Abstract
Emerging zoonotic diseases exert a significant burden on human health and have considerable socioeconomic impact worldwide. In Asia, live animals as well as animal products are commonly sold in informal markets. The interaction of humans, live domestic animals for sale, food products, and wild and scavenging animals, creates a risk for emerging infectious diseases. Such markets have been in the spotlight as sources of zoonotic viruses, for example, avian influenza viruses and coronaviruses, Here, we bring data together on the global impact of live and wet markets on the emergence of zoonotic diseases. We discuss how benefits can be maximized and risks minimized and conclude that current regulations should be implemented or revised, to mitigate the risk of new diseases emerging in the future.
Keywords: zoonoses, live animal market, wet market, food security, One Health, EcoHealth, pandemics
Live and Wet Markets and Emerging Diseases
Zoonotic diseases have potential to cause global pandemics; large-scale outbreaks of zoonoses, resulting in huge numbers of deaths, have caused significant disruption to economies, political order, and societies throughout history [1]. These diseases can spread from animals to humans where there is an interface allowing pathogens to jump species, such as in a farm or a market.
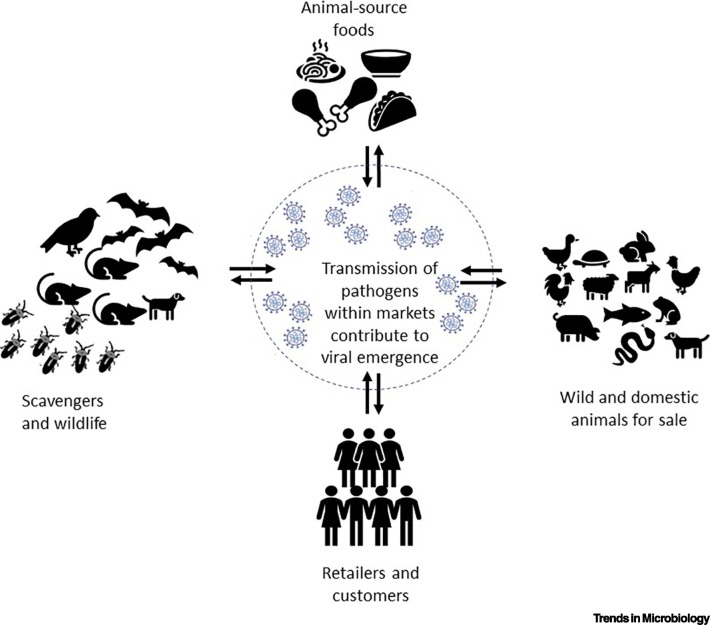
Live and wet markets (LWMs), markets selling live animals, and animal products (sometimes called traditional, as opposed to modern markets), are widespread in the growing cities in low- and middle-income countries (LMICs), including those in Asia and Africa. Many people depend on these markets for their livelihoods and food supply. The interaction of humans, both retailers and customers; live animals for sale; food products, including ready-to-eat food, as well as wild and peri-domestic animals, pose important risk factors for emerging infectious diseases. But LWMs also bring many benefits, for example, their cultural significance has brought numerous international tourists to visit local LWMs [2]. The markets are accessible to local consumers; they often sell traditional and well-liked foods, and they foster personal relations between buyers and sellers. Yet, these markets, while providing customers with animals to consume, or animal-sourced foods, undoubtedly act as an interface for virus exchange with a high risk of cross-species transmission to humans (Figure 1 ). The recent history of outbreaks of coronaviruses (CoVs) and avian influenza viruses (AIVs) has well illustrated that these emerging zoonotic diseases, originating from animals in LWMs, can present threats to human health [3]. The impact on health and economy in LMICs by zoonotic infections spread in LWMs is likely largely underestimated, but perhaps also the importance of these markets on livelihoods, nutrition and psychosocial wellbeing is also underestimated.
Figure 1.
Potential for Viral Emergence in Live and Wet Markets.
These markets bring together humans, both retailers and customers, with animals. Both wild and domestic animals may be for sale live or slaughtered, as well as sold as ready-to-eat foods. In addition, the markets are places that attract scavenging animals and pests. The often-crowded conditions, and lack of sanitation, create optimal circumstances for the spread of zoonotic and foodborne diseases, as well as the emergence of new viruses.
By categorizing known pathogens, one study found that over 60% of human emerging infectious diseases are zoonotic, and the majority of these (72%) originate in wildlife [4], while another estimate was that 75% of emerging pathogens were zoonotic [5]. While estimates vary, there is overall consensus that most emerging viruses originate from animals. Many emerging epizootic and zoonotic virus infections involve single-stranded RNA viruses, such as coronaviruses, capable of causing infections that can be most severe in both animals and humans. Recently 1445 novel RNA viruses were discovered in invertebrates and more than 200 previously unknown viruses were found in vertebrates [6,7]. The true extent of the ‘virosphere’, however, remains largely unknown and still needs to be explored in depth. Most RNA virus populations exist as complex mixtures of genetic and phenotypic variants, as a result of the high RNA polymerase error rate [8]. However, these errors may also be lethal for the virus, which coronaviruses seem to avoid by having an increased proofreading capacity [9,10]. The high rate of mutation creates diverse viral populations, which increases the likelihood that some variants can adapt to a new environment or a new host, and thus facilitate new emerging virus variants. Moreover, changes in natural conditions often drive genetic evolution, such as genetic reassortment and recombination, possibly resulting in the transition of microbes from nonpathogenic to pathogenic, from low virulence to high virulence, thus causing emergence of zoonotic diseases [11]. Cross-species transmission and the ability to sustain many cycles in the new host, which is needed for human-to-human transmission, require that the virus has the ability to interact with receptors in the new host [12]. It has been hypothesized that viral evolution in the new host depends on the trade-off between virulence and transmission [13]. This is exemplified by the different human CoV epidemics, in which Middle East respiratory syndrome (MERS) CoV has a high case fatality, but transmits less frequently than severe acute respiratory syndrome (SARS)-CoV-2, which has lower case fatality [14,15].
Factors that connect naïve hosts with pathogens, including crowding, urbanization, increasing human populations with immunosuppression and comorbidities, deforestation, fragmentation of natural habitats, agricultural intensification, and globalization, could be drivers for viral shifting [16]. These factors are prominent in many LMICs today. The growing population has growing needs for food, particularly in large cities with substantial middle-income classes, with the demand for animal-source food increasing, driven by increasing population, urbanization, and wealth [17., 18., 19.]. Meeting this demand for perishable products is not without its challenges. In countries lacking infrastructure, it can be impossible to transport animal-source foods over long distances, especially in tropical temperatures. Lacking a cold chain, people prefer purchasing live animals or fresh meat, and often animals are trekked or transported across considerable distances from rural to urban areas or from urban and peri-urban farms. However, despite the increasing availability of affordable refrigeration, the demand for live animals or fresh meat in wet markets persists, as people believe that fresh meat is safer, tastier, and more natural [20]. Moreover, even in urban areas, household cold storage may be lacking, and electricity 'outages' or failures are common. Also, in many cultures, households prefer to buy fresh food every day rather than buying in bulk and often value the social interactions experienced in LWMs.
Interspecies Transmission
Unfortunately, LWMs, which supply fresh products to millions of customers in tropical and subtropical regions every day, increase the risk of viruses jumping from animals to humans. In 2002–2003, an outbreak of SARS, caused by a coronavirus later termed SARS-CoV, emerged from a LWM in southern China [3]. Recently, a new pandemic, coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, has been associated with a LWM in Wuhan in the Hubei province of China [21]. CoVs have a wide host range and give rise to important animal and human diseases, including transmissible gastroenteritis virus in pigs, infectious bronchitis in poultry, feline infectious peritonitis and others. The highly transmissible and human-pathogenic CoVs belong to Betacoronavirus: SARS-CoV, MERS-CoV, and SARS-CoV-2. SARS-CoV-2 is the third CoV to emerge within 18 years, which, along with SARS-CoV and MERS-CoV, has spread from their natural hosts to humans [14], mostly via an intermediate host. However, despite the zoonotic origin, SARS-CoV-2 has become independent of animal reservoirs, and is transmitted from human to human, albeit with spillover events occurring to different animals [22]. By late 2020, more than 44 million people in over 208 countries and territories had been reported to have COVID-19, with more than a million deaths (https://covid19.who.int). Many countries have tried to combat rapid community transmission of SARS-CoV-2 through a variety of public health measures, including contact tracing, social distancing, sanitization, and the use of face masks. Due to the devastating impact of COVID-19, many cities and even entire countries have had to be under lockdown [23].
The origin of many important zoonotic viruses appears to be bats, which may not be surprising as they comprise almost a quarter of mammalian species [24] and have both biological and behavioral features facilitating disease emergence, such as close contact in large colonies [25]. Bats have been shown to harbor many different coronaviruses [26] as well as the recently reported influenza A subtypes H17N10 and H18N11 [27]. For these, there is often an amplifying host more closely related to humans which allows a species jump. For MERS-CoV, the main amplifying host close to humans has been identified as dromedaries, while for SARS-CoV and SARS-CoV-2 (Table 1 ), the amplifiers were probably mammals sold at LWMs [28,29]. The animal source of SARS-CoV-2 is thought to be bats or pangolins, potentially the latter being only an intermediary host [30,31]. SARS-CoV-2 has also been shown to be able to infect other mammals, including cats, which can transfer the virus between themselves [32,33], and minks [34], with large outbreaks being reported in mink farms. Other zoonotic viruses, such as Ebola and Nipah virus, may spread directly from bats to humans without an intermediary host [35., 36., 37.].
Table 1.
An Overview of Zoonotic Pathogens with High Potential for Spread through Live Animal and Wet Markets
| Pathogens | Original animal/natural host | Potential for spread in markets | High-risk area |
|---|---|---|---|
| Crimean–Congo hemorrhagic fever virus | Ticks, ruminants | Live ruminants brought to markets could spread virus through body fluids, or through vectors | Africa, the Balkans, the Middle East and Asia |
| Ebola viruses | Bats and/or primates | Sale of live exotic animals or bush meat brings the pathogen close to humans | West and Central Africa |
| Hantaviruses | Rodents, shrews, moles, bats | Reservoir animals may be sold at markets, but scavenging rodents may also bring the pathogens close to the markets and contaminate products | Worldwide |
| Hepatitis E virus | Domestic pigs, wild boars and maybe other animal species | Spread through food products or contacts with live animals at market | Worldwide |
| Avian influenza virus | Wild birds, poultry | Infected birds can transmit the virus to humans | Worldwide (mainly in Southeast Asia and the Middle East) |
| Marburg virus | Fruit bats of the Pteropodidae family | Bats sold at markets, or products contaminated by bats | sub-Saharan Africa |
| Monkeypox virus | Monkey | Through bush meat or live animals sold at markets | West and Central Africa |
| Nipah virus | Fruit bats, pig | Contaminated food products or live animals sold | South and Southeast Asia |
| Rabies virus | Carnivores, bats, dogs | Not transmitted by food, but, by bringing carnivores or bats live to markets, there is a risk of bites. Similarly, markets may attract scavenging dogs, increasing risks for bites | Africa and Asia |
| Coronaviruses | Bats, mammals | Large variety of coronaviruses could be brought by live animals taken to the market; some of these viruses may have zoonotic potential | Worldwide |
| Vector-borne viral disease | Mosquitoes and ticks | Wet markets could provide breeding grounds for mosquitoes and ticks in urban settings | Worldwide |
| Leptospira spp. | Livestock, rodents | Could be brought to markets through infected animals for sale, but also risk of scavenging rodents spreading the pathogen in the environment | Worldwide, with a higher incidence in tropical climates |
Migratory birds are the natural reservoir of influenza A viruses and act as the main route for AIV incursion and spread to different countries. AIVs are classified according to their level of virulence as either highly pathogenic AIVs (HPAIVs) or low-pathogenic AIVs (LPAIVs). Several AIV subtypes – such as HPAIV H5N1, H5N6, H7N7, and LPAIV H6N1, H7N2, H7N3, H7N4, H7N7, H7N9, H9N2, H10N7, and H10N8 – have occasionally crossed species barriers and infected humans [38., 39., 40., 41., 42.]. Several outbreaks by different subtypes of AIV have been linked to live-poultry markets [3,43]. Due to high-density settings and the mix of different species, live-poultry markets have been the source of different novel AIV reassortants that spread to and kill humans [44,45]. This has been exemplified by the recent emergence of zoonotic AIV of subtypes H7N9, H10N8 and H6N1 which were all linked with live-poultry markets [38., 39., 40., 41.] (Table 1).
In addition, the poor hygienic conditions, the presence of live animals kept and butchered on site, and the presence of foodborne bacteria and parasites, such as Campylobacter spp., Salmonella spp., Giardia lamblia, and Escherichia coli [2], have the potential to cause disease outbreaks and are responsible for a considerable disease burden. These endemic diseases are seldom as noteworthy as the viral epidemics, but likely contribute to more morbidity and mortality [46]
Is This the Final Call for Live Animal Market Closure?
In response to the zoonotic outbreaks, various actions targeting LWMs have been carried out. Temporary market closure has a proven effect on reducing the risk of spillover transmission [47,48]. However, long-term or permanent closure of LWMs is still highly controversial [49,50]. Informal LWMs play an important role for the food security and equity in LMICs, with low price and accessibility being the most important reasons for choosing these markets in Asia, where they have been described as indispensable [51,52]. Even though it may be argued that large supermarkets could buy products in higher quantities and thereby reduce prices, informal markets have the advantage of being more easily accessible for the poorest, who can choose to purchase the amount they can afford [53]. While supermarkets may sell staples in large amounts at low prices, informal markets usually offer better prices for perishable food. And while a supermarket shopper may get a better price on a kilogram of pork, many low-income customers would prefer to buy a small piece even though, gram-for-gram, the price is higher, or they may select a less valuable piece of the carcass. Zimmerer and de Haan [54] point to the need for strengthening informal value chains to improve resilience during the pandemic, as small-scale farmers and markets were severely affected by restrictions with negative impact on food supply. In addition, while supermarkets often procure their products from large-scale, intensified, and often international, farms, LWMs represent a key avenue for many smallholder producers, which produce the bulk of the food in LMICs. While it is beyond the scope of this opinion paper to discuss all risks and benefits with livestock intensification, there are risks of zoonotic infections identified in both large-scale and small-scale livestock farming. Close contact with the animals, as well as the lack of proper space, sanitation, resources, and knowledge, may make smallholders more prone to zoonotic infections, while large-scale farms may be at risk of disease outbreaks due to the increased number of animals. Reviews have also found that there is a lack of data on the risks of many pathogens in different farming systems, such as urban livestock keeping, or intensified farming [55,56].
Successful closure of LWMs would be greatly dependent on the local socioeconomic conditions and cannot be performed without including the views of all involved stakeholders, taking into account food security and livelihoods. When LWMs offer so many benefits in terms of price, trust, and availability there is a considerable risk that they would go 'underground' and continue to sell food but with little public service capacity to assess this. Solutions should preserve the benefits that LWMs bring to people while mitigating the risks of disease outbreaks. There is also, perhaps, a false dichotomy between completely shutting down LWMs and allowing them to operate just as they currently do. A compromise could be to decrease the risks associated with LWMs and gradually improve their operation and sanitation; this would not only reduce the risk of emerging diseases but would also have significant impact on endemic foodborne diseases. In addition, there is a risk that completely banning LWMs would force the trade underground, resulting in even more risk since this would make it even more difficult to have sampling and control programs.
There are several questions that need to be addressed in order to understand the LWMs and the zoonotic diseases that may emerge in them, as well as the incentives of the stakeholders involved (see Outstanding Questions). To fully evaluate the zoonotic risks of LWMs, we recommend a scientific-based risk-assessment framework, combining in-field surveillance and risk assessment, enabling regulatory environment and appropriate interventions. More specifically, long-term, large-scale surveillance is imperative to understand the true prevalence of hazards in LWMs, and where the risk for disease emergence is the highest. This would include sampling and interviewing along the value chains, sampling food products and the environment, as well as the consumers and vendors. To identify new emerging pathogens, a pathogen-discovery approach with metagenomics will be necessary, in addition to testing for the most likely suspected pathogens. With this all-round surveillance system, rapid risk assessment aims to examine the environmental and zoonotic context, as well as the transmission potential of the current and future zoonotic outbreaks. The estimated growth rate of the epidemic, timing of the epidemic peak, and demand for hospital resources would support decision makers in government, business, and civil society. Accordingly, risk-based hierarchical controls would leverage the emergency response in high-risk areas and regular interventions in areas with low-to-medium risk.
Outstanding Questions.
We still need much more evidence on the role of LWMs in the risk of emerging diseases, as well as the contribution to livelihoods and food security.
• What are the animal species commonly present in different markets, both wanted and unwanted? It is necessary to do an inventory of different species that occur, and to include surveying different seasons and time periods, to also capture nocturnal guests to market premises, including bats and rodents, which may contaminate surfaces.
• Which pathogens are circulating in different hosts? The animals present in the markets may be reservoirs for potential pathogens, and therefore it may be necessary to screen more broadly than for the zoonoses we already know of.
• What is the level of exposure to known and potential pathogens in high-risk actors, such as the vendors? It may be necessary to conduct microbiological and molecular testing of feces and sputum from vendors, as well as to screen serum for antibodies. Is it common to find seroconversions to coronaviruses and influenza viruses in this population? Is spillover more common than we think?
• How much of the food supplied to an area goes through the live and wet markets, and would a formal market be able to absorb the demand if the other markets close? Is there really an infrastructure ready to provide an area with animal products, and if not, how can this be improved?
• Are vendors and consumers aware of the risks of zoonotic and foodborne diseases, and what measures do they take to protect themselves? We need to understand what is the general level of knowledge, and how products are handled, in order to design possible interventions that could mitigate risks.
• What are the incentives that could motivate consumers and vendors to change their behavior? How can the risky markets be made safer without increasing the price for consumers beyond what they can afford?
Alt-text: Outstanding Questions
The One Health approach that recognizes the interconnectedness of human, animal, and environmental health is accepted as the best approach to address emerging and re-emerging zoonotic diseases. One Health should be integrated throughout the proposed risk assessment framework, incorporating data on ecosystem health, wild-animal disease, and human health. For zoonotic diseases, consistent surveillance should synthesize the animal, human, and environmental indicators, providing a valuable tool for early warning of zoonotic diseases. This could include syndromic surveillance, which could alert the health system of, for example, increased cases of febrile disease, or abortions in animals, and then trigger sampling for diagnosis, as well as environmental parameters such as increased precipitations or waterlogging, which could increase the vector population. Similarly, scientific evaluation tools, such as mathematical models, should initially clarify the animal–human–environment interactions, and subsequently evaluate the effect of a variety of policy options. To date, prevention and control of zoonotic diseases at source are still insufficient, mirroring the limited understanding of the underlying transmission mechanism in the animal reservoir. Given this, existing surveillance and model assessment should be improved for better monitoring and inferring disease dynamics in animal reservoirs. Markets selling live animals or animal products will always be associated with different hazards, but it is important to use a risk-based approach to these to be able to prioritize surveillance and interventions where the risks are the highest [57,58]. By using the One Health approach, lowering the potential of future zoonotic outbreaks would be more sustainable compared with other strict approaches such as market closure.
In reality, the decision to close markets has not been made based on scientific evidence due to the urgency of, and social reaction to, the situation. In China, after the outbreak of COVID-19 linked to a seafood wholesale market in Wuhan in late 2019, China closed this wet market on 1 January 2020, (www.nationalgeographic.com/animals/article/coronavirus-linked-to-chinese-wet-markets), when the virus had already spread and was no longer dependent on an animal host. Even though SARS-CoV2 had a zoonotic origin, the virus is transmitted mainly between humans, with occasional reverse zoonosis events [22]. Shortly after that, China also banned all the trade and consumption of wildlife for food on 26 January 2020. The response of China to COVID-19, regarding wildlife and wet market management, was rapid, but in reality it was unlikely to have affected the spread of the pandemic. Similarly, in July 2020, the neighboring country, Vietnam, also banned wildlife imports and ordered the closure of illegal wildlife markets to protect human health and ecological balance. It is worth mentioning that the practices of trading live animals vary a lot from one context to another. For example, in China and Vietnam, wildlife is actually not allowed in wet markets, and live animals are not permitted to be sold and slaughtered in wet markets in urban and peri-urban settings. However, this still occurs, particularly in more rural areas.
These strong policies to close the LWMs might be useful and effective in the short term to reduce disease transmission if there is still an animal host, such as the case with AIV. However, from a medium- and long-term perspective, decision-makers need to consider the livelihood, nutrition, and socioeconomic impacts of market closure on people whose livelihood relies on LWMs, particularly for the poor who lose their daily income. This also plays an important role in food security because it limits the access to foods. Altogether, the social impact of LWM closure might be greater than the health impact caused by the diseases, and assessments of the socioeconomic consequences of trade restrictions and market closures have been done for smaller outbreaks of emerging infectious diseases, such as Rift Valley fever [59]. However, in the case of the SARS-CoV-2 outbreak, this has not yet been evaluated.
No one can predict exactly when or where the next virus outbreak will appear. However, information is emerging on some of the drivers of novel virus outbreaks [60]. Focus on early identification of emerging or re-emerging outbreaks could be an effective measure of disease preparedness and stopping a virus from taking hold [61]. This implies that LWM surveillance needs to be established in a sustainable and systematic manner, to avoid conducting surveillance only in a response to outbreaks. Here, a collaboration between veterinary and public health authorities is necessary, in addition to a participatory collaboration with the actual stakeholders. Participatory disease surveillance has previously been implemented to achieve control of different infectious diseases, such as avian influenza, in LMICs [62], and in some cases it was found to be sustainable. The principle of participatory disease surveillance includes the population at risk in the prioritization and shaping of a control program so that they can contribute, with their unique knowledge and social context, beyond what technical experts could know. This provides the means for more qualitative, active surveillance [63,64], with high sensitivity allowing for rapid response.
However, increased preparedness for zoonotic emergence in LWMs may not necessarily reduce risk. A gradual improvement of the markets with improved food safety, hygiene standards, reduced crowding and mixing of animals, as well as regular inspections from trustworthy officials with authority to sample and condemn products, is necessary to mitigate public health risks, not only from emerging diseases but also from endemic diseases. However, these changes should not add to the expenses of the vendors, unless they bring additional benefits. In addition, there may be low trust in officials where corruption is common, and the possibility of obtaining objective results, such as laboratory analyses, may be limited. Therefore, there is a need to evaluate participatory research in which interventions are acceptable by the communities, and in which consumers are willing to pay extra for improvements, or governments are prepared to subsidize. There is a slow progression in many countries towards more formal markets, including in Asia [51,52], but still the infrastructure is not sufficient to allow a complete closure of wet markets in many LMICs. Interestingly, in high-income countries, there is increasing interest in open-air farmers' markets or street markets which sell fresh food under sanitary conditions.
Concluding Remarks and Future Perspectives
As argued in the previous text, LWMs are important not only for the food supply in LMICs, they also contribute to the risk of disease emergence. While much attention is given to wildlife as being exotic species deliberately brought to markets, assessing the unwanted peri-domestic wildlife in markets, including rodents, bats, dogs, and birds, is highly recommended and often neglected.
Without efficient vaccines and treatments, the early preparedness and detection of these emerging viruses is extremely important. Thus, it is crucial to reconsider the current regulations and practices in LWMs and develop surveillance systems that take the interests of all stakeholders into account.
Acknowledgments
Acknowledgments
J.L. and J.F.L. was supported by the 'Metropolitan Mosquitoes Project' funded by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas, grant number 2016-00364). M.N. is supported by the Swedish Research Council VR (2018–02569).
Declaration of Interests
There are no interests to declare.
References
- 1.Kruse H., et al. Wildlife as source of zoonotic infections. Emerg. Infect. Dis. 2004;10:2067–2072. doi: 10.3201/eid1012.040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kogan N.E., et al. Wet markets and food safety: TripAdvisor for improved global digital surveillance. JMIR Pub. Health Surveill. 2019;5:e11477. doi: 10.2196/11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster R.G. Wet markets – a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004;363:234–236. doi: 10.1016/S0140-6736(03)15329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones K.E., et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor L.H., et al. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi M., et al. The evolutionary history of vertebrate RNA viruses. Nature. 2018;556:197–202. doi: 10.1038/s41586-018-0012-7. [DOI] [PubMed] [Google Scholar]
- 7.Shi M., et al. Redefining the invertebrate RNA virosphere. Nature. 2016;540:539–543. doi: 10.1038/nature20167. [DOI] [PubMed] [Google Scholar]
- 8.Lauring A.S., Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofer U. Fooling the coronavirus proofreading machinery. Nat. Rev. Microbiol. 2013;11:662–663. doi: 10.1038/nrmicro3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robson F., et al. Coronavirus RNA proofreading: molecular basis and therapeutic targeting. molecular. Cell. 2020;79:710–727. doi: 10.1016/j.molcel.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longdon B., et al. The evolution and genetics of virus host shifts. PLoS Pathog. 2014;10:e1004395. doi: 10.1371/journal.ppat.1004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., et al. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E5135–E5143. doi: 10.1073/pnas.1802879115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acevedo M.A., et al. Virulence-driven trade-offs in disease transmission: A meta-analysis. Evolution. 2019;73:636–647. doi: 10.1111/evo.13692. [DOI] [PubMed] [Google Scholar]
- 14.Guarner J. Three emerging coronaviruses in two decades. Am. J. Clin. Pathol. 2020;153:420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Que J., et al. Psychological impact of the COVID-19 pandemic on healthcare workers: a cross-sectional study in China. Gen. Psychiatry. 2020;33 doi: 10.1136/gpsych-2020-100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindahl J.F., Grace D. The consequences of human actions on risks for infectious diseases: a review. Infect. Ecol. Epidemiol. 2015;5:30048. doi: 10.3402/iee.v5.30048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu Hatab A., et al. Urbanization, livestock systems and food security in developing countries: A systematic review of the literature. Food Security. 2019;11:279–299. doi: 10.3390/pathogens10050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindahl J.F., et al. In: Encyclopedia of Food Security and Sustainability. Ferranti P., et al., editors. Elsevier; 2019. Urban livestock keeping: leveraging for food and nutrition security; pp. 322–325. [Google Scholar]
- 19.Thornton P.K. Livestock production: recent trends, future prospects. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2010;365:2853–2867. doi: 10.1098/rstb.2010.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roesel K., Grace D. 1st edn. Routledge; 2015. Food Safety and Informal Markets. [Google Scholar]
- 21.Wu Y.-C., et al. The outbreak of COVID-19: An overview. J. Chinese Med. Assoc. 2020;83:217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sooksawasdi Na Ayudhya S., Kuiken T. Reverse zoonosis of COVID-19: lessons from the 2009 influenza pandemic. Vet. Pathol. 2020;58:234–242. doi: 10.1177/0300985820979843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaxman S., et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 24.Jones K.E., et al. A phylogenetic supertree of the bats (Mammalia: Chiroptera) Biol. Rev. 2002;77:223–259. doi: 10.1017/s1464793101005899. [DOI] [PubMed] [Google Scholar]
- 25.Han H.-J., et al. Bats as reservoirs of severe emerging infectious diseases. Virus Res. 2015;205:1–6. doi: 10.1016/j.virusres.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu G., et al. Bat-to-human: spike features determining 'host jump' of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23:468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y., et al. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 2014;22:183–191. doi: 10.1016/j.tim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Wit E., et al. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilgenfeld R., Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antivir. Res. 2013;100:286–295. doi: 10.1016/j.antiviral.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam T.T., et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 31.Andersen K.G., et al. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallapaty S. Coronavirus can infect cats – dogs, not so much. Nature. 2020 doi: 10.1038/d41586-020-00984-8. Published online April 1, 2020. [DOI] [PubMed] [Google Scholar]
- 33.Schlottau K., et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe. 2020;1:e218–e225. doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molenaar R.J., et al. Clinical and pathological findings in SARS-CoV-2 disease outbreaks in farmed mink (Neovison vison) Vet. Pathol. 2020;57:653–657. doi: 10.1177/0300985820943535. [DOI] [PubMed] [Google Scholar]
- 35.Leroy E.M., et al. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector-Borne Zoon. Dis. 2009;9:723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- 36.Marí Saéz A., et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol. Med. 2015;7:17–23. doi: 10.15252/emmm.201404792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weatherman S., et al. Transmission of henipaviruses. Curr. Opin. Virol. 2018;28:7–11. doi: 10.1016/j.coviro.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi W., et al. Genesis of the novel human-infecting influenza A(H10N8) virus and potential genetic diversity of the virus in poultry, China. Euro Surveill. 2014;19:20841. doi: 10.2807/1560-7917.es2014.19.25.20841. [DOI] [PubMed] [Google Scholar]
- 39.Wei S.H., et al. Human infection with avian influenza A H6N1 virus: an epidemiological analysis. Lancet Respir. Med. 2013;1:771–778. doi: 10.1016/S2213-2600(13)70221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naguib M.M., et al. Global patterns of avian influenza A (H7): virus evolution and zoonotic threats. FEMS Microbiol. Rev. 2019;43:608–621. doi: 10.1093/femsre/fuz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang T., et al. Human infection with influenza virus A(H10N8) from live poultry markets, China, 2014. Emerg. Infect. Dis. 2014;20:2076–2079. doi: 10.3201/eid2012.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nuñez I.A., Ross T.M. A review of H5Nx avian influenza viruses. Ther. Adv. Vaccines Immunother. 2019;7:1–15. doi: 10.1177/2515135518821625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J., et al. Isolation of H5N6, H7N9 and H9N2 avian influenza A viruses from air sampled at live poultry markets in China, 2014 and 2015. Euro Surveill. 2016;21:30331. doi: 10.2807/1560-7917.ES.2016.21.35.30331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou X., et al. The role of live poultry movement and live bird market biosecurity in the epidemiology of influenza A (H7N9): A cross-sectional observational study in four eastern China provinces. J. Infect. 2015;71:470–479. doi: 10.1016/j.jinf.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Bao C.-j., et al. Live-animal markets and influenza A (H7N9) virus infection. N. Engl. J. Med. 2013;368:2337–2339. doi: 10.1056/NEJMc1306100. [DOI] [PubMed] [Google Scholar]
- 46.Havelaar A.H., et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu H., et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study. Lancet. 2014;383:541–548. doi: 10.1016/S0140-6736(13)61904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi N., et al. Interventions in live poultry markets for the control of avian influenza: a systematic review and meta-analysis. J. Infect. Dis. 2020;221:553–560. doi: 10.1093/infdis/jiz372. [DOI] [PubMed] [Google Scholar]
- 49.Yuan J., et al. Effect of live poultry market closure on avian Influenza A(H7N9) virus activity in Guangzhou, China, 2014. Emerg. Infect. Dis. 2015;21:1784–1793. doi: 10.3201/eid2110.150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y., et al. Closure of live bird markets leads to the spread of H7N9 influenza in China. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorton M., et al. Wet markets, supermarkets and the 'Big Middle' for food retailing in developing countries: evidence from Thailand. World Dev. 2011;39:1624–1637. [Google Scholar]
- 52.Maruyama M., Trung L.V. The nature of informal food bazaars: Empirical results for Urban Hanoi, Vietnam. J. Retail. Consum. Serv. 2010;17:1–9. [Google Scholar]
- 53.Crush J., Frayne B. Supermarket expansion and the informal food economy in Southern African cities: implications for urban food security. J. South. Afr. Stud. 2011;37:781–807. [Google Scholar]
- 54.Zimmerer K.S., de Haan S. Informal food chains and agrobiodiversity need strengthening – not weakening – to address food security amidst the COVID-19 crisis in South America. Food Security. 2020;12:891–894. doi: 10.1007/s12571-020-01088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White R.J., Razgour O. Emerging zoonotic diseases originating in mammals: a systematic review of effects of anthropogenic land-use change. Mammal Rev. 2020;50:336–352. doi: 10.1111/mam.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindahl J., Magnusson U. Zoonotic pathogens in urban animals: Enough research to protect the health of the urban population? Anim. Health Res. Rev. 2020;21:50–60. doi: 10.1017/S1466252319000100. [DOI] [PubMed] [Google Scholar]
- 57.Grace D., et al. Safe food, fair food: participatory risk analysis for improving the safety of informally produced and marketed food in sub-Saharan Africa. Rev. Afr. Santé Prod. Anim. 2010;8:3–11. [Google Scholar]
- 58.Roesel K., Grace D. 1st edn. Routledge; 2014. Food Safety and Informal Markets: Animal Products in sub-Saharan Africa. [Google Scholar]
- 59.Rich K.M., Wanyoike F. An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley Fever outbreak in Kenya. Am. J. Trop. Med. Hyg. 2010;83:52–57. doi: 10.4269/ajtmh.2010.09-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.United Nations Environment Programme (UNEP) and International Livestock Research Institute . UNEP; Nairobi, Kenya: 2020. Preventing the next pandemic: Zoonotic diseases and how to break the chain of transmission. [Google Scholar]
- 61.Holmes E.C., et al. Pandemics: spend on surveillance, not prediction. Nature. 2018;558:180–182. doi: 10.1038/d41586-018-05373-w. [DOI] [PubMed] [Google Scholar]
- 62.Mariner J.C., et al. Experiences in participatory surveillance and community-based reporting systems for H5N1 highly pathogenic avian influenza: a case study approach. Ecohealth. 2014;11:22–35. doi: 10.1007/s10393-014-0916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mariner J.C., et al. Integration of participatory approaches into surveillance systems. Rev. Sci. Tech. 2011;30:653–659. doi: 10.20506/rst.30.3.2065. [DOI] [PubMed] [Google Scholar]
- 64.Smolinski M.S., et al. Participatory disease surveillance: engaging communities directly in reporting, monitoring, and responding to health threats. JMIR Pub. Health Surveill. 2017;3:e62. doi: 10.2196/publichealth.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]