Abstract
There is limited information on the effect of exogenous ghrelin infusion on feed intake (FI) in chickens. Therefore, male broilers were used in 3 factorial experiments to determine the relationships between doses (0, 1, or 4 nM; Dose), frequency (once every two h; 2 h), once every 4th h (4 h) or continuous infusion, and ghrelin forms including acylated-ghrelin (AG) and desacylated-ghrelin (DAG) on FI, ADG, and concentrations of corticosterone and Growth Hormone (GH). Treatments were delivered via a jugular cannula, using programmable pumps for 11 consecutive days. FI and ADG were recorded, and plasma was collected. Data were analyzed using a factorial design. In Experiment 1 the effect of AG pulse frequency and doses were evaluated. There was a linear decrease in FI (P = 0.002) and a linear increase in corticosterone (P = 0.033) and GH (P = 0.011) concentrations when AG was infused. However, ADG decreased with doses (P = 0.011) only when AG was given at 2 h. In Experiment 2 the effect of ghrelin forms and doses given at 2 h was evaluated. There was a linear decrease in FI when AG was infused and a linear increase in FI when DAG was infused (P < 0.05). Birds infused with DAG gained more weight than those infused with AG. There was a linear increase in corticosterone and GH concentrations only when AG was infused (P < 0.01). In Experiment 3 the effect of continuous infusion of 2 doses (0 and 1 nM) of AG and DAG were evaluated. There was a linear decrease in FI and ADG when AG (P < 0.001) was infused and a linear increase in FI and ADG when DAG was infused (P < 0.05). There was an increase in corticosterone concentrations only when AG was infused (P = 0.022). However, GH concentrations were not affected by treatments. We concluded that AG and DAG pulse frequency and doses had a differential effect on FI, ADG, corticosterone, and GH concentrations in broiler chickens.
Key words: ghrelin, broilers, feed intake, infusion
INTRODUCTION
Ghrelin is a growth hormone (GH)-releasing peptide initially purified from rat stomachs (Kojima et al., 1999). In chickens, ghrelin is 26 amino acids peptide highly expressed and secreted primarily from the proventriculus (Kaiya et al., 2002). Two forms of ghrelin circulate in blood. The active form of ghrelin (acylated ghrelin; AG) undergoes a unique acylation process and is the only form that binds to its cognate receptor, a seven-transmembrane G protein-coupled receptor (GHSR-1a). Ghrelin O-acyltransferase is the enzyme that transfers an acyl group from fatty acids to the serine-3 residue of ghrelin, thus making it active (Gutierrez et al., 2008). Desacylated-ghrelin (DAG) does not undergo the acylation process and has a very weak binding ability for GHSR-1a (Gauna et al., 2007; Callaghan et al., 2014).
Systemic or central infusion of ghrelin in mammalian species stimulates feed intake (FI) and induces adiposity (Tschöp et al., 2000; Wren et al., 2001). In avian species, most researchers have shown that intracerebroventricular (ICV) infusion of AG decreases feed intake (Table 1). However, there are conflictive results on the effect of ghrelin on feed intake when injected peripherally. In layer chickens, i.v. injection of ghrelin did not affect feed intake (Kaiya et al., 2007). On the other hand, i.v. injections of ghrelin in broiler chickens inhibited feed intake 30 to 120 min after treatments were applied (Geelissen et al., 2006). Similarly, a short-time inhibitory effect (approximately 30 min) on feed intake was reported when ghrelin was administered peripherally in neonatal broiler chickens (Buyse et al., 2009). However, when a ghrelin receptor agonist (Capromorelin) was administered orally to male broiler chickens, a significant dose-dependent increase in feed intake was observed (Ceron-Romero et al., 2021).
Table 1.
Effect of ghrelin administration on feed intake in different avian species.
| Ghrelin1 | Species2 | Age3 | Route | Dose | Time4 | FI result5 | Reference |
|---|---|---|---|---|---|---|---|
| DAG | Ggd (Layers) | 5d | ICV | 0–100 pM/bird | 30–90 min | ↔ | Tachibana et al. (2011) |
| AG | Ggd (Layers) | 7d | ICV | 0–10 pM/bird | 30–90 min | ↓ | Tachibana et al. (2011) |
| AG | Ggd (Broilers) | 3d | ICV | 0.38–1.5 nM/bird | 30–120 min | ↓ | Saito et al. (2002) |
| mAG | Ggd (Broilers) | 21d | ICV | 0–1.2 nM/bird | 15–180 min | ↓ | Taati et al. (2010) |
| mAG | Ggd (Broilers) | 2d | ICV | 0.38–1.5 nM/bird | 120 min | ↓ | Furuse et al. (2001) |
| AG | Ggd (Broilers) | 5d | ICV | 0.6 nM/100 g | 30–120 min | ↓ | Farrokhi et al. (2021) |
| AG | Ggd (Broilers) | 4d | ICV | 0–20 pM/bird | 30–120 min | ↓ | Saito et al. (2005) |
| GHRP-6 | Ggd (Layers) | 5d | ICV | 0.38–1.5 nM/bird | 30–120 min | ↓ | Khan et al. (2006) |
| mAG | Ccj | Adult | ICV | 0.05–1 nM/bird | 2, 4, and 12 h | ↓ | Shousha et al. (2005) |
| AG | Ggd (Layers) | 8d | i.v. | 0.6 nM/100 g | 30–120 min | ↔ | Kaiya et al. (2007) |
| AG | Ggd (Broilers) | 7 d | i.v. | 1 nM/100 g | 0.5–2 h | ↓ | Geelissen et al. (2006) |
| AG | Ggd (Broilers) | 1 d | i.v. | 1 nM/bird | 30 min | ↓ | Buyse et al. (2009) |
| AG | Sylvia borin | NR | i.p. | 100–500 pM/bird | 18 h | ↔ | Goymann et al. (2017) |
| DAG | Sylvia borin | NR | i.p. | 500 pM/bird | 18 h | ↓ | Goymann et al. (2017) |
| mAG | Ccj | Adult | i.p. | 0.5–1 nM/bird | 12 h | ↑ | Shousha et al. (2005) |
| mAG | Ccj | Adult | i.p. | 3 nM/bird | 12 h | ↓ | Shousha et al. (2005) |
| AG | Ggd (Broilers) | 7 d | i.p. | 0.5 or 2 nM/100 g | 30–120 min | ↓ | Ocłoń and Pietras (2011) |
| Capromorelin | Ggd (Broilers) | 28 d | Oral | 0–12 mg/Kg/d | 0–5 d | ↑ | Ceron-Romero et al. (2021) |
Ghrelin form: DAG, chicken desacylated-ghrelin; AG, chicken acylated-ghrelin; mAG, mammalian AG.
Species: Ggd = Gallus gallus domesticus; Ccj = Coturnix coturnix japonica.
Age: NR = Age not reported by author.
Time: Time at which feed intake measurement was obtained after treatments were applied.
FI results: ↔ = No change in feed intake ↓ = decreased feed intake = ↑ increased feed intake.
The inhibitory effect of AG on feed intake is thought to be associated with the activation of neurons expressing the anorexigenic corticotropin-releasing hormone (CRH). Suppression of feed intake in chickens was induced by ICV injections of ghrelin, but it was attenuated by co-injection of a CRH receptor antagonist (Saito et al., 2005). Similarly, in 1-day-old broiler chickens, a single intravenous injection of chicken ghrelin increased plasma corticosterone levels by almost 400% (Buyse et al., 2009), presumably by the direct effect on endogenous CRH, which in turn activates the hypothalamic–pituitary–adrenal (HPA) axis, leading to increased corticosterone release from the adrenal glands (Kaiya et al., 2002; Saito et al., 2005). Another line of research found that ICV injections of a CRH-like peptide (Urocortin-3; UCN-3) but not CRH increased plasma ghrelin concentration in chickens (Khan et al., 2014). Since UCN-3 has more affinity to the CRH type 2 receptor (Lewis et al., 2001), evidence suggests that the effect of ghrelin in feed intake is at least partly mediated by the CRH system and the two receptors present in the CRH family. Additionally, ghrelin can directly affect the histaminergic system (via H1 receptors) that in turn modulates CRH release (Taati et al., 2010). The GHSR-1a receptor exhibits a highly constitutive activity (Holst et al., 2003; Qin et al., 2022). Consequently, the ability of the ghrelin receptor to retain a certain degree of activity independently of the ligand may also play a role in the differential regulation of ghrelin in feed intake.
Limited information is available on the effect of the two forms of ghrelin in avian species. In migratory garden warblers’ birds, injection (i.p.) of DAG decreased feed intake, whereas injections of AG did not affect feed intake (Goymann et al., 2017). However, in other migratory species (blackbirds) researchers were not able to find correlations between ghrelin and fat levels at departure time (Eikenaar et al., 2018). In layer chickens, ICV injections of DAG did not affect feed intake after 30 min of treatments, whereas AG decreased feed intake (Tachibana et al., 2011).
The physiological pattern of ghrelin secretion is pulsatile in humans with approximately 1 pulse every 1 to 4 h during fasting (Koutkia et al., 2004; Natalucci et al., 2005; Nass et al., 2008). However, in fasting rats, the interpulse interval was found to be more frequent with approximately 1 pulse every 23 min (Bagnasco et al., 2002). The amplitude and width of pulsatile ghrelin secretion appear to increase during regular meal patterns in humans, suggesting that ghrelin rises during meal anticipation (Frecka and Mattes, 2008). The spontaneous pattern of ghrelin secretion indicates that the production could be attributed to neuronal in addition to gastrointestinal signals (Koutkia et al., 2004; Natalucci et al., 2005).
Ghrelin increases the secretion of GH in rats (Date et al., 2000; Hosoda et al., 2000; Wren et al., 2000), humans (Takaya et al., 2000), and in chickens (Ahmed and Harvey, 2002). Acylated-ghrelin pulses are correlated with GH pulses (Nass et al., 2008). However, when total ghrelin was measured, ghrelin pulses did not correlate with GH pulsatility (Koutkia et al., 2004). Acylated-ghrelin stimulates the release of GH after binding GHSR-1a and causing an increase of intracellular calcium concentration via the inositol 1,4,5-trisphosphate signal transduction pathway (Howard et al., 1996; Kohno et al., 2003). When ghrelin was administered in vitro to chicken pituitaries, a downregulation of cGHSR-1a was observed within 15 min (Geelissen et al., 2003). These data suggest that GH and ghrelin provide a feedback mechanism that regulates GHSR-1a.
There is a lack of long-term experiments that evaluates the effect of ghrelin (AG or DAG) on feed intake, ADG, corticosterone and GH concentrations in chickens. Most of the literature on exogenous ghrelin administration in avian species only provides information of measurements taken after a relatively short time post-ghrelin administration (Table 1). Moreover, the effect of different forms of ghrelin, pulse frequency, and doses has not been evaluated. Therefore, the objectives of the experiments presented here were to evaluate the effects of systemic infusion of AG and DAG for 11 consecutive days at different frequencies and doses on FI, ADG, corticosterone and GH concentrations in male broiler chickens.
MATERIALS AND METHODS
Animal Rearing and Treatments
One-hundred-fifty 1-day-old male broiler chickens (Gallus gallus domesticus; Ross 708) were obtained from a commercial hatchery and used in 3 replicated experiments during 3 consecutive years. Birds were randomly placed in 2 battery brooders and reared as recommended by industry standards with ad libitum feed and water consumption. Birds received a standard corn-soybean-based starter diet from 1 to 14 d of age (22 % CP, 1.0 % Lys, 1.2 % Ca, and 0.6 % P) followed by a grower/finisher diet until the end of the experiment (20 % CP, 1.2 % Lys, 1.1 % Ca, and 0.6 % P; Purina, St. Louis, MO). At 3 wk of age, birds (BW 1.5 ± 0.1 Kg) were randomly transferred from the brooders to individual cages (0.6 × 0.5 × 0.5 m) and were fitted with a harness and collar before jugular cannulation. Chickens were housed at the Poultry Building (Alabama A&M University) and exposed to a 23L:1D photoperiod and 22 ± 1.5ºC temperature. The care, treatment, and experimental protocols were approved by the Institutional Animal Care and Use Committee of Alabama A&M University.
Experiment 1
Birds (n = 5/trt; yr 1) were randomly assigned to a 2 × 3 factorial arrangement of treatments (FAT). The first factor in the FAT included the frequency of exogenous acylated ghrelin pulses (Frequency) infused (i.v.) once every 2 h (2 h) or once every 4 h (4 h). The second factor included different nano molar (nM) doses (Dose) of acylated-ghrelin: 0 nM/100 g BW/d (0 nM), 1 nM/100 g BW/d (1 nM), or 4 nM/100 g BW/d (4 nM). Birds in the 0 nM group were considered control birds and received a saline-citrate-gentamycin solution (Saline) via programmable pumps (see below). The experiment was conducted for 11 consecutive days throughout a 24 h period.
Experiment 2
Birds (n = 5/trt; yr 2) were randomly assigned to a 2 × 3 FAT. The first factor in the FAT included the 2 forms of chicken ghrelin (Form): AG, and DAG. The second factor included different doses of ghrelin: 0 nM, 1 nM, and 4 nM given in pulses every 2 h. Birds in the 0 nM group were considered control birds and received Saline via programmable pumps. The experiment was conducted for 11 consecutive days throughout a 24-h period.
Experiment 3
Birds (n = 5/trt; yr 3) were randomly assigned to a 2 × 2 FAT. The first factor in the FAT included the 2 forms of chicken ghrelin: AG and DAG. The second factor included different doses of ghrelin: 0 nM and 1 nM given as a continuous infusion. Birds in the 0 nM group were considered control birds and received Saline via programmable pumps. The total amount of ghrelin given per day in birds receiving 1 nM was the same as the dose receiving 1 nM in Experiment 2. The experiment was conducted for 11 consecutive days throughout a 24-h period.
Jugular Vein Cannulation
Birds were cannulated as previously described (Vizcarra et al., 2004) with modifications. Briefly, 1 wk before cannulation, birds were adapted to wear a harness (Instech Laboratories, PA) and a collar that consisted of a thin piece of cardboard with a hole that fitted the bird's neck. The purpose of the collar was to prevent birds from reaching and removing the cannula. We have previously shown that the cannulation procedure increases corticosterone concentrations during the first 3 to 4 h post cannulation (Vizcarra et al., 2004). Therefore, to avoid any potential stress-related confounding effect, birds were cannulated the day before treatments were applied. After the cannula was inserted into the jugular vein, serological suture glue was applied at the incision point to hold the cannula in place. Cannulas, swivels, needles, and tubing used during the procedure were autoclaved by the provider (Instech Laboratories, Plymouth Meeting, PA). Two KDS220 multi-syringe infusion pumps (KD, Scientific Inc, MA) were placed in a remote location that was out of sight to the birds and easily accessible to deliver treatments (Pump #1) and maintain cannula patency (Pump #2). Pump #1 was programmed to deliver treatments at specified frequencies and doses. Doses were delivered in 2 mL bolus infusions at a rate of 0.5 mL/min when birds were infused with pulses every 2 h or 4 h (Experiment 1 and 2). Pump #2 was programmed to deliver a continuous infusion of Saline at a rate of 0.4 mL/h. The cannula was connected to the harness and pumps via a Y connector (Instech Laboratories) that allowed for continuous delivery of the Saline solution and treatments. When a continuous infusion was used (Experiment 3), only Pump #1 was set to deliver the infusions at a rate of 0.4 mL/h. Fresh supplies of Saline and treatments were provided daily for 11 consecutive days. The cannulation procedure allowed for treatment delivery in an unrestrained bird with continued free access to feed and water.
Feed Intake and BW
Feed intake measurement was obtained every day at 08:00 h, and BW was measured at the beginning and end of the experiments. Individual daily feed intake was measured by recording the weight of feed (g) offered each day minus any unconsumed feed remaining after 24 h. Average feed consumption for each bird during the 11 d was calculated and used in the statistical model. Feed and water were provided ad libitum in all experiments. Individual ADG was calculated by subtracting the initial BW from the final BW and then dividing by 11 (i.e., the number of days between measurements).
Blood Sampling
At the end of each experiment, a single blood sample (3 mL) was collected via the connected cannula from the jugular vein of each bird and placed into a 7-mL tube containing EDTA (Fisher Scientific, Pittsburgh, PA) to prevent clot formation. Aprotinin (500 KIU/mL of blood) was added to the blood immediately after collection to inhibit the activity of proteases. After centrifugation (1,800 × g for 15 min at 4°C), plasma content was collected into 2 mL cryovial tubes and stored at −80 °C until analysis. Concentrations of corticosterone were evaluated using ELISA kits following the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI). Concentrations of GH were evaluated using ELISA kits developed for chickens (Cusabio Biotech, College Park, MD). All samples were run in duplicates in 96-well assays.
Ghrelin Peptide Preparation
Chicken AG (GSS(O_octanoyl) FLSPTYKNIQQQKDTRKPTARLH) and chicken DAG (GSSFLSPTYKNIQQQKDTRKPTARLH) were supplied by (Sigma Aldrich, St. Louis, MO) with a 95% purity. Approximately 100 mg of AG or DAG peptides were dissolved in 25% acetic acid. After solubilization, peptides were further diluted in ultra-pure water to a final concentration of 10 mg/mL (adjusted for purity). The resulting stock solution was aliquoted (1 mL) in cryovial tubes and stored at −80°C. The final treatment doses were prepared in a Saline solution that consisted of sodium citrate (5 mg/mL) and gentamicin sulfate (0.5 mg/mL; Fisher Scientific) that were added to sterile physiological saline to prevent clotting and bacterial contamination.
Statistical Analysis
The criteria used to determine the number of experimental units for the three experiments included a GLMPOWER procedure (SAS, 2009) using the standard deviation and mean of feed intake in chickens from previous research in our laboratory (Taofeek et al., 2018). The number of experimental units was determined using a power (1-β) of 90% and a probability (α) of 1%.
Data from the three experiments were analyzed as a completely randomized design with a factorial arrangement of treatment [PROC MIXED; (SAS version 9.4)]. If a significant two-way interaction existed, polynomial response curves were calculated. When polynomial responses fitted a linear or a quadratic function, significant differences in the intercept and slope were computed by applying dummy variables using a covariance analysis (Steel and Torrie, 1980). When appropriate, orthogonal polynomial contrasts were used to evaluate linear or quadratic effects. PROC IML was used to determine the polynomial coefficients for non-equally spaced doses. Due to limitations in the number of pumps, and space in the environmental controlled physiology laboratory we were able to use 6 cages (experimental units) at a time. Therefore, each experiment was replicated at least 5 times to accommodate all experimental units.
The statistical model for Experiment 1 included the effect of Frequency (2 h or 4 h), Dose (0 nM, 1 Nm, or 4 nM), and the Frequency × Dose interaction. For Experiment 2, the statistical model included the effect of ghrelin Form (AG or DAG), Dose (0 nM, 1 nM, or 4 nM) given in a pulse every 2 h, and the Form × Dose interaction. For Experiment 3 the statistical model included the effect of ghrelin Form (AG or DAG), Dose (0 nM, or 1 nM) given as a continuous infusion, and the Form × Dose interaction.
Doses used in all experiments were carefully selected using information from previous reports (Table 1). Jugular cannula patency ranged from 3 to 15 d. Only birds that completed 11 d of treatment were used in the analysis. Initially, we also tested doses of 0.5 nM/100 g BW/d. However, only a few birds reached the pre-established 11-d treatment. Therefore, data for the 0.5 nM treatment was not included in the statistical analysis and is not reported.
RESULTS
Experiment 1
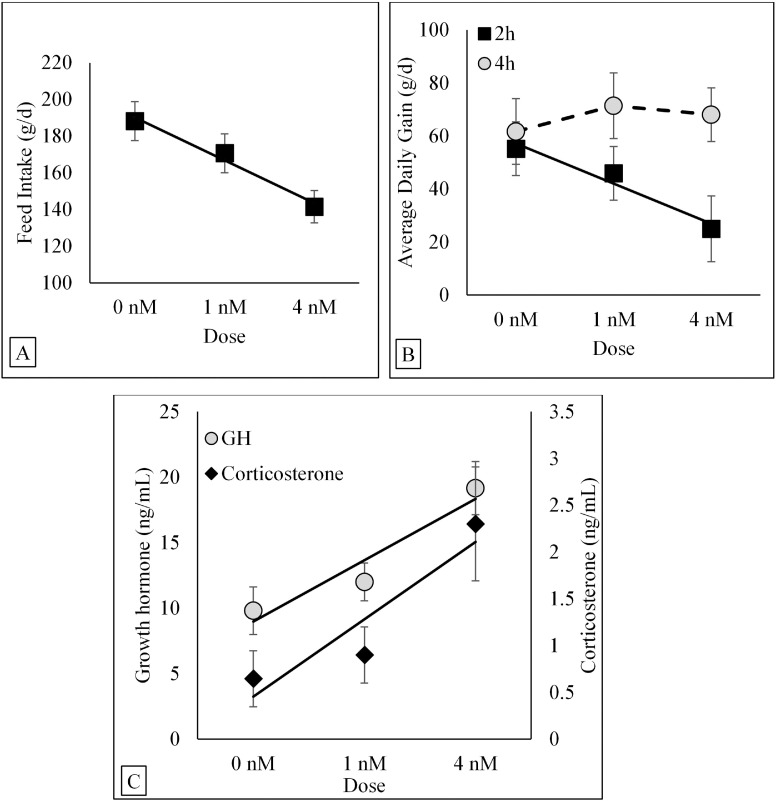
There was no significant Frequency or Frequency × Dose interactions for FI. However, there was a Dose effect (P = 0.003; Figure 1A). Linear orthogonal contrast best described the relationship between AG doses and FI (P = 0.002). Results from the linear regression indicated that there was a decrease of 10.8 ± 3.1 g of FI per each nM/100 g BW/d of exogenous AG systemically infused.
Figure 1.
Least square regressions (solid line) and means (symbols) ± SE for FI (A), ADG (B) and corticosterone and GH concentrations (C) in broiler chickens after systemic infusion of exogenous acylated ghrelin at different doses (0 nM, 1 nM, and 4 nM) and pulse frequencies (2 h or 4 h). There was a Dose effect on FI (P = 0.003). The solid line indicates a dose-dependent decrease of FI. There was a Frequency × Dose interaction on ADG (P = 0.086). The solid line indicates a linear dose-dependent decrease in average daily gain, whereas the broken line indicates the absence of a significant linear or quadratic function. There was a Dose effect on corticosterone (P = 0.033) and GH (P = 0.011) concentrations. The solid lines indicate a linear dose-dependent increase of corticosterone and GH concentrations. Treatments were delivered via programable pumps for eleven consecutive days throughout a 24 h period. Abbreviations: 2h, infused (i.v.) once every 2 h; 4h, infused (i.v.) once every 4 h; GH, growth hormone.
There was a Frequency × Dose interaction (P = 0.086) for ADG (Figure 1B). When polynomial response curves were evaluated, a linear model best described (P = 0.011) the association between Dose and ADG when AG was given at a frequency of 1 pulse every 2 h. Results from the linear regressions indicated that there was a decrease of 8.3 ± 2.3 g in ADG per each nM/100 g BW/d of exogenous AG systemically infused. However, there was no significant polynomial response (linear or quadratic) between Dose and ADG when AG was given at a frequency of 1 pulse every 4 h.
There was no significant Frequency or Frequency × Dose interactions for corticosterone and GH concentrations. However, there were Dose effects for corticosterone (P = 0.033) and GH (P = 0.011) concentrations (Figures 1C). Linear orthogonal contrast best described the relationship between AG doses and corticosterone and GH concentrations. Polynomial contrasts showed that AG doses linearly increased corticosterone and GH. Results from the linear regression indicated that AG increased concentrations of corticosterone (0.6 ± 0.2 ng/mL) and GH (2.2 ± 0.5 ng/mL) per each nM/100 g BW/d of exogenous AG systemically infused.
Experiment 2
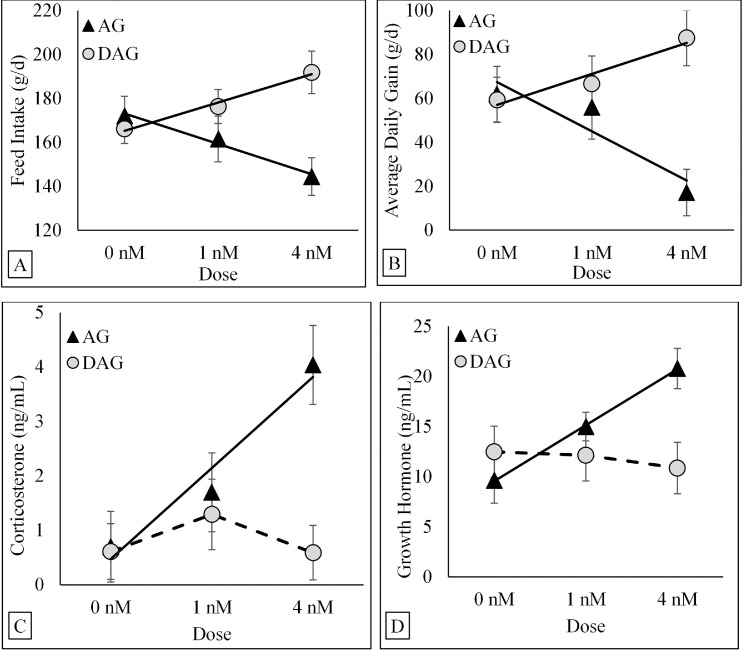
A Form × Dose interaction (P < 0.001) for FI was best described by linear regression equations (Figure 2A). When comparing both regression lines using dummy variables, FI in birds receiving 0 nM/g BW/d (linear intercept) was not influenced by ghrelin forms (166.1 ± 6.4, and 172.0 ± 8.6 g/d; DAG and AG, respectively). However, there were significant differences in the slopes (P = 0.004). Feed intake linearly increased with doses in birds receiving DAG. Results from linear regressions indicated that there was an increase of 6.7 ± 2.5 g of FI per each nM/100 g BW/d of exogenous DAG systemically infused. However, FI linearly decreased with doses in birds receiving AG. Results from linear regressions indicated that there was a decrease of 7.6 ± 3.2 g of FI per each nM/100 g BW/d of exogenous AG systemically infused.
Figure 2.
Least square regression (solid lines) and mean (symbols) ± SE for FI (A), ADG (B), corticosterone (C), and GH concentrations (D) in broiler chickens after systemic infusion of two ghrelin forms (AG and DAG) at different doses (0 nM, 1 nM, and 4 nM) given at a frequency of 1 pulse every 2 h. There was a Form x Dose interaction on FI (P < 0.001) and ADG (P = 0.034). The solid lines indicate a linear dose-dependent increase of FI and ADG when DAG was infused and a linear dose-dependent decrease of FI and ADG when AG was infused. There was a Form × Dose interaction on corticosterone concentrations (P = 0.008). The solid line indicates a linear dose-dependent increase in corticosterone concentrations when AG was infused, whereas the broken line indicates the absence of a significant linear or quadratic function when DAG was infused. There was a Form × Dose interaction on GH concentrations (P < 0.001). The solid line indicates a linear dose-dependent increase in GH concentrations when AG was infused, whereas the broken line indicates the absence of a significant linear or quadratic function when DAG was infused. Treatments were delivered via programable pumps for eleven consecutive days throughout a 24 h period. Abbreviations: AG, chicken acylated-ghrelin; DAG, chicken desacylated-ghrelin.
In congruence with FI data, there was also a Form × Dose interaction (P = 0.034) for ADG. Linear regression equations best described the association between ghrelin forms and doses (Figure 2B). When comparing both regression lines using dummy variables, ADG in birds receiving 0 nM/g BW/d (linear intercept) was not influenced by ghrelin forms (59.3 ± 10.3, and 61.9 ± 12.6 g/d; DAG and AG, respectively). However, there were significant differences in the slopes (P = 0.002). Body weight gain linearly increased with doses in birds receiving DAG. Results from linear regressions indicated that there was an increase of 8.6 ± 4.5 g/d of ADG intake per each nM/100 g BW/d of exogenous DAG systemically infused. On the other hand, ADG linearly decreased with doses in birds receiving AG. Results from linear regressions indicated that there was a decrease of 12.2 ± 2.8 g/d of ADG per each nM/100 g BW/d of exogenous AG systemically infused.
There was a Form × Dose interaction (P = 0.008) for corticosterone concentrations (Figure 2C). When polynomial response curves were evaluated, a linear model best described (P = 0.017) the association between doses and corticosterone concentrations when exogenous AG was given. Results from the linear regression indicated that AG increases the concentration of corticosterone by 0.9 ± 0.3 ng/mL per each nM/100 g BW/d of exogenous AG systemically infused. However, there was no significant polynomial response (linear or quadratic) between doses and corticosterone concentrations when exogenous DAG was given.
Similarly, there was a Form × Dose interaction (P < 0.001) for GH concentrations (Figure 2D). When polynomial response curves were evaluated, a linear model best described (P < 0.001) the association between doses and GH concentrations when exogenous AG was given. Results from the linear regression indicated that AG increased concentrations of GH by 2.8 ± 0.5 ng/mL per each nM/100 g BW/d of exogenous AG systemically infused. However, there was no significant polynomial response (linear or quadratic) between doses and GH concentrations when exogenous DAG was given.
Experiment 3
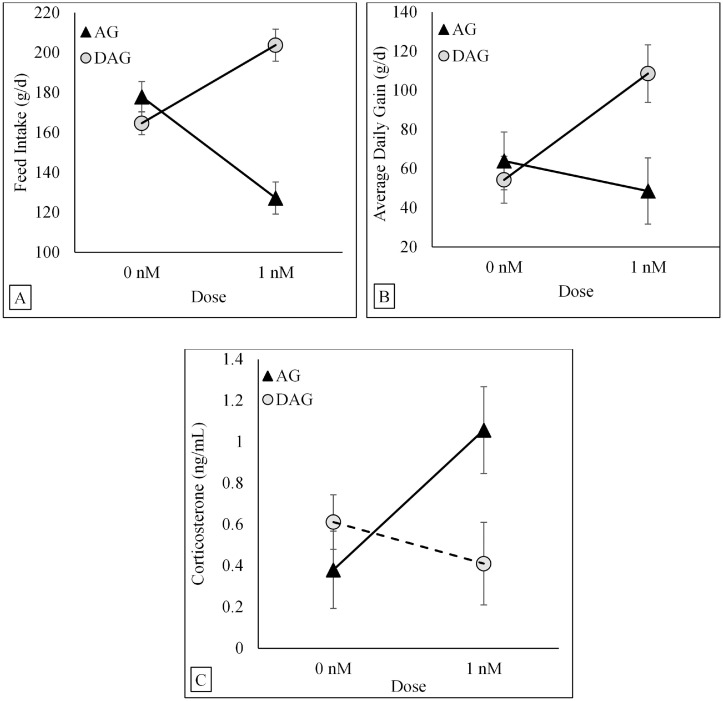
A Form × Dose interaction (P < 0.001) for FI was described by linear regression equations (Figure 3A). When comparing both regression lines using dummy variables, FI in birds receiving 0 nM/g BW/d (linear intercept) was not influenced by ghrelin forms (164.6 ± 5.7 and 177.8 ± 7.7 g/d; DAG and AG, respectively). However, there were significant differences in the slopes (P < 0.001). Feed intake linearly increased with doses in birds receiving a continuous infusion of exogenous DAG (slope = 39.1 ± 9.9 g) whereas FI decreased linearly in birds receiving a continuous infusion of exogenous AG (slope = -50.7 ± 10.9 g).
Figure 3.
Least square regression (solid lines) and mean (symbols) ± SE for FI (A), ADG (B), and corticosterone concentrations (C) in broiler chickens after systemic infusion of two ghrelin forms (AG and DAG) at two doses (0 nM and 1 nM) infused continually. There was a Form × Dose interaction on FI (P < 0.001) and ADG (P = 0.034). The solid lines indicate a linear increase of FI and ADG when DAG was infused and a linear decrease of FI and ADG when AG was infused. There was a Form × Dose interaction on corticosterone concentrations (P = 0.038). The solid line indicates a linear increase in corticosterone concentrations when AG was infused, whereas the broken line indicates the absence of a significant linear function when DAG was infused. Treatments were delivered via programable pumps for eleven consecutive days throughout a 24 h period. Abbreviations: AG, chicken acylated-ghrelin; DAG, chicken desacylated-ghrelin.
A Form × Dose interaction (P = 0.034) for ADG was described by linear regression equations (Figure 3B). When comparing both regression lines using dummy variables, ADG in birds receiving 0 nM/g BW/d (linear intercept) was not influenced by ghrelin forms (54.3 ± 12.0 and 63.9 ± 14.7 g/d; DAG and AG, respectively). However, there were significant differences in the slopes (P = 0.034). Average daily gain linearly increased with doses in birds receiving a continuous infusion of exogenous DAG (slope = 54.3 ± 22.3 g/d) whereas FI decreased linearly in birds receiving a continuous infusion of exogenous AG (slope = −15.4 ± 13.9 g/d).
There was a Form × Dose interaction (P = 0.038) for corticosterone concentrations (Figure 3C). A linear model described (P = 0.022) the association between doses and corticosterone concentrations when exogenous AG was given (slope = 0.68 ± 0.19). However, there was no linear response (slope not different from zero) between doses and corticosterone concentrations when exogenous DAG was given.
There was no significant Form, Dose, or Form × Dose interactions for GH concentrations. On average, concentrations of GH were 16.6 ± 3.6 ng/mL.
DISCUSSION
The data presented in the current study clearly shows that exogenous AG doses resulted in an inhibitory effect of FI when infused as a pulse every 2 h or continuously. When 0.6 nM/100g BW of AG was given (i.v.) to layer chickens, or when 0.1 to 0.5 nM/bird of AG were given (i.p.) to migratory birds, no differences in FI were observed (Kaiya et al., 2007; Goymann et al., 2017). The relative lower doses, treatment delivery, age, and type of bird can explain differences with the present experiment. Nevertheless, the vast majority of authors that reported the effect of exogenous AG on feed intake (Table 1) agree with our data. The anorexigenic effect of AG in chicken broilers was evident in the present experiment and it was associated with decreased ADG. Additionally, there were several orders of magnitude differences in the relationship between AG and FI when exogenous AG was infused continuously (regression slope = −50.7 ± 10.9 g/d) compared with pulsatile infusions (regression slope −10.8 ±3.1 and −7.6 ± 3.2 g/d; Experiment 1 and 2, respectively). A sustained increase in AG appears to be necessary to elicit a physiological response to the anorexic effect of acylated-ghrelin and the associated decrease of ADG.
In contrast to the AG results, DAG doses resulted in increased FI when given as a pulse every 2 h or continuously. Our results are in conflict with the 2 other publications that reported the use of DAG infusions in birds. When 0 to 0.1 nM/bird of DAG were given (ICV) to layer chickens, or when 0.1 to 0.5 nM/bird of DAG were given (i.p.) to migratory birds, no differences (Tachibana et al., 2011) or a decrease (Goymann et al., 2017) in FI was observed. The route of administration, type of bird, treatment delivery, and age of birds (among others) can explain differences between results. Nevertheless, co-injection of DAG and AG attenuated the AG-induced anorexia in chickens (Tachibana et al., 2011). The differential effect DAG and AG on feed intake has also been reported in rats and goldfishes (Chen et al., 2005; Matsuda et al., 2006). In fact, it has been shown that DAG can independently have other physiological effects in mammals, including vasodilation and apoptosis (Chung et al., 2008; Ku et al., 2015).
The orexigenic effect of DAG in chicken broilers was clear in the present experiment and was also associated with increased ADG. We also noted that there were several orders of magnitude differences in the slope of ADG when DAG was infused continuously (slope = 39.1 ± 9.9 g/d) compared with pulsatile infusions (slope = 6.7 ± 2.5 g/d, Experiment 2). Given the low affinity of DAG to GHSR-1a, our data and that from others (Cassoni et al., 2004; Toshinai et al., 2006), points to the presence of a still unknown DAG specific receptor (or another elusive mechanism) that requires a sustained increase in DAG to elicit an effective physiological response.
Corticosterone concentrations were increased when AG was infused with a pulse every 2 h (Experiment 2) or continuously (Experiment 3). These data agree with the direct effect of AG on the secretion of CRH as previously reported (Kaiya et al., 2002; Saito et al., 2005). In turn, adrenocorticotropic hormone stimulates the secretion of corticosterone from the adrenal glands. However, no changes in corticosterone were found when DAG was infused at any dose or frequency (Experiments 2 and 3). As noted above, the incorporation of an octanoic acid in ghrelin is required for the activation of the GHSR-1a receptor, while the affinity of des-acyl-ghrelin to GHSR-1a is minimal. Data suggest that DAG's modus operandi does not involve the HPA axis.
The ability of AG to stimulate GH release is by binding to the GHSR-1a that, in turn, activates the phospholipase C and protein kinase C pathway leading to calcium influx, resulting in GH secretion (Kohno et al., 2003). Findings from this study on the stimulatory effect of AG on GH are in line with other studies (Ahmed and Harvey, 2002). However, the increased secretion of GH was observed only when AG was given as pulse every 2 h (Experiment 2) but not when it was infused continuously (Experiment 3) suggesting a potential downregulation of the receptor as previously reported (Geelissen et al., 2003). Collectively, data from the present experiments indicate that the pulsatile nature of acylated ghrelin is necessary to elicit a physiological response for the release of GH, whereas a sustained increase in AG is more effective in decreasing feed intake. On the other hand, DAG did not elicit an increase in GH when it was infused with a pulse every 2 h (Experiment 2) or continuously (Experiment 3). Taken together, the inability of DAG to trigger the release of corticosterone and GH suggests a mechanism of action independent of AG.
In conclusion, our data demonstrate that doses, pulse frequency, and ghrelin forms differentially affect feed intake, body weight gain, and concentrations of corticosterone and GH in broiler chickens. The endocrine control of appetite in chickens by ghrelin appears to be governed by the 2 forms of ghrelin (i.e., AG and DAG) working in opposite directions. However, only AG has the ability to modulate GH (when infused in pulses every 2 h) and corticosterone secretion, whereas DAG is not involved in the secretion of corticosterone or GH.
ACKNOWLEDGMENTS
This project was supported by Agriculture and Food Research Initiative Competitive Grant #2016-67016-24945 from the USDA National Institute of Food and Agriculture.
DISCLOSURES
No personal, professional or financial conflicts of interest are associated with any of the authors.
REFERENCES
- Ahmed S., Harvey S. Ghrelin: a hypothalamic GH-releasing factor in domestic fowl (Gallus domesticus) J. Endocrinol. 2002;172:117–125. doi: 10.1677/joe.0.1720117. [DOI] [PubMed] [Google Scholar]
- Bagnasco M., Kalra P.S., Kalra S.P. Ghrelin and leptin pulse discharge in fed and fasted rats. Endocrinology. 2002;143:726–729. doi: 10.1210/endo.143.2.8743. [DOI] [PubMed] [Google Scholar]
- Buyse J., Janssen S., Geelissen S., Swennen Q., Kaiya H., Darras V.M., Dridi S. Ghrelin modulates fatty acid synthase and related transcription factor mRNA levels in a tissue-specific manner in neonatal broiler chicks. Peptides. 2009;30:1342–1347. doi: 10.1016/j.peptides.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Callaghan B., Kosari S., Pustovit R.V., Sartor D.M., Ferens D., Ban K., Baell J., Nguyen T.V., Rivera L.R., Brock J.A. Hypotensive effects of ghrelin receptor agonists mediated through a novel receptor. Br. J. Pharmacol. 2014;171:1275–1286. doi: 10.1111/bph.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoni P., Ghé C., Marrocco T., Tarabra E., Allia E., Catapano F., Deghenghi R., Ghigo E., Papotti M., Muccioli G. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur. J. Endocrinol. 2004;150:173–184. doi: 10.1530/eje.0.1500173. [DOI] [PubMed] [Google Scholar]
- Ceron-Romero N., Taofeek N., Thomas A., Vroonland E., Sanmartin K., Verghese M., Heinen E., Vizcarra J. Capromorelin, a ghrelin receptor agonist, increases feed intake and body weight gain in broiler chickens (gallus gallus domesticus) Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., Chao Y., Chang F.Y., Chien E.J., Lee S.D., Doong M.L. Intracisternal des-acyl ghrelin inhibits food intake and non-nutrient gastric emptying in conscious rats. Int. J. Mol. Med. 2005;16:695–699. [PubMed] [Google Scholar]
- Chung H., Seo S., Moon M., Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J. Endocrinol. 2008;198:511–521. doi: 10.1677/JOE-08-0160. [DOI] [PubMed] [Google Scholar]
- Date Y., Murakami N., Kojima M., Kuroiwa T., Matsukura S., Kangawa K., Nakazato M. Central effects of a novel acylated peptide, ghrelin, on growth hormone release in rats. Biochem. Biophys. Res. Commun. 2000;275:477–480. doi: 10.1006/bbrc.2000.3342. [DOI] [PubMed] [Google Scholar]
- Eikenaar C., Hessler S., Ballstaedt E., Schmaljohann H., Kaiya H. Ghrelin, corticosterone and the resumption of migration from stopover, an automated telemetry study. Physiol. Behav. 2018;194:450–455. doi: 10.1016/j.physbeh.2018.06.036. [DOI] [PubMed] [Google Scholar]
- Farrokhi R., Babapour V., Zendehdel M., Asghari A., Gilanpour H. Role of dopaminergic and cannabinoidergic receptors on ghrelin-induced hypophagia in 5-day-old broiler chicken. Arch. Razi Inst. 2021;76:941–954. doi: 10.22092/ari.2020.351261.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frecka J.M., Mattes R.D. Possible entrainment of ghrelin to habitual meal patterns in humans. Am. J. Physiol-Gastro. Liver Physiol. 2008;294:G699–G707. doi: 10.1152/ajpgi.00448.2007. [DOI] [PubMed] [Google Scholar]
- Furuse M., Tachibana T., Ohgushi A., Ando R., Yoshimatsu T., Denbow D.M. Intracerebroventricular injection of ghrelin and growth hormone releasing factor inhibits food intake in neonatal chicks. Neurosci. Lett. 2001;301:123–126. doi: 10.1016/s0304-3940(01)01621-4. [DOI] [PubMed] [Google Scholar]
- Gauna C., Van de Zande B., Van Kerkwijk A., Themmen A.P., van der Lely A.-J., Delhanty P.J. Unacylated ghrelin is not a functional antagonist but a full agonist of the type 1a growth hormone secretagogue receptor (GHS-R) Mol. Cell. Endocrinol. 2007;274:30–34. doi: 10.1016/j.mce.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Geelissen S.M., Beck I.M., Darras V.M., Kuhn E.R., Van der Geyten S. Distribution and regulation of chicken growth hormone secretagogue receptor isoforms. Gen. Comp. Endocrinol. 2003;134:167–174. doi: 10.1016/s0016-6480(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Geelissen S.M., Swennen Q., Geyten S.V., Kuhn E.R., Kaiya H., Kangawa K., Decuypere E., Buyse J., Darras V.M. Peripheral ghrelin reduces food intake and respiratory quotient in chicken. Domest. Anim. Endocrinol. 2006;30:108–116. doi: 10.1016/j.domaniend.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Goymann W., Lupi S., Kaiya H., Cardinale M., Fusani L. Ghrelin affects stopover decisions and food intake in a long-distance migrant. Proc. Natl. Acad. Sci. U. S. A. 2017;114:1946–1951. doi: 10.1073/pnas.1619565114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J.A., Solenberg P.J., Perkins D.R., Willency J.A., Knierman M.D., Jin Z., Witcher D.R., Luo S., Onyia J.E., Hale J.E. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B., Cygankiewicz A., Jensen T.H., Ankersen M., Schwartz T.W. High constitutive signaling of the ghrelin receptor–identification of a potent inverse agonist. Mol. Endocrinol. 2003;17:2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- Hosoda H., Kojima M., Matsuo H., Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- Howard A.D., Feighner S.D., Cully D.F., Arena J.P., Liberator P.A., Rosenblum C.I., Hamelin M., Hreniuk D.L., Palyha O.C., Anderson J. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Kaiya H., Saito E.S., Tachibana T., Furuse M., Kangawa K. Changes in ghrelin levels of plasma and proventriculus and ghrelin mRNA of proventriculus in fasted and refed layer chicks. Domest. Anim. Endocrinol. 2007;32:247–259. doi: 10.1016/j.domaniend.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Kaiya H., Van Der Geyten S., Kojima M., Hosoda H., Kitajima Y., Matsumoto M., Geelissen S., Darras V.M., Kangawa K. Chicken ghrelin: purification, cDNA cloning, and biological activity. Endocrinology. 2002;143:3454–3463. doi: 10.1210/en.2002-220255. [DOI] [PubMed] [Google Scholar]
- Khan M.S., Kaiya H., Tachibana T. Central injection of urocortin-3 but not corticotrophin-releasing hormone influences the ghrelin/GHS-R1a system of the proventriculus and brain in chicks. Domest. Anim. Endocrinol. 2014;47:27–34. doi: 10.1016/j.domaniend.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Khan M.S.I., Dodo K.-I., Yahata K., Nishimoto S., Ueda H., Taneike T., Kitazawa T., Hosaka Y., Bungo T. Intracerebroventricular administration of growth hormone releasing peptide-6 (GHRP-6) inhibits food intake, but not food retention of crop and stomach in neonatal chicks. J. Poult. Sci. 2006;43:35–40. [Google Scholar]
- Kohno D., Gao H.-Z., Muroya S., Kikuyama S., Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Koutkia P., Canavan B., Breu J., Johnson M.L., Grinspoon S.K. Nocturnal ghrelin pulsatility and response to growth hormone secretagogues in healthy men. Am. J. Physiol. Endocrinol. Metab. 2004;287:E506–E512. doi: 10.1152/ajpendo.00548.2003. [DOI] [PubMed] [Google Scholar]
- Ku J.M., Andrews Z.B., Barsby T., Reichenbach A., Lemus M.B., Drummond G.R., Sleeman M.W., Spencer S.J., Sobey C.G., Miller A.A. Ghrelin-related peptides exert protective effects in the cerebral circulation of male mice through a nonclassical ghrelin receptor(s) Endocrinology. 2015;156:280–290. doi: 10.1210/en.2014-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K., Li C., Perrin M., Blount A., Kunitake K., Donaldson C., Vaughan J., Reyes T., Gulyas J., Fischer W. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Miura T., Kaiya H., Maruyama K., Shimakura S., Uchiyama M., Kangawa K., Shioda S. Regulation of food intake by acyl and des-acyl ghrelins in the goldfish. Peptides. 2006;27:2321–2325. doi: 10.1016/j.peptides.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Nass R., Farhy L.S., Liu J., Prudom C.E., Johnson M.L., Veldhuis P., Pezzoli S.S., Oliveri M.C., Gaylinn B.D., Geysen H.M., Thorner M.O. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J. Clin. Endocrinol. Metab. 2008;93:1988–1994. doi: 10.1210/jc.2007-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalucci G., Riedl S., Gleiss A., Zidek T., Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur. J. Endocrinol. 2005;152:845–850. doi: 10.1530/eje.1.01919. [DOI] [PubMed] [Google Scholar]
- Ocłoń E., Pietras M. Peripheral ghrelin inhibits feed intake through hypothalamo-pituitary-adrenal axis-dependent mechanism in chicken. J. Anim. Feed Sci. 2011;20:118–130. [Google Scholar]
- Qin J., Cai Y., Xu Z., Ming Q., Ji S.-Y., Wu C., Zhang H., Mao C., Shen D.-D., Hirata K., Ma Y., Yan W., Zhang Y., Shao Z. Molecular mechanism of agonism and inverse agonism in ghrelin receptor. Nat. Commun. 2022;13:300. doi: 10.1038/s41467-022-27975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H.Kaiya, Takagi T., Yamasaki I., Denbow D.M., Kangawa K., Furuse M. Chicken ghrelin and growth hormone-releasing peptide-2 inhibit food intake of neonatal chicks. Eur. J. Pharmacol. 2002;453:75–79. doi: 10.1016/s0014-2999(02)02393-2. [DOI] [PubMed] [Google Scholar]
- Saito E.S., Kaiya H., Tachibana T., Tomonaga S., Denbow D.M., Kangawa K., Furuse M. Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul. Pept. 2005;125:201–208. doi: 10.1016/j.regpep.2004.09.003. [DOI] [PubMed] [Google Scholar]
- SAS SAS User's Guide, Statistics. 2009 [Google Scholar]
- Shousha S., Nakahara K., Kojima M., Miyazato M., Hosoda H., Kangawa K., Murakami N. Different effects of peripheral and central ghrelin on regulation of food intake in the Japanese quail. Gen. Comp. Endocrinol. 2005;141:178–183. doi: 10.1016/j.ygcen.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Steel R., Torrie J. Principles and procedures of statistics: A biometrical approach. 2nd Ed. McGraw-Hill Higher Education; New York: 1980. [Google Scholar]
- Taati M., Nayebzadeh H., Khosravinia H. The role of the histaminergic system on the inhibitory effect of ghrelin on feed intake in broiler chickens. Iran. J. Vet. Res. 2010;11:38–45. [Google Scholar]
- Tachibana T., Tanaka M., Kaiya H. Central injection of des-acyl chicken ghrelin does not affect food intake in chicks. Gen. Comp. Endocrinol. 2011;171:183–188. doi: 10.1016/j.ygcen.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Takaya K., Ariyasu H., Kanamoto N., Iwakura H., Yoshimoto A., Harada M., Mori K., Komatsu Y., Usui T., Shimatsu A., Ogawa Y., Hosoda K., Akamizu T., Kojima M., Kangawa K., Nakao K. Ghrelin strongly stimulates growth hormone release in humans. J. Clin. Endocrinol. Metab. 2000;85:4908–4911. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- Taofeek N., Vizcarra F., Verghese M., Vizcarra J. International Poultry Scientific Forum Atlanta, GA. 2018. Page 1 in the effect of feed restriction on ghrelin concentrations in male broiler chickens. M3. [Google Scholar]
- Toshinai K., Yamaguchi H., Sun Y., Smith R.G., Yamanaka A., Sakurai T., Date Y., Mondal M.S., Shimbara T., Kawagoe T. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147:2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- Tschöp M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Vizcarra J.A., Kreider D.L., Kirby J.D. Episodic gonadotropin secretion in the mature fowl: serial blood sampling from unrestrained male broiler breeders (Gallus domesticus) Biol. Reprod. 2004;70:1798–1805. doi: 10.1095/biolreprod.103.023143. [DOI] [PubMed] [Google Scholar]
- Wren A., Small C., Ward H., Murphy K., Dakin C., Taheri S., Kennedy A., Roberts G., Morgan D., Ghatei M. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Wren A.M., Seal L.J., Cohen M.A., Brynes A.E., Frost G.S., Murphy K.G., Dhillo W.S., Ghatei M.A., Bloom S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]