Abstract
Background:
No evidence of disease activity (NEDA-3) is a patient-centric outcome increasingly used as the goal of multiple sclerosis treatment.
Objective:
Determine treatment durability of cladribine tablets beyond 2 years considering the variable bridging interval of 0.1–116.0 weeks between CLARITY and CLARITY Extension.
Methods:
Between CLARITY and CLARITY Extension, patients transitioned from cladribine tablets 3.5 mg/kg to placebo (CP3.5 group, n = 98) or continued further treatment with cladribine tablets 3.5 mg/kg (CC7.0 group, n = 186). Treatment assignment was randomized and blinded in both CLARITY and CLARITY Extension.
Results:
The 2-year NEDA-3 in CLARITY Extension (encompassing both years of CLARITY Extension) was 29.6% in the CP3.5 group and 32.8% in the CC7.0 group. There was no evidence that treatment effect differed with varying bridging intervals. For patients in the CP3.5 group with a bridging interval of ⩽48 weeks, 1 year NEDA-3 (the first year of CLARITY Extension) was 44.4% (28/63) compared with 31.4% (11/35) in patients with a bridging interval of >48 weeks.
Conclusion:
Treatment with cladribine tablets in CLARITY, followed by either placebo or cladribine tablets in CLARITY Extension, produced sustained benefits for NEDA-3 and its constituent elements for a follow up period up to 6 years from CLARITY baseline.
Keywords: Cladribine tablets, no evidence of disease activity, CLARITY Extension, bridging interval, relapsing-remitting multiple sclerosis, randomized trial, treatment durability
Introduction
The availability of more effective disease-modifying therapies (DMTs) over recent years has increased the complexity of selecting an appropriate treatment for patients with multiple sclerosis (MS). At the same time, therapeutic aims have evolved in recognition of a need to provide a more comprehensive assessment of treatment outcomes with patient centricity in mind. Consequently, in clinical practice, the composite measure of the absence of relapses, confirmed disability progression, and magnetic resonance imaging (MRI) activity—known as “no evidence of disease activity” (NEDA-3) 1 —is increasingly used as the goal of treatment in MS.2–6 More recently, MRI assessment of brain volume loss has been added to this composite measure (referred to as NEDA-4). 7
In Europe, cladribine tablets 10 mg (MAVENCLAD®; 3.5 mg/kg cumulative dose over 2 years, referred to as cladribine tablets 3.5 mg/kg) are approved for the treatment of relapsing MS with short oral courses of the drug administered at the beginning of Years 1 and 2, with no requirement for further courses of treatment in Years 3 and 4. 8 This unique dosing pattern was derived from the results of the CLARITY and CLARITY Extension studies. 9 CLARITY was a double-blind, randomized, placebo-controlled study that showed significant clinical and MRI efficacy of cladribine tablets. Patients receiving what became the approved cumulative dose of 3.5 mg/kg in CLARITY were re-randomized to placebo or a third and fourth course of cladribine tablets in CLARITY Extension. The latter study showed that clinical outcomes were not significantly different in patients who received further cladribine tablets versus those who received placebo during the extension phase. 9
NEDA-3 with cladribine tablets in Years 1 and 2 has been described previously from the CLARITY study, 10 but a point of interest is the proportion of patients with NEDA-3 in Years 3 and 4 after CLARITY baseline when no further courses of cladribine tablets were administered in comparison to patients who did receive additional courses of cladribine tablets. The current analysis was proposed to investigate this, and reports a post hoc analysis to determine the proportion of patients with NEDA-3 in those who received cladribine tablets 3.5 mg/kg in CLARITY and who were then randomized in CLARITY Extension to either placebo or further treatment with cladribine tablets 3.5 mg/kg.
For administrative reasons there was a delay in initiating the CLARITY Extension study. Consequently, having completed the CLARITY study, there was an interval before many patients could start the extension phase. This variable bridging interval between the CLARITY and CLARITY Extension studies ranged from 0.1 to 116.0 weeks meaning that, after completing the first 2 years of study treatment in CLARITY, some patients going directly into the CLARITY Extension study would finish at 4 years after the baseline of the original study. Other patients, with a bridging interval of 2 years, finished the CLARITY Extension study 6 years after the baseline of CLARITY. By analyzing patients with a bridging interval of ⩽48 weeks or >48 weeks before initiation of treatment in CLARITY Extension, we are therefore able to provide additional insight into the durability of treatment effect with cladribine tablets. A bridging interval of ⩽48 weeks or >48 weeks was chosen because this period corresponds to 1 study year of the 96-week CLARITY and CLARITY Extension studies. Therefore, assessing outcomes in 1 year time frames is relevant to clinical practice and representative of following up a patient after receiving a full course of cladribine tablets. Because the variable bridging interval includes data obtained after Year 4 from CLARITY baseline for some patients, the current analysis therefore provides further insight into the durability of clinical and MRI effects of treatment with cladribine tablets during a time where patients are not expected to receive further DMTs.
Methods
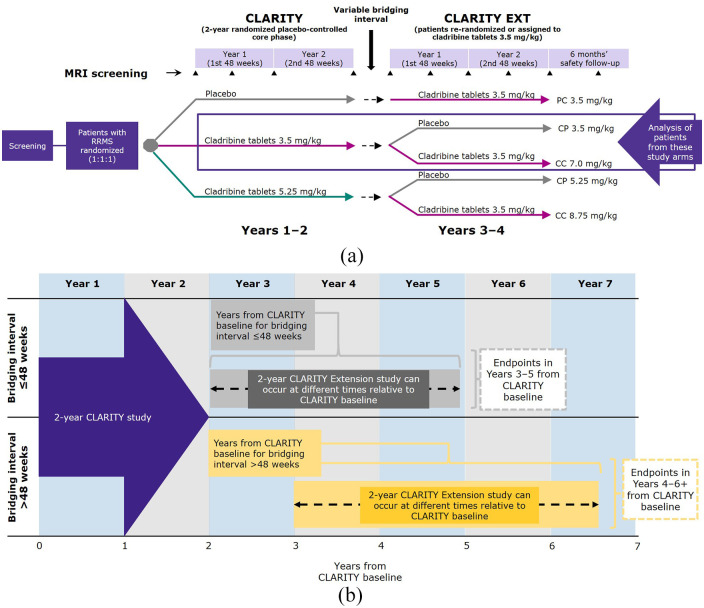
The design and primary outcomes of the CLARITY (NCT00213135) and CLARITY Extension (NCT00641537) studies have been reported previously.11–13 Briefly, patients with relapsing-remitting MS who were placebo recipients in CLARITY received cladribine tablets 3.5 mg/kg in CLARITY Extension. Patients receiving any dose of cladribine tablets in CLARITY were re-randomized to cladribine 3.5 mg/kg or placebo in CLARITY Extension (Figure 1(a)).
Figure 1.
(a) Study design of CLARITY and CLARITY Extension. (b) The impact of variable bridging interval on the CLARITY Extension observation period and endpoints relative to CLARITY baseline.
Bridging interval is the time between completion of CLARITY and the start of CLARITY Extension. Depending on bridging interval duration, the Week 48 (1 year) and Week 96 (2 year) endpoints in CLARITY Extension can therefore occur at different times relative to CLARITY baseline. The variable bridging interval means that some patients in the CLARITY Extension study were at Years 3 and 4, and some at Years 5 and 6 + after initial randomization in the CLARITY study. In this analysis, a cut-off for bridging intervals of ⩽48 weeks or >48 weeks was chosen because this corresponds to 1 study year of the 96-week CLARITY and CLARITY Extension studies and assessing outcomes in 1 year time frames is representative of following up a patient after receiving a full course of cladribine tablets.
RRMS, relapsing-remitting multiple sclerosis.
In CLARITY Extension, clinical examination and recording of Expanded Disability Status Scale (EDSS) score were conducted during the pre-study evaluation, on the first day of the study, and at weeks 13, 24, 36, 48, 60, 72, 84, and 96 of the double-blind treatment period. A qualifying relapse was defined as a 2-grade increase in 1 or more Kurtzke Functional Systems (KFS) score or a 1-grade increase in 2 or more KFS, excluding changes in bowel/bladder or cognition, in the absence of fever, lasting for ⩾24 hours, and preceded by ⩾30 days of clinical stability or improvement. For suspected relapses between study visits, patients were required to attend the study site within 7 days after the onset of symptoms for assessment by the evaluating physician.
Assessments of MRI activity were carried out when patients entered CLARITY Extension and after weeks 24, 48, 72, and 96 of the double-blind treatment period. MRI scans were performed using a standardized operating protocol by operators who were blinded to trial treatment, and patients were scanned using the same machines throughout the trial. A central independent neuroradiology center, also blinded to treatment, was used to assess MRI scans.
Depending on bridging interval duration, the Week 48 (1 year) and Week 96 (2 year) endpoints in CLARITY Extension can occur at different times relative to CLARITY baseline (see Figure 1(b)). Patients were permitted to receive interferon beta or glatiramer acetate during the bridging interval between studies, but discontinued any DMT at least 3 months before the first study day of CLARITY Extension.
Statistical analysis
All analyses were post hoc and exploratory. Two treatment groups are included—patients randomized to cladribine tablets 3.5 mg/kg followed by placebo (hereafter referred to as the CP3.5 group) or cladribine tablets 3.5 mg/kg followed by further treatment with cladribine (referred to as the CC7.0 group).
Patients were analyzed for NEDA-3 (defined as the composite of patients with no qualifying relapse, no confirmed 6-month EDSS progression, and no T1 Gd+ or active T2 lesions), and the individual components of the composite score, at 1 and 2 years of the double-blind period of CLARITY Extension for the CP3.5 and CC7.0 groups. Data were analyzed descriptively for patients in each group depending on the duration of bridging interval between studies (⩽48 weeks and >48 weeks).
As an exploratory analysis, differences in NEDA-3 at 1 and 2 years in CLARITY Extension were assessed between the CP3.5 and CC7.0 groups using logistic regression with treatment and bridging interval duration (continuous variable) as fixed effects. In addition, a logistic regression model with interactions between treatment and bridging interval duration was used to check if the treatment effect was maintained depending on bridging interval duration.
For NEDA-3, patients with no evidence of disease activity on some components and missing data on others are reported as unknown for descriptive analyses but excluded from logistic regression analyses. For individual components of NEDA-3, patients who withdrew early with no disease activity were considered as unknown.
Results
The demographics and characteristics on entry to CLARITY Extension for patients included in the present analysis are shown in Table 1. No patients received any DMT in the 3 months prior to CLARITY Extension, with two patients having received treatment with interferon beta during the bridging interval. Demographics and clinical characteristics were comparable between groups and the median bridging interval between studies were similar. Maximum bridging intervals were over 115 weeks for both groups.
Table 1.
Characteristics of patients included in the analysis at entry into CLARITY extension.
| CP3.5 mg/kg | CC7.0 mg/kg | |
|---|---|---|
| All patients (n = 98) | All patients (n = 186) | |
| Age, years | 40.7 (10.7) | 40.6 (10.5) |
| Female, n (%) | 67 (68.4) | 124 (66.7) |
| Disease duration, a years | 10.1 (6.7) | 10.4 (7.1) |
| Number of T1 Gd+ lesions | 0.27 (0.96) | 0.31 (1.56) |
| T2 lesion volume (103 mm3) | 18.6 (19.1) | 13.7 (14.4) |
| Median EDSS score (min; max) | 2.5 (0; 6.5) | 2.5 (0; 6.5) |
| Prior use of DMT at any time, n (%) | 18 (18.4) | 43 (23.1) |
| DMT use between CLARITY and CLARITY Extension, n (%) | 2 (2.0) | 0 (0) |
| DMT use in 3 months prior to CLARITY Extension, n (%) | 0 (0) | 0 (0) |
| Relapse between CLARITY and CLARITY Extension, n (%) | 9 (9.2) | 17 (9.1) |
| Median interval between studies (min; max), b weeks | 41.3 (0.1; 116.0) | 41.4 (0.4; 115.3) |
SD: standard deviation; EDSS: Expanded Disability Status Scale; DMT: disease-modifying therapy.
Data are mean (SD), unless otherwise stated.
Time from first attack to CLARITY Extension Study Day 1.
Duration of the gap between the last visit date in the CLARITY treatment period and the randomization date in CLARITY Extension.
CC7.0, patients randomized to cladribine tablets 3.5 mg/kg in both CLARITY and CLARITY Extension; CP3.5, patients randomized to cladribine tablets 3.5 mg/kg in CLARITY and placebo in CLARITY Extension; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; Gd+, gadolinium enhanced.
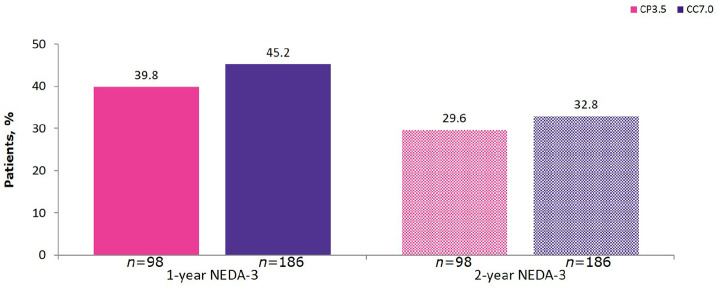
The proportion of patients with NEDA-3 at 1 year (the first year of CLARITY Extension) and 2 years (encompassing both years of CLARITY Extension) in each treatment group is shown Figure 2. Overall, the 2 year NEDA-3 in CLARITY Extension was 29.6% in the CP3.5 group and 32.8% in the CC7.0 group, showing no differences between patients receiving no additional courses of cladribine tablets or additional treatment in CLARITY Extension. Depending on bridging interval duration, this represents data from CLARITY Extension observation periods that could run from the beginning of Year 3 beyond Year 6 relative to CLARITY baseline (see Figure 1(b)).
Figure 2.
1 year and 2 year NEDA-3 in CLARITY Extension.
1 year and 2 year endpoints are from Week 48 and Week 96 of CLARITY Extension, respectively. NEDA-3 was defined as no qualifying relapse, no 6 month Expanded Disability Status Scale progression, and no T1 gadolinium-enhancing or active T2 lesions. CC7.0, patients randomized to cladribine tablets 3.5 mg/kg in both CLARITY and CLARITY Extension; CI, confidence interval; CP3.5, patients randomized to cladribine tablets 3.5 mg/kg in CLARITY and placebo in CLARITY Extension; NEDA-3, no evidence of disease activity.
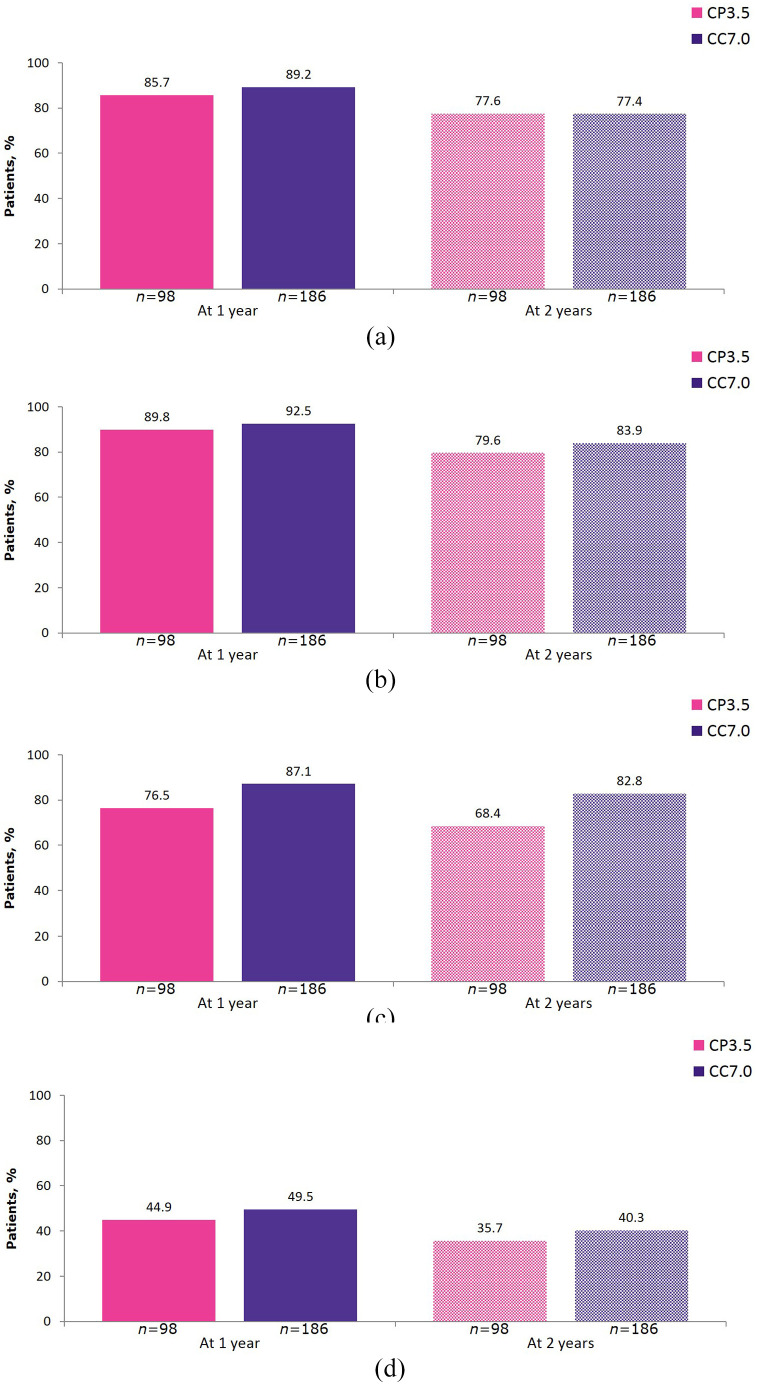
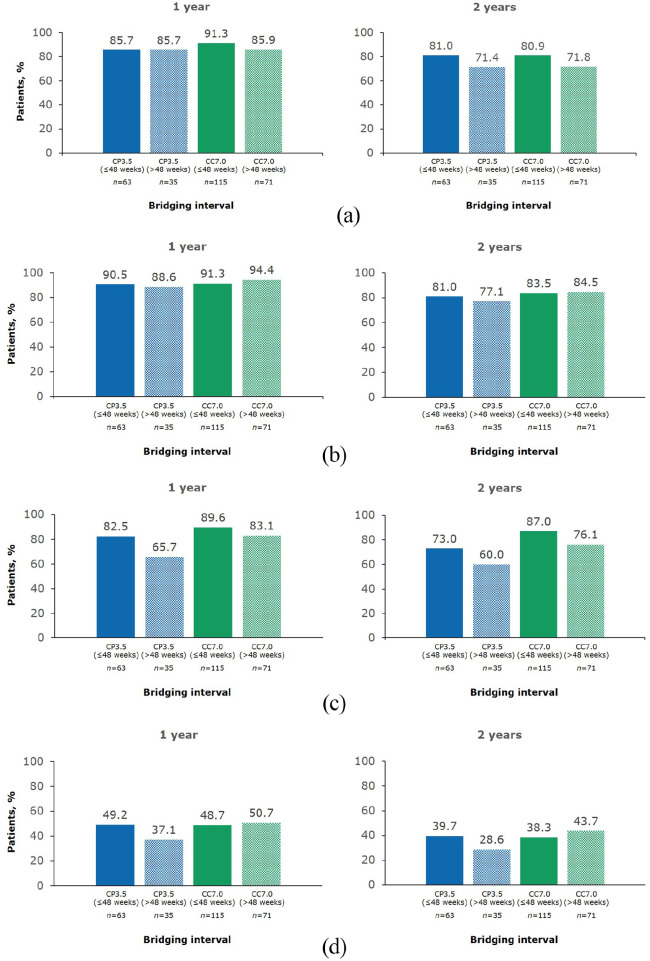
Results for the individual components of NEDA-3 at 1 and 2 years of CLARITY Extension in each treatment group are shown in Figure 3.
Figure 3.
Individual components of 1 year and 2 year NEDA-3 in CLARITY Extension. (a) Freedom from qualifying relapse; (b) Freedom from 6 month EDSS progression; (c) Freedom from T1 Gd+ lesions; and (d) Freedom from active T2 lesions.
1 year and 2 year endpoints are from Week 48 and Week 96 of CLARITY Extension, respectively. CC7.0, patients randomized to cladribine tablets 3.5 mg/kg in both CLARITY and CLARITY Extension; CP3.5, patients randomized to cladribine tablets 3.5 mg/kg in CLARITY and placebo in CLARITY Extension; EDSS, Expanded Disability Status Scale; Gd+, gadolinium enhancing; NEDA-3, no evidence of disease activity.
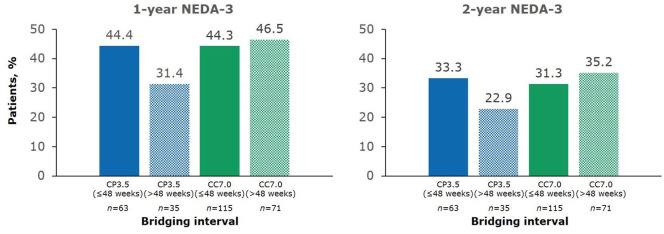
In the current analysis, a cut-off for bridging intervals of ⩽48 weeks or >48 weeks was chosen because this corresponds to 1 study year of the 96-week CLARITY and CLARITY Extension studies, and assessing outcomes in 1 year time frames is representative of following up a patient after receiving a full course of cladribine tablets. For patients who transitioned from cladribine tablets 3.5mg/kg to placebo (CP3.5 group) with a bridging interval of ⩽48 weeks, 1 year NEDA-3 was 44.4% (Figure 4). This represents data collected in Years 3 and 4 after the baseline of the original study. For patients in the CP3.5 group with a bridging interval of >48 weeks, 1 year NEDA-3 was 31.4% (Figure 4). This represents data collected in Years 4 and 5 after the baseline of the original study. The corresponding values for 1 year NEDA-3 in the CC7.0 group were 44.3% for patients with a bridging interval of ⩽48 weeks and 46.5% for patients with a bridging interval of >48 weeks (Figure 4). Findings for the individual components of NEDA-3, according to treatment group and duration of bridging interval, are shown in Figure 5. In the CP3.5 group, the proportion free from T1 Gd+ lesions, active T2 lesions, and relapses was numerically lower for patients with longer bridging intervals than those with shorter bridging intervals. In contrast, the proportions free from 6 month EDSS progression were numerically similar. Somewhat similar observations were made for the CC7.0 groups with respect to clinical outcomes and T1 Gd+ in patients with different bridging interval lengths. However, a numerically higher proportion of patients were free of new T1 Gd+ lesions in the CC7.0 than in the CP3.5. A similar proportion of patients were free or active T2 lesions irrespective of bridging interval length.
Figure 4.
1 year and 2 year NEDA-3 in CLARITY Extension according to bridging interval (⩽48 weeks or >48 weeks) between CLARITY and CLARITY Extension.
1-year and 2-year endpoints are from Week 48 and Week 96 of CLARITY Extension, respectively. NEDA-3 was defined as no qualifying relapse, no 6 month Expanded Disability Status Scale progression, and no T1 gadolinium-enhancing or active T2 lesions. Bridging interval is the time between completion of CLARITY and the start of CLARITY Extension. CC7.0, patients randomized to cladribine tablets 3.5 mg/kg in both CLARITY and CLARITY Extension; CP3.5, patients randomized to cladribine tablets 3.5 mg/kg in CLARITY and placebo in CLARITY Extension; NEDA, no evidence of disease activity.
Figure 5.
Individual components of 1 year and 2 year NEDA-3 in CLARITY Extension according to bridging interval (⩽48 weeks or >48 weeks) between CLARITY and CLARITY Extension. (a) Freedom from qualifying relapse; (b) Freedom from 6 month EDSS progression; (c) Freedom from T1 Gd+ lesions; and (d) Freedom from active T2 lesions.
1 year and 2 year endpoints are from Week 48 and Week 96 of CLARITY Extension, respectively. Bridging interval is the time between completion of CLARITY and the start of CLARITY Extension. CC7.0, patients randomized to cladribine tablets 3.5 mg/kg in both CLARITY and CLARITY Extension; CP3.5, patients randomized to cladribine tablets 3.5 mg/kg in CLARITY and placebo in CLARITY Extension; NEDA, no evidence of disease activity.
Overall, there was no evidence that the treatment effect on NEDA-3 differed with bridging interval duration (p value <0.1 for the treatment by bridging interval duration interaction effect). There was also no effect of bridging interval duration on NEDA-3 results when looking at the p value for bridging interval duration covariate in the model without interaction.
Discussion
NEDA-3 is a relevant outcome that provides useful information and a treatment target for physicians managing patients with relapsing-remitting MS. In particular, a key consideration with NEDA-3 is what happens to this measure over time. The analysis of NEDA-3 after more than 2 years from the start of DMT has been reported in relatively few MS studies, such as the 5-year follow-up from CARE-MS I and CARE-MS II with alemtuzumab14,15 and the 3-year follow-up with dimethyl fumarate. 16 The relevance of looking at NEDA in Years 3 and 4 after starting treatment with cladribine tablets is that patients would not normally receive any DMTs during this time, according to the recommended dosing. 8 It is expected that patients would be regularly monitored by their physicians during Years 3 and 4, and many might judge treatment success by the achievement of NEDA or a reduction in disease activity compared to a period before cladribine tablets were commenced.
The overall results from the current post hoc analysis shows a similar level of NEDA-3 in CLARITY Extension for patients randomized to placebo or additional courses of cladribine tablets. This confirms the original finding from CLARITY Extension of the sustained effect of two annual courses of cladribine tablets, with no significant incremental benefit from further courses of treatment during the extension phase, based on annualized relapse rates. 12 However, overall NEDA-3 in CLARITY Extension represents data over a 2-year period, captured between 3 and 6 years from CLARITY baseline depending on the length of the bridging interval between the core and extension studies. By restricting the observation to Years 3 and 4 from CLARITY baseline, through a combination of bridging interval of 1 year or less and using NEDA over 1 year, NEDA-3 occurred in ~44% of patients randomized to receive placebo or additional cladribine tablets during this time.
One-year NEDA-3 rates with a bridging interval of >48 weeks and 2-year NEDA-3 with a bridging interval of >48 weeks represent data collected progressively later in time from the baseline of CLARITY, with increasing data obtained after Year 4. Numerically, progressively fewer patients achieved NEDA-3 beyond Year 4 if no further courses of cladribine tablets were given in CLARITY Extension. Such an effect was not observed in patients who did receive further courses of cladribine tablets. Examination of the individual components of the composite measure indicates that this effect was mostly related to changes in MRI parameters, which are more sensitive, with less change in clinical parameters. Overall, NEDA-3 at 2 years in CLARITY Extension was comparable for the CP3.5 and CC7.0 groups, which argues against the need for further treatment in Years 3 and 4. However, for patients with a >48-week bridging interval, NEDA-3 was numerically lower in the CP3.5 group compared with the CC7.0 group. Over time, we may therefore identify a subpopulation of patients with new disease activity that require further treatment after having received no treatment with cladribine tablets in Years 3 and 4.
The main limitation of our study is that this was a post hoc analysis of NEDA-3 occurring over 1- or 2 year periods in an extension study, with no correction for patients who had or had not achieved NEDA-3 during the CLARITY study. However, this timing mirrors the usual periodic monitoring utilized during the routine care of people with MS. In addition, the number of patients entering CLARITY Extension from the preceding CLARITY study was reduced, 12 and this should be considered when interpreting the results by bridging interval as the patient group sizes may make it difficult to draw any firm conclusions. Despite this, a recent post hoc analysis of patients in CLARITY showed that most baseline characteristics were similar for those who entered and those who did not enter CLARITY Extension suggesting that the study did not preferentially enroll patients with either less severe disease than at CLARITY baseline, or a greater treatment response to cladribine tablets during CLARITY. 17 Furthermore, the few patients going directly from CLARITY to the CLARITY Extension study without a bridging interval of < 1 month meant that it was not possible to provide data for yearly NEDA-3 and its components for a sufficient number of patients to allow a meaningful analysis, and this necessitated the current analytical approach. Finally, NEDA-3 data for the full study period (CLARITY and CLARITY Extension combined) are not available, because of the treatment switch at the beginning of CLARITY Extension and the fact that MRI and EDSS data were not collected during the bridging interval. Different MRI protocols were used during the CLARITY and CLARITY Extension studies, and there were also differences in individual patient observation periods due to the varying length of the bridging interval. NEDA-3 is an endpoint that is defined for a pre-defined observation period (usually 2 years or shorter) with a standardized measurement schedule. It does not offer any corrections for variations of measurement schedule or patient’s observation period.
Conclusion
In this post hoc analysis, patients treated in CLARITY with cladribine tablets 3.5 mg/kg and with either placebo or cladribine tablets 3.5 mg/kg in CLARITY Extension experienced sustained effects for NEDA-3 and its constituent elements. Overall, the duration of the bridging interval between CLARITY and CLARITY Extension did not have a significant effect on NEDA-3 outcomes. However, there was a tendency that the proportion of patients with NEDA-3 in the CP3.5 group with a longer bridging interval was lower compared to the corresponding CC7.0 group. This leads to the question of whether further treatment courses can be administered to patients after the initial course of treatment, if required. An expert opinion using the Delphi technique did not exclude additional courses of cladribine tablets in patients experiencing new or reappearing disease activity after the full cladribine tablets courses in Years 1 and 2. 18 These results confirm the original finding from CLARITY Extension of the sustained effect of two annual courses of cladribine tablets 3.5 mg/kg with no incremental benefit from further courses of treatment in Years 3 and 4.
Acknowledgments
The authors would like to thank patients and their families, investigators, co-investigators, and the study teams at each of the participating centers and at Merck Healthcare KGaA, Darmstadt, Germany. Medical writing assistance was provided by Mark O’Connor and Claire Mwape of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Merck Healthcare KGaA, Darmstadt, Germany. Statistical analysis was performed by Cytel Inc., Geneva, Switzerland, and was funded by Merck Healthcare KGaA, Darmstadt, Germany.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.G. has received speaker honoraria and consulting fees from AbbVie, Actelion (Janssen/J&J), Atara Bio, Almirall, Bayer, Biogen, Celgene (BMS), FivePrime, GlaxoSmithKline, GW Pharma, Ironwood, Merck & Co., Novartis, Merck, Pfizer Inc., Protein Discovery Laboratories, Roche, Sanofi-Genzyme, Teva Pharmaceutical Industries Ltd, UCB, and Vertex Pharmaceuticals; and has received research support unrelated to this study from Biogen, Ironwood, Merck & Co., Novartis, Merck, and Takeda. B.A.S. has received research support from AbbVie, Alkermes, Biogen, MedImmune, Novartis, Sanofi-Genzyme, and Roche; and speaker honoraria and/or consulting fees from AbbVie, Alexion, Bayer, Biogen, Bristol Myers Squibb, EMD Serono, Inc. (an affiliate of Merck KGaA), Genentech, Greenwich, Janssen, Novartis, Roche, Sanofi-Genzyme, Teva, and TG Therapeutics. D.I. is an employee of Cytel Inc., Geneva, Switzerland. D.J. is an employee of Merck Serono Ltd, Feltham, UK (an affiliate of Merck KGaA). P.V. has received honoraria or consulting fees from AB Science, Biogen, Celgene (BMS), Imcyse, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva; and research support from Novartis, Roche, and Sanofi-Genzyme.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Merck (CrossRef Funder ID: 10.13039/100009945).
Data availability: Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
Contributor Information
Gavin Giovannoni, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Barry A Singer, The MS Center for Innovations in Care, Missouri Baptist Medical Center, St Louis, MO, USA.
Delphine Issard, Department of Biostatistics, Cytel Inc., Geneva, Switzerland.
Dominic Jack, Global Medical Affairs, Neurology and Immunology, Merck Serono Ltd, Feltham, UK (an affiliate of Merck KGaA).
Patrick Vermersch, Univ. Lille, Inserm U1172 LilNCog, CHU Lille, FHU Precise, Lille, France.
References
- 1. Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: A retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 2009; 8(3): 254–260. [DOI] [PubMed] [Google Scholar]
- 2. Giovannoni G, Turner B, Gnanapavan S, et al. Is it time to target no evident disease activity (NEDA) in multiple sclerosis. Mult Scler Relat Disord 2015; 4(4): 329–333. [DOI] [PubMed] [Google Scholar]
- 3. Bevan CJ, Cree BA. Disease activity free status: A new end point for a new era in multiple sclerosis clinical research. JAMA Neurol 2014; 71(3): 269–270. [DOI] [PubMed] [Google Scholar]
- 4. Banwell B, Giovannoni G, Hawkes C, et al. Editors’ welcome and a working definition for a multiple sclerosis cure. Mult Scler Relat Disord 2013; 2(2): 65–67. [DOI] [PubMed] [Google Scholar]
- 5. Lublin FD. Disease activity free status in MS. Mult Scler Relat Disord 2012; 1: 6–7. [DOI] [PubMed] [Google Scholar]
- 6. Stangel M, Penner IK, Kallmann BA, et al. Towards the implementation of “no evidence of disease activity” in multiple sclerosis treatment: The multiple sclerosis decision model. Ther Adv Neurol Disord 2015; 8(1): 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kappos L, De Stefano N, Freedman MS, et al. Inclusion of brain volume loss in a revised measure of “no evidence of disease activity” (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler 2016; 22(10): 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MAVENCLAD 10 mg tablets SmPC. 2021. https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf
- 9. Giovannoni G. Cladribine to treat relapsing forms of multiple sclerosis. Neurotherapeutics 2017; 14(4): 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: A post-hoc and subgroup analysis. Lancet Neurol 2011; 10(4): 329–337. [DOI] [PubMed] [Google Scholar]
- 11. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 12. Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult Scler 2018; 24(12): 1594–1604. [DOI] [PubMed] [Google Scholar]
- 13. Comi G, Cook S, Rammohan K, et al. Long-term effects of cladribine tablets on MRI activity outcomes in patients with relapsing-remitting multiple sclerosis: The CLARITY Extension study. Ther Adv Neurol Disord 2018; 11: 753365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: Efficacy and safety findings. Neurology 2017; 89: 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy. Neurology 2017; 89: 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pilo de la Fuente B, Sabín J, Galán V, et al. Three-Year effectiveness of dimethyl fumarate in multiple sclerosis: A prospective multicenter real-world study. CNS Drugs 2020; 34(12): 1275–1286. [DOI] [PubMed] [Google Scholar]
- 17. Comi G, Soelberg-Sørensen P, Rammohan K, et al. P059: Efficacy outcomes in cladribine tablets-treated patients in CLARITY were similar between patients who did vs. Did not enter CLARITY extension. Mult Scler 2020; 26: 43. [Google Scholar]
- 18. Soelberg Sørensen P, Centonze D, Giovannoni G, et al. Expert opinion on the use of cladribine tablets in clinical practice. Ther Adv Neurol Dis 2020; 13: 935019. [DOI] [PMC free article] [PubMed] [Google Scholar]