Abstract
Ischemic stroke frequently causes motor impairments. Despite exercise can improve motor outcomes, many stroke survivors remain life-long disabled. Understanding the mechanisms associated with motor recovery after a stroke is necessary to develop treatments. Here, we show that endogenous DA transmission is required for optimal motor skill recovery following photothrombotic stroke in rats. Blockade of dopamine D1 and D2 receptors impaired the recovery of a forelimb reaching task and decreased the rats’ motivation to complete full training sessions. Our data indicate that dopamine transmission is important to drive motor rehabilitation after stroke through motivational aspects and ultimately suggest that augmented motivation and reward feedback could be an interesting strategy to increase the effectiveness or rehabilitation.
Keywords: Stroke, Motor skill learning, Dopamine, Motivation, Rehabilitation
Ethical statement
An ethical statement certifying that the experiments have been approved by the Swiis Ethics committee and conducted according to the European and Swiss regulations as well as a certification that all efforts were made to reduce the numbers of animals used and their suffering has been added to the Animals and Study design section.
Introduction
As a response to ischemia, the intact neural tissue around the lesion (periinfarct cortex) transforms into a highly plastic environment at the functional and structural level (Carmichael, 2006, Murphy and Corbett, 2009). Training can likely utilize this environment to mediate recovery of function: functional and structural plasticity in the peri-infarct area and contralateral hemisphere are further enhanced by motor training leading to recovery (Biernaskie et al., 2004, Okabe et al., 2016). To date, movement exercises remain the mainstay of rehabilitation therapies to help recovery of walking and arm use. However, full motor recovery is rarely achieved and most of the patients remain disabled (Kwakkel et al., 2003). Therefore, a better understanding of the mechanisms of motor recovery after stroke is important to enhance rehabilitation interventions.
In the healthy, motor skill learning is enabled by structural and functional plasticity in the motor cortex (Luft et al., 2004a, Luft et al., 2004b, Harms et al., 2008, Guo et al., 2015). The motor cortex in rodents (Hosp et al., 2011, Hosp et al., 2015, Vitrac et al., 2014, Sesack et al., 1995), monkeys (Sesack et al., 1995) and humans (Gaspar et al., 1991) receives a dense dopaminergic (DA) innervation originating in the ventral tegmental area (VTA; Hosp et al., 2011, Hosp et al., 2015). Lesioning the DA innervation increased spine turnover in mice (Guo et al., 2015), leading to unstable spines and impaired motor learning in mice and rats (Molina-Luna et al., 2009, Guo et al., 2015, Li et al., 2017). Li et al. (2017) recorded evoked field potentials (ePSPs) in behaving rats with DA lesion in the motor cortex that were trained in a skilled reaching task. They showed that potentiation of ePSPs during a training session was not maintained between two consecutive sessions compared to sham-operated rats trained in the same task. In brain slices, blockade of D1 receptor (D1R) or D2 receptor (D2R) impaired long-term potentiation (LTP) in rats (Molina-Luna et al., 2009) and in mice (Guo et al., 2015). In behaving mice, dopamine concentration increased in the motor cortex during reward expectation and reward consumption (Patriarchi et al., 2018). Blockade of the D1Rs reduced the number of responsive neurons in the motor cortex of mice and increased the latency to initiate behavior after a cue (Chen et al., 2019). These data indicate that motor cortex neurons can respond to motivational signals. Blockade of the D2Rs reduced motor cortex excitability in anesthetized rats (Hosp et al., 2009) and motor cortex activity in behaving rats (Parr-Brownlie and Hyland, 2005). In addition, blockade of the D2Rs induced bradykinesia in behaving animals (Parr-Brownlie and Hyland, 2005). Thus, DA plays a crucial role in orchestrating motor responses to reward cues and new motor skill learning.
An inverse projection from motor cortex to midbrain DA neurons was identified in mice (Watabe-Uchida et al., 2012). Midbrain DA neurons code for reward uncertainty and probability (Fiorillo et al., 2003), appetitive stimuli (Mirenowicz and Schultz, 1996), and attention/motivation (Saunders et al., 2018). Interestingly, DA neurons projecting from VTA to M1 are only activated during successful motor learning (Leemburg et al., 2018). In addition, DA neurons multiplex sensorimotor parameters with reward and cue signals during a motor task in rodents (Engelhard et al., 2019, Kremer et al., 2020).
In humans, motivation (Galaro et al., 2019), reward probability (Mooshagian et al., 2015), uncertainty (Kapogiannis et al., 2008, Kapogiannis et al., 2011) and value (Freeman and Aron, 2016) modulate motor cortex excitability. As a result, the prospective gain of a reward increased engagement in a motor task (Saunders et al., 2018, Galaro et al., 2019) and motor performance in humans (Galaro et al., 2019) similar to rats (Mosberger et al., 2016). Altogether, these results suggest that the motor cortex and the midbrain communicate to motivate and update behavior during learning.
After a severe stroke in rats transient but massive DA release occurs in the striatum during ischemia (Akiyama et al., 1991, Hashimoto et al., 1994) that resolves quickly after reperfusion (Akiyama et al., 1991). This massive DA release may contribute to the neuronal damage caused by ischemia via activation of D2Rs (Hashimoto et al., 1994) and is followed by a reduced DA release for up to 3 days after stroke (Akiyama et al., 1991). DA concentration remained decreased over 10 weeks after middle cerebral occlusion in the striatum of mice (Kronenberg et al., 2012). The genes coding for DA receptors (DAR) are down-regulated for at least one week post-stroke (Sieber et al., 2014). Consistently, reduced availability of D2R has been demonstrated in the striatum one and two weeks after middle cerebral artery occlusion (Momosaki et al., 2017). These results suggest that stroke is associated with dysfunction of the dopaminergic system whichmust have functional consequences. It may explain why implicit learning is impaired after stroke in humans (Lam et al., 2016). In mice, stroke impaired motivation in a lever-pressing task and reduced the incentive value of reward (Linden et al., 2015). These data suggest that impairment of the DA system after a stroke could be severe enough to have behavioral consequences which may impair recovery. DA-enhancing therapies showed promising effects in rats after stroke (Ruscher et al., 2012), but the results of clinical trials in humans are inconsistent (Stinear, 2019). Nevertheless, Quattrocchi et al., (2017) demonstrated that motor adaptation learning in stroke patients can be improved using reward feedback, suggesting that the DA system could have a role in motor recovery from stroke. However, the knowledge on the role of endogenous DA in motor recovery from stroke is scarce.
In the present study we assessed the influence of DA transmission in the peri-infarct area on motor skill learning after a stroke in the rat motor cortex. We found that blocking DAR in the peri-infarct area after a photothrombotic stroke impaired recovery as compared to saline-treated rats. Our data further suggest that DAR activity is essential for motivation during post-stroke training.
Experimental procedures
Animals and study design
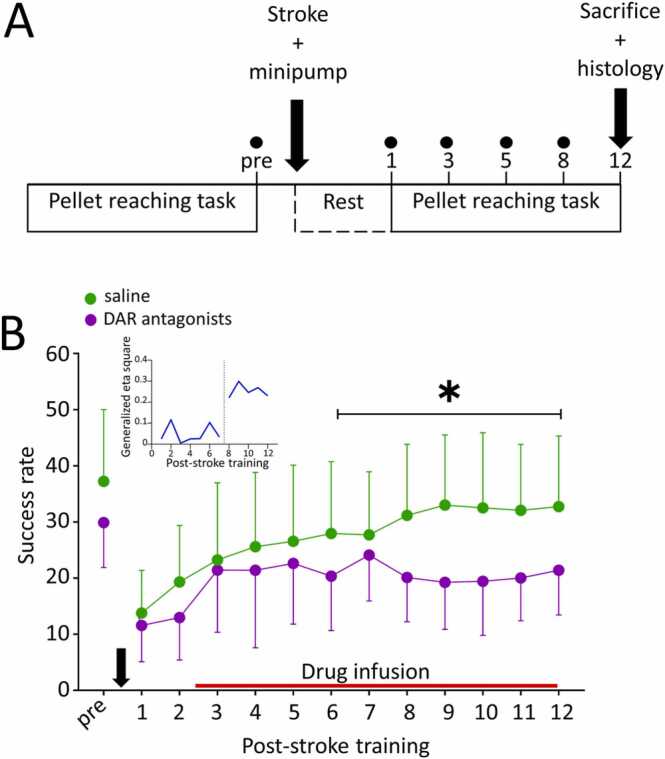
Sprague Dawley male rats (9–10 weeks old at arrival, Janvier) were group housed in IVC cages on a reversed 12-hours light/dark cycle. All experiments were conducted in accordance with the European Council Directive of 24 November 1986 (86/609/EEC), with the Swiss regulations and approved by the Federal Veterinary Office of Switzerland (license ZH011/18). All efforts were made to minimize the number of animals used and their suffering. Rats were trained in the single pellet reaching task to plateau performance. On the following day, rats received a photothrombotic stroke over the motor cortex. At the same time a cannula was placed in the stroke core. The attached minipump was filled with 0.9 % saline, or a mixture of a D1R antagonist (SCH23390) and a D2R antagonist (raclopride) (Fig. 1 A). Rats that did not reach a plateau of at least 20 % success during pre-stroke motor skill training (n = 22) and rats with less than 20 % deficit after stroke (n = 12; 7 saline, 5 antagonists) were excluded from further analysis. Additionally, 6 rats died during the surgery. The data presented here included 18 rats treated with saline and 16 with dopamine antagonists. All drugs were purchased from Sigma-Aldrich unless specified otherwise.
Fig. 1.
Dopamine transmission is necessary for optimal motor recovery from stroke. A: Schematic representation of the experimental protocol. Rats were trained in the pellet reaching task to plateau before receiving a stroke and a minipump infusing either 0.9 % saline (control) or a mixture of D1R and D2R antagonists. Training was resumed 3 days post-stroke and continued for 12 days. Dots represent the days of tape removal test. B: Post-stroke learning curves of DAR antagonist treated (n = 16, purple) and saline treated rats (n = 18, green). Blocking DAR significantly impaired motor recovery starting on training day 8 compared to saline. * : p < 0.05. The red line indicates drug delivery. The black arrow pictures the stroke induction and minipump implantation. The Inset shows that the effect of drug treatment was negligible during the first 8 days of post-stroke training and jumped to much larger values during the second week of training indicating a stronger association between drug and success rate. (For interpretation of the references to colour in this figure, the reader is referred to the web version of this article.)
Single pellet reaching task
Training sessions were performed during the dark phase as previously described (Buitrago et al., 2004). Rats were food-restricted (50 g/kg/day of lab diet) for the entire duration of the training starting 24 h before the first training session. Water was available ad libitum.
Training occurred in a Plexiglas cage with an automated sliding door at the front which opened to give access to a single food pellet (45 mg dustless pellets, BioServ, USA) when the rats nose poked a light sensor located at the back wall. During five days of shaping, rats retrieved the pellets with the tongue from a board located at 0.5 cm distance from the door. Afterwards, pellets were placed on a pedestal 1.5 cm away from the door to enforce the use of the forelimb. During the first training session, the pedestal was placed in the center of the window to determine the rats’ preferred forelimb. The pedestal was shifted 1 cm sideways starting the second session to enforce the exclusive use of the preferred forelimb. Each session lasted 1 h or 100 trials, whichever happened first. A trial was scored as “successful” if the rat could retrieve and eat the pellet or “failed” otherwise.
Pre-stroke training was continued until the rats reached plateau performance and was resumed 3 days after stroke surgery and continued for 12 consecutive days.
Data was expressed as success rate (SR) using following formula:`
Tape removal test
The sensory function was assessed using the tape removal test before stroke, and on post-stroke training day 1, 5 and 8. Each session contained 3 trials per paw. For each trial, a round sticker (6 mm diameter, Avery, Switzerland) was placed on the forepaw, alternating between right and left. The time the rat needed to notice the tape and to remove the tape after having noticed it were recorded in seconds.
Surgical procedures
General surgical procedures
Rats received a stroke and a minipump connected to a cannula in the hemisphere contralateral to their preferred forelimb under anesthesia (induction with isoflurane 3.5–4.5 % in O2, maintenance with isoflurane 2.5–3.5 % in O2). Meloxicam (1 mg/kg) and buprenorphine (0.05 mg/kg, s.c.) were used for analgesia. Lidocaine was injected under the scalp before skin incision. During the surgery, rats’ body temperature was maintained using a heating pad. Vitamin A was systematically applied on the eyes to prevent the corneal drying. Against post-operative infections enrofloxacin (5 mg/kg, s.c.) was injected at the end of the surgery.
Stroke induction
Once anesthetized, rats were placed in a stereotactic frame (Kopf Instruments, USA) and a midline incision was placed to expose the skull. To induce a photothrombotic stroke, a craniotomy (3 mm diameter) was drilled over the motor cortex (2 mm anterior, 2 mm lateral from bregma) and covered with a 2.5 mm diameter aluminum stencil. Rose Bengale (10 mg/kg, 40 mg/ml in sterile water) was injected via the tail vein using a motorized injection pump (Genie, Kent Scientific Corp., USA) for 2 min. At the same time, the motor cortex was illuminated with a cold light source (KL 1500 LCD, Schott, Germany) for 20 min. The injection of rose bengale and the illumination of the brain were started at the same time.
Minipump implantation
After the stroke was induced, a 28 G cannula was implanted in the stroke core 1000 µm deep from the pial surface. An osmotic minipump reservoir (0.25 µL/hr, 200 µL, Alzet model 2204, Alzet, USA) loaded with 0.9 % saline containing 2 mg/ml ascorbic acid or a mixture of SCH23390 (10 µg/µL in 0.9 % saline containing 2 mg/ml ascorbic acid, Tocris, UK) and S(-)-Raclopride L-Tartrate (0.2 µg/µL in 0.9 % saline containing 2 mg/ml ascorbic acid) was implanted subcutaneously under the neck skin and connected to the cannula with a piece of plastic tubing. To prevent DAR antagonists to interfere with the stroke mechanisms early after stroke, we cut the plastic tubing long enough for the drug to reach the brain after the second post-stroke training day. The cannula was maintained with dental cement (VenusFlow, Heraus Kulzer GmBH, Germany) and stabilized with a miniscrew in the occipital bone. Rats were returned to their homecage when fully recovered.
Histology
Section preparation
Rats received an i.p. injection of pentobarbital (Kantonsapotheke Zürich, Switzerland, 150 mg/kg) and were perfused with 150 ml 0.1 M phosphate buffer saline (PBS) followed by 250 ml 4 % paraformaldehyde in PBS (4 % PFA). Brains were harnessed, post-fixed overnight in 4% PFA, switched to 30 % sucrose in PBS at 4 °C until they sunk, and finally frozen at − 20 °C. Brains were serially cut into 30 µm thick floating sections in 10 series with a cryostat (CM 3050 S, Leica Microsystems, Germany). Sections were stored at − 20 °C in antifreeze solution (25 % glycerol, 30 % ethylene glycol, 45 % PBS) until processed.
Stroke volumetry
Two series per rat were mounted on slides, stained with a classical Nissl staining and covered in Depex mounting medium.
Images of individual sections were taken at 5X magnification with an upright microscope (Axioscan Z1, Zeiss, Germany) and analyzed with Fiji (v 1.51 N, NIH, USA). For each rat, the stroke volume in mm3 (S) was estimated from the outlined stroke areas of the stained sections in mm2 and the distance between 2 consecutive sections in mm (d) using the following formula:
| S = ∑stroke areas x d |
Tyrosine Hydroxylase (TH) immunohistochemistry
One series of coronal sections containing the infarct per rat was used for to stain for tyrosine hydroxylase (TH). Washing steps consisted of 3 consecutive baths of 0.1 M PBS for 15 min, dilutions were performed in 0.1 M PBS and incubations at room temperature unless otherwise specified.
After a first washing step, non-specific binding sites were blocked in 0.3 % Triton X-100 % and 5 % bovine serum albumin (BSA) (PBS++) for 90 min and sections were immediately incubated overnight in the primary antibody (Rabbit anti-TH 1:500 in PBS++, AB152, Merck Millipore, USA) at 4 °C. Sections were washed and incubated for 2 h in the secondary antibody (goat anti-rabbit Cy3 1:500 in PBS++, 111–166–003, Jackson Immunoresearch, USA) before being washed. Finally, sections were mounted on slides and coverslipped with Vectashield with DAPI (VectorLabs, USA). Images were taken at 20X magnification with an upright microscope (Axioscan Z1, Zeiss, Germany) and analyzed using Fiji (v 1.51 N, NIH, USA).
Measurement of the TH innervation
Images of individual sections were used to measure the TH density in the peri-infarct area and the contralateral cortex. The TH density was measured in the peri-infarct area, defined as a rim of 800 micrometers around the edge of the stroke core, using a custom-made script in Fiji software. The background was subtracted and thresholded to visualize only the TH-expressing fibers. The area occupied by the TH-expressing fibers was determined within that rim and in the mirrored area in the contralateral cortex. The ratio between the 2 hemispheres was determined to assess possible TH fiber loss in the peri-infarct.
Statistics
All statistics were performed with R v4.0.3 in RStudio v1.3 1093 using chemometrics, Rstatix and WRS2 packages. For all statistical tests, normality of distribution was assessed visually using a QQ plot and confirmed using the Shapiro-Wilk test. Homogeneity of variance was assessed using the Levene test. Sphericity was assessed using the Mauchly test. Anatomical data were analyzed using an unpaired Student t-test in case of a normal distribution and homogeneity of variance or a Wilcoxon rank test for unpaired groups if the assumption of normality was not met. Behavioral data were analyzed using a 2-way repeated measures ANOVA in case of a normal distribution and homogeneity of variance. Violation of the assumption of sphericity was corrected using the Greenhouse-Geisser method. In case the data was not normally distributed or violated the assumption of homogeneity of variance or showed extreme outliers, a robust 2-way repeated measures ANOVA on the 20 % trimmed means was used. Time-to-event analysis was conducted using a log rank test. Statistical significance was set at p < 0.05. Data are presented as mean and SD unless otherwise specified.
Results
Recovery of reaching was impaired in DAR antagonist-treated rats (n = 16) compared to saline-treated controls (n = 18, Fig. 1B, F(day:group, 12, 348) = 2.57, p = 0.003). Both groups showed similar pre-stroke success rates (saline vs antagonists: 37.22 ± 12.78 vs 29.88 ± 7.59 % success, p = 0.42) and a significant drop in success rate on the first day of training after stroke induction (saline: 63 % deficit, 37.22 ± 12.78 vs 13.78 % success, p = 0.0002; antagonists: 61.3 % deficit, 29.88 ± 7.59 vs 11.57 ± 6.46 % success, p = 0.0001). During the first week of re-training, no difference in the success rate between saline and DAR antagonist-treated rats was detected (day 2 to day 7: p = 0.42 to p = 1), suggesting that initially all rats had the same learning and recovery abilities. However, on re-training day 8 DAR antagonist-treated rats started to perform worse than saline-treated rats (p = 0.013 to p = 0.045) indicating that endogenous DA in the peri-infarct area is necessary for optimal motor recovery. Noteworthy, the proportion of variance in the daily performance in the pellet reaching task explained by the drug treatment is about 5 times bigger for the period from day 8 to day 12 than during the first week of re-training (Fig. 1B inset). The treatment accounted only for 5.5 % of the variance (η2g ranging from 0.025 to 0.116) during the first 7 days of re-training jumping to 25.3 % after day 8 (η2g ranging from 0.221 to 0.299) suggesting that motor recovery depended on DA transmission in a later post-stroke phase.
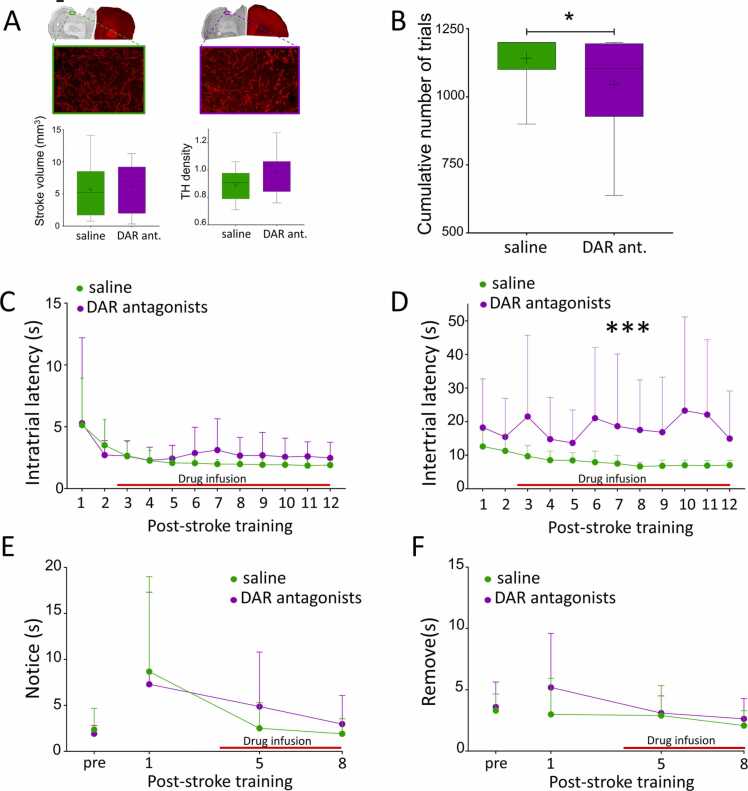
To unravel possible reasons for impaired recovery in the presence of DAR antagonists we first had to ensure that DAR antagonists did not worsen stroke damage. Therefore, we evaluated the stroke volume of both groups (Fig. 2 A, top right). There was no significant difference in stroke volume between saline and DAR antagonist treated rats (saline vs antagonists: 5.73 ± 4.32 mm vs 6.19 ± 3.71, t27 = 0.3, p = 0.77). To assess a potential difference in DA innervation between the groups, we measured the density of TH-expressing fibers and calculated its ratio between peri-infarct and contralateral motor cortex. This ratio was not different between groups (saline vs antagonists: 1.14 ± 0.15 vs 1.04 ± 0.18, t15 = 1.32, p = 0.21; Fig. 2 A, bottom right). These results indicate that blocking DAR did not interfere with stroke damage but rather suggest that impaired recovery results from DAR antagonist treatment.
Fig. 2.
DA antagonists alter motivation after stroke. A. Anatomical characterization of the stroke volume and TH innervation of the peri-infarct area. Analysis of stroke volume and TH innervation of the peri-infarct area (green: saline n = 10, purple: DAR antagonists n = 7) revealed no difference. Scale bar= 1 mm for upper panel, 1 µm for lower panel. B. Effects of DAR antagonists on training intensity. Rats treated with DAR antagonists (purple, n = 16) performed significantly fewer trials during the 12 days of post-stroke training than saline treated rats (green, n = 18). * : p < 0.05. C. Effects of DAR antagonists on intratrial latency. DAR blockade did not impair the speed of execution of a trial compared to saline. D. DAR antagonists increased intertrial latencies. DAR antagonist-treated rats took significantly longer to initiate a new trial than saline treated rats. *** : p < 0.001. E-F. Effect of DAR antagonists on somatosensory function. No difference was detected between saline (green, n = 16) and DAR antagonists (purple, n = 12) on the time to notice (E) or to remove (F) a piece of tape placed on the lesioned forelimb. A, B: Boxes: median, 25th and 75th percentile. Whiskers: 5th and 95th percentile. + : sample mean. C-F: Red line: timecourse of drug infusion. (For interpretation of the references to colour in this figure, the reader is referred to the web version of this article.)
The intensity of post-stroke training is important to promote recovery beyond spontaneously occurring recovery (Jeffers et al., 2018). We compared the total number of trials performed by both groups for the entire 12 days of post-stroke training (Fig. 2B). We found that DAR antagonist-treated rats performed significantly fewer trials than saline-treated rats (saline vs antagonists: 1142 ± 90.53 vs 1044.06 ± 178.09, W = 202, p = 0.038). To test the hypothesis that DAR antagonists impaired movement velocity preventing antagonist-treated rats to complete sessions, we compared the latency to complete a trial (intratrial latency) between the two treatment groups (Fig. 2 C); this showed no detectable difference (F1,18.88 = 2.83, p = 0.11) suggesting that movement velocity was not impaired. However, intertrial latency was longer in DAR antagonists compared to saline-treated rats (Fig. 2D; F(1, 76.19) = 31.25, p = 0). We did not detect a difference in the latency to initiate the first trial between the 2 experimental groups (F(1,82.9) = 0.25, p = 0.62) suggesting that rats treated with DAR antagonists did not forget how to perform the task compared to rats treated with saline. To assess whether the longer intertrial latency in DAR antagonist-treated rats was due to sensory deficits or neglect, we compared saline- and DAR antagonist-treated rats in the tape removal test. DAR antagonists did not impair the ability to either notice (Fig. 2E; F(1,26) = 0.067, p = 0.8, n = 12 DAR antagonists and 16 saline) or remove the tape after having noticed it (Fig. 2 F; F(1,26) = 2.28, p = 0.14). These data indicate that DAR antagonists infused in the peri-infarct cortex did not impair somatosensory function.
Discussion
Here, we showed that blocking DAR in the peri-infarct area after stroke impaired motor skill recovery, likely via impairing the motivation to move.
In healthy rats, learning a new motor skill depends on DA transmission in the motor cortex (Molina-Luna et al., 2009, Guo et al., 2015, Li et al., 2017). DA has been shown to maintain inter-session improvements in rats (Li et al., 2017) and to stabilize spines formed in the motor cortex during training (Guo et al., 2015). Consistent with these findings, our results showed that DA transmission in the peri-infarct cortex is crucial for motor skill recovery after stroke suggesting a common mechanism between learning and recovery. However, unlike in healthy learning (Molina-Luna et al., 2009), DAR antagonists have a 6 days delayed effect on motor skill recovery. This unlikely results from a lack of statistical power because the effect size abruptly changed between training day 7 and 8. This delay suggests that DA transmission is not involved in motor recovery during the early post-stroke phase. The reason for this finding may be a dysfunctional DA system after stroke. Genes coding for DAR are down-regulated for at least one week post-stroke (Sieber et al., 2014). In addition, Momosaki et al. (2017) showed a reduction of D2R bioavailability in the striatum at 7 and 14 days after occlusion of the middle cerebral artery in rat. In the ischemic region after a photothrombotic stroke, D1R bioavailability is also reduced during the first week post-stroke (Rogozinska and Skangiel-Kramska, 2010). These data suggest that the DA system is not fully functional during this period and could explain the lack of a DAR antagonist effect during the first week after stroke. The recovery profiles observed here suggest that rats recover the skill in two phases, a DA-independent first phase, that may rely on structural reorganization of the peri-infarct area (Carmichael, 2006, Murphy and Corbett, 2009), followed by a DA-dependent phase relying on motor learning mechanisms (Rioult-Pedotti et al., 1998, Rioult-Pedotti et al., 2000, Rioult-Pedotti et al., 2007, Molina-Luna et al., 2009).
Interestingly, DAR antagonist treated rats took significantly longer to initiate new trials resulting in the ability to complete their full training session. DAR antagonists infused in the peri-infarct area did not impair somatosensory function compared to saline. Therefore, it is unlikely that sensory deficit or neglect increased the intertrial latency. This might result from a lack of motivation. Blocking DA transmission in the peri-infarct area might have decrease the reward value. However, the similar intratrial latencies between groups suggest that reward pellets were still valued by DA-antagonist treated rats. Operant behaviors like the pellet reaching task are regulated by an effort-benefit tradeoff. Rats chose to exert an effort if the energetic cost of it is outweighed by the reward benefit. In rats trained in a lever pressing task with a progressive ratio, the latency between 2 trials increased with the effort necessary to obtain a reward (Alling and Poling, 1995). Niv et al. (2007) suggested a computational model in which DA signals in the striatum influence the choice of a rat to engage in a task or not, and at which pace, by reflecting the reward rate and value. DA lesions of the nucleus accumbens increased the tendency of rats to take long pauses between trials in a lever pressing task, and decreased the willingness to work at high fixed ratios (Mingote et al., 2005). Experimental data confirmed that the concentration of DA in the ventral striatum affected the rat’s willingness to work (Hamid et al., 2016). Phasic DA signals in the ventral striatum reinforced repetition of a rewarded action whereas tonic DA release motivated engagement in an action (Hamid et al., 2016). Optogenetic activation of the VTA, which innervates the ventral striatum and the motor cortex (Hosp et al., 2015), triggered a strong approach behavior to a previously conditioned light-cue in mice (Saunders et al., 2018). Reward expectation coded by VTA dopamine neurons (Fiorillo et al., 2003), increased the effort exerted by human participants in a motor task and increased motor cortex excitability (Galaro et al., 2019). Motor performance is enhanced by motivation to be rewarded in rats (Mosberger et al., 2016) and humans (Galaro et al., 2019). Recently, Patriarchi et al. (2018) showed that dopamine concentration increased in the motor cortex of mice during a visuomotor association task during reward expectation and consumption. Blocking D2R in the motor cortex of anesthetized rats decreased motor cortex excitability (Hosp et al., 2009). In addition, a single stimulation in the VTA increased ICMS-induced activation of the motor cortex (Kunori et al., 2016). In anesthetized mice, activation of the D2R increased putative pyramidal neurons firing rate (Vitrac et al., 2014). Together, these data suggest that DA signals in the motor cortex could participate in lowering the cost of performing an action for a reward by disinhibiting motor cortex circuits. D1 receptor blockade in the motor cortex reduced the number of cue-responsive neurons in mice performing a cued-licking task (Chen et al., 2019) indicating that motor cortex responds to motivational signals. They also showed that these mice responded later to the cue (Chen et al., 2019). After a stroke, the peri-infarct area reorganizes to take over the lost functions to promote functional recovery (Okabe et al., 2016). Therefore, blocking DAR in the peri-infarct area could reduce the motivation to engage in a task by blocking reward signals. Reduced motivation has been reported in mice after stroke (Kronenberg et al., 2012, Linden et al., 2015) and correlated with a decreased DA concentration in the striatum (Kronenberg et al., 2012). In the same vein, our data suggest that altered DA transmission in the peri-infarct cortex impairs motivation during rehabilitative training.
To conclude, our data showed that DA transmission in the peri-infarct cortex is crucial to recover motor skills after stroke. Rather than a pure reward signal, we suggest that DA in the peri-infarct cortex enhances the willingness to engage in a task and maintains a high motivation during rehabilitation. The cost-benefit tradeoff after stroke could be biased resulting from a dysfunctional DAergic system, modifying patients’ motivation and outcome (Rapolienė et al., 2018). Targeting the DAergic system to enhance motivation would constitute a good strategy to improve the efficiency of rehabilitation. Increasing reward feedback has already been shown promising to alleviate spatial neglect (Li et al., 2016), improve arm rehabilitation (Widmer et al., 2022)and performance as well as retention in a motor adaptation task (Quattrocchi et al., 2017).
CRediT authorship contribution statement
LNK and MI performed the experiments and surgery respectively, CV and MSRP analyzed data and prepared the manuscript. AL initiated and supervised the project and corrected the manuscript.
Acknowledgements
LNK and MI performed the experiments and surgery respectively, CV and MSRP analyzed data and prepared the manuscript. AL initiated and supervised the project and corrected the manuscript.
This work was supported by P&K Pühringer Foundation and the KFSP Stroke (University of Zurich).
Conflict of Interest
none.
References
- Akiyama Y., Ito A., Koshimura K., Ohue T., Yamagata S., Miwa S., Kikuchi H. Effects of transient forebrain ischemia and reperfusion on function of dopaminergic neurons and dopamine reuptake in vivo in rat striatum. Brain Res. 1991;561:120–127. doi: 10.1016/0006-8993(91)90756-l. 〈https://www.sciencedirect.com/science/article/pii/000689939190756L〉 (Available at) [DOI] [PubMed] [Google Scholar]
- Alling K., Poling A. The effects of differing response-force requirements on fixed-ratio responding of rats. J. Exp. Anal. Behav. 1995;63:331–346. doi: 10.1901/jeab.1995.63-331. Available at: 〈https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1334449/〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J., Chernenko G., Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J. Neurosci. 2004;24:1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago M.M., Ringer T., Schulz J.B., Dichgans J., Luft A.R. Characterization of motor skill and instrumental learning time scales in a skilled reaching task in rat. Behav. Brain Res. 2004;155:249–256. doi: 10.1016/j.bbr.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann. Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Chen K., Vincis R., Fontanini A. Disruption of cortical dopaminergic modulation impairs preparatory activity and delays licking initiation. Cereb. Cortex. 2019;29:1802–1815. doi: 10.1093/cercor/bhz005. 〈https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6418393/〉 (Available at) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard B., Finkelstein J., Cox J., Fleming W., Jang H.J., Ornelas S., Koay S.A., Thiberge S.Y., Daw N.D., Tank D.W., Witten I.B. Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature. 2019;570:509–513. doi: 10.1038/s41586-019-1261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo C.D., Tobler P.N., Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Freeman S.M., Aron A.R. Withholding a reward-driven action: studies of the rise and fall of motor activation and the effect of cognitive depletion. J. Cogn. Neurosci. 2016;28:237–251. doi: 10.1162/jocn_a_00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaro J.K., Celnik P., Chib V.S. Motor cortex excitability reflects the subjective value of reward and mediates its effects on incentive-motivated performance. J. Neurosci. 2019;39:1236–1248. doi: 10.1523/JNEUROSCI.1254-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P., Duyckaerts C., Alvarez C., Javoy-Agid F., Berger B. Alterations of dopaminergic and noradrenergic innervations in motor cortex in Parkinson’s disease. Ann. Neurol. 1991;30:365–374. doi: 10.1002/ana.410300308. [DOI] [PubMed] [Google Scholar]
- Guo L., Xiong H., Kim J.-I., Wu Y.-W., Lalchandani R.R., Cui Y., Shu Y., Xu T., Ding J.B. Dynamic rewiring of neural circuits in the motor cortex in mouse models of Parkinson’s disease. Nat. Neurosci. 2015;18:1299–1309. doi: 10.1038/nn.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid A.A., Pettibone J.R., Mabrouk O.S., Hetrick V.L., Schmidt R., Vander Weele C.M., Kennedy R.T., Aragona B.J., Berke J.D. Mesolimbic dopamine signals the value of work. Nat. Neurosci. 2016;19:117–126. doi: 10.1038/nn.4173. 〈https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4696912/〉 (Available at) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms K.J., Rioult-Pedotti M.S., Carter D.R., Dunaevsky A. Transient spine expansion and learning-induced plasticity in layer 1 primary motor cortex. J. Neurosci. 2008;28:5686–5690. doi: 10.1523/JNEUROSCI.0584-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N., Matsumoto T., Abe H.M., Hashitani T., Nishino H. Dopamine has inhibitory and accelerating effects on ischemia-induced neuronal cell damage in the rat striatum. Brain Res. Bull. 1994;33:281–288. doi: 10.1016/0361-9230(94)90195-3. 〈https://www.sciencedirect.com/science/article/pii/0361923094901953〉 (Available at) [DOI] [PubMed] [Google Scholar]
- Hosp J.A., Molina-Luna K., Hertler B., Atiemo C.O., Luft A.R. Dopaminergic modulation of motor maps in rat motor cortex: an in vivo study. Neuroscience. 2009;159:692–700. doi: 10.1016/j.neuroscience.2008.12.056. [DOI] [PubMed] [Google Scholar]
- Hosp J.A., Nolan H.E., Luft A.R. Topography and collateralization of dopaminergic projections to primary motor cortex in rats. Exp. Brain Res. 2015;233:1365–1375. doi: 10.1007/s00221-015-4211-2. [DOI] [PubMed] [Google Scholar]
- Hosp J.A., Pekanovic A., Rioult-Pedotti M.S., Luft A.R. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J. Neurosci. 2011;31:2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers M.S., Karthikeyan S., Gomez-Smith M., Gasinzigwa S., Achenbach J., Feiten A., Corbett D. Does stroke rehabilitation really matter? part B: an algorithm for prescribing an effective intensity of rehabilitation. Neurorehabil Neural Repair. 2018;32:73–83. doi: 10.1177/1545968317753074. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D., Campion P., Grafman J., Wassermann E.M. Reward-related activity in the human motor cortex. Eur. J. Neurosci. 2008;27:1836–1842. doi: 10.1111/j.1460-9568.2008.06147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D., Mooshagian E., Campion P., Grafman J., Zimmermann T.J., Ladt K.C., Wassermann E.M. Reward processing abnormalities in Parkinson’s disease. Mov. Disord. 2011;26:1451–1457. doi: 10.1002/mds.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer Y., Flakowski J., Rohner C., Lüscher C. Context-dependent multiplexing by individual VTA dopamine neurons. J. Neurosci. 2020;40:7489–7509. doi: 10.1523/JNEUROSCI.0502-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G., Balkaya M., Prinz V., Gertz K., Ji S., Kirste I., Heuser I., Kampmann B., Hellmann-Regen J., Gass P., Sohr R., Hellweg R., Waeber C., Juckel G., Hörtnagl H., Stumm R., Endres M. Exofocal dopaminergic degeneration as antidepressant target in mouse model of poststroke depression. Biol. Psychiatry. 2012;72:273–281. doi: 10.1016/j.biopsych.2012.02.026. 〈https://www.biologicalpsychiatryjournal.com/article/S0006-3223(12)00153-9/fulltext〉 (Available at) [DOI] [PubMed] [Google Scholar]
- Kunori N., Kajiwara R., Takashima I. The ventral tegmental area modulates intracortical microstimulation (ICMS)-evoked M1 activity in a time-dependent manner. Neurosci. Lett. 2016;616:38–42. doi: 10.1016/j.neulet.2016.01.047. [DOI] [PubMed] [Google Scholar]
- Kwakkel G., Kollen B.J., van der Grond J., Prevo A.J.H. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- Lam J.M., Globas C., Hosp J.A., Karnath H.-O., Wächter T., Luft A.R. Impaired implicit learning and feedback processing after stroke. Neuroscience. 2016;314:116–124. doi: 10.1016/j.neuroscience.2015.11.051. [DOI] [PubMed] [Google Scholar]
- Leemburg S., Canonica T., Luft A. Motor skill learning and reward consumption differentially affect VTA activation. Sci. Rep. 2018;8:687. doi: 10.1038/s41598-017-18716-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Russell C., Balaji N., Saleh Y., Soto D., Malhotra P.A. The effects of motivational reward on the pathological attentional blink following right hemisphere stroke. Neuropsychologia. 2016;92:190–196. doi: 10.1016/j.neuropsychologia.2016.03.037. 〈https://www.sciencedirect.com/science/article/pii/S0028393216301087〉 (Available at) [DOI] [PubMed] [Google Scholar]
- Li Q., Ko H., Qian Z.-M., Yan L.Y.C., Chan D.C.W., Arbuthnott G., Ke Y., Yung W.-H. Refinement of learned skilled movement representation in motor cortex deep output layer. Nat. Commun. 2017;8:15834. doi: 10.1038/ncomms15834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J., Plumier J.-C., Fassotte L., Ferrara A. Focal cerebral ischemia impairs motivation in a progressive FR schedule of reinforcement in mice. Behav. Brain Res. 2015;279:82–86. doi: 10.1016/j.bbr.2014.10.042. [DOI] [PubMed] [Google Scholar]
- Luft A.R., Buitrago M.M., Kaelin-Lang A., Dichgans J., Schulz J.B. Protein synthesis inhibition blocks consolidation of an acrobatic motor skill. Learn Mem. 2004;11:379–382. doi: 10.1101/lm.72604. [DOI] [PubMed] [Google Scholar]
- Luft A.R., Buitrago M.M., Ringer T., Dichgans J., Schulz J.B. Motor skill learning depends on protein synthesis in motor cortex after training. J. Neurosci. 2004;24:6515–6520. doi: 10.1523/JNEUROSCI.1034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S., Weber S.M., Ishiwari K., Correa M., Salamone J.D. Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions. Eur. J. Neurosci. 2005;21:1749–1757. doi: 10.1111/j.1460-9568.2005.03972.x. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J., Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Molina-Luna K., Pekanovic A., Röhrich S., Hertler B., Schubring-Giese M., Rioult-Pedotti M.-S., Luft A.R. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momosaki S., Ito M., Yamato H., Iimori H., Sumiyoshi H., Morimoto K., Imamoto N., Watabe T., Shimosegawa E., Hatazawa J., Abe K. Longitudinal imaging of the availability of dopamine transporter and D2 receptor in rat striatum following mild ischemia. J. Cereb. Blood Flow. Metab. 2017;37:605–613. doi: 10.1177/0271678X16635183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooshagian E., Keisler A., Zimmermann T., Schweickert J.M., Wassermann E.M. Modulation of corticospinal excitability by reward depends on task framing. Neuropsychologia. 2015;68:31–37. doi: 10.1016/j.neuropsychologia.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosberger A.C., de Clauser L., Kasper H., Schwab M.E. Motivational state, reward value, and pavlovian cues differentially affect skilled forelimb grasping in rats. Learn Mem. 2016;23:289–302. doi: 10.1101/lm.039537.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T.H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. 〈https://www.nature.com/articles/nrn2735〉 (Available at) [DOI] [PubMed] [Google Scholar]
- Niv Y., Daw N.D., Joel D., Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology. 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. (Available at) [DOI] [PubMed] [Google Scholar]
- Okabe N., Shiromoto T., Himi N., Lu F., Maruyama-Nakamura E., Narita K., Iwachidou N., Yagita Y., Miyamoto O. Neural network remodeling underlying motor map reorganization induced by rehabilitative training after ischemic stroke. Neuroscience. 2016;339:338–362. doi: 10.1016/j.neuroscience.2016.10.008. 〈https://www.sciencedirect.com/science/article/pii/S0306452216305176〉 (Available at) [DOI] [PubMed] [Google Scholar]
- Parr-Brownlie L.C., Hyland B.I. Bradykinesia induced by dopamine D2 receptor blockade is associated with reduced motor cortex activity in the rat. J. Neurosci. 2005;25:5700–5709. doi: 10.1523/JNEUROSCI.0523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi T., Cho J.R., Merten K., Howe M.W., Marley A., Xiong W.-H., Folk R.W., Broussard G.J., Liang R., Jang M.J., Zhong H., Dombeck D., von Zastrow M., Nimmerjahn A., Gradinaru V., Williams J.T., Tian L. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science. 2018;360:eaat4422. doi: 10.1126/science.aat4422. 〈https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6287765/〉 (Available at) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchi G., Greenwood R., Rothwell J.C., Galea J.M., Bestmann S. Reward and punishment enhance motor adaptation in stroke. J. Neurol. Neurosurg. Psychiatry. 2017;88:730–736. doi: 10.1136/jnnp-2016-314728. [DOI] [PubMed] [Google Scholar]
- Rapolienė J., Endzelytė E., Jasevičienė I., Savickas R. Stroke patients motivation influence on the effectiveness of occupational therapy. Rehabil. Res Pr. 2018;2018 doi: 10.1155/2018/9367942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti M.-S., Donoghue J.P., Dunaevsky A. Plasticity of the synaptic modification range. J. Neurophysiol. 2007;98:3688–3695. doi: 10.1152/jn.00164.2007. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti M.S., Friedman D., Donoghue J.P. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti M.S., Friedman D., Hess G., Donoghue J.P. Strengthening of horizontal cortical connections following skill learning. Nat. Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rogozinska K., Skangiel-Kramska J. Effect of focal cerebral ischaemia on modulatory neurotransmitter receptors in the rat brain: An autoradiographic study. J. Chem. Neuroanat. 2010;40:232–238. doi: 10.1016/j.jchemneu.2010.06.004. 〈https://www.sciencedirect.com/science/article/pii/S0891061810000827〉 (Available at) [DOI] [PubMed] [Google Scholar]
- Ruscher K., Kuric E., Wieloch T. Levodopa treatment improves functional recovery after experimental stroke. Stroke. 2012;43:507–513. doi: 10.1161/STROKEAHA.111.638767. [DOI] [PubMed] [Google Scholar]
- Saunders B.T., Richard J.M., Margolis E.B., Janak P.H. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 2018;21:1072–1083. doi: 10.1038/s41593-018-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack S.R., Snyder C.L., Lewis D.A. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J. Comp. Neurol. 1995;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Sieber M.W., Guenther M., Jaenisch N., Albrecht-Eckardt D., Kohl M., Witte O.W., Frahm C. Age-specific transcriptional response to stroke. Neurobiol. Aging. 2014;35:1744–1754. doi: 10.1016/j.neurobiolaging.2014.01.012. 〈https://www.sciencedirect.com/science/article/pii/S019745801400013X〉 (Available at) [DOI] [PubMed] [Google Scholar]
- Stinear C.M. Dopamine for motor recovery after stroke: where to from here? Lancet Neurol. 2019;18:514–515. doi: 10.1016/S1474-4422(19)30162-0. 〈https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(19)30162-0/fulltext〉 (Available at) [DOI] [PubMed] [Google Scholar]
- Vitrac C., Péron S., Frappé I., Fernagut P.-O., Jaber M., Gaillard A., Benoit-Marand M. Dopamine control of pyramidal neuron activity in the primary motor cortex via D2 receptors. Front. Neural Circuits. 2014;8:13. doi: 10.3389/fncir.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M., Zhu L., Ogawa S.K., Vamanrao A., Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Widmer M., Held J.P.O., Wittmann F., Valladares B., Lambercy O., Sturzenegger C., Palla A., Lutz K., Luft A.R. Reward during arm training improves impairment and activity after stroke: a randomized controlled trial. Neurorehabil. Neural Repair. 2022;36:140–150. doi: 10.1177/15459683211062898. [DOI] [PMC free article] [PubMed] [Google Scholar]