kingdom (UK) Research Ethics Committee (REC) 13/SW/0255) 15 and ‘Assessment of Bone Marrow-Derived Cellular Therapy in Progressive Multiple Sclerosis (ACTiMuS)’ (NCT01815632; REC 12/SW/0358). 16 Control MSC (C-MSC) were obtained from the discarded femoral head during total hip replacement (REC 10/H102/69); donors were known to have osteoarthritis, but were otherwise healthy and were not receiving drugs associated with myelosuppression. None of the ACTiMuS participants had received disease modifying therapy in the year prior to bone marrow collection although some participants with secondary progressive MS had been exposed to disease modifying therapy previously (see Supplementary Material). Not all samples were available for all experiments; the number of biological replicates is specified in each experiment and details regarding the cohort and which samples were used for each analysis are presented as Supplementary Material.
Isolation of MSC and preparation of MSC-conditioned medium
MSC were isolated using a density gradient and were expanded in vitro as previously described. 17 Cell surface phenotype and mesenchymal differentiation potential were confirmed to be consistent with those expected of MSC. 7 MSC in the logarithmic phase of growth at second (p2) or third passage (p3) were used to produce conditioned medium. 17 The culture flasks were washed twice with Dulbecco’s modified Eagle’s medium (DMEM; Sigma, USA), to remove any residual trophic effect from serum. Minimal medium (MIN) was added to the flasks. This consisted of 48.25 mL DMEM, 500 µL Pen-Strep (Gibco Penicillin–Streptomycin, Ref 15140-122), 500 µL Sato concentrate (containing 100 µg/mL of bovine serum albumin, 0.06 µg/mL progesterone, 16 µg/mL putrescine, 0.04 µg/mL selenite, 0.04 µg/mL thyroxine and 0.04 µg/mL triiodothyronine), 18 500 µL holo-transferrin (Sigma-Aldrich, Ref. T0665) and 250 µL L-glutamine (Sigma Aldrich, Ref. I5500). After 24 hours, conditioned medium was collected from cultures of control MSC (C-MSCcm) or MSC isolated from patients with progressive MS (MS-MSCcm), centrifuged, filtered and stored at −20°C. 17
Isolation of mitochondria
MSC mitochondria were isolated with a commercial kit used according to manufacturer’s instructions (Sigma MITOISO2). Briefly, cells at p3 were detached, washed in ice-cold phosphate-buffered saline (PBS) and lysed on ice for 5 minutes. Extraction buffer was added, and cells centrifuged at 600 × g for 10 minutes. Supernatant was collected and centrifuged at 11,000 × g for 10 minutes to obtain mitochondrial pellet. The pellet was resuspended in either storage buffer for mitochondrial activity assay or CelLytic M cell lysis reagent with protease inhibitors for immunoblotting.
Proteomics
At the University of Bristol Proteomics Facility, liquid chromatography–tandem mass spectrometry (LC-MSMS) of C-MSCcm and MS-MSCcm was performed according to a previously described protocol for tandem mass tagging (Thermo Fisher Scientific, USA). 19
Enzyme-linked immunosorbent assay
Ready to use sandwich enzyme-linked immunosorbent assay (ELISA) for mFH (human mFH: Cusabio Catalogue No. CSB-EL008659HU) was performed on conditioned medium from C-MSC and MS-MSC according to the manufacturer’s instructions. A standard curve was prepared and absorbance read on a spectrophotometer at 450 nm (BMG Labtech Fluostar Optima). Values were interpolated into the curve and multiplied by the dilution factor to obtain the final concentration.
Fumarase activity assay
Fumarase activity was quantified using a commercially available assay according to the manufacturer’s instructions (Sigma-Aldrich, Ref. MAK206). In addition to MSC or mitochondrial lysate, wells contained 50 µL reaction mix which consisted of 36 µL of fumarase assay buffer, 2 µL of fumarase enzyme mix, 10 µL of fumarase developer and 2 µL of fumarase substrate. After adding the reaction mix, the plate was protected from light and mixed using a horizontal shaker. The results were measured using a BMG Labtech Fluostar Optima microplate reader at 450 nm, and MARS data analysis software (kinetic mode for 60 minutes at 37°C with absorbance readings taken every minute). Nicotinamide adenine dinucleotide and hydrogen (NADH) standards were read at the end of the incubation time. To calculate fumarase activity, the absorbance for each well was plotted versus time. Two time points were chosen (T1 and T2) in the linear range of the plot, and the absorbance was determined. Background was corrected by subtracting the measurement obtained for the blank standards. The change in absorbance from T1 to T2 was calculated, and the amount of NADH generated (nmole/well) was obtained. Fumarase activity was ascertained by dividing amount of NADH (nmole) between T1 and T2 by the reaction time multiplied by sample volume added to the well, and the activity was reported as nmole/min/µL or milliunits/µL where one unit of fumarase is the amount of enzyme that generates 1.0 µmole of NADH per minute at pH 9.5 and 37°C.
Immunoblotting
Immunoblotting was performed as previously described.11,20 Briefly, MSC were plated at 5 × 104 cells per well in a six-well plate prior to lysis with universal lysis buffer (Millipore). Protein quantification was performed with Qubit Fluorometer and Quant-iT™ protein assay kit (Invitrogen) to ensure equal loading of samples. Protein lysates were diluted 1:1 with 2 × Laemmli buffer and denatured at 95°C, before loading on Tris HCl 4–20% ready gels (Bio-Rad). Gels were transferred to nitrocellulose membrane and subsequently blocked with 5% bovine serum albumin (Sigma) or 5% milk in tris-buffered saline–Tween for 1 hour. Incubation with primary antibody anti-FH (Abcam, ab95950), anti-nuclear-related (erythroid-derived 2) factor 2 (anti-NRF-2) antibody (R&D, AF3925), anti-hypoxia inducible factor1α (anti-hypoxia inducible factor1α (anti-HIF-1α; Abcam, ab51608)), anti-COX5 (Santa Cruz, SC-376907) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Abcam, ab9484) was performed overnight at 4°C. Amersham ECL Plus™ Western Blotting Detection System (GE Healthcare) was used to visualise specific protein expression patterns by chemiluminescence. The integrated density of bands was measured using ImageJ (National Institute of Health, NIH), and values are expressed relative to GAPDH loading control protein.
Neurotoxicity assays
Rodent cortical neuronal cultures, trophic factor withdrawal (exposure to MIN for 24 hours) and nitric oxide (NO) toxicity assays employing 0.4 mM DETANONOate (Enzo Life Sciences) were established as previously described.10,20 Survival was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. 21 Recombinant human FH was added as described in the relevant experiments (Sigma-Aldrich, Ref. SRP6120).
Statistical analysis
GraphPad PRISM 5 (GraphPad Software) was used for graphical illustrations and statistical analyses not employing multiple regression (*). Where stated, multivariant analyses (#) were performed with STATA v12 (StataCorp).8,10,11 Bar graphs show mean ± standard error of the mean and regression lines were fitted with 95% confidence intervals (CI). Values of p < 0.05 were considered statistically significant.
Results
Reduced mFH secretion in progressive MS is negatively associated with duration of progressive phase of disease
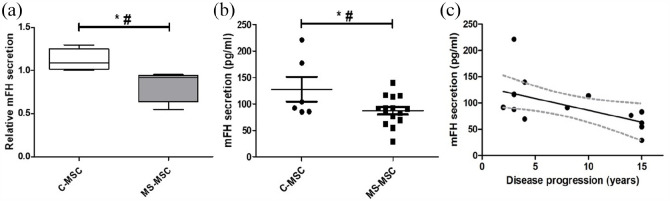
Relative reduction in the concentration of mFH in the secretome of MS-MSC was demonstrated by LC-MSMS (C-MSC: n = 4, MS-MSC: n = 4; *p = 0.048) and reduced concentration was confirmed by ELISA (C-MSC: n = 6, MS-MSC: n = 15; *p = 0.042; Figure 1). Following analysis of the quantitative ELISA data using the regression model to account for effects of age, a statistically significant independent effect of progressive MS was seen (#p = 0.041, CI = −4.614 to −0.097) and there was a negative association with duration of progressive phase of MS (Figure 1; Pearson’s r −0.568, CI = −0.837 to −0.079, *p = 0.027).
Figure 1.
MS-MSC secrete reduced mFH and secretion is negatively associated with duration of progression in MS. (a) Secretion of mFH in C-MSC and MS-MSC as determined by LC-MSMS. (b) Quantitative determination of mFH secretion in MSCs as measured by ELISA. (c) Negative association of mFH secretion with duration of progressive phase of MS.
mFH: mitochondrial fumarate hydratase; C-MSC: control mesenchymal stromal cells; MS-MSC: multiple sclerosis mesenchymal stromal cells.
*p < 0.05, #p < 0.05 multivariant analysis.
FH activity in MS-MSC is preserved when adjusted for age
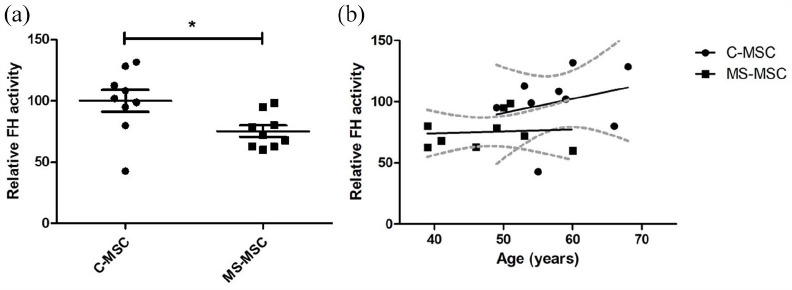
Under basal cell culture conditions, total fumarate hydratase (FH) activity was reduced in MS-MSC (n = 9) compared with C-MSC (n = 9; *p = 0.026; Figure 2). However, a statistically significant effect was not observed when confounding effects of age were taken into account; there was a strong trend towards an increase in FH activity with age (p = 0.059) although the trend was less marked in MS-MSC (Figure 2(b)). There was no association between FH activity in MS-MSC with duration of disease progression.
Figure 2.
MS-MSC have reduced endogenous FH activity, but effect is confounded by differences in age between cohorts. (a) MS-MSC FH activity was reduced in MS-MSC compared with C-MSC although the observed effect does not persist following adjustment for age. (b) There was a strong trend towards an increase in FH activity with increasing age, although this effect was not seen in MS-MSC.
FH: fumarate hydratase; C-MSC: control mesenchymal stromal cells; MS-MSC: multiple sclerosis mesenchymal stromal cells.
*p < 0.05.
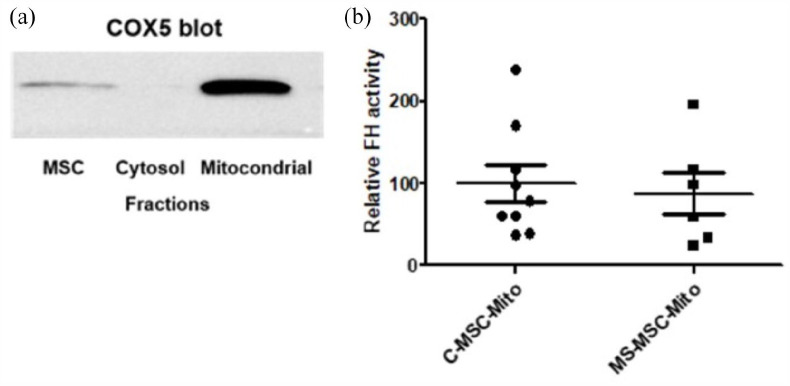
A specific assay for mFH was not available, so that the FH activity assay was used to determine FH activity in mitochondrial cell preparations isolated from C-MSC and MS-MSC. To confirm successful fractionation of the cell preparations, immunoblotting for COX5 was undertaken with equal loading (20 µg) of mitochondrial protein, the cytosolic fraction (negative control) and unfractionated MSC protein. As expected, COX5 expression was greater in the mitochondrial fraction than in the unfractionated MSC lysate and was not detected in the cytosolic fraction (Figure 3(a)). There was no significant difference in FH activity in mitochondria isolated from MS-MSC (n = 6) compared to those from C-MSC (n = 9; p = 0.73; Figure 3(b)).
Figure 3.
mFH activity is preserved in MS-MSC. (a) Immunoblot for COX5 confirms the specificity of MSC mitochondrial fractionation. (b) No difference in mFH activity was seen in mitochondria isolated from C-MSC and MS-MSC.
MSC: mesenchymal stromal cells; COX5: cytochrome c oxidase subunit 5; mFH: mitochondrial fumarate hydratase; C-MSC-Mito: mitochondria isolated from control mesenchymal stromal cells; MS-MSC-Mito: mitochondria isolated from multiple sclerosis mesenchymal stromal cells.
Reduced expression of FH in MS-MSC
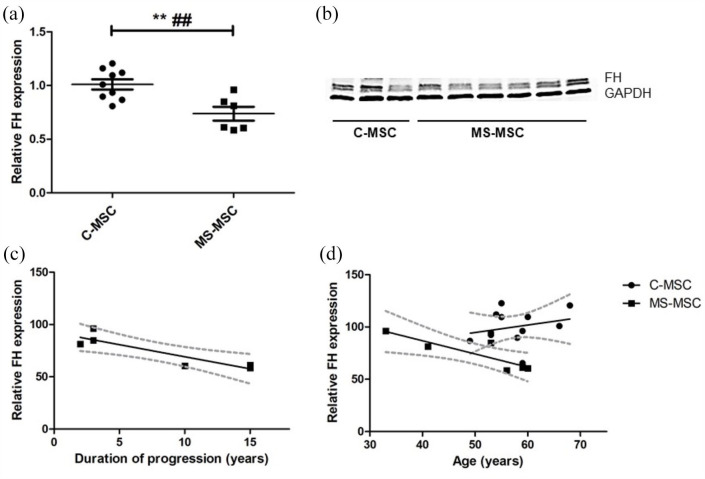
Comparison of total FH expression by C-MSC and MS-MSC was examined by immunoblotting. Western blot analysis of MSC isolated from patients with MS (n = 6) and control subjects (n = 12) demonstrated reduced expression of FH protein by MS-MSC (**p = 0.004). This effect remained following adjustment with multiple regression for age (##p = 0.002, CI = −0.4824343 to −0.1456443; Figure 4(a) and (b)). There was a negative association between FH expression and increasing duration of progression of MS (Pearson’s r = −0.897, p = 0.02, CI = −0.9888 to −0.3138; Figure 4(c)), but this effect did not persist following adjustment for age. In the combined cohorts, there was no significant association between FH protein expression and age although a negative effect of increasing age was seen in MS-MSC (Pearson’s r = −0.872, p = 0.024, CI = −0.9859 to −0.2057). A trend towards a differential effect of age on FH was seen depending on the presence of progressive MS (p = 0.069) with a decrease in FH expression with age being seen only in MS-MSC (Figure 4(d)).
Figure 4.
Reduced expression of FH protein by MS-MSC. (a) Reduced expression of FH protein was seen in MS-MSC and this effect persisted after adjustment for age difference between the cohorts. (b) Representative immunoblotting bands. (c) A reduction in relative FH expression was seen with increasing duration of disease progression in MS, but this effect was not statistically significant following adjustment for age (Pearson’s r = −0.897, p = 0.02, CI = −0.9888 to −0.3138; p > 0.05 following adjustment for age). (d) A differential effect of age on FH protein expression with age was seen between the cohorts; there was a significant negative association in MS-MSC and a strong trend towards a positive association in C-MSC.
FH: fumarate hydratase; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; C-MSC: control mesenchymal stromal cells; MS-MSC: multiple sclerosis mesenchymal stromal cells.
**p < 0.01, ##p < 0.01 multivariant analysis.
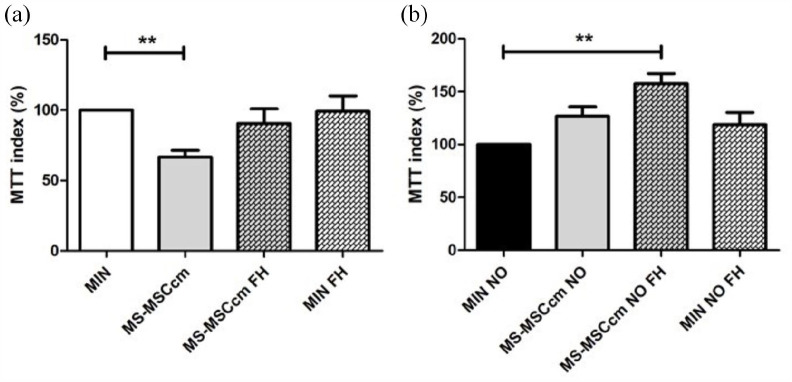
Addition of FH to MS-MSCcm prevents neuronal loss under conditions of trophic factor withdrawal and nitrosative stress
We have previously demonstrated reduced neuronal survival in the presence of MS-MSCcm following trophic factor withdrawal and under conditions of nitrosative stress. 9 Given the observed reduction in FH concentration in MS-MSCcm, we examined whether neuronal loss could be ameliorated by supplementation of MS-MSCcm with FH. Optimum concentration of exogenous FH was determined by a dose response curve which indicated that maximum neuroprotection was observed at concentrations of FH between 500 and 700 pg/mL, and replicates were performed with 500 pg/mL FH.
Following supplementation of MS-MSCcm with FH, neuronal loss was not observed under conditions of trophic factor withdrawal (Figure 5(a)) or nitrosative stress induced by addition of DETANONOate (Figure 5(b)).
Figure 5.
FH supplementation of MS-MSCcm restores neuroprotective potential of MS-MSCcm. (a) Under conditions of trophic factor withdrawal, reduced neuronal survival is observed in the presence of MS-MSCcm (n = 8) compared to minimal media (Kruskal–Wallis with Dunn’s multiple comparison test). However, with administration of exogenous FH to MS-MSCcm, neuronal loss is not observed. (b) Nitrosative stress was induced by application of DETANONOate (NO) and a protective effect of MS-MSCcm (n = 8) was seen only in the presence of exogenous FH (Kruskal–Wallis with Dunn’s multiple comparison test).
FH: fumarate hydratase; C-MSCcm: conditioned medium from control mesenchymal stromal cells; MS-MSCcm: conditioned medium from multiple sclerosis mesenchymal stromal cells; MIN: minimal medium; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NO: nitric oxide; NS: not significant.
**p < 0.01.
Nrf-2 expression is negatively associated with duration of progression in MS
High levels of intracellular fumarate have been associated with a range of downstream effects with potential implication for intracellular metabolic signalling. To begin to explore these, we examined expression of HIF-1α and Nrf-2; upregulation of both has been associated with loss of FH function22,23 and each has been identified as being of potential importance in the pathophysiology of MS.24,25
We have previously demonstrated that, although there is no difference in Nrf-2 expression between C-MSC and MS-MSC, MS-MSC have reduced expression of Nrf-2 protein under standard culture conditions and in response to nitrosative stress. 11 Here, we demonstrated a negative association between Nrf-2 protein expression and duration of disease progression in MS (n = 6, Pearson’s r = −0.9819, p = 0.01, CI = −0.991 to −0.42; Figure 6). An independent effect of age was not observed.
Figure 6.
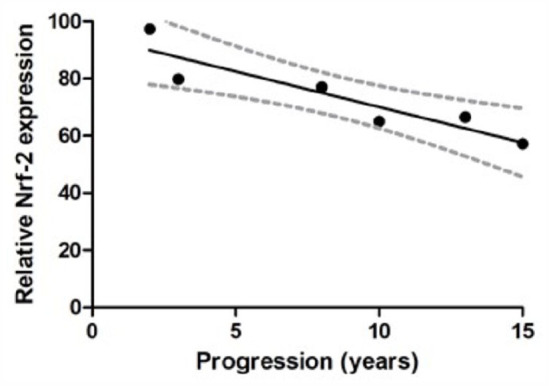
Nrf-2 protein expression. Reduced expression of Nrf-2 protein expression in association with increasing duration of disease progression in MS (Pearson’s r = −0.9819, p = 0.01, CI = −0.991 to −0.42).
Nrf-2: nuclear-related (erythroid-derived 2) factor 2.
Reduced HIF-1α expression in MS-MSC
A strong trend towards reduced expression of HIF-1α protein in MS-MSC was noted on immunoblotting (C-MSC: n = 3, MS-MSC: n = 6; p = 0.056), reaching statistical significance when the effect of age was taken into account (##p = 0.001, CI = −0.4105565 to −0.1046486; Figure 7(a)). An independent effect of duration of progression was not observed.
Figure 7.
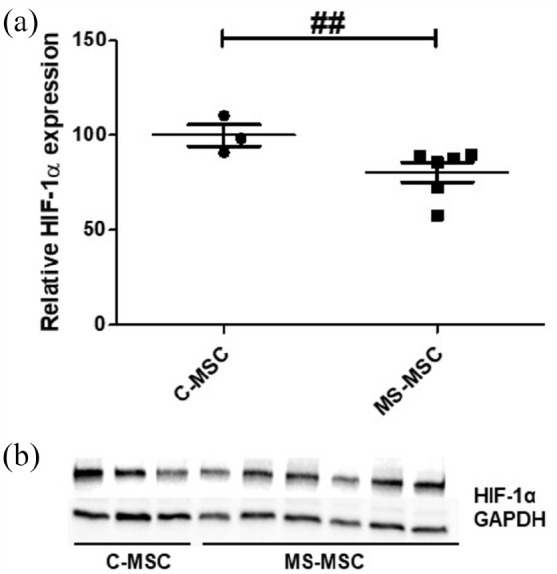
Reduced MS-MSC expression of HIF-1α. (a) HIF-1α protein expression is reduced in MS-MSC when the difference in age between the cohorts is accounted for. (b) Representative immunoblot.
GAPDH: glyceraldehyde 3-phosphate dehydrogenase; HIF-1α: anti-hypoxia inducible factor1α; C-MSC: control mesenchymal stromal cells; MS-MSC: multiple sclerosis mesenchymal stromal cells.
##p < 0.01 multivariant analyses.
Discussion
To investigate the reduced neuroprotective potential of MS-MSC in vitro, we examined the MS-MSC secretome using LC-MSMS and noted reduced mFH secretion by MS-MSC. This was of particular interest given that dimethyl fumarate (DMF), a fumaric acid ester, is a licenced disease modifying therapy for relapsing–remitting MS and a putative neuroprotective effect has been reported. 26 We confirmed reduced secretion of mFH by MS-MSC by ELISA, and there was a negative association with duration of progressive disease. Although reduced FH activity was observed in MS-MSC, this effect did not reach statistical significance after adjustment for differences in age between the cohorts and furthermore, no difference was seen when FH activity was assessed in mitochondrial preparations from MSC isolated from control subjects and people with progressive MS. However, reduced expression of FH was seen in MS-MSC and a negative correlation with duration of MS progression was observed. Exogenous application of FH was neuroprotective in vitro; neuronal survival with exposure to MS-MSCcm under conditions of trophic factor withdrawal and exposure to nitrosative stress increased. Although expression of both Nrf-2 and HIF-1α, downstream targets of FH, are both reduced in MS-MSC, only Nrf-2 expression negatively correlated with duration of progressive MS.
Fumarase deficiency (also known as fumaric aciduria) is a rare, life-limiting, autosomal recessive disorder associated with encephalopathy, hypotonia and seizures. Heterozygous germline mutations of FH are associated with hereditary leiomyomatosis and renal cell cancer (HLRCC). In addition to its role as a tumour suppressor, reduced expression of FH has been implicated in hypertension, 27 type 2 diabetes 28 and diabetic kidney disease. 29 In mice, FH has been identified as a key regulator of metabolism in haematopoietic stem cells and deficiency is associated with aberrant lymphoid differentiation. 30
In the Krebs cycle, fumarate is catalysed to malate by FH. Intracellular accumulation of fumarate in FH deficiency has a multitude of downstream metabolic consequences and the effects are known to vary according to cell type, but include increased oxidative stress and increased cellular senescence, 31 mitochondrial dysfunction and activation of both the pro-oncogenic HIF and anti-oxidant Nrf-2 pathways. 32 The latter are known to be of relevance to pathophysiology in MS33,34 and Nrf-2 has been proposed to underlie putative neuroprotective effects associated with DMF32,35 which is known to be of clinical benefit in relapsing–remitting MS. 36 In our studies however, reduced mFH expression was associated with reduced Nrf-2 expression which may reflect a cell-specific or disease effect.
In many cell types, reduced expression of Nrf-2 is associated with increased HIF-1α and causes a shift to glycolysis. Although blocking aerobic glycolysis might be predicted to be anti-inflammatory, 37 in MSC HIF-1α expression has been reported to promote MSC survival as well as maintenance of differentiation potential and MSC-mediated immunosuppression. 35 Our finding of reduced HIF-1α expression in MS-MSC is therefore notable.
The current study suggests that FH deficiency in MS-MSC contributes to a dysfunctional bone marrow microenvironment in MS with potential significance for metabolic status and immunoregulation that warrants additional investigation to determine whether this is a disease-specific effect with potential for therapeutic intervention.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585211060686 for Reduced expression of mitochondrial fumarate hydratase in progressive multiple sclerosis contributes to impaired in vitro mesenchymal stromal cell-mediated neuroprotection by Pamela Sarkar, Juliana Redondo, Kelly Hares, Steven Bailey, Anastasia Georgievskaya, Kate Heesom, Kevin C Kemp, Neil J Scolding and Claire M Rice in Multiple Sclerosis Journal
Acknowledgments
The authors gratefully acknowledge all marrow donors, colleagues at the Bristol Haematology and Oncology Centre as well as the assistance of Professors Blom and Whitehouse at the Avon Orthopaedic Centre for access to control bone marrow samples. They also thank Alastair Wilkins for his constructive comments on the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: They are grateful for funding support from the Naomi Bramson Charitable Trust, Anne Graham and Steve Scobie (Goldman Sachs Gives) and the Burden Neurological Institute.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs: Neil J Scolding  https://orcid.org/0000-0001-9177-1043
https://orcid.org/0000-0001-9177-1043
Claire M Rice  https://orcid.org/0000-0002-9851-4426
https://orcid.org/0000-0002-9851-4426
Contributor Information
Pamela Sarkar, Clinical Neurosciences, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK; Department of Neurology, North Bristol NHS Trust, Southmead Hospital, Bristol, UK.
Juliana Redondo, Clinical Neurosciences, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Kelly Hares, Clinical Neurosciences, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Steven Bailey, Clinical Neurosciences, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK; Department of Neurology, North Bristol NHS Trust, Southmead Hospital, Bristol, UK.
Anastasia Georgievskaya, Clinical Neurosciences, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Kate Heesom, Bristol Proteomics Facility, Biomedical Sciences, University of Bristol, Bristol, UK.
Kevin C Kemp, Clinical Neurosciences, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Neil J Scolding, Clinical Neurosciences, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK; Department of Neurology, North Bristol NHS Trust, Southmead Hospital, Bristol, UK.
Claire M Rice, Clinical Neurosciences, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK; Department of Neurology, North Bristol NHS Trust, Southmead Hospital, Bristol, UK.
References
- 1. Ben-Hur T, Fainstein N, Nishri Y. Cell-based reparative therapies for multiple sclerosis. Curr Neurol Neurosci Rep 2013; 13(11): 397. [DOI] [PubMed] [Google Scholar]
- 2. Wilkins A, Kemp K, Ginty M, et al. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res 2009; 3(1): 63–70. [DOI] [PubMed] [Google Scholar]
- 3. Bai L, Lennon DP, Caplan AI, et al. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci 2012; 15(6): 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bara JJ, Turner S, Roberts S, et al. High content and high throughput screening to assess the angiogenic and neurogenic actions of mesenchymal stem cells in vitro. Exp Cell Res 2015; 333: 93–104. [DOI] [PubMed] [Google Scholar]
- 5. Mita T, Furukawa-Hibi Y, Takeuchi H, et al. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav Brain Res 2015; 293: 189–197. [DOI] [PubMed] [Google Scholar]
- 6. Munir H, McGettrick HM. Mesenchymal stem cell therapy for autoimmune disease: Risks and rewards. Stem Cells Dev 2015; 24: 2091–2100. [DOI] [PubMed] [Google Scholar]
- 7. Mallam E, Kemp K, Wilkins A, et al. Characterization of in vitro expanded bone marrow-derived mesenchymal stem cells from patients with multiple sclerosis. Mult Scler 2010; 16(8): 909–918. [DOI] [PubMed] [Google Scholar]
- 8. Redondo J, Sarkar P, Kemp K, et al. Reduced cellularity of bone marrow in multiple sclerosis with decreased MSC expansion potential and premature ageing in vitro. Mult Scler 2018; 24(7): 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uccelli A, Laroni A, Brundin L, et al. MEsenchymal StEm cells for Multiple Sclerosis (MESEMS): A randomized, double blind, cross-over phase I/II clinical trial with autologous mesenchymal stem cells for the therapy of multiple sclerosis. Trials 2019; 20: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarkar P, Redondo J, Kemp K, et al. Reduced neuroprotective potential of the mesenchymal stromal cell secretome with ex vivo expansion, age and progressive multiple sclerosis. Cytotherapy 2018; 20(1): 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Redondo J, Sarkar P, Kemp K, et al. Dysregulation of mesenchymal stromal cell antioxidant responses in progressive multiple sclerosis. Stem Cells Transl Med 2018; 7(10): 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Oliveira GL, de Lima KW, Colombini AM, et al. Bone marrow mesenchymal stromal cells isolated from multiple sclerosis patients have distinct gene expression profile and decreased suppressive function compared with healthy counterparts. Cell Transplant 2015; 24(2): 151–165. [DOI] [PubMed] [Google Scholar]
- 13. Mazzanti B, Aldinucci A, Biagioli T, et al. Differences in mesenchymal stem cell cytokine profiles between MS patients and healthy donors: Implication for assessment of disease activity and treatment. J Neuroimmunol 2008; 199: 142–150. [DOI] [PubMed] [Google Scholar]
- 14. Sargent A, Bai L, Shano G, et al. CNS disease diminishes the therapeutic functionality of bone marrow mesenchymal stem cells. Exp Neurol 2017; 295: 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rice CM, Mallam EA, Whone AL, et al. Safety and feasibility of autologous bone marrow cellular therapy in relapsing-progressive multiple sclerosis. Clin Pharmacol Ther 2010; 87: 679–685. [DOI] [PubMed] [Google Scholar]
- 16. Rice CMM, Marks DII, Ben-Shlomo Y, et al. Assessment of bone marrow-derived Cellular Therapy in progressive Multiple Sclerosis (ACTiMuS): Study protocol for a randomised controlled trial. Trials 2015; 16: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kemp K, Hares K, Mallam E, et al. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J Neurochem 2010; 114(6): 1569–1580. [DOI] [PubMed] [Google Scholar]
- 18. Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA 1979; 76(1): 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kittivorapart J, Karamatic Crew V, Wilson MC, et al. Quantitative proteomics of plasma vesicles identify novel biomarkers for hemoglobin E/b-thalassemic patients. Blood Adv 2018; 2: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redondo J, Hares K, Wilkins A, et al. Reductions in kinesin expression are associated with nitric oxide-induced axonal damage. J Neurosci Res 2015; 93(6): 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whone AL, Kemp K, Sun M, et al. Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res 2012; 1431: 86–96. [DOI] [PubMed] [Google Scholar]
- 22. Ooi A, Wong J-C, Petillo D, et al. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell 2011; 20: 511–523. [DOI] [PubMed] [Google Scholar]
- 23. Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell 2005; 8(2): 143–153. [DOI] [PubMed] [Google Scholar]
- 24. Asgari R, Yarani R, Mohammadi P, et al. HIF-1α in the crosstalk between reactive oxygen species and autophagy process: A review in multiple sclerosis. Cell Mol Neurobiol. Epub ahead of print 5 June 2021. DOI: 10.1007/s10571-021-01111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michaličková D, Hrnčíř T, Canová NK, et al. Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur J Pharmacol 2020; 873: 172973. [DOI] [PubMed] [Google Scholar]
- 26. Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011; 134(Pt 3): 678–692. [DOI] [PubMed] [Google Scholar]
- 27. Tian Z, Liu Y, Usa K, et al. Novel role of fumarate metabolism in Dahl-salt sensitive hypertension. Hypertension 2009; 54(2): 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adam J, Ramracheya R, Chibalina MV, et al. Fumarate hydratase deletion in pancreatic β cells leads to progressive diabetes. Cell Rep 2017; 20: 3135–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. You YH, Quach T, Saito R, et al. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J Am Soc Nephrol 2016; 27(2): 466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guitart AV, Panagopoulou TI, Villacreces A, et al. Fumarate hydratase is a critical metabolic regulator of hematopoietic stem cell functions. J Exp Med 2017; 214: 719–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng L, Cardaci S, Jerby L, et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun 2015; 6: 6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang M, Soga T, Pollard PJ, et al. The emerging role of fumarate as an oncometabolite. Front Oncol 2012; 2: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buendia I, Michalska P, Navarro E, et al. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther 2016; 157: 84–104. [DOI] [PubMed] [Google Scholar]
- 34. Trapp BD, Nave KA. Multiple sclerosis: An immune or neurodegenerative disorder? Annu Rev Neurosci 2008; 31: 247–269. [DOI] [PubMed] [Google Scholar]
- 35. Contreras-Lopez R, Elizondo-Vega R, Paredes MJ, et al. HIF1α-dependent metabolic reprogramming governs mesenchymal stem/stromal cell immunoregulatory functions. FASEB J 2020; 34(6): 8250–8264. [DOI] [PubMed] [Google Scholar]
- 36. Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: A multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008; 372: 1463–1472. [DOI] [PubMed] [Google Scholar]
- 37. Kornberg MD. The immunologic Warburg effect: Evidence and therapeutic opportunities in autoimmunity. Wiley Interdiscip Rev Syst Biol Med 2020; 12: e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585211060686 for Reduced expression of mitochondrial fumarate hydratase in progressive multiple sclerosis contributes to impaired in vitro mesenchymal stromal cell-mediated neuroprotection by Pamela Sarkar, Juliana Redondo, Kelly Hares, Steven Bailey, Anastasia Georgievskaya, Kate Heesom, Kevin C Kemp, Neil J Scolding and Claire M Rice in Multiple Sclerosis Journal