Abstract
The beneficial action of probiotics is questioned time and again due to the loss of their survivability under gastrointestinal conditions, particularly gastric acid. In this experiment, a probiotic species was encapsulated to improve its delivery to the distal parts, and its effects on production performance, gut health, and microbial profile in broilers were investigated. A total of 240 Arbor acres (AA) broilers were randomly allotted into 3 treatments with 8 replicate pens per treatment and 10 broilers in each pen for 42 d. Dietary treatments were 1) basal feed without any additives (CON), 2) CON+15 ppm Virginiamycin (ANT), and 3) CON+500 ppm encapsulated Lactobacillus paracaesi (ELP). The result showed that the addition of ELP to the feed did not affect growth performance and carcass characteristics significantly. However, ELP increased the ratio of villus height to crypt depth (P < 0.05) and mRNA expression of ZO-1 (P < 0.05) relative to the CON or ANT group. Similarly, qPCR showed that dietary supplementation of ELP raised gene expression of the anti-inflammatory cytokine and tended to decrease proinflammatory cytokines resulting improve in immunity. Moreover, chicks fed with ELP had lower malondialdehyde (MDA) (P < 0.05) than CON and lower reactive oxygen species (ROS) (P < 0.05) level than ANT in serum. In contrast, the total antioxidant capacity (TAOC) level was tended to increase. The ammonia level of ileum and cecum chyme was decreased (P < 0.05) in the ELP group than CON while the level of propionic acid of cecal content was increased (P < 0.05). 16S rRNA sequencing revealed the dietary treatment modulated the diversity and composition of cecal microflora. At the phylum level, Bacteroidetes was enriched, and Proteobacteria was depleted in the ELP group. At the genus level, ELP increased Bacteroides (P < 0.05) compared to control. The results indicate that oral delivery of probiotics via microcapsule could impart beneficial effects on birds and be used as an alternative to antibiotics.
Key words: microencapsulation, Lactobacillus paracaesi, broiler, gut function, RNA sequencing, microflora
INTRODUCTION
Since the early 1950s, antibiotic growth promoters (AGPs) have been widely used to promote growth performance and protect from diseases in the poultry industry. But, due to extensive use of AGPs, several fatalistic consequences have been detected, including the development of drugs resistant pathogens, antibiotic residues in poultry products, and imbalance of gut microflora; thus, its use as feed additives have been banned in Europe (since 2006), United States (since 2014), and China (since 2020) (Dong et al., 2016; Xu et al., 2021). The diverse and complex microbial community plays an essential role in the digestion of food and absorption of nutrients, exclusion of pathogens, immune system development, and promotes the overall health of the host (Wang et al., 2017; Shang et al., 2018). However, an imbalance of gut microbiota by antibiotics may lead to a pathogenic condition affecting the health conditions of animals. So, to improve the gut microbiota, maximize growth performance and improve the health of poultry, safe and efficacious alternatives such as probiotics, prebiotics, synbiotics, exogenous enzymes, organic acid, antimicrobial peptides, and so on were suggested (Huyghebaert et al., 2011; Yadav and Jha, 2019).
Probiotics are live microbes, when ingested, imparts health benefits by modulating gut microbiota. Previously, from the study of many researches, it is reported that probiotics improve poultry's performance and gut health by modifying the intestinal microbiome community, reducing inflammation, stimulating the immune system, and decreasing the excretion of ammonia and urea (Jha et al., 2020). On the other hand, it is also outlined that the same probiotic species could exhibit variable results, and some couldn't improve performance or gut health (Eugenio Bahule and Natalice Santos Silva, 2021). The variation and inconsistent effects of probiotics might be due to differences in the nature of probiotics used (species, survivability, and adaptability to different altitudes), doses and administration methods, and physiological state of birds (Huyghebaert et al., 2011; Kalia et al., 2017; Yadav and Jha, 2019). During storage or transport, exposure to a higher temperature, oxygen levels, and Relative humidity could decrease the viability of probiotic species (Rodrigues et al., 2011). Similarly, ingestion of probiotics needs to encounter several environmental complexities of the GI tract, including lower pH of gastric fluids, high ionic strength and enzyme activity, bile acid, digestive enzymes in the intestine, and so on, that result to lower viable bacteria population reaching to lower intestine due to reduction of their survivability (Sarao and Arora, 2017; Yao et al., 2020). Thus, the beneficial effects of probiotics on poultry are impaired. Microencapsulation is considered an eminent method to protect bacteria from detrimental surroundings and ameliorate the vitality up to the lower intestine (Cook et al., 2012; Yao et al., 2020).
Briefly, in the microencapsulation technique, probiotic is encapsulated inside a specified substance to form a microcapsule. Majorly, microcapsule holds its structure in the upper GI tract and degrades in the specific target site to release bacteria for colonization (Cook et al., 2012). But, many challenges are still reported in this technique. Some microcapsules may not degrade appropriately in the specific site and can excrete without utilization; some others do not show any beneficial effect to the host but may show a toxic effect. Due to the above facts, it is still challenging to find a proper encapsulating material (Chen et al., 2017; Lee et al., 2019). Previously, the impacts of Lactobacillus paracasei are studied and have shown beneficial effects on the host. However, this strain is found to be sensitive to gastric fluid and lower pH (Corcoran et al., 2005). Owing to this fact, we prepared a microcapsule by encapsulating L. paracasei with polyacrylate resin to deliver the probiotic bacteria to the final destination. Thus, coating of L. paracasei can benefit poultry as our encapsulating material, polyacrylate resin; can dissolve on pH around 7, lower intestinal region of poultry. No previous studies are made in livestock or poultry by using this encapsulating material. Similarly, as per our knowledge, no comparative studies are made between antibiotics and encapsulated L. paracasei.
Thus, in this study, we prepared a microcapsule to improve the delivery of probiotic species on the lower intestine to show a beneficial effect on poultry. To study the impact of encapsulated lactic acid bacteria, we investigated the impacts on growth performance, gut health, along with changes in cecal microflora. Meanwhile, we also compared the prepared microcapsule with the antibiotic effect.
MATERIALS AND METHODS
Bird, Experimental Design and Dietary Treatments
A total of 240 healthy 1-day-old Arbor acres (AA) broilers were randomly divided into 3 treatment groups in a completely randomized design. Each group had 8 replicate pens with 10 chicks each. The three dietary treatment groups included: Control group fed with Basal diet without any additives (CON), Basal diet with 15 ppm antibiotics that is, Virginiamycin (ANT), and Basal diet with 500 ppm encapsulated probiotic (ELP). L. paracaesi, a probiotic strain, was encapsulated with polyacrylate resin by Hefei Ansheng Pharmaceutical Technology Co., Ltd, Hefei, China to get encapsulated probiotic, microcapsule. The feeding trial had 2 phases: starter phase (1–21 days) and grower-finisher phase (21–42 d). The basal diet was based on reference to NRC (1994) and NY/T 33-2004, and prepared as per AA feeding manual. The feed composition and nutrient content of a basal diet is tabulated in Table 1. All birds were raised in an environmentally controlled room with continuous lighting. They were fed and provided with water ad libitum. All the experimental procedures were conducted as per the guidelines approved by the Animal Ethics Committee of the South China Agricultural University, Guangzhou, China.
Table 1.
Ingredients and nutrient composition basal diets.
| Starter diet (1–21 d) | Finisher diet (22–42 d) | |
|---|---|---|
| Raw material composition (%) | ||
| Corn | 58.82 | 57.95 |
| Soyabean meal | 26.8 | 24.89 |
| Cotton meal | 3.5 | 3.5 |
| Rapeseed meal | 2 | 2 |
| Wheat middlings | 2 | 2.5 |
| Soyabean oil | 2.58 | 4.89 |
| Calcium hydrogen phosphate | 1.82 | 1.66 |
| Limestone | 1.32 | 1.13 |
| Salt | 0.28 | 0.26 |
| Lysine | 0.38 | 0.37 |
| Methionine | 0.25 | 0.35 |
| Theronine | 0.03 | 0.15 |
| Premix of trace elements1 | 0.1 | 0.1 |
| Vitamin premix2 | 0.02 | 0.02 |
| Choline chloride | 0.1 | 0.1 |
| Sodium bicarbonate | 0 | 0.13 |
| Total | 100 | 100 |
| Nutritional level | ||
| Metabolizable energy(Kcal/kg) | 2,990 | 3,150 |
| Crude protein (%) | 21.8 | 19.8 |
| Calcium (%) | 1 | 0.9 |
| Available phosphorus (%) | 0.45 | 0.4 |
| Lysine (%) | 1.25 | 1.1 |
| Methionine (%) | 0.58 | 0.5 |
| Methionine+Cystine (%) | 0.92 | 0.83 |
| Theronine (%) | 0.82 | 0.73 |
| Tryptophan (%) | 0.23 | 0.2 |
Premix of trace elements (per kilogram of feed): 8 mg of copper, 75 mg of zinc, 80 mg of iron, 100 mg of manganese, 0.15 mg of selenium, and 0.35 mg of iodine.
Vitamin premix (provided per kilogram of feed): Vitamin A 12,500 IU, Vitamin D3 2,500 IU, Vitamin E 30 IU, Vitamin K3 2.65 mg, Vitamin B12 mg, Vitamin B2 6 mg, Vitamin B12 0.025 mg, Biotin 0.0325 mg, Folic acid 1.25 mg, pantothenic acid 12 mg, niacin 50 mg.
Growth Performance
The bodyweight of broilers for each pen was recorded on d 1. Health status, feed consumption, and death of birds were recorded daily. Similarly, on d 21 and 42, body weight was measured after 12 h of fasting on a pen basis. Growth performance was evaluated by calculating average daily body weight gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) during starter (1–21 d), grower (21–42 d), and overall (1–42 d) phases.
Sample Collection
On d 21 and 42, one bird with a body weight similar to mean body weight was selected from each pen, weighted, and euthanized via cervical dislocation. After opening birds, lymphoid organs (Spleen, bursa of fabricus, and thymus) were separated, weighed, and expressed as organ weight per bird's live weight to investigate immune system development. On d 42, blood samples were collected individually via jugular vein in a 10-mL tube, centrifuged (3,500, 4°C, 10 min), and stored at −20°C for subsequent detection and analysis. After bleeding and defeathering, carcass traits, including carcass weight, chest muscle percentage, thigh muscle percentage, and abdominal fat percentage were measured. The relative weight was calculated as a percentage of the lives weight. Similarly, different intestinal segment and chyme samples from ileum and cecum were collected, transferred to liquid nitrogen, and stored at −80°C for further study. Furthermore, 2-cm long duodenum, jejunum, and ileum segments, taken from the midpoint, were separated, rinsed with saline water, and fixed in formalin for histological study.
Intestinal Histomorphometry
Intestinal tissues collected and placed on formalin were dehydrated, embedded in paraffin, and cut into 5-μm sections. The sections were stained with hematoxylin and eosin by mounting on glass slides and observed under Olympus light microscope (Olympus, Tokyo, Japan) for histological examination. Villus height and crypt depth were measured by Image (Image-Pro Plus 6.1 Media Cybernetics, Rockville, MD), and finally, villus height to crypt depth ratio was calculated. These variables were measured on 8 to 10 well-oriented villi and corresponding crypt from each section of all intestinal segments.
Quantitative PCR
Total RNA was extracted from the duodenal and jejunal section of the intestine with RNA extraction kit (Guangzhou Magen Biotechnology Co., Ltd, China) as per the manufacturer's protocol. The purity and concentration of obtained RNA were determined by Nanodrop spectrophotometer. 2 µg of total RNA was reverse transcribed to complementary DNA (cDNA) in compliance with the kit instructions (Takara Bio Inc., Shiga, Japan). The cDNA was mixed with antisense primers, nucleic acid-free water, and SYBR green Real-Time PCR master Mix to get a qPCR reaction with a total volume of 20 µL. The qPCR was carried out with the Applied Biosystems QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific Waltham, MA, USA ). The housekeeping gene β-actin was used as a reference gene, and the relative gene expression of mRNA was determined by 2− ΔΔCt. The primers sequences used for PCR are provided in Table 2.
Table 2.
Primers used for quantitative PCR.
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) |
|---|---|---|
| β-actin | TTGGTTTGTCAAGCAAGCGG | CCCCCACATACTGGCACTTT |
| Claudin- 1 | ATTAAGTTAGAGCCCGGCGT | TTTGCACGGAAAGGAAGGTG |
| ZO-1 | TCATCCTTACCGCCGCATAT | GTTGACTGCTCGTACTCCCT |
| IL-1β | GCTTCATCTTCTACCGCCTG | ACTTAGCTTGTAGGTGGCGA |
| IL-6 | GGAGAAATGCCTGACGAAGC | ATTGGCGAGGAGGGATTTCT |
| TNF-α | TGTTCTATGACCGCCCAGTT | AGCATCAACGCAAAAGGGAA |
| IFN-γ | GATGCCACCTTCTCTCACGA | GGCTGCTGAGGATTGTGAAG |
| IL-10 | CGCTGTCACCGCTTCTTCA | TCCCGTTCTCATCCATCTTCTC |
Biochemical Analysis
Serum malondialdehyde (MDA) and fecal secretory immunoglobulin A (SIgA) were measured by enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Ruifan Biotechnology Co., Ltd., China) as per manufacture's guidelines. urea nitrogen, uric acid (UA), total protein, serum glutamic pyruvic transaminase (GPT), serum glutamic oxaloacetic transaminase (GOT), serum total bile acids (TBA), serum reactive oxygen species (ROS), and hydrogen peroxide (H2O2) were quantified by using the commercial kits of Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Similarly, total antioxidant capacity (TAOC) was computed by a commercial product of Solrabio Life Science Beijing, China. All the contents were measured and calculated as per the manufacturer's instructions. For the ileal and cecal contents, chyme was diluted with double distilled water at the ratio of 1:5 (weight/volume), mixed thoroughly, and centrifuged at 10,000 rpm and 4°C for 5 min. The supernatant obtained was used to measure different contents by following the protocol of the company.
Lactate and Short Chain Fatty Acid Analysis
Cecal samples were used for quantifying short chain fatty acid (SCFA) and lactate by using high performance liquid chromatography (HPLC). In brief, cecal samples, placed at −80°C, were thawed at 4°C and mixed thoroughly. About 0.2 g of thawed feces was diluted with double distilled water at the ratio of 1:5 (W/V), mixed for 10 min by vortex, and centrifuged (10,000 × g, 4°C, 10 min). The supernatant (about 400 µL) was extracted, filtered via a 0.22-μm filter, and injected into a 4.6 mm × 250 mm dimension glass column. Then, the test was carried out as per the guidelines of the company and the parameters of the machine.
16S rRNA Gene Sequencing Analysis
The cecal samples, collected and placed at −80°C, were used for 16S rRNA gene sequencing analysis which was carried out at Beijing Novogene Co., Ltd, China. The steps and procedures followed are described previously (Zhang et al., 2020). In brief, CTAB/SDS method was used for total genome DNA extraction. After monitoring purity and concentration, DNA was diluted to 1 ng/μL using sterile water. The V3–V4 variable regions of the 16S rRNA gene were amplified with universal primer 338F and 806R with a barcode. All PCR reactions were performed with 15 μLof Phusion High-Fidelity PCR Master Mix (New England Biolabs, Inc., MA, USA), about 10 ng template DNAs and 0.2 μM of forward and reverse primers. Loading buffer containing SYB green was mixed with PCR products in equal volume, operated electrophoresis on 2% agarose gel for detection, and purified with Qiagen Gel Extraction Kit (Qiagen, Germany). The sequencing library was generated using the TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, USA), and quality was assessed using a Qubit 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. The library was sequenced on an Illumina NovaSeq platform, and 250 bp paired-end reads were generated.
Bioinformatic and Statistical Analysis
First, Raw fastq data were qualified using FLASH software (version 1.2.7, http://ccb.jhu.edu/software/FLASH/) (Magoč and Salzberg, 2011), and high quality clean tags were obtained according to QIIME (version 1.9.1, http://cle.org/scripts/split_libraries_fastq.html) (Bokulich et al., 2013) quality control process. After detecting chimera sequencing by UCHIME Algorithm, (http://www.drive5.com/usearch/manual/uchime_algo.html) and removing them, effective tags were obtained (Edgar et al., 2011). For operational taxonomic units (OTU) production, sequences analysis was performed by using Uparse software (Uparse version 7.0.10, http://drive5.com/uparse/). Sequences were assigned to OTU at ≥97% similarity (Edgar, 2013). Representative sequences for each OTU were annotated with taxonomic information as per the Mothur method and SILVA (http://www.arb-silva.de/) database (Quast et al., 2013). OTUs abundance information was normalized using a standard of sequence number corresponding to the sample with the least sequences. Beta diversities of the gut microbiota were analyzed by QIIME software (version 1.7.0).
Graphpad Prism 8.0.1 (Chicago, IL) was used to carry out statistical analysis. The results of experiments are expressed as means ± standard error of the mean (SEM). Methods of statistical analyses were chosen as per the design of each experiment and are mentioned in the figure legends, P values of <0.05 were considered statistically significant.
RESULTS
Effect of Dietary Supplementation of Lactobacillus Paracaesi Microcapsule on Growth Performance
The effect of dietary treatment of encapsulated probiotic (ELP) compared to basal feed (CON), and an antibiotic (ANT) in broiler is tabulated in Table 3. In the starter phase (1–21 d), there was no significant difference in body weight gain and feed intake in the ELP group compared to CON. But, FCR was decreased (P < 0.05). Interestingly, we found a rise in the body weight gain and feed intake in the ELP group in the finisher phase (22–42) compared to CON and ANT without significant difference (P > 0.05). However, there was no notable improvement in FCR. In the overall period, the dietary treatment improved FCR in the ELP group numerically compared to the control. Similarly, the effect on mortality was monitored regularly, and mortality % was found to be least in the ELP group at the end of the experiment.
Table 3.
Effect of encapsulated Lactobacillus paracasei on growth performance and mortality of broilers.
| Days | Dietary treatment |
|||
|---|---|---|---|---|
| CON | ANT | ELP | ||
| Average daily body weight gain (ADG) (g/day) | ||||
| 1-21 d | 40.6±0.94b | 43.32±1.09a | 40.81±1.5b | |
| 22-42 d | 94.1±1.58 | 94.74±3.19 | 95.78±4.55 | |
| 1-42d | 64.71±0.94b | 66.84±1.06a | 65.45±1.5ab | |
| Average daily feed intake (ADFI) (g/day) | ||||
| 1–21 d | 54.09±0.96 | 54.84±2.16 | 53.03±2.08 | |
| 22–42 d | 149.94±3.48 | 147.1±3.62 | 152.4±6.26 | |
| 1–42 d | 96.68±0.96 | 96.97±2.16 | 97.61±2.08 | |
| Feed conversion ratio (FCR)(g:g) | ||||
| 1–21 d | 1.32±0.01a | 1.27±0.02c | 1.3±0.02b | |
| 22–42 d | 1.61±0.02a | 1.56±0.02b | 1.59±0.03ab | |
| 1–42 d | 1.5±0.01a | 1.47±0.02b | 1.49±0.02ab | |
| Mortality % (1–42 d) | 3.33±2.98 | 1.73±1.5 | 0.61±0 | |
Treatments: CON = a basal diet; ANT = CON+0.15 ppm Virginiamycin, ELP = CON+500PPM of encapsulated Lactobacillus paracasei.
Means within a row with different letters are statistically significant (P < 0.05).
Dietary Supplementation of Microcapsule Influenced Lymphoid Organ and Carcass Trait
The effect on lymphoid organ and carcass trait is shown in Table 4. All the chickens were healthy throughout the feeding trial period. No significant differences (P > 0.05) were observed for the relative weight of thymus and bursa of fabricus at any age but, the relative weight thymus was highest among others on both 21 d and 42 d. Similarly, spleen relative weight (P < 0.05) was higher on d 42 in the ELP group. Relative weight was measured per live body weight of an animal. Similarly, we observed a notable increase (P = 0.07) in carcass weight (as a percentage of live body weight) as compared to CON but exhibited no significant influence compared to ANT. Similarly, the relative weight of thigh muscle, liver, and abdominal fat was elevated (P > 0.05) in the ELP group. In addition, weight of chest muscle was higher in the ELP group than CON but was lower to ANT numerically (Table 4).
Table 4.
Effect of encapsulated Lactobacillus paracasei on lymphoid organs, carcass quality of broilers.
| Items | CON | ANT | ELP |
|---|---|---|---|
| Full eviscerated percentage | 74.36±1.13b | 76.53±1.02a | 75.61±1.23ab |
| Chest muscle (%) | 29.07±1.69b | 31.36±0.9a | 30.82±1.57ab |
| Thigh muscle (%) | 26.85±1.1 | 27.44±1.31 | 28.26±1.63 |
| Relative liver weight (%) | 1.73±0.13 | 1.77±0.17 | 1.85±0.17 |
| Abdominal fat (%) | 0.82±0.09 | 0.81±0.13 | 0.95±0.31 |
| Thymus (g/kg) (21 d) | 4.43±0.67 | 4.28±0.68 | 4.56±1.42 |
| Thymus (g/kg) (42 d) | 1.24±0.67 | 1.44±0.68 | 1.47±1.42 |
| Spleen (g/kg) (21 d) | 0.93±0.09 | 0.87±0.1 | 0.81±0.09 |
| Spleen (g/kg) (42 d) | 0.88±0.09a | 0.88±0.1a | 1.15±0.09b |
| Bursa of Fabricus (g/kg) (21 d) | 2.27±0.64 | 2.39±0.31 | 2.59±0.53 |
| Bursa of Fabricus (g/kg) (42 d) | 0.5±0.64 | 0.48±0.31 | 0.46±0.53 |
Treatments: CON = a basal diet; ANT = CON+0.15 ppm Virginiamycin; ELP = CON+500PPM of encapsulated Lactobacillus paracasei.
Means within a row with different letters are statistically significant (P < 0.05).
Effect of Encapsulated Probiotic Supplements on Nitrogen Metabolism
The introduction of ELP increased total serum protein (SP) and decreased the ileal urea nitrogen (IUN). Similarly, urea nitrogen in blood serum (SUN) rose relative to the CON group but was lower to ANT. However, we recorded no significant difference among the 3 groups (P > 0.05) in SP, IUN, SUN, and serum uric acid (SUA) (Table 5). Meanwhile, cecal urea nitrogen was decreased (P < 0.05) relative to CON but was not significant with the ANT group (Table 5). In addition, there was a reduction in ammonia content in both ileum and cecum content in the ELP group, which was significant (P < 0.05) relative to control but, no significant difference was observed with respect to the ANT group.
Table 5.
Effect of encapsulated Lactobacillus paracasei on N-metabolism (d 42).
| Item | Dietary treatment |
||
|---|---|---|---|
| CON | ANT | ELP | |
| SP (g/L) | 25.11±3.96 | 26.17±1.93 | 28.93±2.99 |
| SUN (mmol/L) | 0.49±0.19 | 0.75±0.33 | 0.69±0.18 |
| IUN (mmol/L) | 23.36±6.47 | 23.36±6.66 | 21.54±4.41 |
| CUN (mmol/L) | 17.19±4.05a | 11.58±1.78b | 13.92±1.99b |
| SUA (μmol/L) | 186.86±36.96 | 190.69±50.83 | 190.26±32.94 |
| IA (mmol/L) | 11.71±5.17a | 5.79±2.87b | 5.05±3.12b |
| CA (mmol/L) | 3.69±0.52a | 2.87±0.48b | 3.12±0.43b |
Abbreviations: CA, cecal ammonia (N = 8); CUN, cecal urea nitrogen;; IA, illeal ammonia; IUN, ileal urea nitrogen; SP, serum protein; SUN, serum urea nitrogen; UA, serum uric acid.
Treatments: CON = a basal diet; ANT = CON+0.15 ppm Virginiamycin; ELP = CON+500PPM of encapsulated Lactobacillus paracasei.
Means within a row with different letters are statistically significant (P < 0.05).
Dietary Supplementation of Probiotic Microcapsule Improved Intestinal Morphology and Barrier Integrity
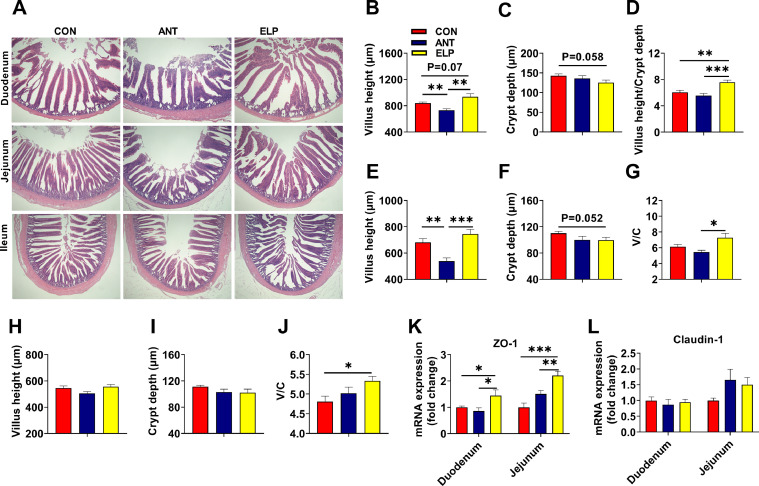
Administration of encapsulated L. paracasei significantly affected the morphometry of intestinal tissues and overall tissues were normal (Figure 1A). Supplementation of encapsulated probiotics increased villus height significantly or numerically in all the intestinal sections over control and antibiotics. Similarly, crypt depth was decreased numerically as compared to CON and ANT groups. Meanwhile, we calculated and found the villus height to crypt depth ratio in the duodenum, jejunum, and ileum section was increased in the ELP group. Moreover, the feeding of encapsulated L. paracasei showed a more significant effect on duodenum and jejunum morphology as compared to the ileum (Figures 1B–1J). Similarly, the expression of junction protein (JP) was quantified by qPCR. The expression of ZO-1 was increased (P < 0.05) in the duodenum of the ELP group (Figure 1K). A similar trend was seen in the jejunum section, where the level of ZO-1 was raised (P < 0.01; Figure 1K). But, the effect of ELP was found to be less effective on another JP, claudin-1. No significant differences were observed in their expression in both duodenum and jejunum sections of the intestine (Figure 1L).
Figure 1.
Effect of dietary supplementation of encapsulated Lactobacillus paracasei on intestinal morphology and barrier integrity: HE staining showing the morphological structure of intestinal tissues (A), villus height, crypt depth and ratio of villus height to crypt depth(V/C) of djuodenum (B–D), jejunum (E–G), and ileum (H–J), expression of tight junction proteins of duodenum and jejunum quantified against housekeeping gene β- actin: ZO-1 (K), Claudin-1 (L) (n = 6–8 per group). The data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Treatments: CON = a basal diet; ANT = CON+0.15 ppm Virginiamycin; ELP = CON+500PPM of encapsulated Lactobacillus paracasei.
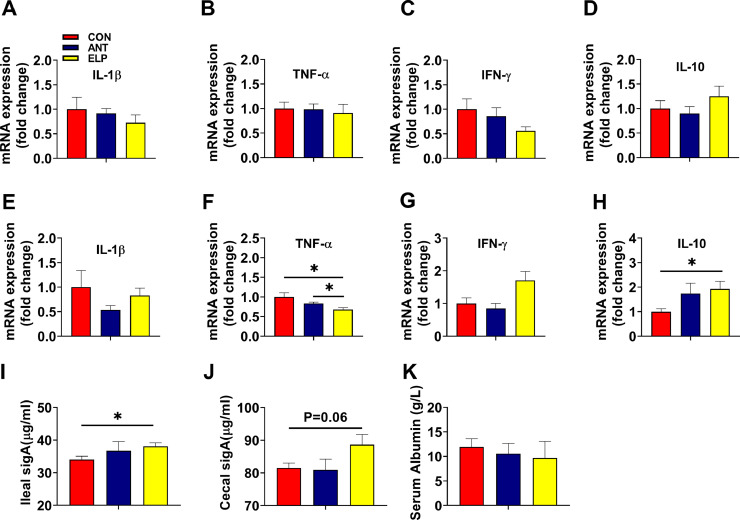
Dietary Supplementation of Probiotic Microcapsule Improved Intestinal Inflammation and Immunity
In the foregut (duodenum), the supplementation of ELP was associated with the downregulation of proinflammatory cytokines, IL-1β, TNF-α, and IFN-γ (Figures 2A–2C). However, the difference among the group was not significant (P > 0.05). Meanwhile, the expression of anti-inflammatory cytokines, IL-10 was upregulated (Figure 2D) but was not statistically different. A similar result was observed in the expression of IL-1β, TNF-α, and IL-10 in the jejunum (Figures 2E, 2F, 2H) but, IFN-γ expression was elevated (Figure 2G). TNF-α expression was notably reduced (P < 0.05) relative to CON and ANT (Figure 2F). Similarly, IL-10 expression was upraised (P < 0.05) in the ELP group compared to CON (Figure 2H). Meanwhile, SIgA and albumin levels in ileal and cecal chyme were measured. Encapsulated Lactobacillus raised the level of Ileal SIgA (P < 0.05) and cecal SIgA (P = 0.06) compared to the CON group, but we didn't notice any significant difference relative to the ANT group (Figures 2I and 2J). Similarly, the level of serum albumin was similar in all three groups (Figure 2K).
Figure 2.
Effects of dietary supplementation of encapsulated Lactobacillus paracasei on inflammation and immunity; mRNA expression of IL-1β, TNF-α, IFN-γ, IL-10:on duodenum (A–D) and jejunum (E–H), Ileal SigA (I), cecal SigA (J), and serum albumin (K). (n=6-8 per group). The data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Treatments: CON = a basal diet; ANT = CON+0.15 ppm Virginiamycin; ELP = CON+500PPM of encapsulated Lactobacillus paracasei.
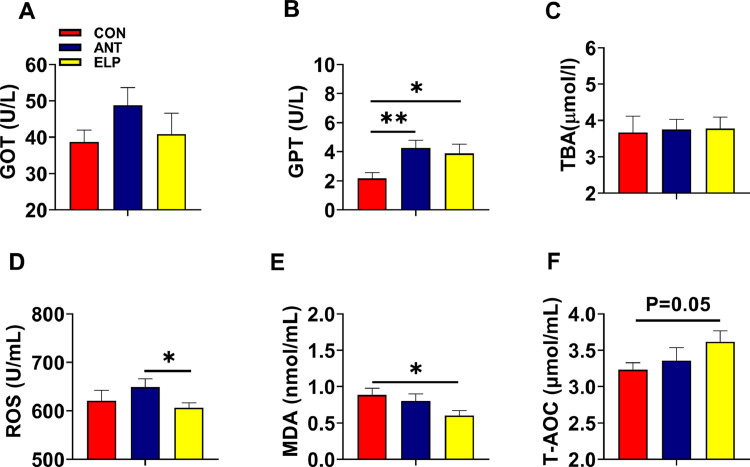
Effect of Encapsulated Probiotic on Liver Enzymes and intestinal redox reaction
We first determined the level of liver-specific enzyme GPT and GOT. We didn't observe any remarkable change in GOT level among the groups, but it was reduced (P > 0.05) in the ELP group relative to the ANT group (Figure 3A). Interestingly, we noticed an elevation of GPT level (P < 0.05) in the ELP group compared to CON (Figure 3B). Similarly, we examined the level of serum bile acid, which is an indicator of liver function and a marker of hepatobiliary diseases, and we found similar in its level among all the groups (Figure 3C). Meanwhile, we observed the content of ROS and MDA were lowest in the ELP group. The value of ROS was reduced (P < 0.05) in the ELP group compared to ANT (Figure 3D), while MDA was downregulated (P < 0.05) compared to control (Figure 3E). When we detected the total antioxidant capacity (TAOC) of serum, we observed that the TAOC level was highest in the ELP group and was relatively higher (P = 0.05) than CON (Figure 3F).
Figure 3.
Effect of dietary supplementation of encapsulated Lactobacillus paracasei on oxidative damage and antioxidant capacity. Serum glutamic oxaloacetic transaminase (GOT) (A), Serum Glutamic Pyruvic Transaminase (GPT) (B), serum total bile acid (TBA) (C), serum reactive oxygen species (ROS) (D), serum Malondialdehyde (MDA) (E), Total antioxidant capacity (T-AOC) (F (n = 6–8 per group). The data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Treatments: CON = a basal diet; ANT = CON+0.15 ppm Virginiamycin; ELP = CON+500PPM of encapsulated Lactobacillus paracasei.
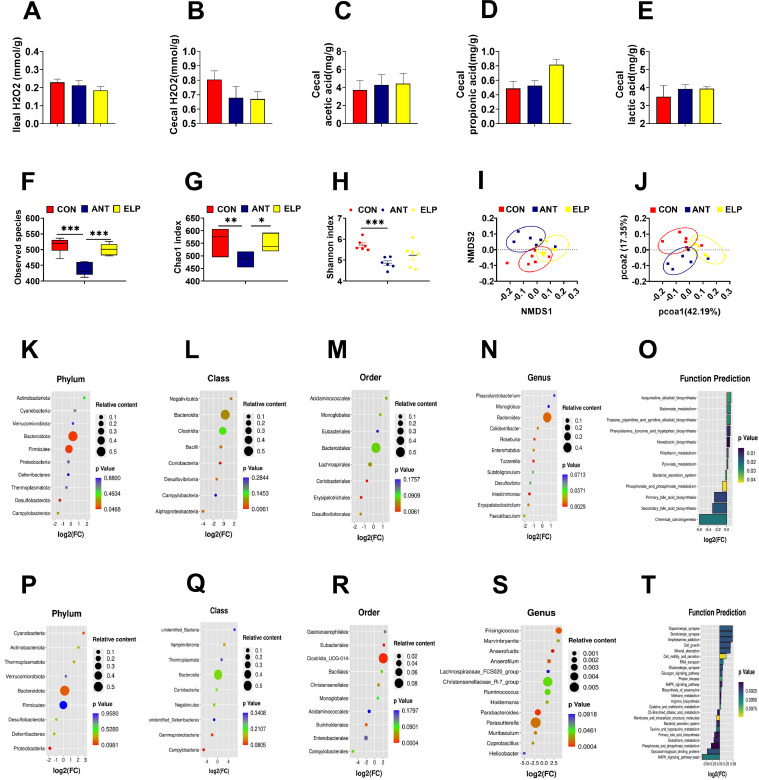
Supplementation of Encapsulated Probiotics Modulated the Diversity and Composition of Cecal Microbiota
We detected the level of hydrogen peroxide (H2O2) of ileal and cecal content, and we didn't notice any significant change (P > 0.05) among the groups, but the level was found to be lower in the ELP group compared to others (Figures 4A–4B). Similarly, the effects of dietary supplementation of encapsulated probiotics on the cecal SCFA and lactate are shown in Figures 4C–4E. The treatment had shown a negligible effect. No distinct differences (P > 0.05) were observed in the concentration of acetate and lactate among the groups but, the propionic acid level was higher (P < 0.05) in the ELP group compared to CON.
Figure 4.
Effect of dietary supplementation of encapsulated Lactobacillus paracasei on cecal microbiota; ileal H2O2 (A), cecal H2O2 (B), cecal lactic acid (C), cecal acetic acid (D, ccecal propionic acid (E) α diversity by observed species (F), Chao index (G) and Shannon index (H), β diversity by nonmetric multidimensional scaling (NMDS) (I), and principal coordinate analysis (PCOA) (J), relative change in compositon of microbiota at different level by using KEGG enrichment analysis: Phylum (K), Class (L), Order (M), Genus (N), Function prediction (0) as per control vs encapsulated Lactobacillus paracasei, Phylum (P), Class (Q), Order (R), Genus (S), Function prediction (T) as per antibiotics vs encapsulated Lactobacillus paracasei, Here, (F-I and K-N), the bubble size represents the relative abundance of bacteria, x-axis shows log2 (FC) that defines fold change, which was calculated by dividing the relative content of bacteria of control/antibiotic group by treatment (encapsulated) to get first value i.e., FC and by excel, log (FC, 2) was calculated to get log2 (FC). Positive log2FC value represents upregulation of bacteria and negative log2FC represents downregulation of bacteria relative to control/antibiotic group, y-axis shows bacteria at different level and different color on the right side shows the significance (P value), only lower P-value in each level were reported . Similarly, (J) and (O) show prediction function of metabolic function at (KEGG level 3) where x-axis shows log2 (FC) that represents fold change, different color on right side shows the significance (P value), and left Y-axis shows different predicted function compared to control (J) and antibiotic (O) on encapsulated group) (n = 6 per group). Treatments: CON = a basal diet; ANT = CON+0.15 ppm Virginiamycin; ELP = CON+500PPM of encapsulated Lactobacillus paracasei.
The effects of feeding a basal diet with encapsulated Lactobacillus on cecal microbiota, based on 16S rRNA sequencing, are presented in Figures 4F–4T. To represent the richness and diversity of a microbial ecosystem, we carried out an alpha diversity analysis by observed species, Chao 1 index, and Shannon index based on OTU. Similarly, Beta diversity analysis was performed by non-metric multidimensional scaling (NMDS) and principal coordinate analysis (PCoA) using the weighted UniFrac distance method. We detected a significant reduction of observed species and Chao1 index in the antibiotics group compared to ELP and CON (Figures 4F–4G). Shannon index showed no remarkable differences between CON and ELP group, and significant reduction (P < 0.001) was observed in the ANT group compared to CON (Figure 4H). Meantime, beta diversity demonstrated that ELP shifted the structure and clustering of cecal microflora as shown in Figures 4I–4J.
We used a Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis to express the enrichment of microbes at a different level. The cecal microbiome was dominated by phyla Bacteroidetes and Firmicutes in all the groups (Figures 4K and 4P) accounting for more than 85% of the total microbial population. We detected administration of coated Lactobacillus could raise the Bacteroidetes (P = 0.07) and downregulate Firmicutes (P = 0.07) relative to the basal diet but, notable differences couldn't be observed compared to antibiotics. Similarly, the relative population of Bacteroidetes was increased in the encapsulated group compared to antibiotics. Meanwhile, the abundance of Proteobacteria and Desulfobacterota phyla was reduced in the ELP group.
The effect of ELP at the class level is shown in Figures 4L and 4Q. Bacteroidia and Clostridia were found to be a dominant class over others. Compared to CON, the relative abundance of Bacteroidia (P = 0.07, 0.4345 vs. 0.5203) in the ELP group was higher. However, we noticed a reduction in Clostridia, Bacilli, and Coriobacteria classes (Figure 4L). In addition, the relative abundance of Bacterioidia (P = 0.18, 0.4577 vs. 0.5203) was elevated, while Clostridia and Bacilli showed no distinct differences in the ELP group compared to ANT (Figure 4Q). Furthermore, the relative abundance of Campylobacteria tended to be lower in the ELP group compared to CON and ANT (Figures 4L and 4Q).
In order, Bacteroidales and Oscillospirales were dominant than others. The relative proportion of Bacteroidales, Acidaminococcales were higher, and Lachnospirales, Coriobacteriales, Erysipelotrichales, and Desulfovibrionales were lower in the ELP group with respect to CON (Figure 4M). However, we didn’t detect any remarkable differences in those orders in the ELP group compared to ANT (Figure 4R). Moreover, Monoglobales and Eubacteriales were elevated in ELP relative to both CON and ANT groups (Figures 4M and 4R). We observed more than 200 genera of bacteria, and Bacteroides were found to be dominant over others in all 3 groups. There was a significant increase (P < 0.05) of Bacteroides in ELP compared to CON (Figure 4N), but no remarkable change was seen relative to the ANT group (P = 0.18). Similarly, Phascolarctobacterium (P = 0.06) and Monglobus (P = 0.07) were upregulated in ELP with respect to CON (Figure 4N). Furthermore, Parabacteroides, Parasutterella, Muribaculum, Helicobacter, and Coprobacillus were downregulated in the ELP group relative to ANT (Figure 4S).
For more understanding of the possible effect of encapsulated probiotic on cecal microbiota, a histogram with FC and P value was used for the prediction functional profile of the microbial community (Figures 4O and 4T). A comparative study was done, on KEGG level 3, and we observed 387 categories of predicted function. Among them, only 12 with significant differences (P < 0.05) were studied between CON and ELP group and 24 between CON and ANT (P < 0.01).
Compared to CON, pathways involved in isoquinoline alkaloid biosynthesis, butanoate metabolism, tropane, piperidine, and pyridine alkaloid biosynthesis, phenylalanine, tyrosine and tryptophan biosynthesis, novobiocin biosynthesis, riboflavin metabolism, and pyruvate metabolism were upregulated in the ELP group, while, bacterial secretion system, phosphonate and phosphinate metabolism, primary bile acid biosynthesis, secondary bile acid biosynthesis, and chemical carcinogenesis were downregulated (Figure 4O). Similarly, pathways related to mineral absorption, RNA transport, methane metabolism, arginine biosynthesis, metabolism of cysteine and methionine, cell motility and secretion, AMPK signaling pathway, and so on were upregulated while C5-Branched dibasic acid metabolism, bacterial secretion system, taurine, and hypotaurine metabolism, primary bile acid biosynthesis, glutathione metabolism, Glycosaminoglycan binding proteins, and so on were decreased in ELP group compared to ANT (Figure 4T).
DISCUSSION
Microencapsulation protects probiotics from a harsh environment and increases mucoadhesive properties, thereby maintaining the viability of probiotics. Briefly, in this technology active components (probiotics) are enclosed by a polymeric wall or matrix to form a capsule that ruptures/dissolves/melts on the particular site under specific conditions to release the contents of the capsule. By this technology, probiotics can be protected from detrimental conditions in an upper region of the GI tract and could release the bacteria in a sufficient amount on a lower region for colonization that benefits the host (Cook et al., 2012; Yao et al., 2020; Pupa et al., 2021). Study regarding microencapsulation technology for probiotics in poultry is limited. Moreover, several encapsulating materials have been practiced to improve the viability of different probiotics species and found to enhance the overall health performance of birds (Zhang et al., 2015; Dong et al., 2016; Trabelsi et al., 2016; Wang et al., 2018). In this study L. paracasei was used as a probiotic strain to encapsulate with polyacrylate resin. L. paracasei is a rod-shaped (bacillus shape) lactic acid bacteria with a width of 2.0 to 4.0 μm and a length of 0.8 to 1.0 μm (Fesseha et al., 2021). It has been found to stimulate the immune system, suppress harmful pathogens (Zagato et al., 2014), and have anti-inflammatory properties (Nutten et al., 2012); thus, it is included in the feed to improve gut health. Limited studies on gut health have been carried out in broiler chickens with this species. Moreover, this species has improved growth performance, intestinal microflora, and overall intestinal health in chicken (Xu et al., 2019; Fesseha et al., 2021). In this study, we observed encapsulation of L. paracasei with polyacrylate resin improved the health performance of broilers.
In our study, all the birds were healthy during the experimental period of 42 d. The supplementation of encapsulated L. paracasei increased body weight gain numerically compared to the basal diet. Our results were similar to the previous study, who supplemented different species of Lactobacillus including Lactobacillus johnsonii, Lactobacillus crispatus, Lactobacillus salivarius via feed to broiler chickens (Olnood et al., 2015). Similarly, it is also outlined that probiotics couldn't show a significant improvement in growth performance on poultry (Lee et al., 2010; Waititu et al., 2014). Contrarily, the supplementation of different probiotics species has improved overall growth performance over a whole period in broilers (Rivera-Pérez et al., 2021; Wang et al., 2021). Variation in the effects on growth performance may be attributed to the use of different strains, doses, breeds of broilers, and methods of administration. Similarly in our study, we revealed the supplementation of encapsulated L. paracasei could increase the mass of different indicators of slaughter performance, including eviscerated yield, chest muscle yield, and thigh muscle yield. Our result was in line with several previous studies (Ghasemi-Sadabadi et al., 2019; Kaushal et al., 2019; Zhang et al., 2021a), which reported that several probiotic species could increase the carcass trait numerically or significantly. The improvement in feed utilization and productivity could be modulated or enhanced by the stimulation protein synthesis. The high level of serum total protein is the expression protein metabolism, which is conductive for promoting growth performance. We found serum protein level was the highest in the ELP group in our study. The breakdown of protein or amino acid leads to the formation of ammonia in the GI tract by the activities of bacterial ureases. A higher level of ammonia formation is toxic to birds and hinders growth performance due to the loss of protein in endogenous secretions (Dibner and Richards, 2005; Gong et al., 2018). The formed ammonia is transformed to uric acid, which is the end product of protein metabolism in the body. In conclusion, encapsulated probiotics could promote growth performance or feed utilization by improving nitrogen metabolism.
The spleen, thymus, and bursa of fabricus are the lymphatic organs that reflect a bird's immune system. Spleens are responsible for the storage and production of lymphocytes and the thymus facilitates the maturation of T cells which orchestrate the adaptive immune system (Thapa and Farber, 2019). Increase in their weight indicated that ELP could exhibit a strong immune system in the broiler (Smith and Hunt, 2004). Similar results were reported on an earlier study by feeding broilers with different species of probiotics (Zhang et al., 2021a). In addition, our qPCR results also revealed that supplementation of ELP could reduce the inflammation by lowering or elevating the gene inflammatory gene expression. Several studies also reported that probiotics reduce inflammation and enhance immunity (Tarradas et al., 2020). Similarly, the rise of sigA, which acts as the first line of defense against enterotoxins and pathogens, in ileum and cecum contents indicate that feeding of ELP play an essential role in immunoregulation, specific immunity and protects intestinal epithelium (Mantis et al., 2011; Li et al., 2020).On the other hand, gut microbiota and its metabolites play an important role in the induction and function of the host immune system and inflammatory signaling, interacting with host immune cells. SCFAs produced by gut microbiota have shown to reduce inflammation and defend against pathogen attack (Yoo et al., 2020). The abundance of Bacteroidetes is correlated with SCFAs content, especially propionate and butyrate (Flint and Duncan, 2014; Wang et al., 2017), that benefit gut health and in our study that elevation of propionate level was investigated. Thus, this study clearly shows that intake of encapsulated L. paracasei protects the gut against inflammation and enhances gut health.
Villus height, crypt depth, and their ratio are the basis of intestinal health, function, and indicator of the absorptive ability of birds (Pluske et al., 1996; Forte et al., 2018) and it could be modulated by the gut microbiota (Biasato et al., 2018). Previously, it was reported that supplementation of different probiotic species, including Lactobacillus could improve intestinal morphology by increasing V or V/C (Forte et al., 2018; He et al., 2019; Rivera-Pérez et al., 2021; Wu et al., 2021). An increase in villus height to crypt depth ratio in all the intestinal tissues in our investigation demonstrates that the dietary ELP promotes the absorptive ability of the intestine in broilers and represents sufficient mature and functionally active epithelium (Jia et al., 2010; Wu et al., 2021). TJs are essential components to the functions of the intestinal physical barrier (Peterson and Artis, 2014; Suzuki, 2020). TJs proteins, including claudin and ZO-1, were found to be elevated in this study, implying that adding ELP to poultry feed could help to maintain intestinal gut barrier integrity. The maintenance of gut barrier integrity and improvement of the transcriptome profile of intestinal epithelial cells might be due to the stability of the microbiome and its metabolites (Pandey and Aich, 2022). Our findings were consistent with the earlier report where the expression of were elevated by feeding the diet with L. acidophilus (Wu et al., 2021). Moreover, a remarkable rise in expression of ZO-1 was reported by supplementing a mixture of various probiotic species to Arbor Acres broilers (He et al., 2019).
The composition of gut microbiota is essential for maintaining intestinal homeostasis and the overall health status of birds. Gut microbiome has a vital role in maintaining gut health and productivity, including digestion of food and its absorption, immune system development, pathogen exclusion, and maintenance of normal physiological functions (Shang et al., 2018; Rychlik, 2020). The gut flora metabolizes proteins and complex carbohydrates, produces a huge variety of metabolic products that can help gut epithelium and immune cells interact. Gut epithelial cells generate a mucosal barrier to segregate bacteria from host immune cells and restrict intestinal permeability as a defensive strategy. Disturbed interaction between the gut microbiota and the mucosal immune system can lead to the weakening of the epithelial barrier and increasing infection susceptibility (Bander et al., 2020; Yoo et al., 2020). Cecum is the most populated region of the GI tract that harbors a complex, diverse, and stable community of microbiota (Rychlik, 2020; Zhu et al., 2020). Thus, 16s rRNA gene sequencing was carried out to find the diversity, composition, and predicted function of microbes present on the cecum. Several studies have shown that supplementation of probiotics could alter and modulate the diversity and composition of gut microbiota, exerting several beneficial effects on health and productivity on broiler (Zhang et al., 2015, 2021b; Zhu et al., 2020; Xu et al., 2021). Reduction of diversity of gut microbiota species may increase the susceptibility of the gut to colonization and can promote the development of antimicrobial resistance (Lange et al., 2016). However, there was no significant difference in abundance or diversity in an encapsulated group compared to the control. Moreover, the microbial community of each group was separated and clustered differently as per NMDS and PCoA, indicating that encapsulated probiotics could modulate gut microbiota.
Bacteroidetes and Firmicutes were dominant phyla in all the groups and our result was consistent with earlier studies, which investigated Bacteroidetes as the dominant phylum and Bacteroides as the dominant genus in the cecum (Xiao et al., 2017; Zhu et al., 2020). But, a study carried out by Singh et al. (2012), reported, Proteobacteria dominated the fecal microbiota of broiler followed by Firmicutes and Bacteroidetes. The difference could be attributed to the breed, age, or environment. Bacteroidetes exhibit a significant ability to respond to the stress imposed by the gut and host environment, and its abundance is correlated with SCFAs content (Flint and Duncan, 2014; Wang et al., 2017). Reduction of Firmicutes reduced H2O2 level in cecum including ileum contents as some member of Firmicutes such as Streptococcus is responsible for their production. A higher level of H2O2 could be toxic to both the microbes and host as it can kill birds’ beneficial microbes and bacteria (Erttmann and Gekara, 2019). Proteobacteria includes wide variety of pathogens, including Salmonella and E. coli. A decrease in the number of Proteobacteria reveals that intake of encapsulated probiotics could reduce the pathogenic bacteria. Previous findings have also outlined that intake of probiotics decreases pathogens, including E. coli or Salmonella (Gao et al., 2017; Zhang et al., 2021a). Similarly, members of genus Bacterioides, including Bacteroides thetaiotaomicron, are involved in carbohydrate metabolism and the maintenance of desmosomes at the epithelial villus, promoting the GI tract’s integrity (Jandhyala et al., 2015).
GI tract is a major source of ROS. The imbalance between antioxidants and ROS leads to oxidative stress that disrupts the redox signaling in the body and causes cell death. Apart from the host, gut microbes, especially in a diseased state, produce ROS, which aids development of the disease (Dam et al., 2019). An elevated level of ROS may promote cellular damage and also leads to lipid peroxidation, and MDA is one of the most used lipid markers of lipid peroxidation (Marí et al., 2010; Wang et al., 2017b). Excess production and accumulation of ROS are removed instantly by the antioxidant system and non-enzymatic defense system, including T-AOC (Marí et al., 2010; Li et al., 2019). We investigated that the supplementation of feed with encapsulated L. paracasei can enhance antioxidant function. Our study is consistent with former findings that probiotics exhibit antioxidant properties (He et al., 2019; Xu et al., 2021; Zhang et al., 2021a).
CONCLUSIONS
In conclusion, dietary supplementation of microcapsule, prepared by encapsulating Lactobacillus paracasei in polyacrylate resin, to broilers imparts health benefits to broiler chicken without affecting on growth performance and carcass characteristics. Meanwhile, broilers fed with microcapsule exhibited increased villus height and ratio of villus height to crypt depth improving morphology of intestine. Moreover, novel microcapsule influenced the immune system by reducing the inflammation and showed a potential effect as an antioxidant by reducing the oxidative damage and elevating total antioxidant capacity. Under our study conditions, microcapsule beneficially modulated the cecal microbiota and reduced the ammonia level. From this result, it can be concluded that the novel microcapsule can be used as an alternative to antibiotics to reduce toxic effect to human and environment without affecting the growth performance of birds.
ACKNOWLEDGMENTS
This study was supported by Guangdong Key Research And Development Program (2019B020218001), the Local Innovative and Research Teams Project of Guangdong province (2019BT02N630), and Quality Control for Feed and Products of Livestock and Poultry Key Laboratory of Sichuan Province (NH2021202206).
Disclosures
The authors declare no conflict of interest
REFERENCES
- Bander Z.Al, Nitert M.D., Mousa A. The gut microbiota and inflammation : an overview. Int. J. Environ. Res. Public Heal. Rev. 2020;17:1–21. doi: 10.3390/ijerph17207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasato I., Ferrocino I., Biasibetti E., Grego E., Dabbou S., Sereno A., Gai F., Gasco L., Schiavone A., Cocolin L., Capucchio M.T. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMC Vet. Res. 2018;14:1–15. doi: 10.1186/s12917-018-1690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang Q., Liu C.M., Gong J. Issues deserve attention in encapsulating probiotics: critical review of existing literature. Crit. Rev. Food Sci. Nutr. 2017;57:1228–1238. doi: 10.1080/10408398.2014.977991. [DOI] [PubMed] [Google Scholar]
- Cook M.T., Tzortzis G., Charalampopoulos D., Khutoryanskiy V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release. 2012;162:56–67. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Corcoran B.M., Stanton C., Fitzgerald G.F., Ross R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005;71:3060–3067. doi: 10.1128/AEM.71.6.3060-3067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam B., Misra A., Banerjee S. Oxidative Stress in Microbial Diseases. Springer; Singapore: 2019. Role of gut microbiota in combating oxidative stress; pp. 43–82. [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Dong Z.L., Wang Y.W., Song D., Hou Y.J., Wang W.W., Qi W.T., Yun T.T., Li A.K. The effects of dietary supplementation of pre-microencapsulated Enterococcus fecalis and the extract of Camellia oleifera seed on growth performance, intestinal morphology, and intestinal mucosal immune functions in broiler chickens. Anim. Feed Sci. Technol. 2016;212:42–51. [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erttmann S.F., Gekara N.O. Hydrogen peroxide release by bacteria suppresses inflammasome-dependent innate immunity. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-11169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenio Bahule C., Natalice Santos Silva T. Advances in Poultry Nutrition Research. IntechOpen; London: 2021. Probiotics as a Promising Additive in Broiler Feed: Advances and Limitations; pp. 1–16. [Google Scholar]
- Fesseha H., Demlie T., Mathewos M., Eshetu E. Effect of lactobacillus species probiotics on growth performance of dual-purpose chicken. Vet. Med. Res. Rep. 2021;12:75–83. doi: 10.2147/VMRR.S300881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H.J., Duncan S.H. Encyclopedia of Food Microbiology. 2nd ed. Academic Press, Elsevier, Ltd.; 2014. Bacteroides and Prevotella. [Google Scholar]
- Forte C., Manuali E., Abbate Y., Papa P., Vieceli L., Tentellini M., Trabalza-Marinucci M., Moscati L. Dietary Lactobacillus acidophilus positively influences growth performance, gut morphology, and gut microbiology in rurally reared chickens. Poult. Sci. 2018;97:930–936. doi: 10.3382/ps/pex396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Wu H., Shi L., Zhang X., Sheng R., Yin F., Gooneratne R. Study of Bacillus subtilis on growth performance, nutrition metabolism and intestinal microflora of 1 to 42 d broiler chickens. Anim. Nutr. 2017;3:109–113. doi: 10.1016/j.aninu.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi-Sadabadi M., Ebrahimnezhad Y., Shaddel-Tili A., Bannapour-Ghaffari V., Kozehgari H., Didehvar M. The effects of fermented milk products (kefir and yogurt) and probiotic on performance, carcass characteristics, blood parameters, and gut microbial population in broiler chickens. Arch. Anim. Breed. 2019;62:361–374. doi: 10.5194/aab-62-361-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Wang B., Mei X., Xu H., Qin Y., Li W., Zhou Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018;89:1561–1571. doi: 10.1111/asj.13089. [DOI] [PubMed] [Google Scholar]
- He T., Long S., Mahfuz S., Wu D., Wang X., Wei X., Piao X. Effects of probiotics as antibiotics substitutes on growth performance, serum biochemical parameters, intestinal morphology, and barrier function of broilers. Animals. 2019;9:1–10. doi: 10.3390/ani9110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Reddy D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8836–8847. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Das R., Oak S., Mishra P. Probiotics (Direct-Fed Microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: a systematic review. Animals. 2020;10:1–18. doi: 10.3390/ani10101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Yan J.Y., Cai J.Y., Wang K.N. Effects of encapsulated and non-encapsulated compound acidifiers on gastrointestinal pH and intestinal morphology and function in weaning piglets. J. Anim. Feed Sci. 2010;19:81–92. [Google Scholar]
- Kalia S., Bharti V.K., Gogoi D., Giri A., Kumar B. Studies on the growth performance of different broiler strains at high altitude and evaluation of probiotic effect on their survivability. Sci. Rep. 2017;7:1–8. doi: 10.1038/srep46074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal S., Sharma R.K., Singh D.V., Shukla S.K., Kumar S., Palod J., Singh M.K. Performance, carcass characteristics and economics of broiler chickens fed dietary enzymes and probiotic. Iran. J. Vet. Res. 2019;20:293–298. [PMC free article] [PubMed] [Google Scholar]
- Lange K., Buerger M., Stallmach A., Bruns T. Effects of antibiotics on gut microbiota. Dig. Dis. 2016;34:260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- Lee S., Kirkland R., Grunewald Z.I., Sun Q., Wicker L., de La Serre C.B. Beneficial effects of non-encapsulated or encapsulated probiotic supplementation on fat fed rats. Nutrients. 2019;11:1–17. doi: 10.3390/nu11091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Lillehoj H.S., Siragusa G.R. Direct-fed microbials and their impact on the intestinal microflora and immune system of chickens. J. Poult. Sci. 2010;47:106–114. [Google Scholar]
- Li Y., Jin L., Chen T., Pirozzi C.J. The Effects of secretory iga in the mucosal immune system. Biomed Res. Int. 2020;2020:1–6. doi: 10.1155/2020/2032057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A., Wang Y., Li Z., Qamar H., Mehmood K., Zhang L., Liu J., Zhang H., Li J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microbial Cell Factories. 2019;18(1):1–12. doi: 10.1186/s12934-019-1161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoč T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis N.J., Rol N., Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marí M., Colell A., Morales A., Von Montfort C., Garcia-Ruiz C., Fernández-Checa J.C. Redox control of liver function in health and disease. Antioxidants and Redox Signaling. 2010;12(11):1295–1331. doi: 10.1089/ars.2009.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutten S., Thierry A., Boudousqui C., Barbier N., Blanchard C., Corth B. Intragastric and Intranasal administration of lactobacillus paracasei NCC2461 modulates allergic airway. Int. J. Inflam. 2012;2012:1–9. doi: 10.1155/2012/686739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olnood C.G., Beski S.S.M., Choct M., Iji P.A. Novel probiotics: their effects on growth performance, gut development, microbial community and activity of broiler chickens. Anim. Nutr. 2015;1:184–191. doi: 10.1016/j.aninu.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey U., Aich P. Postnatal intestinal mucosa and gut microbial composition develop hand in hand: a mouse study. Biomed. J. 2022:1–34. doi: 10.1016/j.bj.2022.03.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Pluske J.R., Thompson M.J., Atwood C.S., Bird P.H., Williams I.H., Hartmann P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996;76:409–422. doi: 10.1079/bjn19960046. [DOI] [PubMed] [Google Scholar]
- Pupa P., Apiwatsiri P., Sirichokchatchawan W., Pirarat N., Muangsin N., Shah A.A., Prapasarakul N. The efficacy of three double-microencapsulation methods for preservation of probiotic bacteria. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-021-93263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Pérez W., Barquero-Calvo E., Chaves A.J. Effect of the use of probiotic Bacillus subtilis (QST 713) as a growth promoter in broilers: an alternative to bacitracin methylene disalicylate. Poult. Sci. 2021;100:1–9. doi: 10.1016/j.psj.2021.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues D., Sousa S., Rocha-Santos T., Silva J.P., Sousa Lobo J.M., Costa P., Amaral M.H., Pintado M.M., Gomes A.M., Malcata F.X., Freitas A.C. Influence of l-cysteine, oxygen and relative humidity upon survival throughout storage of probiotic bacteria in whey protein-based microcapsules. Int. Dairy J. 2011;21:869–876. [Google Scholar]
- Rychlik I. Composition and function of chicken gut microbiota. Animals. 2020;10:1–20. doi: 10.3390/ani10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarao L.K., Arora M. Probiotics, prebiotics, and microencapsulation: a review. Crit. Rev. Food Sci. Nutr. 2017;57:344–371. doi: 10.1080/10408398.2014.887055. [DOI] [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W.K. Chicken gut microbiota: importance and detection technology. Front. Vet. Sci. 2018;5:1–11. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.M., Shah T., Deshpande S., Jakhesara S.J., Koringa P.G., Rank D.N., Joshi C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012;39:10595–10602. doi: 10.1007/s11033-012-1947-7. [DOI] [PubMed] [Google Scholar]
- Smith K.G., Hunt J.L. On the use of spleen mass as a measure of avian immune system strength. Oecologia. 2004;138:28–31. doi: 10.1007/s00442-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Regulation of the intestinal barrier by nutrients: the role of tight junctions. Anim. Sci. J. 2020;91:1–12. doi: 10.1111/asj.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarradas J., Tous N., Esteve-garcia E., Brufau J. The control of intestinal inflammation: a major objective in the research of probiotic strains as alternatives to antibiotic growth promoters in poultry. Microorganisms. 2020;8:1–16. doi: 10.3390/microorganisms8020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa P., Farber D.L. The role of the thymus in the immune response. Thorac. Surg. Clin. 2019;29:123–131. doi: 10.1016/j.thorsurg.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi I., Ktari N., Ben Slima S., Bouchaala K., Ben Salah R. Effects of supplementation with L. plantarum TN8 encapsulated in alginate-chitosan in broiler chickens. Int. J. Biol. Macromol. 2016;89:677–681. doi: 10.1016/j.ijbiomac.2016.05.044. [DOI] [PubMed] [Google Scholar]
- Waititu S.M., Yitbarek A., Matini E., Echeverry H., Kiarie E., Rodriguez-Lecompte J.C., Nyachoti C.M. Effect of supplementing direct-fed microbials on broiler performance, nutrient digestibilities, and immune responses. Poult. Sci. 2014;93:625–635. doi: 10.3382/ps.2013-03575. [DOI] [PubMed] [Google Scholar]
- Wang Y., Dong Z., Song D., Zhou H., Wang W., Miao H., Wang L., Li A. Effects of microencapsulated probiotics and prebiotics on growth performance, antioxidative abilities, immune functions, and caecal microflora in broiler chickens. Food Agric. Immunol. 2018;29:859–869. [Google Scholar]
- Wang B., Gong L., Zhou Y., Tang L., Zeng Z., Wang Q., Zou P., Yu D., Li W. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021;7:829–840. doi: 10.1016/j.aninu.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sun J., Zhong H., Li N., Xu H., Zhu Q., Liu Y. Effect of probiotics on the meat flavour and gut microbiota of chicken. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-06677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Yang K., Zhang A., Chang W., Zheng A., Chen Z., Cai H., Liu G. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poultry Science. 2021;100(9):1–15. doi: 10.1016/j.psj.2021.101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Xiang Y., Zhou W., Chen J., Li K., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- Xu Y., Tian Y., Cao Y., Li J., Guo H., Su Y. Probiotic properties of lactobacillus paracasei subsp. paracasei L1 and its growth performance-promotion in chicken by improving the intestinal microflora. Front. Physiol. 2019;10:1–14. doi: 10.3389/fphys.2019.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Yu Y., Shen Y., Li Q., Lan J., Wu Y., Zhang R., Cao G., Yang C. Effects of bacillus subtilis and bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:1–11. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Xie J., Du H., McClements D.J., Xiao H., Li L. Progress in microencapsulation of probiotics: a review. Compr. Rev. Food Sci. Food Saf. 2020;19:857–874. doi: 10.1111/1541-4337.12532. [DOI] [PubMed] [Google Scholar]
- Yoo J.Y., Groer M., Valeria S., Dutra O., Sarkar A., Mcskimming D.I. Gut microbiota and immune system interactions. Microorganisms. 2020;8:1–22. doi: 10.3390/microorganisms8101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagato E., Mileti E., Massimiliano L., Fasano F., Budelli A., Penna G., Rescigno M. Lactobacillus paracasei CBA L74 metabolic products and fermented milk for infant formula have anti- inflammatory activity on dendritic cells in vitro and protective effects against colitis and an enteric pathogen in vivo. PLoS One. 2014;9:1–14. doi: 10.1371/journal.pone.0087615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li J., Yun T.T., Qi W.T., Liang X.X., Wang Y.W., Li A.K. Effects of pre-encapsulated and pro-encapsulated Enterococcus faecalis on growth performance, blood characteristics, and cecal microflora in broiler chickens. Poult. Sci. 2015;94:2821–2830. doi: 10.3382/ps/pev262. [DOI] [PubMed] [Google Scholar]
- Zhang F., Qi N., Zeng Y., Bao M., Chen Y., Liao J., Wei L., Cao D., Huang S., Luo Q., Jiang Y., Mo Z. The endogenous alterations of the gut microbiota and feces metabolites alleviate oxidative damage in the brain of LanCL1 knockout mice. Front. Microbiol. 2020;11:1–15. doi: 10.3389/fmicb.2020.557342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang R., Jia H., Zhu Z., Li H., Ma Y. Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens. Open Life Sci. 2021;16:311–322. doi: 10.1515/biol-2021-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zhong G., Shao D., Wang Q., Hu Y., Wu T., Ji C., Shi S. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Gong L., Huang K., Li F., Tong D., Zhang H. Effect of heat-inactivated compound probiotics on growth performance, plasma biochemical indices, and cecal microbiome in yellow-feathered broilers. Front. Microbiol. 2020;11:1–16. doi: 10.3389/fmicb.2020.585623. [DOI] [PMC free article] [PubMed] [Google Scholar]