Abstract
A man in his late 50s presented with unilateral pain and discolouration of his fourth and fifth toes suggestive of digital ischaemia. He had a medical history of two unprovoked venous thromboembolisms in the preceding 18 months and a history of monoclonal gammopathy of undetermined significance (MGUS). A CT scan showed evidence of large vessels vasculitis in the absence of circulating antineutrophil cytoplasmic antibodies. Biopsy of the toes showed evidence of light chain and immunoglobulin deposition on immunofluorescence suggesting vasculitis secondary to his haematological diagnosis of MGUS. The patient was treated with high dose glucocorticoids and immunosuppressive treatment with a significant improvement in his symptoms and features of digital ischaemia.
Keywords: Malignant and Benign haematology, Vasculitis, Haematology (incl blood transfusion)
Background
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant condition that is associated with inflammatory conditions and 1% of patients will progress to multiple myeloma or other lymphoproliferative disorders each year.1 2 Its name however is a misnomer with a number of significant consequences occurring as a result of the paraprotein band. More recently, studies have shown associations between plasma cell dyscrasias and autoimmune conditions.1 This case illustrates a rare case of MGUS possible causing large vessel vasculitis and demonstrates approaches to diagnosis, investigation and treatment in such a rare presentation.
Case presentation
This man first presented 5 years ago with lymphadenopathy and fever. He was diagnosed with an internal jugular vein thrombosis and received anticoagulation for 6 months for an unprovoked venous thromboembolisms (VTE). Soon after stopping the anticoagulation, he developed a left arm swelling and was diagnosed with an axillary vein thrombosis. Following the second unprovoked VTE he underwent investigations which identified a raised monoclonal IgG lambda paraprotein band (3.15 g/L). His serum free light chains and skeletal survey were normal. Subsequent bone marrow biopsy was consistent with a diagnosis of MGUS.
Twenty-one months later he complained of progressively worsening pain in his feet over a period of several months. Over the same time period, he had experienced a loss of appetite and weight loss. Importantly, there was an absence of ear nose and throat, cardiorespiratory, gastrointestinal or urinary symptoms. He later presented to hospital and was treated for a community acquired pneumonia. During this admission it was noted that there was significant progressive digital ischaemia and some dry gangrenous lesions on several toes.
Examination findings at the time of presentation showed normal pulses throughout with no bruits. There was evidence of livedo rash over the left truck and lateral aspect of right foot (figure 1) and gangrene on the entire right fourth toe and partially on the left third and fifth toes (figure 2). The rest of his physical examination was largely unremarkable with no cardiovascular, respiratory or neurological signs.
Figure 1.
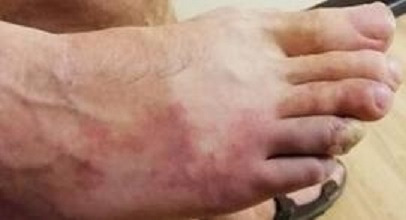
Right foot at time of presentation showing discolouration and early vascular changes.
Figure 2.
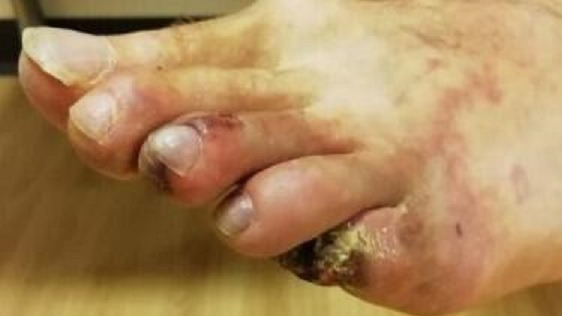
Left foot at time of presentation showing necrotic features of the third toe and evidence of dry gangrene on the fifth toe.
Investigations
Blood results can be seen in the table 1. On arrival the patient had a significantly raised C reactive protein (CRP), leucocytosis, thrombocytosis and normocytic anaemia. His erythrocyte sedimentation rate was significantly raised and his monoclonal IgG lambda paraprotein band remained largely unchanged at 3.71 g/L. A full immunology screen was completed which included, connective tissue screen, complements, antineutrophil cytoplasmic antibodies and lupus serology, which were all negative.
Table 1.
Relevant biochemistry, haematology and immunology results throughout admission
| On admission | Prior to steroid treatment | 7 days after steroids | ||
| Biochemistry | ||||
| Sodium | 138 | 138 | 139 | mmol/L |
| Potassium | 4.0 | 4.0 | 3.5 | mmol/L |
| Urea | 9.7 | 7.2 | 6.1 | mmol/L |
| Creatinine | 99 | 87 | 88 | µmol/L |
| Albumin | 32 | 37 | 39 | g/L |
| CRP | 177.1 | 165.5 | 8.7 | mg/L |
| ESR | 127 | – | – | mm/hour |
| Haematology | ||||
| Haemoglobin | 81 | 76 | 86 | g/L |
| WCC | 18.35 | 13.25 | 16.99 | ×10⁹/L |
| Platelets | 188 | 200 | 295 | ×10⁹/L |
| Neutrophils | 15.07 | 11.45 | 13.73 | ×10⁹/L |
| Immunology | ||||
| Immunoglobulins | IgG 10.24 IgA 1.77 IgM 0.63 |
g/L | Virology | HIV 1 and 2 antibodies not detected Hepatitis B surface antigen not detected Hepatitis C antibody not detected |
| Serum free light chains | Kappa 27.2 Lambda 83.3 |
mg/L | Genetics | No evidence of JAK II Mutation No evidence of calreticlin gene mutation |
| ANCA | PR3 1.9 MPO 0.6 |
IU/ml | Serum amyloid A | 739 mg/mL |
| Complements | C3 0.93 C4 0.20 |
g/L | ||
ANCA, antineutrophil cytoplasmic antibodies; CRP, C -reactive protein; ESR, erythrocyte sedimentation rate; WCC, white cell count.
Other investigations performed later in the admission included virology including HIV, hepatitis B and C which were negative. JAK II mutation and calreticulin gene mutation were checked due to the VTE histology and paroxysmal nocturnal haemoglobinuria was excluded. A bone marrow biopsy was performed which showed features of active haematopoiesis and while plasma cells were not increased, they did show abnormal phenotypes.
Imaging was performed including a chest X-ray which showed patchy pulmonary infiltrates at the left base in keeping with infection. A CT scan was later completed due to persistently raised CRP despite escalating antibiotic treatment. The scan showed evidence of significant distal abdominal aortic wall thickness and proximal peroneal and anterior tibial arteries stenosis on the left, in keeping with inflammatory vasculitis. A PET CT scan was later carried out which identified no metabolic evidence of large vessel vasculitis changes or occult malignancy.
Differential diagnosis
Peripheral vascular disease presenting as an acute ischaemic event was a differential diagnosis at presentation given the feature of dry gangrene and history of hypertension. However, there was no other significant history or risk factors for peripheral vascular disease and distal pulses were intact on examination. An embolic event secondary to hypercoagulable state was another differential given the patient’s history of VTE but this seemed less likely given the patient was being treated with systemic anticoagulation. Vasculitis while rare, was also considered in the differential diagnosis due to the systemic history of weight loss, malaise and VTE.
Treatment
The patient’s inflammatory markers failed to respond to antibiotic treatment alone and his symptoms worsened. Given the initial investigations were suggestive of vasculitis, he was started empirically on 30 mg of oral prednisolone and within 48 hours his CRP improved from 160 mg/L to 40 mg/L and at the end of the first week he had already seen significant improvements in his pain and appearance of his toes.
He was discussed at a multidisciplinary team meeting inclusive of renal physicians, rheumatologists and the vascular surgeons. It was agreed alongside the patient to amputate one of his affected toes, which was sent for biopsy and immunofluorescence.
The biopsy results showed microscopic appearances consistent with vasculitis and immunofluorescence confirmed kappa and lambda chain deposits in the vessel wall along with immunoglobulins and complement. Most interestingly, while his serology showed a predominantly lambda paraprotein, his biopsy showed a large amount of kappa light chain deposition.
Following the biopsy report, remission induction treatment with cyclophosphamide alongside a weaning course of prednisolone was commenced. The CYCLOPS3 protocol was given as a mixture of intravenous and oral treatment due to limitations caused by the COVID-19 pandemic. A cumulative dose of 2750 mg cyclophosphamide was given and the patient has since been started on maintenance azathioprine with no evidence of relapsed disease.
Discussion
MGUS is a preneoplastic plasma cell disorder. It exists on a spectrum of monoclonal plasma cell disorder along with smouldering multiple myeloma and multiple myeloma. The disorder is characterised by a serum monoclonal (M) protein of less than 3 g/dL, bone marrow clonal plasma cells less than 10% and an absence of plasma cell dyscrasia related end organ damage; hypercalcaemia, renal failure, anaemia or lytic lesions.4 5
While the aetiology of MGUS is not fully known, multiple risk factors included increasing age and male sex have been associated.5 Furthermore, MGUS and other plasma cell disorders have been strongly associated with autoimmune diseases,1 ranging from autoimmune haematological disorders such as pernicious anaemia,6 7 autoimmune neurological disorders and rheumatological disorders including Sjogren’s syndrome, systemic lupus erythematous, rheumatoid arthritis and spondyloarthropathies.1 8–10 Due to the rarity of the disease and the multiple ways in which it presents, the published literature is limited and often confined to case reports. This raises the uncertainty as to whether some of these associations are merely coincidental or true associations.10
Vasculitis is a disease process characterised by inflammation and necrosis of vessel walls.11 12 There are several types of vasculitidies which are classified by the size of vessel affected.13 Vasculitis is a rare disorder which can cause life threatening organ damage. The cause if not known but similar to MGUS is thought to be the result of immune dysregulation.1 It is therefore conceivable that conditions relating to immune dysregulation and chronic inflammation are in some way associated and it is not known if there is a causative effect whereby immune mediated conditions may act as a trigger for plasma cell dyscrasia.9
From the literature, there have been several case reports of MGUS causing small vessel vasculitis.14 15 Umemura et al14 describe a case of IgA-associated leukocytoclastic vasculitis associated with MGUS of an IgA lambda chain. Drerup et al15 present two similar cases with typical signs of immune-mediated small vessel vasculitis and demonstrate paraproteinaemia can cause small vessel vasculitis. Furthermore, these case reports also demonstrated a good response to immunosuppressive treatment with a reduction in the paraprotein and vasculitis manifestations.
Case reports looking at MGUS in the context of large vessel vasculitis are seldom. Osório et al16 report a case of MGUS presenting at the same time as giant cell arteritis (GCA). While the temporal biopsy was inconclusive (as it often is), it was hypothesised that the GCA was a result of an immune mediated response to cytokine production and amyloid deposits.16 In our case, the biopsy immunofluorescence showed occlusive deposition of kappa and lambda light chains in the vessel walls, support this theory of pathogenesis.
Treatment options for MGUS related complications are complex. While there is no specific treatment recommended for MGUS, some studies have shown an improvement in monoclonal bands following immunosuppressive treatment for underlying autoimmune conditions.1 This is in contrast to other studies that report medications such as steroids and immunomodulators used in autoimmune conditions have been associated with an increased risk of malignancy.7 17 In our case report, the patient received high dose glucocorticoids and cyclophosphamide. His vasculitis remitted and his monoclonal paraprotein band reduced. Due to the rarity of these conditions, treatment has to be individualised to the patient’s case and clinical presentation, weighing up the adverse risks associated with immunosuppression. In the case of life or organ threatening disease, immunosuppression should be strongly considered.
In conclusion, our case highlights a rare but potentially serious complication of MGUS. Using radiological imaging and histopathology, we have demonstrated a possible causative relationship between MGUS and large vessel vasculitis.
Patient’s perspective.
The patient was involved in the writing of this case and kindly provided the photos seen as figures 1 and 2. I was in pain for about 6 months and none of the doctors I saw knew what was wrong with me until the doctor on the ward noticed my toes and contacted the vasculitis team.
Learning points.
Monoclonal gammopathy of undetermined significance (MGUS) is related to multiple autoimmune disorders including vasculitis. While the aetiology is not completely clear, immune dysregulation and chronic inflammation are risk factors and may act as a trigger for plasma cell dyscrasia.
In patients with features of autoimmune disease, immunology and myeloma screen are important. Imaging and histopathological diagnosis with immunohistochemistry can aid diagnosis.
Treatment of MGUS related complications should be made on an individualised basis, weighing up the risks associated with immunosuppression. In the case of life or organ threatening disease, immunosuppression should be strongly considered.
Acknowledgments
The authors would like to acknowledge the support of Professor Ian Roberts at Oxford University Hospital, NHS Foundation Trust for his histology report and cellular pathology expertise. We would also like to acknowledge the support of the Renal Department at Royal Preston Hospital, Lancashire NHS Foundation Trust.
Footnotes
Contributors: EGA: submitting and corresponding author. Wrote up the case report. LF: edited the case report. Liaised with patient by obtaining consent for the case report and also obtained consent for associated images to be used. AD: main consultant involved in the diagnosis and treatment of the patient. Guided the initial planning of the case report and discussion points.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from the patient(s).
References
- 1.Shimanovsky A, Alvarez Argote J, Murali S, et al. Autoimmune manifestations in patients with multiple myeloma and monoclonal gammopathy of undetermined significance. BBA Clin 2016;6:12–18. 10.1016/j.bbacli.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiner N, Göbel G, Michaeler D, et al. Rheumatologic diseases impact the risk of progression of MGUS to overt multiple myeloma. Blood Adv 2021;5:1746–54. 10.1182/bloodadvances.2020003193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groot K, Harper L, Jayne DRW, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009;150:670–80. 10.7326/0003-4819-150-10-200905190-00004 [DOI] [PubMed] [Google Scholar]
- 4.Landgren O. Advances in MGUS diagnosis, risk stratification, and management: introducing myeloma-defining genomic events. Hematology Am Soc Hematol Educ Program 2021;2021:662–72. 10.1182/hematology.2021000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006;354:1362–9. 10.1056/NEJMoa054494 [DOI] [PubMed] [Google Scholar]
- 6.Baz R, Alemany C, Green R, et al. Prevalence of vitamin B12 deficiency in patients with plasma cell dyscrasias: a retrospective review. Cancer 2004;101:790–5. 10.1002/cncr.20441 [DOI] [PubMed] [Google Scholar]
- 7.McShane CM, Murray LJ, Landgren O, et al. Prior autoimmune disease and risk of monoclonal gammopathy of undetermined significance and multiple myeloma: a systematic review. Cancer Epidemiol Biomarkers Prev 2014;23:332–42. 10.1158/1055-9965.EPI-13-0695 [DOI] [PubMed] [Google Scholar]
- 8.Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012;32:1119 LP–36. [PMC free article] [PubMed] [Google Scholar]
- 9.Brown LM, Gridley G, Check D, et al. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood 2008;111:3388–94. 10.1182/blood-2007-10-121285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikanza I, Akpenyi O. Association of monoclonal gammopathy of undetermined significance with Behcet's disease: a review of shared common disease pathogenetic mechanisms. Mediterr J Rheumatol 2018;29:80–5. 10.31138/mjr.29.2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallenberg CGM. Pathophysiology of ANCA-associated small vessel vasculitis. Curr Rheumatol Rep 2010;12:399–405. 10.1007/s11926-010-0138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savage COS, Harper L, Cockwell P. Vasculitis. BMJ 2000;320. 10.1136/bmj.320.7245.1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jennette JC. Overview of the 2012 revised international chapel Hill consensus conference Nomenclature of vasculitides. Clin Exp Nephrol 2013;17:603–6. 10.1007/s10157-013-0869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umemura H, Yamasaki O, Iwatsuki K. Leukocytoclastic vasculitis associated with immunoglobulin a lambda monoclonal gammopathy of undetermined significance: a case report and review of previously reported cases. J Dermatol 2018;45:1009–12. 10.1111/1346-8138.14466 [DOI] [PubMed] [Google Scholar]
- 15.Drerup C, Metze D, Ehrchen J, et al. Evidence for immunoglobulin-mediated vasculitis caused by monoclonal gammopathy in monoclonal gammopathy of unclear significance prompting oncologic treatment. JAAD Case Rep 2019;5:288–91. 10.1016/j.jdcr.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osório RM, Pina S, Salero T, et al. Association of Multiple Myeloma and Giant Cell Arteritis - A Case Report. Eur J Case Rep Intern Med 2020;7:1360. 10.12890/2020_001360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landgren O, Zhang Y, Zahm SH, et al. Risk of multiple myeloma following medication use and medical conditions: a case-control study in Connecticut women. Cancer Epidemiol Biomarkers Prev 2006;15:2342–7. 10.1158/1055-9965.EPI-06-0097 [DOI] [PubMed] [Google Scholar]


