Abstract
For all primary cutaneous squamous cell carcinomas (cSCCs), physical examination should include full skin examination, recording of tumour diameter and regional lymph-node–basin status. Surgery is the treatment of choice, with a minimal 5-mm margin. For elderly patients with well-differentiated tumours, other surgical modalities can be explored. Surgery for organ-trans-plant recipients should not be delayed. The issue with cSCC is identifying high-risk tumours with staging, as this may alter treatment and follow-up schedules. Adjuvant radiation therapy should be considered for incomplete resection, when re-excision is impossible or there are poor-prognosis histological findings. Recommendations are at least biannual dermatological surveillance for 2 years, but in elderly patients with small, well-differentiated tumours long-term follow-up is not always necessary. In case of positive lymph nodes, radical dissection is needed, with regional postoperative adjuvant radiation. Advanced cSCCs are defined as unresectable local, regional or distant disease requiring systemic treatment. Their only approved treatment is the PD-1 inhibitor, cemiplimab. Trials evaluating adjuvant or neo-adjuvant anti-PD-1 are ongoing. Platin-based chemo or anti-epidermal growth-factor–receptor therapies are possible second-line treatments. For transplant patients, minimizing immunosuppression and switching to sirolimus must be considered at first appearance of cSCC.
Key words: cutaneous squamous cell carcinoma, anti-PD-1, adjuvant treatment
Historically, cutaneous squamous cell carcinoma (cSCC) was the second most common skin cancer after basal cell carcinoma (BCC), but several recent reports on the Australian and US populations have shown a shift in the numbers of cSCCs compared with BCCs. A study of Medicare patients shows a 1:1 ratio of cSCC to BCC (1). The incidence of cSCC has increased markedly over recent decades worldwide, probably because very early cSCC are being resected more often, but also because of increased exposure to the sun (1). cSCC frequency quadrupled for both sexes in Sweden between 1960 and 2004 (2). cSCCs often occur in elderly and male patients. The main risk factors for developing cSCCs are chronic cumulative exposure to ultraviolet (UV), including sunbed use and psoralen and ultraviolet A (UVA), having fair skin or hair, and taking immunosuppressive medication for ≥ 1 month (3–5). Immunocompromised patients, including organ-transplant recipients and human immunodeficiency virus (HIV)-positive patients, are at increased risk of cSCC (6, 7). cSCCs are the most common cancers following organ transplantation, with their risk increasing 100-fold for transplantees (6, 8–10). Oncogenic human papillomavirus, chronic scarring conditions, exposure to arsenic or ionizing radiation, recessive dystrophic epidermolysis bullosa and rare familial syndromes (e.g. xeroderma pigmentosum, albinism and Lynch syndrome) have also been associated with increased risk of cSCCs. Ageing of the population, more organ-transplant recipients, change in attitude toward UV exposure, and increased ascertainment contribute to the increase in incidence of cSCC.
SIGNIFICANCE
This review updates the management of primary resectable cutaneous and advanced cutaneous squamous cell carcinomas. It is important for physicians treating cutaneous squamous cell carcinoma to know that currently available staging systems can help identify high-risk tumours and should guide work-up and treatment. This article describes risk factors and staging methods, along with an overview of current treatments according to disease stage.
Although initial surgical excision cures 95% of patients, a minority of cSCCs recur locally (3–4%) or metastasize (2–4%), usually to regional lymph nodes or, rarely, to distant locations (11, 12). In addition, 1–4% of cSCCs are fatal (13, 14). cSCC-attributed mortality is increasing in Australia. The mortality rate in the southern and central USA approached that of melanoma, emphasizing that cSCC is a critical public health concern (15). Awareness of risk factors for cSCC is essential to improve primary prevention with the objective of containing, and hopefully lowering, the increasing incidence of cSCC. Thus, because sunscreens can prevent cSCC (16), its use should be strongly encouraged, and use of sunbeds should be strongly discouraged. Moreover, high-risk patients, i.e. immunocompromised patients, should undergo regular dermatological monitoring and education about skin self-examination and safe behaviour in the sun.
Application of the currently available staging systems helps to identify patients at high risk of recurrence. The American Joint Committee on Cancer staging 8th edition (AJCC-8) (11) tumour-staging items include tumour diameter, as summarized in Fig. 1a. Lymph-node size, number of positive lymph nodes and their location(s) (ipsilateral, contralateral, bilateral) and extranodal extension. However, the AJCC-8 is relevant only for head-and-neck cSCCs, which might limit its usefulness. The Brigham and Women’s Hospital (BWH)-staging system (17) is based on the presence of 4 risk factors, summarized in Fig. 1b. BWH stage T3 represents only 5% of tumours, but 70% of nodal metastases and 83% of disease-specific deaths. A recent monocentre retrospective study on 186 head-and-neck cSCCs (18) compared the 2 systems and found an overlapping of poor-prognosis predictions.
Fig. 1.
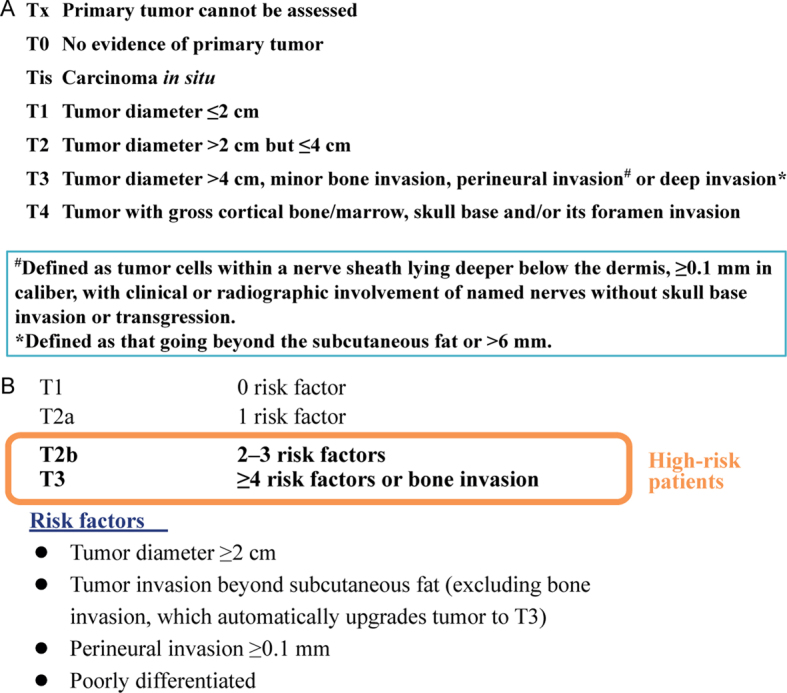
Cutaneous squamous cell carcinoma – staging criteria. (a) American Joint Committee on Cancer 8th edition staging of head-and-neck tumours (adapted from (11). (b) Brigham and Women’s Hospital tumour-staging items (adapted from (17)).
Several other poor-prognosis risk factors are not included in these classifications: high-risk locations (lip, ear), histological thickness or Clark level ≥IV, desmoplastic and adenosquamous histological subtypes or immunosuppression. Organ-transplant recipients’ cSCCs are often aggressive tumours and in view of the presence of multiple viral warts in these patients, which may be difficult to differentiate from early SCC, it is recommended that dedicated dermatology clinics look after these high-risk patients, if possible.
High-risk cSCCs have a higher recurrence, estimated at 16%. Recurrences occur mainly during the first 2 years post-diagnosis (19). However, a review of the literature showed that, for patients with high-risk cSCCs and clearly documented surgical margins, risks of local recurrence, regional metastasis, distant metastasis and disease-specific death were 5%, 5%, 1% and 1%, respectively (20).
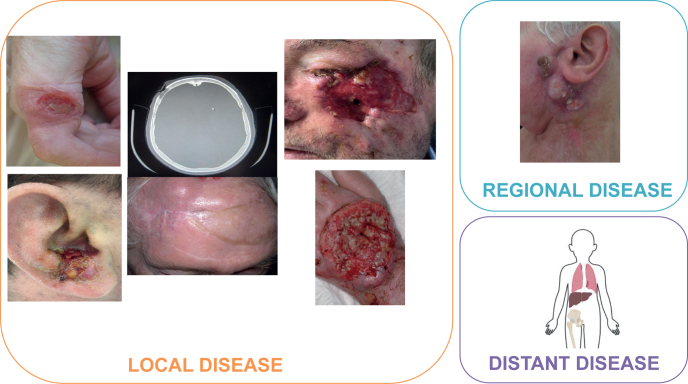
Advanced cSCCs are defined as either locally unresectable, deeply invasive involving muscle, nerve or bone structures, unresectable regional lymph-node disease or multiple distant metastases requiring systemic curative treatment (Fig. 2).
Fig. 2.
The different types of advanced cutaneous squamous cell carcinomas. Local disease (left): local unresectable disease without regional or distant disease. Regional disease (top right): at least regional unresectable disease without distant disease. Distant disease (bottom right): at least one unresectable distant metastasis.
MANAGEMENT OF PRIMARY RESECTABLE CUTANEOUS SQUAMOUS CELL CARCINOMAS
Physical examination and biological staging
Staging should systematically include primary tumour diameter, regional lymph-node–basin status, and search for other skin cancers and chronic inflammatory disorders and previous or current immunosuppression. Rare genetic syndromes, such as xeroderma pigmentosum, albinism and Lynch syndrome have to be ruled out in patients who have early onset and/or multiple cSCC without obvious risk factors.
Imaging studies for staging
Because few studies have addressed cSCC imaging, its value for regional and distant staging is uncertain, even for high-risk cSCCs (21). A meta-analysis of head-and neck tumours evaluating the contributions of computed tomography (CT) scans, magnetic resonance imaging (MRI), ultrasonography (US) and US-guided fine-needle aspiration showed that the last accuracy was the best (22). Ultrasound scanning with fine needle aspiration cytology was found superior to CT in assessing primary SCC of the vulva regional disease status (23). Based on a retrospective series of 98 high-risk patients with BWH-stage T2b or T3 cSCCs, with imaging staging (CT, positron-emission tomography (PET–CT scans or MRI) or without, imaging impacted cSCC management for one-third of them; moreover, patients without imaging staging tended to develop nodal metastases more frequently (p = 0.046) (24). Prospective studies are needed to confirm that an initial imaging work-up can impact management and outcomes, and that imaging should be considered for regional staging in high-risk patients. In 2020, the European Dermatology Forum (EDF), European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC) (EDF–EADO–EORTC) consensus group recommended lymph-node US for high-risk patients (25).
Surgery
Biopsy or limited excision of the tumour is usually performed to confirm a clinically suspected cSCC, but if the tumour is small, a single definitive excision is often performed outright with various margins. Surgery is the treatment of choice. Most primary resectable cSCCs are usually cured by conventional excision. Mohs surgery may be needed for high-risk tumours and/or difficult anatomical sites. Randomized controlled trials on resection-margin widths are lacking, therefore excision margins for SCC are controversial.
Excellent cure rates have been reported in several series. Experience suggests that small well-differentiated tumours, which are slow-growing in elderly patients on sun-exposed sites can be removed by experienced physicians with curettage (http://www.bad.org.uk/healthcare-professionals/clinical-standards/clinical-guidelines). Recurrences were rare in a study on 1,174 cSCC patients and did not differ significantly among tumours treated with electrodessication/curettage destruction, excision or Mohs surgery, respectively: 24.3% of 361 vs. 38.3% of 571, or 37.4% of 556 (26).
The EDF–EADO–EORTC consensus group has recommended surgical resection with a minimal 5-mm margin, even for low-risk tumours, which should be extended to 10 mm for high-risk tumours (Table I) when additional clinical or histological risk factors are present (25). When technically feasible, 1-step resection and closure is preferred; 2-step resection is recommended when a graft or flap reconstruction is planned. If the resection is incomplete, then surgical re-excision should be performed.
Table I.
Summary of treatment options
| Treatment options |
|---|
| Primary resectable cSCCs |
| Surgical resection (5–10 mm margin) |
| Alternative: curative radiation therapy |
| Alternative for low risk small tumours on sun exposed sites: 2 cycles curettage and cautery |
| Adjuvant treatment for primary high-risk cSCCs* |
| Radiation therapy |
| Ongoing immunotherapy trials |
| Neoadjuvant treatment |
| Ongoing immunotherapy trials |
| cSCCs with regional lymph node involvement |
| Radical lymph-nodes dissection |
| Adjuvant radiation therapy |
| Advanced cSCCs |
First line:
|
| Second line: |
Cisplatin-based chemotherapies
|
| Prevention |
| Topical treatments |
| 5% 5-FU cream |
| Alternatives: imiquimod, diclofenac and photodynamic therapy |
| Oral treatments |
| Acitretin, nicotinamide |
| Primary cSCCs in transplant recipients |
| Minimizing immunosuppression and switching to sirolimus |
Incomplete resection, poor-prognosis histological findings.
cSSC: cutaneous squamous cell carcinoma.
In an earlier prospective, multicentre Australian case series of 1,263 cSCC patients, characterized by an elevated percentage of high-risk tumours treated with Mohs micrographic surgery, 5-year recurrence rates were low: 2.6% in patients with primary cSCCs and 5.9% in patients with locally recurrent cSCC, suggesting that this technique achieves a high cure rate for these high-risk cSCCs (12). However, randomized studies comparing Mohs surgery with conventional surgery are lacking.
The pathologist’s report should specify histological differentiation grade, histological subtype, maximum tumour thickness and Clark level, invasion of muscle, cartilage, bone and/or fascia, perineural or lymphatic/ vascular invasion, whether or not the resection was complete with minimal lateral and deep margins.
For high-risk cSCCs with negative regional staging on imaging, a sentinel lymph-node biopsy might be considered an option, but is not standard of care, depending on its potential comorbidities. Indeed, sentinel lymphnode biopsies are positive for one-third of the patients with BWH stage-T2b or -T3 cancers (27). However, the authors of a recent prospective German study found that 6% of a series of sentinel lymph-node-negative patients had distant metastases, suggesting the limited prognostic value of the procedure (28).
Curative radiation therapy
Radiotherapy represents an alternative to primary surgical resection for SCC of the lip and when surgery is not appropriate for cSCCs. However, the risk of cSCC recurrence is higher after radiation therapy compared with surgery. For patients with comorbidities that predispose them to radiation-induced cancers, such as basal cell naevus syndrome or xeroderma pigmentosum, radiotherapy must be avoided. Radiation therapy can cause reversible dermatitis or mucositis. Late side-effects include skin atrophy with loss of hair, reduced sweating and sebaceous secretions, discoloration, telangiectasia, hypodermic sclerosis and/or skin carcinomas so should be avoided in younger patients (29).
Adjuvant radiation therapy for primary high-risk cutaneous squamous cell carcinoma
According to a literature review on cSCCs with perineural invasion treated with surgery (n = 30) or surgery plus adjuvant radiation therapy (n = 44 cases), outcomes were comparable (20). The role of adjuvant radiation therapy for high-risk cSCCs, including those with perineural invasion, remains controversial. However, authors of a recent retrospective study on adjuvant radiation therapy for cSCCs with perineural invasion found it to be associated with prolonged survival (30), suggesting that such patients might benefit from adding radiation to surgery and decisions have to be made on a case-by-case basis.
Other adjuvant or neoadjuvant strategies for primary high-risk cutaneous squamous cell carcinoma
No significant differences were found for retinoic acid and interferon vs. placebo for the time to recurrence or occurrence of second primary cSCCs in patients with high-risk cSCCs enrolled in a randomized phase-3 trial (31).
O’Bryan et al. prescribed adjuvant cetuximab for 7 patients with high-risk cSCCs (32); only 3 experienced disease recurrence. Neoadjuvant gefitinib therapy in a phase-2 study on 22 patients achieved a 45% response rate, including 3 histological complete responses (CRs) (33). However, disease progressed for 32% and the lack of known biomarkers of response highlights the need for further larger studies, including randomized trials. Jenni et al. (34) more recently reported size reduction after 14 days of lapatinib in 2 out of 8 assessable patients, among 10 with resectable cSCCs.
A recent phase-2 study (35), presented at European Society for Medical Oncology (ESMO) 2019, showed that cemiplimab neoadjuvant therapy given to 20 patients induced histological partial responses (PRs) or CRs in 70% of the patients. Moreover, it was well-tolerated. Ongoing trials are evaluating the potential contribution of anti-programmed cell-death protein-1 (PD-1) agents as adjuvant therapy for high-risk cSCCs.
Monitoring
The majority of all recurrences of cSCC occur within 2 years of the initial diagnosis. In high-risk cSCCs the follow up should be at least 2 years and should include palpation of the primary excision site and of the regional lymph node area every 3 or 6 months depending on the initial stage and medical history. Moreover, the entire skin of all patients should be examined once annually or every 6 months in high-risk cSCCs patients (immunosuppression, multiple primary cSCCs, genetic predisposition) as recommended by the current European guidelines (25). However, in elderly patients with small well-differentiated SCC on sun-exposed sites (excluding high-risk sites, such as lips, ears, digits and mucosa), discharge after 3 months is possible.
MANAGEMENT OF CUTANEOUS SQUAMOUS CELL CARCINOMAS WITH REGIONAL LYMPHNODE INVOLVEMENT
Histological examination of fine-needle aspirates or resections of any enlarged nodes is mandatory. Available results of studies on lymph-node involvement of head-and-neck cSCCs indicated positive lymph nodes as a negative factor for survival (36, 37). Extracapsular lymph-node spread is a significant risk factor for recurrence. The most frequently involved lymph-node region is around the parotid. Disease stage should be assessed by imaging studies, including CT or PET–CT scan(s) or MRI. When lymph nodes are histologically positive, they should be subjected to radical dissection. Postoperative adjuvant radiation delivered to the affected lymph-node region is required for head and neck tumours, as it enhances local–regional control and disease-free survival (DFS) and overall survival (OS) of those patients (30).
MANAGEMENT OF ADVANCED CUTANEOUS SQUAMOUS-CELL CARCINOMAS
The PD-1 inhibitor, cemiplimab, is the only approved agent for locally advanced and metastatic cSCCs. Prior conventional treatment for advanced cSCCs, such as cisplatin-based chemotherapies or epidermal growth-factor receptor (EGFR)-targeted therapies, can be used as second-line treatments. Trials evaluating other anti-PD-1 molecules and combinations of anti-PD-1 with other drugs are currently ongoing.
A retrospective study in Europe, completed just before anti-PD-1 became available, described various treatments for patients with advanced cSCCs (38). Among 190 patients (median age 79 years) with locally advanced or metastatic disease, 32% received systemic anti-tumour therapies (excluding anti-PD1), mostly anti-EGFR tyrosine-kinase inhibitors. Half of the patients did not complete systemic therapy as planned. The objective response rate (ORR) was 26% and the mean response duration was 5 months. Among the 152 patients whose survival status was known, 49% had died. The availability of anti-PD-1 agents might allow access to treatment for more patients with cSCC.
Anti-programmed cell-death protein-1
The immune system is important for cSCC, as suggested by the increased risk of cSCCs in transplant recipients (39), the rapid regression of keratoacanthoma, which is characterized by a more active immune response than generally seen in cSCCs (40), and activity of immunotherapy in advanced SCC as combination of interferon and retinoic acid (41). The PD-1 receptor is expressed on T cells, and T cells binding to its ligand (PD-L1) inhibit T-lymphocyte functions. PD-L1 is expressed in 30–50% of cSCCs and its expression was found to correlate with risk of metastases (42). The high mutation rate in cSCCs, as in other UV-induced tumours, is usually a predictor of responsiveness to anti-PD-1 (43).
Cemiplimab (3 mg/kg every 2 weeks) induced a response in approximately half of the 85 patients enrolled in a phase-2 study with locally, regional or distant disease and a phase-1 study with regional or distant disease (44). Those patients were treated, respectively, for up to 48 weeks and up to 96 weeks. Fifty-six to 58% of the patients had received systemic treatment before cemiplimab. Median phase-1 and phase-2 follow-ups were: 11 and 8 months, respectively. Their respective ORRs were 50% and 47%. Median time to response was 2 months for both. In the phase-2 trial, 7% were CRs; median progression-free survival (PFS) and OS had not been reached and median duration of response exceeded 6 months for 16/28 (57%) responders. The most common adverse reactions were fatigue, rash and diarrhoea. Serious adverse events were immune-mediated, such as pneumonitis, hepatitis, colitis, adrenal insufficiency, dysthyroidism, diabetes mellitus and/or nephritis, and, unlike other anti-PD-1 inhibitors, infusion reactions. Treatment was stopped for 7% of patients because of adverse events. Three cemiplimab-related deaths were reported (44). Cemiplimab was approved by the US Food and Drug Administration (FDA) in September 2018 and European Medicines Agency (EMA) in July 2019 for patients with metastatic or locally advanced cSCCs who were not candidates for curative surgery or radiation. The recommended cemiplimab dose and schedule is now 350 mg, infused intravenously over 30 min every 3 weeks. Factors predictive of response are still unknown. Treatment duration needs to be better defined.
Several trials have also assessed pembrolizumab in cSCCs. Interim results of the Keynote 629 study evaluating pembrolizumab (200 mg/3 weeks IV) in advanced cSCC have been presented at the ESMO meeting in 2019 (45). Response rate was 32% in 91 patients receiving pembrolizumab as a second-line treatment and 50% in 14 naïve patients. The median duration of response was not reached. The safety profile was consistent with that of other pembrolizumab monotherapy studies. Interim analysis of the CARSKIN study presented at the ASCO 2019 meeting, showed a response rate of 38.5% in 39 previously untreated patients with advanced cSCC with sustained responses to pembroluzimab (46).
Platin-based chemotherapies
Few prospective trials are available and no treatment regimen has been recommended by health authorities. Because their ORRs are high, platin-based chemotherapies were the first-choice treatment before the anti-PD-1 era, but their administration can be limited by cisplatin toxicity or disease recurrence during treatment. Sadek et al. (47) treated 14 advanced cSCC patients with 1–4 cycles, repeated every 3–4 weeks, of neoadjuvant combination chemotherapy (bolus cisplatin injection, 5-fluorouracil (5-FU) and continuous 5-day bleomycin infusion). The ORR was 78% (4 CRs, 7 PRs). Local control after adjuvant radiation and/or surgery was achieved in 7 (50%) patients. CR lasted >10 months. All patients experienced major toxicities, including grade-3/4 nausea and vomiting; 4 patients had grade-3/4 haematological toxicities and one developed pulmonary fibrosis. In their prospective phase-2 trial, Guthrie et al. treated advanced BCC or locally advanced cSCC patients with cisplatin (75 mg/m2 and doxorubicin 50 mg/m2, every 3 weeks) (48). Among the 12 advanced-cSCC patients, 7 responded (4 CRs and 3 PRs). Based on 7 patients with advanced local-regional or metastatic cSCCs, Khansur et al. reported the activity of cisplatin (100 mg/m2 on day 1) and 5-FU (1 g/m2/day, days 1–4), given every 3 weeks. Six of 7 patients were responders: 3 PRs and 3 CRs (49). The mean duration for CR was one year. Toxicities included grade-1/2 nausea and vomiting. Carboplatin-combination therapy is better tolerated and can be administered as an alternative to patients with comorbidities. Hyperthermic isolated-limb perfusion can be a second-line limb-saving therapy for patients with unresectable disease located on the extremities (50).
Epidermal growth-factor receptor-targeted therapies
EGFR represents a family of proteins, including EGFR and human epidermal growth factor receptor (HER)-2, 3 and 4. Activation of EGFR tyrosine kinase results in autophosphorylation and activation of RAS serine/threonine kinase, murine sarcoma viral oncogene (RAF), mitogen-activated protein (MAP) kinase and phosphatidylinositol 3-kinase (PI3K), AKT protein kinase and mammalian target of rapamycin (mTOR) pathways leading to tumour growth. EGFR is strongly expressed in metastatic cSCCs and its overexpression in primary cSCCs is associated with poor outcome (18). Anti-EGFR therapy consists of monoclonal antibodies, such as cetuximab or panitumumab, which competitively inhibit EGFR, or small molecules, e.g. gefitinib or erlotinib, targeting the intracellular domain of the receptor. EGFR-targeted therapies have been developed and obtained promising ORRs in several clinical trials and retrospective studies on patients with unresectable cSCCs. So far, phase-3 trial results have not yet confirmed their efficacy against cSCCs. Anti-EGFR tyrosine-kinase inhibitors are not approved to treat advanced cSCCs, but cetuximab is listed in the National Comprehensive Cancer Network (NCCN) compendium as a therapy for recurrent and metastatic CSSCs. No biomarker predictive of a cSCC response has been identified.
Cetuximab was evaluated prospectively as first-line monotherapy in a French phase-3 study on 36 patients with metastatic (n = 3), regional (n = 16) or locally advanced (n = 17) cSCCs. The ORR was 28%, including 2 CRs and 8 PRs, and the overall disease-control rate was 69% (25/36 patients). Median PFS lasted 4 months. The median duration of response was 7 months and the mean OS was 8 months. The more frequent severe adverse events were infections (22%) and tumour bleeding (11%). Cetuximab-related adverse events included 2 grade-4 infusion reactions and 1 grade-3 interstitial pneumopathy (51). Cetuximab can be combined with platin-based chemotherapies and this combination might prolong PFS (9.03 vs. 3.55 months), according to a retrospective series of 14 patients treated with cetuximab monotherapy or cetuximab combined with carboplatin (52). Low-grade specific acne-like rash, pruritus and nail changes have been observed. Severe infusion reactions occurred in 3% of patients.
Panitumumab efficacy (6 mg/kg, repeated every 2 weeks) was of the same order of magnitude for 11 Italian patients with advanced penile SCC (53) and 16 Australian patients with advanced cSCC enrolled in a phase-2 study (54). Median PFS and OS, respectively, were 8 and 11 months for cSCC patients, and 2 and 9 months for those with penile SCC. Severe skin rash, mucositis and diarrhoea occurred.
Efficacy of oral small molecules against advanced cSCCs was variable, with ORR of 10–32%. Based on available phase-2 studies, gefitinib or erlotinib alone obtained only poor ORRs of 15% (6/40 patients) and 7% (3/39 patients), respectively (55, 56). Higher ORRs, of the same order of magnitude as those achieved with monoclonal antibodies, were obtained with second-generation irreversible pan-HER tyrosine-kinase inhibitors, such as dacomitinib: in 28% of cSCCs and 32% (9/28 patients) of penile SCC (57, 58). The tolerance profile of small molecules differed, with more diarrhoea and mucositis than with antibodies.
Concurrent radiotherapy with cetuximab did not significantly prolong PFS and OS compared with concurrent radiotherapy and cisplatin-based chemotherapy in a retrospective series of 23 patients with head-and-neck cSCCs (59).
Further prospective studies are needed to determine the characteristics of patients who would benefit from anti-EGFR and to evaluate combinations of anti-EGFR and other drugs to improve outcomes.
PREVENTION
Available topical agents to treat actinic keratosis and cSCC in situ field of cancerization include mainly 5-FU cream, imiquimod, diclofenac and photodynamic therapy. Ingenol metubate (Picato) is now withdrawn because of safety issues. A recent randomized Dutch trial evaluating efficacy of 5% 5-FU cream, 5% imiquimod cream, methyl aminolevulinate photodynamic therapy or 0.015% ingenol mebutate gel in 624 patients with ≥ 5 actinic keratosis lesions on the head and neck showed that 5% 5-FU cream was the most effective in controlling solar keratoses (60). However, it has not been confirmed that it does, in turn, reduce the risk of SCC.
Oral acitretin can prevent the occurrence of new cSCCs in patients with multiple tumours; for example, xeroderma pigmentosum patients or transplant recipients. However, cutaneous adverse events often led patients to discontinuation, which, in turn, allowed quick appearance of new cSCCs.
Oral nicotinamide can be prescribed off-label. Indeed, it was evaluated in a randomized study on 386 patients with a history of 2 or more non melanoma skin cancers. Patients received either nicotinamide (500 mg, 2 times per day) or placebo for one year. The nicotinamide group had 30% significantly fewer new cSCCs (61). However, the long-term benefit remains unknown. Liver toxicity can sometimes occur.
TRANSPLANT RECIPIENTS
All transplant recipients are at high risk of developing cSCCs. These cSCCs are more aggressive, with a 5–10-fold higher risk of metastasis (62, 63). Immunosuppression duration and drug types and doses are involved. Surgery must not be delayed in transplant recipients with resectable tumours.
For transplantees, minimizing immunosuppression and switching to sirolimus should be considered as soon as the first cSCC appears. The benefit of switching to sirolimus is maintained for 5 years, with no negative effect on the graft and patient survival (64). However, administration of mTOR inhibitors remains limited because of poor tolerance. Indeed, 25–40% of patients discontinue sirolimus because of adverse events, e.g. hyperlipidaemia, glucose intolerance, interstitial pneumonia and/or lymphoedema. For transplantees with advanced cSCCs, currently available drugs should be used with caution, as anti-PD-1 agents are associated with a high rate of irreversible allograft rejection, while anti-cutaneous T-lymphocyte antigen-4 (CTLA-4) seems to be better tolerated (65). Moreover, the risk of infections with conventional chemotherapy is higher in immunosuppressed patients. Notably, 2 lung-transplant recipients with metastatic cSCCs died 1–3 weeks after their first infusions of cetuximab due to diffuse alveolar damage (66).
CONCLUSION
Due to the increasing incidence of cSCC, it has become a serious public health concern. All tumours should systematically be staged with AJCC-8 or BWH systems, in order to adapt treatment according to the risk of recurrence. Surgery is the treatment of choice whenever the tumour is resectable. Adjuvant radiation therapy must be considered for high-risk cSCCs. PD-1 inhibition is now the standard-of-care for advanced cSCCs. Platin-based chemotherapy or anti-EGFR can be prescribed in the second-line setting. Factors predictive of cSCC response to anti-PD-1 or anti-EGFR remain to be elucidated. Due to the high rate of irreversible allograft rejection associated with anti-PD-1 in organ-transplant recipients, other, less toxic, anti-CTLA-4 or other approaches warrant investigation. Switching from calcineurin inhibitors to sirolimus, or de-escalating immunosuppression, should always be considered. Because most advanced tumours may not respond to various current treatments, the search for new approaches is warranted. Prevention should not be forgotten. SCC incidence is increasing rapidly because of better screening, therefore most cSCC seen in dermatology or plastic surgery clinics are now detected earlier with better prognosis. Only 1–4% of cSCC are fatal; hence patients with cSCC must be accurately staged, to ensure that they are not over-investigated and do not undergo unnecessary surgical procedures or systemic treatments.
ACKNOWLEDGEMENTS
The authors thank Margot Denis for technical assistance in the preparation of this manuscript and Janet Jacobson for editorial assistance.
EM served as a principal investigator for SANOFI and principal investigator and coordinator for MSD investigations, and received research funding from MSD.
REFERENCES
- 1.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol 2015; 151: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 2.Wassberg C, Thorn M, Johansson AM, Bergstrom R, Berne B, Ringborg U. Increasing incidence rates of squamous cell carcinoma of the skin in Sweden. Acta Derm Venereol 2001; 81: 268–272. [DOI] [PubMed] [Google Scholar]
- 3.Dal H, Boldemann C, Lindelof B. Trends during a half century in relative squamous cell carcinoma distribution by body site in the Swedish population: support for accumulated sun exposure as the main risk factor. J Dermatol 2008; 35: 55–62. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt J, Seidler A, Diepgen TL, Bauer A. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol 2011; 164: 291–307. [DOI] [PubMed] [Google Scholar]
- 5.Christensen GB, Ingvar C, Hartman LW, Olsson H, Nielsen K. Sunbed use increases cutaneous squamous cell carcinoma risk in women: a large-scale, prospective study in Sweden. Acta Derm Venereol 2019; 99: 878–883. [DOI] [PubMed] [Google Scholar]
- 6.Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol 1999; 40: 177–186. [DOI] [PubMed] [Google Scholar]
- 7.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370: 59–67. [DOI] [PubMed] [Google Scholar]
- 8.Garrett GL, Blanc PD, Boscardin J, Lloyd AA, Ahmed RL, Anthony T, et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the United States. JAMA Dermatol 2017; 153: 296–303. [DOI] [PubMed] [Google Scholar]
- 9.Rizvi SMH, Aagnes B, Holdaas H, Gude E, Boberg KM, Bjortuft O, et al. Long-term change in the risk of skin cancer after organ transplantation: a population-based nationwide cohort study. JAMA Dermatol 2017; 153: 1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindelöf B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol 2000; 143: 513–519. [PubMed] [Google Scholar]
- 11.Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol 2014; 32: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J Am Acad Dermatol 2005; 53: 253–260. [DOI] [PubMed] [Google Scholar]
- 13.Czarnecki D. Non-melanoma skin cancer mortality rising in susceptible Australians. J Eur Acad Dermatol Venereol 2017; 31: e286–e287. [DOI] [PubMed] [Google Scholar]
- 14.Osterlind A, Hjalgrim H, Kulinsky B, Frentz G. Skin cancer as a cause of death in Denmark. Br J Dermatol 1991; 125: 580–582. [DOI] [PubMed] [Google Scholar]
- 15.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol 2013; 68: 957–966. [DOI] [PubMed] [Google Scholar]
- 16.Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729. [DOI] [PubMed] [Google Scholar]
- 17.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, Hwang WT, Gelfand JM, Whalen FM, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol 2013; 149: 402–410. [DOI] [PubMed] [Google Scholar]
- 18.Canueto J, Burguillo J, Moyano-Bueno D, Vinolas-Cuadros A, Conde-Ferreiros A, Corchete-Sanchez LA, et al. . Comparing the eighth and the seventh editions of the American Joint Committee on Cancer staging system and the Brigham and Women’s Hospital alternative staging system for cutaneous squamous cell carcinoma: implications for clinical practice. J Am Acad Dermatol 2019; 80: 106–113 e2. [DOI] [PubMed] [Google Scholar]
- 19.Brantsch KD, Meisner C, Schonfisch B, Trilling B, Wehner-Caroli J, Rocken M, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 2008; 9: 713–720. [DOI] [PubMed] [Google Scholar]
- 20.Jambusaria-Pahlajani A, Miller CJ, Quon H, Smith N, Klein RQ, Schmults CD. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg 2009; 35: 574–585. [DOI] [PubMed] [Google Scholar]
- 21.Fox M, Brown M, Golda N, Goldberg D, Miller C, Pugliano-Mauro M, et al. Nodal staging of high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol 2019; 81: 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bondt RB, Nelemans PJ, Hofman PA, Casselman JW, Kremer B, van Engelshoven JM, et al. . Detection of lymph node metastases in head and neck cancer: a meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur J Radiol 2007; 64: 266–272. [DOI] [PubMed] [Google Scholar]
- 23.Land R, Herod J, Moskovic E, King M, Sohaib SA, Trott P, et al. Routine computerized tomography scanning, groin ultra-sound with or without fine needle aspiration cytology in the surgical management of primary squamous cell carcinoma of the vulva. Int J Gynecol Cancer 2006; 16: 312–317. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz ES, Karia PS, Morgan FC, Schmults CD. The positive impact of radiologic imaging on high-stage cutaneous squamous cell carcinoma management. J Am Acad Dermatol 2017; 76: 217–225. [DOI] [PubMed] [Google Scholar]
- 25.Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, Bataille V, Bastholt L, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur J Cancer 2020. Feb 26. pii: S0959–8049(20)30019-8. [DOI] [PubMed] [Google Scholar]
- 26.Chren MM, Linos E, Torres JS, Stuart SE, Parvataneni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol 2013; 133: 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt AR, Brewer JD, Bordeaux JS, Baum CL. Staging for cutaneous squamous cell carcinoma as a predictor of sentinel lymph node biopsy results: meta-analysis of American Joint Committee on Cancer criteria and a proposed alternative system. JAMA Dermatol 2014; 150: 19–24. [DOI] [PubMed] [Google Scholar]
- 28.Jansen P, Petri M, Merz SF, Brinker TJ, Schadendorf D, Stang A, et al. The prognostic value of sentinel lymph nodes on distant metastasis-free survival in patients with high-risk squamous cell carcinoma. Eur J Cancer 2019; 111: 107–115. [DOI] [PubMed] [Google Scholar]
- 29.Cuperus E, Leguit R, Albregts M, Toonstra J. Post radiation skin tumors: basal cell carcinomas, squamous cell carcinomas and angiosarcomas. A review of this late effect of radiotherapy. Eur J Dermatol 2013; 23: 749–757. [DOI] [PubMed] [Google Scholar]
- 30.Harris BN, Pipkorn P, Nguyen KNB, Jackson RS, Rao S, Moore MG, et al. Association of adjuvant radiation therapy with survival in patients with advanced cutaneous squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg 2019; 145: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brewster AM, Lee JJ, Clayman GL, Clifford JL, Reyes MJ, Zhou X, et al. Randomized trial of adjuvant 13-cis-retinoic acid and interferon alfa for patients with aggressive skin squamous cell carcinoma. J Clin Oncol 2007; 25: 1974–1978. [DOI] [PubMed] [Google Scholar]
- 32.O’Bryan K, Sherman W, Niedt GW, Taback B, Manolidis S, Wang A, et al. . An evolving paradigm for the workup and management of high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol 2013; 69: 595–602 e1. [DOI] [PubMed] [Google Scholar]
- 33.Lewis CM, Glisson BS, Feng L, Wan F, Tang X, Wistuba, II, et al. . A phase II study of gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res 2012; 18: 1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenni D, Karpova MB, Muhleisen B, Mangana J, Dreier J, Hafner J, et al. A prospective clinical trial to assess lapatinib effects on cutaneous squamous cell carcinoma and actinic keratosis. ESMO Open 2016; 1: e000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross N, Ferrarotto R, Nagarajan P, Bell D, El-Naggar A, Johnson JM, et al. Phase II study of neoadjuvant cemiplimab prior to surgery in patients with stage III/IV (M0) cutaneous squamous cell carcinoma of the head and neck (cSCC-HN). Presented at the European Society for Medical Oncology meeting, Barcelona, September 28–30, 2019. [Google Scholar]
- 36.Veness MJ, Morgan GJ, Palme CE, Gebski V. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope 2005; 115: 870–875. [DOI] [PubMed] [Google Scholar]
- 37.Ch’ng S, Maitra A, Allison RS, Chaplin JM, Gregor RT, Lea R, et al. . Parotid and cervical nodal status predict prognosis for patients with head and neck metastatic cutaneous squamous cell carcinoma. J Surg Oncol 2008; 98: 101–105. [DOI] [PubMed] [Google Scholar]
- 38.Hillen U, Leiter U, Haase S, Kaufmann R, Becker J, Gutzmer R, et al. Advanced cutaneous squamous cell carcinoma: a retrospective analysis of patient profiles and treatment patterns – results of a non-interventional study of the DeCOG. Eur J Cancer 2018; 96: 34–43. [DOI] [PubMed] [Google Scholar]
- 39.Moloney FJ, Comber H, O’Lorcain P, O’Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol 2006; 154: 498–504. [DOI] [PubMed] [Google Scholar]
- 40.Kambayashi Y, Fujimura T, Aiba S. Comparison of immunosuppressive and immunomodulatory cells in keratoacanthoma and cutaneous squamous cell carcinoma. Acta Derm Venereol 2013; 93: 663–668. [DOI] [PubMed] [Google Scholar]
- 41.Lippman SM, Parkinson DR, Itri LM, Weber RS, Schantz SP, Ota DM, et al. 13-cis-retinoic acid and interferon alpha-2a: effective combination therapy for advanced squamous cell carcinoma of the skin. J Natl Cancer Inst 1992; 84: 235–241. [DOI] [PubMed] [Google Scholar]
- 42.Slater NA, Googe PB. PD-L1 expression in cutaneous squamous cell carcinoma correlates with risk of metastasis. J Cutan Pathol 2016; 43: 663–670. [DOI] [PubMed] [Google Scholar]
- 43.Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res 2014; 20: 6582–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018; 379: 341–351. [DOI] [PubMed] [Google Scholar]
- 45.Grob JJ, Gonzales Mendoza R, Basset Seguin N, Vornicova O, Schachter J, Joshi A, et al. Pembrolizumab for recurrent/ metastatic cutaneous squamous cell carcinoma (cSCC): efficacy and safety results from the Phase 2 KEYNOTE-629 study. Presented at the European Society for Medical Oncology meeting, Barcelona, September 28–30, 2019. [Google Scholar]
- 46.Maubec E, Boubaya M, Petrow P, Basset-Seguin N, Grob JJ, Dreno B, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma: Phase 2 results from CARSKIN. Presented at the American Society of Clinical Onclology meeting, Chicago, May 31 – June 4, 2019. [Google Scholar]
- 47.Sadek H, Azli N, Wendling JL, Cvitkovic E, Rahal M, Mamelle G, et al. Treatment of advanced squamous cell carcinoma of the skin with cisplatin, 5-fluorouracil, and bleomycin. Cancer 1990; 66: 1692–1696. [DOI] [PubMed] [Google Scholar]
- 48.Guthrie TH, Jr, Porubsky ES, Luxenberg MN, Shah KJ, Wurtz KL, Watson PR. Cisplatin-based chemotherapy in advanced basal and squamous cell carcinomas of the skin: results in 28 patients including 13 patients receiving multimodality therapy. J Clin Oncol 1990; 8: 342–346. [DOI] [PubMed] [Google Scholar]
- 49.Khansur T, Kennedy A. Cisplatin and 5-fluorouracil for advanced locoregional and metastatic squamous cell carcinoma of the skin. Cancer 1991; 67: 2030–2032. [DOI] [PubMed] [Google Scholar]
- 50.Huis In‘t Veld EA, Grunhagen DJ, Deroose JP, Nijsten TEC, Wouters M, Verhoef C, et al. . Isolated limb perfusion for unresectable extremity cutaneous squamous cell carcinoma; an effective limb saving strategy. Br J Cancer 2018; 119: 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maubec E, Petrow P, Scheer-Senyarich I, Duvillard P, Lacroix L, Gelly J, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol 2011; 29: 3419–3426. [DOI] [PubMed] [Google Scholar]
- 52.Dereure O, Missan H, Girard C, Costes V, Guillot B. Efficacy and tolerance of cetuximab alone or combined with chemotherapy in locally advanced or metastatic cutaneous squamous cell carcinoma: an open study of 14 patients. Dermatology 2016; 232: 721–730. [DOI] [PubMed] [Google Scholar]
- 53.Necchi A, Giannatempo P, Lo Vullo S, Raggi D, Nicolai N, Colecchia M, et al. Panitumumab treatment for advanced penile squamous cell carcinoma when surgery and chemotherapy have failed. Clin Genitourin Cancer 2016; 14: 231–236. [DOI] [PubMed] [Google Scholar]
- 54.Foote MC, McGrath M, Guminski A, Hughes BG, Meakin J, Thomson D, et al. Phase II study of single-agent panitumumab in patients with incurable cutaneous squamous cell carcinoma. Ann Oncol 2014; 25: 2047–2052. [DOI] [PubMed] [Google Scholar]
- 55.William WN, Jr, Feng L, Ferrarotto R, Ginsberg L, Kies M, Lippman S, et al. . Gefitinib for patients with incurable cutaneous squamous cell carcinoma: a single-arm phase II clinical trial. J Am Acad Dermatol 2017; 77: 1110–1113 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gold KA, Kies MS, William WN, Jr, Johnson FM, Lee JJ, Glisson BS. Erlotinib in the treatment of recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase 2 clinical trial. Cancer 2018; 124: 2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Necchi A, Lo Vullo S, Perrone F, Raggi D, Giannatempo P, Calareso G, et al. First-line therapy with dacomitinib, an orally available pan-HER tyrosine kinase inhibitor, for locally advanced or metastatic penile squamous cell carcinoma: results of an open-label, single-arm, single-centre, phase 2 study. BJU Int 2018; 121: 348–356. [DOI] [PubMed] [Google Scholar]
- 58.Cavalieri S, Perrone F, Miceli R, Ascierto PA, Locati LD, Bergamini C, et al. Efficacy and safety of single-agent pan-human epidermal growth factor receptor (HER) inhibitor dacomitinib in locally advanced unresectable or metastatic skin squamous cell cancer. Eur J Cancer 2018; 97: 7–15. [DOI] [PubMed] [Google Scholar]
- 59.Lu SM, Lien WW. Concurrent radiotherapy with cetuximab or platinum-based chemotherapy for locally advanced cutaneous squamous cell carcinoma of the head and neck. Am J Clin Oncol 2018; 41: 95–99. [DOI] [PubMed] [Google Scholar]
- 60.Jansen MHE, Kessels J, Nelemans PJ, Kouloubis N, Arits A, van Pelt HPA, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med 2019; 380: 935–946. [DOI] [PubMed] [Google Scholar]
- 61.Chen AC, Martin AJ, Dalziell RA, McKenzie CA, Lowe PM, Eris JM, et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br J Dermatol 2016; 175: 1073–1075. [DOI] [PubMed] [Google Scholar]
- 62.Lindelöf B, Jarnvik J, Ternesten-Bratel A, Granath F, Hedblad MA. Mortality and clinicopathological features of cutaneous squamous cell carcinoma in organ transplant recipients: a study of the Swedish cohort. Acta Derm Venereol 2006; 86: 219–222. [DOI] [PubMed] [Google Scholar]
- 63.Genders RE, Osinga JAJ, Tromp EE, O’Rourke P, Bouwes Bavinck JN, Plasmeijer EI. Metastasis risk of cutaneous squamous cell carcinoma in organ transplant recipients and immunocompetent patients. Acta Derm Venereol 2018; 98: 551–555. [DOI] [PubMed] [Google Scholar]
- 64.Dantal J, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. Sirolimus for secondary prevention of skin cancer in kidney transplant recipients: 5-year results. J Clin Oncol 2018; 36: 2612–2620. [DOI] [PubMed] [Google Scholar]
- 65.Aguirre LE, Guzman ME, Lopes G, Hurley J. Immune check-point inhibitors and the risk of allograft rejection: a com-prehensive analysis on an emerging issue. Oncologist 2019; 24: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leard LE, Cho BK, Jones KD, Hays SR, Tope WD, Golden JA, et al. Fatal diffuse alveolar damage in two lung transplant patients treated with cetuximab. J Heart Lung Transplant 2007; 26: 1340–1344. [DOI] [PubMed] [Google Scholar]