Abstract
Imaging markers of cerebrovascular disease and Alzheimer’s disease (AD) are implicated in mobility impairment in older adults, but few studies have examined these relationships longitudinally in a racially-diverse population-based sample. At Visit 5 (2011–13) of the ARIC Study, 1859 participants had usual pace gait speed (cm/s) assessed and brain MRI (mean age = 76.3, 28.5% Black) and PET (n = 343; mean age = 75.9, 42.6% Black) measures including total/regional brain volume (cm3), white matter hyperintensities (WMH; cm3), infarcts (present/absent), microbleeds (count) and global beta-amyloid (Aβ). Participants returned at Visit 6 (n = 1264, 2016–17) and Visit 7 (n = 1108, 2018–19) for follow-up gait speed assessments. We used linear regression to estimate effects of baseline infarct presence, higher microbleed count, and a one interquartile range (IQR) poorer measures of continuous predictors (−1 IQR total brain volume, temporal-parietal lobe meta region of interest(ROI); +1 IQR WMH volume, global Aβ SUVR) on cross-sectional gait speed and change in gait speed adjusting for age, sex, education, study site, APOE e4, estimated intracranial volume, BMI, and cardiovascular risk factors. Cross-sectionally, slower gait speed outcome was associated with higher WMH volume, −3.38 cm/s (95%CI:−4.71, −2.04), infarct presence, −5.60 cm/s (−7.69, −3.51), microbleed count, −2.20 cm/s (−3.20, −0.91), smaller total brain volume, −9.26 cm/s (−12.1, −6.43), and smaller temporal-parietal lobe ROI −6.28 cm/s (−8.28, −4.28). Longitudinally, faster gait speed outcome decline was associated with higher WMH volume, −0.27 cm/s/year, (−0.51, −0.03) and higher global Aβ SUVR, −0.62 cm/s/year (−1.20, −0.03). Both cerebrovascular and AD pathology may contribute to mobility decline commonly seen with aging.
Keywords: Physical function, Neuroimaging, Cerebrovascular disease, Amyloid
Introduction
Mobility impairment among older adults is highly prevalent and the most common form of disability, affecting an estimated 26.9% of US adults over age 65 (Okoro et al. 2018). In addition to contributing to loss of functional independence in daily living, mobility impairment is associated with negative health outcomes, including higher risk of falls (Ambrose et al. 2013), lower quality of life (Davis et al. 2015; La Grow et al. 2013), worsening disability (Guralnik et al. 1995), cognitive decline (Savica et al. 2017), and mortality (Studenski et al. 2011; White et al. 2013). While mobility impairment can result from a number of different pathologies including musculoskeletal and cardiopulmonary diseases, the brain has been increasingly implicated in age-related mobility decline, potentially resulting from abnormalities related to cerebrovascular disease, Alzheimer’s disease (AD), and neurodegeneration.
Better cardiovascular health is associated with better mobility in older adults (Welmer et al. 2013), including influence of midlife cardiovascular risk factors and health on late-life physical function (Windham et al. 2017a; Windham et al. 2017b). Cerebrovascular disease markers, identified with magnetic resonance imaging (MRI), have also been associated with mobility impairment in older adults (Smith et al. 2015). White matter hyperintensities (WMH), an age-associated marker of cerebral small vessel disease, have been related to worse physical function, including slower gait speed and faster decline in gait speed (Zheng et al. 2011). Infarcts have been associated with mobility disability and impaired gait (Ince et al. 2017; Rosano et al. 2007; Rosano et al. 2005). Brain volume loss, expected to some degree with age, is exacerbated by several pathological etiologies including cerebrovascular disease and AD, and has also been associated with worse physical performance in older adults (Camicioli et al. 1999; Nadkarni et al. 2014; Rosano et al. 2012). Less is known about the relationship between physical function and beta-amyloid (Aβ), a neuropathological hallmark of AD. Evidence suggests that brain Aβ is associated with slower concurrent gait speed (Del Campo et al. 2016; Nadkarni et al. 2017; Wennberg et al. 2017), with a few studies suggesting higher Aβ burden may predict faster decline in gait speed (Tian et al. 2017; Wennberg et al. 2018).
Despite this body of evidence, gaps remain relating imaging markers of neuropathology to physical function in older adults. Many previous studies utilized clinic-based samples of convenience, a historical necessity given the high expense and eligibility requirements of neuroimaging procedures, but which may lower generalizability to the broader population of older adults (Ganguli et al. 2018). Few studies have investigated these associations in racially diverse samples, a critical gap in knowledge considering Black older adults have a higher prevalence of mobility disability, cardiovascular disease, and AD (Demirovic et al. 2003; Kurian and Cardarelli 2007; Okoro et al. 2018). Potentially, greater markers of neuropathology in Black older adults might explain poorer mobility function compared to White older adults. Lastly, previous studies of Aβ relationships to physical function have been limited by cross-sectional designs or small sample sizes.
To address these gaps, we investigated the association between multiple imaging-based indices of neuropathology with gait speed cross-sectionally and longitudinally in the community-based Atherosclerosis Risk in Communities (ARIC) study. In an exploratory approach to address racial disparities of mobility disability, we also tested whether associations differed by race. We hypothesized that imaging-based neuropathological markers including WMH, total and regional brain volumes, infarcts, microbleeds, and Aβ would be associated with slower concurrent gait speed and steeper decline in gait speed at follow-up visits.
Methods
Study design
The ARIC Study is a community-based prospective cohort study of cardiovascular disease that recruited an initial sample of 15,792 adults (ages 45–64) from four U.S. communities (Washington County, Maryland; Forsyth County, North Carolina; suburbs of Minneapolis, Minnesota; and Jackson, Mississippi) for a baseline visit from 1987 to 1989. Participants self-reported race, age, and education at baseline. Physical function, including usual-pace gait speed, was assessed at visit 5 (2011–13), visit 6 (2016–17) and visit 7 (2018–19).
At visit 5, a subsample (n = 1978, 30.3% of visit 5 participants), was recruited for a brain MRI study, of whom 1859 (mean age = 76.3, 60% female, 28.5% Black) were included in this analysis after excluding 5 participants who self-reported race as non-Black or non-White, and for consistency with the PET imaging sample, 114 participants with adjudicated dementia (Knopman et al. 2016). Dementia-free participants who underwent brain MRI were recruited into a PET study at three of the four study sites (Washington County, Maryland; Forsyth County, North Carolina; and Jackson, Mississippi)(Gottesman et al. 2016). The PET sample included 343 participants (mean age = 76.0, 57% female, 42.6% Black). Figure S1 presents a flow-chart of inclusion into the MRI and PET analysis samples from the total ARIC visit 5 sample. Table S1 displays characteristics of the total ARIC visit 5 sample with the MRI and PET analysis samples. The ARIC study was approved by each site’s institutional review board, and written informed consent was obtained from all participants.
Neuroimaging procedure and predictors
Brain MRI scans were acquired at 3 T and images were analyzed at the Mayo Clinic Imaging Reading Center. WMH volume (cm3) and infarct presence (yes/no) were assessed on axial T2 fluid-attenuated inversion recovery (FLAIR) sequences (5-mm slices)(Schneider et al. 2017). Microbleeds (count, up to 3+) were assessed on axial gradient recalled echo T2-weighted sequences (T2*GRE; 4-mm slices), each defined as ≤5 mm lesions. Brain volume measures (total and temporal-parietal lobe meta region of interest [ROI]; cm3) and total intracranial volume (mm3) were measured on magnetization-prepared rapid gradient-echo (MPRAGE) sequences (1.2-mm slices) using Freesurfer version 5.1. Temporal-parietal lobe ROI volume, a meta-ROI comprised of regions susceptible to but not indicative of AD, was defined as the total summed volume of the parahippocampal gyrus, entorhinal cortex, inferior parietal lobule, hippocampus, precuneus, and cuneus (Schwarz et al. 2016).
Florbetapir PET scans were performed within one year of MRI scans to estimate brain Aβ burden. Images were acquired from 50 to 70 min post-injection in a series of four 5-min frames. MPRAGE sequences were used to coregister PET images to MRI. Standardized uptake value ratios (SUVRs) were quantified globally and regionally using a cerebellum reference region. Global Aβ burden was estimated using a weighted average of the frontal cortices, precuneus, anterior and posterior cingulate, and the parietal, lateral temporal, and occipital lobes. Aβ burden was operationalized as a continuous measure of global SUVR, as well as a dichotomized Aβ positivity status (Aβ(+)), defined as a global SUVR >1.2 (Gottesman et al. 2016). Further details regarding standardized protocol implementation for MRI and PET scanning across study sites have been reported previously (Gottesman et al. 2016; Knopman et al. 2015).
Gait speed outcomes
Usual pace gait speed (cm/s) was measured over 4 m, with the faster of two trials used. Gait speed was first measured at visit 5, with follow-up measurements at visit 6 (average follow-up period 4.87 years from visit 5 gait assessment) and visit 7 (average follow-up period 6.51 years from visit 5 gait assessment) using an identical protocol. The present study examined gait speed cross-sectionally (visit 5) and longitudinally (change in gait speed [cm/s/year] from visit 5 to last gait assessment).
Covariates
Model covariates were apriori determined based on theoretical importance and potential to confound the relationship between physical function and neuropathology. Participant race, sex, and education (<High School, High School, >High School), were self-reported at ARIC visit 1. Covariates measured at visit 5 included age, body mass index (BMI; kg/m2), APOE e4 carrier status, (TaqMan assay; Applied Biosystems), hypertension (systolic blood pressure > 140 mmHg, or diastolic blood pressure > 90 mmHg, or use of antihypertensive medication), diabetes (HbA1c ≥ 6.5%, use of diabetes medication, or self-report of physician diagnosis), coronary heart disease (adjudicated using medical records) alcohol consumption (current, former, never), statin use, heart failure (adjudicated using medical records), and estimated total intracranial volume (mm3).
Statistical analysis
Volumetric measures were log transformed to account for skewed distributions. Each neuropathology marker was modeled separately to examine associations with gait speed and change in gait speed. Associations with continuous predictors are presented per one interquartile range (IQR) instead of per one standard deviation, as this scale allows better comparison across effect sizes between both continuous and categorical predictors. Increases in WMH volume and global Aβ SUVR indicate poorer (worse) health, and therefore their associations were scaled to +1 IQR. Lower total brain volume and temporal-parietal lobe ROI volume indicate poorer (worse) health, and therefore their associations were scaled to −1 IQR. For categorical variables, associations are estimated for the presence of any infarcts, presence of Aβ(+), and one-unit microbleed count increases (up to 3+).
We used linear regression models to estimate cross-sectional associations of imaged neuropathology markers with concurrent gait speed (cm/s). We used linear regression with generalized estimating equations (GEE) to estimate associations of neuropathology markers with longitudinal (yearly) changes in gait speed (cm/s/year). All models included race interaction terms for neuropathology markers to test for effect-modification by race, as well as race interaction terms with all adjusters, which replicates stratified model results. All analyses were adjusted for age, sex, education, study site, APOE e4, estimated intracranial volume, BMI, hypertension, diabetes, coronary heart disease, alcohol consumption, statin use, and heart failure. GEE models used exchangeable working correlation structures with Huber-White robust standard error estimates.
We ran sensitivity analyses to account for potential floor effects in gait speed decline by repeating longitudinal models in a subsample of 597 participants (103 of whom received PET scans) with baseline gait speed performance indicative of healthy aging (≥100 cm/s). Additional analyses tested for selection bias for inclusion in the MRI sample and longitudinal drop-out using weighted models. Cross-sectional sensitivity analyses for inclusion in the visit 5 MRI sample was conducted using inverse selection weighting analyses (Qiao et al. 2017). Sensitivity analyses for longitudinal attrition was conducted using inverse proportional weighting (IPW) (Gottesman et al. 2014). Additionally, primary results for all analyses were consistent when we tested additional models using a parsimonious covariate set (backwards elimination using α < .10 criterion).
Results
Visit 5 sample characteristics are displayed by race in Table 1. Sample characteristics at follow-up (Visits 6 and 7) are displayed in Table S2. In both the MRI and PET samples, Black participants tended to be younger, have poorer baseline physical function, and worse cardiovascular health in comparison to White participants (Table 1). Table 2 presents descriptive statistics for each of the imaging-based neuropathology markers by race. Figure 1 illustrates examples of our cross-sectional and longitudinal analyses using WMH and global Aβ as predictors of both concurrent gait speed and gait speed decline.
Table 1.
Baseline (Visit 5) Characteristics by Race for MRI and PET Samples
| MRI Sample | PET Sample | ||||||
|---|---|---|---|---|---|---|---|
| Total (N=1859) | White (N=1330) | Black (N=529) | Total (N=343) | White (N=197) | Black (N=146) | ||
| Age (years) | 76.0 [72.0,80.0] | 77.0 [72.0,81.0] | 74.0 [71.0,79.0] | 76.0 [71.0,80.0] | 77.0 [71.0,81.0] | 75.0 [71.0,79.0] | |
| Male (Y/N) | 744 (40%) | 562 (42%) | 182 (34%) | 149 (43%) | 92 (47%) | 57 (39%) | |
| Education | <HS | 249 (13%) | 108 (8%) | 141 (27%) | 57 (17%) | 27 (14%) | 30 (21%) |
| HS | 769 (41%) | 619 (47%) | 150 (28%) | 147 (43%) | 94 (48%) | 53 (36%) | |
| >HS | 839 (45%) | 601 (45%) | 238 (45%) | 139 (41%) | 76 (39%) | 63 (43%) | |
| Gait speed (cm/s) | 91.3 [77.9,105.5] | 93.5 [81.0,107.8] | 85.5 [71.0,100.0] | 91.2 [79.1,103.4] | 93.5 [83.0,104.4] | 86.2 [71.9,102.6] | |
| APOE e4 (Y/N) | 509 (28%) | 321 (25%) | 188 (37%) | 103 (30%) | 50 (26%) | 53 (37%) | |
| BMI (kg/m2) | 27.7 [24.7,31.3] | 27.2 [24.4,30.5] | 29.1 [26.2,33.5] | 28.6 [25.1,31.8] | 27.7 [24.5,31.2] | 29.3 [26.3,33.6] | |
| Hypertensive (Y/N) | 1384 (75%) | 930 (71%) | 454 (86%) | 246 (72%) | 125 (64%) | 121 (83%) | |
| Diabetes (Y/N) | 609 (33%) | 395 (30%) | 214 (41%) | 123 (36%) | 72 (37%) | 51 (35%) | |
| CHD (Y/N) | 167 (9%) | 136 (10%) | 31 (6%) | 26 (8%) | 20 (10%) | 6 (4%) | |
| Statin Use (Y/N) | 938 (51%) | 680 (51%) | 258 (49%) | 167 (49%) | 97 (49%) | 70 (48%) | |
| Heart failure (Y/N) | 60 (3%) | 35 (3%) | 25 (5%) | 12 (3%) | 6 (3%) | 6 (4%) | |
| Alcohol use | Current | 846 (47%) | 730 (57%) | 116 (22%) | 129 (38%) | 100 (52%) | 29 (20%) |
| Former | 522 (29%) | 299 (23%) | 223 (43%) | 119 (35%) | 47 (24%) | 72 (50%) | |
| Never | 438 (24%) | 254 (20%) | 184 (35%) | 90 (27%) | 46 (24%) | 44 (30%) | |
Note: MRI = Magnetic Resonance Imaging; PET = Positron Emission Tomography; HS = high school; BMI=Body Mass Index; CHD = Coronary Heart Disease
Cells contain Median [Q1,Q3] for continuous variables and n (%) for categorical variables
Participant race, sex, and education are self-reported from ARIC Visit 1 (1993–95). All other variables were collected at Visit 5 (2011–13)
Table 2.
Imaging-based Neuropathology Markers by Race
| Total (N=1859) | White (N=1330) | Black (N=529) | ||
|---|---|---|---|---|
| MRI Neuropathology Markers | ||||
| Temporal-Parietal Lobe Meta-ROI (cm3) | 58.88 [54.32,63.68] | 60.09 [55.76,64.82] | 55.64 [51.54,60.55] | |
| Temporal-Parietal Lobe Meta-ROI (log2cm3) | 5.88 [5.76,5.99] | 5.91 [5.80,6.02] | 5.80 [5.69,5.92] | |
| Infarct Presence (Y/N) | 460 (25%) | 316 (24%) | 144 (27%) | |
| WMH Volume (cm3) | 11.53 [6.46,21.16] | 11.61 [6.41,21.06] | 11.39 [6.70,21.40] | |
| WMH Volume (log2cm3) | 3.53 [2.69,4.40] | 3.54 [2.68,4.40] | 3.51 [2.74,4.42] | |
| Microbleeds | 0 | 1398 (76%) | 1012 (77%) | 386 (73%) |
| 1 | 269 (15%) | 182 (14%) | 87 (17%) | |
| 2 | 75 (4%) | 54 (4%) | 21 (4%) | |
| 3+ | 99 (5%) | 67 (5%) | 32 (6%) | |
| PET Neuropathology Markers | PET Total (N=343) | PET White (N=197) | PET Black (N=146) | |
| Global Cortical Aβ (SUVR) | 1.20 [1.12,1.40] | 1.17 [1.10,1.30] | 1.24 [1.15,1.51] | |
| Global Cortical Aβ (log2SUVR) | 0.26 [0.16,0.48] | 0.22 [0.13,0.38] | 0.31 [0.22,0.58] | |
| Aβ(+) (SUVR>1.2) | 175 (51%) | 82 (42%) | 93 (64%) |
Note: MRI = Magnetic Resonance Imaging; ROI = region of interest; WMH=White Matter Hyperintensity; PET = Positron Emission Tomography; Aβ = beta-amyloid; SUVR = Standardized Uptake Value Ratio
Cells contain Median [Q1,Q3] for continuous variables and n (%) for categorical variables
Fig. 1.
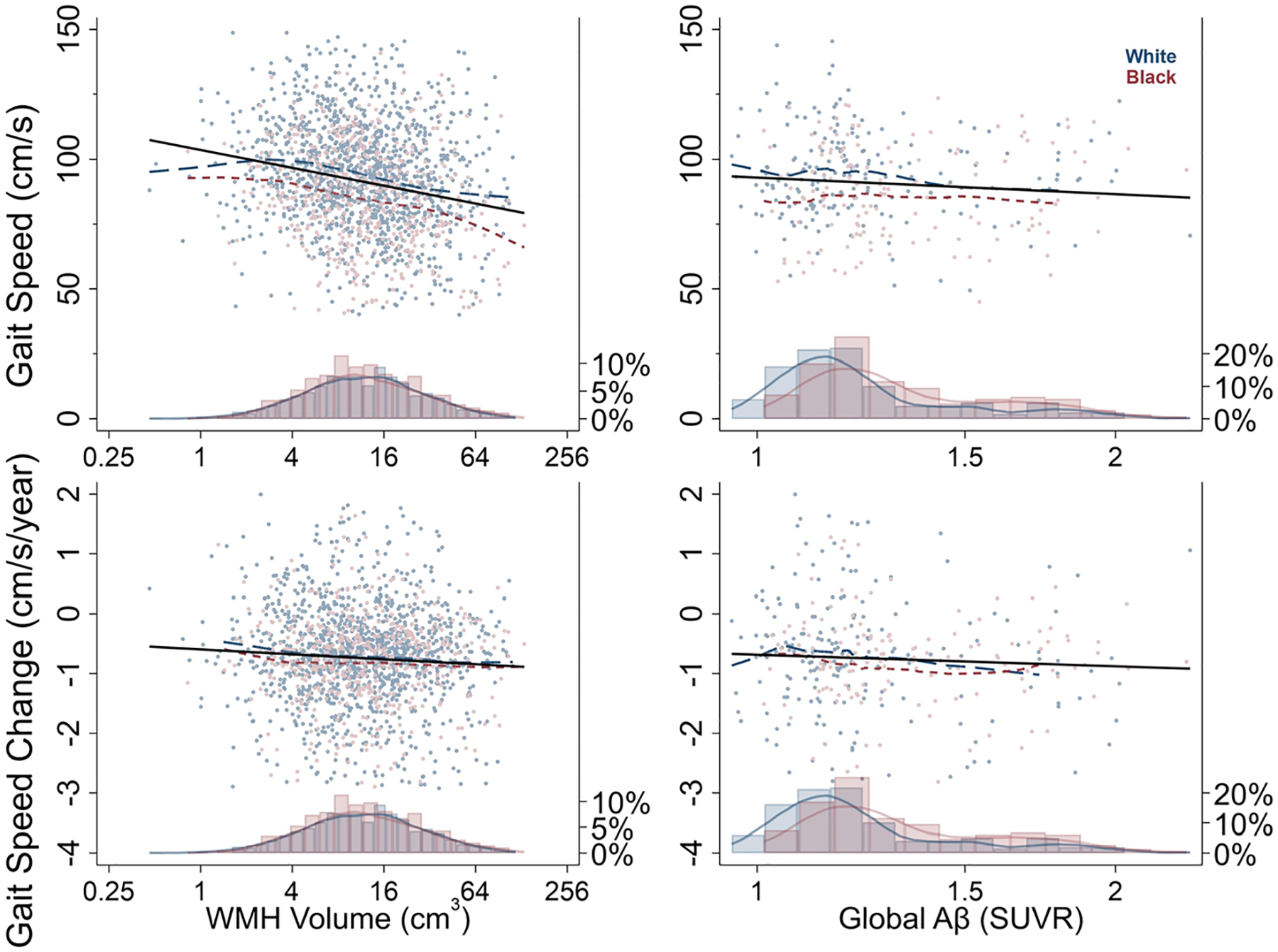
Gait Speed (cm/s) and Gait Speed Change (cm/s/year) by White Matter Hyperintensity (WMH) Volume (cm3) and Global beta-amyloid (Aβ; SUVR) and by Race. Histograms display proportions of sample by race at given value of WMH Volume and Global Aβ
Cross-sectional associations of imaging-based Neuropathology markers and gait speed
Figure 2 displays pooled and race-stratified cross-sectional associations of neuropathological markers with concurrent gait speed. All MRI neuropathology predictors were associated with slower concurrent walking speed. For example, a one IQR lower brain volume (−150.1 cm3) was associated with a 9.26 cm/s slower gait speed on average (95% CI:−12.09, −6.43) p < 0.001, while a one IQR higher WMH volume (+14.7 cm3) was associated with a 3.38 cm/s slower gait speed (95% CI: −4.71, −2.04) p < 0.001. Cross-sectional gait speed associations with Aβ were not supported, whether treated as a continuous variable, Global Cortical Aβ SUVR: 0.16 cm/s (−2.49, 2.80) p = 0.907, or as categorical Aβ(+) status: −1.02 cm/s (−5.04, 3.00) p = 0.618. Effect-modification by race was unsupported for all neuropathological markers and associations were similar in White and Black participants (See Table S3 for all model estimates including interaction terms). Results were consistent when including inverse-section weighting for inclusion in the MRI sample (See Table S4).
Fig. 2.
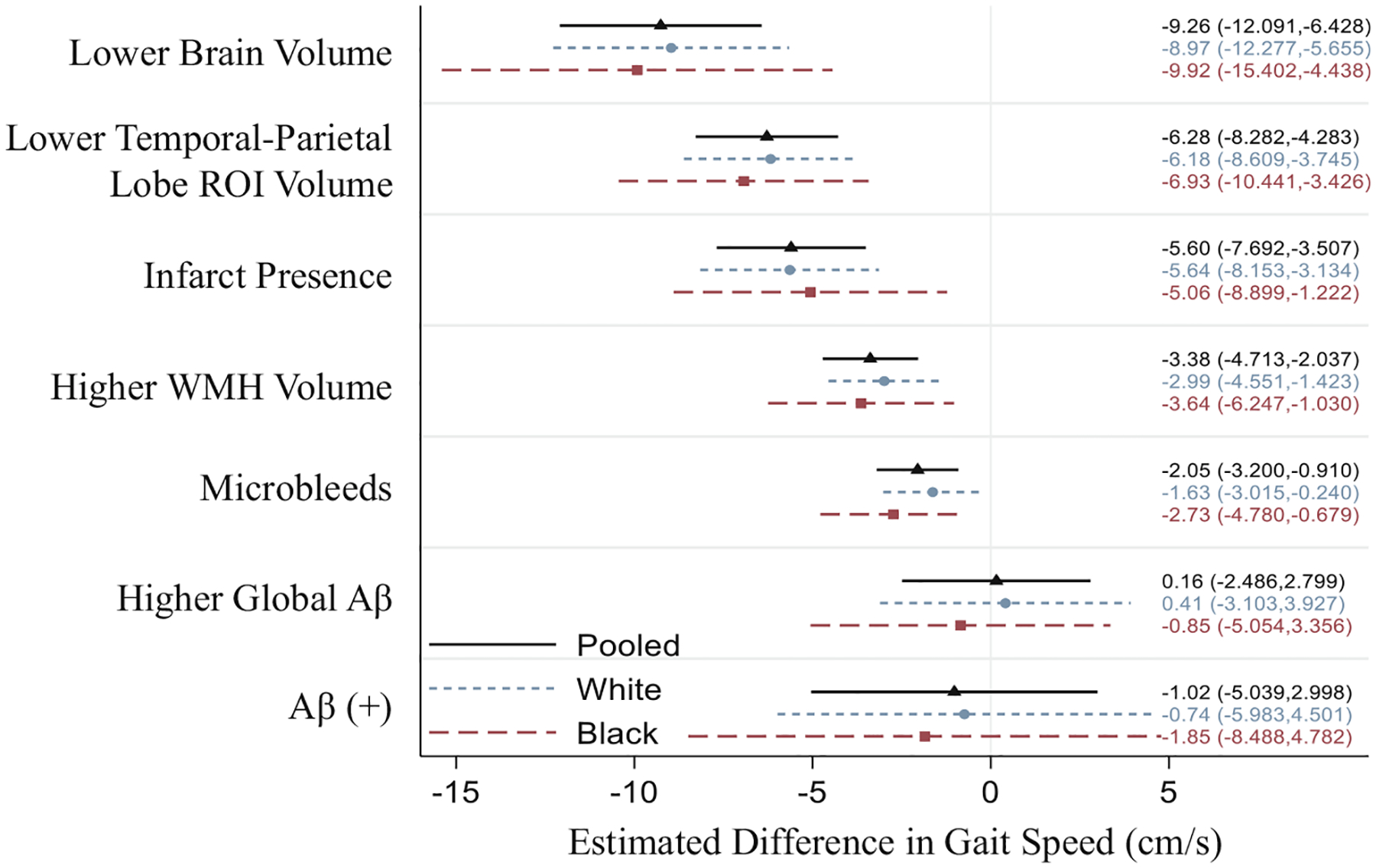
Adjusted cross-sectional associations of Neuropathology with Gait Speed (cm/s) by Race. Estimates β (95% confidence interval) correspond to differences in gait speed (cm/s) associated with a 1 interquartile range (IQR) change in continuous predictors (+1 IQR in WMH Volume, Aβ SUVR; −1 IQR in Brain Volume, AD Region Volume) and presence (Infarcts, Aβ(+)) or count (Microbleeds, up to 3+) of categorical predictors. ROI = region of interest, WMH = White Matter Hyperintensity, Aβ = beta-amyloid, SUVR = Standardized Uptake Value Ratio. Estimates adjusted for age, sex, education, study site, APOE e4, BMI, hypertension, diabetes, coronary heart disease, alcohol consumption, statin use, heart failure, and estimated total intracranial volume
Longitudinal Association of Imaging-based Neuropathology Markers and Gait Speed Change
Figure 3 displays associations of neuropathological markers with changes in gait speed (cm/s/year) over a mean follow-up of 6.51 years. Of the MRI markers, only higher baseline WMH was significantly associated with faster decline in gait speed over time, estimated at −0.27 cm/s/year (95% CI:−0.51, −0.03) p = 0.028 for an IQR increase (+14.7 cm3). Increased global Aβ burden was also associated with faster declines in gait speed, estimated at −0.62 cm/s/year (95% CI:−1.20, −0.03) p = 0.040 for an IQR increase (+0.3 SUVR). Other longitudinal associations were unsupported, including all race interaction terms (See Table S5). Results were consistent when including IPW to account for attrition (See Table S6). Sensitivity analyses for potential floor effects showed some stronger longitudinal associations of neuropathology markers with gait speed change in a subsample of participants (n = 597) with baseline gait speed performance ≥100 cm/s (Table S7).
Fig. 3.

Adjusted longitudinal associations of Neuropathology with Gait Speed Change by Race. Estimates β (95% confidence interval) correspond to gait speed change (cm/s/year) associated with a 1 interquartile range (IQR) change in continuous predictors (+1 IQR in WMH Volume, Aβ SUVR; −1 IQR in Brain Volume, AD Region Volume) and presence (Infarcts, Aβ(+)) or count (Microbleeds, up to 3+) of categorical predictors. ROI = region of interest, WMH = White Matter Hyperintensity, Aβ = beta-amyloid, SUVR = Standardized Uptake Value Ratio. Estimates adjusted for age, sex, education, study site, APOE e4, BMI, hypertension, diabetes, coronary heart disease, alcohol consumption, statin use, heart failure, and estimated total intracranial volume
Discussion
In this biracial, community-based sample of older adults, we observed detrimental effects of multiple imaging-based indices of neuropathology on mobility. All imaging-based pathological markers examined, excluding Aβ, were significantly associated with slower concurrent gait speed. In longitudinal analyses, global Aβ SUVR was associated faster decline gait speed decline, whereas WMH burden was the only MRI marker to be associated with faster decline. The longitudinal estimates were greater when limiting analyses to persons with faster gait speeds at baseline, suggesting that potential floor effects may have attenuated associations. Our findings suggest these associations between mobility and imaging markers are clinically relevant, as several estimates approached or met a threshold of meaningful change in gait speed (4–5 cm/s)(Perera et al. 2006). Moderations by race were not supported in any of the associations between neuropathology and gait speed.
Studies investigating the relationship between PET-measured Aβ and mobility longitudinally are scarce. We observed that Aβ was not associated with concurrent gait speed but was related to faster decline in gait speed at follow-up. Our findings contrast with previous cross-sectional studies that have reported associations of higher regional Aβ with slower adjusted gait speed in the Multidomain Alzheimer Preventative Trial PET Study (Del Campo et al. 2016) and in the Ginkgo Evaluation of Memory study (GEMS)(Nadkarni et al. 2017), although the association in the GEMS study was not observed after adjustment for APOE genotype. In the Mayo Clinic Study of Aging (MCSA), regional Aβ was associated with poorer concurrent gait speed and a number of other physical function measures primarily in women (Wennberg et al. 2017). However, our findings are consistent with two other studies relating Aβ burden to faster decline in gait speed. In a separate MCSA analysis, global Aβ was associated with gait speed decline over an average 15-month follow-up (Wennberg et al. 2018). Higher Aβ burden was also associated with greater decline in gait speed in the Baltimore Longitudinal Study of Aging among similar aged participants with comparable follow-up time to the present study, although with a largely White sample (Tian et al. 2017). Our observation that Aβ was associated with worsening physical function is generally consistent with data suggesting mobility impairment often co-occurs with AD (Scherder et al. 2007; Suttanon et al. 2012). By design, our PET sample excluded participants with dementia. This may have contributed to lack of cross-sectional associations with physical performance.
Regarding MRI markers, our findings are consistent with several previous reports associating cerebrovascular pathology and brain volume measures with poor mobility in older adults (de Laat et al. 2010; Zheng et al. 2011), particularly longitudinal associations of WMH with gait speed decline (Callisaya et al. 2013). These associations have been observed even in subclinical levels of neuropathology (Pinter et al. 2017; Rosano et al. 2005; Smith et al. 2015). The proposed mechanisms linking vascular and volumetric brain health with mobility function are multi-faceted (Rosso et al. 2013; Sorond et al. 2015), and include physiological injury to motor-related brain regions, but may also relate to cognitive function, which shares many of the same neuropathological risk factors. The intersection of cognitive function with mobility has long been proposed as an important consideration in the development and progression of dementia (Buracchio et al. 2010; Scherder et al. 2007).
Our study is among the first to report magnitudes of association between neuropathology that included Aβ and gait speed in a relatively large community-based sample of Black older adults. Biologically, we did not anticipate different relationships of neuropathology with gait outcomes, but investigated race effects given that Black older adults are at higher risk for mobility disability (Okoro et al. 2018), cerebrovascular disease, and AD (Demirovic et al. 2003; Kurian and Cardarelli 2007). We observed no significant interaction terms to suggest the relationship between any imaging-based pathological marker and gait speed differed by race cross-sectionally or longitudinally. Racial disparities in cardiovascular health including hypertension, diabetes, and obesity, all of which were observed in this sample, likely account for some racial disparities in mobility performance, potentially influencing physical function through deleterious effects on brain health (Knopman et al. 2011; Windham et al. 2017c).
While age-related mobility impairment and decline are often synonymous with the aging process, cerebrovascular damage, AD pathology, and brain volumetric loss, which dramatically increase with age (Peters 2006), may explain some mobility decline independent of age. Data from the community-based Prospective Urban Rural Epidemiologic (PURE) study suggested associations between brain MRI markers of vascular disease with mobility function were not moderated by age, and were observed in participants as young as midlife, much younger than our sample (Smith et al. 2015). Relationships of these markers with modifiable risk factors suggest the potential to mitigate mobility dysfunction through prevention or treatment strategies targeting factors such as obesity, diabetes, and hypertension (DeCarli et al. 1999; Macpherson et al. 2017; Pruzin et al. 2018). Results from the Systolic Blood Pressure Intervention Trial (SPRINT) suggested that more intensive lowering of systolic blood pressure was associated with less progression of WMH volume and brain volume loss in a sample of older adults over approximately three years follow-up (The SPRINT MIND Investigators for the SPRINT Research Group 2019). However, it was not associated with less gait speed decline (Odden et al. 2017). Potentially, earlier targeting of risk factors and longer follow-up periods may further understanding of the relationship between risk factors, brain health, and physical function. Our study, in combination with others, provides further evidence that routine, inexpensive measures of gait may indicate concurrent neuropathology with the potential to inform further steps to maximize brain health.
Our study has some limitations to consider. First, although the ARIC study has multiple time points of physical function measurement from Visit 5 to Visit 7, PET measured Aβ and 3 T MRI brain markers were available only at one time point, preventing an examination of relations of change in brain pathology. Furthermore, although we investigated decline in gait speed longitudinally, we cannot strictly establish temporality in brain pathology and declining physical function as these may occur in parallel. Second, longitudinal analyses investigating gait speed decline could have been limited by floor effects given the advanced age and relatively slow baseline gait speed, as suggested by the sensitivity analyses in high performers (≥100 cm/s). Potentially, these floor effects may have been differential by race, as Black participants performed worse at baseline gait speed assessments. However, no race interactions were statistically supported in the high performers sensitivity analysis, although we may have been underpowered to detect differences in this smaller subsample. Additional studies of larger, racially representative samples of higher functioning adults across regions are needed to shed light on the contributions of neuropathological processes on aging-associated mobility decline. Third, selection bias remains a potential limitation, as participants were survivors who returned to ARIC visit 5. Lastly, we were limited in our ability to disentangle race differences since the majority of Black participants in ARIC and all Black participants who underwent PET imaging were from one site; therefore, differences by race could be due to regional and cultural influences.
In conclusion, mitigating mobility decline with aging may require interventions that target multiple aspects of brain health, including cerebrovascular disease markers and AD pathology. Effective strategies and timing of interventions remain to be elucidated, although physical function measures may be useful as an inexpensive measure and early marker signaling neuropathology.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions. Author contributions included study conceptualization and manuscript authorship (KJS, BGW, MEG, and THM), data analysis (RR, DS, and MEG), and critical revision of the manuscript for intellectual content (BRN, TMH, CEH, SNL, DFW, CRJ, and RFG).
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data and imaging data is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917, from the NIH (NHLBI, NINDS, NIA and NIDCD) and R01 AG040282 and K24AG052573 from the NIA. Avid Radiopharmaceuticals provided the florbetapir isotope for the ARIC-PET study but had no role in study design or interpretation.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11682-020-00435-y.
Conflict of interest Dr. Wong reports non-monetary assistance from Avid and Eli Lilly on NIH grants. Dr. Jack serves on an independent data monitoring board for Roche, has served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr. Gottesman recently served as the Associate Editor for the journal Neurology. All other authors have no conflicts of interest to disclose.
References
- Ambrose AF, Paul G, & Hausdorff JM (2013). Risk factors for falls among older adults: A review of the literature. Maturitas, 75(1), 51–61. 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Buracchio T, Dodge HH, Howieson D, Wasserman D, & Kaye J (2010). The trajectory of gait speed preceding mild cognitive impairment. Archives of Neurology, 67(8), 980–986. 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Beare R, Phan TG, Blizzard L, Thrift AG, Chen J, & Srikanth VK (2013). Brain structural change and gait decline: A longitudinal population-based study. Journal of the American Geriatrics Society, 61(7), 1074–1079. 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Sexton G, Howieson DB, & Kaye JA (1999). Age-related brain changes associated with motor function in healthy older people. Journal of the American Geriatrics Society, 47(3), 330–334. 10.1111/j.1532-5415.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Davis JC, Bryan S, Best JR, Li LC, Hsu CL, Gomez C, Vertes KA, & Liu-Ambrose T (2015). Mobility predicts change in older adults’ health-related quality of life: Evidence from a Vancouver falls prevention prospective cohort study. Health and Quality of Life Outcomes, 13, 101. 10.1186/s12955-015-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat KF, van Norden AG, Gons RA, van Oudheusden LJ, van Uden IW, Bloem BR, et al. (2010). Gait in elderly with cerebral small vessel disease. Stroke, 41(8), 1652–1658. 10.1161/STROKEAHA.110.583229. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, & Carmelli D (1999). Predictors of brain morphology for the men of the NHLBI twin study. Stroke, 30(3), 529–536. 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- Del Campo N, Payoux P, Djilali A, Delrieu J, Hoogendijk EO, Rolland Y, et al. (2016). Relationship of regional brain beta-amyloid to gait speed. Neurology, 86(1), 36–43. 10.1212/WNL.0000000000002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirovic J, Prineas R, Loewenstein D, Bean J, Duara R, Sevush S, & Szapocznik J (2003). Prevalence of dementia in three ethnic groups: The South Florida program on aging and health. Annals of Epidemiology, 13(6), 472–478. 10.1016/s1047-2797(02)00437-4. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Albanese E, Seshadri S, Bennett DA, Lyketsos C, Kukull WA, Skoog I, & Hendrie HC (2018). Population neuroscience: Dementia epidemiology serving precision medicine and population Health. Alzheimer Disease and Associated Disorders, 32(1), 1–9. 10.1097/WAD.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen-Roche K, Coker LH, Coresh J, Couper DJ, Griswold ME, Heiss G, Knopman DS, Patel MD, Penman AD, Power MC, Selnes OA, Schneider ALC, Wagenknecht LE, Windham BG, Wruck LM, & Mosley TH (2014). Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: The ARIC neurocognitive study. American Journal of Epidemiology, 179(8), 956–966. 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, Schneider AL, Zhou Y, Chen X, Green E, Gupta N, et al. (2016). The ARIC-PET amyloid imaging study: Brain amyloid differences by age, race, sex, and APOE. Neurology, 87(5), 473–480. 10.1212/WNL.0000000000002914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, & Wallace RB (1995). Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. The New England Journal of Medicine, 332(9), 556–561. 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince PG, Minett T, Forster G, Brayne C, Wharton SB, Medical Research Council Cognitive, F, & Ageing Neuropathology, S. (2017). Microinfarcts in an older population-representative brain donor cohort (MRC CFAS): Prevalence, relation to dementia and mobility, and implications for the evaluation of cerebral small vessel Disease. Neuropathology and Applied Neurobiology, 43(5), 409–418. 10.1111/nan.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, & Mosley TH Jr. (2011). Vascular risk factors and longitudinal changes on brain MRI: The ARIC study. Neurology, 76(22), 1879–1885. 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, et al. (2015). Vascular imaging abnormalities and cognition: Mediation by cortical volume in nondemented individuals: Atherosclerosis risk in communities-neurocognitive study. Stroke, 46(2), 433–440. 10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider ALC, Hengrui S, Alonso A, Coresh J, Albert MS, & Mosley TH Jr. (2016). Mild cognitive impairment and dementia prevalence: The atherosclerosis risk in communities neurocognitive study (ARIC-NCS). Alzheimers Dement (Amst), 2, 1–11. 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian AK, & Cardarelli KM (2007). Racial and ethnic differences in cardiovascular disease risk factors: A systematic review. Ethnicity & Disease, 17(1), 143–152. [PubMed] [Google Scholar]
- La Grow S, Yeung P, Towers A, Alpass F, & Stephens C (2013). The impact of mobility on quality of life among older persons. Journal of Aging and Health, 25(5), 723–736. 10.1177/0898264313490198. [DOI] [PubMed] [Google Scholar]
- Macpherson H, Teo WP, Schneider LA, & Smith AE (2017). A life-long approach to physical activity for brain Health. Frontiers in Aging Neuroscience, 9, 147. 10.3389/fnagi.2017.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni NK, Nunley KA, Aizenstein H, Harris TB, Yaffe K, Satterfield S, et al. (2014). Association between cerebellar gray matter volumes, gait speed, and information-processing ability in older adults enrolled in the Health ABC study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(8), 996–1003. 10.1093/gerona/glt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni NK, Perera S, Snitz BE, Mathis CA, Price J, Williamson JD, DeKosky ST, Klunk WE, & Lopez OL (2017). Association of Brain Amyloid-beta with Slow Gait in elderly individuals without dementia: Influence of cognition and Apolipoprotein E epsilon4 genotype. JAMA Neurology, 74(1), 82–90. 10.1001/jamaneurol.2016.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odden MC, Peralta CA, Berlowitz DR, Johnson KC, Whittle J, Kitzman DW, et al. (2017). Effect of intensive blood pressure control on gait speed and mobility limitation in adults 75 years or older: A randomized clinical trial. JAMA Internal Medicine, 177(4), 500–507. 10.1001/jamainternmed.2016.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoro CA, Hollis ND, Cyrus AC, & Griffin-Blake S (2018). Prevalence of disabilities and Health care access by disability status and type among adults - United States, 2016. MMWR. Morbidity and Mortality Weekly Report, 67(32), 882–887. 10.15585/mmwr.mm6732a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera S, Mody SH, Woodman RC, & Studenski SA (2006). Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society, 54(5), 743–749. 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Peters R (2006). Ageing and the brain. Postgraduate Medical Journal, 82(964), 84–88. 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter D, Ritchie SJ, Doubal F, Gattringer T, Morris Z, Bastin ME, del C. Valdés Hernández M, Royle NA, Corley J, Muñoz Maniega S, Pattie A, Dickie DA, Staals J, Gow AJ, Starr JM, Deary IJ, Enzinger C, Fazekas F, & Wardlaw J (2017). Impact of small vessel disease in the brain on gait and balance. Scientific Reports, 7, 41637. 10.1038/srep41637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzin JJ, Nelson PT, Abner EL, & Arvanitakis Z (2018). Review: Relationship of type 2 diabetes to human brain pathology. Neuropathology and Applied Neurobiology, 44(4), 347–362. 10.1111/nan.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Suri FK, Zhang Y, Liu L, Gottesman R, Alonso A, Guallar E, & Wasserman BA (2017). Racial differences in prevalence and risk for intracranial atherosclerosis in a US Community-based population. JAMA Cardiology, 2(12), 1341–1348. 10.1001/jamacardio.2017.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr., & Newman AB (2005). Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. Journal of the American Geriatrics Society, 53(4), 649–654. 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- Rosano C, Brach J, Studenski S, Longstreth WT Jr., & Newman AB (2007). Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology, 29(3–4), 193–200. 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT Jr., & Newman AB (2012). Slower gait, slower information processing and smaller prefrontal area in older adults. Age and Ageing, 41(1), 58–64. 10.1093/ageing/afr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, Black SE, Camicioli R, Carlson MC, Ferrucci L, Guralnik JM, Hausdorff JM, Kaye J, Launer LJ, Lipsitz LA, Verghese J, & Rosano C (2013). Aging, the central nervous system, and mobility. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 68(11), 1379–1386. 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savica R, Wennberg AM, Hagen C, Edwards K, Roberts RO, Hollman JH, et al. (2017). Comparison of gait parameters for predicting cognitive decline: The Mayo Clinic study of aging. Journal of Alzheimer’s Disease, 55(2), 559–567. 10.3233/JAD-160697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherder E, Eggermont L, Swaab D, van Heuvelen M, Kamsma Y, de Greef M, van Wijck R, & Mulder T (2007). Gait in ageing and associated dementias; its relationship with cognition. Neuroscience and Biobehavioral Reviews, 31(4), 485–497. 10.1016/j.neubiorev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Schneider ALC, Selvin E, Sharrett AR, Griswold M, Coresh J, Jack CR Jr., Knopman D, Mosley T, & Gottesman RF (2017). Diabetes, Prediabetes, and brain volumes and subclinical cerebrovascular Disease on MRI: The atherosclerosis risk in communities neurocognitive study (ARIC-NCS). Diabetes Care, 40(11), 1514–1521. 10.2337/dc17-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Wiste HJ, Przybelski SA, Weigand SD, Ward CP, et al. (2016). A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin, 11, 802–812. 10.1016/j.nicl.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, O’Donnell M, Dagenais G, Lear SA, Wielgosz A, Sharma M, et al. (2015). Early cerebral small vessel disease and brain volume, cognition, and gait. Annals of Neurology, 77(2), 251–261. 10.1002/ana.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Cruz-Almeida Y, Clark DJ, Viswanathan A, Scherzer CR, De Jager P, et al. (2015). Aging, the central nervous system, and mobility in older adults: Neural mechanisms of mobility impairment. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70(12), 1526–1532. 10.1093/gerona/glv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, & Guralnik J (2011). Gait speed and survival in older adults. JAMA, 305(1), 50–58. 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttanon P, Hill KD, Said CM, Logiudice D, Lautenschlager NT, & Dodd KJ (2012). Balance and mobility dysfunction and falls risk in older people with mild to moderate Alzheimer disease. American Journal of Physical Medicine & Rehabilitation, 91(1), 12–23. 10.1097/PHM.0b013e31823caeea. [DOI] [PubMed] [Google Scholar]
- The SPRINT MIND Investigators for the SPRINT Research Group. (2019). Association of Intensive vs standard blood pressure control with cerebral White matter lesions. JAMA, 322(6), 524–534. 10.1001/jama.2019.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Resnick SM, Bilgel M, Wong DF, Ferrucci L, & Studenski SA (2017). Beta-amyloid burden predicts lower extremity performance decline in cognitively unimpaired older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72(5), 716–723. 10.1093/gerona/glw183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welmer AK, Angleman S, Rydwik E, Fratiglioni L, & Qiu C (2013). Association of cardiovascular burden with mobility limitation among elderly people: A population-based study. PLoS One, 8(5), e65815. 10.1371/journal.pone.0065815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg AMV, Savica R, Hagen CE, Roberts RO, Knopman DS, Hollman JH, Vemuri P, Jack CR Jr., Petersen RC, & Mielke MM (2017). Cerebral amyloid deposition is associated with gait parameters in the Mayo Clinic study of aging. Journal of the American Geriatrics Society, 65(4), 792–799. 10.1111/jgs.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg AMV, Lesnick TG, Schwarz CG, Savica R, Hagen CE, Roberts RO, Knopman DS, Hollman JH, Vemuri P, Jack CR Jr., Petersen RC, & Mielke MM (2018). Longitudinal association between brain amyloid-Beta and Gait in the Mayo Clinic study of aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 73(9), 1244–1250. 10.1093/gerona/glx240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DK, Neogi T, Nevitt MC, Peloquin CE, Zhu Y, Boudreau RM, Cauley JA, Ferrucci L, Harris TB, Satterfield SM, Simonsick EM, Strotmeyer ES, & Zhang Y (2013). Trajectories of gait speed predict mortality in well-functioning older adults: The Health, aging and body composition study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 68(4), 456–464. 10.1093/gerona/gls197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham BG, Griswold ME, Wang W, Kucharska-Newton A, Demerath EW, Gabriel KP, Pompeii LA, Butler K, Wagenknecht L, Kritchevsky S, & Mosley TH Jr. (2017a). The importance of mid-to-late-life body mass index trajectories on late-life gait speed. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72(8), 1130–1136. 10.1093/gerona/glw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham BG, Harrison KL, Lirette ST, Lutsey PL, Pompeii LA, Gabriel KP, Koton S, Steffen LM, Griswold ME, & Mosley TH Jr. (2017b). Relationship between midlife cardiovascular Health and late-life physical performance: The ARIC study. Journal of the American Geriatrics Society, 65(5), 1012–1018. 10.1111/jgs.14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham BG, Lirette ST, Fornage M, Benjamin EJ, Parker KG, Turner ST, Jack CR Jr., Griswold ME, & Mosley TH (2017c). Associations of brain structure with adiposity and changes in adiposity in a middle-aged and older biracial population. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72(6), 825–831. 10.1093/gerona/glw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JJ, Delbaere K, Close JC, Sachdev PS, & Lord SR (2011). Impact of white matter lesions on physical functioning and fall risk in older people: A systematic review. Stroke, 42(7), 2086–2090. 10.1161/STROKEAHA.110.610360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


