Abstract
Purpose:
The Breast Cancer Surveillance Consortium (BCSC) model is a widely-used risk model that predicts five- and ten-year risk of developing invasive breast cancer for healthy women aged 35–74 years. Women with high BCSC risk may also be at elevated risk to develop interval cancers, which present symptomatically in the year following a normal screening mammogram. We examined the association between high BCSC risk (defined as the top 2.5% by age) and breast cancers presenting as interval cancers.
Methods:
We compared the mode of detection and tumor characteristics of patients in the top 2.5% BCSC risk by age with age-matched (1:2) patients in the lower 97.5% risk. We constructed logistic regression models to estimate the odds ratio (OR) of presenting with interval cancers, and poor-prognosis tumor features, between women from the top 2.5% and bottom 97.5% of BCSC risk.
Results:
Our analysis included 113 breast cancer patients in the top 2.5% of risk for their age and 226 breast cancer patients in the lower 97.5% of risk. High-risk patients were more likely to have presented with an interval cancer within one year of a normal screening, OR 6.62 (95% CI 3.28–13.4, p<0.001). These interval cancers were also more likely to be larger, node positive, and higher stage.
Conclusion:
Breast cancer patients in the top 2.5% of BCSC risk for their age were more likely to present with interval cancers. The BCSC model could be used to identify healthy women who may benefit from intensified screening.
Keywords: breast cancer, screening, breast density, supplemental screening, interval cancer
Introduction
Interval cancers are invasive breast cancers that present symptomatically within 12 months of a normal screening mammogram. These cancers include both those that develop after a mammogram and those that were not detected (but did exist) at the previous screening mammograms. Interval cancers tend to be more aggressive and faster-growing than screen-detected cancers.[1–4] Identifying women who are at increased risk for interval breast cancers could inform screening strategies, as these women may benefit from supplemental or more frequent screening and risk reduction. However, no consensus regarding how to risk-stratify women for interval breast cancer risk exists. The Breast Cancer Surveillance Consortium (BCSC) model is a validated and widely used risk prediction tool that predicts five- and ten-year risk of developing invasive breast cancer for women age 35–74.[5] It bases risk prediction on age, race/ethnicity, presence of first degree relative with breast cancer, prior biopsies/benign breast disease, and Breast Imaging-Reporting and Data System (BI-RADS) breast density.[5,6] Past work by Kerlikowske et al. has suggested that the combination of BCSC risk and BI-RADS breast density is one method upon which risk-stratification for interval cancer could be based.[7]
However, both the BCSC model and breast density itself are correlated with age: as age increases, BCSC score increases and breast density decreases. Providers may be wary of basing recommendations for screening frequency and modality on risk models (such as BCSC) that may enrich for increased screening as age increases. Further, tumor characteristics and morbidity vary by age, with younger women being at increased risk of developing poor prognosis tumors and interval cancers.[7–9] In contrast to using an absolute risk cutoff to identify high risk women, an alternative method is to use age-specific cutoffs. Age-specific BCSC risk distributions are generated directly by the BCSC, and aggregate 5-year age groups have been described in the literature.[5] The WISDOM study, run by the Athena Breast Health Network, uses these distributions to establish a threshold of the top 2.5% of risk for each age group to initiate counseling on prevention interventions and annual screening. Prior thresholds were not sufficiently high to motivate interest in embarking on risk reduction strategies.[10] The top 2.5% by age threshold consistently identifies women with lifetime risk of 23–28%, and 20% of these women elect to pursue prevention interventions. This is why it was chosen for the high risk threshold to trigger for annual screening and prevention counseling in WISDOM.[10]
This study’s primary aim is to validate this top 2.5% by age threshold by determining if these women are more likely to present with interval cancers rather than screen-detected cancers. We also evaluated whether these interval cancers have more aggressive features to confirm the clinical relevance of detecting interval cancers.
Patients and Methods
Patients
We conducted a case-case analysis of women treated for invasive breast cancer at the University of California San Francisco Breast Care Center (UCSF BCC). This study included only women with a confirmed diagnosis of invasive breast cancer previously undergoing standard mammography screening. Between 2013 and 2017, 896 patients completed the Athena intake questionnaire (described in Measures) in the BCC and had available BI-RADS breast density and pathology data. We identified the 180 women in the top 2.5% of BCSC risk for their age. Women were excluded from the study if they deviated from standard screening intervals by having an increased screening frequency (more than 1 mammogram per year) or if they had an “interval” cancer detected more than one year after their prior clear mammogram. Ultimately, information on mode of detection (screen detected versus interval breast cancer) was available for 113 women in the top 2.5% of risk for their age. We then used a random number generator to select age-matched (±1 year) women from the lower 97.5% who also had method of detection available. (Figure 1).
Figure 1:
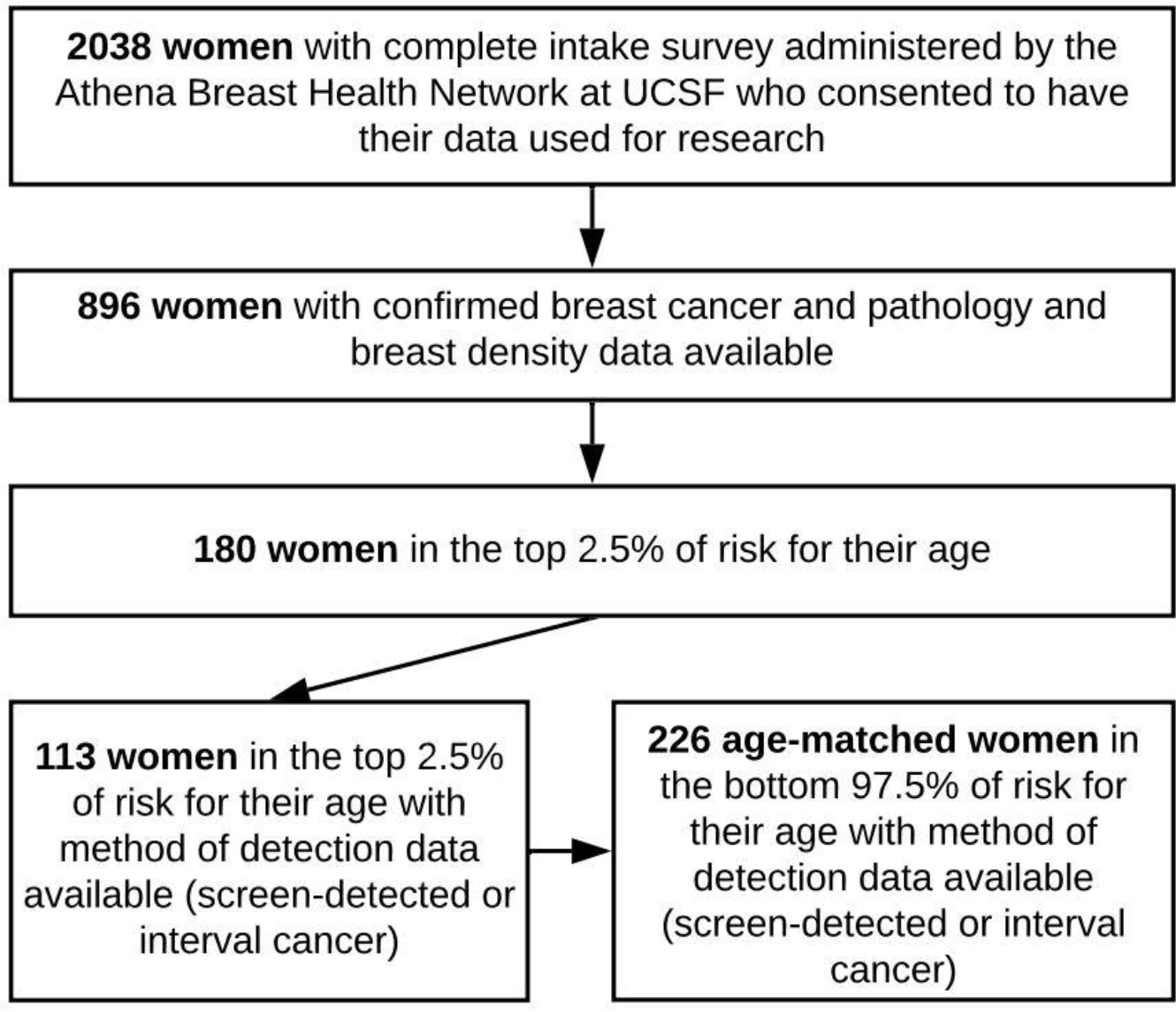
Selection of the study group from women seen at the UCSF Breast Care Center from 2013–2017. Top 2.5% threshold determined from distributions of BCSC 5-year risk estimates.
Measures
The UCSF BCC is part of the Athena Breast Health Network, a breast cancer clinical care and research collaborative that includes breast care clinics from five University of California hospitals and Sanford Health in South Dakota.[11] Athena collects patient characteristics and outcome data across the entire care spectrum from screening and prevention to treatment and survivorship. At the UCSF BCC, questionnaires are distributed to all patients presenting with a new breast problem.
The Athena intake questionnaire at the BCC collects race/ethnicity, family history, personal cancer history, history of prior biopsies, presence of comorbidities, and psychosocial and physical quality of life metrics.[12] These questionnaires contain all the variables included in the BCSC model (http://tools.bcsc-scc.org/BC5yearRisk/) except for BI-RADS density. Using the electronic medical record, we exported BI-RADS density based on the last negative screening mammogram prior to diagnosis and used it for the BCSC risk assessment. While the BCSC model is intended for women without a history of breast cancer, this allowed for a retrospective estimate of each patient’s 5-year risk of developing cancer at the approximate time of their diagnosis with the assumption that breast density stayed relatively stable between the last negative mammogram and density.[13] The 97.5th BCSC risk percentile for each age (Supplementary Table A) was estimated by applying the BCSC risk calculator to data collected from more than six million mammograms from eight breast imaging registries across the country.[14]
The BCSC risk score was calculated for eligible women (those between the ages of 40–74 without a diagnosis of breast cancer prior to the current diagnosis) who completed the online intake questionnaire and whose BI-RADS density was available (Figure 1). These BCSC scores were based on the patient’s age at time of intake. Medical records for all patients in the top 2.5% of risk for their age and two age-matched (±1 year) cases from the bottom 97.5% of risk were reviewed to determine method of cancer detection.
The UCSF Cancer Center registry contains pathology and outcome data linked to state and national registries, and has been described previously.[15,16] We collected information on each patient’s histology, grade, stage, nodal involvement, hormone receptor status, and tumor size from the Registry. If data were not available for a patient, we imported these fields from the UCSF surgical registry. The UCSF BCC maintains an internal surgical registry that is updated weekly with pathology reports from recent surgeries. This dataset, updated in near real-time, was included to capture data that were not yet reported in other registries.
Outcomes
Our primary outcome focused on interval cancers, defined as invasive breast cancers that presented within one year of a normal mammogram, BI-RADS score 1 or 2. Tumor characteristics including hormone receptor status, grade, size, nodal involvement and stage were imported from the registries based on patient medical record number and approximate diagnosis date.
Statistical Analysis
We compared the proportion of interval cancers between the two age-matched groups using conditional logistic regressions in R. We also used logistic regressions to compare tumor characteristics between interval cancers and screen-detected cancers. All tests were two-sided with alpha of 0.05.
In addition to comparing patients in the top 2.5% of risk for their age to patients from the lower 97.5%, we examined two additional risk stratification criteria from the literature: patients with extremely dense breasts (BI-RADS d) or a very high BCSC score irrespective of age (>4.00% 5-year risk of developing breast cancer).[7] This was an adjunct analysis included to address potential questions from the reader. However, it is important to note that the sample used in this study is not matched based on these two criteria.
Results
Patient Characteristics
Of the 339 patients included in the final analysis, 113 fell in the top 2.5% of risk for their age, and they were compared to 226 from the lower 97.5% of risk (Figure 1). Table 1 summarizes demographic information from the patients included in the analysis. Women in the top 2.5% of risk for their age tended to have higher breast density and more frequently reported a first degree relative with breast cancer and a personal history of breast biopsy (p<0.001 for all comparisons).
Table 1:
Baseline characteristics and demographic data for women in the top 2.5% of risk for their age (n=113) and age-matched women from the lower 97.5% (n=226).
| Characteristic | BCSC High Risk (Top 2.5% by Age) |
BCSC Lower Risk (Lwr 97.5% by Age) |
P-value |
|---|---|---|---|
|
| |||
| Mean age (range) | 57 (40–73) | 57 (40–73) | Matched |
|
| |||
| Mean BCSC score (SD) | 3.9 (1.3) | 2.0 (0.72) | <0.001 *** |
|
| |||
| BCSC score distribution | <0.001 *** | ||
| Low (0%–<1.00%) | 0 (0%) | 23 (10%) | |
| Average (1.00%–1.66%) | 0 (0%) | 65 (29%) | |
| Intermediate (1.67%–2.49%) | 13 (11%) | 92 (41%) | |
| High (2.50%–3.99%) | 54 (48%) | 46 (20%) | |
| Very high (≥4.00%) | 46 (41%) | 0 (0%) | |
|
| |||
| Breast density distribution | <0.001*** | ||
| BI-RADS a (mostly fatty) | 1 (1%) | 20 (9%) | |
| BI-RADS b | 26 (23%) | 87 (39%) | |
| BI-RADS c | 62 (55%) | 98 (43%) | |
| BI-RADS d (extremely dense) | 24 (21%) | 21 (9%) | |
|
| |||
| First-degree relative with breast cancer | 59 (52%) | 36 (16%) | <0.001 *** |
|
| |||
| History of breast biopsy | 72 (64%) | 88 (39%) | <0.001 *** |
|
| |||
| Mean body mass index (SD) | 23.9 (5.2) | 25.9 (6.1) | 0.003 ** |
|
| |||
| Race: | 0.004** | ||
| White | 92 (81%) | 157 (70%) | |
| Asian | 8 (7%) | 41 (18%) | |
| Black or African American | 0 (0%) | 9 (4%) | |
| Mixed race or other | 13 (12%) | 19 (8%) | |
= p < 0.05
= p < 0.01
= p < 0.001; Standard Deviation (SD)
Interval cancer risk by BCSC risk group
Patients from the top 2.5% of risk for their age were more likely to present with an interval cancer within one year of a normal screening mammogram compared to patients in the lower 97.5% of risk, OR 6.62 (95% CI 3.28–13.4, p<0.001) (Table 2). Similar results were seen when we expanded the analysis to include “late-interval” cancers, those discovered within two years of a normal screening mammogram.
Table 2:
Association between three risk stratification criteria and interval cancers. The three risk stratification criteria included the BCSC top 2.5%, BI-RADS d (extremely dense), or BCSC 5-year cancer risk >4.00% (very high).
| Primary Risk Stratification Criteria | Interval Cancers in High-risk Group | Interval Cancers in Low-risk Group | Odds Ratio | Confidence Interval | P-value |
|---|---|---|---|---|---|
| BCSC top 2.5% n=113/339 Avg age=57 Avg BCSC (SD)=3.9 (1.3) |
40 (35%) | 21 (9%) | 6.62† | 3.28 – 13.4 | <0.001*** |
| Alternative Risk Stratification Criteria ‡ | |||||
| BI-RADS d N=45/339 Avg age=51 Avg BCSC (SD)=2.6 (1.2) |
18 (40%) | 43 (15%) | 3.89 | 1.98 – 7.67 | <0.001*** |
| BCSC very high, >4.00% n=46/339 Avg age=62 Avg BCSC (SD)=5.2 (1.1) |
15 (30%) | 46 (16%) | 2.60 | 1.30 – 5.19 | 0.007* |
= p < 0.05
= p < 0.01
= p < 0.001; Standard Deviation (SD)
= derived from conditional linear regression, unadjusted odds ratio 5.35
= samples not age-matched
We also compared the top 2.5% by age threshold to two other common risk stratification criteria: extremely dense breasts (BI-RADS d) or a very high BCSC score irrespective of age (>4.00% 5-year risk of developing breast cancer) (Table 2). The BCSC top 2.5% by age threshold was most strongly associated with interval cancer risk. The mean age for the BCSC top 2.5% threshold was between that of extremely dense breasts and 4% 5-year BCSC risk. Furthermore, a substantial number of women in the top 2.5% of risk for their age would not have been identified by these other risk cutoffs. Specifically, 49 of 113 (43%) women would only be flagged for increased risk using the top 2.5% by age threshold – and these women show a similarly high percentage of interval cancers (32.7%).
Tumor characteristics of interval cancers
Interval cancers had more aggressive features than cancers detected via screening mammogram. Interval cancers were more likely to be lymph node positive (odds ratio, OR 3.24, 95% CI 1.76 – 5.96, p<0.001) and larger than two centimeters (OR 3.49, 95% CI 1.82 – 6.70, p<0.001). Thus, they were more likely to be stage II or higher (OR 4.88, 95% CI 2.34 – 10.2, p<0.001). Likewise, interval cancers tended to be grade 3 and hormone receptor negative, although these trends were not statistically significant (Table 3).
Table 3:
Tumor characteristics of interval cancers compared to screen-detected cancers from 339 breast cancer patients seen at the UCSF Breast Care Center. Certain components of pathology were not available for all patients, most notably tumor size. The ratios represent number of patients with the characteristic per those with data available.
| Characteristic | Interval Cancers (n=61) | Screen-detected Cancers (n=278) | Odds Ratio | Confidence Interval | Difference |
|---|---|---|---|---|---|
| Size > 2 cm | 27/48 | 52/193 | 3.49 | 1.82 – 6.70 | <0.001** |
| Lymph node invasion present | 24/61 | 43/258 | 3.24 | 1.76 – 5.96 | <0.001*** |
| Stage > 1 | 37/48 | 73/179 | 4.88 | 2.34 – 10.2 | <0.001*** |
| Grade > 2 | 22/60 | 71/270 | 1.62 | 0.90 – 2.93 | 0.108 |
| Hormone receptor negative | 9/61 | 29/269 | 1.43 | 0.64 – 3.21 | 0.382 |
= p < 0.05
= p < 0.01
= p < 0.001; Standard Deviation (SD)
Discussion
In this study, we compared breast cancer patients in the BCSC top 2.5% of risk for their age to patients from the remaining 97.5%. We found that women in the top 2.5% of risk for their age, who have double the risk of getting breast cancer relative to the average women, had more than six-fold higher odds of presenting with interval cancers. Furthermore, the interval cancers detected in this study were of clinical relevance as they followed trends outlined in the literature and tended to have more aggressive features.
Our study extends the literature by validating an alternative approach to risk stratification, which considers the distribution of risk among similarly aged women, as a predictor of interval cancer risk.[17] This allows providers to identify women at high risk without selecting certain age groups, as would BCSC score or density alone. A numeric threshold, identical for all ages, also fails to recognize the range of risk in each age group and does not account for lifetime risk. A 1.5% 5-year risk in a 40-year-old, for example, is associated with a much higher lifetime risk than a 1.5% 5-year risk in a 75-year-old. Many patients in the top 2.5% of risk for their age have extremely dense or heterogeneously dense breasts, which may mask tumors and contribute to interval cancer prevalence. However, if density alone drove this effect, we would expect to see the highest interval cancer prevalence in patients with BI-RADS d density. To the contrary, the data presented in this manuscript demonstrate that the top 2.5% by age threshold had the highest proportion of interval cancers when compared to other previously reported risk stratification criteria such as extremely dense breasts (BIRADS d) or a 4% absolute 5-year risk. However, it is important to recognize that this study was not designed to compare these criteria, and in creating the BIRADS d or 4% absolute risk groups age-matching was broken. Further research is necessary to effectively compare risk-stratification criteria; this analysis was included to address common questions from readers but is largely beyond the scope of this work.
We also replicated previous work showing interval cancers to be enriched for aggressive features and linked to poor prognosis.[7,18] In a large case-case study of 431,480 women, Kirsh et al. found interval cancers were more likely to be higher stage, higher grade, estrogen receptor negative, and progesterone receptor negative when compared to screen-detected tumors. We replicated these findings for stage, and while our study may not have been sufficiently powered to detect significant differences in grade and hormone receptor status, it should be noted that trends in our results were aligned with previous findings in the literature.[1,2]
Our work should be interpreted in light of several limitations. First, this was a case-case analysis and our sample size may have limited the precision of our estimates and ability to detect small differences between groups. Larger cohort studies in multiracial/multiethnic populations are needed to validate our main findings. Such studies would also make our work more generalizable, given our study predominately included white patients. Second, we did not review the most recent mammogram to confirm that the tumor represented a “true” interval cancer – rather than merely a missed tumor due to human error in the initial reading. However, missed interval cancers have also been shown to have more aggressive features compared to screen-detected cancers, although to a lesser extent.[1] Furthermore, these data reflect the limits of what is understood in clinical practice. Ultimately, if this sampling includes tumors that should have been screen-detected, it should only underestimate the unique characteristics of interval cancers. Third, women with higher risk are often offered more intensive screening due to the presence of risk factors such as dense breasts or positive family history. This may also bias these results, but we expect the bias to be toward the null, given that we expect increased screening to decrease interval cancer prevalence in high-risk groups.
Our results have several important clinical implications. Since interval cancers tend to present at later stages and lead to worse prognosis, it follows that a goal of breast cancer screening should be to detect interval cancers at an earlier, more treatable stage. However, increasing screening frequency for all women would likely lead to unsustainable resource usage and unintended effects such as false positives. As such, there is a clear need for risk stratification criteria that can identify women at elevated risk of interval cancers so that they can receive targeted screening and prevention. However, providers may be wary of using existing criteria that tend to select specific age groups for a variety of reasons – such as the prevalence of indolent tumors in older women.[19,20] Our results suggest that a simple top 2.5% by age threshold, based on a widely used risk-assessment tool, may effectively identify women with higher odds of developing interval cancers. This threshold is already being used to target preventative efforts (such as chemoprevention and lifestyle changes) by providers in the Athena Breast Health Network and in the WISDOM (Women Informed to Screen Depending on Measures of risk) Study, a randomized trial of personalized versus annual breast cancer screening that uses the BCSC model as well as genetic predisposition (mutations and polygenic risk).[21,22] Women in the personalized arm who are in the top 2.5% of risk for their age are assigned to annual screening and active outreach for risk reduction counseling; those whose 5-year risk is over 6% get screening every 6 months, alternating annual mammography with annual MRI.
Future work should aim to validate whether the top 2.5% by age threshold is associated with a similar increase in the likelihood of interval cancers in large cohort studies. These studies may also determine that a different sensitivity is optimal, such as top 1% or 5% by age. Cohort studies should ideally be powered to compare alternative risk-stratification criteria and examine the link between BCSC score and other features of aggressiveness, such as HER2 positivity, triple-negative/basal subtype, or high grade or proliferation.
Implications
Breast cancer patients whose BCSC risk, at the time they were diagnosed with breast cancer, was in the top 2.5% of predicted breast cancer risk for their age are significantly more likely to have their cancers detected in the interval between screening mammograms. These interval cancers were more likely to be higher grade and later stage, and thus may be linked to poor prognosis. Women in this elevated-risk category may benefit from tailored screening strategies or preventative interventions such as chemoprevention. A prospective validation is underway in the WISDOM study.
Supplementary Material
Acknowledgments
We are extremely grateful to Karla Kerlikowske and her team at the San Francisco Mammography Registry (SFMR) for their guidance contextualizing this research and their willingness to collaborate. The SFMR provided access to data that was not ultimately used in this study. We would also like to thank Ann Griffin from the UCSF Cancer Registry and Patrick Wang from the UCSF Breast Care Center Internship Program. Data collection and sharing was supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium (HHSN261201100031C). You can learn more about the BCSC at: http://www.bcsc-research.org/. Yiwey Shieh was supported by funding from the National Cancer Institute (1K08CA237829) and the MCL consortium. Dr. Esserman is supported by funding from the NCI MCL consortium (U01CA196406). We would also like to thank the dedicated Athena investigators and advocates for their continued work and support.
Funding:
Yiwey Shieh was supported by funding from the National Cancer Institute (1K08CA237829) and the MCL consortium. Laura Esserman is supported by funding from the NCI MCL consortium (U01CA196406).
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
Ethics approval: This work was approved by the UCSF Institutional Review Board and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate: All participants consented to have their data used for research that may result in publication.
Consent for publication: All participants consented to have their data used for research that may result in publication.
Availability of data and material: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability: Code used in this analysis will be made available from the corresponding author on reasonable request.
References
- 1.Kirsh VA, Chiarelli AM, Edwards SA, O’Malley FP, Shumak RS, Yaffe MJ, Boyd NF (2011) Tumor characteristics associated with mammographic detection of breast cancer in the Ontario breast screening program. J Natl Cancer Inst 103:942–950. 10.1093/jnci/djr138 [DOI] [PubMed] [Google Scholar]
- 2.Holm J, Humphreys K, Li J, Ploner A, Cheddad A, Eriksson M, Törnberg S, Hall P, Czene K (2015) Risk Factors and Tumor Characteristics of Interval Cancers by Mammographic Density. J Clin Oncol. 10.1200/JCO.2014.58.9986 [DOI] [PubMed] [Google Scholar]
- 3.Henderson LM, Miglioretti DL, Kerlikowske K, Wernli KJ, Sprague BL, Lehman CD (2015) Breast Cancer Characteristics Associated with Digital Versus Film-Screen Mammography for Screen-Detected and Interval Cancers. AJR Am J Roentgenol 205:676–684. 10.2214/AJR.14.13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PAM50 and risk of recurrence scores for interval breast cancers. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5984721/. Accessed 18 Sep 2019 [DOI] [PMC free article] [PubMed]
- 5.Tice JA, Miglioretti DL, Li C-S, Vachon CM, Gard CC, Kerlikowske K (2015) Breast Density and Benign Breast Disease: Risk Assessment to Identify Women at High Risk of Breast Cancer. J Clin Oncol 33:3137–3143. 10.1200/JCO.2015.60.8869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sickles EA, D’Orsi CJ, Bassett LW. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston: American College of Radiology; 2013. Mammography. [Google Scholar]
- 7.Kerlikowske K, Zhu W, Tosteson ANA, Sprague BL, Tice JA, Lehman CD, Miglioretti DL (2015) Identifying Women with Dense Breasts at Highi Risk of Interval Cancers. Ann Intern Med 162:673–681. 10.7326/M14-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long-term outcome in young women with breast cancer: a population-based study. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5050247/. Accessed 18 Sep 2019 [DOI] [PMC free article] [PubMed]
- 9.Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: a study from the Japanese Breast Cancer Registry. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5050233/. Accessed 18 Sep 2019 [DOI] [PMC free article] [PubMed]
- 10.Huilgol YS, Keane H, Shieh Y, Tice JA, Ziv E, Madlensky L, Sabacan L, Acerbi I, Che M, Fiscalini AS, Anton-Culver H, Borowsky A, Hunt S, Naiem A, Parker B, van ‘t Veer LJ, Athena Investigators and Advocate Partners, Esserman LJ. Breast Cancer Risk Thresholds as a Predictor of Chemoprevention Uptake in the Athena Breast Health Network. San Antonio Breast Cancer Symposium (SABCS). [Google Scholar]
- 11.Elson SL, Hiatt RA, Anton-Culver H, Howell LP, Naeim A, Parker BA, Van’t Veer LJ, Hogarth M, Pierce JP, Duwors RJ, Hajopoulos K, Esserman LJ, Athena Breast Health Network (2013) The Athena Breast Health Network: developing a rapid learning system in breast cancer prevention, screening, treatment, and care. Breast Cancer Res Treat 140:417–425. 10.1007/s10549-013-2612-0 [DOI] [PubMed] [Google Scholar]
- 12.Wong EC, Kaplan CP, Dreher N, Hwang J, Van’t Veer L, Melisko ME (2018) Integration of Health Questionnaire Systems to Facilitate Supportive Care Services for Patients at an Academic Breast Care Center. JCO Clin Cancer Inform 2:1–13. 10.1200/CCI.18.00018 [DOI] [PubMed] [Google Scholar]
- 13.Lokate M, Stellato RK, Veldhuis WB, Peeters PHM, van Gils CH (2013) Age-related changes in mammographic density and breast cancer risk. Am J Epidemiol 178:101–109. 10.1093/aje/kws446 [DOI] [PubMed] [Google Scholar]
- 14.Risk Factors Dataset: BCSC. https://www.bcsc-research.org/data/rf. Accessed 18 Sep 2019
- 15.Chien AJ, Duralde E, Hwang R, Tsung K, Kao C-N, Rugo HS, Melisko ME, Esserman LJ, Munster PN, Cedars M, Kerlikowske K, McCulloch CE, Rosen MP (2015) Association of tamoxifen use and ovarian function in patients with invasive or pre-invasive breast cancer. Breast Cancer Res Treat 153:173–181. 10.1007/s10549-015-3511-3 [DOI] [PubMed] [Google Scholar]
- 16.Engmann NJ, Scott CG, Jensen MR, Winham S, Miglioretti DL, Ma L, Brandt K, Mahmoudzadeh A, Whaley DH, Hruska C, Wu F, Norman AD, Hiatt RA, Heine J, Shepherd J, Pankratz VS, Vachon CM, Kerlikowske K (2019) Combined effect of volumetric breast density and body mass index on breast cancer risk. Breast Cancer Res Treat 177:165–173. 10.1007/s10549-019-05283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozanne EM, Esserman LJ (2004) Evaluation of breast cancer risk assessment techniques: a cost-effectiveness analysis. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 13:2043–2052 [PubMed] [Google Scholar]
- 18.Kerlikowske K, Sprague BL, Tosteson ANA, Wernli KJ, Rauscher GH, Johnson D, Buist DSM, Onega T, Henderson LM, O’Meara ES, Miglioretti DL (2019) Strategies to Identify Women at High Risk of Advanced Breast Cancer During Routine Screening for Discussion of Supplemental Imaging. JAMA Intern Med. 10.1001/jamainternmed.2019.1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundquist M, Thorstenson S, Brudin L, Wingren S, Nordenskjöld B (2002) Incidence and prognosis in early onset breast cancer. The Breast 11:30–35. 10.1054/brst.2001.0358 [DOI] [PubMed] [Google Scholar]
- 20.Syed BM, Green AR, Paish EC, Soria D, Garibaldi J, Morgan L, Morgan D a. L, Ellis IO, Cheung KL (2013) Biology of primary breast cancer in older women treated by surgery: with correlation with long-term clinical outcome and comparison with their younger counterparts. Br J Cancer 108:1042–1051. 10.1038/bjc.2012.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shieh Y, Eklund M, Madlensky L, Sawyer SD, Thompson CK, Stover Fiscalini A, Ziv E, Van’t Veer LJ, Esserman LJ, Tice JA, Athena Breast Health Network Investigators (2017) Breast Cancer Screening in the Precision Medicine Era: Risk-Based Screening in a Population-Based Trial. J Natl Cancer Inst 109. 10.1093/jnci/djw290 [DOI] [PubMed] [Google Scholar]
- 22.Esserman LJ (2017) The WISDOM Study: breaking the deadlock in the breast cancer screening debate. Npj Breast Cancer 3:1–7. 10.1038/s41523-017-0035-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


