Abstract
Major advances have recently defined functions for human mono-ADP-ribosylating PARP enzymes (mono-ARTs), also opening up potential applications for targeting them to treat diseases. Structural biology combined with medicinal chemistry has allowed the design of potent small molecule inhibitors which typically bind to the catalytic domain. Most of these inhibitors are at the early stages, but some have already a suitable profile to be used as chemical tools. One compound targeting PARP7 has even progressed to clinical trials. In this review, we collect inhibitors of mono-ARTs with a typical “H–Y−Φ” motif (Φ = hydrophobic residue) and focus on compounds that have been reported as active against one or a restricted number of enzymes. We discuss them from a medicinal chemistry point of view and include an analysis of the available crystal structures, allowing us to craft a pharmacophore model that lays the foundation for obtaining new potent and more specific inhibitors.
Introduction
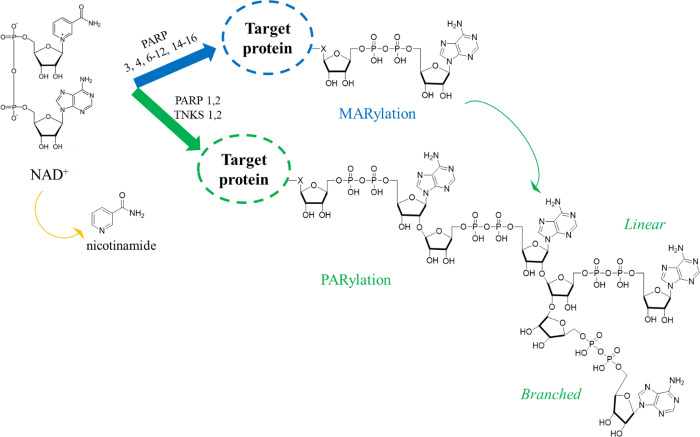
ADP-ribosyltransferases catalyze the covalent attachment of ADP-ribose units post-translationally on a variety of amino acid residues of target proteins.1 ADP-ribosylation is found in bacteria2 as well as in eukaryotes, and indeed, human PARPs, previously referring to “poly-ADP-ribose polymerase”, and tankyrases (TNKS) form a family of diphtheria-toxin-like ADP-ribosyltransferases (ARTDs). The family name comes from the similarities of their catalytic domain with that of diphtheria toxin,3 which exerts its pathogenic mechanism by ADP-ribosylating the specific diphthamide residue of the elongation factor 2.4 Similarly, many other bacterial toxins specifically label target proteins affecting the host cell functions.5 Since the 1960s, when the poly-ADP-ribosylation activity was discovered,6 different nomenclatures have been used for these enzymes, as reported in Table 1. In this review, we will use the recent recommended nomenclature3 with the term “PARP” (or TNKS) only used in association with the exact number of PARP or TNKS referred to and the terms mono-ARTs and poly-ARTs to indicate the two subfamilies of the mono-ADP-ribosylating (MARylating) and poly-ADP-ribosylating (PARylating) enzymes, respectively.
Table 1. Overview of the PARP and TNKS Enzymes of the ARTD Family.
| activity | protein | other nomenclature | catalytic motif | localization | exemplary functions | noncatalytic domainsa |
|---|---|---|---|---|---|---|
| poly-ART | PARP1 | ARTD1, PARS, ADPRT1 | H–Y–E | nuclear/cytosolic | DNA damage repair | 3 × ZnF; BRCT; WGR; HD |
| PARP2 | ARTD2 | H–Y–E | nuclear | DNA damage repair | WGR | |
| HD | ||||||
| TNKS1 | PARP5a; ARTD5 | H–Y–E | nuclear/cytosolic | telomere elongation and Wnt/β-catenin signaling | 5 × ARC; SAM | |
| TNKS2 | PARP5b; ARTD6 | H–Y–E | nuclear/cytosolic | telomere elongation and Wnt/β-catenin signaling | 5 × ARC; SAM | |
| mono-ART | PARP3 | ARTD3 | H–Y–E | nuclear | DNA damage repair | WGR |
| HD | ||||||
| PARP4 | vPARP; ARTD4 | H–Y–E | nuclear/cytosolic | unknown role in vault particles | BRCT; HD; vWA; MVP BD; VIT | |
| PARP6 | ARTD17 | H–Y–I | cytosolic | G2-M cell cycle progression, neurodevelopment | ||
| PARP7 | tiPARP; ARTD14 | H–Y–I | nuclear/cytosolic | gene regulation, immune response | ZnF; WWE | |
| PARP8 | ARTD16 | H–Y–I | nuclear/cytosolic | cellular apoptosis pathway | ||
| PARP10 | ARTD10 | H–Y–I | nuclear/cytosolic | immune response, cell proliferation, DNA damage repair | 3 × UIM; 2 × RRM | |
| PARP11 | ARTD11 | H–Y–I | cytosolic | immune response, nuclear pore formation | WWE | |
| PARP12 | ARTD12, ZC3HDC1 | H–Y–I | cytosolic | immune response, stress granule formation, vesicle trafficking | 4 × ZnF; 2 × WWE | |
| PARP14 | BAL2; ARTD8 | H–Y–L | nuclear/cytosolic | gene regulation, immune response | RRM; WWE; 3 × MD | |
| PARP15 | BAL3; ARTD7 | H–Y–L | nuclear/cytosolic | stress granule formation | 2 × MD | |
| PARP16 | ARTD15 | H–Y–Y | cytosolic | ER stress response | PRD | |
| inactive | PARP9b | BAL1; ARTD9 | Q–Y–T | nuclear/cytosolic | DNA damage repair and immune response | 2 × MD; DeBD |
| PARP13 | ARTD13, ZAP, ZC3HAV1 | Y–Y–V | cytosolic | immune and stress response | 4 × ZnF; WWE |
ZnF: zinc finger. BRCT: BRCA1 carboxy terminal domain. WGR: W, G, R domain. HD: helical regulatory domain. ARC: ankyrin repeat cluster. SAM: sterile alpha motif. vWA: von Willebrand factor type A domain. MVP BD: major vault particle binding domain. VIT: vault protein interalpha-trypsin domain. WWE: W, W, E, domain. UIM: ubiquitin interaction motif. RRM: RNA recognition domain. MD: macrodomain. PRD: putative regulatory domain. DeBD: Deltex binding domain.
PARP9 complex with an E3 ubiquitin ligase DTX3L is actually able to MARylate ubiquitin.
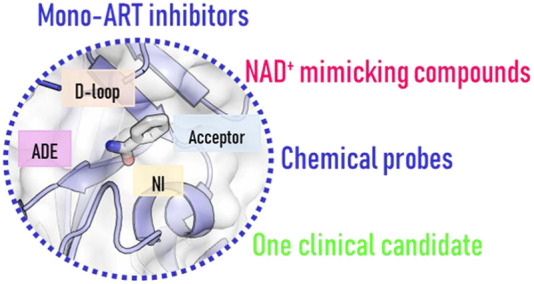
Different classifications can be used for the various ART subfamilies based on their functions or on the catalytic activity. PARP1, PARP2, and PARP3 can be also defined as DNA-dependent enzymes; TNKS1 and TNKS2 (previously known also as PARP5a and PARP5b) belong to the class of tankyrases; three members contain CCCH zinc fingers (PARP7, PARP12, and PARP13); three enzymes contain two or three macrodomains (PARP9, PARP14, and PARP15).7 All of the members share a common catalytic domain, which binds the substrate β-nicotinamide adenine dinucleotide (NAD+) and transfers the ADP-ribose units of NAD+ to target macromolecules. The nicotinamide portion of the substrate is released as a byproduct (Figure 1). The catalytic domain can be divided into a donor site binding substrate NAD+ and an acceptor site where the target macromolecule or the PAR chain to be elongated binds. The donor site is composed of a nicotinamide binding pocket (NI site), a phosphate binding site, and an adenine ribose binding site (ADE site). The NI site is characterized by a highly conserved motif constituted by two tyrosine residues that generate π–π interactions with the nicotinamide ring and serine and glycine residues responsible for two essential hydrogen bonds utilized also by most of the developed pan-PARP inhibitors.8,9 The donor site and acceptor site are surrounded by two different loops, a donor loop (D-loop) and an acceptor loop, respectively. The D-loop is not conserved among the subfamilies and influences their catalytic activity. While the catalytic domain is shared by all of the subfamilies, additional noncatalytic domains are found in various PARPs that modulate the enzymatic activity, macromolecular interactions, and protein localization.10,11
Figure 1.
MARylation and PARylation ART mediated as a post-translational modification of proteins with concomitant release of nicotinamide from NAD+ as a byproduct. In some cases, MARylated proteins can be also further modified by poly-ARTs PARP1–2 and TNKS1–2 that can elongate the chain.
According to their catalytic activity, ARTs can be classified into mono-ARTs, poly-ARTs, and inactive proteins. Poly-ARTs (PARP1–2 and TNKS1–2) iteratively catalyze the covalent attachment of multiple ADP-ribose units, resulting in poly-ADP-ribosylation (called PARylation for conciseness, Figure 1).
The polymer is formed by α(2–1) O-glycosidic bonds, allowing linear (2′–1′′ ribose ribose glycosidic bond) or branched (2″–1″′ ribose ribose glycosidic bond) chains, and can span from a few up to 200 ADP-ribose units.12 The PARylation activity mainly results from the presence of a triad of amino acids “H–Y–E” (Table 1) in which histidine and tyrosine are responsible for the correct orientation of NAD+, while the glutamate is essential for the elongation of the chain.13,14 The glutamate is also involved in stabilizing the oxocarbenium ion transition state of NAD+.13
The mono-ARTs (PARP3–4, -6–8, -10–12, and -14–16) are not capable of generating polymers but catalyze the addition of a single ADP-ribose unit (MARylation, Table 1, Figure 1). Typically, mono-ARTs present the triad “H–Y−Φ”, in which Φ corresponds to an isoleucine, leucine, or tyrosine residue, thus losing the glutamate critical for the PARylation reaction. In the mono-ART enzymes like PARP10, the oxocarbenium ion is stabilized by a glutamate belonging to the substrate protein, making the modification a substrate-assisted catalysis.15 However, the presence of the H–Y–E triad is not a sufficient condition to PARylate the substrate, as demonstrated by PARP3 and PARP4 that, despite the presence of an intact triad, lost the polymerase activity,16 suggesting that additional structural features like the donor site lining D-loop could limit the activity of PARP3–4 to MARylation.14
The catalytic domains of PARP9 (triad Q–Y–T) and PARP13 (triad Y–Y–V) do not have a histidine that is essential for the binding of NAD+, making them inactive (Table 1).7 The inactivity of PARP13 has been explained also by a closed and tightly packed active site,17 and it should be noted that the PARP9 complex with an E3 ubiquitin ligase is actually able to MARylate ubiquitin.18 The C-terminus of DTX3L is capable of doing this reaction alone,19 but the role of PARP9 in modulating the activity is unclear.20
The lysine, glutamate, aspartate, serine, and cysteine residues of the target proteins have been found to be MARylated and PARylated through the generation of O-, N-, or S-glycosidic bonds. A large amount of proteins has been identified as ADP-ribosylation targets21−23 of which some are specific because they are modified by a single enzyme, while others can be a substrate of a variety of enzymes. PARPs are also able to automodify themselves, which could limit their activity and affect their localization and stability.24 In addition to proteins, nucleic acids also have been demonstrated to be modified by ADP-ribosylation (reviewed recently by Feijs, Zaja et al.25). PARylation adds a large negative charge to the target proteins and subsequently modulates its interactions with other macromolecules but simultaneously acts as a localization signal for, e.g., DNA repair proteins26 and tags proteins for ubiquitination and subsequent proteasomal degradation.27 MARylation, despite being a smaller modification, causes similar phenomena and can also act as a priming modification for PARylation.28
ADP-ribosylation is a reversible process, and several enzymes not only bind the modification but can also hydrolyze it, limiting the signaling event (reviewed recently by Ahel et al.29). PAR glucohydrolase (PARG) and ADP-ribosyl hydrolase 3 (ARH3) are able to cleave ribose–ribose bonds and degrade the polymer. PARG cannot remove the proximal serine MARylation prominent in DNA repair, and ARH3 is the enzyme responsible for hydrolyzing this last ADP-ribose unit.30 ARH1 can remove the arginine MARylation, while TARG, MacroD1, and MacroD2 hydrolyze the ester bond between the ribose and the acidic amino acids.31 Finally, the NUDIX family of proteins is also involved in the degradation by hydrolyzing the phosphodiester bond in the protein-proximal ADP-ribose unit, but they do not remove the modification completely.32
DNA-activated PARP1–3 play critical roles in maintaining our genomes. They differ in the various DNA damages they detect and by the resulting modification as PARP1–2 are poly-ARTs, while PARP3 is a mono-ART. The DNA damage recognizing part of the proteins also differs; PARP1 has specialized zinc fingers ZnF1, ZnF2, and ZnF3, the last essential for DNA-dependent activation. PARP2–3 lack these and rely on the WGR domain which is present in all of these proteins. The role of the various domains in PARP1–3 has been recently reviewed by de Oliveira and colleagues.10 PARP1 is the main DNA damage sensor and signal transducer and responsible for approximately 90% of PAR chain formation, while PARP2–3 have more specialized roles in the process as reviewed by Lavrik et al.33 PARP1–3 are autoinhibited by a helical regulatory domain, which undergoes conformational changes allowing substrate NAD+ binding and binding of a histone PARylation factor HPF1.34,35 HPF1 forms a joint active site with PARP1–2 and changes the residue specificity of the PARylation to serine, which is the major PARylated residue in DNA repair.36 PARylation of PARP itself and of histone tails leads to chromatin remodeling, recruitment of DNA repair factors through their PAR binding module, and release of PARP from the DNA damage site.37
PARP1–3 are activated by single-strand breaks (SSBs) that are repaired and do not progress to double-strand breaks (DSBs). Inhibition of these PARPs would prevent this repair, and a major advancement in using this property in cancer therapy came when it was discovered that PARP inhibition was synthetically lethal with the BRCA deficiency (breast cancer type 1/2 susceptible).38,39 BRCA1/2 are critical enzymes in the resolution of DNA DSBs by promoting the homologous recombination repair (HRR), and as BRCA deficiency is a common feature in multiple cancer cells, including breast and ovarian cancers, PARP inhibition appeared as a magic bullet to target these tumors alone or in combination with DNA damage causing therapy.40−42 On the basis of the essential role played by PARP1 in tumor progression and the discovered synthetic lethality, multiple academic and industrial efforts were initiated to improve the early PARP inhibitors (PARPi) toward clinically approved drugs,43 and patent literature on new PARPi has expanded44 since the PARP1 inhibitor development culminated with the approval of olaparib/Lynparza (AstraZeneca) by the FDA and EMA in 2014 for the treatment of BRCA-deficient ovarian cancers (Figure 2).45 This opened the way to a new anticancer treatment that expanded the precision medicine approach, and subsequently, talazoparib46 (Pfizer), rucaparib47 (Pfizer/Clovis), and niraparib48 (Merck/Tesaro) have been approved for the treatment of breast, ovarian, fallopian, peritoneal, pancreatic, and prostate cancers. In addition, veliparib, which is still in phase 3 clinical trials, has been approved for use by the EMA and FDA under an orphan designation for ovarian cancer.49 Multiple investigations on the approved drugs and new inhibitors are ongoing to expand the use of PARPi toward a range of other oncologies, including lung cancer and neuroblastoma.50−55
Figure 2.
PARPi currently in use as anticancer agents and in phase III of clinical trials, and examples of dual PARP1–2/TNKS1–2 inhibitors in clinical studies and TNKS inhibitors in the preclinical phase.
Besides the role of PARP1, TNKS1–2 poly-ARTs are currently being investigated as potential therapeutic targets in cancer mainly due to their role in controlling the Wnt/β-catenin signaling pathway. Readers are directed to the following reviews.56−59 TNKS1–2 control protein complexes and protein stability through PARylation and targeting proteins to proteasomal degradation. In Wnt/β-catenin signaling they control the so-called “β-catenin destruction complex” consisting of axin, casein kinase 1, adenomatous polyposis coli (APC), glycogen synthase kinase 3 GSK3, and protein phosphatase 2A. TNKSs PARylate axin, which is a limiting component of the complex, leading to reduced phosphorylation of β-catenin. TNKS inhibition thus stabilizes the complex and prevents β-catenin transport to the nucleus. Mutations in the destruction complex proteins, especially in APC, are frequent in cancers, and therefore, TNKS inhibitors could be used as a therapy for treating a range of cancers60 including colon,61 lung,62 liver,63 ovarian,64 and brain.65 Another essential role of these enzymes is explicated in the Hippo signaling. Indeed, they promote the activity of the oncoprotein YAP by suppressing the antagonist angiomotin (AMOT) family proteins involved in the YAP degradation. Elevated expression of YAP has been identified in various human cancers, and as YAP inhibitor development is very limited, TNKS inhibition could be a valid alternative for hampering YAP oncogenic properties.66 Several TNKS inhibitors have been developed67,68 such as compounds E744969 and STP100270 (structure undisclosed), which have progressed in clinical studies, even if compound E7449 behaves as a dual TNKS1–2 and PARP1–2 inhibitor. Other TNKS inhibitors such as RK287107 (1)71 and OM-153 (2)72 are being evaluated in preclinical studies.
While the development of poly-ART inhibitors is widely pursued with new compounds continuing to appear in the literature,44,67,73−75 the mono-ART inhibitor development is still in the early stages. However, remarkable advances have been achieved during recent years. The approved PARPi are not selective toward PARP1,76 especially the early inhibitors that also inhibit mono-ART enzymes; however, next generation of selective PARP1 inhibitors is emerging.77 Also the H–Y–E containing PARP3–4 are typically inhibited by the known PARPi78,79 although efforts have been recently made to develop selective inhibitors also for these enzymes.80,81 More attention is now paid toward assessing inhibitor selectivity as this is crucial information when using the discovered inhibitors as chemical probes to study the functions of the rather recently discovered and often understudied mono-ARTs.
Many human enzymes with mono-ART activity have been implied to have critical roles in cancer progression and other diseases, and their potential as drug targets is emerging.82−84 The first mono-ART inhibitors appeared about 10 years ago, but interest has increased in recent years with many compounds that have been just published, prompting us to collect all of them in this review. In particular, the inhibitors of mono-ARTs “H–Y−Φ” that have been reported as active against one enzyme or restricted toward the mono-ARTs subfamily will be described. We will discuss the identification strategy, the hit-to-lead optimization phase, and the possible SAR studies. When available, the cocrystal structures will be analyzed attempting to highlight the structural features leading to mono-ART selectivity and to derive a pharmacophore model differentiating mono-ART inhibitors from the PARPi described in the earlier literature.
Mono-ART Inhibitors
Mono-ART inhibitor discovery has often focused on some particular PARP, while efforts have been made to profile the inhibitors also against other enzymes of the family. This has revealed an overlap that can be somewhat expected due to the conserved catalytic domains. Often the discovery has taken advantage of previously described early PARP1 inhibitors, providing nicotinamide mimetic scaffolds that after (structure-guided) optimization led to discover selective/specific mono-ARTs inhibitors. The screening of large compound libraries in some cases permitted one to enrich the scaffold armamentarium. In the following we will address each human mono-ART separately, indicating possible selectivity data that are available for the compounds described in the literature. Many inhibitors have been reported for the best studied PARP10 and PARP14 that will be discussed first. Most of the other mono-ARTs, for which only one paper has been published, will be reported based on structure similarity and biological functions in the following order: PARP11, PARP15, PARP6, PARP12, and PARP16. The review will culminate describing the only clinical candidate, RBN-2397 (76), that selectively inhibits the PARP7.
PARP10 Inhibitors To Modulate Cell Proliferation, Inflammation, and DNA Repair
PARP10 was the first ARTD family member demonstrated to catalyze only mono-ADP-ribosylation.15 It is a 150 kDa protein localized both in the cytoplasm and in the nucleus, shuttling between the compartments.85 Its interaction partners, e.g., c-Myc and histones, are situated in the nucleus and others such as NEMO in the cytoplasm and some that shuttle between the compartments, similarly to PARP10, like proliferating cell nuclear antigen (PCNA).86 Both the catalytic activity as well as the ubiquitin interaction motifs of PARP10 are required for some of the cellular functions of the protein.87 PARP10 is able to automodify itself and other proteins on acidic residues. Even if the role of PARP10 is not completely elucidated, it is clear that it is a partner of the proto-oncoprotein c-Myc that functions as a transcriptional regulator involved in cellular apoptosis or proliferation.88 The interaction was confirmed by coimmunoprecipitation experiments that highlighted the involvement of the C-terminal half of PARP10 in the c-Myc binding. The PARP10 catalytic domain interacts with a PCNA linking PARP10 to the DNA replication progression under cellular stress conditions.86 In particular, upon replication fork arrest, the monoubiquitination of PCNA at its Lys164 generates a cascade of events, essential to restart the stalled fork. A downregulation of PARP10 is responsible for a reduction of the PCNA ubiquitination, thus inhibiting the replication.89 Multiple potential protein targets for PARP10 were identified through protein assays.90 Validated ones include GSK3β, an enzyme known for its involvement in Wnt signaling, metabolism, immunity, apoptosis, and tumorigenesis.90,91 PARP10 is differently expressed in the various tissues with enhanced expression in the thymus and spleen or adipose tissue and liver, suggesting potential roles in innate immunity and metabolism, respectively. On the other hand, PARP10 overexpression in various cancer cell lines along with the known PARP10 target proteins led to the hypothesis that PARP10 promotes cancer proliferation and acts as an oncogene.92
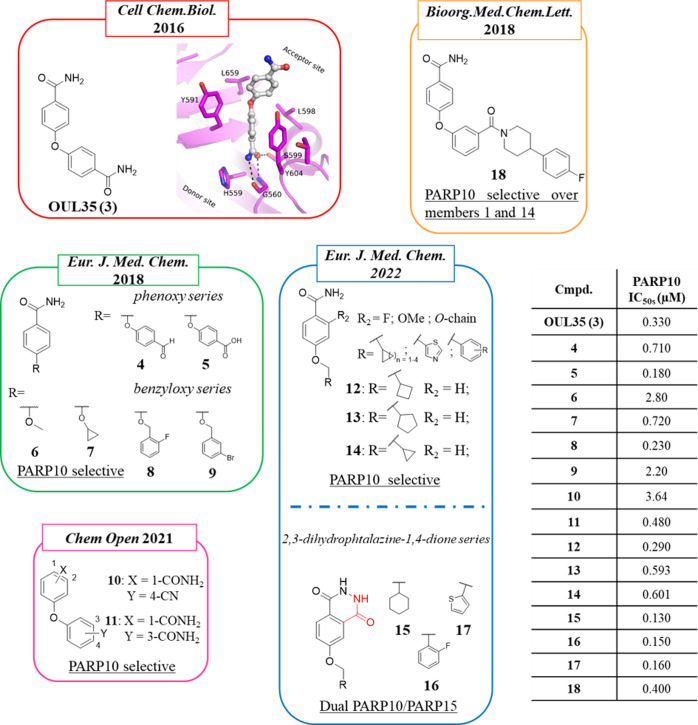
The first potent and selective PARP10 inhibitor is OUL35 (3) (Figure 3), which was identified in 2016 by Lehtiö et al.93 The authors developed an activity-based assay that was exploited to screen a library of 2638 compounds from the open chemical repository of the National Cancer Institute (NCI) against PARP10. The 19 hits identified were then tested in a dose–response assay, and a few of them showed nanomolar inhibitory activity with compound 3 emerging as one of the most interesting, exerting the desired inhibitory effect without showing significant cytotoxicity against HeLa cells. With an IC50 of 330 nM (Table 2), compound 3 was 13–300-fold selective for PARP10 over the other 12 mono- and poly-ARTs tested. Differential scanning fluorimetry (DSF) confirmed the inability of the compound to bind the inactive PARP9 and PARP13, while it stabilized the catalytic domain of PARP10. However, 3 also inhibited PARP14 and PARP15, although at lower potency (IC50 = 23 and 4.2 μM, respectively). Compound 3 inhibited PARP10 by interacting with its nicotinamide binding site through the benzamide moiety, as confirmed in DSF by the loss of the activity against a PARP10 inactive mutant in which the NI site is not accessible. Docking studies using PARP10 indicated that 3 would made hydrogen bonds with Gly888 and Ser927 and π-stacking with Tyr919 and Tyr932. In contrast to known PARPi, it extended toward the acceptor site, making hydrophobic interactions with Ile987 and Leu926. The crystal structure of 3 bound to the nicotinamide pocket of PARP15 mutant, gain-of-binding surrogate, confirmed its binding mode (Figure 3).
Figure 3.
Structure of OUL35 (3)93 along with its crystal structure with PARP15 gain-of-binding mutant94 and its analogues developed by Lehtiö et al.94 (in the green box), Schuler et al.95 (in the orange box), Tabarrini et al.96 (in the blue box), and Lüscher et al.97 (in the pink box) along with the IC50.
Table 2. Selectivity Profile of OUL35 (3) and Its Analogues against a Panel of ARTs (μM IC50s) and Activity in the Cell-Based Assay (μM).
| OUL35 (3)a | 8b | 9b | 10c | 11c | 12d | 15d | 16d | 17d | 18e | |
|---|---|---|---|---|---|---|---|---|---|---|
| PARP10 | 0.330 | 0.230 | 2.4 | 3.64 | 0.480 | 0.290 | 0.140 | 0.150 | 0.160 | 0.400 |
| PARP15 | >10f | 3.3 | 1.2 | 11.0 | 1.7 | 2.4 | 0.400 | 0.400 | 0.370 | n.t.g |
| PARP14 | 23 | 10 | 0.630 | >100 | 41 | ≫10 | ≫10 | ≫10 | ≫10 | 5.2 |
| TNKS1 | >100 | >100 | n.t. | >100 | 21 | >100 | >100 | >100 | >100 | n.t. |
| TNKS2 | >100 | >100 | >100 | >100 | 6.5 | >100 | >100 | >100 | >100 | n.t. |
| PARP1 | >100 | >100 | n.t. | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| PARP2 | >100 | >100 | >100 | 27 | 1.7 | 38 | >100 | >100 | >100 | n.t. |
| cell-based assayh | 1.35 | 2 | n.t. | 1.7 | 1.8 | 0.59 | 1.5 | 1.6 | 0.95 | n.t. |
Compound 3 was also able to enter HeLa cells and rescued the cells in a clonogenic assay from induced PARP10 overexpression in a dose-dependent manner with an IC50 of 1.35 μM, while the inactive analogs did not have this effect. The assay is based on the observation that overexpression of wild-type PARP10 but not the catalytically inactive mutant leads to cell death.98 Finally, in the presence of 5 μM of 3, HeLa cells were sensitized to hydroxyurea-induced DNA damage in accordance to the data reported for PARP10 knockdown. The potent and selective PARP10 inhibition replicated also in cells coupled with an unconventional binding mode extending toward the acceptor site made 3 a valid starting point for successive SAR investigations.94,95,97
In a follow-up study performed by Lehtiö, Tabarrini et al. in 2018, two series of 34 analogues (mainly phenoxy and benzyloxy derivatives) were purchased and synthesized maintaining one benzamide moiety of hit 3 while exploring the ether linker as well as the second benzamide portion (Figure 3).94 When the second amide was replaced by substituents such as the aldehyde (4) and carboxylic acid (5), good PARP10 inhibition was maintained (710 and 180 nM, respectively). Unfortunately, derivative 5 was completely inactive in a colony formation assay, most probably due to its unsuitable pharmacokinetic properties related to the negative charge. The replacement of one of the two benzamide moieties of 3 with smaller substituents was well tolerated. In particular, a methyl group was sufficient to maintain the activity (6, IC50 = 2.8 μM), but the presence of the cyclobutyl (7) gave nanomolar inhibitory activity (PARP10, IC50 = 720 nM) (Figure 3). From the linker exploration it emerged that the oxygen can be fruitfully replaced by a more flexible −OCH2. This linker did not tolerate a C-4′-substituted aromatic moiety, but other positions could be functionalized with the o-F benzene derivative 8 emerging as the best with an IC50 of 230 nM (Figure 3). Compound 8 was profiled against a panel of 13 additional PARP/TNKS enzymes, emerging as highly selective (from 1.5- to >435-fold selective), and the activity was also maintained in cells (IC50 = 2 μM). Also, the m-bromo substituent was suitable with 9 emerging as a weak PARP10 inhibitor (IC50 = 2.2 μM) but endowed with better PARP14 and PARP15 inhibitory activity, as reported in a successive paper (IC50 of 630 nM and 1.2 μM, respectively).99 Without any PARP2 and TNKS2 inhibition, the compound stood out as selective mono-ARTs inhibitor.
Two additional SAR studies on 3 were published successively (Figure 3, Table 2). In 2021, Lüscher, Lehtiö et al. reported 32 close analogues where the diphenyl ether core was variously decorated in the para and/or meta positions of both rings.97 The compounds were initially evaluated for their ability to inhibit PARP10 automodification, and then, the active compounds were tested in a colony formation assay in HeLa cells.98 Few compounds emerged as active, and the two best derivatives were the 4-phenoxybenzamide bearing a p-cyano group in the second ring, compound 10, and the 3-phenoxybenzamide 11 (Figure 3), a strict positional analogue of 3, also reported by Schuler and Ferraris in 2018 (see below).95 While compounds 10 and 11 inhibited PARP10 with IC50 values of 3.64 μM and 480 nM, respectively, when tested in a cellular context a comparable PARP10 inhibition was detected at values of 1.7 and 1.8 μM. This is likely due to differences on the uptake or the stability of the compounds. They were then tested against a panel of 10 other enzymes, showing some selectivity toward PARP10, followed by PARP15 and PARP2 inhibition in the low micromolar range. Worthy of note is that while the compounds inhibited PARP2, the enzyme with a highly similar catalytic domain, PARP1, was not inhibited up to 100 μM.
On the basis of the good profile of benzyloxy derivative 8, Tabarrini, Lehtiö et al. in 2022 prepared a series of 15 p-methoxy benzamide analogues by mainly using cycloalkyls as a C-4 substituent.96 With the aim to lock the amide through intramolecular hydrogen bonds and extend the compounds along the NAD+ binding cleft, substituents of different sizes were also placed at the C-2 position. When tested against PARP10, most of the compounds showed inhibitory activity in the submicromolar range with the best compound represented by the cyclobutyl derivative 12 (IC50 = 290 nM) followed by cyclopentyl derivative 13 (IC50 = 593 nM) (Figure 3). Compound 12 maintained very good activity also in HeLa cells with an IC50 of 590 nM, better than those reported for hit compounds 3 and 8, without showing any significant toxicity. None of the synthesized compounds inhibited the poly-ARTs PARP2 and TNKS2 as well as the mono-ART PARP14. On the contrary, most of the PARP10 inhibitors also showed activity against PARP15 even if at 2–9-fold higher IC50 values. In the same work, with the aim to rigidify the amide covalently, a series of 2,3-dihydrophthalazine-1,4-dione derivatives 15–17, variously functionalized at the C-6 position, were prepared. Many of the compounds inhibited PARP10 in the nanomolar range with IC50s ranging from 130 to 160 nM, thus emerging as the most potent inhibitors reported to date for PARP10. Their profiling against a panel of PARPs highlighted that the inhibitory activity also extended toward PARP15 with nanomolar activity (IC50s from 370 to 400 nM), making them dual PARP10/15 inhibitors. 2,3-Dihydrophthalazine-1,4-diones maintained the PARP10 inhibitory activity also in HeLa cells with IC50s from 0.95 to 1.6 μM (Table 2).
Compound 3 was also used by Schüler and colleagues in a paper published in 2018 aimed at identifying PARP10 and/or PARP14 inhibitors (see PARP14).95 In this work, the amide diarylether scaffold of 3 was decorated at the C-3 or C-4 position with bulky substituents such as 4′-arylpiperidines/piperazines, previously discovered by the same authors as suitable to impart selective PARP14 inhibition.100 Only 1 out of 21 compounds, the 3-phenylpiperidine 18 (Figure 3), emerged as a PARP10-selective inhibitor with an IC50 of 400 nM with 15-fold selectivity over PARP14 and without any PARP1 inhibition at 100 μM concentration.
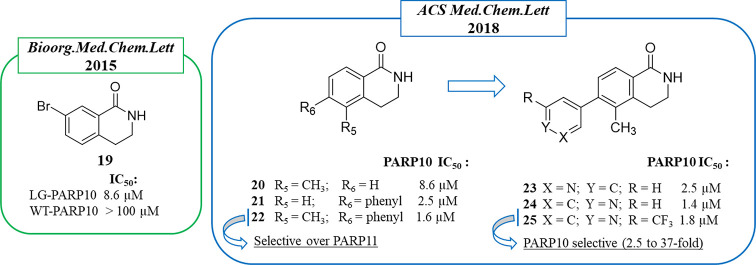
In 2018, Cohen et al.101 synthesized a series of 24 3,4-dihydroisoquinolin-1(2H)-one (dq) derivatives with the aim of extending the compounds toward a hydrophobic pocket characterizing PARP10 and composed by Ile987 and amino acids Tyr914, Val913, and Ala911 of the D-loop that is less conserved among the various PARP subfamilies. The work moved from a previous study102 where the authors applied the chemical genetic strategy of the “bump hole”. In the bump hole approach, the target protein is engineered by removing a bulky residue and thus creating a unique hole where proper substituents can interact. Thus, they prepared two PARP10 mutants, LA-PARP10 and LG-PARP10, against which compounds based on the dq nucleus, already known for its ability to inhibit PARP1,43,103 were tested. 7-Bromo derivative 19 (Figure 4) showed the best activity, inhibiting LG-PARP10 with an IC50 of 8.6 μM, while no activity against PARP10 wild type and PARP1 was observed up 100 μM. Starting from compound 19, the C-5 and C-6 positions were explored by introducing different aromatic and nonaromatic substituents (Figure 4). The 5-methyl group emerged as the best, as in compound 20 (PARP10, IC50 8.6 μM), while bulkier substituents, such as the phenyl group, were well tolerated at the C-6 position, as in compound 21 (PARP10, IC50 2.5 μM). By merging the best C-5 and C-6 substituents, disubstituted derivatives were prepared, giving compound 22 that inhibited PARP10 at 1.6 μM with 17-fold selectivity over the other mono-ART tested, PARP11. In order to overcome some aqueous solubility issues, the phenyl ring was replaced by heterocycles, including pyridine, quinolone, 1H-indole, and 1H-pyrrolo[2,3-b]pyridine; the pyridin-3-yl and pyridin-4-yl derivatives (23 and 24) showed a similar profile of 22 in terms of both potency and PARP10/PARP11 selectivity. A further structural investigation led to compound 25, characterized by a 2-CF3 pyridin-4-yl at the C-6 position, which maintained an IC50 of 1.8 μM against PARP10 and showed a selectivity from 2.5-fold over PARP16 to ≫37-fold over the other 12 ARTs tested. Compound 25 inhibited in a dose-dependent manner both auto-MARylation of PARP10 and MARylation of endogenous PARP10 proteins in HeLa cells.
Figure 4.
PARP10 3,4-dihydroisoquinolin-1(2H)-one (dq)-based inhibitors identified by Cohen et al. in 2015102 (in the green box) and 2018101 (in the blue box) along with the IC50 values against PARP10.
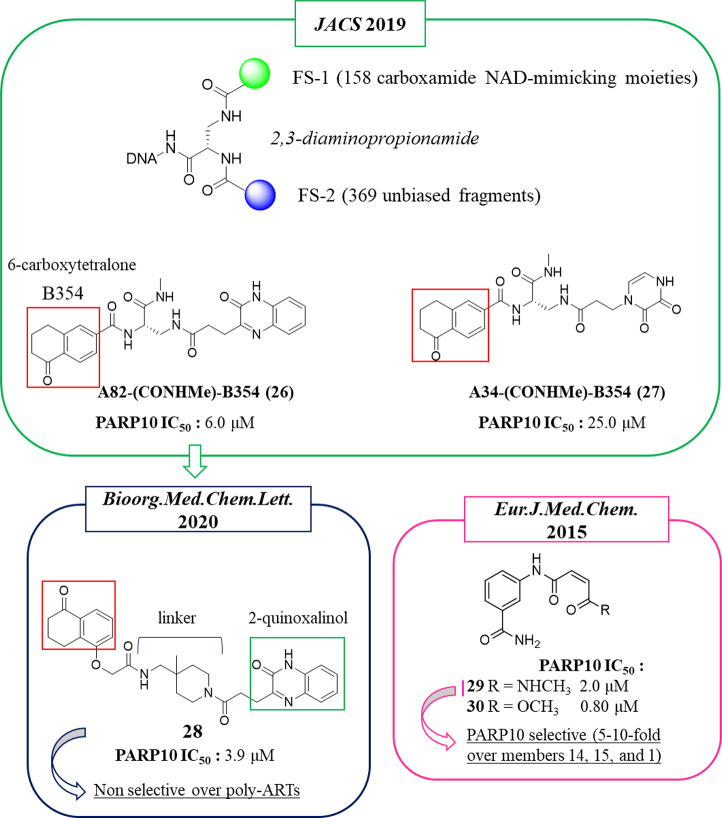
In 2019, Franzini et al. applied the DNA-encoded chemical library (DECL) approach104 to identify NAD+-dependent enzyme inhibitors. This approach was initially validated to identify PARP1 inhibitors. A 2,3-diaminopropionamide core was functionalized by two fragment sets, FS1 and FS2 (Figure 5). The 158 FS1, which mimicked the carboxamide group of NAD+ or other groups usually present in known inhibitors, were combined with 369 unbiased FS2 to give 58 302 DNA-tagged compounds. Overall, the compounds mimicked the NAD+ shape, and for this reason they could interact with NAD+-dependent enzymes (not only PARPs but also sirtuins or oxidoreductases). By screening the DECL against various NAD+-dependent enzymes, disparate compounds emerged that bind mainly the mono-ARTs PARP10, PARP12, or PARP15. Thus, the DNA-free compounds were synthesized and tested against the emerged PARPs. In particular, for PARP10, the well-known benzamide group was identified as a NI mimicking moiety, validating the approach. In addition, the 6-carboxytetralone fragment B354 (red box, Figure 5), never seen before within PARP10 inhibitors, was also identified. This fragment characterized the two inhibitors A82(CONHMe)-B354 (26) and A34(CONHMe)-B354 (27) that showed IC50s of 6.0 and 25.0 μM, respectively (Figure 5).
Figure 5.
General structure of the compounds belonging to the NAD+-mimicking DECL, and structures of the two 6-carboxytetralone derivatives initially identified (in the green box) and its derivative (in the blue box) reported by Franzini et al.104,105 6-Carboxytetralone fragment B354 is underlined with a red box. Structure of 3-aminobenzamide derivatives identified by Schuler et al.106 (in the pink box) along with the IC50 values against PARP10.
Starting from the hit compound 26, in 2020105 10 different tetralones were virtually coupled with three distinct 2-quinoxalinols through 350 diamine linkers, used to increase drug-like properties, resulting in 10 500 virtual compounds. The designed derivatives were screened in silico using the PARP10 crystal structure. On the basis of the best glide score, chemical feasibility, and diversified linkers, 10 different compounds were synthesized and tested against PARP10. Compound 28, characterized by a 4-(aminomethyl)-4-(methyl)piperidine linker, emerged with the best glide score (−12.20) and activity against PARP10 with an IC50 of 3.9 μM (Figure 5). When evaluated against the other mono-ARTs, PARP3–4, -6, -8–12, -14, and -15, the compound maintained good selectivity for PARP10. It however also showed 100% inhibition of poly-ART PARP2 at 10 μM.
Two additional PARP10 inhibitors, 29 and 30, were identified by Schüler and colleagues in 2015106 while searching for PARP14 inhibitors (see PARP14 for details). The compounds were based on the 3-aminobenzamide nucleus bearing a cis-double bond in the side chain and differ for a methyl amide (29) or an ester (30) as the terminal moiety (Figure 5). This small structural modification made compound 29 slightly less active (IC50 = 2.0 μM) on PARP10 with respect to compound 30 (IC50 = 0.80 μM) but markedly more selective over PARP14, PARP15, and PARP1.
PARP14 Inhibitors for Multiple Cancers
PARP14 (BAL2) is a mono-ART constituted by three ADP-ribosyl binding macrodomains, likely iso-ADP-ribose binding, a WWE domain and a catalytic domain.107 The typical catalytic glutamate of PARP1 in PARP14 is replaced by a smaller hydrophobic residue, Leu1782. PARP14 is overexpressed in a series of cancers such as diffuse large B cell lymphoma (DLBCL),108 multiple myeloma,109 prostate cancer,110 and hepatocellular carcinoma (HCC).111 The multiple roles of PARP14 in cancer have been recently reviewed.112 In cancer cell lines, the overexpressed PARP14 promotes the transcription of genes involved in the growth and cancer proliferation due to its modulation of the IL-4-STAT6 signaling pathway. Indeed, in the absence of IL-4, PARP14 binds the gene promoter and silences the transcription. On the contrary, in the presence of IL-4, STAT6 is activated, and this also promotes PARP14 activation, which in turn dissociates from promoters and enables gene transcription. In this context, PARP14 MARylates histone deacetylases 2 and 3 (HDAC2 and HDAC3) and successively itself, facilitating the activation of transcription cofactors. Another less explored mechanism that correlates PARP14 to the cancer disease is the JNK2–PARP14–JNK1 axis, which seems to be pivotal for malignant multiple myeloma progression. While JNK2 is related to a protective effect of multiple myeloma, JNK1 promotes its apoptosis. Recently, it emerged that in 80% of these tumors, JNK2 regulates the overexpression of PARP14. In turn, overexpressed PARP14 binds JNK1 through its C-terminal domain, thus preventing JNK1-dependent apoptosis.109,112 Furthermore, PARP14 emerged as an important effector of the Warburg effect in the HCC. Indeed, PARP14 blocked JNK1-dependent phosphorylation of pyruvate kinase M2 in HCC cells, leading to an aerobic glycolysis promotion. In the absence of PARP14, instead, PKM2 is phosphorylated by JNK1, glucose is converted into pyruvate, and apoptotic processes are enhanced.111 Besides the cancer disease, PARP14 dysregulation is also related to other pathological states such as allergic inflammation or atherosclerosis.113
Among the mono-ART enzymes, PARP14 is one of the most studied also in the context of inhibitor design. In 2012 Schüler, Linusson et al.114 performed a structure-based virtual screening of 8050 compounds in order to identify PARP14 and PARP15 inhibitors. Among the 111 compounds that emerged as initial hits, 14 stabilized PARP14 and 2 stabilized PARP15 in DSF with 4 of them showing selectivity for PARP14 (not 15) over PARP1. On the basis of the docking pose, the authors suggested that the high number of compounds that interacted with PARP14 in comparison to PARP15 could be due by the unique position of Tyr576 in PARP15 that partially constricted the NAD+ binding site. The active compounds belong to different classes, but all of them showed the benzamide moiety or its bioisoster triazole. Derivative 31 (Figure 6) showed high selectivity for PARP14 as measured in DSF assay (ΔTm > 3 °C) over PARP15 (ΔTm < 1 °C) and also over PARP1 (ΔTm < 0.5 °C). This compound was based on the classical 3-aminobenzamide nucleus in which the amino group was decorated with a side chain having a double bond with an (E) configuration ending with a carboxylic acid. The (Z) isomer, compound 32, was also synthesized to be studied in parallel (Figure 6).
Figure 6.
Structures of 3-aminobenzamides reported by Schüler, Linusson et al.114 (in the green box) and by Ferraris et al.100 (in the blue box) and of diaryl ether compounds reported by Ferraris et al.95 (in the pink box) along with the IC50 values against PARP14.
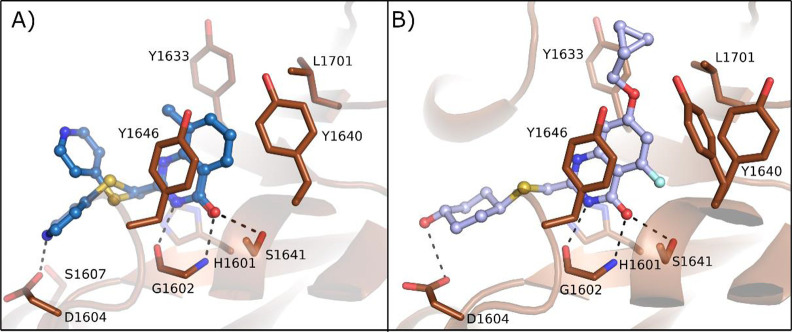
Through isothermal titration calorimetry (ITC) measurement it emerged that compounds 31 and 32 bound PARP14 with kd = 11.2 and 7.6 μM, respectively. They were then cocrystallized with PARP14 with a resolution of 1.9 and 2.8 Å, respectively (Figure 7). From the structures, it emerged that both isomers interacted with the NAD+ binding site extending toward the D-loop. 31 showed one binding mode (Figure 7A), whereas 32 showed two binding modes (Figure 7B and 7C) with different conformations, both having an intramolecular interaction between the carboxylic moiety and the amino group. Two tyrosine residues (1633 and 1646) were involved in stacking interactions, and Gly1602 formed typical hydrogen bonds with the amide of conformer 1 that is also involved in an interaction with the hydroxyl group of Ser1641. Conformer 1 interacted also through two hydrogen bonds: the amide carbonyl with Asn1624 of the D-loop and the carboxylic acid with Tyr1640. Furthermore, the acid also had a water-mediated interaction with Val1626 (Figure 7B). However, based on our interpretation, the amide of conformer 2 is too far (3.6 Å) and therefore not able to create a hydrogen bond to Gly1602 (Figure 7C). The selectivity of compound 32 could be explained based on the binding mode of the compounds that showed an interaction with the less conserved part of the NAD+ binding site. Indeed, Tyr1640, involved in the interaction, is replaced by Lys903 in PARP1; another difference is represented by Leu1701 that is instead replaced by Glu988 in PARP1.114
Figure 7.
Binding modes of compounds 31 (A) and 32 (B, conformer 1; C, conformer 2) bound to PARP14 (PDB IDs 4F1Q and 4F1L). Residues are numbered according to isoform 1 of PARP14 (UniProt Q460N5-1), and this numbering has been used in all of the PARP14 crystal structures presented in the review.
When compounds 31 and 32 were assayed enzymatically, contrasting results emerged. In 2015, they were reported as inactive in PARP14 with IC50 > 20 μM while showing some activity on PARP1, -10, and -15. A successive paper reported for compound 32 an IC50 of 7.6 μM on PARP14.106 In the same paper, aiming at extending the compound from the NI site to the less conserved ADE subsite or the adjacent D-loop, it was elaborated giving the piperidine derivative 33 and piperazine analogue 34, which emerged as active against PARP14 with IC50 values of 7.7 and 11 μM, respectively, even if they were deprived of selectivity over PARP1 (Figure 6). Some analogues of both piperidine and piperazine derivatives were synthesized that improved the compounds. The activity increased in both series when a bulky substituent was placed at the 4 position of the heterocyclic rings such as the p-chlorophenyl in the piperazinyl derivative 36, which emerged as the most potent for PARP14 but with only 5-fold selectivity over TNKS1 and unselective for PARP1. A better selectivity profile was shown by p-fluorophenylpiperidine 35, which showed an IC50 of 0.27 μM against PARP14, 30-fold selective over TNKS1 even if it was still active against PARP1 at the same range. Starting from compound 32 and replacing the carboxylic acid with a simple methyl amide moiety, the PARP10 inhibitor 29 was obtained, as previously mentioned (Figure 3).
Schüler, Ferraris et al. continued to work on these compounds by designing derivatives aimed to discern the SAR against the two related mono-ARTs, PARP14 and PARP10.95 To this aim, their 3-aminobenzamide PARP14 inhibitors were merged with PARP10 inhibitor 3(93) (Figure 3). Among the 21 hybridized compounds, all characterized by a benzamide bearing a p-phenyl ether, variously functionalized at the 3 or 4 position, the phenylpiperidine derivative 18 emerged as selective for PARP10, as previously mentioned (Figure 3). On the other hand, when the piperidine was replaced by a piperazine, as in compound 37 (Figure 6), the activity was shifted from PARP10 (IC50 of 1.4 μM) to PARP14 (IC50 of 0.78 μM) without inhibiting PARP1. The fluorine analogue 38 showed a similar profile (Figure 6). The authors also tested the metabolic stability of 18 and 37 to evaluate the potential of compounds based on the p-phenyl ether benzamide. The piperazine 37 showed better metabolic stability in mouse liver microsomes than the piperidine analogue 18, which was almost completely metabolized after 2 h (85%).
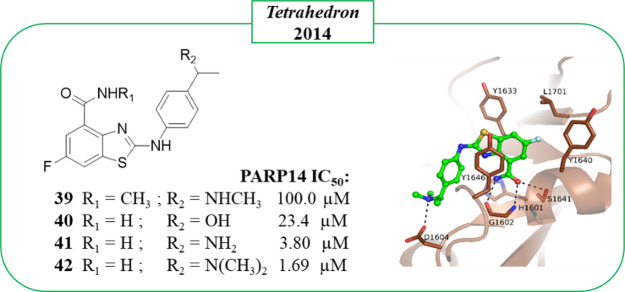
In 2014, Zhang et al.115 developed an efficient Pd-catalyzed reaction for the direct coupling of 2-aminobenzothiazole with aryl halide for obtaining 2-arylaminobenzothiazoles. Exploiting this synthetic procedure, a small library of 30 derivatives was prepared, and 4 of them were evaluated for their inhibitory activity in ELISA against PARP14. The compounds are functionalized with a C-6 fluorine atom and C-4 amide coupled with different aminoaryl groups at the C-2 position (Figure 8). With the exception of 39 characterized by a methylated amide, the other derivatives, 40–42, showed good inhibitory activity with the N,N-dimethylamino derivative 42 emerging as the best (IC50 = 1.69 μM, Figure 8). Crystallographic studies confirmed that compound 42 interacted with the NAD+ binding site. However, the authors deposited a model where there are clear errors in the compound as well as poor electron density to support the binding mode. We therefore describe here our interpretation of the binding mode (Figure 8). The compound binds to the NI site as other PARP inhibitors and forms typical hydrogen bonds to the glycine and serine residues. It extends along the donor site, and the authors noted that the N,N-dimethylamino group formed a hydrogen bond with Asp1604. Although compound 42 showed very interesting activity against PARP14, the authors did not explore its selectivity profile.
Figure 8.
2-Aminobenzothiazole-4-carboxamides synthesized by Zhang et al.115 along with PARP14 IC50 values; crystal structure of compound 42 in complex with PARP14 catalytic domain as observed in monomer B in the asymmetric unit (PDB ID 4PY4). It is important to note that the amide group of 42 was flipped because it was incorrect in the deposited model and resulted in clashes, and shown in the figure is the corrected model. Error in the original model also pushed the compound out from the very poor density observed in monomer A that was used by the authors to analyze the interaction.
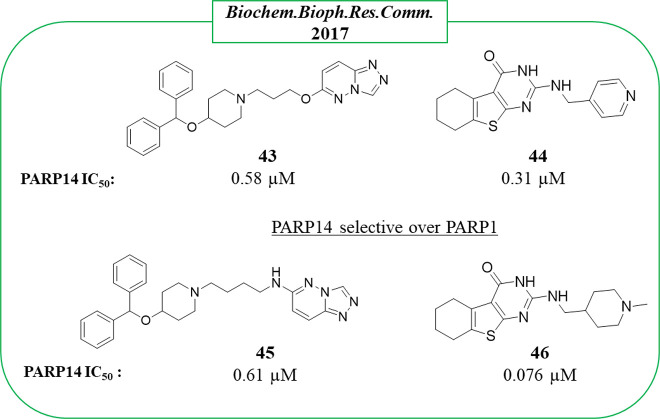
By screening a library of 500 000 small compounds from Takeda Pharmaceutical Co. using a RapidFire high-throughput mass spectrometry method (which calculated the nicotinamide released from NAD+), novel PARP14 inhibitors were identified by Yoneyama-Hirozane in 2017.116 Compounds 43 based on a triazolo[4,3-b]pyridazine and 44 based on 5,6,7,8-tetrahydro[1]benzothieno[2,3-d]pyrimidin-4-one (Figure 9) inhibited PARP14 without significant activity against PARP1 (IC50 > 26 μM). The ADP-ribosylation measured by immunoradiometric assay confirmed a submicromolar activity with IC50 = 0.58 and 0.31 μM, for 43 and 44, respectively. Two strict analogues, 45 and 46 (Figure 9), were also tested as active with compound 46 showing the best activity (IC50 = 76 nM). Unfortunately, the selectivity for these new compounds was not studied.
Figure 9.
Structures and PARP14 IC50s of triazolo[4,3-b]pyridazine 43, benzothieno[2,3-d]pyrimidin-4-one 44, and their derivatives 45 and 46 reported by Yoneyama-Hirozane et al.116 along with the IC50 values against PARP14.
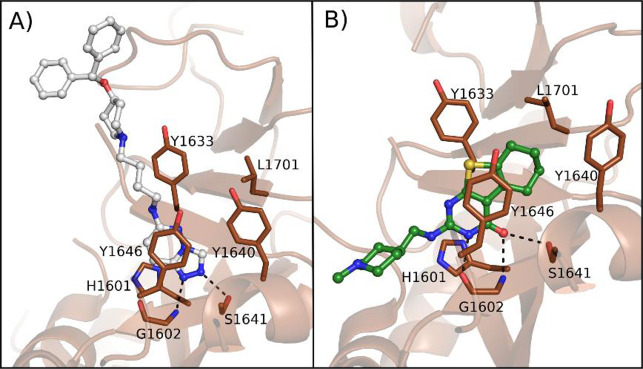
From their structures with PARP14 it emerged that the compounds interacted with the catalytic domain (Figure 10). Compound 45 made two key hydrogen bonds between two of the three nitrogen atoms of triazole and Ser1641 and Gly1602 residues (Figure 10A). The aliphatic chain and two terminal phenyl rings extend to solvent. Compound 46 formed hydrogen bonds with the same residues of Ser1641 and Gly1602 through the carbonyl group. Gly1602 was also involved in another hydrogen bond with pyrimidine nitrogen (Figure 10B). The terminal methylpiperidine extends the compound toward the ADE site. A comparison with the PARP1 structure showed that the compounds would clash with the residues of the autoinhibitory regulatory domain only present in PARP1–4, and this could explain the reduced activity against PARP1. Compounds 43–46 were also cell permeable and recognized intracellular PARP14, as monitored in the intracellular PARP14 stability assay performed in A549 lung cells by accumulation of NanoLuciferase-fused PARP14 (PARP14–NanoLuc) with pEC50 values showing correlation with the pIC50 values measured with the enzymatic assay. The studies revealed the triazole ring as a suitable replacer of the classic benzamide as a nicotinamide mimic.
Figure 10.
(A) Compound 45 (PDB ID 5V7T) and (B) compound 46 (PDB ID 5V7W) in complex with PARP14.116
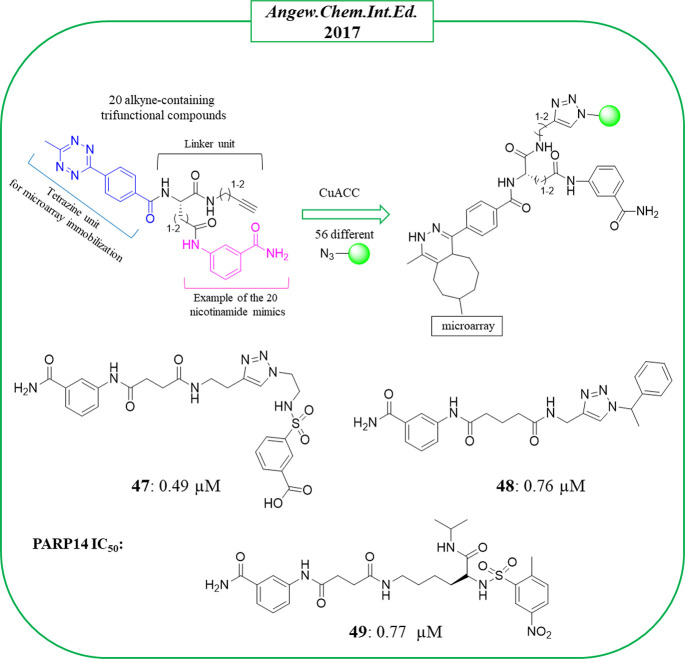
In the same year, bidentate PARP14 inhibitors were identified by Schüler, Yao et al.117 The compounds, similarly to inhibitors of TNKS or PARP1/2, were designed to extend from the NI binding site to the close site of ADE. There were 1120 compounds generated through an on-chip CuI-catalyzed azide–alkyne cycloaddition (CuAAC) coupling 20 alkyne-containing trifunctional compounds with 56 different azides (Figure 11). The trifunctional compounds were synthesized starting from five different ligands known for their ability to bind the PARP nicotinamide binding pocket and a tetrazine unit useful for the small molecules microarray immobilization. The two portions were connected through four amino acid-like linkers variable in length and flexibility. The 56 different azides included adenine-mimicking compounds derived from known kinase inhibitors. The microarray was performed against four enzymes (PARP10, PARP14, and TNKS1–2), and 20 hits were identified as potential PARP14 inhibitors. Successively, the corresponding tetrazine-free compounds were synthesized and tested in an in vitro enzymatic assay. Compounds 47 and 48 (Figure 11) emerged as the best, inhibiting PARP14 with IC50 values of 0.49 and 0.76 μM and with 24- and 6-fold selectivity over PARP1 and 18- and 4-fold over TNKS1, respectively. They were based on the typical 3-aminobenzamide moiety that from the cocrystallographic studies (on 47) resided in the NI binding site, as expected based on previously described PARPi. The inhibitor extended toward the ADE binding site with the benzenesulfonamide portion (Figure 12). Compound 47 was then subjected to structural modifications of the triazole ring in order to improve the flexibility. Among the 22 new derivatives, compound 49 (Figure 11), with an opened triazole ring, showed a similar activity of the hit compound against PARP14 with IC50 = 0.77 μM. Both 47 and 49 showed good cell permeability and hydrolytic stability. They inhibited the endogenous PARP14 from hepatocellular carcinoma cell line (HepG2) at 10 μM, while they did not inhibit PARP1 in the same cells. A synergism with doxorubicine in HepG2 and dexamethasone in multiple myeloma cell line (RMPI-8226) was also reported.
Figure 11.
Bidentate PARP14 inhibitors identified by Yao et al.117 along with the IC50 values against PARP14.
Figure 12.
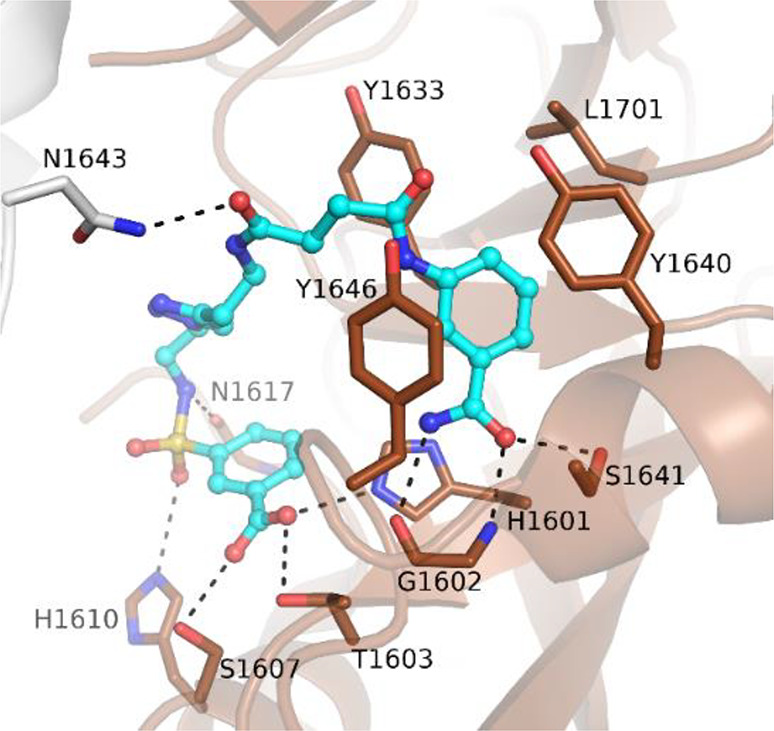
Binding of compound 47 to the PARP14 active site (PDB ID 5LYH).117 Asn1643 from a neighbor molecule is colored in gray.
In 2020, Schweiker et al.118 performed docking studies using the catalytic domain of PARP14 (PDB ID 3SMI) with 60 natural products, already known for various biological activities, which led to the identification of a few virtual compounds. Of them, epigallocatechin-3-gallate (EGCG, 50), already reported as a mono-ART inhibitor for PARP16,119 and quercetin (51) (Figure 13) completely inhibited PARP14 at 20 μM with 50 that still maintained the activity at 10 μM. Although these natural compounds could gain attention due to the unusual scaffolds, the lack of a selectivity profile along with the probable promiscuity and possible PAINS behavior could cause alarm regarding their possible progression.120
Figure 13.
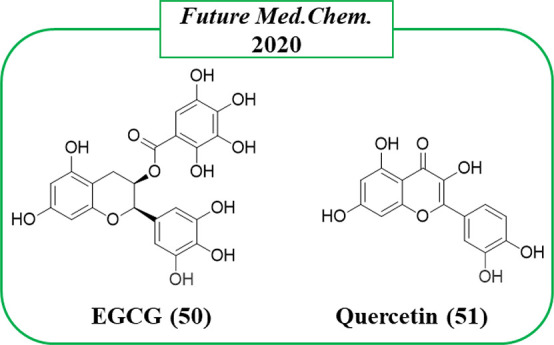
Natural compounds identified as PARP14 inhibitors by Schweiker et al.118
In 2021, Ribon Therapeutics published two papers on quinazolinone-based compounds, one reporting the first proteolysis targeting chimera (PROTAC) (see the Alternative Strategies To Inhibit Mono-ARTs section)121 and the other that identified the most promising PARP14 inhibitor reported so far, RBN012759 (52) (Figure 14).122 The study that led to 52(122) started from compound 53, which emerged by screening a PARP-targeted compound collection through PARP14 automodification DELFIA (dissociation-enhanced lanthanide fluorescence immunoassay).
Figure 14.
PARP14 inhibitors developed by Ribon Therapeutics122 along with the IC50 values against PARP14.
Compound 53 inhibited PARP14 at 1 μM, but when it was profiled against all of the human PARPs and TNKS enzymes, it emerged as an unspecific inhibitor. Compound 53 bound the NAD+ binding pocket (Figure 15A) with the quinazolinone NH that interacted through a hydrogen bond with Gly1602 and the carbonyl with OH of Ser1641; π–π interactions were also performed with Tyr1646. The C-2 thiopyridine substituent instead extended the compound toward the D-loop, very close to Ser1607 and Asp1604, which are unique residues of PARP14 and PARP15. These two residues interacted each other with a hydrogen bond that was hypothesized to be displaced by a proper substituent to achieve selective inhibition. With this aim, the C-2 position was explored with different thioether groups giving 2-trans-cyclohexanol 54 (Figure 14) that showed the best inhibitory activity against PARP14 with IC50 = 0.3 μM, while it was less active on most of the studied PARPs and above all very selective over PARP15 (IC50 = 30 μM) (Figure 14). Additional hydrophobic substituents were successively placed in the benzene ring to reduce the affinity with poly-ARTs that are characterized by a catalytic glutamate, a strategy already applied for the same scaffold.123 Compound 52, having a 7-cyclopropylmethoxy group coupled with a 5-fluorine atom, reached an IC50 of 3 nM against PARP14 (Figure 14) with >300-fold selectivity over the other mono-ARTs and >1000-fold selectivity over the poly-ARTs. The cocrystallographic structure (Figure 15B) showed that compound 52 has the same binding mode as 53. In addition, as planned, the OH interacted with Asp1604, while the C-7 substituent generated a series of van der Waals interactions with the hydrophobic region of PARP14 (Figure 15B). Additional studies showed that compound 52 was endowed with good solubility and permeability properties along with a low MDR1-mediated efflux. It entered the cells and inhibited intracellular PARP14 in a dose-dependent manner with the same IC50 value. In addition, compound 52 inhibited PARP14 auto-MARylation in human primary macrophage and in CFPAC-1 (ductal pancreatic adenocarcinoma cells), the latter characterized by a high endogenous level of PARP14, and the PARP14 engagement was confirmed in an in vivo model.122
Figure 15.
PARP14 crystal structures in complex with (A) 53 and (B) 52. PDB IDs 6WE4 and 6WE2, respectively.122
PARP11 Inhibitors To Reveal Its Cellular Roles
In the catalytic domain an isoleucine residue occupies the third position of the triad. Preceding the catalytic domain PARP11 contains a WWE domain which is involved in the binding of terminal ADP-ribose of poly-ADP-ribosylated proteins.124 Very little is known about the physiological and pathological roles of PARP11, but it is involved in the regulation of a nuclear pore complex through the MARylation of some proteins such as Nup98–Nup96.125 In 2019, it emerged that MARylation of ubiquitin E3 ligase β-transducin repeat-containing protein (β-TrCP) mediated by PARP11 modulated the activity of type I interferon in the antiviral response.126 In particular, mono-ADP-ribosylated β-TrCP promotes IFNα/β receptor subunit 1 (IFNAR1) degradation through a ubiquitination-mediated mechanism. PARP11 is mainly upregulated in response to Zika infections, and by cooperating with PARP12 it suppressed Zika virus replication.127
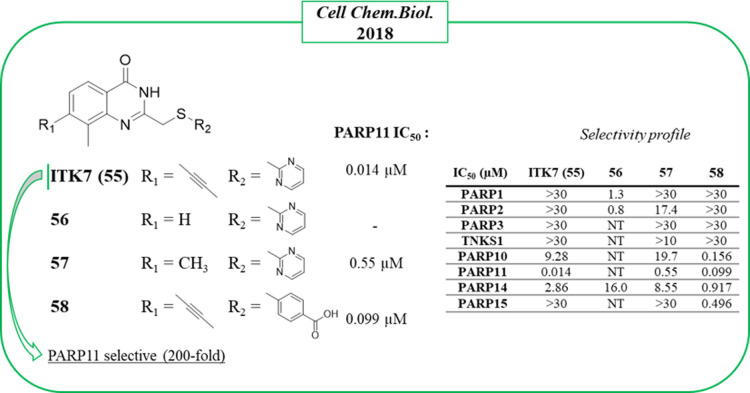
ITK7 (55) (Figure 16) is the sole potent and selective PARP11 inhibitor reported to date. It was identified by Cohen et al. in 2018,123 who explored the quinazolinon-4(3H)-one scaffold, the same largely exploited also by Ribon Therapeutics to obtain PARP14 inhibitors, as mentioned above. The study started with compound 56, which inhibited PARP1 and PARP2 at low micromolar concentrations, and to shift the selectivity in favor of mono-ARTs, small and hydrophobic substituents were placed at the C-7 position. As planned, 7-methylated 57 (Figure 16) did not inhibit any poly-ARTs at concentrations below 10 μM but was active on PARP11 with an IC50 of 0.55 μM. A clear preference for mono-ARTs was achieved by inserting a bigger propynyl group coupled with a 2-p-benzoic acid, as in compound 58, which reached PARP11 with nanomolar activity. Selective PARP11 inhibition was then achieved when the propynyl group was coupled with a 2-pyrimidine ring, as in compound 55, which showed an IC50 = 14 nM, 200-fold selective over other five mono-ARTs, and without inhibiting the three poly-ARTs at all. Its selective activity was also maintained in a cellular context with EC50 = 13 nM, preventing the auto-MARylation of PARP11 in HeLa cells in a dose-dependent manner without inhibiting PARP1 or PARP10. Given its selectivity and potency, 55 was also exploited as a chemical probe in order to elucidate the enzymatic localization of PARP11 that was identified at the nuclear envelope.123
Figure 16.
Structural optimization of the quinazolinone scaffold up to the identification of PARP11 inhibitor 55 by Cohen et al.123 along with the IC50 values against PARP11 and selectivity profile.
PARP15 Inhibitors To Reveal Its Cellular Roles
PARP15 (BAL3) contains ADP-ribosyl binding macrodomains at the N-terminus, allowing likely localization and protein–protein interactions enabling the C-terminal catalytic domain to potentially modify the target macromolecules.107,128 However, the enzyme has not been studied much, and the cellular roles of PARP15 are still elusive. It is associated with stress granule formation,129 and recently, it has been shown that it is able to ADP-ribosylate 5′-phosphorylated ssRNA.130 PARP15 is overexpressed in B-aggressive lymphoma,107 and there are some implications in the literature that could play a role in acute myeloid leukemia.131
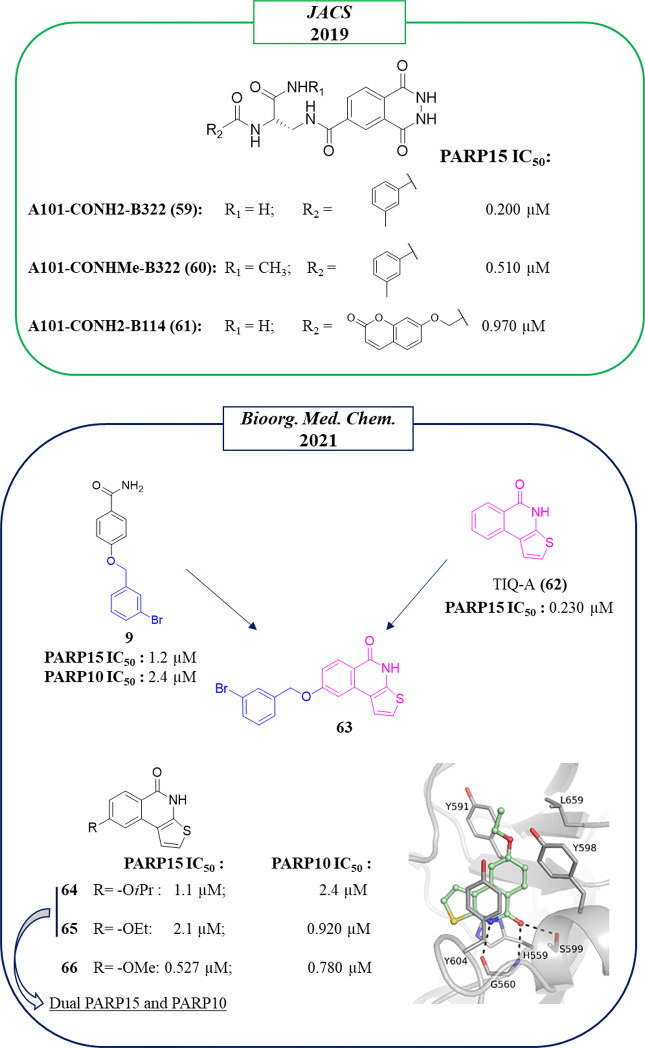
In the work of 2019 in which Franzini et al.104 through the DECL approach identified the already discussed PARP10 inhibitors, anti-PARP15 compounds also emerged (Figure 17). In particular, the 2,3-dihydrophthalazine-1,4-dione fragment was identified as suitable to inhibit PARP15, conferring submicromolar activity to the compounds A101-CONH2-B322 (59), A101-CONHMe-B322 (60), and A101-CONH2-B114 (61) with IC50s of 200, 510, and 970 nM, respectively. Molecular docking studies indicated that compound 59 would bind to the nicotinamide pocket, but the binding mode was not experimentally determined, and the selectivity of the compounds was not evaluated.
Figure 17.
Structures of compounds identified from DECL by Franzini et al. (in the green box)104 and of chimera compounds identified by Lehtiö et al. (in the blue box)99 along with the IC50 values against PARP15. Crystal structure of compound 65 in complex with PARP15 catalytic domain (PDB ID 7OUX).
The 2,3-dihydrophthalazine-1,4-dione nucleus was confirmed as particularly suitable to inhibit PARP15 also by compounds 15–17 (Figure 3). As mentioned before, these compounds were conceived as PARP10 inhibitors, but they actually behave as dual PARP10/PARP15 inhibitors, inhibiting both enzymes with IC50s ranging from 150 to 400 nM. The cocrystallographic structures revealed that they work as nicotinamide-mimicking compounds that extend toward the acceptor site and interact with PARP15 through hydrogen bonds with Gly560 and Ser599 and π–π interaction with Tyr604.96
Another series of dual PARP10/PARP15 inhibitors was identified by Lehtiö et al,99 in a paper aimed at improving the selectivity toward mono-ARTs again by reaching the acceptor site. Through a hybridization approach, TIQ-A (62),132 known to inhibit PARP1 but also other enzymes in the nanomolar range including PARP15, and the selective mono-ART inhibitor m-Br derivative 9 (Figure 17) were merged.94,133 Unfortunately, the synthesized chimeric compound 63 (Figure 17) was completely inactive. However, when a small aliphatic substituent was placed at the C-8 position, the thieno[2,3-c]isoquinolin-5(4H)-ones 64 and 65 emerged as dual PARP15/PARP10 inhibitors in the low micromolar range. The presence of the smallest methoxy substituent gave 66, the most potent compound on PARP10/PARP15 but at the expense of selectivity, also being active on TNKS2 (IC50 = 160 nM). The modest activity of 64 and 65 against TNKS2 is justified by the crystallographic structures that highlight a steric clash between the C-8 substituent and the catalytic Glu1138 of TNKS2 (Leu659 in PARP15, Figure 17).
PARP6 Inhibitors To Interfere with Mitosis and Cause Cancer Cell Apoptosis
Little is known about the functions of PARP6. The first study aimed at understanding its role was performed by Cohen et al.,134 which identified PARP6 as the most relevant mono-ART enzyme involved in the regulation of hippocampal dendrite morphogenesis. Indeed, high levels of PARP6 are present in the brain, particularly in the hippocampus region with an essential role in neurodevelopment from late embryonic to the early postnatal stage. This role also suggested that defective PARP6 could contribute to the pathogenesis of several diseases such as autism and Rett’s syndrome.134,135
Successively, identification of the first, even orally bioavailable, PARP6 inhibitor AZ0108 (67) by AstraZeneca also shed light on its role in cellular replication.136 In particular, it was found that PARP6 MARylates checkpoint kinase 1 (chk1) downregulating its phosphorylation. When PARP6 is inhibited, the chk1 hyperphosphorylation led to the generation of supernumerary centrosomes during mitosis, resulting in a multipolar spindle (MPS), which is toxic for the cancerous cells (mitotic catastrophe). This highlights the important role of PARP6 in mitosis, in particular, in the G2-M phase progression.136 The PARP6 inhibition or its knockdown in some breast cell lines, such as HCC1806 or MDA-MB-468, induced strong apoptosis that could represent a valid therapeutic approach.136
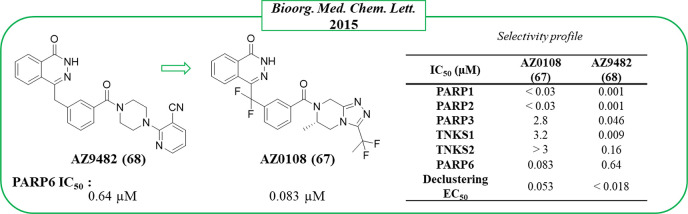
On the basis of the known role of TNKS2 and PARP16 in the centrosome clustering, in 2015 AstraZeneca assayed their quinazolinone- and phthalazinone-based PARP inhibitors collection in the phenotypic declustering assay using HeLa cells.137 This assay evaluates the ability of small molecules to block the centrosome clustering causing MPS. From the screening, compound 68 (Figure 18), based on the same phthalazinone nucleus of olaparib developed by the same company, emerged as a potent inhibitor with an EC50 < 18 nM. When the compound was tested against a restricted panel of enzymes (PARP1, PARP2, PARP3, TNKS1, TNKS2, and PARP6), it resulted as a pan inhibitor in the nanomolar range. An optimization campaign led to 67, which is orally bioavailable with an excellent in vivo pharmacokinetic profile transversally on mouse, rat, and dog. 67 showed a slightly decreased declustering activity (EC50 = 53 nM, Figure 18) and a different PARP inhibition profile, becoming much less active on TNKS1, TNKS2, and PARP3 and 6-fold more active on PARP6. Since olaparib tested in parallel showed a very low declustering activity, the authors suggested that the PARP6 inhibition mainly contributed to the declustering of compound 67. The effect of the PARP6 inhibition by 67 in cell death was evaluated against a series of 18 breast cancer cell lines, active only in HCC1806 and MDA-MB-468.136 The activity was confirmed in in vivo xerographic models with more efficacy in the MDA-MB-468 model using a daily oral dosing of 10 mg/kg. Samples from the treated tumors exhibited mitotic defects such as disorganized spindle and MPS, confirming the mechanism of the antitumor activity.
Figure 18.
PARP6 inhibitors developed by AstraZeneca (in green box)137 along with the IC50 values against PARP6, and the table with their selectivity profile.
PARP12 Inhibitors To Effect Cell Stress and Cancers
PARP12 is a member of mono-ARTs containing 5 CCCH zinc fingers, which recognizes viral and cytoplasmic RNAs.138,139 It also contains a putative iso-ADP-ribose binding WWE domain preceding the catalytic C-terminal domain which is H–Y–I. The cellular roles of PARP12 are still elusive. Under steady-state conditions, PARP12 is localized in the Golgi complex, while under stress conditions, it translocates to stress granules in a PARP1-dependent manner. Indeed, several PARylated nuclear proteins move to the cytoplasm where they interact with the PARP12 WWE domain, thus promoting the PARP12 accumulation in cytoplasmic stress granules.129,140 Herein, PARP12 regulates mRNA translation and stability as a response to a stress conditions.129 This translocation is, however, a reversible condition, and after the stress response, PARP12 relocalizes in the Golgi complex.141 PARP12 ADP-ribosylates Golgin-97 thereby regulates the basolateral transport of proteins.142 The function of PARP12 in cancer remains highly controversial. While a deficiency of PARP12 in HCC was found to be associated with a promotion of migration and invasion of HCC,143 in other tumors it seems to be highly upregulated.144 In particular, it was recently reported that in MCF7 breast cancer cell lines PARP12 silencing potentiated the effect of the alkylating agent mafosfamide by reducing cell survival and cancer regrowth.144 In the latter case, the use of a PARP12 inhibitor could be a good selective strategy to counteract the cancer proliferation.
Only modest PARP12 inhibitors have been reported that came from the already mentioned work of Franzini et al.104 Combination of the benzamide fragment A68 (FS-1, in the red box) and the 3-(4-pyridinyl)-1,2,4-oxazole B259 (FS-2, in the red box) led to A68-(CONH2)-B259 (69), which showed inhibitory activity against PARP12, IC50 of 38 μM (Figure 19). When the CONH2 moiety in the linker was replaced with CONHPr, similar activity was displayed by A68-(CONHPr)-B259 (70); on the other hand, the presence of a H atom gave the inactive A68-(H)-B259 (71) (Figure 19).
Figure 19.
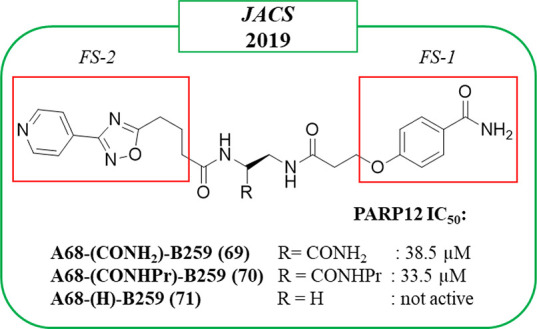
Structures of compounds identified from DECL by Franzini et al.104 along with the IC50 values against PARP12. Benzamide and 3-(4-pyridinyl)-1,2,4-oxazole fragments are highlighted in red boxes.
PARP16 Inhibitors To Control Unfolded Protein Response
PARP16 is another member of mono-ARTs in which the catalytic glutamate is replaced by isoleucine. It is the only member that, to date, was demonstrated to be associated with the nuclear envelope and endoplasmic reticulum through a C-terminal transmembrane domain.145,146 It plays essential roles in the regulation of unfolded protein response and in response to stress.7 Worthy of note, PARP16 deletion is associated with the formation of toxic protein aggregates that could reduce cancer cell growth.147
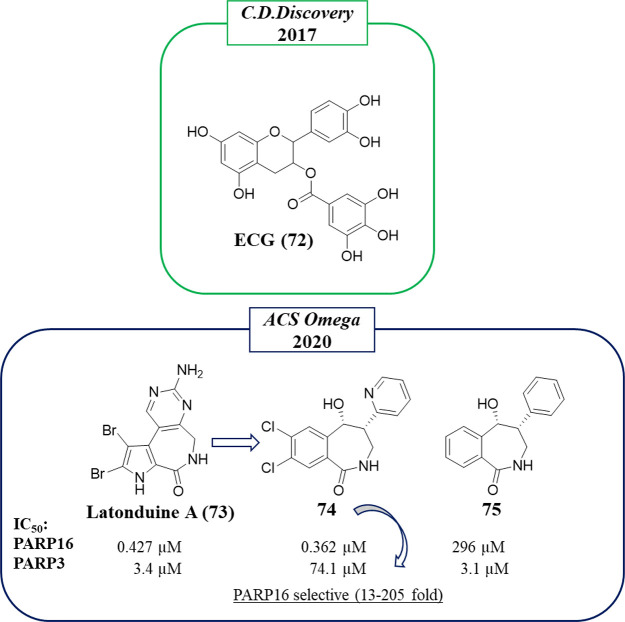
To identify PARP16 inhibitors, in 2017,119 3375 small molecules were screened using a microarray, including natural compounds from traditional Chinese medicine, FDA-approved drugs, and known inhibitors. Nineteen showed high affinity for PARP16, and this activity was confirmed at 0.5 mM by an in vitro auto-ADP-ribosylation assay with the natural compound epicatechin-3-gallate (ECG, 72) (Figure 20) that completely inhibited the catalytic activity. A binding affinity assay showed for this compound Kd = 3.41 nM, 2-fold more potent than its strict analogue 50 (Figure 13) assayed in parallel (Kd = 6.16 nM). The inhibitory activity of PARP16 was then evaluated in vitro, and 50 showed an improved inhibitory activity with an IC50 of 14.52 μM compared to 72 that was 47.18 μM. There is however a large discrepancy between the reported dissociation constant and the IC50 values.
Figure 20.
Structures of natural compounds identified by Wu et al. (in the green box)119 and of derivatives reported by Andersen et al. (in the blue box)148 along with the IC50 values against PARP16 and PARP3.
As the reported potencies in binding assays and in enzymatic assays differ substantially, further studies should be needed to confirm the inhibition mechanisms. Compound 50 was however used as a chemical tool for better understanding the PARP16 role.119 To this aim, the relationship between PARP16 activity and two ER stress sensors, PERK and IREα phosphorylation, was investigated. From the data it emerged that PARP16 is essential for PERK phosphorylation, and any PERK activation was instead observed in the presence of 50 as well as in PARP16-deficient QGY-7703 cell lines. In addition, 50 was also responsible for a 10% increase of death in wild-type cell lines, in agreement with the knowledge that a PARP16 deficiency made the cells more susceptible to the ER stress; indeed, no apoptosis was detected in the negative PARP16-deficient cells after treatment with 50, used as a control.119
Other natural products were found to be active against PARPs, such as latonduine A (73) (Figure 20), from a marine source, that was identified as a PARP3 and PARP16 inhibitor,149 and this inhibition was translated into the ability to correct a F508del-cystic fibrosis transmembrane regulator (CFTR), a chlorine ion channel blocked in cystic fibrosis. In pathologic conditions, chlorine ions cannot flux out of the cells, and this determines the accumulation of thick mucus in the lungs. In order to separate the PARP16 and PARP3 inhibitory properties of 73, in 2020, Thomas, Andersen et al.148 synthesized a library of ∼30 analogues characterized by a simplified structure based on a 2,3,4,5-tetrahydro-H-benzo[c]azepin-1-one core differently decorated on the two rings (Figure 20). All of the compounds were preliminary tested at 10 μM against PARP3 and PARP16. Two compounds emerged as active with an opposite selectivity profile. Indeed, while compound 74 was 205-fold selective for PARP16 (IC50 = 362 nM) over PARP3, compound 75 emerged as selective for PARP3. Profiling compound 74 against a restricted panel of ARTD family enzymes (PARP1, PARP2, TNKS1,2, and PARP4), it emerged to be from 13- to 70-fold selective. Surprisingly, neither compound 74 nor 75 separately showed F508del-CFTR corrector activity, while an activity comparable to 73 was recovered using a combination of the two compounds. Thus, the dual PARP inhibition of 73 justified its effect as a corrector potentially useful to treat cystic fibrosis.
PARP7 Inhibitors as Anticancer Drugs
PARP7 is a mono-ART also known as tetrachlorodibenzo-p-dioxin (TCDD)-inducible poly(ADP-ribose) polymerase (TiPARP). Besides the catalytic domain with H–Y–I, it is characterized by a N-terminal nuclear localization signal (NLS), CCCH-type zinc finger domain able to bind RNA, and WWE domain that interacts with iso-ADP-ribose and can mediate protein–protein interactions.150 PARP7 is involved in a variety of cellular processes including innate immune regulation, cellular stress response, and transcription factor regulation. Its expression in most of the human tissues is regulated by aryl hydrocarbon receptor (AHR),151 liver X receptor (LXR),152 and type I interferon (IFN-I).153 In 2021, two important papers154,155 were published around the PARP7 involvement in cancer, even if they highlighted two opposite PARP7 engagements. Indeed, while Rasmussen et al. demonstrated that PARP7 functions as a tumor suppressor in E2-responsive breast cancer cells,154 a PARP7 inhibitor reached clinical trials for its promising antitumor activity.155 In the first study, the authors provided evidence that PARP7 is a fundamental member of the negative feedback that regulates estrogen receptor α (ERα). Since ERα represents the main regulator of estrogens in breast tissue and is one of the principal targets for breast cancer treatment, its negative regulation mediated by PARP7 could be beneficial in this kind of cancer.154 On the contrary, the downregulation of INF-I response in which PARP7 is involved could be responsible for a reduced T-cell-mediated antitumor activity.155 Thus, PARP7 could represent an immunotherapeutic target, and through its inhibition, anticancer activity can be achieved.
Another important role of PARP7 has been recently identified also in ovarian cancer, in which PARP7 contributes to the cancer proliferation. Indeed, PARP7 MARylated various cytoskeletal proteins, including α-tubulin, thus facilitating cell growth; when PARP7 was depleted, a reduction in cancer cell growth is observed.156
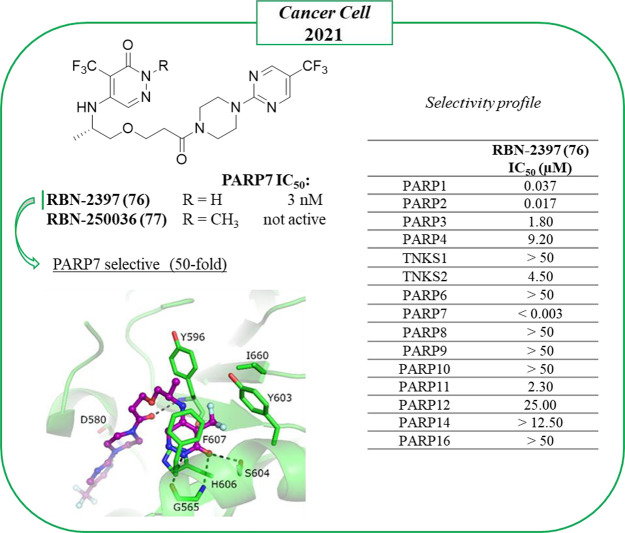
Ribon Therapeutics developed the first PARP7 inhibitor, RBN-2397 (76) (Figure 21),155 which is based on the pyridazinone nucleus, previously identified by fragment screening as a privileged structure to inhibit mainly mono-ARTs and used to obtain NAD+ competitive probes for PARP enzymes.157 Compound 76 inhibited PARP7 by interacting with the NAD+ binding pocket of the enzyme with an IC50 < 3 nM and a kd of 0.22 nM. It was >50-fold selective over all of the other catalytically active human PARPs and showed an IC50 of 2 nM in SK-MES-1 cells. The PARP7 inhibition mediated by the compound completely abolished the MARylation of TBK1, and this restored the IFN-I signaling. On the contrary, using the inactive methylated analogue RBN-250036 (77) (Figure 21), no effect was detected.
Figure 21.
Development of 76 by Ribon Therapeutics, currently under clinical investigation,155 along with the IC50 values against PARP7, selectivity profile, and crystal structure with a mutated PARP12 mimicking PARP7 (PDB ID 6V3W).
The binding mode of 76 was determined with a complex structure of a mutated PARP12 mimicking PARP7. The compound makes five hydrogen bond interactions with residues Gly565, Tyr596, Asp580, and Ser604. In addition, one of the mutated residues, Phe607, creates a π–π interaction with the compound. The antitumor effect of 76 was confirmed using CT26 tumor-bearing mice. Significant tumor growth inhibition was detected at all doses with complete and durable regression after treatment with 30 mg/kg for 6 weeks. Since it was speculated that the PARP7 inhibition led to an immunomodulation responsible for the anticancer activity, also immunodeficient NOG mice with CT26 were treated with 76, and as expected, no tumor regression was observed. CD8 T cells emerged as being responsible for much of the antitumor immunity induced by the compound. The anticancer effect was also observed in most xenograft models with tumor growth inhibition ranging from 49% to 69% and complete tumor regression in NCI-H1373 lung cancer xenograft. The promising preclinical results led to 76 being investigated in phase I clinical trials that are currently ongoing in patients with advanced or metastatic solid tumors (NCT04053673).155
Alternative Strategies To Inhibit Mono-ARTs
A generally known challenge in PARP inhibitor development is to gain selectivity due to the high sequence similarity among ARTs family NAD+ binding pockets. To overcome this issue, alternative ways to mediate and modulate ADP-ribosylation signaling are emerging. A valid approach that can be applied without the difficulties of obtaining inhibitors specific for individual ADP-ribosyltransferase domains could rely on interfering with accessory domains through allosteric modulators.158
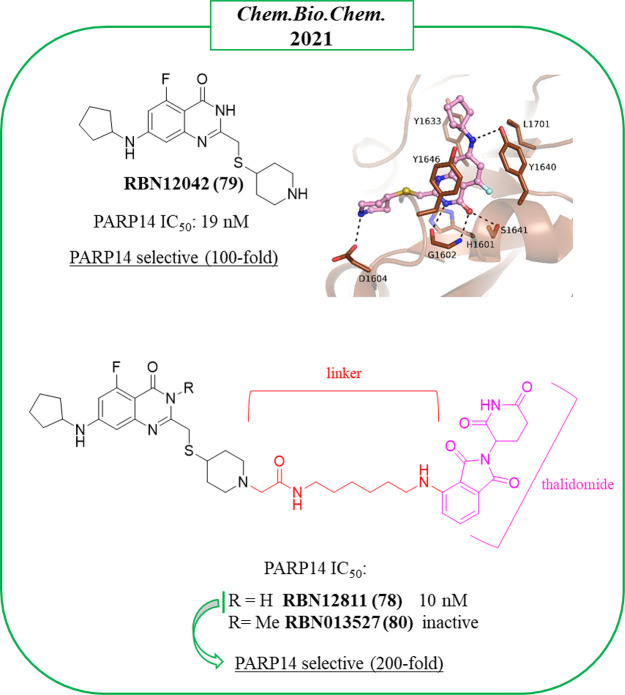
An alternative way to interfere with the enzyme would be through reducing the proteins levels with a PROTAC approach. Several PROTACs for poly-ARTs have been already reported as potent and efficacious to treat cancer,159−161 while only one degrader, has been developed for mono-ART PARP14 by Ribon Therapeutics,121 RBN12811 (78). The etherobifunctional degrader 78 (Figure 22) was obtained by tethering the selective PARP14 inhibitor RBN12042 (79) to thalidomide through an appropriate linker. Compound 79, which was developed by the same company, inhibited PARP14 with an IC50 of 19 nM, more than 100-fold selective over all of the other human ART enzymes. The 7-NH of 79 makes an interaction with Tyr1640 while Tyr1646 is involved in a stacking interaction with the bicyclic ring. The carboxamide generates hydrogen bonds with Ser1641 and Gly1602. Asp1604 also makes a hydrogen bond with the pyrrolidine group (Figure 22). PROTAC 78 was as active as 79 with an IC50 of 10 nM and more than 200-fold selective over all of the other ARTs. The compound was tested in KYSE-270 cells (human esophageal squamous cell carcinoma) where degradation of endogenous PARP14 was detected without affecting the levels of the other ARTs. The degradation was also confirmed in MDA-MB-231 (human breast adenocarcinoma) and JJN-3 (lymphoma cell line) as well as in HEK293T and in human macrophages. The methylated analogue RBN013527 (80) was inactive, highlighting the need for the secondary benzamide moiety. Degrader 78 was also tested in the presence of three different proteasome inhibitors, confirming that the compound mediated the destruction of PARP14 via a ubiquitin-proteasome pathway. Thus, 78 represents the founder of mono-ART PROTACs, but recent identification of potent and selective inhibitors of a single family could definitely expand this strategy.
Figure 22.
(Top) Compound 79 with its structure in complex with PARP14 (PDB ID 7L9Y) and (bottom) its PROTAC derivative 78(121) along with the IC50 values against PARP14 and their selectivity profile.
PARPs are known to interact with multiple other macromolecules. Protein–protein interaction (PPI) disruptors could provide alternative ways to inhibit these enzymes. PPIs are critical regulatory events in many biological processes representing a precious source for possible new therapeutics, but PPIs are difficult to target, and compounds binding to these interfaces are not commonly found in HTS because PPI inhibitors and traditional drug-like compounds occupy quite different chemical spaces. While still largely underexplored, a few examples have already been reported as PARP-1 inhibitors able to interact with the BRCT domain.162 Efforts are also ongoing to prevent interaction of TNKS with their target protein peptides.163,164 PPI inhibition could be a potential new avenue to explore also for mono-ARTs as we know more about their target proteins and regulatory mechanisms.
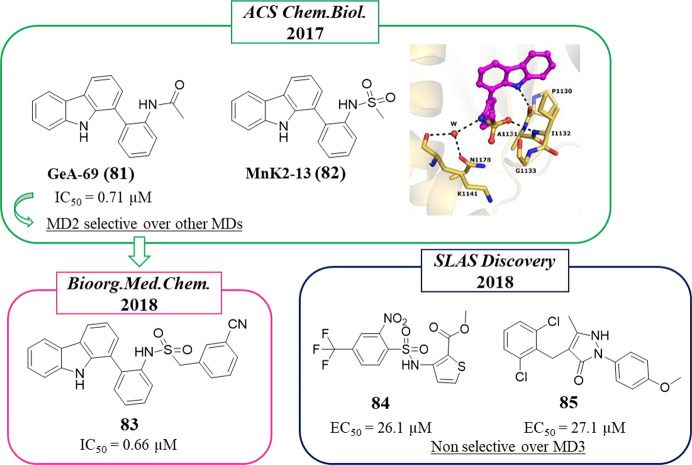
Mono-ARTs contain multiple accessory domains enabling the enzymes to bind to mono- (macrodomains) and poly-ADP-ribosylated targets (WWE domains), allowing them to possibly carry out further modifications on different residues by their catalytic domains. Small molecules have been discovered in two papers that inhibited the macrodomain 2 (MD2) of PARP14.165,166 The inhibition of MDs is very promising to obtain selective inhibition, since these accessory domains characterize only three ARTs (PARP9, -14, and -15).167 The inhibitors would interfere with the localization and PPIs while not necessarily affecting the catalytic activity itself.
The first allosteric inhibitor dates back to 2017 when Knapp et al.165 described a screening of 48 000 small molecules (3000 in-house kinase inhibitors and 45 000 form Novartis) against MD2 using an AlphaScreen assay that led to the identification of the carbazole derivative GeA-69 (81) (Figure 23). This compound, at 80 μM, fully inhibited the binding of ADP-ribose to MD2 with a calculated IC50 of 0.71 μM. In order to evaluate the binding mode of the compound, the authors cocrystallized the corresponding methanesulfonamide compound MnK2–13 (82) with a PARP14 MD2 mutant. From the structure solved at 1.6 Å, it emerged that it was bound to a hydrophobic site adjacent to the ADP-ribose binding site of MD2 (Figure 23). The interaction determined that a loop from Pro1130 to Pro1140 was pushed into the ADP-ribose site, thereby displacing ADP-ribose. A hydrogen bond was formed between the carbazole NH and the backbone carbonyl of Pro1130 and another between the sulfonamide carbonyl and amino groups of Ile1132. Compound 81 was tested against 11 other MDs, confirming its selectivity in inhibiting only PARP14 MD2. In the presence of a high concentration of 81 (250 μM), the recruitment of PARP14 MD2 to the DNA damage was completely prevented, confirming the inhibitory activity of the compound also in cells (U-2-OS). However, the compound showed some cytotoxicity at about 50 μM against HeLa, U-2-OS, and HEK293T cells after 72 h of incubation. Thirty analogues of 81 were successively synthesized,168 highlighting the essential role of the NH of the carbazole and leading to the identification of a new derivative, the 3-cyanophenylmethanesulfoamide carbazole 83 (Figure 23), displaying similar PARP14 MD2 inhibition (IC50 = 0.66 μM). Unfortunately, the cytotoxicity was not tested.
Figure 23.
PARP14 macrodomain-2 (MD2) inhibitors developed by Knapp et al. (in the green and pink boxes)165,168 and Ekblad et al. (in the blue box)166 along with the their inhibitory activity against MD2 and their selectivity. Crystal structure (PDB ID 5O2D) shows the binding mode of 82.
In the same year and using again an AlphaScreen method, different PARP14 MD2 small molecule inhibitors were identified by Ekblad et al.166 On testing 1584 compounds provided by Chemical Biology Consortium Sweden, 17 compounds were active and were then evaluated by SPR, leading to the identification of the thiophene derivative 84 and the pyrazole derivative 85, which showed kd = 8 and 162 μM, respectively, with respective AlphaScreen EC50 values of 26.1 and 27.1 μM, respectively (Figure 23). These compounds also inhibited PARP14 MD3.
Concluding Remarks and Future Perspectives
The discovery and therapeutic application of PARPi represents a successful approach to targeted cancer treatment due to their excellent ability to selectively kill tumors with deficiency in DNA repair proteins through the so-called synthetic lethality.38 After olaparib was introduced to clinical use for the treatment of tumors harboring a defective HRR pathway,45 other PARPi reached the market and many other compounds are at various stages of clinical studies, where they are being investigated as a single agent or in combination against diverse cancer types.52 PARP1 is generally considered the major target of the FDA-approved PARPi, but due to the structural similarity of the targeted NAD+-binding domain, they also inhibit the activity of other PARPs, mainly PARP2. The effect of a polypharmacology in this context is still unknown, and selectivity over the other PARPs may not even be in all cases the most beneficial property of a drug candidate in a clinical setting.169 On the contrary, to be useful as a chemical probe for the analysis of the biological response and in proof-of-concept in vivo studies, high specificity of the inhibitor is important. The lack of access to a complete panel of sensitive, high-throughput PARP assays117,170−172 to fully evaluate selectivity is a common weakness that leads to profiling of PARP inhibitors only against a handful of family members, rarely including representative mono-ARTs. This also emerged from the present perspective, where only a few research teams profiled their compounds against a larger panel of PARPs and TNKS, but the recent assay development efforts will likely make the profiling against a PARP panel more routine in the future.173,174 The lack of selective inhibitors has until now hampered the unveiling of the pathophysiological roles of many mono-ARTs.
The first study that aimed at identifying selective inhibitors of a mono-ART, PARP14, dates back less than 10 years ago.114 Since then, 28 papers have been published with an increasing trend during the past few years. In this review, we collected the literature and focused the discussion on those molecules that have been designed or were found to be active against one enzyme or to some extent restricted toward the mono-ARTs class. The SAR studies and hit-to-lead optimization phase have taken advantage of the crystal structures, which in many cases were solved in complex with the inhibitors and the target or a surrogate mono-ART. The selectivity profile against focused or a wide panel of mono- and poly-ARTs was almost always recorded, but the cytotoxicity was tested in parallel only in some cases. Similarly, while the activity against the enzymes was confirmed in cells for many compounds, the pharmacological effects were rarely tested, likely due to the still modest potency and unspecificity of the disclosed early compounds. Most of the small molecules herein reported are indeed still in their infancy as the pharmacokinetic profile has been studied only for the most advanced inhibitors coming from the pharmaceutical companies.
With the exception of TNKS inhibitors, all of the potent PARPi with defined binding modes share the key features170,175 described for the early PARP1 inhibitors. These include the requirements of hydrogen bond donors and acceptors allowing interactions with the backbone amide and carbonyl of a glycine as well as side chain hydroxyl of serine located at the bottom of the NI site. The NI binding pocket is also lined with aromatic tyrosine residues packing against NI of NAD+ or the competitive inhibitors. From the efforts described here to discover inhibitors specific toward mono-ARTs, this pharmacophore can be extended to include additional features: some shared with poly-ARTs to increase inhibitor potency and some to gain selectivity toward mono-ARTs through structure-based design (Figure 24).
Figure 24.
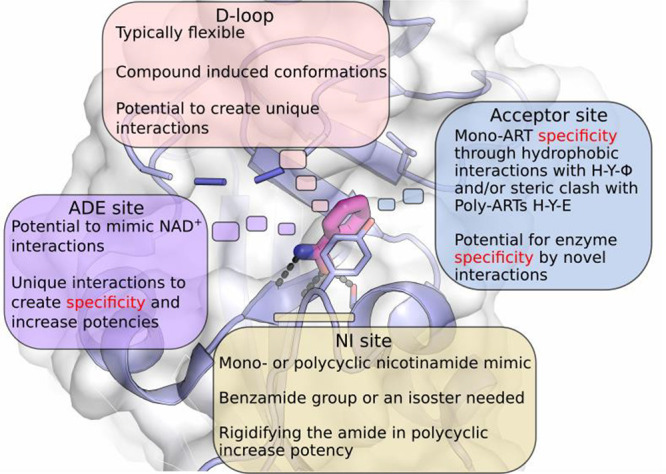
Extended PARP inhibitor pharmacophore highlighting the routes to improve mono-ART inhibitor selectivity while targeting the conserved NI binding site of the catalytic domain.
Analogously to the inhibitors of poly-ARTs, most of the mono-ART inhibitors interact with the NI binding site (Figure 24). Thus, they possess a nicotinamide mimic moiety, usually represented by a bicyclic or polycyclic ring, but a monocycle is also tolerated in mono-ART inhibitors like in OUL35 (3) when coupled with the essential primary carboxamide (Figure 24). The carboxamide can be also included in a cycle. This rigidifies the amide by fixing it to a certain conformation and providing entropy gains, a strategy often used in PARPi development. A few structures have emerged as particularly privileged to obtain mono-ART inhibitors such as quinazolinones and phthalazinones that although widespread also among poly-ARTs inhibitors when properly functionalized permitted one to achieve potent and selective RBN012759 (52) (PARP14), ITK7 (55) (PARP11), and dual compounds 15–17 (PARP10/PARP15). In a very few cases, the typical benzamide group is missing, such as in PARP10 tetralone-based inhibitors, 26–28, and PARP14 inhibitors, 43 and 45, in which the benzamide moiety was replaced by the triazole bioisoster group. The typical feature of mono-ARTs, the “H–Y−Φ” motif replacing H–Y–E of poly-ARTs, provides a way used to restrict the activity toward mono-ARTs due to unfavorable interactions with the negatively charged glutamate present in PARP1–4 and TNKS. PARP10 inhibitor OUL35 (3) contains a p-ether benzamide and extends toward the acceptor site and toward the hydrophobic Ile987 (Figure 24). Similarly, in the quinazolinone derivatives RBN012759 (52) and ITK7 (55), the presence of a hydrophobic substituent in C-7 imparted selectivity against PARP14 or PARP11, respectively, by interacting with Leu1782 (PARP14) or Leu1701 (PARP11).
PARPi, like olaparib, extend from the NI site toward the ADE site and span the whole NAD+ binding cleft of the ART domain. Analogously to poly-ARTs inhibitor design, also some mono-ART inhibitors extend beyond the NI site, reaching the ADE site. This design would make the compounds larger in terms of MW but would also increase potencies through new interactions and potentially also selectivity by targeting unique residues. As an example, a bidentate compound 47 extends to the ADE site, giving potent and selective PARP14 inhibition. Also, other mono-ART inhibitors were designed to interact with the unique residues in PARP14, such as RBN012759 (52), where the trans-cyclohexanol interacted with Asp1604, displacing the intra-hydrogen bond with Ser1607 and becoming selective over the other mono-ARTs. Similarly, RBN12042 (79) forms interactions with the Asp1604 at the ADE site.
The D-loop lining the NAD+ binding cleft is often found to be flexible and disordered in the structures, even when the binding pocket is occupied by a ligand.76,167 The possible interactions observed with this region have to be therefore carefully evaluated for using them in inhibitor design. In addition, this region is often involved in crystal contacts with the adjacent protein contributing to the observed conformation (Figure 12). However, these features could also be utilized efficiently in the design of specific inhibitors as shown by compounds 31 and 32, which inhibited PARP14 (Figure 7) by capturing specific conformation of the D-loop.
There is still a long way to go to study in a more precise and systematic manner the implications of mono-ARTs in the development of cancer as well as in other diseases. Significant advances have been reported, and parallel work of multiple laboratories has elucidated possibilities to create specific inhibitors. Mono-ART inhibitors are now emerging that satisfy Frye’s five criteria for quality chemical probes for interrogating their biological roles in cells and in animal models,176 possibly validating these enzymes as drug targets for human diseases.
Glossary
Abbreviations Used
- ADE site
adenine ribose binding site
- AHR
aryl hydrocarbon receptor
- AMOT
anigomotin
- APC
adenomatous polyposis coli
- ARH3
ADP-ribosyl hydrolase 3
- ARTD
diphtheria toxin-like ADP ribosyltransferase
- CFTR
cystic fibrosis transmembrane regulator
- chk1
checkpoint kinase 1
- CuAAC
CuI-catalyzed azide–alkyne cycloaddition
- DECL
DNA-encoded chemical library
- DELFIA
dissociation-enhanced lanthanide fluorescence immunoassay
- DLBCL
diffuse large B cell lymphoma
- D-loop
donor loop
- DSB
double-stranded break
- DSF
differential scanning fluorimetry
- dq
3,4-dihydroisoquinolin-1-(2H)-one
- ECG
epicatechin-3-gallate
- EGCG
epigallocatechin-3-gallate
- EMA
European Medicines Agency
- ERα
estrogen receptor α
- FDA
Food and Drug Administration
- HCC
hepatocellular carcinoma
- HDAC
histone deacetylase
- HPF1
histone PARylation factor 1
- HRR
homologous recombination repair
- IFN-I
type I interferon
- ITC
isothermal titration calorimetry
- LXR
liver X receptor
- MD
macrodomain
- MPS
multipolar spindle
- NAD+
β-nicotinamide adenine dinucleotide
- NCI
National Cancer Institute
- NI
nicotinamide
- NLS
nuclear localization signal
- PARP
poly-ADP-ribose polymerase
- PARG
poly-ADP-ribose glucohydrolase
- PARPis
PARP inhibitors
- PCNA
proliferating cell nuclear antigen
- PPI
protein–protein interaction
- SSB
single-stranded break
- SAR
structural–activity relationship
- TCDD
tetrachlorodibenzo-p-dioxin
- TNKS
tankyrase
- ZnF
zinc finger
Biographies
Maria Giulia Nizi graduated in Pharmacy (February 2018) at the University of Perugia and started her Ph.D. studies in Medicinal Chemistry in November 2018 at the same University. The main focus of her Ph.D. project was the design and synthesis of small molecules as potent and specific inhibitors for mono-ARTs. In 2020, she spent 6 months at the Faculty of Biochemistry and Molecular Medicine & Biocenter Oulu, University of Oulu, as a visiting Ph.D. student under the supervision of Prof. Lari Lehtiö, acquiring the skills of protein purification, biochemical assays, and crystallography.
Mirko M. Maksimainen received his M.Sc. degree in Organic Chemistry from the University of Joensuu in 2008. He received his Ph.D. degree from the University of Eastern Finland in 2012, where his Ph.D. thesis focused on structural studies of β-galactosidases by protein crystallography. He continued research as a postdoctoral scientist in 2012–2015 and expanded his expertise on mass spectrometry methods. In 2015, he joined Prof. Lehtiö’s research group at the University of Oulu, where he studies the structures and functions of ADP-ribosyltransferases and develops small molecule inhibitors for mono-ADP-ribosyltransferases and ADP-ribosylhydrolases.
Lari Lehtiö received his B.Sc. and M.Sc. degrees in Biochemistry from the University of Turku in 2000. He received his Ph.D. degree from the University of Helsinki in 2006, where he used protein crystallography as the main research tool. Subsequently, he worked as a postdoctoral scientist in the Structural Genomics Consortium at Karolinska Institutet, Sweden. He established his research team in Åbo Akademi University in 2009 and moved to the Biocenter Oulu and University of Oulu in 2012. He worked as an Academy of Finland research fellow and was appointed as Full Professor of Structural Biology in 2019. His research focuses on ADP-ribosylation signaling, and he studies the structures and functions of the proteins involved as well as develops small molecule inhibitors for readers, writers, and erasers of ADP-ribosylation.
Oriana Tabarrini is a full professor of Medicinal Chemistry at the Department of Pharmaceutical Sciences (University of Perugia, Italy). After receiving her degree in Pharmaceutical Chemistry and Technology, she has worked for several years with research grants from pharmaceutical industries. In 2002, she was promoted to Associate Professor. Her research focuses on developing small molecules as pharmacological tools and potential chemotherapeutic agents with particular focus on antivirals and more recently anticancers. She has published over 120 research articles in leading peer-reviewed journals, including some invited reviews and patents.
The authors declare the following competing financial interest(s): L.L. holds a patent on mono-ART inhibitors, and L.L., M.M., O.T., and M.G.N. all are inventors in a patent application on PARP inhibitors.
References
- Gibson B. A.; Kraus W. L. New Insights into the Molecular and Cellular Functions of Poly(ADP-Ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 2012, 13 (7), 411–424. 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- Mikolčević P.; Hloušek-Kasun A.; Ahel I.; Mikoč A. ADP-Ribosylation Systems in Bacteria and Viruses. Comput. Struct. Biotechnol. J. 2021, 19, 2366–2383. 10.1016/j.csbj.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B.; Ahel I.; Altmeyer M.; Ashworth A.; Bai P.; Chang P.; Cohen M.; Corda D.; Dantzer F.; Daugherty M. D.; Dawson T. M.; Dawson V. L.; Deindl S.; Fehr A. R.; Feijs K. L. H.; Filippov D. V.; Gagné J. P.; Grimaldi G.; Guettler S.; Hoch N. C.; Hottiger M. O.; Korn P.; Kraus W. L.; Ladurner A.; Lehtiö L.; Leung A. K. L.; Lord C. J.; Mangerich A.; Matic I.; Matthews J.; Moldovan G. L.; Moss J.; Natoli G.; Nielsen M. L.; Niepel M.; Nolte F.; Pascal J.; Paschal B. M.; Pawłowski K.; Poirier G. G.; Smith S.; Timinszky G.; Wang Z. Q.; Yélamos J.; Yu X.; Zaja R.; Ziegler M.. ADP-Ribosyltransferases, an Update on Function and Nomenclature. FEBS J. 2021. 10.1111/febs.16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness B. G.; Howard J. B.; Bodley J. W. ADP-Ribosylation of Elongation Factor 2 by Diphtheria Toxin. Isolation and Properties of the Novel Ribosyl-Amino Acid and Its Hydrolysis Products. J. Biol. Chem. 1980, 255 (22), 10717–10720. 10.1016/S0021-9258(19)70366-4. [DOI] [PubMed] [Google Scholar]
- Simon N. C.; Aktories K.; Barbieri J. T. Novel Bacterial ADP-Ribosylating Toxins: Structure and Function. Nat. Rev. Microbiol. 2014, 12 (9), 599–611. 10.1038/nrmicro3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P.; Weill J. D.; Mandel P. Nicotinamide Mononucleotide Activation of New DNA-Dependent Polyadenylic Acid Synthesizing Nuclear Enzyme. Biochem. Biophys. Res. Commun. 1963, 11 (1), 39–43. 10.1016/0006-291X(63)90024-X. [DOI] [PubMed] [Google Scholar]
- Vyas S.; Chesarone-Cataldo M.; Todorova T.; Huang Y.-H.; Chang P. A Systematic Analysis of the PARP Protein Family Identifies New Functions Critical for Cell Physiology. Nat. Commun. 2013, 4, 2240–2253. 10.1038/ncomms3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen J. D.; Brody J. R.; Armen R. S.; Pascal J. M. Structural Implications for Selective Targeting of PARPs. Front. Oncol. 2013, 3, 301. 10.3389/fonc.2013.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier M. F.; Zandarashvili L.; Aguiar P. M.; Black B. E.; Pascal J. M. NAD + Analog Reveals PARP-1 Substrate-Blocking Mechanism and Allosteric Communication from Catalytic Center to DNA-Binding Domains. Nat. Commun. 2018, 9 (1), 844–857. 10.1038/s41467-018-03234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beek L.; Mcclay É.; Patel S.; Schimpl M.; Spagnolo L.; Maia De Oliveira T. PARP Power: A Structural Perspective on PARP1, PARP2, and PARP3 in DNA Damage Repair and Nucleosome Remodelling. Int. J. Mol. Sci. 2021, 22 (10), 5112. 10.3390/ijms22105112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A. F.; Schüler H. Comparative Structural Analysis of the Putative Mono-ADP-Ribosyltransferases of the ARTD/PARP Family. Curr. Top. Microbiol. Immunol. 2014, 384, 153–166. 10.1007/82_2014_417. [DOI] [PubMed] [Google Scholar]
- Miwa M.; Ishihara M.; Takishima S.; Takasuka N.; Maeda M.; Yamaizumi Z.; Sugimura T.; Yokoyama S.; Miyazawa T. The Branching and Linear Portions of Poly(Adenosine Diphosphate Ribose) Have the Same Alpha(1 Leads to 2) Ribose-Ribose Linkage. J. Biol. Chem. 1981, 256 (6), 2916–2921. 10.1016/S0021-9258(19)69701-2. [DOI] [PubMed] [Google Scholar]
- Ruf A.; Rolli V.; De Murcia G.; Schulz G. E. The Mechanism of the Elongation and Branching Reaction of Poly(ADP-Ribose) Polymerase as Derived from Crystal Structures and Mutagenesis. J. Mol. Biol. 1998, 278 (1), 57–65. 10.1006/jmbi.1998.1673. [DOI] [PubMed] [Google Scholar]
- Vyas S.; Matic I.; Uchima L.; Rood J.; Zaja R.; Hay R. T.; Ahel I.; Chang P. Family-Wide Analysis of Poly(ADP-Ribose) Polymerase Activity. Nat. Commun. 2014, 5, 4426–4455. 10.1038/ncomms5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine H.; Poreba E.; Lesniewicz K.; Hassa P. O.; Hottiger M. O.; Litchfield D. W.; Shilton B. H.; Lüscher B. Substrate-Assisted Catalysis by PARP10 Limits Its Activity to Mono-ADP-Ribosylation. Mol. Cell 2008, 32 (1), 57–69. 10.1016/j.molcel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Loseva O.; Jemth A. S.; Bryant H. E.; Schüler H.; Lehtiö L.; Karlberg T.; Helleday T. PARP-3 Is a Mono-ADP-Ribosylase That Activates PARP-1 in the Absence of DNA. J. Biol. Chem. 2010, 285 (11), 8054–8060. 10.1074/jbc.M109.077834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg T.; Klepsch M.; Thorsell A.-G.; Andersson C. D.; Linusson A.; Schüler H. Structural Basis for Lack of ADP-Ribosyltransferase Activity in Poly(ADP-Ribose) Polymerase-13/Zinc Finger Antiviral Protein. J. Biol. Chem. 2015, 290 (12), 7336–7344. 10.1074/jbc.M114.630160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. S.; Jividen K.; Spencer A.; Dworak N.; Ni L.; Oostdyk L. T.; Chatterjee M.; Kuśmider B.; Reon B.; Parlak M.; Gorbunova V.; Abbas T.; Jeffery E.; Sherman N. E.; Paschal B. M. Ubiquitin Modification by the E3 Ligase/ADP-Ribosyltransferase Dtx3L/Parp9. Mol. Cell 2017, 66, 503–516. 10.1016/j.molcel.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrin C.; Gabrielsen M.; Buetow L.; Nakasone M. A.; Ahmed S. F.; Sumpton D.; Sibbet G. J.; Smith B. O.; Huang D. T. Structural Insights into ADP-Ribosylation of Ubiquitin by Deltex Family E3 Ubiquitin Ligases. Sci. Adv. 2020, 6 (38), 418–423. 10.1126/sciadv.abc0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok Y.; Vela-Rodríguez C.; Yang C.; Alanen H. I.; Liu F.; Paschal B. M.; Lehtiö L. Reconstitution of the DTX3L-PARP9 Complex Reveals Determinants for High-Affinity Heterodimerization and Multimeric Assembly. Biochem. J. 2022, 479 (3), 289–304. 10.1042/BCJ20210722. [DOI] [PubMed] [Google Scholar]
- Rodriguez K. M.; Buch-Larsen S. C.; Kirby I. T.; Siordia I. R.; Hutin D.; Rasmussen M.; Grant D. M.; David L. L.; Matthews J.; Nielsen M. L.; Cohen M. S. Chemical Genetics and Proteome-Wide Site Mapping Reveal Cysteine MARylation by PARP-7 on Immune-Relevant Protein Targets. Elife 2021, 10, 1–94. 10.7554/eLife.60480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson B. A.; Kraus W. L. Identification of Protein Substrates of Specific PARP Enzymes Using Analog-Sensitive PARP Mutants and a ‘Clickable’ NAD+ Analog. Methods Mol. Biol. 2017, 1608, 111–135. 10.1007/978-1-4939-6993-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskiewicz M. J.; Palazzo L.; Hughes R.; Ahel I. Progress and Outlook in Studying the Substrate Specificities of PARPs and Related Enzymes. FEBS J. 2021, 288 (7), 2131–2142. 10.1111/febs.15518. [DOI] [PubMed] [Google Scholar]
- Luscher B.; Butepage M.; Eckei L.; Krieg S.; Verheugd P.; Shilton B. H. ADP-Ribosylation, a Multifaceted Posttranslational Modification Involved in the Control of Cell Physiology in Health and Disease. Chem. Rev. 2018, 118, 1092–1136. 10.1021/acs.chemrev.7b00122. [DOI] [PubMed] [Google Scholar]
- Weixler L.; Schäringer K.; Momoh J.; Lüscher B.; Feijs K. L. H.; Žaja R. ADP-Ribosylation of RNA and DNA: From in Vitro Characterization to in Vivo Function. Nucleic Acids Res. 2021, 49 (7), 3634–3650. 10.1093/nar/gkab136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H.; Yu X. Targeting DePARylation Selectively Suppresses DNA Repair-Defective and PARP Inhibitor-Resistant Malignancies. Sci. Adv. 2019, 5 (4), 4340–4355. 10.1126/sciadv.aav4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivelo C. A.; Ayyappan V.; Leung A. K. L. Poly(ADP-Ribose)-Dependent Ubiquitination and Its Clinical Implications. Biochem. Pharmacol. 2019, 167, 3. 10.1016/j.bcp.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhorova E.; Agnew T.; Wondisford A. R.; Tellier M.; Kaminski N.; Beijer D.; Holder J.; Groslambert J.; Suskiewicz M. J.; Zhu K.; Reber J. M.; Krassnig S. C.; Palazzo L.; Murphy S.; Nielsen M. L.; Mangerich A.; Ahel D.; Baets J.; O’Sullivan R. J.; Ahel I. Unrestrained Poly-ADP-Ribosylation Provides Insights into Chromatin Regulation and Human Disease. Mol. Cell 2021, 81 (12), 2640–2655. 10.1016/j.molcel.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack J. G. M.; Palazzo L.; Ahel I. (ADP-Ribosyl)Hydrolases: Structure, Function, and Biology. Genes Dev. 2020, 34, 263–284. 10.1101/gad.334631.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrision D.; Gravells P.; Thompson R.; Bryant H. E. Poly(ADP-Ribose) Glycohydrolase (PARG) vs. Poly(ADP-Ribose) Polymerase (PARP) - Function in Genome Maintenance and Relevance of Inhibitors for Anti-Cancer Therapy. Front. Mol. Biosci. 2020, 7, 191–212. 10.3389/fmolb.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Ma Y.; Li Y.; Dong Y.; Yu L. L.; Wang H.; Guo L.; Wu C.; Yu X.; Liu X. Molecular Basis for the MacroD1-Mediated Hydrolysis of ADP-Ribosylation. DNA Repair (Amst). 2020, 94, 102899. 10.1016/j.dnarep.2020.102899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo L.; Thomas B.; Jemth A.-S.; Colby T.; Leidecker O.; Feijs K. L.H.; Zaja R.; Loseva O.; Puigvert J. C.; Matic I.; Helleday T.; Ahel I. Processing of Protein ADP-Ribosylation by Nudix Hydrolases. Biochem. J. 2015, 468 (2), 293–301. 10.1042/BJ20141554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzov M. M.; Belousova E. A.; Ilina E. S.; Lavrik O. I. Impact of PARP1, PARP2 & PARP3 on the Base Excision Repair of Nucleosomal DNA. Adv. Exp. Med. Biol. 2020, 1241, 47–57. 10.1007/978-3-030-41283-8_4. [DOI] [PubMed] [Google Scholar]
- Obaji E.; Maksimainen M. M.; Galera-Prat A.; Lehtiö L. Activation of PARP2/ARTD2 by DNA Damage Induces Conformational Changes Relieving Enzyme Autoinhibition. Nat. Commun. 2021, 12 (1), 3479–3487. 10.1038/s41467-021-23800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilokapic S.; Suskiewicz M. J.; Ahel I.; Halic M. Bridging of DNA Breaks Activates PARP2-HPF1 to Modify Chromatin. Nature 2020, 585 (7826), 609–613. 10.1038/s41586-020-2725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskiewicz M. J.; Zobel F.; Ogden T. E. H.; Fontana P.; Ariza A.; Yang J. C.; Zhu K.; Bracken L.; Hawthorne W. J.; Ahel D.; Neuhaus D.; Ahel I. HPF1 Completes the PARP Active Site for DNA Damage-Induced ADP-Ribosylation. Nature 2020, 579 (7800), 598–602. 10.1038/s41586-020-2013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Chaudhuri A.; Nussenzweig A. The Multifaceted Roles of PARP1 in DNA Repair and Chromatin Remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18 (10), 610–621. 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant H. E.; Schultz N.; Thomas H. D.; Parker K. M.; Flower D.; Lopez E.; Kyle S.; Meuth M.; Curtin N. J.; Helleday T. Specific Killing of BRCA2-Deficient Tumours with Inhibitors of Poly(ADP-Ribose) Polymerase. Nature 2005, 434 (7035), 913–917. 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- Farmer H.; McCabe H.; Lord C. J.; Tutt A. H. J.; Johnson D. A.; Richardson T. B.; Santarosa M.; Dillon K. J.; Hickson I.; Knights C.; Martin N. M. B.; Jackson S. P.; Smith G. C. M.; Ashworth A. Targeting the DNA Repair Defect in BRCA Mutant Cells as a Therapeutic Strategy. Nature 2005, 434 (7035), 917–921. 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Walsh C. S. Two Decades beyond BRCA1/2: Homologous Recombination, Hereditary Cancer Risk and a Target for Ovarian Cancer Therapy?. Gynecol. Oncol. 2015, 137 (2), 343–350. 10.1016/j.ygyno.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Shah G. M.; Robu M.; Purohit N. K.; Rajawat J.; Tentori L.; Graziani G. PARP Inhibitors in Cancer Therapy: Magic Bullets but Moving Targets. Front. Oncol. 2013, 3, 00279. 10.3389/fonc.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. W.; Tewari K. S. New Targeted Agents in Gynecologic Cancers: Synthetic Lethality, Homologous Recombination Deficiency, and PARP Inhibitors. Curr. Treat. Options Oncol. 2016, 17 (3), 12. 10.1007/s11864-015-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris D. V. Evolution of Poly(ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors. From Concept to Clinic. J. Med. Chem. 2010, 53 (12), 4561–4584. 10.1021/jm100012m. [DOI] [PubMed] [Google Scholar]
- Velagapudi U. K.; Patel B. A.; Shao X.; Pathak S. K.; Ferraris D. V.; Talele T. T. Recent Development in the Discovery of PARP Inhibitors as Anticancer Agents: A Patent Update (2016–2020). Expert Opin. Ther. Pat. 2021, 31 (7), 609–623. 10.1080/13543776.2021.1886275. [DOI] [PubMed] [Google Scholar]
- Deeks E. D. Olaparib: First Global Approval. Drugs 2015, 75 (2), 231–240. 10.1007/s40265-015-0345-6. [DOI] [PubMed] [Google Scholar]
- FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer; FDA; https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer (accessed Jan 7, 2022).
- Shirley M. Rucaparib: A Review in Ovarian Cancer. Target. Oncol. 2019, 14 (2), 237–246. 10.1007/s11523-019-00629-5. [DOI] [PubMed] [Google Scholar]
- Heo Y. A.; Duggan S. T. Niraparib: A Review in Ovarian Cancer. Target. Oncol. 2018, 13 (4), 533–539. 10.1007/s11523-018-0582-1. [DOI] [PubMed] [Google Scholar]
- EU/3/14/1339: Orphan designation for the treatment of Dravet syndrome; European Medicines Agency; https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu310830 (accessed Apr 25, 2022).
- Home; https://clinicaltrials.gov/ (accessed Feb 2, 2021).
- Paluch-Shimon S.; Cardoso F. PARP Inhibitors Coming of Age. Nat. Rev. Clin. Oncol. 2020 182 2021, 18 (2), 69–70. 10.1038/s41571-020-00452-2. [DOI] [PubMed] [Google Scholar]
- Chan C. Y.; Tan K. V.; Cornelissen B. PARP Inhibitors in Cancer Diagnosis and Therapy. Clin. Cancer Res. 2021, 27 (6), 1585–1594. 10.1158/1078-0432.CCR-20-2766. [DOI] [PubMed] [Google Scholar]
- Risdon E. N.; Chau C. H.; Price D. K.; Sartor O.; Figg W. D. PARP Inhibitors and Prostate Cancer: To Infinity and Beyond BRCA. Oncologist 2021, 26 (1), e115–e129. 10.1634/theoncologist.2020-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Carvajal R.; Irving E.; Lukashchuk N.; Forment J. V. Preventing and Overcoming Resistance to PARP Inhibitors: A Focus on the Clinical Landscape. Cancers 2022, 14 (1), 44. 10.3390/cancers14010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby S. J.; Chalmers A. J.; Carruthers R. D. Radiotherapy-Poly(ADP-Ribose) Polymerase Inhibitor Combinations: Progress to Date. Semin. Radiat. Oncol. 2022, 32 (1), 15–28. 10.1016/j.semradonc.2021.09.005. [DOI] [PubMed] [Google Scholar]
- Haikarainen T.; Krauss S.; Lehtiö L. Tankyrases: Structure, Function and Therapeutic Implications in Cancer. Curr. Pharm. Des. 2014, 20 (41), 6472–6488. 10.2174/1381612820666140630101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtiö L.; Chi N. W.; Krauss S. Tankyrases as Drug Targets. FEBS J. 2013, 280 (15), 3576–3593. 10.1111/febs.12320. [DOI] [PubMed] [Google Scholar]
- Riffell J. L.; Lord C. J.; Ashworth A. Tankyrase-Targeted Therapeutics: Expanding Opportunities in the PARP Family. Nat. Rev. Drug Discovery 2012 1112 2012, 11 (12), 923–936. 10.1038/nrd3868. [DOI] [PubMed] [Google Scholar]
- Mariotti L.; Pollock K.; Guettler S. Regulation of Wnt/β-Catenin Signalling by Tankyrase-Dependent Poly(ADP-Ribosyl)Ation and Scaffolding. Br. J. Pharmacol. 2017, 174 (24), 4611–4636. 10.1111/bph.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio-Martinez E.; Herrera-Campos A.; Muñoz A.; Rodríguez-Vargas J.; Oliver F. Tankyrases as Modulators of Pro-Tumoral Functions: Molecular Insights and Therapeutic Opportunities. J. Exp. Clin. Cancer Res. 2021, 40 (1), 144–159. 10.1186/s13046-021-01950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatoff E. M.; Goswami S.; Zafra M. P.; Foronda M.; Shusterman M.; Leach B. I.; Katti A.; Diaz B. J.; Dow L. E. Distinct Colorectal Cancer-Associated APC Mutations Dictate Response to Tankyrase Inhibition. Cancer Discovery 2019, 9 (10), 1358–1371. 10.1158/2159-8290.CD-19-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.; Wang Y.; Neri S.; Zhen Y.; Fong L. W. R.; Qiao Y.; Li X.; Chen Z.; Stephan C.; Deng W.; Ye R.; Jiang W.; Zhang S.; Yu Y.; Hung M. C.; Chen J.; Lin S. H. Tankyrase Disrupts Metabolic Homeostasis and Promotes Tumorigenesis by Inhibiting LKB1-AMPK Signalling. Nat. Commun. 2019, 10 (1), 4363. 10.1038/s41467-019-12377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.; Wang X.; Jia T.; Wei W.; Chua M.-S.; So S. Tankyrase Inhibitors Attenuate WNT/β-Catenin Signaling and Inhibit Growth of Hepatocellular Carcinoma Cells. Oncotarget 2015, 6 (28), 25390–25401. 10.18632/oncotarget.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Ren S.; Xu F.; Ma Z.; Liu X.; Wang L. Tankyrase Promotes Aerobic Glycolysis and Proliferation of Ovarian Cancer through Activation of Wnt/ β-Catenin Signaling. BioMed Res. Int. 2019, 2019, 5763602. 10.1155/2019/5763602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B.; Wang J.; Fang J.; Jiang B.; Zhang M.; Wang Y.; Yang Z. Expression of TNKS1 Is Correlated with Pathologic Grade and Wnt/β-Catenin Pathway in Human Astrocytomas. J. Clin. Neurosci. 2012, 19 (1), 139–143. 10.1016/j.jocn.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Wang W.; Li N.; Li X.; Tran M. K.; Han X.; Chen J. Tankyrase Inhibitors Target YAP by Stabilizing Angiomotin Family Proteins. Cell Rep. 2015, 13 (3), 524–532. 10.1016/j.celrep.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta C. C.; Bhatt H. G. Tankyrase Inhibitors as Antitumor Agents: A Patent Update (2013 - 2020). Expert Opin. Ther. Pat. 2021, 31 (7), 645–661. 10.1080/13543776.2021.1888929. [DOI] [PubMed] [Google Scholar]
- Yu M.; Yang Y.; Sykes M.; Wang S. Small-Molecule Inhibitors of Tankyrases as Prospective Therapeutics for Cancer. J. Med. Chem. 2022, 65, 5244–5273. 10.1021/acs.jmedchem.1c02139. [DOI] [PubMed] [Google Scholar]
- Plummer R.; Dua D.; Cresti N.; Drew Y.; Stephens P.; Foegh M.; Knudsen S.; Sachdev P.; Mistry B. M.; Dixit V.; McGonigle S.; Hall N.; Matijevic M.; McGrath S.; Sarker D. First-in-Human Study of the PARP/Tankyrase Inhibitor E7449 in Patients with Advanced Solid Tumours and Evaluation of a Novel Drug-Response Predictor. Br. J. Cancer 2020, 123 (4), 525–533. 10.1038/s41416-020-0916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First-In-Human Dose-Escalation Study of STP1002 in Patients With Advanced-Stage Solid Tumors; https://clinicaltrials.gov/ct2/show/NCT04505839 (accessed Dec 2, 2021).
- Mizutani A.; Yashiroda Y.; Muramatsu Y.; Yoshida H.; Chikada T.; Tsumura Y.; Okue M.; Shirai F.; Fukami T.; Yoshida M.; Seimiya H. RK-287107, a Potent and Specific Tankyrase Inhibitor, Blocks Colorectal Cancer Cell Growth in a Preclinical Model. Cancer Sci. 2018, 109 (12), 4003–4014. 10.1111/cas.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders R. G. G.; Brinch S. A.; Sowa S. T.; Amundsen-Isaksen E.; Galera-Prat A.; Murthy S.; Aertssen S.; Smits J. N.; Nieczypor P.; Damen E.; Wegert A.; Nazaré M.; Lehtiö L.; Waaler J.; Krauss S. Development of a 1,2,4-Triazole-Based Lead Tankyrase Inhibitor: Part II. J. Med. Chem. 2021, 64, 17936–17949. 10.1021/acs.jmedchem.1c01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesi L.; Rugo H. S.; Jackisch C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target. Oncol. 2021, 16, 255–282. 10.1007/s11523-021-00796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis E. S.; Gomella L. G.; Petrylak D. P. When and How to Use PARP Inhibitors in Prostate Cancer: A Systematic Review of the Literature with an Update on On-Going Trials. Eur. Urol. Oncol. 2020, 3 (5), 594–611. 10.1016/j.euo.2020.07.005. [DOI] [PubMed] [Google Scholar]
- Damale M.; Pathan S.; Shinde D.; Patil R.; Arote R.; Sangshetti J. Insights of Tankyrases: A Novel Target for Drug Discovery. Eur. J. Med. Chem. 2020, 207, 112712. 10.1016/j.ejmech.2020.112712. [DOI] [PubMed] [Google Scholar]
- Wahlberg E.; Karlberg T.; Kouznetsova E.; Markova N.; Macchiarulo A.; Thorsell A.-G.; Pol E.; Frostell Å.; Ekblad T.; Öncü D.; Kull B.; Robertson G. M.; Pellicciari R.; Schüler H.; Weigelt J. Family-Wide Chemical Profiling and Structural Analysis of PArP and Tankyrase Inhibitors. Nat. Biotechnol. 2012, 30 (3), 283–288. 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]
- Johannes J. W.; Balazs A.; Barratt D.; Bista M.; Chuba M. D.; Cosulich S.; Critchlow S. E.; Degorce S. L.; Di Fruscia P.; Edmondson S. D.; Embrey K.; Fawell S.; Ghosh A.; Gill S. J.; Gunnarsson A.; Hande S. M.; Heightman T. D.; Hemsley P.; Illuzzi G.; Lane J.; Larner C.; Leo E.; Liu L.; Madin A.; Martin S.; Mcwilliams L.; O’connor M. J.; Orme J. P.; Pachl F.; Packer M. J.; Pei X.; Pike A.; Schimpl M.; She H.; Staniszewska A. D.; Talbot V.; Underwood E.; Varnes J. G.; Xue L.; Yao T.; Zhang K.; Zhang A. X.; Zheng X. Discovery of 5-{4-[(7-Ethyl-6-Oxo-5,6-Dihydro-1,5-Naphthyridin-3-Yl)Methyl]Piperazin-1-Yl}-N-Methylpyridine-2-Carboxamide (AZD5305): A PARP1-DNA Trapper with High Selectivity for PARP1 over PARP2 and Other PARPs. J. Med. Chem. 2021, 64, 14498–14512. 10.1021/acs.jmedchem.1c01012. [DOI] [PubMed] [Google Scholar]
- Lehtiö L.; Jemth A. S.; Collins R.; Loseva O.; Johansson A.; Markova N.; Hammarström M.; Flores A.; Holmberg-Schiavone L.; Weigelt J.; Helleday T.; Schüler H.; Karlberg T. Structural Basis for Inhibitor Specificity in Human Poly(ADP-Ribose) Polymerase-3. J. Med. Chem. 2009, 52 (9), 3108–3111. 10.1021/jm900052j. [DOI] [PubMed] [Google Scholar]
- Thorsell A.-G.; Ekblad T.; Karlberg T.; Löw M.; Pinto A. F.; Trésaugues L.; Moche M.; Cohen M. S.; Schüler H. Structural Basis for Potency and Promiscuity in Poly(ADP-Ribose) Polymerase (PARP) and Tankyrase Inhibitors. J. Med. Chem. 2017, 60 (4), 1262–1271. 10.1021/acs.jmedchem.6b00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby I. T.; Person A.; Cohen M. Rational Design of Selective Inhibitors of PARP4. RSC Med. Chem. 2021, 12 (11), 1950–1957. 10.1039/D1MD00195G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren A. E. G.; Karlberg T.; Thorsell A. G.; Hesse M.; Spjut S.; Ekblad T.; Andersson C. D.; Pinto A. F.; Weigelt J.; Hottiger M. O.; Linusson A.; Elofsson M.; Schüler H. PARP Inhibitor with Selectivity toward ADP-Ribosyltransferase ARTD3/PARP3. ACS Chem. Biol. 2013, 8 (8), 1698–1703. 10.1021/cb4002014. [DOI] [PubMed] [Google Scholar]
- Challa S.; Stokes M. S.; Kraus W. L. MARTs and MARylation in the Cytosol: Biological Functions, Mechanisms of Action, and Therapeutic Potential. Cells 2021, 10, 313–334. 10.3390/cells10020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa E. S.; Fabrizio G.; Di Girolamo M. A Role of Intracellular Mono-ADP-Ribosylation in Cancer Biology. FEBS J. 2013, 280 (15), 3551–3562. 10.1111/febs.12290. [DOI] [PubMed] [Google Scholar]
- Hopp A.-K.; Hottiger M. O. Uncovering the Invisible: Mono-ADP-Ribosylation Moved into the Spotlight. Cells 2021, 10 (3), 680. 10.3390/cells10030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine H.; Herrmann A.; Lamark T.; Forst A. H.; Verheugd P.; Lüscher-Firzlaff J.; Lippok B.; Feijs K. L. H.; Herzog N.; Kremmer E.; Johansen T.; Müller-Newen G.; Lüscher B. Dynamic Subcellular Localization of the Mono-ADP-Ribosyltransferase ARTD10 and Interaction with the Ubiquitin Receptor P62. Cell Commun. Signal. 2012, 10 (1), 28–45. 10.1186/1478-811X-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M.; Feijs K. L. H.; Lüscher B. Function and Regulation of the Mono-ADP-Ribosyltransferase ARTD10. Curr. Top. Microbiol. Immunol. 2014, 384, 167–188. 10.1007/82_2014_379. [DOI] [PubMed] [Google Scholar]
- Verheugd P.; Forst A. H.; Milke L.; Herzog N.; Feijs K. L. H.; Kremmer E.; Kleine H.; Lüscher B. Regulation of NF-ΚB Signalling by the Mono-ADP-Ribosyltransferase ARTD10. Nat. Commun. 2013, 4, 1683. 10.1038/ncomms2672. [DOI] [PubMed] [Google Scholar]
- Yu M.; Schreek S.; Cerni C.; Schamberger C.; Lesniewicz K.; Poreba E.; Vervoorts J.; Walsemann G.; Grötzinger J.; Kremmer E.; Mehraein Y.; Mertsching J.; Kraft R.; Austen M.; Lüscher-Firzlaff J.; Lüscher B. PARP-10, a Novel Myc-Interacting Protein with Poly(ADP-Ribose) Polymerase Activity, Inhibits Transformation. Oncogene 2005, 24 (12), 1982–1993. 10.1038/sj.onc.1208410. [DOI] [PubMed] [Google Scholar]
- Nicolae C.; Aho E. R.; Vlahos A. H.; Choe K. N.; De S.; Karras G. I.; Moldovan G. L. The ADP-Ribosyltransferase PARP10/ARTD10 Interacts with Proliferating Cell Nuclear Antigen (PCNA) and Is Required for DNA Damage Tolerance. J. Biol. Chem. 2014, 289 (19), 13627–13637. 10.1074/jbc.M114.556340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijs K. L.; Kleine H.; Braczynski A.; Forst A. H.; Herzog N.; Verheugd P.; Linzen U.; Kremmer E.; Lüscher B. ARTD10 Substrate Identification on Protein Microarrays: Regulation of GSK3β by Mono-ADP-Ribosylation. Cell Commun. Signal. 2013, 11 (1), 5–16. 10.1186/1478-811X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R. S.; Yuskaitis C. J.; Beurel E. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem. Res. 2007, 32, 577–595. 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher E. M.; Galvan A. M.; Imamura-Kawasawa Y.; Moldovan G. L.; Nicolae C. M. PARP10 Promotes Cellular Proliferation and Tumorigenesis by Alleviating Replication Stress. Nucleic Acids Res. 2018, 46 (17), 8908–8916. 10.1093/nar/gky658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkannagari H.; Verheugd P.; Koivunen J.; Haikarainen T.; Obaji E.; Ashok Y.; Narwal M.; Pihlajaniemi T.; Lüscher B.; Lehtiö L. Small-Molecule Chemical Probe Rescues Cells from Mono-ADP-Ribosyltransferase ARTD10/PARP10-Induced Apoptosis and Sensitizes Cancer Cells to DNA Damage. Cell Chem. Biol. 2016, 23 (10), 1251–1260. 10.1016/j.chembiol.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Murthy S.; Desantis J.; Verheugd P.; Maksimainen M. M.; Venkannagari H.; Massari S.; Ashok Y.; Obaji E.; Nkizinkinko Y.; Lüscher B.; Tabarrini O.; Lehtiö L. 4-(Phenoxy) and 4-(Benzyloxy)Benzamides as Potent and Selective Inhibitors of Mono-ADP-Ribosyltransferase PARP10/ARTD10. Eur. J. Med. Chem. 2018, 156, 93–102. 10.1016/j.ejmech.2018.06.047. [DOI] [PubMed] [Google Scholar]
- Holechek J.; Lease R.; Thorsell A. G.; Karlberg T.; McCadden C.; Grant R.; Keen A.; Callahan E.; Schüler H.; Ferraris D. Design, Synthesis and Evaluation of Potent and Selective Inhibitors of Mono-(ADP-Ribosyl)Transferases PARP10 and PARP14. Bioorg. Med. Chem. Lett. 2018, 28 (11), 2050–2054. 10.1016/j.bmcl.2018.04.056. [DOI] [PubMed] [Google Scholar]
- Nizi M. G.; Maksimainen M. M.; Murthy S.; Massari S.; Alaviuhkola J.; Lippok B. E.; Sowa S. T.; Galera-Prat A.; Prunskaite-Hyyryläinen R.; Lüscher B.; Korn P.; Lehtiö L.; Tabarrini O. Potent 2,3-Dihydrophthalazine-1,4-Dione Derivatives as Dual Inhibitors for Mono-ADP-Ribosyltransferases PARP10 and PARP15. Eur. J. Med. Chem. 2022, 237, 114362. 10.1016/j.ejmech.2022.114362. [DOI] [PubMed] [Google Scholar]
- Korn P.; Classen A.; Murthy S.; Guareschi R.; Maksimainen M. M.; Lippok B. E.; Galera-Prat A.; Sowa S. T.; Voigt C.; Rossetti G.; Lehtiö L.; Bolm C.; Lüscher B. Evaluation of 3- and 4-Phenoxybenzamides as Selective Inhibitors of the Mono-ADP-Ribosyltransferase PARP10. ChemistryOpen 2021, 10, 939–948. 10.1002/open.202100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog N.; Hartkamp J. D. H.; Verheugd P.; Treude F.; Forst A. H.; Feijs K. L. H.; Lippok B. E.; Kremmer E.; Kleine H.; Lüscher B. Caspase-Dependent Cleavage of the Mono-ADP-Ribosyltransferase ARTD10 Interferes with Its pro-Apoptotic Function. FEBS J. 2013, 280 (5), 1330–1343. 10.1111/febs.12124. [DOI] [PubMed] [Google Scholar]
- Maksimainen M. M.; Murthy S.; Sowa S. T.; Galera-Prat A.; Rolina E.; Heiskanen J. P.; Lehtiö L. Analogs of TIQ-A as Inhibitors of Human Mono-ADP-Ribosylating PARPs. Bioorg. Med. Chem. 2021, 52, 116511–116517. 10.1016/j.bmc.2021.116511. [DOI] [PubMed] [Google Scholar]
- Upton K.; Meyers M.; Thorsell A. G.; Karlberg T.; Holechek J.; Lease R.; Schey G.; Wolf E.; Lucente A.; Schüler H.; Ferraris D. Design and Synthesis of Potent Inhibitors of the Mono(ADP-Ribosyl)Transferase, PARP14. Bioorg. Med. Chem. Lett. 2017, 27 (13), 2907–2911. 10.1016/j.bmcl.2017.04.089. [DOI] [PubMed] [Google Scholar]
- Morgan R. K.; Kirby I. T.; Vermehren-Schmaedick A.; Rodriguez K.; Cohen M. S. Rational Design of Cell-Active Inhibitors of PARP10. ACS Med. Chem. Lett. 2019, 10 (1), 74–79. 10.1021/acsmedchemlett.8b00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. K.; Carter-O’Connell I.; Cohen M. S. Selective Inhibition of PARP10 Using a Chemical Genetics Strategy. Bioorg. Med. Chem. Lett. 2015, 25 (21), 4770–4773. 10.1016/j.bmcl.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto M. J.; Turner W. R.; Arundel-Suto C. M.; Werbel L. M.; Sebolt-Leopold J. S. Dihydroisoquinolinones: The Design and Synthesis of a New Series of Potent Inhibitors of Poly(ADP-Ribose) Polymerase. Anti-Cancer Drug Des. 1991, 6, 107–117. [PubMed] [Google Scholar]
- Yuen L. H.; Dana S.; Liu Y.; Bloom S. I.; Thorsell A.-G.; Neri D.; Donato A. J.; Kireev D.; Schüler H.; Franzini R. M. A Focused DNA-Encoded Chemical Library for the Discovery of Inhibitors of NAD+-Dependent Enzymes. J. Am. Chem. Soc. 2019, 141 (13), 5169–5181. 10.1021/jacs.8b08039. [DOI] [PubMed] [Google Scholar]
- Lemke M.; Ravenscroft H.; Rueb N. J.; Kireev D.; Ferraris D.; Franzini R. M. Integrating DNA-Encoded Chemical Libraries with Virtual Combinatorial Library Screening: Optimizing a PARP10 Inhibitor. Bioorg. Med. Chem. Lett. 2020, 30 (19), 127464–127470. 10.1016/j.bmcl.2020.127464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad T.; Lindgren A. E. G.; Andersson C. D.; Caraballo R.; Thorsell A. G.; Karlberg T.; Spjut S.; Linusson A.; Schüler H.; Elofsson M. Towards Small Molecule Inhibitors of Mono-ADP-Ribosyltransferases. Eur. J. Med. Chem. 2015, 95, 546–551. 10.1016/j.ejmech.2015.03.067. [DOI] [PubMed] [Google Scholar]
- Aguiar R. C. T.; Takeyama K.; He C.; Kreinbrink K.; Shipp M. A. B-Aggressive Lymphoma Family Proteins Have Unique Domains That Modulate Transcription and Exhibit Poly(ADP-Ribose) Polymerase Activity. J. Biol. Chem. 2005, 280 (40), 33756–33765. 10.1074/jbc.M505408200. [DOI] [PubMed] [Google Scholar]
- Camicia R.; Bachmann S. B.; Winkler H. C.; Beer M.; Tinguely M.; Haralambieva E.; Hassa P. O. BAL1/ARTD9 Represses the Anti-Proliferative and pro-Apoptotic IFNγ-STAT1-IRF1-P53 Axis in Diffuse Large B-Cell Lymphoma. J. Cell Sci. 2013, 126, 1969–1980. 10.1242/jcs.118174. [DOI] [PubMed] [Google Scholar]
- Barbarulo A.; Iansante V.; Chaidos A.; Naresh K.; Rahemtulla A.; Franzoso G.; Karadimitris A.; Haskard D. O.; Papa S.; Bubici C. Poly(ADP-Ribose) Polymerase Family Member 14 (PARP14) Is a Novel Effector of the JNK2-Dependent pro-Survival Signal in Multiple Myeloma. Oncogene 2013, 32 (36), 4231–4242. 10.1038/onc.2012.448. [DOI] [PubMed] [Google Scholar]
- Bachmann S. B.; Frommel S. C.; Camicia R.; Winkler H. C.; Santoro R.; Hassa P. O. DTX3L and ARTD9 Inhibit IRF1 Expression and Mediate in Cooperation with ARTD8 Survival and Proliferation of Metastatic Prostate Cancer Cells. Mol. Cancer 2014, 13 (1), 125–149. 10.1186/1476-4598-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iansante V.; Choy P. M.; Fung S. W.; Liu Y.; Chai J.-G.; Dyson J.; Del Rio A.; D’Santos C.; Williams R.; Chokshi S.; Anders R. A; Bubici C.; Papa S. PARP14 Promotes the Warburg Effect in Hepatocellular Carcinoma by Inhibiting JNK1-Dependent PKM2 Phosphorylation and Activation. Nat. Commun. 2015, 6 (1), 7882. 10.1038/ncomms8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W.; Wu H.-J.; Cao L.-Q.; Li H.-J.; He C.-X.; Zhao D.; Xing L.; Li P.-Q.; Jin X.; Cao H.-L. Research Progress on PARP14 as a Drug Target. Front. Pharmacol. 2019, 10, 172–184. 10.3389/fphar.2019.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra P.; Riley J. P.; Patel R.; Li F.; Voss L.; Goenka S. PARP-14 Functions as a Transcriptional Switch for Stat6-Dependent Gene Activation. J. Biol. Chem. 2011, 286 (3), 1767–1776. 10.1074/jbc.M110.157768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson C. D.; Karlberg T.; Ekblad T.; Lindgren A. E. G.; Thorsell A.-G.; Spjut S.; Uciechowska U.; Niemiec M. S.; Wittung-Stafshede P.; Weigelt J.; Elofsson M.; Schüler H.; Linusson A. Discovery of Ligands for ADP-Ribosyltransferases via Docking-Based Virtual Screening. J. Med. Chem. 2012, 55 (17), 7706–7718. 10.1021/jm300746d. [DOI] [PubMed] [Google Scholar]
- Wang P.; Li J.; Jiang X.; Liu Z.; Ye N.; Xu Y.; Yang G.; Xu Y.; Zhang A. Palladium-Catalyzed N-Arylation of 2-Aminobenzothiazole-4-Carboxylates/Carboxamides: Facile Synthesis of PARP14 Inhibitors. Tetrahedron 2014, 70 (35), 5666–5673. 10.1016/j.tet.2014.06.064. [DOI] [Google Scholar]
- Yoneyama-Hirozane M.; Matsumoto S. I.; Toyoda Y.; Saikatendu K. S.; Zama Y.; Yonemori K.; Oonishi M.; Ishii T.; Kawamoto T. Identification of PARP14 Inhibitors Using Novel Methods for Detecting Auto-Ribosylation. Biochem. Biophys. Res. Commun. 2017, 486 (3), 626–631. 10.1016/j.bbrc.2017.03.052. [DOI] [PubMed] [Google Scholar]
- Peng B.; Thorsell A.-G.; Karlberg T.; Schüler H.; Yao S. Q. Small Molecule Microarray Based Discovery of PARP14 Inhibitors. Angew. Chemie Int. Ed. 2017, 56 (1), 248–253. 10.1002/anie.201609655. [DOI] [PubMed] [Google Scholar]
- Schweiker S. S.; Tauber A. L.; Levonis S. M. In Silico Identification and in Vitro Activity of Natural Products as ADP-Ribosyl Transferase Member 8 Inhibitors. Futur. Med. Chem. 2020, 12 (19), 1729–1741. 10.4155/fmc-2020-0138. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhu C.; Song D.; Xia R.; Yu W.; Dang Y.; Fei Y.; Yu L.; Wu J. Epigallocatechin-3-Gallate Enhances ER Stress-Induced Cancer Cell Apoptosis by Directly Targeting PARP16 Activity. Cell Death Discovery 2017, 3 (1), 1–9. 10.1038/cddiscovery.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson J.; McAlpine J. B.; Friesen J. B.; Chen S. N.; Graham J.; Pauli G. F. Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery?. J. Med. Chem. 2016, 59 (5), 1671–1690. 10.1021/acs.jmedchem.5b01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle T. J.; Ren Y.; Molina J. R.; Blackwell D. J.; Schenkel L. B.; Swinger K. K.; Kuplast-Barr K.; Majer C. R.; Church W. D.; Lu A. Z.; Mo J.; Abo R.; Cheung A.; Dorsey B. W.; Niepel M.; Perl N. R.; Vasbinder M. M.; Keilhack H.; Kuntz K. W. Targeted Degradation of PARP14 Using a Heterobifunctional Small Molecule. ChemBioChem. 2021, 22 (12), 2107–2110. 10.1002/cbic.202100047. [DOI] [PubMed] [Google Scholar]
- Schenkel L. B.; Molina J. R.; Swinger K. K.; Abo R.; Blackwell D. J.; Lu A. Z.; Cheung A. E.; Church W. D.; Kunii K.; Kuplast-Barr K. G.; Majer C. R.; Minissale E.; Mo J.-R.; Niepel M.; Reik C.; Ren Y.; Vasbinder M. M.; Wigle T. J.; Richon V. M.; Keilhack H.; Kuntz K. W. A Potent and Selective PARP14 Inhibitor Decreases Protumor Macrophage Gene Expression and Elicits Inflammatory Responses in Tumor Explants. Cell Chem. Biol. 2021, 28 (8), 1158–1168. 10.1016/j.chembiol.2021.02.010. [DOI] [PubMed] [Google Scholar]
- Kirby I. T.; Kojic A.; Arnold M. R.; Thorsell A. G.; Karlberg T.; Vermehren-Schmaedick A.; Sreenivasan R.; Schultz C.; Schüler H.; Cohen M. S. A Potent and Selective PARP11 Inhibitor Suggests Coupling between Cellular Localization and Catalytic Activity. Cell Chem. Biol. 2018, 25 (12), 1547–1553. 10.1016/j.chembiol.2018.09.011. [DOI] [PubMed] [Google Scholar]
- He F.; Tsuda K.; Takahashi M.; Kuwasako K.; Terada T.; Shirouzu M.; Watanabe S.; Kigawa T.; Kobayashi N.; Güntert P.; Yokoyama S.; Muto Y. Structural Insight into the Interaction of ADP-Ribose with the PARP WWE Domains. FEBS Lett. 2012, 586 (21), 3858–3864. 10.1016/j.febslet.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Carter-O’Connell I.; Jin H.; Morgan R. K.; Zaja R.; David L. L.; Ahel I.; Cohen M. S. Identifying Family-Member-Specific Targets of Mono-ARTDs by Using a Chemical Genetics Approach. Cell Rep. 2016, 14 (3), 621–631. 10.1016/j.celrep.2015.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T.; Zuo Y.; Qian L.; Liu J.; Yuan Y.; Xu K.; Miao Y.; Feng Q.; Chen X.; Jin L.; Zhang L.; Dong C.; Xiong S.; Zheng H. ADP-Ribosyltransferase PARP11 Modulates the Interferon Antiviral Response by Mono-ADP-Ribosylating the Ubiquitin E3 Ligase β-TrCP. Nat. Microbiol. 2019, 4 (11), 1872–1884. 10.1038/s41564-019-0428-3. [DOI] [PubMed] [Google Scholar]
- Li L.; Shi Y.; Li S.; Liu J.; Zu S.; Xu X.; Gao M.; Sun N.; Pan C.; Peng L.; Yang H.; Cheng G. ADP-Ribosyltransferase PARP11 Suppresses Zika Virus in Synergy with PARP12. Cell Biosci. 2021, 11, 116–129. 10.1186/s13578-021-00628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace S. R.; Chihab L. Y.; Yamasaki M.; Yoshinaga B. T.; Torres Y. M.; Rideaux D.; Javed Z.; Turumella S.; Zhang M.; Lawton D. R.; Fuller A. A.; Carter-O’connell I. Rapid Analysis of ADP-Ribosylation Dynamics and Site-Specificity Using TLC-MALDI. Cite This ACS Chem. Biol. 2021, 16, 2137–2143. 10.1021/acschembio.1c00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. E.; Vyas S.; Rood J. E.; Bhutkar A.; Sharp P. A.; Chang P. Poly(ADP-Ribose) Regulates Stress Responses and MicroRNA Activity in the Cytoplasm. Mol. Cell 2011, 42 (4), 489–499. 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnur D.; Bartlett E.; Mikolčević P.; Kirby I. T.; Rack J. G. M.; Mikoč A.; Cohen M. S.; Ahel I. Reversible ADP-Ribosylation of RNA. Nucleic Acids Res. 2019, 47 (11), 5658–5669. 10.1093/nar/gkz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. K.; Cheong H. S.; Koh Y.; Ahn K. S.; Yoon S. S.; Shin H. D. Genetic Association of PARP15 Polymorphisms with Clinical Outcome of Acute Myeloid Leukemia in a Korean Population. Genet. Test. Mol. Biomarkers 2016, 20 (11), 696–701. 10.1089/gtmb.2016.0007. [DOI] [PubMed] [Google Scholar]
- Chiarugi A.; Meli E.; Calvani M.; Picca R.; Baronti R.; Camaioni E.; Costantino G.; Marinozzi M.; Pellegrini-Giampietro D. E.; Pellicciari R.; Moroni F. Novel Isoquinolinone-Derived Inhibitors of Poly(ADP-Ribose) Polymerase-1: Pharmacological Characterization and Neuroprotective Effects in an in Vitro Model of Cerebral Ischemia. J. Pharmacol. Exp. Ther. 2003, 305, 943–949. 10.1124/jpet.103.048934. [DOI] [PubMed] [Google Scholar]
- Venkannagari H.; Fallarero A.; Feijs K.; Lüscher B.; Lehtiö L. Activity-Based Assay for Human Mono-ADP-Ribosyltransferases ARTD7/PARP15 and ARTD10/PARP10 Aimed at Screening and Profiling Inhibitors. Eur. J. Pharm. Sci. 2013, 49 (2), 148–156. 10.1016/j.ejps.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Huang J. Y.; Wang K.; Vermehren-Schmaedick A.; Adelman J. P.; Cohen M. S. PARP6 Is a Regulator of Hippocampal Dendritic Morphogenesis. Sci. Rep. 2016, 6, 18512. 10.1038/srep18512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermehren-Schmaedick A.; Huang J. Y.; Levinson M.; Pomaville M. B.; Reed S.; Bellus G. A.; Gilbert F.; Keren B.; Heron D.; Haye D.; Janello C.; Makowski C.; Danhauser K.; Fedorov L. M.; Haack T. B.; Wright K. M.; Cohen M. S. Characterization of PARP6 Function in Knockout Mice and Patients with Developmental Delay. Cells 2021, 10 (6), 1289. 10.3390/cells10061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Grosskurth S. E.; Cheung T.; Petteruti P.; Zhang J.; Wang X.; Wang W.; Gharahdaghi F.; Wu J.; Su N.; Howard R. T.; Mayo M.; Widzowski D.; Scott D. A.; Johannes J. W.; Lamb M. L.; Lawson D.; Dry J. R.; Lyne P. D.; Tate E. W.; Zinda M.; Mikule K.; Fawell S. E.; Reimer C.; Chen H. Pharmacological Inhibition of PARP6 Triggers Multipolar Spindle Formation and Elicits Therapeutic Effects in Breast Cancer. Cancer Res. 2018, 78, 6691–6702. 10.1158/0008-5472.CAN-18-1362. [DOI] [PubMed] [Google Scholar]
- Johannes J. W.; Almeida L.; Daly K.; Ferguson A. D.; Grosskurth N. E.; Guan H.; Howard T.; Ioannidis S.; Kazmirski S.; Lamb M. L.; Larsen N. A.; Lyne P. D.; Mikule K.; Ogoe C.; Peng B.; Petteruti P.; Read J. A.; Su N.; Sylvester M.; Throner S.; Wang W.; Wang X.; Wu J.; Ye Q.; Yu Y.; Zheng X.; Scott D. A. Discovery of AZ0108, an Orally Bioavailable Phthalazinone PARP Inhibitor That Blocks Centrosome Clustering. Bioorg. Med. Chem. Lett. 2015, 25 (24), 5743–5747. 10.1016/j.bmcl.2015.10.079. [DOI] [PubMed] [Google Scholar]
- Guo X.; Carroll J.-W. N.; MacDonald M. R.; Goff S. P.; Gao G. The Zinc Finger Antiviral Protein Directly Binds to Specific Viral MRNAs through the CCCH Zinc Finger Motifs. J. Virol. 2004, 78 (23), 12781–12787. 10.1128/JVI.78.23.12781-12787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.; Ma J.; Sun J.; Gao G. The Zinc-Finger Antiviral Protein Recruits the RNA Processing Exosome to Degrade the Target MRNA. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (1), 151–156. 10.1073/pnas.0607063104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catara G.; Grimaldi G.; Schembri L.; Spano D.; Turacchio G.; Lo Monte M.; Beccari A. R.; Valente C.; Corda D. PARP1-Produced Poly-ADP-Ribose Causes the PARP12 Translocation to Stress Granules and Impairment of Golgi Complex Functions. Sci. Rep. 2017, 7 (1), 14035–14052. 10.1038/s41598-017-14156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G.; Corda D. ADP-Ribosylation and Intracellular Traffic: An Emerging Role for PARP Enzymes. Biochem. Soc. Trans. 2019, 47 (1), 357–370. 10.1042/BST20180416. [DOI] [PubMed] [Google Scholar]
- Grimaldi G.; Filograna A.; Schembri L.; Lo Monte M.; Di Martino R.; Pirozzi M.; Spano D.; Beccari A. R.; Parashuraman S.; Luini A.; Valente C.; Corda D. PKD-Dependent PARP12-Catalyzed Mono-ADP-Ribosylation of Golgin-97 Is Required for E-Cadherin Transport from Golgi to Plasma Membrane. Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2026494119. 10.1073/pnas.2026494119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C.; Qiu Y.; Liu J.; Feng H.; Shen S.; Saiyin H.; Yu W.; Wei Y.; Yu L.; Su W.; Wu J. PARP12 (ARTD12) Suppresses Hepatocellular Carcinoma Metastasis through Interacting with FHL2 and Regulating Its Stability. Cell Death Dis. 2018, 9, 856–870. 10.1038/s41419-018-0906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston J.; Cheradame L.; Yvonnet V.; Deas O.; Poupon M. F.; Judde J. G.; Cairo S.; Goffin V. Intracellular STING Inactivation Sensitizes Breast Cancer Cells to Genotoxic Agents. Oncotarget 2016, 7 (47), 77205–77224. 10.18632/oncotarget.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jwa M.; Chang P. PARP16 Is a Tail-Anchored Endoplasmic Reticulum Protein Required for the PERK and IRE1α-Mediated Unfolded Protein Response. Nat. Cell Biol. 2012, 14 (11), 1223–1230. 10.1038/ncb2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola S.; Micaroni M.; Di Tullio G.; Buccione R.; Di Girolamo M. PARP16/ARTD15 Is a Novel Endoplasmic-Reticulum-Associated Mono-ADP-Ribosyltransferase That Interacts with, and Modifies Karyopherin-SS1. PLoS One 2012, 7 (6), e37352. 10.1371/journal.pone.0037352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J.; Gao J.; Li Q. Ribosome ADP-Ribosylation: A Mechanism for Maintaining Protein Homeostasis in Cancers. Cell Biol. Int. 2022, 46 (3), 333–335. 10.1002/cbin.11745. [DOI] [PubMed] [Google Scholar]
- Centko R. M.; Carlile G. W.; Barne I.; Patrick B. O.; Blagojevic P.; Thomas D. Y.; Andersen R. J. Combination of Selective PARP3 and PARP16 Inhibitory Analogues of Latonduine A Corrects F508del-CFTR Trafficking. ACS Omega 2020, 5 (40), 25593–25604. 10.1021/acsomega.0c02467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlile G. W.; Robert R.; Matthes E.; Yang Q.; Solari R.; Hatley R.; Edge C. M.; Hanrahan J. W.; Andersen R.; Thomas D. Y.; Birault V. Latonduine Analogs Restore F508del-Cystic Fibrosis Transmembrane Conductance Regulator Trafficking through the Modulation of Poly-ADP Ribose Polymerase 3 and Poly-ADP Ribose Polymerase 16 Activity. Mol. Pharmacol. 2016, 90, 65–79. 10.1124/mol.115.102418. [DOI] [PubMed] [Google Scholar]
- Gomez A.; Bindesbøll C.; Satheesh S. V.; Grimaldi G.; Hutin D.; MacPherson L.; Ahmed S.; Tamblyn L.; Cho T.; Nebb H. I.; Moen A.; Anonsen J. H.; Grant D. M.; Matthews J. Characterization of TCDD-Inducible Poly-ADP-Ribose Polymerase (TIPARP/ARTD14) Catalytic Activity. Biochem. J. 2018, 475 (23), 3827–3846. 10.1042/BCJ20180347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson L.; Tamblyn L.; Rajendra S.; Bralha F.; Mcpherson J. P.; Matthews J. Tetrachlorodibenzo-p-Dioxin Poly(ADP-Ribose) Polymerase (TiPARP, ARTD14) Is a Mono-ADP-Ribosyltransferase and Repressor of Aryl Hydrocarbon Receptor Transactivation. Nucleic Acids Res. 2013, 41, 1604–1621. 10.1093/nar/gks1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindesbøll C.; Tan S.; Bott D.; Cho T.; Tamblyn L.; MacPherson L.; Grønning-Wang L.; Nebb H. I.; Matthews J. TCDD-Inducible Poly-ADP-Ribose Polymerase (TIPARP/PARP7) Mono-ADP-Ribosylates and Co-Activates Liver X Receptors. Biochem. J. 2016, 473 (7), 899–910. 10.1042/BJ20151077. [DOI] [PubMed] [Google Scholar]
- Yamada T.; Horimoto H.; Kameyama T.; Hayakawa S.; Yamato H.; Dazai M.; Takada A.; Kida H.; Bott D.; Zhou A. C.; Hutin D.; Watts T. H.; Asaka M.; Matthews J.; Takaoka A. Constitutive Aryl Hydrocarbon Receptor Signaling Constrains Type I Interferon-Mediated Antiviral Innate Defense. Nat. Immunol. 2016, 17 (6), 687–694. 10.1038/ni.3422. [DOI] [PubMed] [Google Scholar]
- Rasmussen M.; Tan S.; Somisetty V. S.; Hutin D.; Olafsen N. E.; Moen A.; Anonsen J. H.; Grant D. M.; Matthews J. PARP7 and Mono-ADP-Ribosylation Negatively Regulate Estrogen Receptor α Signaling in Human Breast Cancer Cells. Cells 2021, 10 (3), 623–642. 10.3390/cells10030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozgit J. M.; Vasbinder M. M.; Abo R. P.; Kunii K.; Kuplast-Barr K. G.; Gui B.; Lu A. Z.; Molina J. R.; Minissale E.; Swinger K. K.; Wigle T. J.; Blackwell D. J.; Majer C. R.; Ren Y.; Niepel M.; Varsamis Z. A.; Nayak S. P.; Bamberg E.; Mo J.-R.; Church W. D.; Mady A. S. A.; Song J.; Utley L.; Rao P. E.; Mitchison T. J.; Kuntz K. W.; Richon V. M.; Keilhack H. PARP7 Negatively Regulates the Type I Interferon Response in Cancer Cells and Its Inhibition Triggers Antitumor Immunity. Cancer Cell 2021, 39, 1214. 10.1016/j.ccell.2021.06.018. [DOI] [PubMed] [Google Scholar]
- Palavalli Parsons L. H; Challa S.; Gibson B. A; Nandu T.; Stokes M. S; Huang D.; Lea J. S; Kraus W L. Identification of PARP-7 Substrates Reveals a Role for Marylation in Microtubule Control in Ovarian Cancer Cells. Elife 2021, 10, e60481. 10.7554/eLife.60481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle T. J.; Blackwell D. J.; Schenkel L. B.; Ren Y.; Church W. D.; Desai H. J.; Swinger K. K.; Santospago A. G.; Majer C. R.; Lu A. Z.; Niepel M.; Perl N. R.; Vasbinder M. M.; Keilhack H.; Kuntz K. W. In Vitro and Cellular Probes to Study PARP Enzyme Target Engagement. Cell Chem. Biol. 2020, 27 (7), 877–887. 10.1016/j.chembiol.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Fu W.; Yao H.; Bütepage M.; Zhao Q.; Lüscher B.; Li J. The Search for Inhibitors of Macrodomains for Targeting the Readers and Erasers of Mono-ADP-Ribosylation. Drug Discovery Today 2021, 26 (11), 2547–2558. 10.1016/j.drudis.2021.05.007. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Lan T.; Su S.; Rao Y. Induction of Apoptosis in MDA-MB-231 Breast Cancer Cells by a PARP1-Targeting PROTAC Small Molecule. Chem. Commun. 2019, 55 (3), 369–372. 10.1039/C8CC07813K. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Chang X.; Zhang C.; Zeng S.; Liang M.; Ma Z.; Wang Z.; Huang W.; Shen Z. Identification of Probe-Quality Degraders for Poly(ADP-Ribose) Polymerase-1 (PARP-1). J. Enzyme Inhib. Med. Chem. 2020, 35 (1), 1606. 10.1080/14756366.2020.1804382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C.; Yang J.; Chen Y.; Zhou P.; Wang Y.; Du W.; Zhao L.; Chen Y. Discovery of SK-575 as a Highly Potent and Efficacious Proteolysis-Targeting Chimera Degrader of PARP1 for Treating Cancers. J. Med. Chem. 2020, 63 (19), 11012–11033. 10.1021/acs.jmedchem.0c00821. [DOI] [PubMed] [Google Scholar]
- Na Z.; Peng B.; Ng S.; Pan S.; Lee J.-S.; Shen H.-M.; Yao S. Q. A Small-Molecule Protein-Protein Interaction Inhibitor of PARP1 That Targets Its BRCT Domain. Angew. Chemie Int. Ed. 2015, 54 (8), 2515–2519. 10.1002/anie.201410678. [DOI] [PubMed] [Google Scholar]
- Sowa S. T.; Vela-Rodríguez C.; Galera-Prat A.; Cázares-Olivera M.; Prunskaite-Hyyryläinen R.; Ignatev A.; Lehtiö L. A FRET-Based High-Throughput Screening Platform for the Discovery of Chemical Probes Targeting the Scaffolding Functions of Human Tankyrases. Sci. Rep. 2020, 10 (1), 12357–12369. 10.1038/s41598-020-69229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock K.; Liu M.; Zaleska M.; Meniconi M.; Pfuhl M.; Collins I.; Guettler S. Fragment-Based Screening Identifies Molecules Targeting the Substrate-Binding Ankyrin Repeat Domains of Tankyrase. Sci. Rep. 2019, 9 (1), 19130–1950. 10.1038/s41598-019-55240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller M.; Riedel K.; Gibbs-Seymour I.; Uth K.; Sieg C.; Gehring A. P.; Ahel I.; Bracher F.; Kessler B. M.; Elkins J. M.; Knapp S. Discovery of a Selective Allosteric Inhibitor Targeting Macrodomain 2 of Polyadenosine-Diphosphate-Ribose Polymerase 14. ACS Chem. Biol. 2017, 12 (11), 2866–2874. 10.1021/acschembio.7b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad T.; Verheugd P.; Lindgren A. E.; Nyman T.; Elofsson M.; Schüler H. Identification of Poly(ADP-Ribose) Polymerase Macrodomain Inhibitors Using an AlphaScreen Protocol: SLAS Discovery. SLAS Discov. 2018, 23 (4), 353–362. 10.1177/2472555217750870. [DOI] [PubMed] [Google Scholar]
- Schweiker S. S.; Tauber A. L.; Sherry M. E.; Levonis S. M. Structure, Function and Inhibition of Poly(ADP-Ribose)Polymerase, Member 14 (PARP14). Mini Rev. Med. Chem. 2018, 18 (19), 1659–1669. 10.2174/1389557518666180816111749. [DOI] [PubMed] [Google Scholar]
- Moustakim M.; Riedel K.; Schuller M.; Gehring A. P.; Monteiro O. P.; Martin S. P.; Fedorov O.; Heer J.; Dixon D. J.; Elkins J. M.; Knapp S.; Bracher F.; Brennan P. E. Discovery of a Novel Allosteric Inhibitor Scaffold for Polyadenosine-Diphosphate-Ribose Polymerase 14 (PARP14) Macrodomain 2. Bioorg. Med. Chem. 2018, 26 (11), 2965–2972. 10.1016/j.bmc.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu D.; Antolin A. A.; Cox A. R.; Jones A. M. Identification of Different Side Effects between PARP Inhibitors and Their Polypharmacological Multi-Target Rationale. Br. J. Clin. Pharmacol. 2022, 88 (2), 742–752. 10.1111/bcp.15015. [DOI] [PubMed] [Google Scholar]
- Kirby I. T.; Cohen M. S. Small-Molecule Inhibitors of PARPs: From Tools for Investigating ADP-Ribosylation to Therapeutics. Curr. Top. Microbiol. Immunol. 2018, 420, 211–231. 10.1007/82_2018_137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. Z.; Abo R.; Ren Y.; Gui B.; Mo J. R.; Blackwell D.; Wigle T.; Keilhack H.; Niepel M. Enabling Drug Discovery for the PARP Protein Family through the Detection of Mono-ADP-Ribosylation. Biochem. Pharmacol. 2019, 167, 97–106. 10.1016/j.bcp.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Wigle T. J.; Church W. D.; Majer C. R.; Swinger K. K.; Aybar D.; Schenkel L. B.; Vasbinder M. M.; Brendes A.; Beck C.; Prahm M.; Wegener D.; Chang P.; Kuntz K. W. Forced Self-Modification Assays as a Strategy to Screen MonoPARP Enzymes. SLAS Discovery 2020, 25 (3), 241–252. 10.1177/2472555219883623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa S.; Stokes M. S.; Kraus W. L. Cells MARTs and MARylation in the Cytosol: Biological Functions, Mechanisms of Action, and Therapeutic Potential. Cells 2021, 10 (2), 313. 10.3390/cells10020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glumoff T.; Sowa S. T.; Lehtiö L. Assay Technologies Facilitating Drug Discovery for ADP-Ribosyl Writers, Readers and Erasers. Bioessays 2022, 44 (1), 2100240. 10.1002/bies.202100240. [DOI] [PubMed] [Google Scholar]
- Curtin N. J. PARP Inhibitors for Cancer Therapy. Expert Rev. Mol. Med. 2005, 7 (4), 1–20. 10.1017/S146239940500904X. [DOI] [PubMed] [Google Scholar]
- Frye S. V. The Art of the Chemical Probe. Nat. Chem. Biol. 2010, 6 (3), 159–161. 10.1038/nchembio.296. [DOI] [PubMed] [Google Scholar]