Graphical abstract
Keywords: Fluoride; Europium; Sonochemistry; Luminescence; Crystal Phase, pH
Highlights
-
•
We reveal the significance of sonochemistry in the design of luminescent fluorides.
-
•
The study offers the key to synthesizing both the α- and δ-phases of KY3F10.
-
•
A landmark methodology for the preparation of yttrium hydroxyfluorides is reported.
-
•
The reaction mechanisms underlying the formation of the compounds are unraveled.
-
•
The Eu3+-doping endows the materials with highly tunable optical properties.
Abstract
In this study Eu3+-doped yttrium fluorides were designed by ultrasound-assisted processes at different pH values (4.0–9.0). This novel strategy has enabled to obtain materials with intriguing morphologies and modulated crystal structures: α-KY3F10, δ–KY3F10·xH2O, and Y(OH)3–xFx. To date, the literature has primarily focused only on the α-phase of KY3F10. Yet, explaining the formation of the mostly uncharted δ-phase of KY3F10 remains a challenge. Thus, this paper offers the key to synthesizing both the α and the δ-phases of KY3F10 and also reports the first ultrasound-assisted process for the preparation of yttrium hydroxyfluorides. It is also unraveled the connection between the different pH-dependent reactions and the formation mechanisms of the compounds. In addition to this, the unique features of the Eu3+ ion have allowed to conduct a thorough study of the different materials and have endowed the compounds with photoluminescent properties. The results underscore a highly tunable optical response, with a wide gamut of color emissions (from orangish to red hues), lifetimes (from 7.9 ms to 1.1 ms) and quantum efficiencies (98–28%). The study unveils the importance of sonochemistry in obtaining luminescent fluorides with controlled crystal structures that can open up new avenues in the synthesis and design of inorganic materials.
1. Introduction
Sonochemistry has emerged as a powerful strategy in the synthesis and design of novel inorganic materials by virtue of its unique features [1], [2]. Sonochemical reactions are induced by ultrasound radiations that generate local hot spots which can reach temperatures above 5000 K, pressures exceeding 1000 atm, and heating and cooling rates higher than 1010 K/s [3]. These extreme, transient conditions allow the synthesis of chemical compounds with controllable structures, shapes, and crystallinity at room temperature, without the need to use high pressures or high temperatures that would entail an important economic cost. It comes as no surprise, therefore, that ultrasound-assisted processes to develop nano/micron-sized materials at the expense of other conventional methods have aroused a great deal of enthusiasm [4], [5].
As a series of important luminescent materials, fluoride hosts doped with lanthanide ions (Ln3+) are attracting a growing amount of attention owing to their remarkable applications in photonics, solar energy, medicine, bioanalytics, or environmental science [6], [7], [8], [9], [10]. The extraordinary optical response of these materials arises from their electronic characteristics: sharp f-f transitions, low phonon energy (usually below 600 cm−1), and high viability of energy transfers with other ions [11], [12]. Particularly, fluoride crystal lattices with trivalent yttrium cations are the most common due to the ease with which they replace Y3+ ions by isovalent Ln3+ ions [13]. Moreover, Eu3+ has been widely appreciated for its adequacy as a site-sensitive structural probe, while the great similarity of its ionic radius to that of Y3+ ensures a perfect incorporation of the Ln3+ ion into the host matrix [14].
To date, KY3F10 and its Ln3+-doped counterparts have gained much attention for their important applications in several fields of materials science [15], [16], [17], [18], [19]. However, the studies in the literature have only focused on the common α-phase of KY3F10 in detriment of the mostly unknown δ-phase, which was discovered in 2000 by Le Berre et al. [20]. These authors reported the crystallographic description of this new structure and performed a detailed analysis of its zeolitic and thermal behavior. As a drawback, it is important to note that a great amount of hydrofluoric acid (HF) was required during the synthesis process. Even so, as far as we are aware, no additional research about this material had been conducted until the results of a recent study by our laboratory were published [21]. It was reported a simple high-yield method based on a sonochemical approach to obtain the compound δ-KY3F10·xH2O. For the first time, the growth mechanism of this structure and its perfect adequacy for optical applications were studied. Moreover, the alternative method did not require the use of HF, which greatly reduced the safety hazards.
Despite the aforementioned signs of progress, explaining the mechanism underlying the formation of such an uncharted and promising phase of KY3F10 remains a challenge. In addition, further studies to exploit the δ-phase as host lattice for luminescent ions are required. The zeolitic behavior of this compound could also be essential for different photonic applications since it could help to modulate the incorporation/substitution of different cations inside the structure. With this in mind, in this paper, the significance of sonochemistry in the design of Eu3+-doped fluorides is revealed. The ultrasound-assisted processes enabled to obtain materials with intriguing morphologies and modulated crystal structures: α-KY3F10, δ–KY3F10·xH2O, and Y(OH)3–xFx. Hence, this study not only offers the key to synthesizing both the α- and δ-phases of KY3F10 but also reports a landmark methodology for the preparation of yttrium hydroxyfluorides. It also explains how the different pH–dependent reactions govern the formation mechanisms of the compounds. Further to this, the incorporation of Eu3+ into the host lattice endows the material with important optical properties that are also highly tunable, depending on the compound.
Therefore, this study can contribute to a further comprehension of the δ-phase of KY3F10, while at the same time opening up novel avenues in the synthesis and design of inorganic materials for important photonic and associated applications.
2. Experimental section
2.1. Materials
The reagents used were yttrium(III) nitrate hexahydrate [Y(NO3)3·6H2O 99.9%, Alfa Aesar], europium(III) nitrate hexahydrate [Eu(NO3)3·6H2O 99.9%, Strem Chemicals], potassium hydroxide [KOH 85%, Labkem], and potassium tetrafluoroborate [KBF4 96%, Sigma-Aldrich]. All reagents were used without further purification.
2.2. Synthesis of Eu3+-doped compounds
The powders were prepared following a sonochemical process based on some previous studies of our research group detailed in references [21], [22]. Calculations were performed in order to obtain approximately 0.25 g of the α/δ-phase of KY3F10. In the synthesis procedure, 1.5 mmol of Ln(NO3)3·6H2O were dissolved in 30 mL of water (Ln = Y, Eu; 1 mol% Eu3+) with a resulting pH of 4.0. Then, 3.0 mmol of KBF4 were added to the above solution and the pH of the medium was adjusted to the desired value (pH: 4.0, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0) by adding dropwise 0.1 or 2 M KOH aqueous solutions (for pH > 7.0, a whitish precipitate appeared and it underwent redissolution/precipitation processes with the reaction time). The mixture was stirred for 5 min and the final volume was adjusted to 50 mL with water. The whole system was transferred into a Bandelin Sonorex ultrasonic bath operating at a frequency of 35 kHz for 24 h. The as-formed precipitate was then centrifuged, washed several times with water, and dried under an infrared lamp. For convenience, the abbreviations used for the different samples were denoted as “S-n”, where n indicates the initial pH of the reaction medium. A general scheme of the experimental procedure can be found in Fig. 1 of the Supplementary Information.
Fig. 1.
Coordination polyhedra of Y3+ in the different crystal structures together with the corresponding coordination numbers (CN) and local symmetries (LS). Some symmetry elements have been included for clarification purposes. The labels F1, F2, F3… refer to fluoride anions situated at different Wyckoff positions.
2.3. Characterization
All the characterization was performed at room temperature. Powder X-ray diffraction (XRD) was performed using a Bruker-AX D8-Advance X-ray diffractometer with CuKα1 radiation from 2θ = 15° to 90° at a scan speed of 2.25°/min. The microstructure of the samples was observed using a JEOL 7001F scanning electron microscope (SEM) operating with an acceleration voltage of 30 kV, a measuring time of 20 s, and a working distance of 10 mm. For the microstructural characterization, the powders were deposited on double-sided carbon stickers (previously adhered to the surface of aluminum stubs) and were sputtered with platinum. Moreover, the FT-IR spectra of the powders were recorded using an Agilent Cary 630 FT-IR spectrometer in transmission mode. Chemical equilibrium diagrams were generated with the Database-Spana software package, which is based on the SOLGASWATER and HALTAFALL algorithms [23], [24].
The optical properties were studied with an Eclipse Fluorescence Spectrophotometer (Varian). Excitation spectra were recorded in the range 250–500 nm with an emission wavelength of 593 nm, while emission spectra were performed upon excitation at 395/397 nm (depending on the compound) in the 500–750 nm range. All the spectra were normalized to unity. The asymmetry ratio and the Judd-Ofelt parameters ( and ) were calculated from the emission spectra. In addition, the CIE coordinates of the Eu3+-doped materials were calculated from the spectra using the GoCIE software package [25]. Time-resolved luminescence measurements were also performed to obtain the lifetimes and calculate the corresponding quantum efficiencies. In these experiments, the emission wavelength was set to 593 nm and the excitation was monitored at 395/397 nm (depending on the compound).
3. Results and discussion
3.1. Structural characterization
Prior to the discussion on the structural characterization, a brief description of the different crystal structures is provided in order to properly understand some concepts that can be helpful in this and subsequent sections of the paper. The compound α–KY3F10 crystallizes in a cubic structure belonging to the space group (SG) with 8 formula units per unit cell (Z = 8) [26], while δ-KY3F10·xH2O presents a cubic crystal system with SG = and Z = 16 [27]. Conversely, Y(OH)3–xFx is a hexagonal structure with SG = and Z = 2 [28]. The coordination polyhedra of Y3+ in the different crystal structures are depicted in Fig. 1. All structures were plotted with VESTA software [29]. The coordination number (CN) and the local symmetry (LS) of Y3+ are also shown. For the sake of clarity, different colors have been assigned to the crystal structures and have been maintained throughout all the graphics in the article: red (α-KY3F10), blue (δ-KY3F10·xH2O), and green (Y(OH)3–xFx). When a mixture of α- and δ-phases exists, the color purple is used.
It is important to mention that the ideal LS of Y3+ in Y(OH)3–xFx is C3h. Yet, this symmetry would only be strictly true for the fluoride-free compound, i.e., Y(OH)3, since the partial substitution of OH– by F– generates some local distortions and a lack/descent in symmetry is expected. The C3h point group is thus lowered to other subgroups of symmetry such as C3v, Cs, or C1, depending on the local environment of Y3+.
The XRD patterns of the Eu3+-doped powders prepared at different pH values following the sonochemical synthesis procedure are depicted in Fig. 2 and the results are summarized in Table 1. As can be appreciated, there is an extreme influence of the pH in the resulting crystal phase. Sample S-4.0 exhibits all the peaks corresponding to the single phase of cubic α-KY3F10 (ICSD card 00–040-9643). However, the increase in pH produces a coexistence between the α-KY3F10 and δ-KY3F10·xH2O phases in samples S-6.0 and S-6.5. Indeed, the peaks corresponding to the cubic δ-phase of KY3F10 become more prominent as the pH increases (see the blue stars in Fig. 2). Surprisingly, as presented in a recent publication [21], when the initial pH of the synthesis is set to 7.0 (sample S-7.0) the XRD pattern exhibits only the peaks corresponding to δ-KY3F10·xH2O (ICDD card 04–016-7073) [27], with no traces of α–KY3F10 secondary phase or other impurities.
Fig. 2.
XRD patterns for the Eu3+-doped compounds prepared at different pH values by a sonochemical process. The blue stars in samples S-6.0 and S-6.5 highlight the presence of peaks corresponding to the δ–KY3F10·xH2O structure. For the sake of greater clarity, the crystal phases of each powder have been indicated in brackets as abbreviations: α for α-KY3F10, δ for δ-KY3F10·xH2O, and YOHF for Y(OH)3–xFx. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Initial and final pH values of the reaction medium for the different Eu3+-doped compounds. The crystal phase and the weight of the synthesized powders are also indicated.
| Sample | Initial pH | Final pH | Powder Weight (g) | Crystal Phase |
|---|---|---|---|---|
| S-4.0 | 4.0 | 1.0 | 0.24 | α-KY3F10 |
| S-6.0 | 6.0 | 1.0 | 0.24 | α-KY3F10 + δ-KY3F10·xH2O |
| S-6.5 | 6.5 | 1.1 | 0.25 | α-KY3F10 + δ-KY3F10·xH2O |
| S-7.0 | 7.0 | 1.1 | 0.25 | δ-KY3F10·xH2O |
| S-7.5 | 7.5 | 6.4 | 0.22 | Y(OH)3–xFx |
| S-8.0 | 8.0 | 6.5 | 0.14 | Y(OH)3–xFx |
| S-8.5 | 8.5 | 6.6 | 0.06 | Y(OH)3–xFx |
| S-9.0 | 9.0 | 7.2 | 0.05 | Y(OH)3–xFx |
For the rest of the samples synthesized with a pH ≥ 7.5, another substantial change occurs. At first glance, the most notable visual change is the absence of peaks between 20 and 25° 2θ (present in the previous samples), a fact that affirms that the crystal structure of these compounds is different and, probably, with a different spatial group and/or crystal system. The XRD patterns of samples S-(7.5–9.0) are in good agreement with the hexagonal structure Y(OH)3–xFx (ICDD card 00–080-2008, x = 1.43). The reflection peaks of these powders are slightly shifted toward a higher angle relative to the JCPDS data (Figure S2, Supplementary Information), a behavior previously noted in several studies [30], [31], [32], [33], [34]. In this series of samples, it is important to note that the increase in the pH produces a broadening and a decrease in the intensity of the peaks, resulting in a more amorphous character.
In addition, the initial pH has an extreme influence not only on the crystal phase(s) obtained, but also on the weight of the final product and on the final pH of the reaction medium (measured at the end of the ultrasonic process, that is, 24 h after the reaction started), which are summarized in Table 1 as well. The results will be discussed in the following section because of their direct connection with the reaction mechanisms. To further characterize the materials, the FT-IR spectra of some representative compounds were recorded (Figure S3, Supplementary Information). All the spectra exhibited the characteristic bands associated with the internal vibrations of the different crystal structures. Besides, the presence of some absorbed water was noted.
3.2. Reaction mechanism
In order to elucidate the phenomena that govern the reaction mechanisms, some emphasis must first be placed on the equilibria that take place in the aqueous medium. Hence, it has been noted that one of the keystones is the hydrolysis of the tetrafluoroborate ion, which can be written as [35]:
BF4– + 3H2O ⇄ 3H+ + 4F– + B(OH)3 (1).
In addition, the equilibrium reactions of hydrofluoric acid and the species formed with boric acid [36], [37] also have to be considered:
HF ⇄ H+ + F– (2).
B(OH)3 + xF– + (x – 1)H+ ⇄ [BFx(OH)4-x]– + (x – 1)H2O (3).
Moreover, there is an equilibrium between the deprotonated [BFx(OH)4-x]– species and their respective protonated forms HBFx(OH)4-x, although the latter compounds dissociate very quickly and only HBF3(OH) has a significant concentration in the system at pH < 0, so they can be neglected. The equilibrium constant for the reverse reaction of (1), i.e., the formation of BF4–, takes the value Kf = 1019.21 at 25 °C. Consequently, the hydrolysis of BF4– is not favored due to the small value of the equilibrium constant of the forward reaction (1), Kh = 10–19.21, which has also been established to be very slow [35], [38].
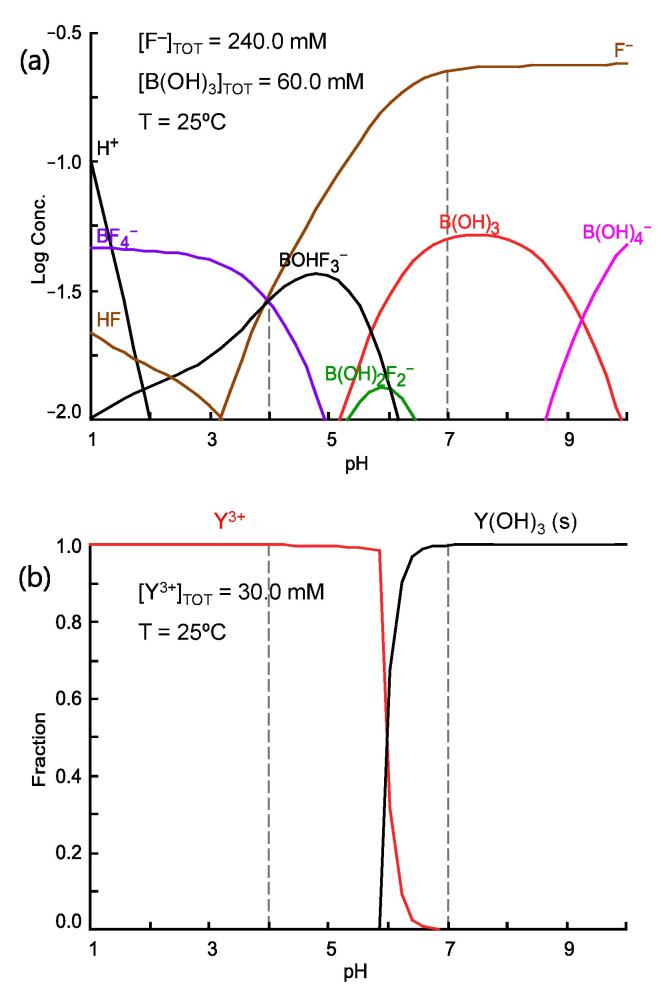
In order to properly understand the reaction mechanisms, the theoretical chemical equilibrium diagrams were generated with the Database-Spana software package and are presented in Fig. 3. Default parameter values (temperature and total concentrations) were set according to the experimental procedure followed in the synthesis of the materials. It should be noted that these diagrams take into account the thermodynamic behavior of the species, while neither the kinetics nor the sonochemical effects are contemplated. Hence, the diagrams can be useful to discuss some aspects of the reactions but they are not representative of the final products. Bearing this in mind, yttrium was considered separately because its reactivity with fluoride anions depends on the degree of hydrolysis of BF4–.
Fig. 3.
(a) Speciation in the boric acid–hydrofluoric acid system as a function of pH. (b) Fractions of the dominant species of yttrium as a function of pH.
Fig. 3(a) depicts the speciation in the B(OH)3–HF system as a function of pH, see reactions (1)–(3). At pH ≈ 7, an inflection point in the F– curve is observed with a plateau for pH > 7. By that stage BF4– hydrolysis should be completed. Fig. 3(b) presents the fractions of the dominant species of yttrium as a function of pH. It is clear that in the pH range 6–7, Y(OH)3 starts to become the dominant species. In contrast to the hydrolysis and reactivity of BF4– (slow and requires activation by sonochemical processes), the solid formation of Y(OH)3 is immediate. This fact is in clear agreement with the experimental procedure since in the basic medium a whitish precipitate was formed, thus indicating the formation of yttrium hydroxide, which will be redissolved, as highlighted in the following. As per the above considerations, it can be distinguished different reaction pathways (addressed in the following sections) that determine the formation of the final product. Furthermore, for a better comprehension of the results, the evolution of the final pH and the weight of the powders synthesized at different initial pH values are depicted in Fig. 4. This will also be discussed below because of their direct connection with the reaction mechanisms.
Fig. 4.
Evolution of the final pH and the weight of the powders synthesized at different initial pH values.
3.2.1. Formation of compounds with initial pH ≤ 7
As can be observed in Fig. 4, all the samples synthesized with an initial pH ≤ 7 had a very similar behavior taking into account the weight of the powders (0.24/0.25 g) and the final pH of the reaction medium (1.0/1.1). The amounts of the powders produced indicate that the reactions have a high yield since the calculations were performed so as to obtain approximately 0.25 g. The final pH values agree with the protons released during the successive hydrolysis of BF4–, see reaction (1). These facts lead to feel that the general reaction for these compounds must be very similar and can be represented as:
K+ + 3Y3+ + 10F– + xH2O → (α/δ)-KY3F10·xH2O (4).
The most intriguing issue to understand is perhaps the evolution from the α-phase of KY3F10 toward the formation of the δ-phase as the initial pH is increased. It should be noted that both phases crystallize in a cubic system with very similar space groups ( for the α-phase, and for the δ-phase). Therefore, these similarities may explain the possible coexistence of the two phases (as in samples S-6.0 and S-6.5) or the tendency of the system to form only one crystal structure in detriment of the other one (samples S-4.0 and S-7.0).
To explain the single-phase formation of δ-KY3F10·xH2O (sample S-7.0), more focus must be put again on Fig. 3(b). As mentioned earlier, yttrium hydroxide precipitates at pH ≈ 7. Moreover, the equilibrium of reaction (1) is expected to be shifted to the right by the addition of OH–, which partially neutralizes the H+. In fact, during the synthesis of this sample, when the pH value was initially set to 7.0, the solution became turbid, thereby indicating the precipitation of a whitish solid that corresponded to Y(OH)3. However, within a few minutes after putting the mixture in the ultrasonic bath, the pH of the medium decreased (which is in agreement with the successive hydrolysis of BF4–) and the precipitate was redissolved (see our previous publication for further details [21]). It also has to be noted that for samples synthesized with pH ≤ 6.5, this effect was not observed and no precipitate was initially formed. Therefore, the incipient precipitation of Y(OH)3 and its posterior redissolution could be crucial to control the fluoride and yttrium concentrations that would lead the system toward the formation of δ–KY3F10·xH2O.
Additionally, the formation of [BFx(OH)4-x]– species might also play a pivotal role because they could affect the solvation shell of Y3+ ions and make the reaction advance through different pathways. Indeed, at pH = 4, in Fig. 3(a) the confluence of curves corresponding to BF4–, [BF3(OH)]– and F– can be appreciated.
3.2.2. Formation of compounds with initial pH > 7
The mechanistic explanations for these compounds are easier because all the samples present the same crystal structure: Y(OH)3–xFx. According to Fig. 3(b), Y(OH)3 is the dominant species in a basic medium. During the synthesis procedure, the fast precipitation of Y(OH)3 yields an amorphous solid that can progressively react with fluoride anions released by BF4– hydrolysis. The reaction that takes place to form the final product may be understood by means of an anion-exchange process [39]:
Y(OH)3 + xF– ⇄ Y(OH)3–xFx + xOH– (5).
For these samples, at the initial stage (before being ultrasonicated), two phases were clearly distinguished in the reaction medium: a solid phase at the bottom of the bottle containing mainly the as-formed Y(OH)3, and a transparent aqueous phase at the top of it. As time went by, the distinction between the two phases started to disappear and only one phase was appreciated at the end of the reaction with the final product dispersed (as in the rest of the samples in this work).
Nonetheless, the amount of product decreased as the medium became more basic (see Fig. 4). Hence, the sonochemical processes involved in the formation of crystalline yttrium hydroxyfluorides are not favored in highly basic media. A plausible account of it might be found considering that some redissolution processes can occur promoted by ultrasonication. Additionally, the decrease in the powder weight may also be explained considering the kinetics of reaction (5), which is influenced by the pH. Thus, further analysis about the formation of hydroxyfluorides at different reaction times could complement this work and be the scope of future studies. Be that as it may, it is important to note that, to the best of our knowledge, this is the first publication in which an ultrasound-assisted process is described for the fabrication of yttrium hydroxyfluorides. Particularly, these materials can be used as precursors to produce the greatly appreciated YOF compounds by topotactic transformations or thermal decompositions [40], [41].
3.3. Morphological characterization
Fig. 5 presents the SEM images of the different Eu3+-doped materials and a schematic representation to illustrate the evolution of the morphology in a simple way. Table 2 summarizes the morphological results (shapes and approximate sizes of the particles and their respective subunits).
Fig. 5.
SEM images of the Eu3+-doped samples prepared at different pH values by a sonochemical process, and schematic representation of the morphology of the particles.
Table 2.
Representative morphologies and approximate sizes of the different particles and subunits.
| Sample | Morphology | Particle size (μm)a | Subunit | Subunit size (Ø nm)a |
|---|---|---|---|---|
| S-4.0 | Spherical | 0.2 – 0.7 (Ø) | Nanospheres | 20 |
| S-6.0 | Spherical + Truncated Triangular Nanoplates | 0.2 – 0.7 (Ø) | Nanospheres | 20 |
| S-6.5 | Spherical + Truncated Triangular Nanoplates | 0.2 – 0.7 (Ø) | Nanospheres | 20 |
| S-7.0 | Truncated Triangular Nanoplates | 0.1 (thickness), 0.2 (long edge) | – | – |
| S-7.5 | Spheroidal | 1.0 (long axis), 0.6 (short axis) | Nanorods | 30 |
| S-8.0 | Spheroidal | 0.8 (long axis), 0.3 (short axis) | Nanorods | 40 |
| S-8.5 | Spheroidal | 0.9 (long axis), 0.3 (short axis) | Nanorods | 50 |
| S-9.0 | Amorphous | Aggregates | – | – |
For samples S-6.0 and S-6.5, the particle/subunit size refers to the spherical particles.
Sample S-4.0 (α-KY3F10 structure) consists of spherical particles. A close-up observation (Figure S4(a), Supplementary Information) shows that these particles resulted from the self-assembly of nano-sized subunits around 20 nm in diameter (Ø). The same morphology is representative of samples S-6.0 and S-6.5 (α-KY3F10 + δ–KY3F10·xH2O structures), although some truncated triangular nanoplates were also observed in a very small proportion (Figure S4(b), Supplementary Information). The major particles (spherical) are attributed to the α-phase, while the minor ones (nanoplates) can be assigned to the δ-phase. These remarks suggest that the δ–phase is in the minority. In contrast, sample S-7.0 (δ-KY3F10·xH2O structure) only exhibits truncated triangular nanoplates, which is consistent with the existence of a single-phase rather than a mixture as in samples S-6.0 and S-6.5. It was also noted that the aggregation of some nanoplates occurred in order to reduce their surface energy.
As explained in the previous sections, substantial changes take place in materials with a Y(OH)3–xFx structure. The SEM micrographs show that samples S-(7.5–8.5) exhibit spheroidal particles that are composed of aligned nanorods as subunits (a magnification can be found in Figure S4(c,d) of the Supplementary Information). These nanorods were observed both on the surface and in the interior of the architectures and they aggregated side-by-side. Similar morphologies were obtained by Xianghong et al. [34] for Y(OH)3–xFx compounds synthesized hydrothermally. Curiously, the spheroidal particles tend to elongate with an increase in the pH. Lastly, the particle aggregates of sample S-9.0 evidence its amorphous character, as observed in the XRD pattern. Hence, the evolution of the crystal phase is also linked to the evolution of the morphology of particles (from spherical to truncated triangular nanoplates and to spheroidal). This fact can be explained taking into account that the activation energy required to form each compound is different, so the ultrasound radiation affects the morphology in different manners as well.
3.4. Photoluminescence studies
3.4.1. Excitation and emission spectra
The room temperature excitation and emission spectra of the Eu3+-doped samples recorded with a delay time (DT) of 0.2 ms are shown in Fig. 6. All the excitation spectra present similar spectra profiles, exhibiting the common bands associated with the different transitions of Eu3+ from the ground 7F0 level to higher excited levels with a clear dominance of the 7F0→5L6 transition. For samples S-(4.0–7.0), with (α/δ)–KY3F10·xH2O structures, the maximum of the band corresponding to the above transition is observed at 395 nm, while for samples S-(7.5–9.0), with a Y(OH)3–xFx structure, the band is slightly shifted toward the low-energy side (397 nm). Therefore, the corresponding emission spectra were recorded upon excitation at 395/397 nm, depending on the sample.
Fig. 6.
Room temperature excitation and emission spectra obtained with a DT = 0.2 ms for samples prepared at different pH values. The blue stars in the emission spectra indicate bands associated with 5D1→7FJ transitions from the higher excited level 5D1. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The most significant differences are found in the emission spectra of the phosphors, the profiles of which depend on the crystal lattice of the materials and thus the resulting color emission is affected, as will be discussed later on. All the samples present the typical 5D0→7FJ transitions of Eu3+ but interesting changes occur, taking into account the presence of transitions from higher excited levels, the splitting and relative intensities of bands, and their broadening.
Samples S-4.0 (α-KY3F10 structure) and S-(6.0, 6.5), with a mixture of α-KY3F10 + δ–KY3F10·xH2O structures, have the same emission profile. These results suggest that Eu3+ ions are mainly incorporated into the crystal lattice of α-KY3F10 in samples where the coexistence of α/δ-phases exists (S-6.0 and S-6.5). For these first samples, S-(4.0, 6.0, 6.5), the whole spectra are dominated by the 5D0→7F1 magnetic dipole transition and notable contributions from transitions that take place from the higher excited state 5D1 are observed (highlighted with blue stars in Fig. 6). The absence of the 5D0→7F0 transition is justified by its overlapping with the 5D0→7F1 band and because of its typical low intensity. The interpretation of the emission spectra recorded with a DT = 0.2 ms can be difficult due to the mixing of 5D0,1→7FJ transitions. Therefore, the photoluminescence measurements of these samples were also carried out increasing the detector delay time to 10 ms, since the Eu3+ lifetimes associated with the transitions occurring from the 5D1 higher energy level are lower than those corresponding to the 5D0 ground level. As a result, these experiments allowed to observe only the contribution of 5D0→7FJ transitions and ensure that the assignation of bands was accurate (Figure S5 of the Supplementary Information). Further information about the assignation of the emission bands to their respective transitions can also be found in Section 3 of the Supplementary Information.
A remarkable contrast is observed in sample S-7.0 (with a single δ-KY3F10·xH2O phase). In this case, the spectrum is dominated by the 5D0→7F2 electric dipole transition, and 5D1→7FJ transitions (blue stars) are almost absent, probably quenched by the existence of crystalline water molecules in the host lattice. However, although this contribution was very small and may not be of substantial importance for the subsequent calculation of physical parameters, the emission spectrum of sample S-7.0 was also collected with a DT = 10 ms.
The last series of samples S-(7.5–9.0) presents the typical photoluminescence profile of Eu3+-doped Y(OH)3–xFx compounds [34], and also exhibits broad bands and is dominated by the 5D0→7F2 electric dipole transition. For these samples, there is a complete absence of transitions from higher excited levels due to the presence of hydroxyl groups in the crystalline matrix. Besides, the observation of the 5D0→7F0 transition is clearer, albeit with low intensity.
Lastly, the CIE coordinates of the materials are plotted in Fig. 7. Interestingly, the color emissions of the phosphors can be tuned from orangish to red hues through the different crystal structures (higher pH values imply an evolution toward reddish tonalities). However, it is important to note that samples with the same crystal phase have virtually the same color emission regardless of the pH.
Fig. 7.
CIE chromaticity diagram of the Eu3+-doped samples prepared at different pH values.
3.4.2. Asymmetry ratio and Judd-Ofelt parameters
The 5D0→7F1 transition has a magnetic dipole character and is considered independent of the chemical surroundings of Eu3+ ions. Moreover, the 5D0→7F2 transition of Eu3+ presents an electric dipole character, and is therefore hypersensitive to the local symmetry of the dopant [42], [43]. As a consequence, the relative intensities of these transitions are widely used to obtain information about the site symmetry of Eu3+ ions [44]. For that purpose, it is usual to define the asymmetry ratio as the quotient between the integrated intensities of the 5D0→7F2 and 5D0→7F1 transitions.
On the other hand, the Judd-Ofelt (JO) parameters are extremely useful to characterize the local structure and bonding of lanthanide ions and, in the particular case of Eu3+, they can be calculated directly from the emission spectra [45]. In the literature, it is well known that the short-range JO parameter () provides information about the polarizability and crystal environment of Eu3+ in the lattice, whereas the long-range JO parameter () is sensitive to macroscopic properties of the hosts such as viscosity and rigidity [46]. A direct relation between and the asymmetry ratio can be established [47]. Hence, larger values of point to stronger polarizability and higher asymmetry of Eu3+ ions in the crystal lattice [48], [49]. The physicochemical parameters mentioned above are listed in Table 3, while the equations employed to calculate them are described in Sections 4 and 5 of the Supplementary Information.
Table 3.
Asymmetry ratio () values, Judd-Ofelt parameters (, ), 5D0 lifetimes () and quantum efficiencies () for the different Eu3+-doped phosphors. For samples S-(7.5–9.0), corresponds to the effective lifetime.
| Sample | (10-20 cm2) | (10-20 cm2) | (ms) | (ms) | (%) | |
|---|---|---|---|---|---|---|
| S-4.0 | 0.99 | 1.77(2) | 1.65(1) | 7.88(1) | 8.07(4) | 98 |
| S-6.0 | 0.97 | 1.74(2) | 1.77(2) | 7.73(2) | 8.10(4) | 95 |
| S-6.5 | 0.97 | 1.72(2) | 1.80(2) | 7.52(2) | 7.99(4) | 94 |
| S-7.0 | 1.43 | 2.52(3) | 1.93(3) | 4.93(1) | 6.80(3) | 73 |
| S-7.5 | 1.84 | 3.17(4) | 1.96(3) | 1.82(5) | 4.25(3) | 43 |
| S-8.0 | 1.79 | 3.09(4) | 2.16(3) | 1.40(6) | 4.23(3) | 33 |
| S-8.5 | 1.71 | 2.95(4) | 2.30(3) | 1.20(4) | 4.28(4) | 28 |
| S-9.0 | 1.85 | 3.19(4) | 2.56(3) | 1.11(3) | 3.99(2) | 28 |
The values of the asymmetry ratio are linked to the incorporation of Eu3+ ions in different crystal structures: ≈ 1.0 for α-KY3F10 as the main phase (pH ≤ 6.5), ≈ 1.4 for δ-KY3F10·xH2O (pH = 7.0), and ≈ 1.8 for Y(OH)3–xFx (pH ≥ 7.5). The successive increase of indicates the higher asymmetry of Eu3+, which is in excellent agreement with the expected site symmetry for the dopant (see Fig. 1). The asymmetry ratio and are directly related, and therefore the same reasoning can be applied to this JO parameter. It is also observed that increases as the pH rises, which can be attributed to the different host lattices and morphologies of the particles, since is ascribed to bulk properties.
3.4.3. Time-resolved luminescence and quantum efficiencies
The decay curves for the 5D0→7F1 emission of Eu3+ were measured to allow further analysis of the luminescence properties of the samples (Figure S7, Supplementary Information) and to extract the observed lifetimes (). Interestingly, a small rising part at the beginning of the decay profiles was observed for samples synthesized with pH ≤ 7.0 (samples with α/δ-phase of KY3F10). This effect is ascribed to the Eu3+ population feeding from the upper 5D1 excited level. The rising phenomenon was more notable for samples S-(4.0–6.5), which also presented the most important contribution of 5D1→7FJ emissions (see the emission spectra in Fig. 6). The decay curves were thus fitted using a single exponential model with a modified pre-exponential factor that modulates the population of Eu3+ ions [50], [51]. However, the decay curves of samples synthesized with a pH ≥ 7.5, Y(OH)3–xFx compounds, were best fitted with a double exponential model without the presence of a rising part. This change in the fitting expression might well be attributed to different local environments of Eu3+ affected by the surrounding OH– and F– anions.
In order to evaluate the quantum efficiencies (the intrinsic quantum yields,) of the materials, the theoretical radiative lifetimes () of Eu3+ ions for the 5D0 level were also calculated from the emission spectra. Extra details about all the equations used can be found in Section 6 of the Supplementary Information. The observed lifetimes, the calculated radiative lifetimes, and the quantum yields are presented in Table 3. All the parameters present a monotonic decrease with the rise in pH. Lifetimes vary from 7.9 ms to 1.1 ms, whereas extremely different quantum yields are also obtained (98–28%). The key point that allows these values to be obtained is the sequential evolution of the crystal phase. For instance, the crystalline water molecules in the host lattice of the δ-phase of KY3F10 (sample S-7.0) can partially quench the radiative emission, thus yielding notably lower quantum efficiencies in comparison to the α–phase of KY3F10 (sample S-4.0). Following the same line of reasoning, the presence of hydroxyls in Y(OH)3–xFx compounds has a strong impact on the quenching process. For this last series of samples, a higher degree of suppression also occurs while increasing the pH. This can be attributed to the increasing amorphous character of the particles (as noted in the lack of resolution in the photoluminescence spectra) and to the possible presence of residual hydroxyls in the surface of the particles.
By all accounts, the results underscore the highly tunable optical response of the materials, with a wide gamut of color emissions, lifetimes, and quantum efficiencies. These parameters have shown a direct connection with the modulated crystal structures and morphologies of the compounds. The detailed analysis of the photoluminescence properties has allowed to further confirm the formation of the different crystal structures due to the unique site-selective characteristics of the Eu3+ ion. Hence, the optical study has also corroborated the postulated formation mechanisms of the materials.
4. Conclusions
In order to shed some light on the formation of the different crystal phases of KY3F10, Eu3+-doped yttrium fluorides were designed by ultrasound-assisted processes at a wide range of pH values (4.0–9.0). The major conclusions that can be extracted are as follows:
-
▪
The crystal phase and the morphology of the materials are critically dependent upon the initial pH of the reaction medium.
-
▪
A description of plausible reaction mechanisms has underlined the fact that the hydrolysis of the tetrafluoroborate ion and the incipient precipitation of Y(OH)3 and posterior redissolution/precipitation processes could play a crucial role.
-
▪
The unique site-selective characteristics of the Eu3+ ion have allowed to describe the optical performance of the materials and to establish a relationship with their chemical structure, as has been observed with the calculations of the asymmetry ratio and the Judd-Ofelt parameters.
-
▪
The emission profiles of the phosphors depend on the crystal lattice of the materials, which exhibit 5Di→7FJ transitions with different splitting, broadening, and relative intensities of bands. The resulting color can thus be tuned from orangish to red hues.
-
▪
The modulated optical response of the phosphors is also evidenced by the lifetime values (7.9–1.1 ms) and quantum efficiencies (98–28%).
Based on the aforementioned points, the present novel strategy can contribute to an in-depth comprehension of these materials, especially of the uncharted δ–KY3F10·xH2O structure. Furthermore, the study unveils the importance of sonochemistry in obtaining luminescent fluorides with controlled crystal structures that can open up new avenues in the synthesis and design of inorganic materials for important photonic and associated applications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the Spanish Ministerio de Ciencia e Innovación (Project PID2020-116149 GB-I00) and the Universitat Jaume I (Project UJI-B2019-41). P. Serna also thanks the Spanish Ministerio de Ciencia, Innovación y Universidades for an FPU predoctoral contract.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106059.
Contributor Information
Pablo Serna-Gallén, Email: pserna@uji.es.
Héctor Beltrán-Mir, Email: mir@uji.es.
Eloísa Cordoncillo, Email: cordonci@uji.es.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gedanken A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004;11:47–55. doi: 10.1016/j.ultsonch.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 2.Pokhrel N., Vabbina P.K., Pala N. Sonochemistry: Science and Engineering. Ultrason. Sonochem. 2016;29:104–128. doi: 10.1016/j.ultsonch.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Hangxun X., Zeiger B.W., Suslick K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013;42:2555–2567. doi: 10.1039/c2cs35282f. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L., Liu Y., Fan X., Yang D., Cao X. Rapid synthesis of single-crystalline TbF3 with novel nanostructure via ultrasound irradiation. Mater. Res. Bull. 2011;46:252–257. doi: 10.1016/j.materresbull.2010.11.003. [DOI] [Google Scholar]
- 5.Li Z., Zhuang T., Dong J., Wang L., Xia J., Wang H., Cui X., Wang Z. Sonochemical fabrication of inorganic nanoparticles for applications in catalysis. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J., Wang J., Lin J., Xiao Y., Yue G., Huang M., Lan Z., Huang Y., Fan L., Yin S., Sato T. Dual functions of YF3:Eu3+ for improving photovoltaic performance of dye-sensitized solar cells. Sci. Rep. 2013;3:1–5. doi: 10.1038/srep02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tissue B.M. Synthesis and Luminescence of Lanthanide Ions in Nanoscale Insulating Hosts. Chem. Mater. 1998;10:2837–2845. doi: 10.1021/cm9802245. [DOI] [Google Scholar]
- 8.Grzyb T., Wȩcławiak M., Pȩdziński T., Lis S. Synthesis, spectroscopic and structural studies on YOF, LaOF and GdOF nanocrystals doped with Eu3+, synthesized via stearic acid method. Opt. Mater. (Amst) 2013;35:2226–2233. doi: 10.1016/j.optmat.2013.06.007. [DOI] [Google Scholar]
- 9.Podhorodecki A., Noculak A., Banski M., Sojka B., Zelazo A., Misiewicz J., Cichos J., Karbowiak M., Zasonska B., Horak D., Sikora B., Elbaum D., Dumych T., Bilyy R., Szewczyk M. Lanthanides Fluorides Doped Nanocrystals for Biomedical Applications. ECS Trans. 2014;61:115–125. doi: 10.1149/06105.0115ecst. [DOI] [Google Scholar]
- 10.Jain A., Fournier P.G.J., Mendoza-Lavaniegos V., Sengar P., Guerra-Olvera F.M., Iñiguez E., Kretzschmar T.G., Hirata G.A., Juárez P. Functionalized rare earth-doped nanoparticles for breast cancer nanodiagnostic using fluorescence and CT imaging. J. Nanobiotechnology. 2018;16:1–18. doi: 10.1186/s12951-018-0359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sassoye C., Patriarche G., Mortier M. High yield syntheses of reactive fluoride K1–x(Y, Ln)xF1+2x nanoparticles. Opt. Mater. (Amst) 2009;31:1177–1183. doi: 10.1016/j.optmat.2008.12.013. [DOI] [Google Scholar]
- 12.Auzel F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004;104(1):139–174. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 13.Silva E.N., Ayala A.P., Gesland J.Y., Moreira R.L. Vibrational spectrum and lattice dynamics of KY3F10 single crystals. Vib. Spectrosc. 2005;37:21–26. doi: 10.1016/j.vibspec.2004.05.004. [DOI] [Google Scholar]
- 14.Cascales C., Fernández J., Balda R. Investigation of site-selective symmetries of Eu3+ ions in KPb2Cl5 by using optical spectroscopy. Opt. Express. 2005;13:2141–2152. doi: 10.1364/OPEX.13.002141. [DOI] [PubMed] [Google Scholar]
- 15.Zhu L., Meng J., Cao X. Sonochemical synthesis of monodispersed KY3F10:Eu3+nanospheres with bimodal size distribution. Mater. Lett. 2008;62:3007–3009. doi: 10.1016/j.matlet.2008.01.096. [DOI] [Google Scholar]
- 16.Cao C., Yang H.K., Moon B.K., Choi B.C., Jeong J.H., Kim K.H. Hydrothermal synthesis, phase evolution, and optical properties of Eu3+-doped KF-YF3 system materials. J. Mater. Res. Soc. 2012;27(23):2988–2995. [Google Scholar]
- 17.Goderski S., Runowski M., Lis S. Synthesis of luminescent KY3F10 nanopowder multi-doped with lanthanide ions by a co-precipitation method. J. Rare Earths. 2016;34:808–813. doi: 10.1016/S1002-0721(16)60098-4. [DOI] [Google Scholar]
- 18.Runowski M. Color-tunable up-conversion emission of luminescent-plasmonic, core/shell nanomaterials-KY3F10:Yb3+, Tm3+/SiO2-NH2/Au. J. Lumin. 2017;186:199–204. doi: 10.1016/j.jlumin.2017.02.032. [DOI] [Google Scholar]
- 19.Chen M., Loiko P., Serres J.M., Veronesi S., Tonelli M., Aguiló M., Díaz F., Choi S.Y., Bae J.E., Rotermund F., Dai S., Chen Z., Griebner U., Petrov V., Mateos X. Fluorite-type Tm3+:KY3F10: A promising crystal for watt-level lasers at ∼1.9 μm. J. Alloys Compd. 2020;813 doi: 10.1016/j.jallcom.2019.152176. [DOI] [Google Scholar]
- 20.Le Berre F., Boucher E., Allain M., Courbion G. Synthesis, stability and zeolitic behavior of δ-ALn3F10, xH2O and γ-ThLn2F10, H2O phases (Ln=lanthanide) J. Mater. Chem. 2000;10:2578–2586. doi: 10.1039/b002520h. [DOI] [Google Scholar]
- 21.Serna-Gallén P., Beltrán-Mir H., Cordoncillo E. The unexplored δ-phase of KY3F10: toward novel Eu3+-doped nanoplates with a ‘super-diamond’ structure for optical applications. J. Mater. Res. Technol. 2021;15:6940–6946. doi: 10.1016/j.jmrt.2021.11.060. [DOI] [Google Scholar]
- 22.Serna-Gallén P., Beltrán-Mir H., Cordoncillo E. Tuning the optical and photoluminescence properties of high efficient Eu3+-doped KY3F10 phosphors by different synthetic approaches. Opt. Laser Technol. 2021;136 doi: 10.1016/j.optlastec.2020.106734. [DOI] [Google Scholar]
- 23.Eriksson G. An algorithm for the computation of aqueous multi-component, multiphase equilibria. Anal. Chim. Acta. 1979;112:375–383. doi: 10.1016/S0003-2670(01)85035-2. [DOI] [Google Scholar]
- 24.Ingri N., Kakolowicz W., Sillén L.G., Warnqvist B. High-speed computers as a supplement to graphical methods—V1Haltafall, a general program for calculating the composition of equilibrium mixtures. Talanta. 1967;14:1261–1286. doi: 10.1016/0039-9140(67)80203-0. [DOI] [PubMed] [Google Scholar]
- 25.J.T. K. R., GoCIE V2, (2009). http://faculty.iitr.ac.in/∼krjt8fcy/index.html.
- 26.P. Villars, K. Cenzual, eds., KY3F10 Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition – 2012” in SpringerMaterials (https://materials.springer.com/isp/crystallographic/docs/sd_0552093), (n.d.).
- 27.P. Villars, K. Cenzual, eds., δ-KY3F10·xH2O (KY3F10[H2O]) Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition – 2012” in SpringerMaterials (https://materials.springer.com/isp/crystallographic/docs/sd_1004004), (n.d.).
- 28.P. Villars, K. Cenzual, eds., Y(OH)1.57F1.43 (Y[OH]2F) Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition – 2012” in SpringerMaterials (https://materials.springer.com/isp/crystallographic/docs/sd_1900591), (n.d.).
- 29.Momma K., Izumi F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011;44:1272–1276. doi: 10.1107/S0021889811038970. [DOI] [Google Scholar]
- 30.Liu B.-Q., Guo K., Wang J., Zhang Z.-J., Tao Y., Huang Y., Zhao J.-T. Mild hydrothermal synthesis and photoluminescence of needle-like Y(OH)1.1F1.9:Tb3+ Mater. Lett. 2013;100:245–247. doi: 10.1016/j.matlet.2013.03.024. [DOI] [Google Scholar]
- 31.Guo L., Wang Y., Han L., Qiang Q., Zeng W., Zou Z., Wang B., Guo X. Band structure, shape controllable synthesis and luminescence properties of the precursor and final product Lu6O5F8:Eu/Tb/Ce/Dy nano/microstructures. J. Mater. Chem. C. 2013;1:7952–7962. doi: 10.1039/c3tc31838a. [DOI] [Google Scholar]
- 32.Liu L., Chen H., Liu B., Zhang H., Tang B., Feng X., Sun Z., Zhao J. Synthesis, structure and luminescent properties of a new Vernier phase Lu7O6F9 doped by Eu3+ as potential scintillator with unique lath tube architecture. J. Rare Earths. 2014;32:686–690. doi: 10.1016/S1002-0721(14)60126-5. [DOI] [Google Scholar]
- 33.Shao B., Zhao Q., Jia Y., Lv W., Jiao M., Lü W., You H. Facile large-scale synthesis of monodisperse REF3 (RE = Y, Ce, Nd, Sm-Lu) nano/microcrystals and luminescence properties. J. Mater. Chem. C. 2014;2:7666–7673. doi: 10.1039/c4tc00808a. [DOI] [Google Scholar]
- 34.He X., Yan B. Yttrium hydroxide fluoride based monodisperse mesocrystals: Additive-free synthesis, enhanced fluorescence properties, and potential applications in temperature sensing. CrystEngComm. 2015;17:621–627. doi: 10.1039/c4ce01823k. [DOI] [Google Scholar]
- 35.Zeebe R.E., Rae J.W.B. Equilibria, kinetics, and boron isotope partitioning in the aqueous boric acid–hydrofluoric acid system. Chem. Geol. 2020;550 doi: 10.1016/j.chemgeo.2020.119693. [DOI] [Google Scholar]
- 36.Wamser C.A. Equilibria in the System Boron Trifluoride-Water at 25°. J. Am. Chem. Soc. 1951;73:409–416. doi: 10.1021/ja01145a134. [DOI] [Google Scholar]
- 37.Mesmer R.E., Palen K.M., Baes C.F., Jr. Fluoroborate Equilibria in Aqueous Solution. Inorg. Chem. 1973;12:89–95. [Google Scholar]
- 38.Anbar M., Guttmann S. The isotopic exchange of fluoroboric acid with hydrofluoric acid. J. Phys. Chem. 1961;64:1896–1899. doi: 10.1021/j100841a021. [DOI] [Google Scholar]
- 39.Tian L., Jiang W., Sun Q., Liu J. Synthesis and characterization of branched yttrium hydroxide fluoride microcrystals with hierarchical tubular structure. J. Rare Earths. 2012;30:378–382. doi: 10.1016/S1002-0721(12)60054-4. [DOI] [Google Scholar]
- 40.Shao B., Zhao Q., Lv W., Jiao M., Lü W., You H. Novel Two-step topotactic transformation synthetic route towards monodisperse LnOF:Re,3+ (Ln = Y, Pr-Lu) Nanocrystals with down/upconversion luminescence properties. Adv. Opt. Mater. 2015;3:583–592. doi: 10.1002/adom.201400638. [DOI] [Google Scholar]
- 41.Li J., Wang X., Zhu Q., Kim B.N., Sun X., Li J.G. Interacting layered hydroxide nanosheets with KF leading to Y/Eu hydroxyfluoride, oxyfluoride, and complex fluoride nanocrystals and investigation of photoluminescence. RSC Adv. 2017;7:53032–53042. doi: 10.1039/c7ra10508h. [DOI] [Google Scholar]
- 42.Vuković K., Medić M., Sekulić M., Dramićanin M.D. Analysis of Eu3+ emission from Mg2TiO4 nanoparticles by Judd-Ofelt theory. Adv. Condens. Matter Phys. 2015;2015:1–7. doi: 10.1155/2015/736517. [DOI] [Google Scholar]
- 43.Cantelar E., Sanz-García J.A., Sanz-Martín A., Muñoz Santiuste J.E., Cussó F. Structural, photoluminescent properties and Judd-Ofelt analysis of Eu3+-activated CaF2 nanocubes. J. Alloys Compd. 2020;813 doi: 10.1016/j.jallcom.2019.152194. [DOI] [Google Scholar]
- 44.Hassairi M.A., Garrido Hernández A., Kallel T., Dammak M., Zambon D., Chadeyron G., Potdevin A., Boyer D., Mahiou R. Spectroscopic properties and Judd-Ofelt analysis of Eu3+ doped GdPO4 nanoparticles and nanowires. J. Lumin. 2016;170:200–206. doi: 10.1016/j.jlumin.2015.10.054. [DOI] [Google Scholar]
- 45.B.M. Walsh, Judd-Ofelt theory: principles and practices, in: B. Di Bartolo, O. Forte (Eds.), Adv. Spectrosc. Lasers Sens., Springer, Dordrecht, 2006: pp. 403–433. 10.1007/1-4020-4789-4_21.
- 46.S.K. Gupta, M.A. Penilla Garcia, J.P. Zuniga, Y. Mao, pH induced size tuning of Gd2Hf2O7:Eu3+ nanoparticles and its effect on their UV and X-ray excited luminescence, J. Lumin. 228 (2020) 117605. 10.1016/j.jlumin.2020.117605.
- 47.Serna-Gallén P., Beltrán-Mir H., Cordoncillo E., West A.R., Balda R., Fernández J. Site-selective symmetries of Eu3+-doped BaTiO3 ceramics: a structural elucidation by optical spectroscopy. J. Mater. Chem C. 2019;7:13976–13985. doi: 10.1039/c9tc03987b. [DOI] [Google Scholar]
- 48.Patel D.K., Vishwanadh B., Sudarsan V., Kulshreshtha S.K., McKittrick J. Difference in the Nature of Eu3+ Environment in Eu3+-Doped BaTiO3 and BaSnO3. J. Am. Ceram. Soc. 2013;96(12):3857–3861. [Google Scholar]
- 49.de Mello Donegá C., Junior S.A., de Sá G.F. Synthesis, luminescence and quantum yields of Eu(III) mixed complexes with 4,4,4-trifluoro-1-phenyl-1,3-butanedione and 1,10-phenanthroline-N-oxide. J. Alloys Compd. 1997;250(1-2):422–426. [Google Scholar]
- 50.Yamase T., Kobayashi T., Sugeta M., Naruke H. Europium(III) Luminescence and Intramolecular Energy Transfer Studies of Polyoxometalloeuropates. J. Phys. Chem. A. 1997;101(28):5046–5053. [Google Scholar]
- 51.Debasu M.L., Ananias D., Macedo A.G., Rocha J., Carlos L.D. Emission-decay curves, energy-transfer and effective-refractive index in Gd2O3:Eu3+ nanorods. J. Phys. Chem. C. 2011;115:15297–15303. doi: 10.1021/jp205093x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.