Highlights
-
•
The hot spots of PSS were distributed around the central region of cerebral cortex.
-
•
We observed differences between the distribution of hot spots among patients with early-PSS and those with late-PSS.
-
•
The specific regions of the brain are significantly associated with the development of PSS after cerebral infarction.
-
•
The location of infarction could help clinicians assess the risk of PSS in specific post-stroke stages.
Keywords: Post-stroke epilepsy, Epileptogenesis, Risk factors, Cerebral infarction, Ischemic stroke
Abstract
Post-stroke seizure (PSS) can have a strong negative impact on functional recovery after stroke. Researchers have identified numerous risk factors of PSS; however, the relationship between infarction location and PSS remains unclear. We recruited patients who presented with an acute cerebral infarction between 2012 and 2017 and suffered from seizures within 1 year after stroke (PSS group). PSS group was subgrouped into early-PSS and late-PSS groups based on the interval between seizure and stroke. We also recruited an equal number of acute cerebral infarction patients without post-stroke seizures during the follow-up period (Non-PSS group). All brain MRIs from the two groups were processed, whereupon normalized infarct maps from the PSS and Non-PSS groups were compared via voxel- and volumetric-based analyses. A total of 132 subjects were enrolled in the study, including PSS (n = 66, consisting of 31 early-PSS and 35 late-PSS) and Non-PSS (n = 66) patients. No significant differences were observed between the two groups in terms of stroke lateralization or severity. Image analysis revealed that the volume of infarction was larger in the PSS group than in the Non-PSS group; however, the difference did not reach the level of significance. Unlike the Non-PSS group, the PSS group presented hot spots over the left central region, left superior parietal lobule, and right frontal operculum. We observed differences between the distribution of hot spots among patients with early-PSS and those with late-PSS. We found that some brain regions were significantly associated with the development of PSS after ischemic stroke, and these regions differed between cases of early and late PSS. It appears that the location of infarction could help clinicians assess the risk of PSS in specific post-stroke stages.
1. Introduction
Post-stroke seizure (PSS) is an important complication in cases of stroke. Researchers have identified risk factors for PSS, including age, severity of stroke, stroke location, and whether the stroke is hemorrhagic or cortical. Evidence suggests that younger patients and patients who suffered a hemorrhagic stroke face a higher risk of PSS (Chi et al., 2018, Slapø et al., 2006). In addition, multivariate analysis showed that cortical stroke was associated with an elevated risk of PSS (Bladin et al., 2000).
The brain regions that are prone to epileptic seizures vary in accordance with the underlying disease. For example, patients with cavernous malformation in the temporal lobe also face a higher risk of epilepsy than those with cavernous malformations in other brain regions (Rosenow et al., 2013). Only a small number of studies on PSS have explored the relationship between stroke location and the likelihood of epileptic seizures. One pediatric study reported that stroke location was unrelated to the occurrence of seizures (López-Espejo et al., 2018). Another study provided evidence that PSS was more likely in cases where stroke occurred in the frontal lobe (Yamada et al., 2020). Overall, the link between stroke location and the occurrence of PSS remains unclear. In the current study, we investigated this issue by comparing the location of acute infarction in brain MRIs from patients with and without PSS.
2. Methods
2.1. Subjects
This retrospective study was exempted from a full review by the Institutional Research Ethics Committee of Taipei Veterans General Hospital, Taipei, Taiwan. Analysis was based on a review of the stroke registration database at Taipei Veterans General Hospital. Patients who experienced acute cerebral infarction between January 1, 2012 and December 31, 2017 were enrolled. Exclusion criteria included the following: (1) patients younger than 20 years of age; (2) diagnosis of epilepsy before stroke; (3) history of brain disease, including intracranial hemorrhage, brain tumor, traumatic brain injury, or encephalitis; (4) history of brain surgery; (5) lack of brain magnetic resonance imaging (MRI) within 30 days after stroke; (6) acute infarct only present in the infratentorial region, including the brainstem and cerebellum; (7) acute infarct with hemorrhagic transformation.
Patients who experienced epileptic seizures within 1 year after stroke were assigned to the Post-stroke Seizure (PSS) group and further divided into early-PSS and late-PSS groups according to the interval between seizure and stroke (i.e., ≤7 and > 7 days, respectively). We selected an equal number of patients as control, called Non-PSS group, with match of infarct side and stroke severity to minimize their effects. The patients in Non-PSS group did not experience seizures after stroke during the follow-up period (mean 62.1 months, range from 32 to 145 months). We recorded the basic data (including age, gender, habit of smoke and alcohol), past medical history (including diabetes, hypertension, dyslipidemia), follow-up duration (from the date of acute stroke to the most recent clinical visit), medication (thrombolytic treatment, antiplatelets, anticoagulants, statin) and factors related to stroke, including lateralization of infarct, National Institute of Health Stroke Scale (NIHSS) score, and modified ranking scale (mRS) score.
2.2. Image acquisition protocol
All brain MRIs were acquired using a Signa HDxt 1.5 T, Optima edition (GE Healthcare, Waukesha, WI). Images included DWI data (TR/TE/Flip angle = 4000–8200 ms/64.8–75.8 ms/90°, FOV = 100 × 100 mm2, matrix = 256 × 256, in-plane resolution = 0.9375 × 0.9375 mm, 30 axial slices, 5 mm slice thickness); T1WI data (TR/TE/Flip angle = 250–683.332 ms/8–13.048 ms/90°, FOV = 75 × 75 mm2, matrix = 512 × 512, in-plane resolution = 0.4688 × 0.4688 mm, 30 axial slices, 5 mm slice thickness); and an ADC map with 1000 s/mm2.
2.3. Processing of MR images
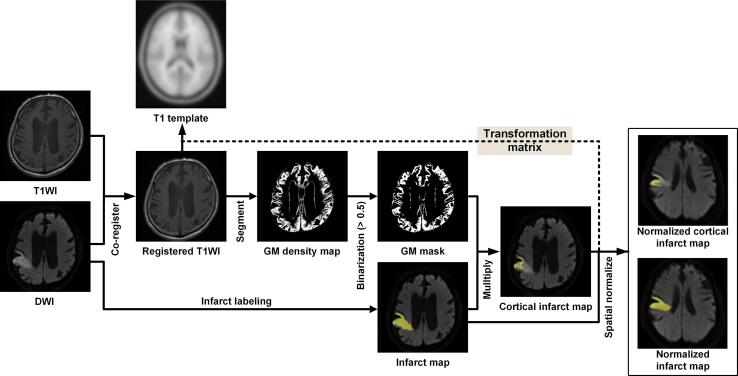
The preprocessing of the MR images was implemented in MATLAB (MathWorks, Inc., Natick, MA, USA) using the Statistical Parametric Mapping program SPM12 (Functional Imaging Laboratory, Institute of Neurology, University College London, London, UK). As shown in Fig. 1, the processing of MR Images was implemented in multiple steps, as described below.
Fig. 1.
Flowchart illustrating DWI and T1WI image processing employed to obtain normalized infarct maps and cortical infarct maps. The steps shown include co-registration, infarct labeling, GM segmentation, GM mask creation, and spatial normalization.
Step 1. DICOM to NIfTI conversion.
The original DICOM file format of the T1WI and DWI was converted into the three-dimensional NIfTI-1 (Neuroimaging Informatics Technology Initiative) file format to enable the use of SPM12.
Step 2. Resetting the MR image orientation.
After Step 1, the origin of the T1WI and DWI were shifted to align roughly with the anterior commissure of the individual brain space in order to facilitate the normalization of T1WI and DWI to the standard brain space via SPM12 in a subsequent step.
Step 3. Registration.
To correct for differences due to patient head movement, the T1WI was registered to the DWI via rigid registration based on normalized mutual information (Maes et al., 1997).
Step 4. Infarct region segmentation.
Segmentation of infarct regions in the DWI was conducted by three experienced neurologists (CC Chou, YC Shih, and HH Chiu) to obtain precise information pertaining to the infarct location and volume.
Step 5. Segmentation of cortical infarct regions.
The unified segmentation module of SPM12 was applied to the registered T1WI in order to create a probabilistic map of gray matter (GM) (Ashburner and Friston, 2005). A binary GM mask was then generated by assigning a threshold of 0.5 to the GM probability map. The cortical infarct map was obtained using a multiply operation involving the binary GM mask and the infarct map, and the cortical infarct volume was calculated from this map.
Step 6. Spatial normalization to MNI space.
Registered T1WI images were spatially aligned to the MNI152 template of the MNI (Montreal Neurological Institute) space using the SPM12 normalization tool. The infarct map was also normalized to a standard MNI space based on a transformation matrix. During this step, the dimensions of the infarct maps changed from the original 256 × 256 × 30 voxels to 61 × 73 × 61 voxels.
Step 7. Spatial smoothing of normalized infarct maps.
The normalized infarct maps underwent spatial smoothing using a spatially stationary Gaussian filter. To facilitate between-group statistical analysis, we increased the signal-to-noise ratio by selecting a Gaussian smooth kernel with 4-mm FWHM (Full-Width at Half-Maximum). In the event that a patient with PSS had experienced multiple strokes within one year, all normalized infarct maps were added together.
2.4. Statistical analysis
The AlphaSim provides a way of estimating the probability of a false detection within the whole brain mask and is performed using Monte Carlo simulation (Ledberg et al., 1998). The probability of a false positive detection is identified from the frequency count of cluster sizes based on the combination of individual voxel probability thresholding and minimum cluster size thresholding. A random-effect analysis was applied to normalized infarct maps from the PSS and Non-PSS groups using a one-tailed one-sample t-test with a combined height threshold of p < 0.05 and a minimum cluster size of 154 voxels, as determined via AlphaSim correction using REST software (Song et al., 2011). Differences in normalized infarct maps between (1) PSS and Non-PSS, (2) early-PSS and Non-PSS, and (3) late-PSS and Non-PSS groups were identified by performing two-sample t-test group comparisons using data from the two groups of interest (covariates: infarct volume and lateralization of infarction). In so doing, a double statistical threshold was used (a combined height threshold of p < 0.05 and a minimum cluster size of 154 voxels, as determined via AlphaSim correction).
Statistical analysis for clinical variables in the PSS and non-PSS groups was conducted using univariate and multivariate logistic regression analysis, as well as Mann-Whitney U tests. For these, the level of statistical significance was set at p < 0.05. The IBM Statistical Package for the Social Sciences (SPSS, version 25.0) was employed for all data analysis.
3. Results
3.1. Demographic information
Table 1 lists the demographic data of the 132 subjects in this study, including PSS (n = 66) and Non-PSS (n = 66) patients. The PSS group included 31 patients with early-PSS and 35 patients with late-PSS. Recurrent stroke was more frequent in the Non-PSS group, which is the only factor with significant difference in univariate analysis (p = 0.03). From the results of multivariate logistic regression analysis, male gender and recurrent stroke had lower risk having post-stroke seizures (please see the Table S1). The mean age at the time of the 1st stroke was as follows: PSS group (74.5 years; range 34–94) and Non-PSS group (76.7 years; range 43–93). The prevalence of infarcts over bilateral hemispheres among patients was 28.8% in the PSS group and 31.8% in the Non-PSS group. Most of the patients underwent MRI within 3 days after stroke: PSS group (81.8%; n = 54) and Non-PSS group (71.2%; n = 47).
Table 1.
. Patient demographics.
| PSS group (n = 66) |
Non-PSS group (n = 66) |
|
|---|---|---|
| Age | ||
| Mean ± SD (y)Range (y) |
74.5 ± 13.7 34–94 |
76.7 ± 11.6 43–93 |
| Gender | ||
| Male: Female | 39: 27 | 49: 17 |
| Past history DM HTN Dyslipidemia |
25 48 32 |
27 50 40 |
| Personal history | ||
| Smoke Alcohol |
22 17 |
21 13 |
| Management Thrombolytic Antiplatelet Anticoagulant Statin |
3 62 28 37 |
3 60 19 45 |
| NIHSS score | ||
| Mean ± SD Range |
15.9 ± 10.6 0–38 |
13.4 ± 9.6 1–34 |
| mRS score | ||
| Mean ± SD Range |
4.4 ± 0.8 1–5 |
4.3 ± 0.8 1–5 |
| Infarct side | ||
| L: R: B | 24: 23: 19 | 24: 21: 21 |
| Cortical involvement | 58 | 50 |
| Recurrent stroke | 7 | 17 |
| Days between stroke and MRI | ||
| Mean ± SD (d) Range |
2.2 ± 3.2 0–15 |
2.6 ± 4.0 0–28 |
SD: standard deviation; DM: diabetes mellitus; HTN: hypertension; NIHSS: NIH stroke scale; mRS: modified ranking scale; L: left; R: right; B: bilateral; MRI: magnetic resonance imaging.
3.2. Infarct location in PSS and Non-PSS groups
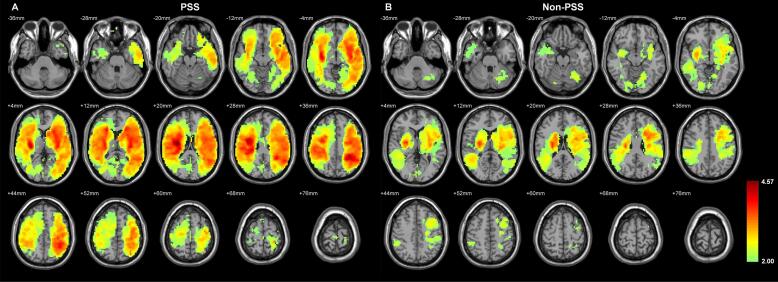
Total infarct maps from PSS and Non-PSS groups are shown in Fig. 2. In PSS patients, locations of infarcts were widely distributed over both cerebral hemispheres, and particularly common in the territory of the middle cerebral artery (Fig. 2A). In the Non-PSS group, infarcts tended to be located in both cerebral hemispheres; however, their distribution was more asymmetric, as well as more in the deep grey matter or subcortical region. Infarcts over the left hemisphere were more distributed in the frontal region, whereas those over the right hemisphere were more distributed in the temporal region (Fig. 2B).
Fig. 2.
Total infarct maps in the (A) PSS and (B) Non-PSS groups. The significance threshold was set at 0.05 (via AlphaSim correction) for multiple comparisons. The left side of the MRI corresponds to the right hemisphere of the brain. The color bar indicated the T-score.
3.3. Infarct volume in PSS and Non-PSS groups
The median volume of infarcts was as follows: PSS group (26.38 cm3; IQR = 91.10) and Non-PSS group (16.38 cm3; IQR = 41.25) (p = 0.139). The median volume of cortical infarcts was as follows: PSS group (15.45 cm3; IQR = 60.18) and Non-PSS group (8.39 cm3; IQR = 31.14) (p = 0.261). We observed no differences between the two groups in terms of total infarct volume or cortical infarct volume.
3.4. Hot spots of post-stroke seizure
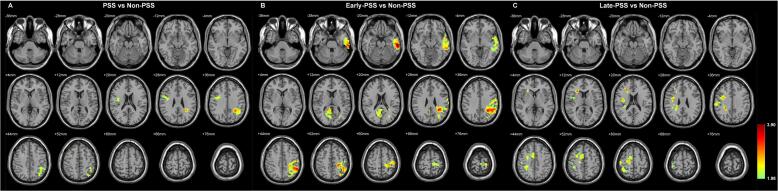
Compared to the Non-PSS group, the infarcts in the PSS group were more frequently localized over the left central region (surrounding the central sulcus), the left superior parietal lobule, and the right frontal operculum (Fig. 3A). After dividing PSS patients into early-PSS and late-PSS groups, the distribution of hot spots was found to be different. Most hot spots in the early-PSS group were located over the left central region, left superior parietal lobule, left lateral temporal cortex, and right medial occipital cortex (Fig. 3B), whereas hot spots in the late-PSS group were located over the right superior frontal cortex and right frontal operculum (Fig. 3C).
Fig. 3.
Differences in distribution of infarct between the Non-PSS group and the (A) PSS, (B) early-PSS, and (C) late-PSS groups. A two-sample t-test group comparison with infarct volume and lateralization of infarction as covariates was performed between the Non-PSS group and each other group. For this, a double statistical threshold (a height threshold of p < 0.05 and a minimum cluster size of 154 voxels, as determined using AlphaSim correction) was used. The color bar indicated the T-score.
4. Discussion
This is the first study to use a quantitative analysis of infarction in brain MRIs to investigate the relationship between the location of acute infarction and post-stroke seizure. We identified hot spots associated with an elevated risk of PSS. The location of these hot spots differed according to the timing of post-stroke seizures (early or late). These results could potentially be used to inform clinicians about the risk of PSS in patients after ischemic stroke.
In this study, the hot spots of PSS were distributed around the central region of cerebral cortex. We posited that the distribution of PSS hot spots in this study was related to two factors: First, the semiology of epileptic seizures in the central region mostly comprised simple, elementary motor symptoms (e.g. myoclonus of limbs or face) (Luders, 2008). These motor symptoms are more easily identifiable with epileptic seizures, compared to non-motor seizures (e.g. staring, behavior arrest, déjà vu, visual hallucinations) originate in temporal and occipital regions of the brain. Second, we defined the PSS in this study that it occurred within 1 year after stroke because the greatest risk for PSS was during the first follow-up year. Insults far away from central cortex might need longer period to generate epileptogenic networks and develop PSS (Pitkänen et al., 2016).
Epileptic seizures caused by acute brain insult can be classified as early- or late-onset. Early PSS is one kind of acute symptomatic seizures, while late PSS can be considered as post-stroke epilepsy by new diagnostic criteria of epilepsy in ILAE. Early and late post-stroke seizures differ in terms of epileptogenesis. Cellular biochemical dysfunction during acute ischemic cell injuries play an important role in early post-stroke seizures (Slapø et al., 2006). Conversely, development of late poststroke seizure requires both cellular dysfunction and the formation of an epileptogenic network (de Palma et al., 2020). The location of the brain insult is crucial to epileptogenesis, due to its influence on the duration over which epileptic symptoms develop (Löscher et al., 2015). Therefore, it is reasonable that the hot spots of early PSS are different from those of late PSS. In the study from Yamada et al, late PSS was significantly related to acute stroke in frontal lobe (Yamada et al., 2020). The hot spots of late PSS were more specifically localized in fronto-operculum-insula in our study. Stroke located in the posterior cerebral artery territory associated with a higher risk of early post-stroke seizures was found in a multicenter Study (Ferreira-Atuesta et al., 2021). In our study, the hot spots of early PSS were not only in the posterior cerebral artery territory but also in the middle cerebral artery territory. However, we found that temporal and occipital cortex were only related to early PSS but not late PSS.
Nearly 90% (58/66) patients in the PSS group had infarction with cortical involvement in this study. Epilepsy is generally considered as a disease of cerebral cortex with hyperexcitability. We supposed two possible factors to explain why not all patients having cortical infarction. First, we didn’t review their brain images when the patients had PSS. Therefore, we didn’t know if there is new cortical lesion occurring between the stroke we recorded and PSS. Second, subcortical ischemia (so called leukoaraiosis) might play a role in epileptogenesis. In the Gasparini’s study, the patients with only leukoaraiosis suffered from temporal lobe epilepsy (Gasparini et al., 2015). It suggested that leukoaraiosis might damage the temporal lobe networks and further develop epilepsy.
This study was subject to limitations that should be considered in the interpretation of our results. First, our analysis was based on a relatively small number of cases due to our focus on the issue of epilepsy after cerebral infarction. We also excluded patients with intracranial hemorrhage and infarction with hemorrhagic transformation, both of which are associated with an elevated risk for post-stroke epilepsy. Second, the patients in this study were elderly, and the onset of seizure was restricted to within one year after stroke. Note that both patient age and the latent period of post-stroke epilepsy may have had a profound impact on our results. Thus, the distribution of hot spots we observed in the PSS group is not necessarily applicable to younger patients or in cases where PSS onset occurs more than one year after cerebral infarction. Third, there is a potential selection bias because we excluded the subjects who lack of brain MRI within 30 days after stroke as well as selected the control group with match of infarct side and stroke severity. Match of the stroke severity might explain paradoxically higher percentage of recurrent stroke in the Non-PSS group.
In conclusion, some specific regions of the brain are significantly associated with the development of PSS after cerebral infarction. In combination with other well-known risk factors for PSS, this knowledge could enhance the accuracy of PSS-related predications and could alter treatment strategies after stroke.
5. Funding sources
The study was supported in part by Taipei Veterans General Hospital (V109B-033), Taiwan Ministry of Science and Technology (MOST 109–2314-B-075–042, 110–2221-E-038–008, 110–2314-B-075–036-MY2), and the Vivian W. Yen Neurological Foundation (2020 research grant to C.C.C.). The study was also financially supported of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. We are grateful to our research assistants, Li- Chun Wang and Hsiang-Ting Chou, for data recording and transcription.
CRediT authorship contribution statement
Chien-Chen Chou: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Validation, Writing – original draft. Yen-Cheng Shih: Data curation, Validation, Writing – review & editing. Hsu-Huai Chiu: Data curation, Validation, Writing – review & editing. Hsiang-Yu Yu: Data curation, Resources, Supervision. I-Hui Lee: Data curation, Resources, Supervision. Yung-Yang Lin: Resources, Supervision. Cheng-Chia Lee: Resources, Supervision. Syu-Jyun Peng: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103069.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bladin C.F., Alexandrov A.V., Bellavance A., Bornstein N., Chambers B., Coté R., Lebrun L., Pirisi A., Norris J.W. Seizures after stroke: A prospective multicenter study. Arch. Neurol. 2000;57:1617–1622. doi: 10.1001/archneur.57.11.1617. [DOI] [PubMed] [Google Scholar]
- Chi N.F., Kuan Y.C., Huang Y.H., Chan L., Hu C.J., Liu H.Y., Chiou H.Y., Chien L.N. Development and validation of risk score to estimate 1-year late poststroke epilepsy risk in ischemic stroke patients. Clin. Epidemiol. 2018;10:1001–1011. doi: 10.2147/CLEP.S168169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Palma L., De Benedictis A., Specchio N., Marras C.E. Epileptogenic network formation. Neurosurg. Clin. N. Am. 2020;31:335–344. doi: 10.1016/j.nec.2020.03.012. [DOI] [PubMed] [Google Scholar]
- Ferreira-Atuesta C., Döhler N., Erdélyi-Canavese B., Felbecker A., Siebel P., Scherrer N., Bicciato G., Schweizer J., Sinka L., Imbach L.L., Katan M., Abraira L., Santamarina E., Álvarez-Sabín J., Winklehner M., von Oertzen T.J., Wagner J.N., Gigli G.L., Serafini A., Janes F., Merlino G., Valente M., Gregoraci G., Conrad J., Evers S., Lochner P., Roell F., Brigo F., Bentes C., Peralta A.R., Melo T.P.E., Keezer M.R., Duncan J.S., Sander J.W., Tettenborn B., Koepp M.J., Galovic M. Seizures after ischemic stroke: A matched multicenter study. Ann. Neurol. 2021;90:808–820. doi: 10.1002/ana.26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S., Ferlazzo E., Beghi E., Sofia V., Mumoli L., Labate A., Cianci V., Fatuzzo D., Bellavia M.A., Arcudi L., Russo E., De Sarro G., Gambardella A., Aguglia U. Epilepsy associated with leukoaraiosis mainly affects temporal lobe: A casual or causal relationship? Epilepsy Res. 2015;109:1–8. doi: 10.1016/j.eplepsyres.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Ledberg A., Akerman S., Roland P.E. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8:113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- López-Espejo M., Hernández-Chávez M., Huete I. Clinical and radiological risk factors for poststroke epilepsy in childhood. Epilepsy Behav. 2018;88:113–116. doi: 10.1016/j.yebeh.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Löscher W., Hirsch L.J., Schmidt D. The enigma of the latent period in the development of symptomatic acquired epilepsy - traditional view versus new concepts. Epilepsy Behav. 2015;52:78–92. doi: 10.1016/j.yebeh.2015.08.037. [DOI] [PubMed] [Google Scholar]
- Luders H. CRC Press; Boca Raton, Fla: 2008. Textbook of epilepsy surgery. [Google Scholar]
- Maes F., Collignon A., Vandermeulen D., Marchal G., Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans. Med. Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- Pitkänen A., Roivainen R., Lukasiuk K. Development of epilepsy after ischaemic stroke. Lancet Neurol. 2016;15:185–197. doi: 10.1016/S1474-4422(15)00248-3. [DOI] [PubMed] [Google Scholar]
- Rosenow, F., Alonso-Vanegas, M.A., Baumgartner, C., Blümcke, I., Carreño, M., Gizewski, E.R., Hamer, H.M., Knake, S., Kahane, P., Lüders, H.O., Mathern, G.W., Menzler, K., Miller, J., Otsuki, T., Ozkara, C., Pitkänen, A., Roper, S.N., Sakamoto, A.C., Sure, U., Walker, M.C., Steinhoff, B.J., Surgical Task Force, Commission on Therapeutic Strategies of the ILAE, 2013. Cavernoma-related epilepsy: Review and recommendations for management--report of the surgical task force of the ILAE commission on therapeutic strategies. Epilepsia. 54, 2025-2035. [DOI] [PubMed]
- Slapø G.D., Lossius M.I., Gjerstad L. Poststroke epilepsy: occurrence, predictors and treatment. Expert Rev. Neurother. 2006;6:1801–1809. doi: 10.1586/14737175.6.12.1801. [DOI] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., Li S.F., Zuo X.N., Zhu C.Z., He Y., Yan C.G., Zang Y.F. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Nakagawa I., Tamura K., Nishimura F., Motoyama Y., Park Y.S., Nakase H. Investigation of poststroke epilepsy (INPOSE) study: A multicenter prospective study for prediction of poststroke epilepsy. J. Neurol. 2020;267:3274–3281. doi: 10.1007/s00415-020-09982-2. [DOI] [PubMed] [Google Scholar]
Further reading
- Kammersgaard L.P., Olsen T.S. Poststroke epilepsy in the copenhagen stroke study: Incidence and predictors. J. Stroke Cerebrovasc Dis. 2005;14:210–214. doi: 10.1016/j.jstrokecerebrovasdis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Lossius M.I., Rønning O.M., Mowinckel P., Gjerstad L. Incidence and predictors for post-stroke epilepsy. A prospective controlled trial. the akershus stroke study. Eur. J. Neurol. 2002;9:365–368. doi: 10.1046/j.1468-1331.2002.00415.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.