Abstract
Solid masses of the ovaries raise the suspicion of malignancy or metastasis and require histological diagnosis. Extramedullary haematopoesis (EMH) is a rare histological finding of a mass of the adnexa. The sonographic pattern of EMH has rarely been described in the literature. Transvaginal biopsy of EMH has not been reported in the literature. We present a case of adnexal EMH in a patient affected with β-thalassaemia, and we performed a narrative review. Only in our case, the sonographic pattern was described, and a transvaginal ultrasound-guided core biopsy was used. Assessing patients’ medical history and correlating it to the findings of diagnostic imaging is of paramount importance when evaluating patients with adnexal masses. The correct interpretation of sonographic images can avoid unnecessarily invasive procedures. A transvaginal biopsy could be a safe, easy and well-tolerated method to gain definite histological diagnosis in cases where a primary ovarian malignancy is not suspected.
Keywords: Obstetrics, gynaecology and fertility; Haematology (drugs and medicines); Ultrasonography; Radiology
Background
β-thalassaemia is a clinical condition arising from gene mutations affecting β-globin chain production and resulting in inefficient erythropoiesis, chronic transfusion-dependent anaemia and iron overload. Consequently, compensatory extramedullary haematopoiesis (EMH) can arise in different parts of the body. Usually, EMH involves the liver, spleen and paraspinal regions of the thorax.1 EMH involving the adnexa is an extremely rare finding. We report a case with two solid adnexal lesions in a patient affected by β-thalassaemia intermedia (β-TI) diagnosed as EMH trough transvaginal biopsy. Moreover, we present a narrative review of the literature about adnexal EMH in the context of different haematological pathologies. An extensive literature search of PubMed, Cochrane Library and GoogleScholar for articles published to date was performed using the keywords ‘pelvic extramedullary haematopoiesis’, ‘ovary extramedullary haematopoiesis’, ‘ovary myeloid metaplasia’, ‘extramedullary haematopoiesis thalassaemia’ and ‘extramedullary haematopoiesis ultrasound female pelvis’, to collect all studies reporting cases of adnexal EMH. No language restrictions were applied, and reference lists of all included studies were manually searched for other potentially eligible studies. Our search results revealed that no case-control or cohort studies of adnexal EMH have been published. We identified nine published case reports of adnexal EMH (tables 1 and 2). One has been excluded because EMH was not related to a haematological disease.2 Of the included publications, one was available only as an abstract.3
Table 1.
Case reports of adnexal EMH
| Reference | Year | Age | Disease | Diagnostic tool | Sites of EMH |
| Our case report | 2020 | 40 | β-TI pre-existing | Transvaginal US | Vertebral bodies, lungs and ovary |
| Gadomski et al9 | 2017 | 31 | CML pre-existing | CT scan, abdominal and transvaginal US | Ovaries |
| Palatnik et al10 | 2012 | 43 | CML newly diagnosed | CT scan (suspicious of pyosalpinges) | Uterus, tubes and ovaries |
| Rabischong et al11 | 2010 | 50 | MF pre-existing | CT scan | Ovaries, tubes and lungs |
| Khen-Dunlop et al12 | 2006 | 15 | β-TI pre-existing | US (suspicious of dermoid cyst at the beginning) and CT scan (suspicious of EMH) | Ovary and vertrebral bodies |
| Eapen et al (Abstract)3 |
2004 | 43 | CML newly diagnosed | CT scan (suspicious of pyosalpinges or tubo-ovarian abscesses) |
Ovary |
| Inoue et al13 | 1998 | 2 | Granulocytic sarcoma/newly diagnosed MML after spontaneous remission of MF | CT scan | Ovaries and paraaortic |
| Hillman et al14 | 1968 | 2 | MF newly diagnosed | Autopsy | Ovary, spleen, liver and bones |
| Lieberman et al15 | 1965 | 37 | MF on pre-existing regional enteritis and essential thrombocythaemia | Autopsy | Ovary, spleen, liver, scalp, small intestine, lungs, lymph nodes and spine |
CML, chronic myeloid leukaemia; CT scan, computerized tomography scan; EMH, extramedullary haematopoiesis; MF, myelofibrosis; MML, myelomonocytic leukaemia; US, ultrasound; β-TI, β-thalassaemia intermedia.
Table 2.
Case reports of adnexal extramedullary haematopoiesis
| Reference | Clinical presentation | Therapy | Outcome |
| Our case report | Incidental finding | HDU, transfusion, deferasirox and luspatercept | SD |
| Gadomski et al9 | Abdominal pain, nausea, vomiting, fatigue and anaemia | Transfusion, BSO and evacuation of ascites, palliative RT and paracentesis | DOD |
| Palatnik et al10 | Abdominal pain | TAH-BSO +imatinib and allopurinol | Unknown |
| Rabischong et al11 | Abdominal pain, diarrhoea, nausea and vomiting | Laparoscopic oophorectomy and HDU | PD with pulmonary and spinal involvement |
| Khen-Dunlop et al12 | Abdominal pain | Transfusion and HDU. Three years later laparotomy with enucleation because of growing mass and pain | CR |
| Eapen et al (Abstract)3 |
Abdominal pain | TAH-BSO +imatinib | Unknown |
| Inoue et al13 | Pancytopenia, oral mucosal bleeding and hepatosplenomegalie | Surgery +CT with cytosine arabinoside, 6-thioguanine and daunorubicin | DOD after R 3½ months after the end of CT and bone marrow transplant |
| Hillman et al14 | Pallor, fever and oral ulcers (Down’s syndrome). | Transfusion and steroids | DOD due to PD |
| Lieberman et al15 | Ileitis, essential thrombocytopenia and hepatosplenomegaly | RT, CT with chlorambucil. Then laparotomy due to intestinal bleeding |
DOD |
BSO, bilateral salpingo-oophorectomy; CR, complete response; CT, chemotherapy; DOD, died of disease; HDU, hydroxyurea; PD, progression of disease; R, relapse; RT, radiotherapy; SD, stable disease; TAH-BSO, total abdominal hysterectomy and bilateral salpingo-oophorectomy.
Case presentation
A woman in her 40s, gravida 2, para 1 of Southeast Asian origin, with a history of transfusion-dependent heterozygote β-TI with a compound heterozygosity Beta0/HbE (Deletion c126-129; -CTTT) was referred from haematology to our department of gynaecological ultrasound for the evaluation of two suspicious adnexal lesions. The patient reported that adnexal masses had been observed 4 years earlier during her caesarean delivery and had not been removed, examined nor treated thereafter.
The patient always had a regular cycle; she had no dyspareunia, no dysuria, no dyschezia and did not complain about abdominal pain. She had one spontaneous abortion. A family history of breast and ovarian cancer was denied.
The patient was already known for having EMH in the lungs, as well as in the paravertebral regions, which led to a caudal paraplegic syndrome due to cord compression 3 years before. The lesions regressed after radiation, oral therapy with N-hydroxyurea and regular transfusions with complete regression of the symptomatology. In addition, she had hepatosplenomegaly with hepatic siderosis, suspected functional asplenia and a dilatative cardiopathy.
She was currently on daily treatment with deferasirox 180 mg (2-0-1), an oral tridentate chelator that mobilises iron stores by binding selectively to the ferric form of iron, and N-hydroxyurea 500 mg/day for 4 days a week, an antineoplastic agent and fetal hemoglobin enhancer that increases hemoglobin levels, reduces blood transfusion dependency, decreases skeletal deformities and splenomegaly.
Diagnosis and treatment
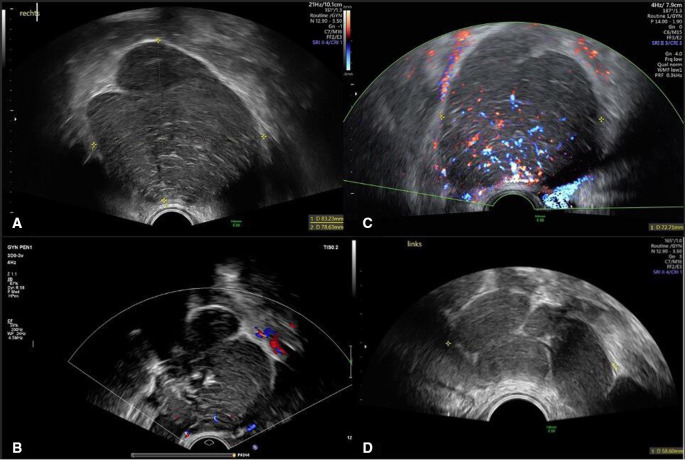
The transvaginal ultrasound showed a normal uterus with a homogeneous hypoechogenic endometrium of 7.7 mm. There was a well-circumscribed, isoechogenic to hypoechogenic, inhomogeneous solid mass involving the right ovary measuring 83×87×77 mm (volume 291 mL). Thick hyperechogenic septa divided the lesion in smaller round compartments which were then further subdivided by thinner septa. There were acoustic shadows and normal residual ovarian tissue of 23 mm of length at the outer rim of the mass (figure 1A, B). The blood flow within the solid lesion was moderate (colour score 3) (figure 1C). A smaller second mass measuring 45×28×48 mm (volume 32 mL) with the same sonographic features was observed contiguous to the left ovary (figure 1D). No free fluid was detected in the pouch of Douglas. No further solid lesions were detected intra-abdominally, and there were no signs of pelvic lymphadenopathy.
Figure 1.
(A) 2D grey scale ultrasound image of the solid lesion of the right adnexa. (B) 2D colour ultrasound image of the solid lesion of the right adnexa with acoustic shadows. (C) 2D colour doppler ultrasound image of the solid lesion of the right adnexa with moderate blood flow (colour score 3). (D) Ultrasound image of the solid lesion attached to the left ovary with the same features.
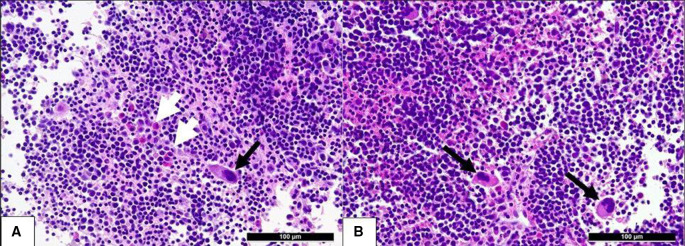
The tumour marker CA125 was normal (13.9 U/mL). No further tumour marker was determined. We assessed the lesions according to the criteria of the International Ovarian Tumour Analysis (IOTA) group.4 IOTA simple rules were inconclusive, as no benign nor malignant features applied.5 The risk calculation with the IOTA Assessment of Different NEoplasias in the adneXa (ADNEX) model (the only one with the advantage of differentiating between histological subtypes) suggested a benign adnexal tumour, with the highest relative risk (1.3) as well as a patient specific risk of 90.9% for a benign lesion,6 classifying the tumour as Ovarian-Adnexal Reporting & Data System (O-RADS 3) (low risk of malignancy).7 Furthermore, the combination of CA125 and the described ultrasound findings resulted in a low risk of malignancy index (1×3×13.9=41.7).8 The medical history of the patient and the sonographic features as interpreted by expert pattern recognition were suggestive of a possible site of EMH. After an interdisciplinary discussion with the haematologists and the gynaecological oncologists, a transvaginal ultrasound-guided core needle biopsy of the right adnexal mass was performed during the 3-week follow-up scan. The procedure was carried out in the lithotomy position, using a high-resolution endovaginal probe (GE Voluson E10, 7.5–12 MHz) and a core biopsy instrument (Bard Mission Disposable Core Biopsy Instrument 18G×25 cm—semiautomatic). No anaesthesia was needed, and no periprocedural nor postprocedural complications were observed. The patient did not complain about any pain. The tissue obtained was placed in formalin and sent for pathological examination. Histology confirmed the presence of trilinear EMH with a predominance of erythropoiesis and proliferation of eosinophilic granulocytes and megakaryocytes (figure 2A, B). The haematologist and the gynaecological oncologist opted against the removal of the lesions and adnexectomy, as the patient was asymptomatic and has a systemic disease which is already under treatment.
Figure 2.
The histological specimen (H&E): extramedullary haematopoiesis with predominance of erythropoiesis ((A, B) dark, round cells), single megakaryocytes ((A, B) black arrows) and eosinophils ((B) white arrows) in the biopsy specimen of the adnexal mass in our patient with β-thalassaemia intermedia.
Outcome and Follow-up
Four months after the biopsy ultrasound imaging of the adnexal masses is unchanged. The patient is stable and undergoes regular follow-ups with her haematologist. She is under treatment with luspatercept (recombinant fusion protein that binds transforming growth factor beta) to enhance erythroid maturation and reduce transfusion burden.
Synthesis of the narrative review
Clinical features of the cases
From the analysis of the nine included case reports (tables 1 and 2),3 9–15 the ages of the adult patients ranged from 31 to 50 years with a median of 41.5 years. One patient was a young girl in her early 10s.12 Two patients were infants in their 2's, one of whom was affected by trisomy 21.13 14 Four patients (44.4%) were of African-American ethnicity,3 9 10 12 one of Asian ethnicity (our case report) and another one of American-Indian ethnicity.15 For three patients, the ethnic group was not reported.11 13 14 The most frequent clinical presentation was severe abdominal pain described in six patients (66.6%).3 9–12 15 The two infants presented oral ulcers and mucosal bleeding.13 14 In our case, the adnexal lesions were incidental findings on ultrasound.
Haematological diseases
Adnexal EMH may occur in the context of various haematological pathologies. Three patients (33.3%) were affected by chronic myeloid leukaemia,3 9 10 three by myelofibrosis (33.3%)11 14 15 and one by myelomonocytic leukaemia with granulocytic sarcoma.13 Our patient and the 15-year-old girl described by Khen-Dunlop et al12 (20%) were affected by β-TI.
Diagnosis and treatment
CT scan was selected for imaging in four cases (44.4%),3 10 11 13 and pyosalpinges were suspected in two of them.3 10 In two cases, both CT scan and ultrasound were used;9 12 in one case, ultrasound suspected a dermoid cyst.12 In four cases (44.4%), laparotomy or laparoscopy was performed for histological diagnosis.3 10 11 13 In none of the cases described, other than ours, transvaginal ultrasound-guided core biopsy was used as a diagnostic method.
In five cases including ours (55.5%), no primary surgical management was chosen; however, a conservative approach with transfusions only, transfusions and hydroxyurea in two cases, transfusions and steroids in one case, and radiotherapy and chemotherapy in the last case was started.9 12 14 15 Despite this, three of these cases were refractory to treatment and underwent surgery later on.9 12 15 Particularly interesting was the course of the patient reported by Gadomski et al.9 After several unsuccessful attempts to treat the severe anaemia of the patient with continuous blood transfusions and abdominal distention with paracentesis, surgery remained the only option to relieve the abdominal pain, avoid further intra-abdominal blood loss from the adnexal lesions and stabilise her clinical condition. In their experience, early operative intervention should be considered to prevent further drops in haemoglobin and/or platelets’ count. Furthermore, haemodynamic relevant preoperative anaemia should prompt surgery.9
In three cases, the ovarian mass was the first finding that led to the diagnosis of the haematological disease.3 10 13 In four further cases including ours, the diagnosis of ovarian EMH was superimposed on a known pre-existing haematological disorder.9 11 12 In the case described by Hillman and Forrester in 1968,14 and in the one of Liebermann et al in 1965,15 the findings of adnexal EMH were detected histologically during postmortem examination of a 2-year-old girl with Down’s syndrome and a 37-year-old woman both affected by myelofibrosis.
Long-term outcome
For six out of eight cases of EMH described in the literature, data about patients’ outcome were available.
The case observed by Khen-Dunlop et al12 had a complete response to medical and surgical therapy and no sign of relapse. In one case (Rabischong et al11), the authors reported disease progression despite surgery and treatment with hydroxyurea. Four patients (44.4%) died due to complications of the haematological disease.9 13–15 For two of them, diagnosis of adnexal EMH occurred postmortem during autopsy.
Discussion
β-TI is a clinical disorder whose severity can vary widely, depending on the type of genetic defect involved. Ineffective erythropoiesis, chronic anaemia and iron overload may drive to clinical complications like splenomegaly, extramedullary erythropoiesis, iron accumulation, leg ulcers, thrombophilia, skeletal abnormalities, osteopenia, heart failure and endocrine disorders.16
Due to this broad clinical spectrum, patients affected by β-TI need tailored therapies and continuous follow-up. At the moment, available treatments vary from occasional transfusions, modulation of γ-globulin chain production with hydroxyurea or other drugs, iron chelation, up to splenectomy and in some cases stem cell transplantation.1 16
EMH is a compensatory reaction of the body to ineffective erythropoiesis and chronic anaemia. This mechanism is a common feature of different haematological diseases that usually develop in the liver, spleen, lymph nodes and paraspinal zones.1 16 EMH of the reproductive organs in patients with haematological disorders has been sporadically described in the literature and even more rarely in the presence of β-TI.
Several studies have demonstrated the accuracy and safety of transvaginal ultrasound-guided core biopsy for the diagnosis of pelvic lesions in selected cases.17 18 Laparoscopy or laparotomy, as well as CT-guided procedures with prolonged waiting times, could be avoided.
The first and only sonographic description in the literature of cases of abdominal EMH in female and male patients (not of the adnexa) had been published by Shawker et al in 1987.19 At sonography, common features were the presence of solid, smooth and hypoechogenic masses, as in our case.19 Technological advances give us now the opportunity to revise and newly describe imaging characteristics of these tumours, helping in their pattern recognition. Correct differential diagnosis is particularly useful in patients’ selection for upfront surgery, ultrasound-guided core biopsy or conservative management.
The poor outcomes of the clinical courses described in this review could suggest a more aggressive evolution of disease in cases of adnexal EMH in patients with both pre-existing and newly diagnosed haematological disease. Moreover, adnexal EMH could be a sign of advanced disease. Further studies are needed to confirm this hypothesis.
This review is limited by the rarity of such adnexal lesions and the paucity of reports in the literature. Therefore, long-term follow-up and a significant comparison of the clinical aspects are not possible.
We must also consider that therapeutic options for haematological malignancies have tremendously improved during the last decades.
Our aim was to sensitise clinicians about the possible presence of EMH masses of the adnexa, especially in patients with pre-existing haematological disorders. Misinterpretation of ultrasound findings can lead to overly invasive diagnostic choices in already severely impaired patients. This points out the importance of a correct interpretation of the ultrasound images which is feasible today. In selected cases, transvaginal ultrasound-guided core biopsy could be a safe and minimally invasive option to gain an accurate diagnosis.
Learning points.
Ultrasound-guided transvaginal biopsy is a well-tolerated, low-risk procedure that can be used as an effective method to provide histological diagnosis.
Physicians should include adnexal extramedullary haematopoiesis in the differential diagnosis of patients with solid ovarian masses concurrently affected by haematological disorders.
A correct interpretation of the ultrasound images, and in selected cases transvaginal biopsy, can avoid the need for unnecessarily invasive procedures in already severely impaired patients.
Acknowledgments
The authors would like to thank Prof Dr V Heinzelmann, Chief of the Department of Gynaecology and Obstetrics of the University Hospital of Basel for her continuous support and inspiration in work and research. Prof Dr J Passweg, Chief of the Department of Haematology of the University Hospital of Basel, for giving us valuable information about the patient and her haematological disease. Prof Dr A Tzankov of the Institute of Medical Genetics and Pathology of the University Hospital of Basel for providing the histological images.
Footnotes
Contributors: CM visited the patient first and gave us details about the ultrasound features. VF and HR conceived the presented idea. VF collected and interpreted the data. VF, HR and GMB drafted the article. GMB made a critical revision of the article and approved the final version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Asadov C, Alimirzoeva Z, Mammadova T, et al. β-Thalassemia intermedia: a comprehensive overview and novel approaches. Int J Hematol 2018;108:5–21. 10.1007/s12185-018-2411-9 [DOI] [PubMed] [Google Scholar]
- 2.Huang C-W, Hsueh S, Chang M-Y. Agnogenic myeloid metaplasia in an ovarian steroid cell tumor with virilization: a case report. J Reprod Med 2004;49:765–8. [PubMed] [Google Scholar]
- 3.Eapen SS, Narayan R, Khan A. A case of unusual extra-medullary hematopoiesis. Journal of Clinical Oncology 2004;22:6698. 10.1200/jco.2004.22.90140.6698 [DOI] [Google Scholar]
- 4.Timmerman D, Valentin L, Bourne TH, et al. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International ovarian tumor analysis (iota) group. Ultrasound Obstet Gynecol 2000;16:500–5. 10.1046/j.1469-0705.2000.00287.x [DOI] [PubMed] [Google Scholar]
- 5.Timmerman D, Van Calster B, Testa A, et al. Predicting the risk of malignancy in adnexal masses based on the simple rules from the International ovarian tumor analysis group. Am J Obstet Gynecol 2016;214:424–37. 10.1016/j.ajog.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 6.Van Calster B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ 2014;349:g5920. 10.1136/bmj.g5920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreotti RF, Timmerman D, Strachowski LM, et al. O-RADS us risk stratification and management system: a consensus guideline from the ACR Ovarian-Adnexal reporting and data system Committee. Radiology 2020;294:168–85. 10.1148/radiol.2019191150 [DOI] [PubMed] [Google Scholar]
- 8.Manegold-Brauer G, Buechel J, Knipprath-Mészaros A, et al. Improved detection rate of ovarian cancer using a 2-step triage model of the risk of malignancy index and expert sonography in an outpatient screening setting. Int J Gynecol Cancer 2016;26:1062–9. 10.1097/IGC.0000000000000718 [DOI] [PubMed] [Google Scholar]
- 9.Gadomski TE, Rogg K, Kidd L, Robinson WR R, et al. New adnexal masses in a patient with a history of chronic myelogenous leukemia: a case report. medical-oncology 2017;02. 10.35841/medical-oncology.2.2.24-26 [DOI] [Google Scholar]
- 10.Palatnik A, Narayan R, Walters M. Extramedullary hematopoiesis involving uterus, fallopian tubes, and ovaries, mimicking bilateral tuboovarian abscesses. Int J Gynecol Pathol 2012;31:584–7. 10.1097/PGP.0b013e31825183ad [DOI] [PubMed] [Google Scholar]
- 11.Rabischong B, Larraín D, Charpy C, et al. Extramedullary hematopoiesis and myeloid metaplasia of the ovaries and tubes in a patient with myelofibrosis: case report and Concise review of the reported cases. J Clin Oncol 2010;28:e511–2. 10.1200/JCO.2010.29.6442 [DOI] [PubMed] [Google Scholar]
- 12.Khen-Dunlop N, Girot R, Brunelle F, et al. Surgical treatment of an unusual case of pelvic extramedullary hematopoiesis. J Pediatr Surg 2006;41:e13–15. 10.1016/j.jpedsurg.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 13.Inoue S, Limsuwan A, McQueen R. Spontaneous resolution of myelofibrosis and pancytopenia followed by the development of acute myeloid leukemia with an extramedullary mass. J Pediatr Hematol Oncol. May 1998;20:268–70. [DOI] [PubMed] [Google Scholar]
- 14.Hillman F, Forrester RM. Myelofibrosis simulating acute leukaemia in a female infant with Down's syndrome. Ir J Med Sci 1968;7:167–73. 10.1007/BF02946511 [DOI] [PubMed] [Google Scholar]
- 15.Lieberman PH, Rosvoll RV, Ley AB. Extramedullary myeloid tumors in primary myelofibrosis. Cancer 1965;18:727–36. [DOI] [PubMed] [Google Scholar]
- 16.Karimi M, Cohan N, De Sanctis V, et al. Guidelines for diagnosis and management of beta-thalassemia intermedia. Pediatr Hematol Oncol 2014;31:583–96. 10.3109/08880018.2014.937884 [DOI] [PubMed] [Google Scholar]
- 17.Mascilini F, Quagliozzi L, Moro F, et al. Role of transvaginal ultrasound-guided biopsy in gynecology. Int J Gynecol Cancer 2020;30:128–32. 10.1136/ijgc-2019-000734 [DOI] [PubMed] [Google Scholar]
- 18.Zikan M, Fischerova D, Pinkavova I, et al. Ultrasound-guided tru-cut biopsy of abdominal and pelvic tumors in gynecology. Ultrasound Obstet Gynecol 2010;36:767–72. 10.1002/uog.8803 [DOI] [PubMed] [Google Scholar]
- 19.Shawker TH, Hill M, Hill S, et al. Ultrasound appearance of extramedullary hematopoiesis. J Ultrasound Med 1987;6:283–90. 10.7863/jum.1987.6.6.283 [DOI] [PubMed] [Google Scholar]