Abstract
Few studies have evaluated whether topical anesthetic cream reduces pain during pneumococcal vaccination. This is crucial, since effective pain management should be evidence‐based. Previous studies have shown that topical lidocaine‐prilocaine (EMLA®) reduces vaccination‐related pain, measured using pain‐rating instruments and observation of crying time. This intervention study aimed to compare the efficacy of topical lidocaine‐prilocaine cream with that of the standard of care on the expression of pain during the first pneumococcal vaccination administered at age 3 months under the Swedish national vaccination program. A randomized controlled trial included 72 infants receiving their first pneumococcal vaccination (Prevenar 13®). The study showed that topical lidocaine‐prilocaine before pneumococcal vaccination significantly reduced infants’ expression of pain according to the Face, Legs, Activity, Cry, Consolability (FLACC) score (P = .006) and increased latency to cry (P = .001). There were no statistically significant differences in the total crying time (P = .146) between the groups. Topical lidocaine‐prilocaine cream reduced pain expression and increased latency to cry in infants receiving their first pneumococcal vaccine. Systematic efforts are needed to successfully implement the use of topical anesthetic cream and other effective non‐pharmacological pain‐relieving strategies during infant vaccination procedures.
Keywords: infants, randomized controlled study, topical anesthetic, vaccine‐related pain
This intervention study aimed to compare the efficacy of topical lidocaine‐prilocaine cream (EMLA®) to the standard of care for the first pneumococcal vaccination administered at the age of 3 months. Topical lidocaine‐prilocaine cream for pneumococcal vaccination significantly reduced infants' expression of pain according to FLACC and latency to cry. Topical anesthetic cream and other effective non‐pharmacological pain‐relieving strategies during infant vaccination procedures are needed.

1. INTRODUCTION
Infants’ ability to convey their personal experience of pain depends on their level of cognitive development and previous pain experience. 1 Pain in infants should be treated with appropriate effective pain management strategies. 2 In this regard, nurses, along with other healthcare team members and organizations, have a responsibility to effectively manage pain. 3 , 4 Particularly needles have been identified as a major source of anxiety, and routine vaccination is the most common reason for using needles with infants. 5 , 6 , 7 In Sweden, the Child Health Services (CHS) offer vaccination following the established national vaccination program for children. 8 Moreover, pediatric healthcare is free and financed through local tax revenue. 9 Therefore, vaccination coverage in Sweden is high, and severe infections among children are uncommon. 9 , 10
Parents should ideally be informed about vaccine‐related pain strategies for their infants. 11 Healthcare workers should ensure that evidence‐based pain treatments are used, as vaccination‐related pain causes distress and discomfort to the infant, their parents, and even the healthcare staff. 12 , 13
Vaccination is associated with procedure‐related pain, which is caused by the pricking of the skin, the sensation of the vaccine, 5 , 7 , 14 and the intramuscular injection technique. 15 Several studies have shown that topical lidocaine‐prilocaine, applied as a cream or patch, reduced vaccination‐related pain, measured using a pain‐rating instrument and observation of crying time, without affecting the antibody response. 5 , 7 , 14 , 16 , 17 , 18 , 19 The Face, Legs, Activity, Cry, Consolability (FLACC) scale, used for pain assessment, has been validated for use in infants aged 2 months to 7 years. 20 Despite its efficacy, the topical lidocaine‐prilocaine cream should be applied at least 1 hour in advance to achieve its pain‐relieving effect, 21 which can be an obstacle to its use in the CHS. Moreover, although topical lidocaine‐prilocaine is a well‐studied drug that has been available for decades, it is not routinely used for infant vaccinations. 5 , 6 , 7 , 18 , 22 , 23 Moreover, previous studies have shown that non‐pharmacological pain management methods, including breastfeeding, skin‐to‐skin contact, small volumes of sweet solutions, and distraction, helped reduced pain experienced during vaccination. 24 , 25 , 26
Based on their observations of infants’ pain expressions, healthcare professionals believed that pneumococcal vaccination was more painful than other vaccines in the national vaccination program. More specifically, infants were more difficult to comfort, and their crying was louder and had a higher pitch. Therefore, it was deemed important to evaluate whether topical lidocaine‐prilocaine (EMLA®) was effective in reducing vaccination‐related pain from pneumococcal vaccination.
This study aimed to investigate the efficacy of topical lidocaine‐prilocaine cream compared with no pain management (standard of care at Swedish CHS) at the first pneumococcal vaccination, administered at 3 months of age under the Swedish national vaccination program.
Primary outcome: The effect of EMLA on infants’ pain expression during pneumococcal vaccination, as measured using FLACC.
Secondary outcome: The effect of EMLA on infants’ pain expression during pneumococcal vaccination, as measured using the visual analog scale (VAS) reported by the infant's parents, crying time (latency to cry and total crying time), and physiological parameters (heart rate and oxygen saturation).
Research questions:
Was pain expression, measured using FLACC during pneumococcal vaccination at 3 months, lower when topical lidocaine‐prilocaine cream was used compared with standard of care (no treatment)?
Was latency to cry significantly longer and total crying time significantly shorter in the lidocaine‐prilocaine group compared with the standard of care group?
Were physiological responses (heart rate and oxygen saturation) significantly different between groups during vaccination?
Did parent‐reported pain intensity, measured using an observational visual analog scale, differ significantly between groups?
2. METHODS
This study was a randomized controlled trial 27 conducted at two child welfare centers in central Sweden.
2.1. Selection and description of participants
The present study was performed at two CHS locations in one municipality in central Sweden. The parents of the infants were invited to participate consecutively in the study, and these infants were then randomized into two groups via the simple envelope model and were each assigned a code. Specifically, one group (36 infants) received the topical lidocaine‐prilocaine cream at the site for vaccine administration, and the other (36 infants) received the standard of care (placebo cream) (Figure 1). No evidence‐based pain management during vaccination was provided in the placebo group, as per the standard of care in Sweden.
FIGURE 1.
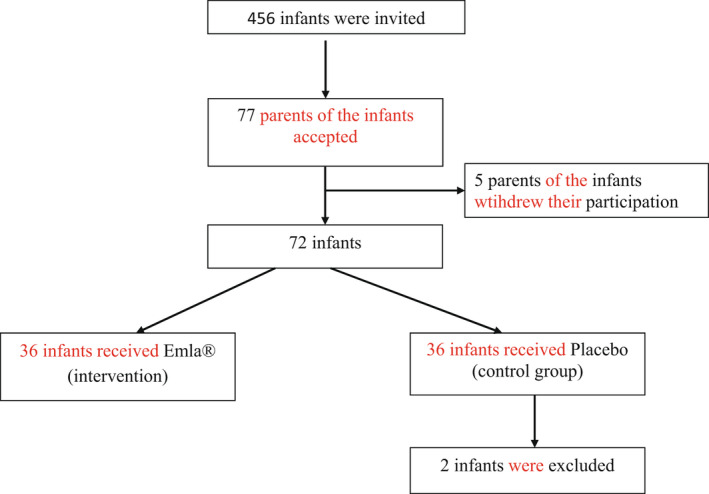
Consort flow diagram for the study ‐ Efficacy of topical lidocaine‐prilocaine (EMLA®) for management of infant pain during pneumococcal vaccination: a randomized controlled trial
The inclusion criteria were healthy infants, born vaginally or by Cesarean section after week 37, whereas the exclusion criterion was infants who had received neonatal care. Furthermore, the only allowable test before vaccination in this study was the routine phenylketonuria test administered to all neonates in Sweden.
2.2. Technical information
The data collected for the study were infants’ reaction to pain according to FLACC score, latency to cry, total crying time, heart rate, and oxygen saturation. Furthermore, the VAS score as assessed by the infants’ parents was also analyzed. The FLACC scale is an interval scale for measuring pain by quantifying pain behaviors and communicating infants’ pain expression. Its score ranges between 0 = no pain behavior and 10 = most pain behavior possible. The five behavior categories included on the scale are facial expression, leg movement, activity, crying, and consolability. 20 The VAS is a 100‐mm scale divided into ten parts, where 0 = no pain and 10 = worst pain. Using this scale, patients mark their current pain perception. 28 In the current study, parents of the infants assessed the infants’ pain according to the VAS. If both parents were present, they had to agree on the VAS rating. At the time of the study, the infants were administered two different vaccines at two separate injection sites. Sixty minutes before vaccination, the infant was administered either 1 g of topical lidocaine‐prilocaine cream or 1 g of placebo cream (Miniderm®). Specifically, Research Nurse 1 marked the injection site on the outside of the infant's left thigh using a circular template, which was identical in diameter to the topical lidocaine‐prilocaine patch (3.5 cm). After this, Research Nurse 2 selected an envelope to determine whether the infant would be randomized to the topical lidocaine‐prilocaine or placebo group. The cream was then applied to the drawn circle on the infant's thigh and fixed using a Tegaderm patch™ (3M) by Research Nurse 2, who also removed the patch and wiped away the cream immediately before the vaccination. All parents, irrespective of which group their infant was randomized to, were instructed to feed the infant 30 minutes prior to vaccination in order to minimize the effect of hunger on the pain response. Information regarding the age, and gender of the infant, as well as the time of application and removal of the patch, was noted in the study protocol. All vaccinations were performed by Research Nurse 1, who has extensive experience vaccinating infants. Research Nurse 1 also used a doll to instruct parents in how to hold the infant during the vaccination procedure. According to the instructions, the infant must be seated upright in the parent's lap with their left side facing the research nurse. The infant's left leg must be stretched out, with the parent fixing it with their right hand above the infant's kneecap. The parent's left arm must then be wrapped over the infant's left arm, thereby fixing the infant's body by holding it tight to the parent's body. This was done to allow greater maneuverability for Research Nurse 1 in order to optimally administer the injection. The vaccine was injected intramuscularly using a 23‐g syringe (0.6 mm, 25 mm length) without aspiration. During this entire procedure, the vaccination process was video‐recorded by Research Nurse 2 after the infant had been situated correctly. A saturation probe was also attached to the infant's foot, allowing data on heart rate and oxygen saturation to be collected in real time by Research Nurse 1 every 15, 30, and 45 seconds pre‐ and post‐vaccination. When pre‐ and post‐vaccination heart rate and oxygen saturation were collected, the parents were allowed to stand up as they comforted the infant. Once the infant stopped crying, the video camera was switched off. Thereafter, the parents were asked to assess their infant's pain scores on a VAS. Latency to cry, total crying time, and FLACC score were also assessed from the video‐recorded material. In addition to the parents VAS, one independent person with long‐standing experience with infants and knowledge of pain rating analyzed the infant's pain reaction 45 seconds both pre‐ and post‐vaccination using the FLACC scale. Following this, collected data on latency to cry and total crying time (from when the infant started crying) were calculated by both research nurses. The second vaccine (Infanrix® Hexa) was then injected after all the study data had been collected.
2.3. Statistics
All data were analyzed using descriptive and comparative statistics. Means (m) and ±standard deviations (SD) were utilized for latency to cry, total crying time, VAS score, heart rate, and oxygen saturation. Medians (md) and interquartile ranges (IQR) were used to analyze differences in the FLACC scores between the two groups. A t test was used to analyze the differences in latency to cry, total crying time, VAS score, heart rate, and oxygen saturation between the groups, whereas Mann‐Whitney's U test was used to analyze the differences in the FLACC scores. For all analyses, statistical significance was set at P < .05, and the SPSS version 22.0 program was used for data analysis.
2.4. Ethical considerations
This study was approved by the ethical board (2010/1762‐31/4, 2011/1879‐32, 2012/2084‐32) and the Medical Products Agency (151:2012/46775, EU. No. 2010‐021406‐38) and was registered at ClinicalTrials.gov (NCT number 01 802 086). The study was conducted according to clinical practice guidelines. 27 As the infants were unable to give informed consent to participate, this concern was directed at their parents. The parents received written information about the study and gave informed consent before their infants were enrolled in the study, according to the principles established by the Declaration of Helsinki. 29 The parents were also informed that they could refuse participation at any time with no negative consequences for their infant and without the need to provide any reason for this. 29 Furthermore, the parents received an adverse‐event report to take home and were instructed to record any side effects for the duration of one week.
3. RESULTS
The parents of 456 infants were invited to participate in the study. Among them, 77 (17%) agreed to participate; however, five parents decided to withdraw their infant from the study after having initially given consent but before their appointment at the CHS. This resulted in a total of 72 infants participating in the study. Furthermore, two infants from the placebo group had to be excluded: One of them had been admitted for neonatal care, and the other's crying time may have been influenced by extraneous disturbances (a sudden loud telephone signal). Therefore, a total of 70 infants were ultimately included and were then randomized to receive topical lidocaine‐prilocaine (n = 36) or placebo (n = 34). The included infants were between the ages of 13 and 15 weeks when they received their first pneumococcal vaccination (Prevenar 13®) and comprised 38 girls and 32 boys. No statistically significant differences were observed between the groups according to gender, age, or other background variables.
The application time for both the placebo cream and the topical lidocaine‐prilocaine cream was (m) 60.53 min (P = .99). Data were collected from May 2013 to November 2015.
3.1. Pain rating using the FLACC scale
The pre‐vaccination FLACC score did not differ between the groups [(md) 0, IQR 2 (n = 36) in the topical lidocaine‐prilocaine group; (md) 0, IQR 4.50 (n = 34) in the placebo group] (P =.977). Post‐vaccination, the FLACC score was (md) 7, IQR 3 (n = 36) in the topical lidocaine‐prilocaine group and (md) 9, IQR 2 (n = 34) in the placebo group (P = .006) (Figure 2).
FIGURE 2.
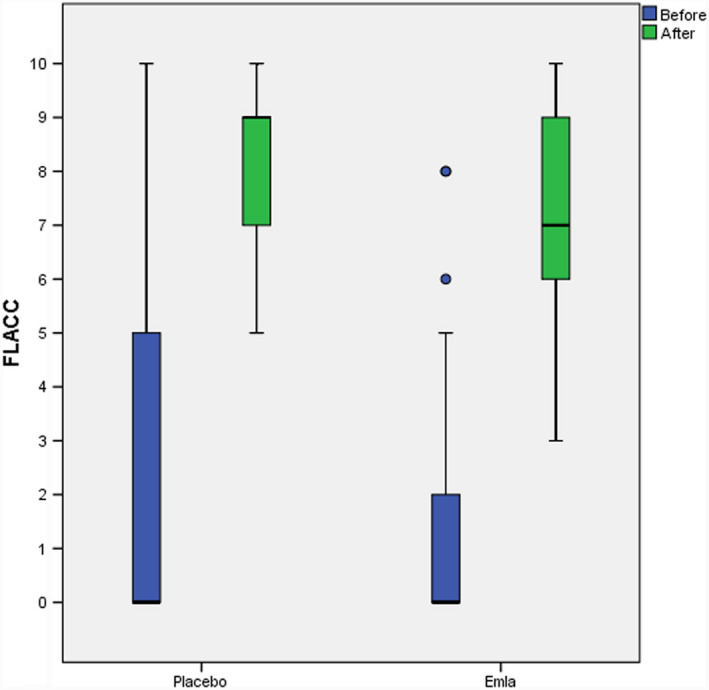
FLACC score pre‐ and post‐vaccination. The figure show infants’ reaction to vaccination according to the FLACC scale. The boxes comprise the interval between Q1 and Q3. The horizontal line inside the box represents the median value. The whiskers stretch to the highest and lowest scores, as long as they lie within 1.5 IQR from the nearest quartile. More extreme observations are illustrated by circles
3.2. Latency to cry and total crying time
Latency to cry was significantly longer for infants who received topical lidocaine‐prilocaine cream [(m) 2.77 seconds, SD ± 0.80 (n = 36)] than for those who received placebo cream [(m) 2.05 seconds, SD ± 0.978 (n = 34)] (P =.001). No significant difference in total crying time was observed between the topical lidocaine‐prilocaine group [(m) 78.74 s, SD ± 45.866 (n = 36)] and the placebo group [(m) 100.44 seconds, SD ± 73.307 (n = 34)] (P = .146) (Figure 3).
FIGURE 3.
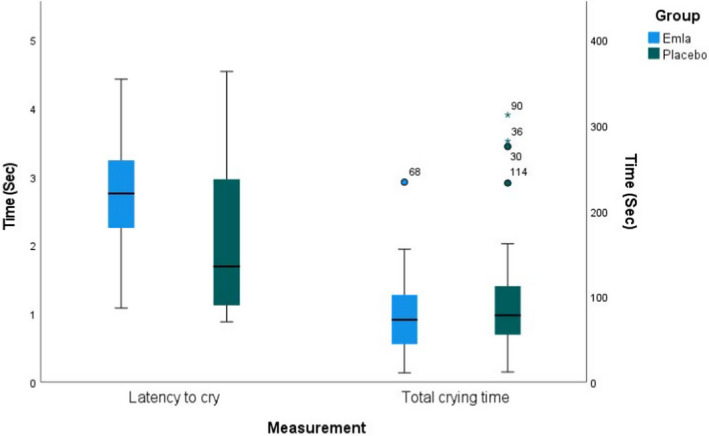
Latency to cry and Total crying time. The figure shows the infants’ Latency to cry and Total crying time according to vaccination in both groups. The boxes comprise the interval between Q1 and Q3. The horizontal line inside the box represents the median value. The whiskers stretch to the highest and lowest scores, as long as they lie within 1.5 IQR from the nearest quartile. More extreme observations are illustrated by circles or stars
3.3. Effect on heart rate and oxygen saturation
Pre‐vaccination heart rate was (m) 148.31 SD + 17.33 (n = 36) in the topical lidocaine‐prilocaine group and (m) 147.93 SD ± 17.95 (n = 34) in the placebo group (P = .928). Post‐vaccination heart rate was (m) 159.59 SD ± 15.53 (n = 36) in the topical lidocaine‐prilocaine group and (m) 160.46 SD ± 17.36 (n = 34) in the placebo group (P = .827).
Pre‐vaccination oxygen saturation was (m) 99.59 SD ± 0.82 (n = 36) in the topical lidocaine‐prilocaine group and (m) 99.78 SD ± 0.43 (n = 34) in the placebo group (P = .224). Post‐vaccination oxygen saturation was (m) 98.86 SD ± 1.22 (n = 36) in the topical lidocaine‐prilocaine group and (m) 98.76 SD ± 2.06 (n = 34) in the placebo group (P = .815).
3.4. Adverse event report
During the data collection, no adverse events occurred. Three parents in the topical lidocaine‐prilocaine group reported that their infant experienced tenderness at the injection site, whereas six in the placebo group reported the same.
4. DISCUSSION
4.1. Discussion of results
The present study showed that topical anesthetics used in conjunction with pneumococcal vaccination reduced infants’ expression of pain, which was consistent with former studies regardless of the vaccine or pain scale used. 2 , 3 , 5 , 7 , 16 , 17 , 18 , 19 , 20 , 30 , 31 , 32
In the present study, local anesthetics significantly lengthened latency to cry, although total crying time was not significantly reduced. These results conformed with those of Lindh et. al., 30 and Taddio, 7 both of which showed that infants who received topical lidocaine‐prilocaine cream started crying later after needle puncture. However, the current study revealed a wide standard deviation in total crying time. Previous research has similarly reported different results regarding total crying time. For instance, Mörelius et al 31 observed no significant differences in how long the infants cried, whereas Taddio 7 showed that the total crying time was shorter when infants were administered topical lidocaine‐prilocaine. Therefore, total crying time may not be a good indicator of infants’ expression of pain. 32
The FLACC scores in both groups can be regarded as relatively high. Thus, it is important to consider that more than one pain‐relief management approach should be used to address pain during vaccination. All children have the right to receive pain relief in connection to vaccination, and no infant's pain response should be ignored or left untreated. 13 , 22 , 23 , 33 , 34
In this study, there was no significant difference in heart rate response or oxygen saturation between the groups either before or after vaccination, which can be interpreted as a normal finding. When experiencing a painful procedure, the body strives to maintain homeostatic control. 35 Lindh et al 30 argued that it was difficult to assess pain based on heart rate frequency. In their study, they were unable to demonstrate that heart rate frequency alone could distinguish pain between infants who received topical lidocaine‐prilocaine cream and glucose or placebo cream and water before their first diphtheria, tetanus, and pertussis vaccination at the age of 3 months.
In low‐income countries, the use of topical anesthetic creams before vaccination can be an obstacle. Even in upper and middle‐income countries, not all infants receive pain relief for vaccination. 26 As such, non‐pharmacological evidence‐based strategies are important. Therefore, the implementation of other evidence‐based management strategies for the reduction of vaccination‐related pain is also important. 3 , 13 , 17 , 36 , 37 , 38
4.2. Ethical discussion
Since the aim here was to study infants’ pain reaction to pneumococcal vaccination, this vaccine was administered first. Thus, the “one injection at a time” approach was used. This consecutive approach was one of two normal routines being used at the study site at the time the study was conducted. Previous studies suggest that the least painful vaccine should be administrated first. 39 However, there is no comparative research on which of the two vaccines Prevenar 13® and Infanrix® Hexa are more painful. Therefore, it is unclear whether injecting Prevenar 13® first caused greater pain to the infant, as compared to injecting Infanrix® Hexa first.
The choice to not feed the infants during vaccination may have negatively affect their pain experience. New international guidelines recommend that sweet solution administration or breastfeeding always be performed during vaccination. 26 However, these guidelines were published in September 2015 whereas data collection was completed before this, and the study was concluded in November 2015. Therefore, the study could not be modified. 26 For future research, it is strongly recommended that small amounts of sweet solution, breastfeeding, or skin‐to‐skin contact be administered along with pharmacological treatment during vaccination.
4.3. Methodological discussion
Some limitations of the present study included the low inclusion rate and the lack of a previous pilot study. In order to include 72 infants, 456 parents were asked to participate, corresponding to an inclusion rate of 17%. The low inclusion rate may have carried a risk of selection bias. An aspect of the standardization was that a single nurse performed all the vaccinations in the study. Therefore, some parents did not meet their usual nurse, which was stated as a reason for unwillingness to participate. Furthermore, we noted that parents with more than one child were more inclined to participate in the study.
Pneumococcal vaccination was performed through a quick injection without aspiration, 1 which has been reported to significantly lower the pain response in infants. 33 , 39 However, the administration of an injection also involves the risk of over‐ or under‐penetration of the thigh muscle. Although under‐penetration is the more common of the two, both can cause more pain and discomfort for the infant. To mediate this, the needle's gauge, length, and angle are important for optimal intramuscular injection; for instance, a 90° angle has been recommended. 15 Healthcare professionals who perform intramuscular injections should apply their clinical experience in choosing the needle's length and angle in their practice. 15
A hypothetical reason that may influence the feasibility and acceptability of this study is that topical lidocaine‐prilocaine cream must be applied 1 hour before vaccination, implying prolonged time spent at the healthcare center for families. Providing parents with validated instructions in how to apply topical lidocaine‐prilocaine cream before vaccination, or using lidocaine (Maxilene®), 40 which has a shorter duration to onset, are two less time‐consuming alternatives.
To assess the infants’ pain expression, the FLACC scale, latency to cry, total crying time, VAS assessed by the parents, heart rate, and oxygen saturation were analyzed. While there are no gold standard methods for measuring pain in infants, FLACC has been reported to be a validated and frequently used scale for pain assessment. 20 Numerous studies have shown different results regarding post‐vaccination crying time, resulting in non‐conclusive findings. 7 , 30 , 31 , 32 Crying is used in research as a measure of pain and often as a complement to pain ratings; however, there are many factors that contribute to an infant's crying. While crying is not an unequivocal measure of pain, it can still be assessed as an expression of pain. A validated pain‐rating instrument that measures what is intended to be measured is sufficient for obtaining a decent idea of the child's response to vaccination‐related pain. 20 , 32
It is important for healthcare professionals to use a validated pain scale to measure infants’ pain response during vaccination, as total crying time is an insufficient indicator of an infant's response to pain. 32 Systematic efforts are needed to successfully implement the use of topical anesthetic creams, in addition to other effective non‐pharmacological pain‐relieving strategies, during the vaccination procedure for infants in middle‐ and upper‐income countries. Currently, the standard of care in Sweden lacks sufficient pain‐relieving strategies, and those that are available are not mandatory. 41 We believe that all infants should receive pain relief during vaccination. Although studies over more than two decades have shown that the topical lidocaine‐prilocaine cream was effective in reducing vaccine‐related pain, the use of anesthetic cream has not been standardized. Therefore, there is a need for implementation studies that include mandatory documentation regarding pain‐relief strategies in vaccination, and take‐home instructions for parents regarding the application of the anesthetic cream. In addition to a validation of this study's results, further studies are also needed to evaluate the pain intensity associated with different vaccines and whether a consecutive or simultaneous injection technique is best for the infant.
5. CONCLUSION
Topical lidocaine‐prilocaine cream reduced pain expression and increased latency to cry in infants receiving their first pneumococcal vaccine. However, as infants in the intervention group still presented with relatively high pain scores, the use of additional pain‐relieving strategies during vaccination is warranted.
AUTHOR CONTRIBUTIONS
Beatrice Olsson Duse contributed to conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, project administration, and funding acquisition. Ylva Sporrong contributed to conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, project administration, and funding acquisition. Marco Bartocci contributed to conceptualization, methodology, validation, formal analysis, resources, data curation, and supervision. Karin Skoglund contributed to validation, formal analysis, data curation, writing—review and editing, and supervision.
ACKNOWLEDGMENTS
Thanks to Nicklas Pihlström, statistician at the Centre for Clinical Research Sörmland, for the excellent statistics support. The study was funded by the Center for Clinical Research Sörmland, Uppsala University, Sweden, and by a scholarship from Linde Healthcare through the Swedish Pediatric Pain Association.
Olsson Duse B, Sporrong Y, Bartocci M, Skoglund K. Efficacy of topical lidocaine‐prilocaine (EMLA®) for management of infant pain during pneumococcal vaccination: A randomized controlled trial. Paediatr Neonatal Pain. 2022;4:53–60. doi: 10.1002/pne2.12070
[Correction added on 4 January 2022, after initial online publication. Third author Marcco Bartocc spell error corrected to Marco Bartocc.]
REFERENCES
- 1. Murat I, Gall O, Tourniaire B. Procedural pain in children: evidence‐based best practice and guidelines. Reg Anesth Pain Med. 2003;28(6):561‐572. [DOI] [PubMed] [Google Scholar]
- 2. Abdel Razek A, AZ El‐Dein N. Effect of breast‐feeding on pain relief during infant immunization injections. Int J Nurs Pract. 2009;15(2):99‐104. [DOI] [PubMed] [Google Scholar]
- 3. Taddio A, Appleton M, Bortolussi R, et al. Reducing the pain of childhood vaccination: an evidence‐based clinical practice guideline (summary). CMAJ. 2010;182(18):1989‐1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children's memory for pain: overview and implications for practice. J Pain. 2004;5(5):241‐249. [DOI] [PubMed] [Google Scholar]
- 5. Halperin SA, McGrath P, Smith B, Houston T. Lidocaine‐prilocaine patch decreases the pain associated with the subcutaneous administration of measles‐mumps‐rubella vaccine but does not adversely affect the antibody response. J Pediatr. 2000;136(6):789‐794. [PubMed] [Google Scholar]
- 6. Taddio A, Ipp M, Thivakaran S, et al. Survey of the prevalence of immunization non‐compliance due to needle fears in children and adults. Vaccine. 2012;30(32):4807‐4812. [DOI] [PubMed] [Google Scholar]
- 7. Taddio A, Nulman I, Goldbach M, Ipp M, Koren G. Use of lidocaine‐prilocaine cream for vaccination pain in infants. J Pediatr. 1994;124(4):643‐648. [DOI] [PubMed] [Google Scholar]
- 8. Folkhälsomyndigheten (2020). Barnvaccinationsprogram ‐ Allmänt program för barn. [Public health agency of Sweden. National vaccination programme for children]. Downloaded Sept. 3rd, 2020, from https://www.folkhalsomyndigheten.se/smittskydd‐beredskap/vaccinationer/vaccinationsprogram/allmant‐program‐for‐barn
- 9. Wettergren B, Blennow M, Hjern A, Söder O, Ludvigsson JF. Child health systems in Sweden. J Pediatr. 2016;177S:S187‐S202. [DOI] [PubMed] [Google Scholar]
- 10. Folkhälsomyndigheten (2020). Vaccinationsstatistik från barnhälsovården . [Public health agency of Sweden. Vaccination register and vaccination coverage]. Downloaded March 2nd, 2021 from https://www.folkhalsomyndigheten.se/globalassets/statistik‐uppfoljning/vaccinationsstatistik/vc/vaccinationsstatistik‐fran‐bhv‐2020_rapport.pdf
- 11. Taddio A, Smart S, Sheedy M, et al. Impact of prenatal education on maternal utilization of analgesic interventions at future infant vaccinations: a cluster randomized trial. Pain. 2014;155(7):1288‐1292. [DOI] [PubMed] [Google Scholar]
- 12. Rikshandboken barnhälsovård för professionen (2019). Smärtlindring vid vaccinering [The National Handbook for Child Health Services. Pain‐relieving in connection to vaccination]. Downloaded Febr. 2nd, 2020 from https://www.rikshandboken‐bhv.se/vaccination/smartlindring‐vid‐vaccinering/
- 13. Taddio A, Riddell RP, Ipp M, et al. Relative effectiveness of additive pain interventions during vaccination in infants. CMAJ. 2017;189(6):E227‐E234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uhari M. A eutectic mixture of lidocaine and prilocaine for alleviating vaccination pain in infants. Pediatric. 1993;92(5):719‐721. [PubMed] [Google Scholar]
- 15. Lippert WC, Wall EJ. Optimal intramuscular needle‐penetration depth. Pediatrics. 2008;122(3):e556–e563. [DOI] [PubMed] [Google Scholar]
- 16. Abuelkheir M, Alsourani D, Al‐Eyadhy A, Temsah MH, Meo SA, Alzamil F. EMLA® cream: a pain‐relieving strategy for childhood vaccination. J Int Med Res. 2014;42(2):329‐336. [DOI] [PubMed] [Google Scholar]
- 17. Dilli D, Küçük IG, Dallar Y. Interventions to reduce pain during vaccination in infancy. J Pediatr. 2009;154(3):385‐390. [DOI] [PubMed] [Google Scholar]
- 18. Halperin BA, Halperin SA, Mcgrath P, Smith B, Houston T. Use of lidocaine‐prilocaine patch to decrease intramuscular injection pain does not adversely affect the antibody response to diphtheria‐tetanus‐acellular pertussis‐inactivated poliovirus‐Haemophilus influenzae type b conjugate and hepatitis B vaccines in infants from birth to six months of age. Pediatr Infect Dis J. 2002;21(5):399‐405. [DOI] [PubMed] [Google Scholar]
- 19. Gupta NK, Upadhyay A, Agarwal A, Goswami G, Kumar J, Sreenivas V. Randomized controlled trial of topical EMLA and breastfeeding for reducing pain during wDPT vaccination. Eur J Pediatr. 2013;172(11):1527‐1533. [DOI] [PubMed] [Google Scholar]
- 20. Manworren RC, Hynan LS. Clinical validation of FLACC: preverbal patient pain scale. Pediatr Nurs. 2003;29(2):140‐146. [PubMed] [Google Scholar]
- 21. Läkemedelsindustriföreningen (2021). [Equivalent to The United States Pharmacopeia Drug Information, USP DI] EMLA® Kräm 25 mg/g + 25 mg/g. I FASS Vårdpersonal. Downloaded March 2nd, 2021 from https://www.fass.se/LIF/product?userType=0&nplId=19841101000029
- 22. International Association for the Study of Pain (IASP) . Declaration of Montreal‐ Declaration that Access to Pain Management Is a Fundamental Human Right [Internet]. 2018. Downloaded Febr. 2nd, 2020 from https://www.iasp‐pain.org/Advocacy/Content.aspx?ItemNumber=1821&navItemNumber=582
- 23. Schofield P, Hadjistavropoulos T. Guidelines for the management of pain in vulnerable populations. International Association for the Study of Pain (IASP) [Internet]. 2019. Downloaded Febr. 2nd, 2020 from https://s3.amazonaws.com/rdcms‐iasp/files/production/public/1_Guidelines_for_the_Management_of_Pain_in_Vulnerable_Populationsedited.pdf
- 24. Harrison D, Reszel J, Bueno M, et al. Breastfeeding for procedural pain in infants beyond the neonatal period. Cochrane Database Syst Rev. 2016;10(10):CD011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kassab M, Almomani B, Nuseir K, Alhouary AA. Efficacy of sucrose in reducing pain during immunization among 10‐ to 18‐month‐old infants and young children: a randomized controlled trial. J Pediatr Nurs. 2020;50:e55‐e61. [DOI] [PubMed] [Google Scholar]
- 26. World health organization (WHO) . Reducing pain at the time of vaccination: WHO position paper. 2015. Downloaded May 23rd, 2019 from https://www.who.int/wer/2015/wer9039.pdf?ua=1
- 27. Frieman LM, Furberg Curt D, DeMets DL. Fundamentals of Clinical Trials. 4th ed. Springer; 2010. [Google Scholar]
- 28. Willame C, Henry O, Lin L, Vetter V, Baril L, Praet N. Pain caused by measles, mumps, and rubella vaccines: a systematic literature review. Vaccine. 2017;35(42):5551‐5558. 10.1016/j.vaccine.2017.08.068. PMID: 28893478. [DOI] [PubMed] [Google Scholar]
- 29. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191‐2194. [DOI] [PubMed] [Google Scholar]
- 30. Lindh V, Wiklund U, Blomquist HK, Håkansson S. EMLA cream and oral glucose for immunization pain in 3‐month‐old infants. Pain. 2003;104(1‐2):381‐388. [DOI] [PubMed] [Google Scholar]
- 31. Mörelius E, Theodorsson E, Nelson N. Stress at three‐month immunization: parents' and infants' salivary cortisol response in relation to the use of pacifier and oral glucose. Eur J Pain. 2009;13(2):202‐208. [DOI] [PubMed] [Google Scholar]
- 32. Gupta NK, Upadhyay A, Dwivedi AK, Agarwal A, Jaiswal V, Singh A. Randomized controlled trial of topical EMLA and vapocoolant spray for reducing pain during wDPT vaccination. World J Pediatr. 2017;13(3):236‐241. 10.1007/s12519-017-0004-y. Epub 2017 Jan 19 PMID: 28101779 [DOI] [PubMed] [Google Scholar]
- 33. Taddio A, McMurtry CM, Shah V, et al. Reducing pain during vaccine injections: clinical practice guideline. Can Med Assoc J. 2015;187(13):975‐982. 10.1503/cmaj.150391. Epub 2015 Aug 24. PMID: 26303247; PMCID: PMC4577344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eccleston C, Fisher E, Howard RF, et al. Delivering transformative action in paediatric pain: a Lancet Child & Adolescent Health Commission. Lancet Child Adolesc Health. 2021;5(1):47‐87. [DOI] [PubMed] [Google Scholar]
- 35. Verriotis M, Chang P, Fitzgerald M, Fabrizi L. The development of the nociceptive brain. Neuroscience. 2016;338:207‐219. [DOI] [PubMed] [Google Scholar]
- 36. Ravikiran SR, Kumar PM, Meundi AD. Pain response in newborns to the order of injecting BCG and Hepatitis‐B vaccines: a randomized trial. Indian J Pediatr. 2011;78(6):693‐697. [DOI] [PubMed] [Google Scholar]
- 37. Taddio A, Ipp M, Vyas C, et al. Teaching parents to manage pain during infant immunizations: laying the foundation for better pain management practices. Clin J Pain. 2014;30(11):987‐994. [DOI] [PubMed] [Google Scholar]
- 38. Taddio A, Shah V, McMurtry CM, et al. Procedural and physical interventions for vaccine injections: systematic review of randomized controlled trials and quasi‐randomized controlled trials. Clin J Pain. 2015;31(10 Suppl):S20‐S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ipp M, Parkin PC, Lear N, Goldbach M, Taddio A. Order of vaccine injection and infant pain response. Arch Pediatr Adolesc Med. 2009;163(5):469‐472. [DOI] [PubMed] [Google Scholar]
- 40. Läkemedelsindustriföreningen (2021). [Equivalent to The United States Pharmacopeia Drug Information, USP DI]. Maxilene Kräm 40 mg/g. I FASS Vårdpersonal. Downloaded March 2nd, 2021 from https://www.fass.se/LIF/product?userType=0&nplId=20131122000013
- 41. Socialstyrelsen (2014). Vägledning för barnhälsovården . [the National Board of Health and Welfare. Guidance for child health service]. Downloaded May 23rd, 2019 from https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelkatalog/vagledning/2014‐4‐5.pdf


