Abstract
Purpose
To describe a case of local recurrence of uveal melanoma with concomitant brain metastases after secondary enucleation.
Observations
A 73 year-old patient presented with dizziness and gait instability. MRI of the orbits and brain showed an anophthalmic socket with an orbital implant and an associated optic nerve mass as well as multiple mass lesions in the brain. The patient's history was significant for secondary enucleation for uveal melanoma recurrence seven years prior to presentation. Histopathology of the enucleated eye revealed no signs of extrascleral extension or optic nerve invasion. Biopsy of the optic nerve mass confirmed recurrent uveal melanoma with somatic mutations in GNAQ (Q209L) and the telomerase (TERT) promoter (c.1–124C > T) found on targeted next-generation sequencing (NGS). The same mutations were found in the primary tumor in the patient's archived enucleation samples.
Conclusions
Local recurrence of uveal melanoma can occur after enucleation and is associated with an increased risk of systemic metastases. It is important for clinicians to monitor patients for local recurrence and systemic metastases even after enucleation. Genetic biomarkers may play an important role in identifying tumors at highest risk of local recurrence and metastasis. To our knowledge, this is the first case study to describe the TERT promoter mutation c.1–124C > T in the setting of recurrent uveal melanoma.
Keywords: Melanoma, Enucleation, Local recurrence, Orbit, Uveal melanoma
Abbreviations: MRI, Magnetic resonance imaging; CT, Computed tomography; IV, Intravenous
1. Introduction
Uveal melanoma is the most common primary intraocular malignancy in adults, with an overall mean age-adjusted incidence of 5.1 cases per million per year.1 Over 90% of uveal melanoma arises from the choroid.2 If left untreated or inadequately treated, uveal melanoma would invariably lead to death through the development of systemic metastases.3 While the Collaborative Ocular Melanoma Study (COMS) has established iodine-125 plaque brachytherapy as the standard of care for the treatment of medium-sized uveal melanomas,4 enucleation remains the standard of care for large uveal melanomas (>10 mm in apical height and >16 mm in basal diameter), those with poor visual potential and those with extraocular extension.5
However, as demonstrated in COMS, the cumulative 10-year mortality rate from confirmed or suspected melanoma metastasis after enucleation was 21%,4 with the liver being the most frequent site of metastases.6 The rate of local recurrence after enucleation for uveal melanoma is not well documented but may be as low as 1%.7 We report a case of orbital recurrence of uveal melanoma seven years after enucleation associated with a rare activating mutation in the telomerase gene (TERT) promoter. To our knowledge, this is the first case study to describe the TERT promoter mutation c.1–124C > T in the setting of recurrent uveal melanoma.
2. Case report
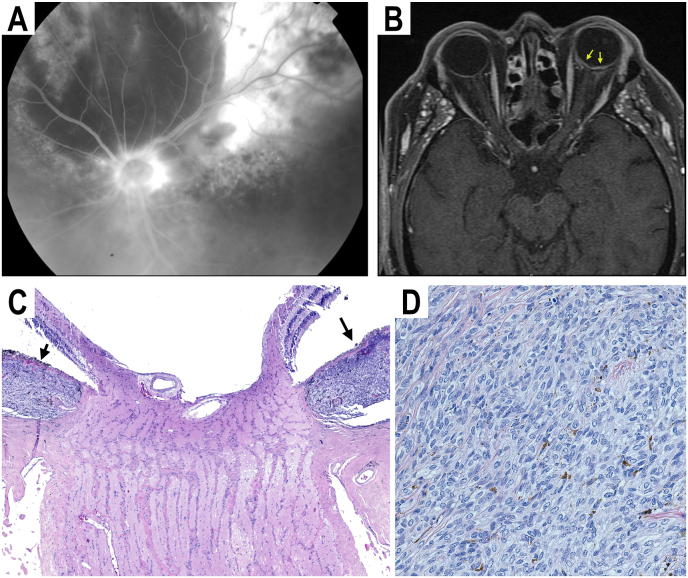
A 73-year-old man with a history of choroidal melanoma in his left eye was treated with four cycles of transpupillary thermotherapy (TTT) with partial regression of the choroidal melanoma. Five months after TTT, he was referred to our institution for growth of the choroidal melanoma. Fundoscopic examination at that time revealed a circumpapillary choroidal melanoma measuring 19 × 16 × 3.5 mm with orange pigment and extensive subretinal fluid (Fig. 1A). MRI of his orbits did not show any extraocular extension of the choroidal melanoma (Fig. 1B). Given the tumor's large size and circumpapillary location, the patient underwent enucleation. On histologic examination of the enucleated eye, a pigmented choroidal melanoma was found to surround the optic disc but did not penetrate the lamina cribrosa (Fig. 1C). There were no signs of extrascleral extension, and the optic nerve margin appeared free of tumor. The tumor was composed of a mixed cell type (Fig. 1D). The patient was lost to follow up after attending his post-operative visit two months after his enucleation, and therefore did not undergo surveillance imaging of his brain and orbits.
Fig. 1.
(A) Fluorescein angiogram image of a large circumpapillary choroidal melanoma (B) T1 axial image of MRI orbits showing the large circumpapillary choroidal melanoma (arrows) without evidence of extraocular extension (C) H&E-stained section (4x magnification) of enucleated left eye showing large choroidal melanoma (arrows) surrounding optic nerve. (D) H&E-stained section (40x magnification) of choroidal melanoma showing both spindle and epithelioid cell types.
Seven years after the enucleation of the patient's left eye, he presented to the emergency department with dizziness and gait instability. CT of his brain showed a high-density mass in the pons and an intermediate-attenuation mass in the suprasellar cistern concerning for metastases. The Ophthalmology service was consulted due to the patient's history of choroidal melanoma. On examination, the patient's best-corrected visual acuity was 20/20 in his right eye with an abduction deficit concerning for a right cranial nerve VI palsy. Anterior segment and dilated fundus exam of the right eye were otherwise unremarkable. The left anophthalmic socket was found to have an orbital implant with healthy overlying conjunctiva and was unremarkable.
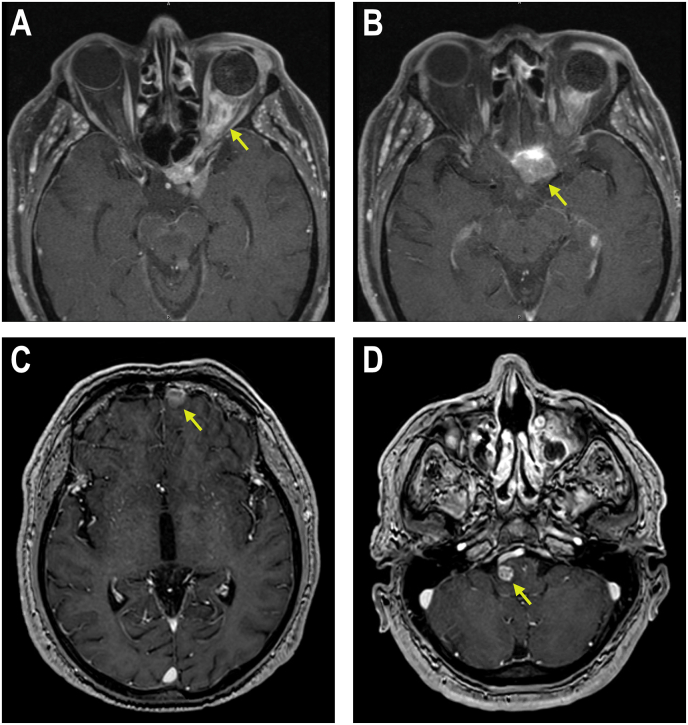
MRI of his orbit showed an infiltrative mass with irregular enhancement encasing the entire left optic nerve sheath, closely apposed to the orbital implant in the left orbit (Fig. 2A). The mass involved the cavernous sinus, extended through the orbital apex and into the sella (Fig. 2B) with abutment of the infundibulum and further extension into the planum sphenoid valley. MRI of his brain showed additional masses in the left frontal lobe (Fig. 2C), the left superior cerebellum and the right side of the pons (Fig. 2D). CT of his chest, abdomen and pelvis with IV contrast did not show any other systemic metastasis.
Fig. 2.
(A) T1 axial image of MRI orbits showing a mass encasing the optic nerve sheath and extending through the orbital apex (arrow). (B) T1 axial image of MRI orbits showing a large mass in the sellar region (arrow) continuous with the optic nerve sheath mass in 2A. (C) T1 axial image of MRI brain showing mass in left frontal lobe (arrow). (D) T1 axial image of MRI brain showing mass in right pons (arrow).
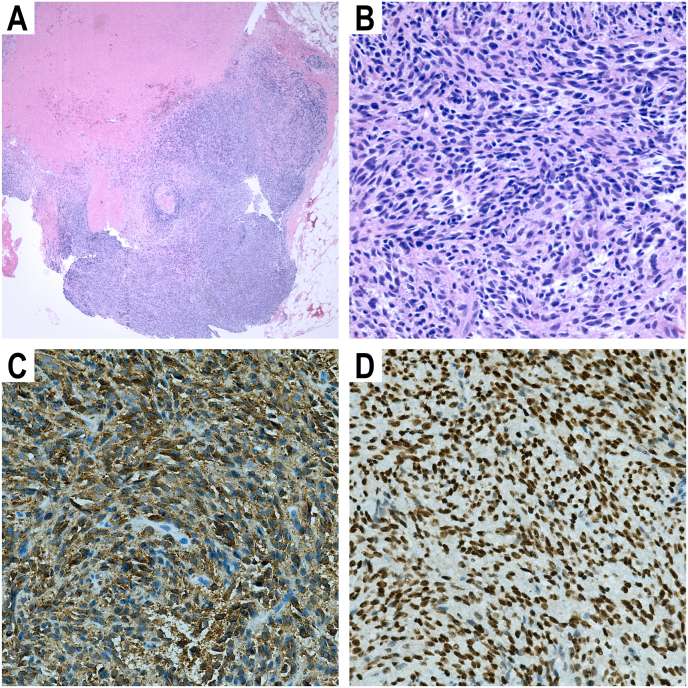
A left orbitotomy via an eyelid crease incision was performed to obtain a biopsy of the optic nerve mass with preservation of the orbital implant (Fig. 3A). Histopathology demonstrated recurrent malignant melanoma of mixed cell type with highly-cellular spindle cell proliferation (Fig. 3B). Tumor cells stained positive for Melan-A (Fig. 3C) and SOX10 (Fig. 3D) on immunohistochemistry, confirming the diagnosis of malignant melanoma. Tumor profiling by targeted next-generation sequencing (NGS) of 146 cancer-related genes (Oncomine Comprehensive Assay Version 3, ThermoFisher Scientific, USA) was significant for the somatic mutations in GNAQ (Q209L, 36% allelic fraction) and the telomerase (TERT) promoter (c.1–124C > T, 72% allelic fraction). There were no other mutations detected in the other genes tested including BAP1 and SF3B1. Profiling of archived enucleation samples by the same targeted NGS assay revealed the same GNAQ Q209L (29% allelic fraction) and TERT c.1–124C > T (31% allelic fraction) mutations in the primary tumor.
Fig. 3.
(A) H&E-stained section (4x magnification) of biopsy sample of left optic nerve mass. (B) H&E-stained section (40x magnification) of biopsy showing both spindle and epithelioid cell types. (C) Positive Melan-A immunohistochemical staining of biopsy sample (40x magnification). (D) Positive SOX10 immunohistochemical staining of biopsy sample (40x magnification).
The patient underwent gamma knife radiation to his brain metastases and fractionated radiation to the left optic nerve and suprasellar space. He was subsequently treated with four cycles of ipilimumab/nivolumab and four cycles of maintenance nivolumab. At last follow-up seven months after presentation, the optic nerve/suprasellar mass remained stable and the brain metastases decreased in size on MRI.
3. Discussion
This case demonstrated local recurrence of a large circumpapillary choroidal melanoma seven years after enucleation with concomitant brain metastases despite no histological evidence of extrascleral extension or optic nerve invasion in the enucleated specimen. Local recurrence of uveal melanoma is a rare event. Starr and Zimmerman reported a 3% local recurrence rate in a study of 1872 cases of ciliary body and choroidal melanoma.8 In a case series by Sanke et al., of 542 patients with choroidal melanoma who underwent enucleation, six patients (1.11%) had local recurrence.7
Local recurrence after enucleation is usually associated with extrascleral extension.7,9,10 In the presence of extrascleral extension, the risk of local recurrence was found to be as high as 23% 5 years after enucleation.11 The presence of extrascleral extension does not seem to correlate with the time from enucleation to local recurrence,7 which can vary widely from 3.5 months7 to 40 years.12 It is worth noting that our patient had no histologic evidence of extrascleral extension or optic nerve invasion at time of enucleation. Similarly, Sanke et al. reported two patients with local recurrence of uveal melanoma after enucleation who did not have histological evidence of extrascleral extension.7 Regardless of the initial treatment modality, local recurrence of uveal melanoma is associated with systemic metastases and mortality.13
It is also important to consider the role of transpupillary thermotherapy in our patient's tumor recurrence. TTT is a form of targeted infrared radiation used as primary treatment for a very select group of small pigmented choroidal melanomas, such as those in close proximity to the fovea or optic disc.14 The risk of local treatment failure with transpupillary thermotherapy is well known. In a systematic review by Chang et al.,15 TTT had the highest rate of local treatment failure among non-surgical, globe-sparing modalities at 20.8%. Furthermore, Shields et al. found that patients requiring more than three TTT sessions and those with circumpapillary tumors, like our patient, were at the greatest risk of local treatment failure.14 Therefore, it is plausible that the initial delay in enucleation by attempting transpupillary thermotherapy (TTT) may have facilitated the microscopic spread of tumor cells in our patient.
Local recurrence of uveal melanoma is most frequently of the mixed cell type,7,9,12 as seen in our patient. The advent of tumor genetic profiling has enabled molecular characterization of uveal melanomas and the potential derivation of insights into their pathophysiology. Like most uveal melanomas, the recurrent choroidal melanoma in this case carried a mutation (GNAQ Q209L) associated with constitutive activation of the Gαq pathway.16 A high allelic fraction of an activating mutation (c.1–124C > T) in the telomerase (TERT) gene promoter was also found. Activating TERT promoter mutations increase telomerase activity by abolishing TERT gene silencing even in differentiated cells.17 This enables continuous cell proliferation without additional gene mutations by maintaining abnormally long telomeres.17 It is plausible that the TERT promoter mutation arose in a subset of melanoma cells in our patient and conferred these cells with an unrestricted proliferative capacity, which allowed them to act as “cancer stem cells”. The increase in allelic fraction of the TERT c.1–124C > T mutation from 31% in the primary tumor to 72% in the recurrent tumor suggests that microscopic spread of tumor cells with the TERT c.1–124C > T mutation to the proximal optic nerve might have occurred prior to enucleation and contributed to the recurrent tumor in our patient. To our knowledge, none of the previous reports on local recurrence of uveal melanoma documented the genetic profile of both the primary and recurrent tumors.
In ocular tissues, TERT promoter mutations are typically associated with conjunctival melanoma18,19 and correlate with an increased risk of metastasis.20 On the other hand, TERT promoter mutations are exceedingly rare in uveal melanoma.18,19,21 We found only two other reported cases of uveal melanoma with TERT promoter mutations19,21 that were not recurrent tumors. Koopmans et al. reported a TERT promoter (250C > T) mutation in 1 out of 102 cases of uveal melanoma (1%) that underwent DNA extraction and analysis.19 In contrast, the authors found TERT promoter mutations in 16 of 39 cases of conjunctival melanoma (41%).19 In a cohort study of 50 cases of uveal melanoma that underwent enucleation, Dono et al. identified a TERT promoter (228C > T) mutation in only one case of uveal melanoma.21 Interestingly, both cases of uveal melanoma described by Koopmans et al.19 and Dono et al.21 had low-risk genetic profiles (no mutations in BAP1 with disomy of chromosome 3) and did not metastasize. Overall, given the rarity of TERT mutations in uveal melanoma, it is difficult to assess the potential effects of TERT mutations on uveal melanoma progression. Additional studies on genetic biomarkers are needed to identify uveal melanomas at high risk of local recurrence and metastasis. Such biomarkers may also offer opportunities for rational therapeutic strategies. For instance, the telomerase inhibitor Imetelstat22 could be used to target uveal melanomas with activating telomerase mutations as in the case of our patient.
4. Conclusions
Local recurrence of uveal melanoma can occur after enucleation and is associated with an increased risk of systemic metastases. It is important for clinicians to monitor patients for local recurrence even after enucleation. Genetic biomarkers may play an important role in identifying tumors at highest risk of local recurrence.
Patient consent
The patient consented to publication of the case in writing.
Funding
Connecticut Lions Eye Research Foundation.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None.
Acknowledgements
The authors thank Dr. Zenta Walther, MD, PhD, for her assistance in performing and analyzing the Oncomine Comprehensive Assay.
References
- 1.Singh A.D., Turell M.E., Topham A.K. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011 Sep 1;118(9):1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Shields C.L., Furuta M., Thangappan A., et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009 Aug 1;127(8):989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 3.Kujala E., Mäkitie T., Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003 Nov;44(11):4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Ocular Melanoma Study Group The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006 Dec;124(12):1684–1693. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- 5.Shields J.A., Shields C.L. Management of posterior uveal melanoma: past, present, and future: the 2014 charles L. Schepens lecture. Ophthalmology. 2015 Feb 1;122(2):414–428. doi: 10.1016/j.ophtha.2014.08.046. [DOI] [PubMed] [Google Scholar]
- 6.The Collaborative Ocular Melanoma Study Group Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the collaborative ocular melanoma study (COMS): COMS report No. 15. Arch Ophthalmol. 2001 May 1;119(5):670–676. doi: 10.1001/archopht.119.5.670. [DOI] [PubMed] [Google Scholar]
- 7.Sanke R.F., Collin J.R., Garner A., Packard R.B. Local recurrence of choroidal malignant melanoma following enucleation. Br J Ophthalmol. 1981 Dec;65(12):846–849. doi: 10.1136/bjo.65.12.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starr H.J., Zimmerman L.E. Extrascleral extension and orbital recurrence of malignant melanomas of the choroid and ciliary body. Int Ophthalmol Clin. 1962 Jun;2(2):369–385. [Google Scholar]
- 9.Allen J.C., Jaeschke W.H. Recurrence of malignant melanoma in an orbit after 28 years. Arch Ophthalmol. 1966 Jul 1;76(1):79–81. doi: 10.1001/archopht.1966.03850010081016. [DOI] [PubMed] [Google Scholar]
- 10.Simonsz H.J., Löffler K. Enlargement of the bony orbit by orbital recurrence of choroidal melanoma 21 years after enucleation. Orbit. 1992 Jan 1;11(1):7–10. [Google Scholar]
- 11.Shammas H.F., Blodi F.C. Orbital extension of choroidal and ciliary body melanomas. Arch Ophthalmol. 1977 Nov 1;95(11):2002. doi: 10.1001/archopht.1977.04450110096010. –5. [DOI] [PubMed] [Google Scholar]
- 12.Menicacci C., Al-Jamal R.T., De Francesco S., et al. Very late orbital recurrence of choroidal melanoma four decades post enucleation. Eur J Ophthalmol. 2021 Mar;11 doi: 10.1177/11206721211001266. [DOI] [PubMed] [Google Scholar]
- 13.Ophthalmic Oncology Task Force Local recurrence significantly increases the risk of metastatic uveal melanoma. Ophthalmology. 2016 Jan;123(1):86–91. doi: 10.1016/j.ophtha.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Shields C.L., Shields J.A., Perez N., Singh A.D., Cater J. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002 Feb;109(2):225–234. doi: 10.1016/s0161-6420(01)00902-2. [DOI] [PubMed] [Google Scholar]
- 15.Chang M.Y., McCannel T.A. Local treatment failure after globe-conserving therapy for choroidal melanoma. Br J Ophthalmol. 2013 Jul;97(7):804–811. doi: 10.1136/bjophthalmol-2012-302490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Raamsdonk C.D., Griewank K.G., Crosby M.B., et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010 Dec 2;363(23):2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiba K., Johnson J.Z., Vogan J.M., Wagner T., Boyle J.M., Hockemeyer D. Cancer-associated TERT promoter mutations abrogate telomerase silencing. de Lange T. Elife. 2015 Jul 21;4 doi: 10.7554/eLife.07918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griewank K.G., Murali R., Schilling B., et al. TERT promoter mutations in ocular melanoma distinguish between conjunctival and uveal tumours. Br J Cancer. 2013 Jul;109(2):497–501. doi: 10.1038/bjc.2013.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopmans A.E., Ober K., Dubbink H.J., et al. Prevalence and implications of TERT promoter mutation in uveal and conjunctival melanoma and in benign and premalignant conjunctival melanocytic lesions. Investig Ophthalmol Vis Sci. 2014 Sep 24;55(9):6024–6030. doi: 10.1167/iovs.14-14901. [DOI] [PubMed] [Google Scholar]
- 20.van Poppelen N.M., van Ipenburg J.A., van den Bosch Q., et al. Molecular genetics of conjunctival melanoma and prognostic value of TERT promoter mutation analysis. Int J Mol Sci. 2021 Jan;22(11):5784. doi: 10.3390/ijms22115784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dono M., Angelini G., Cecconi M., et al. Mutation frequencies of GNAQ, GNA11, BAP1, SF3B1, EIF1AX and TERT in uveal melanoma: detection of an activating mutation in the TERT gene promoter in a single case of uveal melanoma. Br J Cancer. 2014 Feb 18;110(4):1058–1065. doi: 10.1038/bjc.2013.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tefferi A., Lasho T.L., Begna K.H., et al. A pilot study of the telomerase inhibitor Imetelstat for myelofibrosis. N Engl J Med. 2015 Sep 3;373(10):908–919. doi: 10.1056/NEJMoa1310523. [DOI] [PubMed] [Google Scholar]