Abstract
Loss of functional mitochondrial complex I (MCI) in the dopaminergic neurons of the substantia nigra is a hallmark of Parkinson’s disease1. Yet, whether this change contributes to Parkinson’s disease pathogenesis is unclear2. Here we used intersectional genetics to disrupt the function of MCI in mouse dopaminergic neurons. Disruption of MCI induced a Warburg-like shift in metabolism that enabled neuronal survival, but triggered a progressive loss of the dopaminergic phenotype that was first evident in nigrostriatal axons. This axonal deficit was accompanied by motor learning and fine motor deficits, but not by clear levodopa-responsive parkinsonism—which emerged only after the later loss of dopamine release in the substantia nigra. Thus, MCI dysfunction alone is sufficient to cause progressive, human-like parkinsonism in which the loss of nigral dopamine release makes a critical contribution to motor dysfunction, contrary to the current Parkinson’s disease paradigm3,4.
Parkinson’s disease (PD) is the second most common neurodegenerative disease, affecting millions of individuals5. The cardinal motor symptoms of PD are caused by the loss of dopaminergic neurons in the substantia nigra (SN) that modulate basal ganglia circuits regulating habitual and goal-directed movement. Although the pathogenesis of PD is undoubtedly multifactorial, several lines of evidence point to the importance of mitochondrial dysfunction. For example, loss of function mutations in PARK7, PARK6 and PARK2—all of which encode proteins that are involved in maintaining mitochondrial health—cause early-onset forms of PD6. Mutations in genes associated with autosomal dominant forms of PD have also been linked to mitochondrial dysfunction7. Moreover, alpha-synuclein, a major component of Lewy pathology in PD, inhibits MCI, as do environmental toxins that increase PD risk8. The selective vulnerability of dopaminergic neurons to mitochondrial dysfunction is attributable in part to the metabolic demands placed on them by a massive, highly branched axonal arbor with hundreds of thousands of transmitter release sites1,9. Maintaining this arbor and the autonomous regenerative activity characteristic of SN dopaminergic neurons leads to sustained stimulation of mitochondrial oxidative phosphorylation (OXPHOS) to power the cell’s biochemical machinery. However, sustained stimulation comes at the cost of mitochondrial oxidant stress, damage and increased mitochondrial turnover rates in SN dopaminergic neurons10. Indeed, post-mortem examination of the human SN has uncovered an association between PD, loss of mitochondrial deoxyribonucleic acid (mtDNA) integrity in the SN and loss of functional MCI1.
What is less clear is whether acquired damage to MCI is simply a PD tombstone (a disease by-product) or whether it is a driver of late-stage pathogenesis. Partial disruption of MCI function by deleting its Ndufs4 subunit in dopaminergic neurons does not result in a Parkinsonian phenotype, nor does it alter the sensitivity to environmental toxins linked to PD, suggesting that MCI dysfunction is simply a PD tombstone2. However, the lack of a clear phenotype in this mouse may simply reflect the ability of neurons to compensate for partial disruption of OXPHOS. To assess the consequences of a more complete disruption of MCI function, the gene encoding an essential subunit of the MCI catalytic core, Ndufs2, was deleted in dopaminergic neurons using intersectional genomics11. These conditional Ndufs2-knockout mice displayed progressive, axon-first, levodopa-responsive parkinsonism, resembling that seen in humans12. Importantly, the staging of pathology enabled us to assess the consequences of regional dopamine (DA) depletion on motor behaviour. Surprisingly, although disruption of striatal dopaminergic signalling impaired motor learning and fine motor skills, it did not cause gross deficits in ambulation. These PD features emerged only late in the evolution of pathology with loss of SN dopaminergic signalling. In fact, selectively boosting SN conversion of systemic levodopa to DA significantly alleviated motor disability in late-stage Parkinsonian mice.
Loss of MCI induces metabolic reprogramming
To delete Ndufs2 specifically from dopaminergic neurons, mice harbouring floxed Ndufs2 (Ndufs2fl/fl) were crossed with mice expressing Cre recombinase (Cre) under the control of the DA transporter (DAT, encoded by Slc6a3) promoter (Dat-cre+/−)11 (Extended Data Fig. 1a, b and Supplementary Table 1). The efficacy of the Ndufs2 deletion was confirmed by isolating actively transcribed mRNA from SN dopaminergic neurons using the RiboTag method13 and then performing quantitative PCR with reverse transcription (RT–qPCR) assays (Fig. 1a–c). Through weaning (at around postnatal day 20 (P20)), conditional Ndufs2-knockout (cNdufs2−/−) mice were normal in appearance and gross motor behaviour. As MCI proteins commonly have lifetimes of 20–40 days14, the absence of a phenotype in this early postnatal period was not surprising. However, between P20 and P30, the mitochondria in SN dopaminergic neurons became net consumers, rather than producers, of adenosine triphosphate (ATP). This shift was evident in the sensitivity of the inner mitochondrial membrane (IMM) potential—measured using the potentiometric dye tetramethylrhodamine—to blockade of the adenine nucleotide transporter (ANT) (Fig. 1d–f). In ex vivo brain slices from wild-type mice, dopaminergic neuron IMM potential increased with ANT blockade, as the dissipative activity of complex V was presumably inhibited by slowing of ATP transport out of the matrix (Fig. 1e, f). However, in cNdufs2−/− dopaminergic neurons, ANT blockade caused the IMM potential to collapse, as it prevented mitochondria from importing ATP and running complex V in reverse (Fig. 1e, f and Extended Data Fig. 1c).
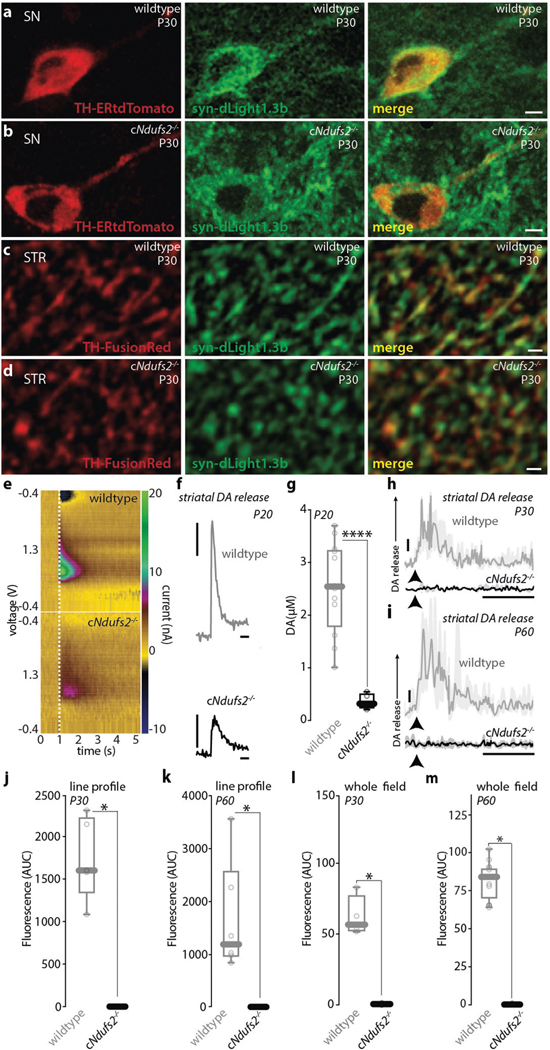
Fig. 1 |. Loss of NDUFS2 function triggers metabolic adaptations in SN DA neurons.
a, Schematic of a coronal section of the midbrain highlighting the sampled region of the SN pars compacta (SNc; orange). Schematic modified from the Allen Mouse Brain Atlas, online version 1, 2008 (http://mouse.brain-map.org ). SNr, SN pars reticulata; ml, medial lemniscus; VTA, ventral tegmental area. b, Meta-analysis of RNA-sequencing (RNA-seq) data obtained by Cre-dependent RiboTag mRNA precipitation from P40 wild-type mice. The heat map shows enrichment of dopaminergic-specific markers (N = 5), where N indicates the number of mice. GLUT, glutamatergic; GABA, gabaergic. c, Effective Ndufs2 knockout was verified by qPCR analysis of RiboTag-collected mRNA. N = 5 (wild type (WT)) and N = 5 (cNdufs2−/−). d, TMRM-labelled mitochondria of an SN DA neuron in an ex vivo brain slice. Scale bar, 10 μm. e, Representative TMRM fluorescence time-series analysis after the application of carboxyatractyloside, an ANT inhibitor (iANT). f, Box plots of TMRM after blocking ANT and depolarizing mitochondria with FCCP. Wild type: n = 8 (P40); cNdufs2−/−: n = 4 (P20) and n = 5 (P40), where n indicates the number of cells. g, h, Heat maps of expression determined using RNA-seq show the downregulation of OXPHOS (g) and upregulation of glycolysis (h) in cNdufs2−/− mice. N = 5 (wild type) and N = 6 (cNdufs2−/−). i, Representative image of SN DA neurons expressing PercevalHR and stained for TH in an ex vivo brain slice. Scale bar, 15 μm. N = 4. ROI, region of interest. j, Representative time-lapse measurements of the PercevalHR fluorescence ratio. Oligomycin and 2-deoxyglucose (2-DG) were applied to determine OXPHOS and glycolytic contributions to the ATP/ADP ratio. Scale bar, 10 min. k, The OXPHOS index (OXPHOS/(OXPHOS + glycolysis)). n = 6 (wild type) and n = 10 (cNdufs2−/−). Statistical analysis was performed using two-tailed Mann–Whitney U-tests (c and k) and one-tailed Kruskal–Wallis tests with Dunn’s correction for multiple comparisons (f); *P < 0.05, **P < 0.01, ***P < 0.001. For the boxplots in c, f and k, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range.
To determine whether the mitochondrial OXPHOS deficit triggered changes in mitochondrial morphology or density, SN dopaminergic neurons were retrogradely labelled by injecting Fluoro-Gold into the striatum, and were then examined using transmission electron microscopy. Consistent with the proposition that mitochondria were maintaining their membrane potential, somatic mitochondrial density was normal in cNdufs2−/− dopaminergic neurons (Extended Data Fig. 1d, e). Thus, mitochondria were not sufficiently depolarized to induce mitophagy15. However, in many cNdufs2−/− dopaminergic neurons, the structure of the mitochondrial cristae was altered (Extended Data Fig. 1f), consistent with the downregulation in electron transport chain genes16. This modest structural change stands in contrast to the gross mitochondrial pathology and turnover seen in dopaminergic neurons in the MitoPark mouse, in which mitochondrial gene transcription is widely disrupted17.
To get a better picture of the epigenetic changes induced by disruption of mitochondrial OXPHOS, mRNA from wild-type and cNdufs2−/− SN dopaminergic neurons was isolated using the RiboTag approach and then sequenced13. This analysis revealed a substantial metabolic reprogramming—a Warburg-like effect—in cNdufs2−/− dopaminergic neurons. That is, there was an upregulation of genes encoding proteins that promote glycolysis and downregulation of those genes associated with OXPHOS. Genes encoding inhibitors of glycolysis were also downregulated (such as TP53-inducible glycolysis and apoptosis regulator) (Fig. 1g, h, Extended Data Figs. 2 and 3 and Supplementary Tables 2 and 3). Indeed, measurement of the cytosolic ATP/adenosine diphosphate (ADP) ratio using the genetically encoded sensor PercevalHR18, revealed that, in contrast to wild-type dopaminergic neurons in which inhibition of mitochondrial complex V by oligomycin caused a precipitous drop in ATP levels, in cNdufs2−/− dopaminergic neurons, this ratio fell only in response to inhibition of glycolysis (Fig. 1i–k and Extended Data Fig. 1g–j).
Early axonal dysfunction after Ndufs2 deletion
In addition to triggering metabolic reprogramming, loss of Ndufs2 induced substantial changes in the expression of genes related to axonal growth and transport (such as Tubb3, Uchl1, Wnt5a, Sema3g, Nefl and Prkca), synaptic transmission (such as Syt1, Syt3, Syt17, Syn2 and Scna), DA synthesis/storage (such as Th and Vamp2) and presynaptic regulation (such as Drd2, Chrna4 and Chrna6) (Fig. 2a and Supplementary Tables 4–6). Tyrosine hydroxylase (TH) protein levels were downregulated in the dorsal striatum by around P30, paralleling the loss of mitochondrial OXPHOS (Fig. 2b–d). By contrast, TH expression was much less affected in the ventral striatum (Fig. 2b, c) and the number of TH-immunoreactive neurons in the SN and ventral tegmental area (VTA) was unchanged at this time point (Fig. 2b–d and Extended Data Fig. 4). To determine the functional significance of these changes, DA release evoked by electrical stimulation was measured in ex vivo brain slices using fast-scan cyclic voltammetry or the genetically encoded, optical DA sensor dLight19. By P30, DA release by dopaminergic axons in the dorsolateral striatum—which were measured using both approaches—was close to zero. By contrast, somatodendritic DA release in the SN (measured with dLight) was not detectably different (Fig. 2e–g and Extended Data Fig. 5). The deficit in evoked striatal DA release was validated using liquid chromatography and mass spectrometry analysis of striatal tissue, confirming that there was a considerable drop in striatal DA synthesis in cNdufs2−/− mice (Extended Data Fig. 6a–d).
Fig. 2 |. Loss of Ndufs2 induces early axonal dysfunction.
a, Heat map of RNA-seq analysis shows the downregulation of dopamine release and synaptic function in cNdufs2−/− mice. N = 5 (wild type) and N = 6 (cNdufs2−/−), where N indicates the number of mice. b, c, Representative images showing a significant reduction in TH staining in the dorsal striatum but not in the SNc in P30 wild-type (b) and cNdufs2−/− (c) mice. Scale bars, 1 mm (top) and 200 μm (bottom). TH-IR, immunoreactivity. d, Quantification of TH expression in the dorsal striatum, SNc and VTA. N = 5 (wild type) and N = 5 (cNdufs2−/−). e, Striatal (top) and dendritic (bottom) dopamine release measured with fast-scan cyclic voltammetry and dLight1.3b measurements. The solid lines represent the median trace and the shaded area indicates the 95% confidence intervals. Scale bars, 1 μM dopamine (vertical), 1 s (horizontal) (top); and 5% Δf/f0 (vertical) 10 s (horizontal) (bottom). f, g, Quantification of dopamine release at P30 in the dorsal striatum (f; N = 4 (wild type) and N = 5 (cNdufs2−/−) and in the SN (g; N = 6 (wild type) and N = 9 (cNdufs2−/−). AUC, area under the curve. h, Cell-attached recordings from identified wild-type and cNdufs2−/− SN DA neurons. Scale bars, 10 pA (vertical) and 1 s (horizontal). i, Cumulative probability plot of autonomous discharge rates. n = 21 (wild type) and n = 21 (cNdufs2−/−), where n indicates the number of cells. j, Representative reconstruction of a Fura-2-filled SN neuron (left). Scale bar, 20 μm. Right, whole-cell recording and Fura-2 Ca2+ imaging of SN DA neurons (n = 5) at P30. Scale bars, 20 mV (vertical) and 1 s (horizontal). Statistical analysis was performed using two-tailed Mann–Whitney U-tests (d, f and g) and a one-tailed Mann–Whitney U-test (i); **P < 0.01. For the box plots in d, f and g, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range.
Although somatodendritic release of DA by SN dopaminergic neurons was not discernibly altered by Ndufs2 deletion at this point in time, the physiology of this subcellular region was changed. Wild-type SN dopaminergic neurons have ion channels that drive slow, autonomous pacemaking with broad spikes20,21. But in cell-attached recordings from P30–40 SN dopaminergic neurons from cNdufs2−/− mice, pacemaking had slowed or stopped (Fig. 2h, i). The translatomes of these cells revealed an upregulation in background K+ channels (such as TASK-1 and TREK-1 channels) that might suppress pacemaking (Extended Data Fig. 6e and Supplementary Table 7). Furthermore, expression of Mink-related peptide 1 (Kcne2), which positively regulates the function of hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels22, was downregulated, as was expression of the Hcn2 and Hcn3 subunits (Extended Data Fig. 6f and Supplementary Table 7). Indeed, HCN channel currents, which help to drive pacemaking, were reduced in cNdufs2−/− SN dopaminergic neurons (Extended Data Fig. 6g, h). Another important pacemaking-related current flows through Cav1.3 channels and is responsible for large cytosolic Ca2+ transients that drive mitochondrial OXPHOS7. In cNdufs2−/− SN dopaminergic neurons, the expression of mRNA for the pore-forming subunit of Cav1.3 channels was downregulated and cytosolic Ca2+ transients evoked by spiking were nearly abolished (Fig. 2j, Extended Data Fig. 6i, j and Supplementary Table 7). By contrast, the expression of mRNAs encoding glutamate receptors that drive burst spiking appeared to be unchanged in cNdufs2−/− neurons (Supplementary Table 8). Indeed, the dendritic arbors of ~P40 cNdufs2−/− SN dopaminergic neurons were normal in appearance (Fig. 2j) and two-photon uncaging of glutamate along them evoked bursts of spikes. Interestingly, these bursts were higher in frequency and longer in duration compared with those evoked in wild-type neurons, reflecting in part the substantial shortening of spike duration (Extended Data Fig. 6k–n).
Somatodendritic shift after Ndufs2 deletion
By P60, the loss of axonal proteins associated with dopaminergic signalling expanded to include the ventral striatum (Fig. 3a, b and Extended Data Fig. 4). Moreover, TH expression in the somatodendritic region of cNdufs2−/− SN dopaminergic neurons was decreased to around half that of age-matched controls (Fig. 3d–f and Extended Data Fig. 4). TH expression also decreased in cNdufs2−/− VTA dopaminergic neurons (Extended Data Fig. 4). In parallel, the release of DA in the SN fell by approximately 75% (Fig. 3g–i and Extended Data Fig. 5). To determine whether these functional changes were attributable to the degeneration of dopaminergic axons and cell bodies, the retrogradely transported marker Fluoro-Gold was injected into the striatum of P60 (±4 d) cNdufs2−/− mice; then, 5 d later, animals were euthanized for histological analysis. Many cNdufs2−/− SN neurons were labelled. In fact, the numbers of retrogradely labelled neurons were similar in cNdufs2−/− and wild-type mice (wild-type cells = 235, interquartile range = 303; cNdufs2−/− cells = 207, interquartile range = 317.5; N = 4 per group, where N indicates the number of mice; Fig. 3j). Moreover, the somatic mitochondrial density in cNdufs2−/− dopaminergic neurons was unchanged at this time point (Extended Data Fig. 7a–e). Thus, at this stage in the evolution of pathology in the cNdufs2−/− mice, the loss of markers for dopaminergic signalling reflected a phenotypic downregulation, rather than obvious neurodegeneration; that said, it remains to be determined whether the branching morphology of nigrostriatal axons was retained.
Fig. 3 |. Progressive loss of the somatodendritic phenotype, but not neuronal death.
a, b, Representative images showing TH immunostaining in the striatum in wild-type (a) and cNdufs2−/− (b) mice at P60. White dashed line encloses the dorsal striatum; yellow dotted lines indicate areas used for quantification of TH expression Scale bar, 1 mm. c, Quantification of TH expression in the dorsal striatum. N = 5 (wild type) and N = 5 (cNdufs2−/−), where N indicates the number of mice. d, e, Representative images showing a significant reduction in TH staining in the SNc of cNdufs2−/− mice (e) compared with wild-type mice (d) at P60. Scale bars, 200 μm (top) and 15 μm (higher-magnification images of the SNc showing DA neurons in wild-type and cNdufs2−/− mice). f, Quantification of TH expression in the SNc. N = 5 (wild type) and N = 5 (cNdufs2−/−). g, Striatal (top) and dendritic (bottom) dopamine release measured using fast-scan cyclic voltammetry and dLight1.3b measurements. The solid lines represent the median trace and the shaded area shows the 95% confidence intervals. Scale bars, 1 μM of dopamine (vertical), 1 s (horizontal) (top); and 5% Δf/f0 (vertical), 10 s (horizontal) (bottom). h, i, Quantification of dopamine release at P60 in the dorsal striatum (h; n = 4 (wild type) and n = 5 (cNdufs2−/−)) and in the SN (i; n = 5 (wild type) and n = 9 (cNdufs2−/−)), where n indicates the number of cells. j, Diagram of Fluoro-Gold injection for the analysis of retrograde transport. Representative images showing phenotypic downregulation but not cell loss in brain sections obtained from wild-type (N = 4) and cNdufs2−/− (N = 4) mice at P60. Scale bars, 20 μm. Statistical analysis was performed using two-tailed Mann–Whitney U-tests (c, f, h and i); *P < 0.05, **P < 0.01. For the box plots in c, f, h and i, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range.
To determine whether the changes in somatodendritic TH expression and DA release were matched by alterations in electrophysiology, dopaminergic neurons were labelled by injecting an adenoassociated virus (AAV) carrying a Th-promoter-driven FusionRed reporter construct into the SN of P30 mice. At P60 (±4 d), FusionRed-labelled neurons in ex vivo brain slices from cNdufs2−/− mice were studied (Extended Data Fig. 7f–h). As was the case at P30, many cNdufs2−/− SN dopaminergic neurons were silent and those that did spike did so irregularly (Extended Data Fig. 7i, j). Nevertheless, as at P30, the dendritic arbor of cNdufs2−/− SN dopaminergic neurons appeared to be intact and dendritic uncaging of glutamate evoked a robust, high-frequency burst of somatic spikes (Extended Data Fig. 7k–l). Thus, the decline in the somatodendritic transmitter phenotype of cNdufs2−/− SN dopaminergic neurons was not closely matched by a loss in responsiveness to extrinsic excitatory stimuli.
Emergence of parkinsonism
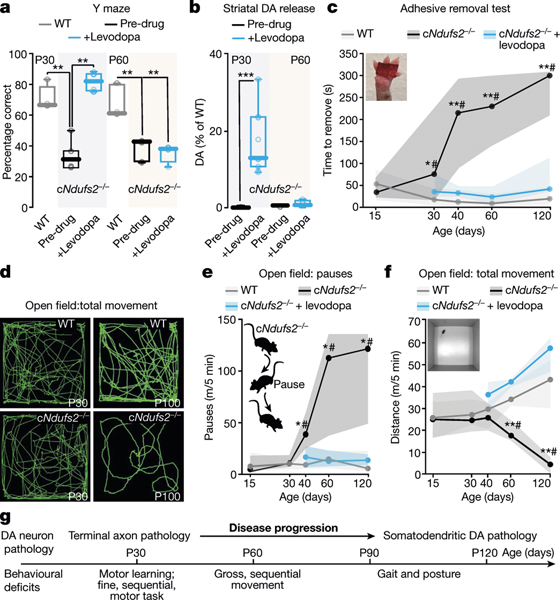
In contrast to conventional PD models in which DA is depleted rapidly throughout the basal ganglia, the staging of pathology in cNdufs2−/− mice enabled an assessment of how regional deficits in DA release are coupled to behaviour. Again, through weaning, cNdufs2−/− mice were indistinguishable from their littermate controls. However, as dorsal striatal DA release declined to near detection thresholds at around P30, cNdufs2−/− mice lost the ability to perform an associative learning task that is thought to rely on DA-dependent striatal synaptic plasticity21. Interestingly, this memory task was restored by systemic levodopa treatment (6 mg kg−1) at P30, but not by treatment later at P60 (Fig. 4a and Extended Data Fig. 8a). As associative synaptic plasticity in this task is widely thought to depend on appropriately timed phasic DA release23, the ability of levodopa treatment to restore evoked striatal DA release was determined in ex vivo brain slices from mice at these two ages. In striatal tissue from P30 mice, but not from P60 mice, levodopa treatment was able to restore the evoked DA release (Fig. 4b).
Fig. 4 |. cNdufs2−/− mice present progressive, levodopa-responsive parkinsonism.
a, The percentage of correct choices (Y-maze test) for wild-type mice (N = 8 (P30) and N = 6 (P60)), cNdufs2−/− mice (N = 8 (P30) and N = 6 (P60)) and cNdufs2−/− mice treated with 6 mg kg−1 levodopa (N = 8 (P30) and N = 6 (P60)), where N indicates the number of mice. b, Striatal dopamine release measured using fast-scan cyclic voltammetry at P30 and P60. P30: N = 5 (vehicle) and N = 9 (levodopa); P60: N = 4 (vehicle) and N = 6 (levodopa). c, Adhesive removal test from P15 to P120. N = 10 (wild type), N = 11 (cNdufs2−/−) and N = 10 (cNdufs2−/− + 6 mg kg–1 levodopa). d, Open-field traces in wild-type and cNdufs2−/− mice at P30 and P100. e, The number of pauses during the open-field test. N = 10 (wild type), N = 11 (cNdufs2−/−) and N = 10 (cNdufs2−/− + 6 mg kg−1 levodopa). m/5 min, metres per 5 min. f, The total distance travelled in 5 min. N = 11 (wild type), N = 13 (cNdufs2−/−) and N = 11 (cNdufs2−/− + 6 mg kg−1 levodopa). g, Schematic of the behavioural differences between young and older cNdufs2−/− mice. For a, c, e and f, statistical analysis was performed using one-tailed Kruskal–Wallis tests with Dunn’s correction for multiple comparisons; *P < 0.05, **P < 0.01 (wild type versus cNdufs2−/−); #P < 0.01 (cNdufs2−/− versus cNdufs2−/− + 6 mg kg−1 levodopa). For b, statistical analysis was performed using two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test; ***P < 0.001. For the box plots in a and b, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range. For c, e and f, the solid lines represent median trace and the shaded area shows the range.
Although deficits in striatal motor learning were profound at P30, mice did not exhibit gross impairments in motor performance. That said, fine motor skill, as assessed by the time taken by mice to remove an adhesive from their forepaw, was significantly slower in cNdufs2−/− mice at this age and became progressively worse with time. In contrast to the situation with the associative learning task, levodopa treatment improved the speed of adhesive removal regardless of age (Fig. 4c). By P40, open-field, exploratory behaviour of cNdufs2−/− mice began to be interrupted by brief pauses, although the total distance travelled during the testing period was normal. Later, the open-field behaviour of cNdufs2−/− mice became progressively more impaired and the total distance travelled began to decrease (Fig. 4d–g). As expected of a disability rooted in dopaminergic signalling, levodopa treatment improved the performance of mice in this task (Fig. 4e, f and Extended Data Fig. 8b, c). Another common exploratory behaviour of mice involves rearing. When placed in a tall glass cylinder, mice rear, land and rear again in a different part of the cylinder; over a period of 3 min, mice typically rear and land 15–20 times. At P30, the rearing behaviour of cNdufs2−/− mice was normal but, by P60, cNdufs2−/− mice appeared to have difficulty transitioning between rearing and landing, spending much more time stuck in an elevated posture. Interestingly, levodopa treatment did not lessen this deficit (Extended Data Fig. 8d–f). After the appearance of these gross motor deficits, the weight of cNdufs2−/− mice stabilized and diverged from that wild-type mice, possibly due to difficulty feeding (Extended Data Fig. 8g, h).
Despite the slowing of movement in the open field, cNdufs2−/− mice at around P60 exhibited only subtle impairments in gait when placed on a treadmill. However, by P100, cNdufs2−/− mice had splayed hindlimbs, abnormal paw placement and alterations in stride when forced to ambulate on the treadmill. Levodopa ameliorated some, but not all, of these deficits (Extended Data Fig. 9a–n). Interestingly, in this timeframe (P120–P150), approximately 40% of SN dopaminergic neurons appeared to have been lost, suggesting that phenotypic downregulation preceded obvious cell death (Extended Data Fig. 9o–q). As mice became progressively more impaired beyond P150, they died of unknown causes (Methods, ‘Animals’ subsection). Because cNdufs2−/− mice manifest clear, levodopa-responsive parkinsonism that is attributable to MCI dysfunction, we refer to them as MCI-Park mice.
Nigral determinants of motor disability
The late emergence of gross motor deficits in MCI-Park mice, which paralleled the changes in SN DA release rather than release in the dorsal striatum, poses a conceptual problem for the theory of network dysfunction underlying PD motor symptoms that has been in place for over 30 years3. This network theory posits that the imbalance between the activity of direct and indirect striatal efferent pathways created by striatal DA depletion is the prime driver of clinical PD symptoms (bradykinesia, rigidity). Although there is unequivocal clinical evidence that striatal DA depletion is necessary for bradykinesia and rigidity in patients with PD4, the sufficiency of striatal pathophysiology has never been adequately tested because commonly used models induce rapid DA depletion throughout the basal ganglia. The staging of DA depletion in MCI-Park mice suggests that, although the loss of dorsal striatal DA release is sufficient to produce motor learning and fine movement deficits, it is not sufficient to bring about a state resembling clinical PD, in agreement with several lines of study24–26.
To test this hypothesis, an AAV carrying an expression construct for aromatic-l-amino acid decarboxylase (AADC), which converts levodopa to DA, was stereotaxically delivered into either the dorsal striatum or the SN of Parkinsonian MCI-Park mice and the impact of a low (1.5 mg kg−1) systemic dose of levodopa on ambulation assessed (P100). In agreement with previous research27, striatal AADC expression enhanced ambulation in response to levodopa (Fig. 5a–d, i, k and Extended Data Fig. 10a–d). As DA does not cross the blood–brain barrier and has a limited ability to diffuse in the brain parenchyma28, this effect is consistent with the proposition that striatal DA depletion is necessary for the ambulatory impairment in MCI-Park mice and in patients with PD. However, as predicted by the behavioural staging, SN expression of AADC was just as effective in boosting the impact of a low dose of levodopa on open-field ambulation (Fig. 5e–h, j, k and Extended Data Fig. 10e–h). This effect was not attributable to elevation of striatal conversion of levodopa to DA, as SN AADC expression did not increase evoked striatal DA release or DA after levodopa treatment (Extended Data Fig. 10i–o). These results are consistent with the conclusion that both striatal and SN DA depletion are necessary for the emergence of PD-like deficits in ambulation26 (Fig. 5l and Extended Data Fig. 10p).
Fig. 5 |. Boosting mesencephalic dopamine levels reverses motor deficits in cNdufs2−/− mice.
a, Schematic of the conversion of levodopa to dopamine by AADC (top). Bottom, the experimental timeline for the AADC experiments. b, Schematic of the striatal injection strategy. c, Confocal image of a coronal slice showing GFP expression in the striatum of cNdufs2−/−mice (N = 4) injected with AAV2-GFP-AADC, where N indicates the number of mice. Scale bar, 200 μm. d, Magnified view of the dorsal striatum showing the expression of AADC–GFP in medium spiny neurons in cNdufs2−/− mice. Scale bar, 20 μm. e, Schematic of the SN injection strategy. AAV2-GFP-AADC or AAV2-GFP was bilaterally injected into the SN of cNdufs2−/− mice at P60. f, Confocal image of a coronal slice showing the expression of AADC–GFP in the SN of cNdufs2−/− mice (N = 4) at P100. Scale bar, 500 μm. g, h, Magnified views of the SN showing GFP+ neurons in cNdufs2−/− mice. Scale bars, 100 μm (g) and 20 μm (h). i, j, Representative traces of locomotor activity in the open-field test for striatal AADC (i) and SN AADC (j) before and after levodopa treatment (1.5 mg kg−1). k, The total distance travelled during 5 min for cNdufs2−/− mice infected with AAV2-GFP (black) or AAV2-GFP-AADC (red). A single AAV injection was performed for the SN. For the striatum, two injection strategies were tested: a single AAV injection (striatal) or three AAV injections to cover the whole striatum (×3 striatal). −AADC: N = 5 (striatal), N = 6 (SN) and N = 6 (×3 striatal); +AADC: N = 6 (striatal), N = 6 (SN) and N = 5 (×3 striatal). l, Schematic of our hypothesis of the cascade of events in the prediagnostic and clinical phase of PD. For k, statistical analysis was performed using two-way ANOVA followed by Tukey’s post hoc test; *P < 0.05, ***P < 0.001. For the boxplots in k, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range. The schematics in b and e are modified from the Allen Mouse Brain Atlas, online version 1, 2008 (http://mouse.brain-map.org ).
Taken together, the studies presented support two far-reaching conclusions. The first is that the loss of MCI function in dopaminergic neurons is sufficient to trigger a progressive, axon-first loss of function and levodopa-responsive parkinsonism. The slow loss of MCI function induced by Ndufs2 deletion revealed the metabolic plasticity of dopaminergic neurons, as they switched from being strongly dependent on mitochondrial OXPHOS for ATP production to being entirely reliant on glycolysis. In parallel, neurons remodeled their physiology by suppressing pacemaking, narrowing spikes and downregulating Ca2+ entry and signalling. The goal of this remodeling could have been to lower bioenergetic demand, but it is also possible that these features were shed because they were part of mitochondrial control mechanism that was no longer needed29. This plasticity makes the resilience of SN dopaminergic neurons to partial loss of MCI function more understandable2,11.
Although adequate to maintain somatodendritic integrity and excitability, the Warburg-like metabolic switch led to an early loss of axonal function, in agreement with previous research suggesting that the bioenergetic demands associated with this highly specialized region of SN dopaminergic neurons could be met only by mitochondrial OXPHOS9,30,31. Importantly, our results provide a framework for understanding how declining mitochondrial OXPHOS capacity with aging could result in the early loss of nigrostriatal axons characteristic of idiopathic PD32.
With time, dopaminergic traits were lost in the somatodendritic region of SN dopaminergic neurons in MCI-Park mice. Why this happened is not clear. It did not appear to be due to a bioenergetic shortfall, as neurons retained the ability to spike and respond to glutamatergic stimulation long after the switch to glycolysis. One possibility is that axonal dysfunction significantly reduced retrograde transport of striatally released trophic factors33. It is also possible that network dysfunction triggered by striatal DA depletion led to elevated excitatory input to SN dopaminergic neurons, leading to periods of bioenergetic crisis in vivo and suppression of anabolic processes34. A third possibility is that alterations in mitochondrial retrograde signalling induced by MCI dysfunction were responsible for phenotypic repression35. Despite this phenotypic downregulation, many once-dopaminergic neurons remained in place, very similar to the situation in the brains of individuals with PD36.
The second conclusion to be drawn from our studies is that DA depletion in the dorsolateral striatum is necessary but not sufficient to produce the gross movement deficits that are associated with clinical parkinsonism. For 30 years, the prevailing hypothesis has been that striatal DA depletion produces an imbalance in the excitability of direct and indirect striatal efferent pathways, resulting in disinhibition of basal ganglia output nuclei and the bradykinesia of clinical PD3. Although there is ample support for the proposition that striatal DA depletion creates a pathway imbalance37,38, whether it is sufficient to cause symptoms has not been rigorously tested23. In the MCI-Park mouse, selective striatal DA depletion produced expected deficits in associative motor learning and fine sequential motor tasks23,39, but not the gross motor impairment characteristic of PD. Levodopa-responsive parkinsonism appeared only when somatodendritic SN DA release fell. This release was first described in the 1970s40, but its functional role has been obscure. The staging of motor deficits in MCI-Park mice and the impact of regional AADC expression suggest that, in the prodromal stages of PD, dendritic DA release may rebalance direct and indirect pathway control of SN pars reticulata output created by striatal depletion. In so doing, it may prevent basal ganglia pathophysiology from driving a ‘toxic’ patterning of SN pars reticulata activity and enable other motor circuits to compensate. However, when this rebalancing capacity is lost, the pathophysiology may spread resulting in the ambulatory and gait features of PD4,41.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41586-021-04059-0.
Methods
Animals
All housing, breeding and procedures were performed according to the Northwestern University Animal Studies committee and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were group housed with food and water provided ad libitum under a 12 h–12 h light–dark cycle and temperatures of 65–75 °F with 40–60% humidity. Male and female mice were used for experiments. No significant differences were observed between both groups. We obtained Dat-cre mice (B6.SJL-Slc6a3tm1.1(cre)Bkmn/J) from the Jackson Laboratories and mice with the floxed Ndufs2 allele were provided by J. López Barneo (University of Sevilla, Spain). Dat-cre−/−-Ndufs2fl/fl mice were used as controls and Dat-cre+/−-Ndufs2fl/fl mice were referred to as cNdufs2−/− mice (MCI-Park mice). Dat-cre+/− mice were used as controls for RiboTag and dLight experiments. MCI-Park mice developed normally but died of unknown causes at 5–6 months of age. The premature death may be linked to the emergence of a profound sleep disturbance (fragmented slow-wave sleep and excessive daytime sleepiness) (P. Jiang, P.G.-R., F.W. Turek and D.J.S., unpublished observations).
Stereotaxic injections
Mice were anaesthetized using an isoflurane precision vapourizer (Smiths Medical) and placed in a stereotaxic frame (David Kopf Instruments) with a Cunningham adaptor (Harvard Apparatus) to maintain anaesthesia delivery throughout surgery. After exposing the skull, a small hole was bored with a micro drill bit and 360 nl of viral vector was delivered through a glass micropipette (Drummond Scientific Company) pulled on a Sutter P-97 puller. The SNc was targeted at the following coordinates (mm): anterioposterior (AP) = −3.10, mediolateral (ML) = 1.30 and dorsoventral (DV) = −4.50 (all (AP, ML, DV) relative to the bregma) and the striatum was targeted at the following coordinates: AP = 1, ML = 2.30 and DV = −3.40. For AADC experiments to cover the whole striatum (3×) the coordinates were: AP = 1, ML = 2.30, and DV = −3.5 and −3.1; and AP = 0.34, ML = 1.70, and DV = −3.60 and 3.00). All surgeries were performed in wild-type and/or cNdufs2−/− mice. Source of coordinates and diagrams: Allen Mouse Brain Atlas, online version 1, 2008 (http://mouse.brain-map.org). Experiments in animals with stereotaxic delivery of AAV viral vectors were performed starting 10 d after virus injection for all of the experiments with the exception of RiboTag and AADC experiments, in which mice were analysed 4 weeks after virus injection. All mouse brains were verified for proper construct expression after experiments. Data from mice with failed expression were excluded.
Recombinant AAV vectors
AAV serotype 2 vectors encoding AADC and the reporter gene GFP under the control of the CAG promoter were generated using an AAV helper-free system. For in vivo experiments, the viral titre was adjusted to 109 viral genomic particles per microlitre. The recombinant AAV vector was provided by M. G. Kaplitt (Weill Cornell Medical College, New York, NY, USA).
Ex vivo brain slice preparation
Mice were anaesthetized with a mixture of ketamine (50 mg kg−1) and xylazine (4.5 mg kg−1) then transcardially perfused with ice-cold, oxygenated modified artificial cerebrospinal fluid (aCSF) containing 125 mM sucrose, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 0.5 mM CaCl2, 10 mM MgCl2 and 25 mM glucose, pH 7.3 (315–320 mOsm l−1). Once perfused, the brain was rapidly removed and either sagittal or coronal slices containing the striatum (thickness, 275 μm) or the SNc (thickness, 220 μm) were sectioned using a vibratome (VT1200S Leica Microsystems). Brain slices were incubated in oxygenated modified aCSF containing 135.75 mM NaCl, 2.5 mM KCl, 25 mM NaHCO3, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2 and 3.5 mM glucose at 34 °C for 30 min, then at room temperature for another 30 min before the experiments. All solutions were pH 7.4, 310–320 mOsm l−1 and were continually bubbled with 95% O2/5% CO2. Experiments were performed at 32–33 °C.
2PLSM imaging
Two-photon laser scanning microscopy (2PLSM) was performed as follows. Fluorescence was measured using an Ultima Laser Scanning Microscope System (Prairie Technologies) with a DODT contrast detector to provide bright-field transmission images, with an Olympus ×60/1.0 NA water-dipping objective lens. A Chameleon Ultra series tunable (690–1,040 mm) Ti: sapphire laser system (Coherent laser group) provided the 2P excitation source. Laser power attenuation was achieved using two Pockels cell electro-optic modulators (M35080–02-BK and M350–50-02-BK, Con Optics) in series controlled by PrairieView v.5.3–5.5. Non-descanned emission photons were detected with GaAsP photomultiplier tube (PMT) (green, 490 nm to 560 nm) and multi-alkali PMT (red, 585 nm to 630 nm).
2PLSM: mitochondrial membrane potential
To monitor changes in mitochondrial membrane potential, dopaminergic neurons (visually identified on the basis of size and somatodendritic morphology) were loaded with 100 nM tetramethylrhodamine (TMRM) for 30 min (15 min at 34 °C and 15 min at room temperature), washed and maintained in 5 nM TMRM during imaging. Fluorescence was measured using 2PLSM imaging as described above. Signals were acquired using an 830 nm light in a fixed plane of focus with a pixel size 0.2 mm (256 × 256; zoom 4). Time-series scanning (60 frames) at a fixed plane was performed with a 12 μs dwell time at 3 frames per second (f.p.s.). Fluorescence measurements in a ROI were monitored to confirm a stable signal; samples with a drifting baseline (due to photobleaching of dye) were discarded. After imaging approximately 30 min to establish stable baselines, the adenine nucleotide translocase inhibitor carboxyatractyloside (10 μM) was added to perfusion buffer for the remainder of the imaging. Then, 40 min later, cells were treated with 10 μM of the ionophore carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) and imaging was continued for an additional 30 min to fully depolarize the mitochondria and obtain the minimum TMRM signal. Four to five ROIs in the cell body and one ROI in the nucleus were monitored in a typical experiment, and changes in TMRM fluorescence were plotted.
2PLSM: ex vivo ATP/ADP measurements
Fluorescence was measured using 2PLSM as described above. The genetically encoded ATP/ADP sensor PercevalHR was expressed by mesencephalic stereotaxic injection of an AAV9 vector carrying the expression construct under the control of the TH promoter fragment. Then, 10 d later, mice were euthanized and ex vivo brain slices were prepared as described above. To estimate the ATP/ADP ratio using PercevalHR, the probe was excited with 950 nm and 820 nm light in rapid succession18. Green channel (490–560 nm) fluorescent emission signals for both wavelengths were detected using a non-descanned Hamamatsu H7422P-40 select GaAsP PMT. Two time series of 5 frames (rate of 3–4 f.p.s., 0.195 × 0.195 mm pixels and 12 μs px−1 dwell time) were acquired for each wavelength. Time series were analysed offline using FIJI. Multiple cytosolic ROIs and a background ROI were measured, the background was subtracted, and the 950/820 ratio was calculated for each ROI at each time point. The contribution of mitochondria to the bioenergetic status of each cell (the OXPHOS index) was estimated by comparing the drop in the PercevalHR ATP/ADP ratio induced by bath application of oligomycin (10 μM) with the drop in the ratio after superfusion with a modified aCSF with oligomycin (10 μM) and glucose replaced with the non-hydrolyzable 2-deoxy-glucose (3.5 mM).
Electrophysiology, 2PLSM Ca2+ imaging and laser uncaging
Conventional tight-seal (>2 GΩ) patch-clamp recordings were made from SN dopaminergic neurons10. To identify neurons in older cNdufs2−/− mice, an AAV9 vector carrying a TH-FusionRed expression construct was injected into the SN 10 d before experiments. For most recordings, electrophysiological signals were filtered at 1–4 kHz and digitized at 10 kHz using a Digidata 1400 (Molecular Devices). For Ca2+ imaging experiments, a Prairie Ultima system running Prarie View v.5.3 (Bruker Nano Fluorescence) was used. For both cell-attached and whole-cell current-clamp recordings, the patch electrode solution contained 135 mM KMeSO4, 5 mM KCl, 5 mM HEPES, 0.05 mM EGTA, 10 mM Na2PCr (phosphocreatine disodium), 2 mM ATP-Mg and 0.5 mM GTP-Na, the pH adjusted to 7.3 (290–300 mOsm l−1). For Ca2+ imaging, EGTA was replaced with Fura-2 (100 μM). The liquid junction potential in our recording aCSF was ~7 mV and was not corrected. Synaptic blockers (10 μM DNQX, 50 μM D-AP5, 10 μM GABAzine, 1 μM CGP55845) were bath-applied for all recordings except for glutamate uncaging. Ca2+ imaging was performed with 2PLSM as previously described10. Fura-2 was excited with a 780 nm light. [Ca2+] was estimated using the equation: [Ca2+] = Kd[1 – f(t)/fmax]/[f(t)/fmax – 1/Rf], where Kd is the dissociation constant, Rf is the indicator’s dynamic range, and Fmax is the maximum fluorescence. The three parameters of the equation (Kd, Rf and fmax) were estimated as previously described10. Glutamate uncaging experiments were performed by superfusing MNI-glutamate (5 mM; Tocris, Cookson) over the slice using a minipump (P720 peristaltic pump, INSTECH). Glutamate was uncaged near a dendrite 10–20 μm from the soma using 3 pulses of 20 ms of 720 nm light; pulses were typically 10–20 mW at the sample plane. The 720 nm sample average power for photolysis was tuned via a third Pockels’ cell modulator (M350–50-02-BK, Conoptics).
2PLSM: DA release with dLight1.3b
Mice were stereotaxically injected with 250 nl of each AAV9-syn-dLight1.3b and AAV9-TH-ERtdTomato into the SNc at P20 or P50. As the endoplasmic reticulum (ER) is difficult to visualize in axons by light microscopy, mice were injected with AAV-TH-FusionRed for striatum DA release. Then, 10 d after surgery, sagittal (striatal) or coronal (SN) slices were prepared as previously described. For AADC experiments in the striatum (Extended Data Fig. 10), P60–P70 mice were injected with 810 nl of AAV9-CAG-dLight1.3b into the striatum. Then, 40 d after surgery, coronal slices were prepared as previously described.
dLight imaging was performed with 920 nm light using the 2PLSM system, as defined above. Both the green and red fluorescence channels were collected. dLight samples were imaged with 100 μm diameter field of view (continuous spiral scan with 95% duty cycle using 84,267 px, 5.2 μs pixel dwell time at 2.3 f.p.s. (SNc) and subsequently with a 16-px-wide line profile with a resolution of 3.4 ms or better (striatum) or imaged with 50 μm square imaging (32 × 32 px, 11.4 μs dwell time at 40 f.p.s.)). SNc analysis used tdTomato-channel-defined ROIs, which were then applied to the dLight green channel images. All ROI signals were summed per frame. The striatum line profile data (outside of the circle towards the inside of the circle) has 220 ms of prestimulation baseline (f0, with laser blocked background counts subtracted). The time-series plot displays Δf/f0. For square xy raster scan, the fluorescence over the entire field (50 × 50 μm) was measured for each frame and plotted against time. DA release was evoked by electrical stimulation (350 μA, 2 ms pulse width, 1P (striatum) or 5P (SN) 100 Hz). To amplify the signal in the SN, slices were preincubated for 1 h in 100 μM levodopa with 1.7 mM sodium ascorbate to prevent oxidation. FIJI was used for image analysis.
DA release measured using fast-scan cyclic voltammetry
Custom-built carbon fibre microelectrodes (80–100 μm exposed carbon fibre in silica) were inserted 100 μm into the dorsolateral striatum of sagittal brain slices, opposite a pronged stimulation electrode. A cyclic waveform sufficient to oxidize and reduce DA (−0.3 to 1.4 V, 10 Hz, 400 V s−1) was applied to the carbon fibre microelectrode (Dagan ChemCLAMP) amplifier/headstage driven by the Axon Digidata 1440A system). DA release was elicited through electrical stimulation (single pulse, 350 μA, 2 ms, 4 min interstimulus interval) and measured as the current generated at the surface of the carbon fibre microelectrode. For levodopa experiments, cNdufs2−/− mice were injected 1 h before euthanasia with 6 mg kg−1 levodopa + 12 mg kg−1 benserazide. Data were analysed using custom software that is available at GitHub (https://github.com/surmeierlab/imagej_macros )42.
Behavioural tests
Mice were habituated to the dimly lit and sound-proofed testing room for 60 min before the start of each assay.
Open-field test
Locomotor activity in the open field (56 cm × 56 cm box) was monitored during a 5 min test session using LimeLight software (ACTIMETRICS). The total distance travelled, number of pauses and speed were measured. For levodopa experiments, wild-type mice were injected with saline and cNdufs2−/− mice were injected with 1.5 mg kg−1, 3 mg kg−1 or 12 mg kg−1 levodopa and 12 mg kg−1 benserazide 45 min before test.
Gait analysis
The ambulatory gait of mice was quantified using the DigiGait Imaging System (Mouse Specifics). A video camera mounted below a motorized transparent treadmill belt captures the ventral side of each mouse while walking. DigiGait software generates digital paw prints and dynamic gait signals, representing the temporal record of paw placement relative to the treadmill belt. Numerous postural and kinematic metrics of gait dynamics were determined by dissecting the time that each limb spent in various portions of the walking phase. After a brief acclimation period, mice were placed in the arena and the treadmill was started. The belt speed was kept between 5 cm s−1 and 80 cm s−1. Speed for analysis was 17 cm s−1. A testing session lasted for approximately 5 min to collect several bouts (4 s) of gait that were interspersed with periods of rest. Mouse tails were coloured with a black Sharpie marker to improve contrast for automated analysis.
Rearing test
Mice were placed in a cylinder (600 ml glass beaker) and filmed for 5 min. The number of times mice reared and the time taken to transition between stances was measured in a 3 min interval.
Adhesive removal test
On day 1, mice were placed in the 500 ml beaker for a habituation period of 30 min. On day 2, two adhesive tape strips were applied on each animal paw with equal pressure. The total time to remove the adhesive tape for both paws was measured as the mouse felt the tape (mouse bringing its paws to its mouth). Maximum duration given to the mouse to remove the two adhesives was 300 s (ref. 43).
Y maze
To motivate mice, they were food-deprived overnight and then maintained on a restricted diet (0.7 g chow per mouse per day) for the duration of the experiment (8 d). Body weight was monitored daily to ensure that mice did not fall below 90% initial body weight. Mice were introduced into a Y-shaped arena (45-cm-long arms with a width of 3.2 cm at the bottom and 13.5 cm at the top; the side walls have a width of 18 cm). The centre region is a triangular space with 3.2-cm-long sides) and the trial began when the animal exited the start arm. On day 1, each mouse was placed in the maze for 5 min for exploration and then removed back into its home cage. During the training period (day 2 to day 6), 3 trials per mouse per day were performed. Ethanol 70% was used to clean the floor of the maze between uses in order to eliminate any olfactory cues present in the platform. Also, the floor of the maze was covered with sawdust, which was mixed after each individual trial to equate differential olfactory stimuli. Trials were spaced at 20 min apart. Median values of correct choices were calculated from 3 trials over 3 d per mouse. The levodopa experiment was performed on days 7 and 8. For levodopa experiments, the mice were injected 45 min before experiments with 6 mg kg−1 levodopa + 12 mg kg−1 benserazide. Three trials per mouse per day were performed and the percentage of correct choices was quantified. Mice were given 5 min to make a choice. There were three possibilities: (1) they went to the food-containing arm; (2) they went to the empty arm; or (3) they stayed at the choice point of the Y. Choices 2 and 3 were counted as incorrect. Dyskinesia was not observed at any of the levodopa doses used in behaviour experiments.
Fluorogold and transmission electron microscopy
Mice were anaesthetized, positioned onto stereotaxic apparatus and injected with 300 nl of 1% Fluorogold (Santa Cruz) dissolved in saline at a rate of 0.1 μl min−1 into the striatum at the following coordinates: 0.5 mm anterior and 1.8 mm lateral to bregma, at a depth of 3 mm from dura. Then, 5 d later, mice were perfused at room temperature with 0.9% saline, followed by a fixative containing 2% paraformaldehyde, 1.25% glutaraldehyde and 0.1 M phosphate buffer, pH 7.3. Brain slices (thickness, 300 μm) were visualized under the fluorescence microscope to localize areas containing cells labelled with retrogradly transported Flurogold. Dissected areas were then postfixed in buffered 2% OsO4, rinsed and stained in 1% uranyl acetate, dehydrated and embedded in EMBed 812 (EMS, 14120). Ultrathin sections (90 nm) were contrasted with lead citrate and uranyl acetate, and analysed under a JEOL 1230 transmission electron microscope operated at 80 kV with a Gatan Orius CCD camera with the Digital Micrograph software. Neurons displaying electron-dense lysosome-like structures44 were used for further analysis. The nuclei, cytoplasm and mitochondria were manually outlined and measured using FIJI (v.2.0.0). For analysis, mitochondria were classified as abnormal when they appeared swollen and cristae integrity was compromised. For each cell, the percentage of abnormal mitochondria was calculated as the ratio between the area occupied by abnormal mitochondria over the total area occupied by mitochondria.
Immunofluorescence
Mice were perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were dissected and serial coronal sections (80 μm) were cut throughout the striatum and the midbrain. For fluorescence immunohistochemistry, mouse monoclonal anti-TH antibodies (Immunostar, 22941) were applied to sections at a dilution of 1:1,000 at 4 °C for 24 h. After rinsing with PBS, the sections were incubated with goat anti-mouse Alexa 594 secondary antibodies (Thermo Fisher Scientific, A-11005) at 1:400 dilution for 30 min at room temperature. Sections were mounted with Vectashield (Vector Laboratories, H-1000–10). Confocal fluorescence microscopy images were acquired using a laser-scanning confocal microscope (FV10i; Olympus) using ×10/0.4 NA (dry) and ×60/1.35 NA (oil) objectives. The obtained images were analysed using Imaris v.8.2. TH immunoreactive neurons with detected nuclei were counted from three representative levels of SNc and VTA using the spots function of Imaris. For TH fibre density, the Baseline Subtraction function of Imaris was used to separate the signal from the background (the measured values were corrected for non-specific background by subtracting values obtained from the corpus callosum) and the signal was then measured using the Surface function of Imaris. Counts of Fluorogold-positive cells were irrespective of cellular morphology using the Spots function of Imaris.
Confocal imaging
Coronal or sagittal slices (80–100 μm) were mounted onto glass slides. Images were acquired on a confocal microscope (FV 10i, Olympus), using the Metamorph software (MetaMorph). Images were adjusted in ImageJ (US National Institutes of Health) for brightness, contrast and pseudocolouring.
RiboTag profiling
Myc-DDK-tagged mouse ribosomal protein L22 like 1 (Rpl22l1) cDNA was purchased from Origene (MR200606 representing NM_026517) and packaged into AAV9-EF1a-DIO-Rpl22l1-Myc-DDK-2A-tdTomato-WPRE by Virovek with titres of 2.24 × 1013 viral genomes per ml. AAV viral vector carrying RiboTag was stereotaxically injected into the SNc in Dat-cre mice and cNdufs2−/− mice. Four weeks after the injection, the mice were euthanized and the SNc tissue expressing RiboTag was dissected and placed at −80 °C. RiboTag immunoprecipitation was performed as described previously45. In brief, tissue was homogenized in cold homogenization buffer (50 mM Tris (pH 7.4), 100 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol, 100 μg ml−1 cycloheximide, protease inhibitors and recombinant RNase inhibitors, and 1% NP-40). Homogenates were centrifuged 10,000g for 10 min, and the resulting supernatant was precleared with protein G magnetic beads (Thermo Fisher Scientific) for 1 h at 4 °C with constant rotation. Immunoprecipitations were carried out with anti-Flag magnetic beads (Sigma-Aldrich) at 4 °C overnight with constant rotation. Four washes were carried out with high-salt buffer (50 mM Tris (pH 7.4), 350 mM KCl, 10 mM MgCl2, 1% NP-40, 1 mM dithiothreitol and 100 μg ml−1 cycloheximide). RNA extraction was performed using the RNA-easy Micro RNA extraction kit (QIAGEN) according to the manufacturer instructions.
RT–qPCR
RNA was isolated from the SNc tissue collected by microdissection of the injected area using the RNAeasy mini kit (QIAGEN). Synthesis of complementary DNA was performed using the Superscript IV VILO Master Mix (Applied Biosystems). Each cDNA was preamplified for 10 cycles using the TaqMan PreAmp Master Mix and a pool of TaqMan Gene Expression Assays (Applied Biosystems). The resulting product was diluted and then used for PCR with the corresponding TaqMan Gene Expression Assay and TaqMan Fast Advanced Master Mix. Data were normalized to Hprt using the comparative Ct (2−ΔΔCt) method. The following Taqman probes were used for PCR amplification of the Hprt (Mm01318746_g1), Cav1.3 (Mm00551392_m1) Hcn2 (Mm00468538_m1) and Ndufs2 (custom made; Up, GTCTGGGATCCGCTTGCCAA; Low, GGGTACATGACAGCTCCCGAA; Probe, CTGCCACTGCCGAGCACCT) genes. Experimental Ct values were normalized to Hprt Ct values using the formula: ΔCt = Ct(Ndufs2) – Ct(Hprt). The final expression levels were shown as ΔCt values.
RNA-seq analysis
The quality of reads, in FASTQ format, was evaluated using FastQC. Reads were trimmed to remove Illumina adapters from the 3′ ends using cutadapt46. Trimmed reads were aligned to the Mus musculus genome (mm10) using STAR47. Read counts for each gene were calculated using htseq-count48 in conjunction with a gene annotation file for mm10 obtained from Ensembl (http://useast.ensembl.org/index.html). Normalization and differential expression were calculated using DESeq2, which uses the Wald test49. The cut-off for determining significantly differentially expressed genes was a false-discovery-rate-adjusted P value of less than 0.05 using the Benjamini–Hochberg method. Metascape (http://metascape.org)50, specifically the Express Analysis module, was used to generate Extended Data Figs. 2 and 3.
HPLC–MS methods
Method for sample reconstitution.
Extraction solution was dried using SpeedVac. 60% acetonitrile was added to the tube for reconstitution following by vortexing for 30 s. Sample solution was then centrifuged for 30 min at 20,000g and 4 °C. The supernatant was collected for LC–MS analysis.
Method for metabolomics.
Samples were analysed using high-performance liquid chromatography (HPLC) and high-resolution mass spectrometry and tandem mass spectrometry. Specifically, the system consisted of a Thermo Q-Exactive system in line with an electrospray source and an Ultimate3000 (Thermo Fisher Scientific) series HPLC system consisting of a binary pump, degasser and auto-sampler outfitted with an Xbridge Amide column (Waters; dimensions, 3.0 mm × 100 mm and a particle size of 3.5 μm). The mobile phase A contained 95% (v/v) water, 5% (v/v) acetonitrile, 10 mM ammonium hydroxide, 10 mM ammonium acetate, pH 9.0; B was 100% acetonitrile. The gradient was as follows: 0 min, 15% A; 2.5 min, 30% A; 7 min, 43% A; 16 min, 62% A; 16.1–18 min, 75% A; 18–25 min, 15% A with a flow rate of 150 μl min−1. The capillary of the electrospray ionization source was set to 275 °C, with sheath gas at 35 arbitrary units, auxiliary gas at 5 arbitrary units and the spray voltage at 4.0 kV. In positive/negative polarity switching mode, an m/z scan range from 60 to 900 was chosen and MS1 data were collected at a resolution of 70,000. The automatic gain control target was set at 1 × 106 and the maximum injection time was 200 ms. The top five precursor ions were subsequently fragmented, in a data-dependent manner, using the higher energy collisional dissociation cell set to 30% normalized collision energy in MS2 at a resolution power of 17,500. In addition to matching m/z, metabolites were identified by matching either the retention time with analytical standards and/or the MS2 fragmentation pattern.
Method for absolute quantification of DA.
After reconstitution in 60% acetonitrile spiked with internal standards, samples were analysed using HPLC and triple-quadruple mass spectrometry and tandem mass spectrometry. Specifically, the system consisted of a TSQ system (Thermo Fisher Scientific) in line with an electrospray source and a Vanquish (Thermo Fisher Scientific) UHPLC consisting of a binary pump, degasser and auto-sampler outfitted with a XBridge Amide column (Waters; dimensions, 3.0 mm × 50 mm and a particle size of 3.5 μm). The mobile phase A contained 95% (v/v) water, 5% (v/v) acetonitrile, 10 mM ammonium hydroxide, 10 mM ammonium acetate, pH 9.0; B was 100% acetonitrile. The gradient was as follows: 0 min, 15% A; 3 min, 45% A; 10 min, 60% A; 10.1–11 min, 75% A; 11.1 min, 15% A; 11.1–15 min, 15% A with a flow rate of 150 μl min−1. In positive/negative polarity switching mode, the capillary of the electrospray ionization source was set to 300 °C, with sheath gas at 35 arbitrary units, auxiliary gas at 5 arbitrary units and the spray voltage at 3.5 kV. The selective reaction of the protonated precursor ion and the related product ions were monitored. The transitions are listed as follows: dopamine (+) 154→137, dopamine_D4(+) 158→141. The peak area was integrated. Data acquisition and analysis were carried out using Xcalibur v.4.1 and Tracefinder v.4.1, respectively (both from Thermo Fisher Scientific).
Stereological estimation of dopaminergic neurons.
Mice were perfused through the aorta, first with 0.9% NaCl and then with 4% paraformaldehyde in 0.1 M phosphate buffer. The brains were removed and cryoprotected in 30% sucrose/PBS overnight at 4 °C. Serial coronal sections (30 μm) were cut throughout the midbrain and processed for neuronal nuclei (NeuN) immunohistochemistry. After overnight incubation of free-floating sections with primary rabbit anti-NeuN antibodies (1:400 dilution, EMD Millipore, ABN78) at 4 °C, the sections were incubated with goat anti-mouse Alexa 594 secondary antibodies (Thermo Fisher Scientific, A-11005) at 1:400 dilution for 30 min at room temperature. The sections were mounted with Vectashield (Vector Laboratories, H-1000).
Estimates of the number of TH-positive cells in the SNc and VTA were obtained using the optical fractionator method and unbiased counting rules51. The borders of the SNc at all levels in the rostrocaudal axis were defined according to Ilijic52. In brief, the medial border was defined by a vertical line passing through the medial tip of the cerebral peduncle and by the medial terminal nucleus of the accessory nucleus of the optic tract, when present in sections, thereby excluding the TH-positive cells in the VTA. The ventral border excluded the TH-positive cells in the SN pars reticulata. Sampling was performed using the Zeiss Axioplan 2 Imaging microscope (Cals Zeiss MicroImaging) with assistance from a computerized stereology system (Stereo Investigator, MicroBrightField). From each animal, the SNc and VTA were delineated at ×4 magnification in every sixth section with counting areas of 135 μm × 150 μm. The counting frame (50 μm × 50 μm) was placed randomly on the first counting area and systematically moved through all of the fields. The estimate of the total number of neurons was calculated according to the optical dissector formula (for more details, see ref. 53). The coefficient of error was calculated according to Gundersen and Jensen54, and values of 0.10 were accepted. NeuN-positive cells were counted using a ×63/1.4 NA objective only when the nucleus could be identified.
Data analysis
Data analysis was performed using Clampfit 10.3 (Molecular Devices), Igor Pro 5.0 (WaveMetrics), GraphPad Prism 8.0 (GraphPad Software), Metascape or FIJI. Statistical analyses were performed using GraphPad Prism 8. Data are presented as box-and-whisker plots depicting the median, quartiles and range. Datasets were not assumed to be normal and were therefore analysed using nonparametric statistics (Mann–Whitney and Kruskal–Wallis tests with Dunn’s post hoc analyses for comparison of multiple groups). For DigiGait data, normality was assessed using the Shapiro–Wilk test and P values were calculated using one-way ANOVA followed by Tukey’s post hoc test. The probability threshold for statistical significance was P < 0.05. n indicates the number of cells, N indicates the number of mice. Reproducibility: all key experiments were independently reproduced by different co-authors. Each experiment was performed multiple times across multiple mice as described in the figure legends. All of the findings in this manuscript were replicated across animals/brain slices/cells, and data were pooled for analysis and presentation. Representative images/traces and statistical analysis were based on at least four replicates.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
RNA-seq reads have been deposited at the NCBI Sequence Read Archive under BioProject accession number PRJNA752682. DA release data measured using fast-scan cyclic voltammetry were analysed using custom software that is available at GitHub (https://github.com/surmeierlab/imagej_macros)42. The mouse model will be made available on request. All data generated or analysed in this study are included in this published Article and the Supplementary Information). Source data are provided with this paper.
Extended Data
Extended Data Fig. 1 |. Metabolic remodeling in cNdufs2−/− mice.
a, b, Ndufs2 was ablated specifically in dopaminergic neurons by selective breeding of mice expressing Cre under the control of the dopamine transporter (DAT) promoter with mice containing a floxed allele of the Ndufs2 gene. c, Cartoon representing the activity of the ETC (electron transport chain) and ANT following Ndufs2 deletion. d, Electron micrographs of SN DA neurons. The nucleus and the mitochondria are highlighted in green and red, respectively. Scale bar: 1μm. e, Box plots showing no differences in mitochondrial density (wildtype, n=21; cNdufs2−/−, n=21) where n is the number of cells. f, Box plots showing mitochondrial morphology in wildtype and MCI-Park SN neurons (wildtype, n=21; cNdufs2−/−, n=21). Insets: representative electron micrographs of mitochondria showing intact (top) or abnormal (bottom) morphology (wildtype, n=21; cNdufs2−/−, n=21). The percentage of abnormal mitochondria was calculated as the ratio between the area occupied by abnormal mitochondria over the total area occupied by mitochondria for each cell. Scale bar: 0.2μm. g, The schematic on top is a sagittal view of the brain, and the red line indicates the position at 3.52mm from bregma, which is shown as a coronal section in the bottom panel (modified from Allen Mouse Brain Atlas, online version 1, 2008). h, Box plots indicate that OXPHOS index (OXPHOS/ (OXPHOS + glycolysis)) is lower in Ndufs2 deficient neurons (wildtype, n=5; cNdufs2−/−, n=7). i, Confocal image of dopaminergic terminals in wildtype mice expressing PercevalHR in ex vivo brain slice at P40. Scale bar: 20μm. j, Box plots show decrease in the OXPHOS index in dopaminergic terminals of cNdufs2−/− mice (wildtype, n=4; cNdufs2−/−, n=5). Wildtype (grey); cNdufs2−/− (black). Two-tailed Mann-Whitney test; (e), (f), (h), (j). For the boxplots, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range. *P < 0.05; ****P < 0.0001.
Extended Data Fig. 2 |. Bar graph of enrichment analysis in MCI-Park mice.
Bar graph for viewing top 20 enrichment clusters, one per cluster, using a discrete color scale to represent statistical significance (Metascape). Wildtype, N=5; cNdufs2−/−, N=6 where N is the number of mice. a, Enriched Ontologycluster analysis of down-regulated genes. b, Gene Ontology biological processes analysis of down-regulated genes. c, Enriched Ontology cluster analysis of up-regulated genes. d, Gene Ontology biological processes analysis of up-regulated genes.
Extended Data Fig. 3 |. Enrichment network visualization in MCI-Park mice.
Network of enriched terms colored by cluster identity, where nodes that share the same cluster identity are typically close to each other (Metascape). Wildtype, N=5; cNdufs2−/−, N=6 where N is the number of mice. a, Enrichment network visualization in down-regulated genes in MCI-Park mice. b, Enrichment network visualization in up-regulated genes in MCI-Park mice.
Extended Data Fig. 4 |. TH expression in SNc and VTA dopaminergic neurons in wildtype and cNdufs2−/− mice.
a, Quantification of TH expression in SNc dopaminergic neurons at P30 and P60 (wildtype, N=5; cNdufs2−/−, N=5) where N is the number of mice. b, Quantification of TH expression in VTA dopaminergic neurons at P30 and P60 (wildtype, N =5; cNdufs2−/−, N =4). c, Representative images showing TH-IR in VTA and SN dopaminergic neurons in wildtype mouse at P60. Scale bar: 200μm. d, Magnified VTA region showing dopaminergic neurons in wildtype at P60. Scale bar: 15μm. e, Representative images showing TH-IR in VTA and SN dopaminergic neurons in cNdufs2−/−mouse at P60. Scale bar: 200μm. f, Magnified VTA region showing dopaminergic neurons in cNdufs2−/−mouse at P60. Scale bar: 15μm. Wildtype (grey); cNdufs2−/− (black). Two-tailed Mann-Whitney test (a) and (b). For the boxplots, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range. *P < 0.05; **P < 0.01.
Extended Data Fig. 5 |. Dopamine release is reduced in cNdufs2−/− mice.
Representative images from SN in P30 wildtype (a) and cNdufs2−/− (b) mice are shown. Scale =5μm. Representative images from dorsal striatum in wildtype (c) and cNdufs2−/− (d) mice are shown. Scale= 5μm (wildtype, N=5; cNdufs2−/−, N=5) where N is number of mice. Note that at P30, the TH promoter (not TH expression) was effectively driving the expression of a fluorescent reporter (ERtdTomato) in dopaminergic neurons, despite the down-regulation in TH expression in the dorsolateral striatum (shown in Fig. 2c). e–g, Dopamine release was measured by fast-scan cyclic voltammetry in wildtype and cNdufs2−/− mice at P20. Representative colorplots (e) and traces (f) show dramatic reduction in evoked (1p, 350 nA, 2 ms) release in dorsal striatum of cNdufs2−/− mice. Scale: vertical =0.5 μM dopamine, horizontal = 1s. g, Summary data demonstrate dopamine release is significantly decreased by P20 (wildtype, N= 12, cNdufs2−/−, N= 4). Striatal dopamine release measured with dLight1.3b at P30 (h, j, l) and P60 (i, k, m). Traces are ΔF/F0 over time. Solid lines represent median trace, shaded area is 95% CI. Scale bars: (h, i), vertical =200 % ΔF/F0, horizontal= 500ms. Quantification of dopamine release at P30 (j, l) and P60 (k, m) in dorsal striatum; striatal dLight1.3b responses were analyzed either by defining 16-pixel-wide line profiles, which provided high temporal resolution measurements of selected regions (j,k), or by averaging the entire field of view, with lower temporal resolution but broader sampling area (l, m). (j, wildtype N=5; cNdufs2−/− N=5; k, wildtype N=6; cNdufs2−/− N=6; l, wildtype N=4; cNdufs2−/− N=5; m, wildtype N=9; cNdufs2−/− N= 4). Wildtype (grey); cNdufs2−/− (black). Two-tailed Mann-Whitney test: (g), (j), (k), (l) and (m). For the boxplots, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range. *P < 0.05, ****P ≤ 0.0001.
Extended Data Fig. 6 |. Physiology remodeling in cNdufs2−/− mice.
Dopamine (a), DOPAC (b), serotonin (c), and acetylcholine (d) separated from wildtype and cNdufs2−/− striatum tissue lysate (P30 and P60, wildtype, N=4, cNdufs2−/−, N=4) where N is the number of mice. Note that elevation of striatal serotonin was detected at P120. This is a common feature of rodent PD models55. e, Heat maps illustrating the remodeling of ion channels in cNdufs2−/− mice; repeated samples are grouped horizontally (wildtype, N=5; cNdufs2−/−, N=6). f, qPCR analysis of RiboTag harvested mRNA showing a drop in hcn2 mRNA in cNdufs2−/− neurons (wildtype, N=4; cNdufs2−/−, N=4). g, Whole-cell somatic recording showing hyperpolarization-activated, cyclic nucleotide-gated currents from a wildtype and cNdufs2−/− neuron at P30. Scale bars: 100pA, 200ms. h, Cumulative probability plot of peak current from wildtype and cNdufs2−/− SN dopaminergic neurons (wildtype, n=12; cNdufs2−/−, n=10) where n is the number of cells. i, qPCR analysis of RiboTag harvested mRNA showing a drop in Cav1.3 mRNA in cNdufs2−/− SN dopaminergic neurons (wildtype, N=4; cNdufs2−/−, N=4). j, Cumulative probability plot of peak [Ca2+] at proximal dendrite (wildtype, n=8; cNdufs2−/−, n=6). k, Whole-cell somatic recordings showing the response to glutamate uncaging in wildtype (left) and cNdufs2−/− (right) mice. Scale bars: 20mV, 1s. Representative SN DA neuron filled with Alexa Flour 594 showing the location for uncaging in blue. Scale bar: 20μm. l, Spikes/burst - peak spiking rate plot showing the difference in response to uncaged glutamate between wildtype (n=5) and cNdufs2−/− (n=5). m, Representative traces showing spike width in SN neurons from wildtype and cNdufs2−/− at P30. n, Box plots indicate AP half width in wildtype and cNduf2−/− at P30 (wildtype n=6; cNdufs2−/−n=7). Wildtype (grey); cNdufs2−/− (black). Two-tailed Mann-Whitney test: (a-d, f and i). One tailed Mann-Whitney test: (h), (j), (l) and (n). For the boxplots, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range. *P < 0.05; **P < 0.01, ***P < 0.001.
Extended Data Fig. 7 |. Physiological characterization of SN dopaminergic neurons in cNdufs2−/− mice at P60.
a, b, Electron micrographs of SN dopaminergic neurons at P60. The nucleus and the mitochondria are highlighted in green and red, respectively. Scale bar: 2μm. c, Box plots showing no differences in mitochondrial density (wildtype, n=14; cNdufs2−/−, n=23) where n is the number of cells. d, Box plots showing abnormal morphology in MCI-Park mitochondria (wildtype, n=14; cNdufs2−/−, n=23). e, Representative images showing normal (top) and abnormal (bottom) mitochondria. The percentage of abnormal mitochondria was calculated as the ratio between the area occupied by abnormal mitochondria over the total area occupied by mitochondria for each cell. Scale bar: 500nm. f, Schematic diagram of injection site (modified from Allen Mouse Brain Atlas, online version 1, 2008). TH-Fusion Red reporter was bilaterally injected into the SN of cNdufs2−/−mouse at P50. Experiments were done at P60 (± 4 days). Representative image showing TH-Fusion Red expression in wildtype (g) and cNdufs2−/− (h) mice at P60. Scale bar: 20μm (wildtype, N=5; cNdufs2−/−, N=5) where N is the number of mice. i, Cell attached recordings from identified wildtype and cNdufs2−/− SN DA neurons at P60. Scale bars: 10pA, 1s. j, Cumulative probability plot of autonomous discharge rates (wildtype n=20; cNdufs2−/− n=25 cells). k, Whole-cell somatic recordings from a cNdufs2−/− SN DA neurons at P60 showing the response to glutamate uncaging. Representative SN DA neuron filled with Alexa Flour 594 is showing the location for uncaging in blue (n=4). Scale bars: 20mV, 2s, 20μm. l, Spikes/burst - peak spiking rate plot showing the difference in response to uncaged glutamate between wildtype (n=4) and cNdufs2−/− (n=5). Wildtype (grey); cNdufs2−/− (black). Two-tailed Mann-Whitney test (c, d). One-tailed Mann-Whitney test (j) and (l). For the boxplots, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range. *P < 0.05.
Extended Data Fig. 8 |. Behavioural phenotypes in cNdufs2−/− mice.
a, Schematic diagram of the experimental protocol for Y maze test. The scoring of 2 and 3 was counted as incorrect. b, Open-field traces in wildtype and cNdufs2−/− mice at P60 and P120 with and without levodopa (3mg/Kg) treatment. c, Representatives traces showing the effect of levodopa treatment on the speed in wildtype (N=5) and cNdufs2−/− (N=5) mice at P120 where N is the number of mice. d, Rearing test performance. e, Number of rearings in a 3-minute period in wildtype, cNdufs2−/− and cNdufs2−/− + 3mg/kg levodopa mice at different ages (P20-P120). Number of rearings begin to be impaired at P40. Levodopa did not rescue this deficit (N=11 per group) f, Rearing time in wildtype, cNdufs2−/− and cNdufs2−/− + 3mg/kg levodopa mice at different ages (P20-P120). At P60, cNdufs2−/− mice show difficulty transitioning between rearing and landing, spending much more time ‘stuck’ in an elevated posture. Levodopa did not rescue this deficit (N=11 per group). (g, h) Body weight was analyzed from P20 to P120. g, Body weight development of wildtype (N=10) and cNdufs2−/− (N=11) mice in males. h, Body weight development of wildtype (N=10) and cNdufs2−/− (N=11) mice in females. Wildtype (gray), cNdufs2−/− (black), cNdufs2−/− plus levodopa (red). One tailed Kruskal-Wallis with Dunn’s correction for multiple comparisons: (e, f). One-way ANOVA followed by Tukey’s post hoc test: (g, h). Data are presented as median and range (shaded area). *P < 0.05; **P < 0.01.
Extended Data Fig. 9 |. Gait analysis in cNdufs2−/− mice.
a, Pictures of wildtype and cNdufs2−/− mice footprints: RF (Right Fore), RH (Right Hind), LF (Left Fore) and LH (Left Hind) in yellow. Body length measurements (b) and hind limb stance width (c) for wildtype (N=7), cNdufs2−/− (N=7) and cNdufs2−/−+levodopa (N=7) where N is the number of mice. d, Representative graph of right hind paw area. Stride length (e) and swing duration (f) for wildtype N=7, cNdufs2−/− N=7 and cNdufs2−/−+levodopa N=7. g, Step sequence for wildtype (N=7), cNdufs2−/− (N=7) and cNdufs2−/−+levodopa (N=7). h, Stance duration for wildtype (N=7), cNdufs2−/− (N=7) and cNdufs2−/−+levodopa (N=7). i, Brake duration for wildtype (N=7), cNdufs2−/− (N=7) and cNdufs2−/−+levodopa (N=7). j, Paw area for wildtype (N=7), cNdufs2−/− (N=7) and cNdufs2−/−+levodopa (N=7). k, Stride frequency for wildtype (N=7), cNdufs2−/− (N=7) and cNdufs2−/−+levodopa (N=8). l, Propulsion time for wildtype (N=7), cNdufs2−/− (N=8) and cNdufs2−/−+levodopa (N=7). m, Gait symmetry for wildtype (N=7) and cNdufs2−/− (N=8) mice. n, Fore limb stance width for wildtype (N=7) and cNdufs2−/− (N=7) mice. o, Box-plots summarizing stereological estimate of the numbers of NeuN-immunopositive neurons in the SNC (o) and VTA (p), wildtype (N=5) and cNdufs2−/− (N=5). q, Representative images showing NeuN immunostaining in the midbrain in wildtype (top panel) and cNdufs2−/− mouse (bottom panel) in P120–150 mice. Scale bar:100μm. Wildtype(grey); cNdufs2−/−(black); cNdufs2−/−+levodopa (red). One-way ANOVA followed by Tukey’s post hoc test: (b), (c), (e–n), two-tailed Mann–Whitney test (o, p). Data from right hind paw: (b), (c), (e–n). Levodopa dosage: 6mg kg−1. For the boxplots, (b), (c), (e), (f), (o) and (p), the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range. (g–n): Solid lines represent median trace shaded area is the range. *P < 0.05; **P < 0.01.
Extended Data Fig. 10 |. SN expression of AADC did not induce DA release in P100 cNdufs2−/− mice even after perfusion with dopamine.
a, Schematic diagram of injection site. AAV2-GFP-AADC was bilaterally injected into the striatum of cNdufs2−/−mouse at P60. Confocal image of coronal slice containing striatum (b) or SN (c) from cNdufs2-/ mouse. Scale bar: 200μm. d, Magnified view of SN showing no expression of AADC-GFP. Scale bar: 20μm. e, Schematic diagram of injection site. AAV2-GFP-AADC was bilaterally injected into the SN of cNdufs2−/−mouse at P60. Confocal image of coronal slice containing striatum (f) or SN (g) from cNdufs2−/−mouse. Scale bar: 200μm. h, Magnified view of SN showing expression of AADC- GFP. Scale bar: 20μm. Wildtype (N=5), cNdufs2−/−(N=5) where N is the number of mice. i, Quantification of dopamine in striatal tissue from wildtype (N=4), cNdufs2−/−+low levodopa (1.5mg/kg) with (N=4) or without AADC (N=4) and cNdufs2−/−+high levodopa (12mg kg−1) (N=4). j, Schematic diagram of the AAV-AADC injection into the SN and AAV-dLight injection into the striatum (P60). k, dLight fluorescence (raw in thin lines and average in thick lines) in response to a single electrical stimulus (350μA, 2ms). l, Summary of dLight responses (wildtype n=16, cNdufs2−/−-AADC n=9, cNdufs2−/−+AADC n=12 where n is the number of slices). m, An example srecording of dLight fluorescence response upon bath application of dopamine (100μM) followed by washout in a cNdufs2−/− mouse injected with AADC in the SN. n-o, Example recordings of dLight fluorescence responses (raw in thin lines and average in thick lines) upon single electrical stimulus after dopamine washout. cNdufs2−/− mouse injected with AADC (n, n=4) or GFP (o, n=4) into the SN. Wildtype (grey); cNdufs2−/− (black); cNdufs2−/−+AADC (red); cNdufs2−/− +high levodopa (blue). (i), two-way ANOVA followed by Tukey’s post hoc test, *P< 0.05; **P< 0.01; (l), one-tailed Kruskal-Wallis with Dunn’s correction for multiple comparisons, ****P<0.0001. Fossr the boxplots, the centre line indicates the median, the box limits indicate the first and third quartiles, and the whiskers indicate the data range. p, Schematic showing the cascade of events following to Ndufs2 deletion in MCI-Park mice. DA (dopamine), DLS (dorsolateral striatum). The schematics in (a), (e) and (j) are modified from the Allen Mouse Brain Atlas, online version 1, 2008 (http://mouse.brain-map.org).
Supplementary Material
Acknowledgements
We thank the staff at the Metabolomic Core Facility at Robert H. Lurie Comprehensive Cancer Center of Northwestern University for HPLC-MS experiments and staff at the NUSeq Core Facility for RNA-seq analysis; D.G. Galtieri for the original scripts in the Surmeier lab GitHub; and G. Yellen for insights on PercevalHR experiments. Electron microscopy tissue processing and imaging was performed at the Northwestern University Center for Advanced Microscopy, supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center. This study was supported by grants from the Michael J. Fox Foundation (to D.J.S.), the JPB Foundation (to D.J.S.), the IDP Foundation (to D.J.S.), the Flanagan Fellowship (to P.G.-R.) and the European Research Council ERC Advanced Grant PRJ201502629 (to J.L.-B.).
Footnotes
Competing interests The authors declare no competing interests.
Additional information
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41586-021-04059-0.
References
- 1.Surmeier DJ, Obeso JA & Halliday GM Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci 18, 101–113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HW et al. Genetic reduction of mitochondrial complex I function does not lead to loss of dopamine neurons in vivo. Neurobiol. Aging 36, 2617–2627 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albin RL, Young AB & Penney JB The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989). [DOI] [PubMed] [Google Scholar]
- 4.Wichmann T.Changing views of the pathophysiology of parkinsonism. Mov. Disord 34, 1130–1143 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Poewe W.et al. Parkinson disease. Nat. Rev. Dis. Primers 3, 17013 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Beilina A.& Cookson MR Genes associated with Parkinson’s disease: regulation of autophagy and beyond. J. Neurochem 139, 91–107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haelterman NA et al. A mitocentric view of Parkinson’s disease. Annu. Rev. Neurosci 37, 137–159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faustini G.et al. Mitochondria and α-synuclein: friends or foes in the pathogenesis of Parkinson’s disease? Genes 8, 377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolam JP & Pissadaki EK Living on the edge with too many mouths to feed: why dopamine neurons die. Mov. Disord 27, 1478–1483 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzman JN et al. Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J. Clin. Invest 128, 2266–2280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Agüera MC et al. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 22, 825–837 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Kordower JH & Burke RE Disease modification for Parkinson’s disease: axonal regeneration and trophic factors. Mov. Disord 33, 678–683 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Sanz E.et al. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl Acad. Sci. USA 106, 13939–13944 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fornasiero EF et al. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun 9, 4230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narendra D, Walker JE & Youle R.Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harb. Perspect. Biol 4, 11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker N, Patel J.& Khacho M.Linking mitochondrial dynamics, cristae remodeling and supercomplex formation: how mitochondrial structure can regulate bioenergetics. Mitochondrion 49, 259–268 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Ekstrand MI et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl Acad. Sci. USA 104, 1325–1330 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tantama M.et al. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat. Commun 4, 2550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patriarchi T.et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, 6396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paladini CA & Roeper J.Generating bursts (and pauses) in the dopamine midbrain neurons. Neuroscience 282, 109–121 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Roeper J.Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 36, 336–342, (2013). [DOI] [PubMed] [Google Scholar]
- 22.Yu H.et al. MinK-related peptide 1: a beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ. Res 88, E84–E87 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Klaus A.et al.What, if, and when to move: basal ganglia circuits and self-paced action initiation. Annu. Rev. Neurosci 42, 459–483 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Baker KA et al. Simultaneous intrastriatal and intranigral dopaminergic grafts in the Parkinsonian rat model: role of the intranigral graft. J. Comp. Neurol 426, 106–116 (2000). [PubMed] [Google Scholar]
- 25.Mukhida K.et al. Enhancement of sensorimotor behavioural recovery in hemiparkinsonian rats with intrastriatal, intranigral, and intrasubthalamic nucleus dopaminergic transplants. J. Neurosci 21, 3521–3530 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson GS & Robertson HA D1 and D2 dopamine agonist synergism: separate sites of action? Trends Pharmacol. Sci 8, 295–299 (1987). [Google Scholar]
- 27.Coune PG, Schneider BL & Aebischer P.Parkinson’s disease: gene therapies. Cold Spring Harb. Perspect. Med 2, a009431 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarre S.et al. Biotransformation of l-DOPA to dopamine in the substantia nigra of freely moving rats: effect of dopamine receptor agonists and antagonists. J. Neurochem 70, 1730–1739 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Diederich NJ et al. Parkinson’s disease: is it a consequence of human brain evolution? Mov. Disord 34, 453–459 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacelli C.et al. Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr. Biol 25, 2349–2360 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Berthet A.et al. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J. Neurosci 34, 14304–14317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collier TJ, Kanaan NM & Kordower JH Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat. Rev. Neurosci 12, 359–366 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kordower JH & Bjorklund A.Trophic factor gene therapy for Parkinson’s disease. Mov. Disord 28, 96–109 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez MC et al. Subthalamic nucleus-mediated excitotoxicity in Parkinson’s disease: a target for neuroprotection. Ann. Neurol 44, S175–S188 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Reyes I. & Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun 11, 102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kordower JH et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136, 2419–2431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovaleski RF et al. Dysregulation of external globus pallidus‐subthalamic nucleus network dynamics in Parkinsonian mice during cortical slow‐wave activity and activation. J. Physiol 598, 1897–1927 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kravitz AV et al. Regulation of Parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerfen CR & Surmeier DJ Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci 34, 441–466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheramy A, Leviel V.& Glowinski J.Dendritic release of dopamine in the substantia nigra. Nature 289, 537–542 (1981). [DOI] [PubMed] [Google Scholar]
- 41.Cáceres-Chávez VA et al. Acute dopamine receptor blockade in substantia nigra pars reticulata: a possible model for drug-induced parkinsonism. J. Neurophysiol 120, 2922–2938 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Galtieri DJ & Estep CM Neurphsy. https://github.com/surmeierlab/neurphys (2014). [Google Scholar]
- 43.Bouet V.et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat. Protoc 4, 1560–1564 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Schmued L.et al. Collateralization and GAD immunoreactivity of descending pallidal efferents. Brain Res. 487, 131–142 (1989) [DOI] [PubMed] [Google Scholar]
- 45.Heiman M.et al. Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nat. Protoc 9, 1282–1291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin M.et al. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J. 17, 10–12 (2011). [Google Scholar]
- 47.Dobin A.et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anders S, Pyl PT & Huber W.HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Love MI et al. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550–558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y.et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun 10, 1523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.West MJ, Slomianka L.& Gundersen HJG, Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec 231, 482–497 (1991). [DOI] [PubMed] [Google Scholar]
- 52.Ilijic E.et al. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis. 43, 364–371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oorschot DE Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J. Comp. Neurol 366, 580–599 (1996). [DOI] [PubMed] [Google Scholar]
- 54.Gundersen HJG & Jensen EB, The efficiency of systematic sampling in stereology and its prediction. J. Microsc 147, 229–263 (1987). [DOI] [PubMed] [Google Scholar]
- 55.Zhou, et al. Serotonergic sprouting is induced by dopamine-lesion in substantia nigra of adult rat brain. Brain Res. 556, 108–116 (1991). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq reads have been deposited at the NCBI Sequence Read Archive under BioProject accession number PRJNA752682. DA release data measured using fast-scan cyclic voltammetry were analysed using custom software that is available at GitHub (https://github.com/surmeierlab/imagej_macros)42. The mouse model will be made available on request. All data generated or analysed in this study are included in this published Article and the Supplementary Information). Source data are provided with this paper.