Abstract
Purpose
To analyze long-term results of two multicenter prospective single-arm trials (ARO-2010-01 and ARO-2013-04) investigating adjuvant hypofractionated radiotherapy (HF) with simultaneous integrated boost (SIB) after breast-conserving surgery (BCS).
Methods
Eligible patients had histopathologically confirmed unifocal breast cancer planned for whole breast irradiation plus boost radiotherapy to the tumor bed. In both studies, a total dose of 40 Gy was applied to the whole breast and of 48 Gy to the tumor bed in 16 fractions of 2.5 and 3.0 Gy. Radiotherapy could be given either as three-dimensional conformal radiotherapy (3D-CRT) or as intensity-modulated radiotherapy (IMRT). The primary study objectives were feasibility and security within an observation period of six months. The current investigation focuses on long-term efficacy and toxicities.
Results
Between 2011 and 2014, both trials enrolled 300 patients in total. Data from 274 of these patients could be used for the current analysis. The median follow-up time was 60 months and the 5-year disease-free survival 92.1%. Three patients suffered a local recurrence (after 36–72 months) while a regional recurrence occurred in one patient (after 17 months). The 5-year local control rate in the breast was 99.6%. 63.5% of all patients did not report any late radiation-related toxicity, 28.5% reported grade 1 and 7.3% grade 2 toxicities. The highest late toxicity was grade 3 in 2 women (0.7%, telangiectasia and lymphedema of the breast).
Conclusion
Our analysis demonstrates favorable efficacy and low rates of long-term side effects of HF with SIB after BCS. Randomized controlled phase III trials are ongoing.
Keywords: Moderate hypofractionation, Local recurrence, Late toxicity, Boost irradiation, SIB
Highlights
-
•
Hypofractionated breast radiotherapy with SIB was safe and feasible.
-
•
The local control rate at 5 years was 99.6%.
-
•
The rate of late grade 3 toxicity was 0.7%.
1. Introduction
Post-operative radiotherapy is an important part of breast cancer therapy after breast-conserving surgery. Conventionally fractionated whole-breast radiotherapy (WBRT) administers a total dose of 50–50.4 Gy in 25–28 fractions. An additional targeted irradiation of the tumor bed, also known as boost, is usually administered to patients at increased risk for local recurrence. Traditionally, this boost has been administered sequentially after completion of WBRT. With advances in radiotherapy techniques, the possibility arose to shorten treatment time by administering the boost to the tumor bed simultaneously with WBRT. During the past decade, moderate hypofractionation (HF) with 40–42.5 Gy in 15–16 fractions over 3 weeks has replaced conventionally fractionated radiotherapy as standard of care for WBRT [1]. Numerous randomized controlled trials and a Cochrane meta-analysis have demonstrated equivalent outcome in terms of local tumor control, overall survival and late toxicity with the benefit of shorter overall treatment time [[2], [3], [4], [5], [6], [7]]. Another meta-analysis has suggested lower rates of acute toxicity such as radiation dermatitis and late normal tissue effects such as breast edema and telangiectasia with moderate HF [8].
It should be noted that not all of these trials used boost irradiation. In the Canadian trial by Whelan et al. [7], boost irradiation was prohibited while its use was permitted and used as a stratification factor in the START-trials [2]. In the randomized controlled trials included in the above mentioned meta-analyses, the boost to the tumor bed, if indicated, was administered sequentially after HF-WBRT. A simultaneous integrated boost (SIB) has dosimetric advantages, in particular an improved dose homogeneity within the breast with a reduction of overdosages outside the boost volume [[9], [10], [11]]. For conventionally fractionated radiotherapy, data on the use of SIB are available from two randomized controlled trials that demonstrated similar outcome in terms of local control, late toxicity and cosmetic outcome [[12], [13], [14], [15]].
The combination of HF and SIB promises a further shortening of the overall treatment time for breast cancer patients and also has economic advantages, e.g., reduced costs and savings of resources for radiotherapy facilities. Two multicenter prospective trials of the ARO (Arbeitsgemeinschaft Radiologische Onkologie, German Radiation Oncology Working Group), ARO-2010-01 and ARO-2013-04, have demonstrated the short-term feasibility and safety of HF plus SIB in early breast cancer patients [16,17]. The ARO-2010-01 trial, which focused on short-term feasibility of HF with SIB, achieved a protocol-conform therapy in 89% (95% CI: 87–96%). The ARO-2013-04 trials primary focus was on the acute toxicity of the method. Overall, 14.7% of the patients experienced a toxicity of grade 2 or higher. No grade 4 toxicity was reported and the most frequent grade 3 toxicities were hot flashes (11%) [17] .
Previous reports from other groups have studied various HF-WBRT regimens with SIB [[18], [19], [20], [21], [22], [23]], however many of these reports were restricted by small sample size and/or limited follow up. Here we report long-term results of both ARO-trials with a special emphasis on efficacy and long-term toxicity.
2. Materials and methods
2.1. Study overview
ARO 2010–01 and ARO 2013–04 were both multicenter prospective single-arm trials investigating the feasibility (ARO 2010–01) and safety (ARO 2013–04; NCT01948726) of adjuvant HF with a SIB in patients after breast conserving surgery. Both trials had an observation period of six months after completion of radiotherapy; these results have been published [16,17]. The current observation was not pre-specified in the study protocols and therefore not considered in calculation of the original sample size [16,17]. The current observation has collected data beyond the original observation period to analyze long-term side effects and long-term events in the patients of both trials.
2.2. Eligibility criteria
The eligibility criteria included women aged 18 years or older with histopathologically confirmed unifocal breast cancer. Patients had undergone BCS with clear margins and had to have an indication for post-operative radiotherapy of the breast (without regional irradiation) including boost radiotherapy. There were no protocol-specified criteria or risk factors for boost irradiation. The decision to use boost irradiation was left to the discretion of the treating physician. The main exclusion criteria were mastectomy, no indication for boost irradiation, no clear identification of the tumor bed on planning computed tomography (CT), prior radiation therapy which could impair the treatment investigated in trial, extensive seroma in the resection bed before radiotherapy, indication for regional nodal irradiation, poor general condition, pregnancy, other conditions limiting protocol-specific administration of radiotherapy and lack of informed consent. These criteria were identical in both trials. The later ARO 2013–04 trial protocol specified additional criteria, mainly linguistical and mental ability to fill out quality of life questionnaires. One more exclusion criterion was the participation in other trials at the same time which could influence the effect of radiotherapy.
Pretreatment evaluation included a complete history and physical examination with acquisition of relevant comorbidities. Relevant comorbidities were defined as morbidities which may impair treatment conduct (e.g., unstable cardiac disease). The tumor bed had to be detectable on the planning CT.
2.3. Radiation therapy
The treatment protocol was similar in both trials. The whole breast received a dose of 40 Gy in 16 fractions of 2.5 Gy. The SIB to the tumor bed had a total dose of 48 Gy with additional daily doses of 0.5 Gy to the boost planning target volume (PTV). This hypofractionation approach yields an equivalent dose in 2 Gy-fractionation (EQD2) of 43.6 Gy for the whole breast and 56.7 Gy to the tumor bed using an α/β-value of 3.5 for tumor control [2].
Radiotherapy was to be delivered by a linear accelerator with minimal energy of 6 MeV using either photon/electron or photon/photon combination depending on optimal PTV coverage. Either threee-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT) technique could be used. Upper dose limits were defined for organs at risk. These were median heart dose of <5 Gy, median LAD dose of <15 Gy, heart and LAD maximum dose of ≤40 Gy, median contralateral breast dose of <3 Gy and additionally in ARO 2013-04 median ipsilateral lung dose of <10 Gy. It was specified in the study protocol that the planning target volume (PTV) for the boost should not be larger than 20% of the breast PTV. No specific constraints for breast PTV minus boost PTV were provided in the protocol. Generally, V95% ≥ 90% for the PTV breast and PTV boost was intended.
2.4. Follow up analysis
The recruitment periods were 2011 through 2013 (ARO-2010-01) and 2013 through 2014 (ARO-2013-04). This unplanned analysis was conducted in 2020 after a maximum follow-up of 8.5 years and observed disease events including local recurrences, regional recurrences, metastases, second malignancies (including contralateral breast cancers) and deaths. Moreover, late side effect of radiation therapy, in particular fibrosis of the tumor bed, hyperpigmentation and telangiectasia of the irradiated skin, lung fibrosis, heart disease, plexopathy of the shoulder on the irradiated side and breast or arm lymphedema, were analyzed. Data were retrospectively collected from the participating sites. All participating trial sites were contacted, and the follow-up was evaluated for all patients, and, if necessary, completed. Participating sites were asked to obtain missing data through either additional follow-up examinations or telephone interviews. If neither in-person follow-up or telephone interviews were feasible, patients were contacted via mailed questionnaires. The questionnaire was designed in such a way to be descriptive and understandable for the patients (see supplementary material).
Late toxicities, in particular fibrosis, telangiectasia and hyperpigmentation of the skin, were classified based on the RTOG/EORTC Late Radiation Morbidity Scoring Criteria [24]. Plexopathy of the arm was classified according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4 and lung fibrosis according to RTOG/EORTC Late Morbidity Scoring Criteria. Lymph edema of the arm and the breast were scored separately with a non-validated classification based on the severity of symptoms and the need of therapy. Grade 1 included mild symptoms with no need for therapy, grade 2 moderate swelling with a need for occasional manual lymphatic drainage, grade 3 severe swelling with a need for continuous elastic compression or an operation and grade 4 included ulcerations or necrosis. In addition, the frequency of different toxicities was compared between patients treated with 3D-CRT versus IMRT.
2.5. Statistical methods
Disease-free survival-rate as well as local and regional control-rate was calculated with the Kaplan-Meier method. Disease-free survival included local in-breast recurrence, regional lymph node recurrence, distant metastases, secondary malignancies and death from any cause as events. Patients that did not experience an event were censored at the last follow-up visit. For estimation of local and locoregional control, only local and locoregional recurrences were rated as an event, patients were censored at the last available follow-up or death. 95% confidence intervals (95% CI) were estimated. The difference in frequency of radiation side effects between 3D-CRT and IMRT were tested for significance via chi-squared tests with a two-sided significance level of 5%.
3. Results
3.1. Patient characteristics
In total, 300 patients were enrolled at 15 participating centers (academic institutions, local hospitals, private facilities) between 2011 and 2014. Two hundred eighty of these patients completed the treatment per protocol and participated in six months of follow-up. Sixteen patients did not start treatment, e.g. because of withdrawn consent, and four patients had to be excluded because they did not attend six months of follow-up. In the current analysis six patients had to be excluded due to insufficient follow-up information, leaving 274 patients in total. The median follow-up time was 60 months (range 11–104 months). The median age was 61.5 years (range 33–85 years) and the median tumor size 13 mm. Most women had a nodal status of N0, and there were no cases of distant metastases at diagnosis. Hormone receptor positive tumors were dominant, and most patients did not receive chemotherapy. The distribution between 3D-CRT and IMRT was similar (43.8% vs. 54.7%). The patients’ characteristics are listed in Table 1.
Table 1.
Baseline characteristics. BMI = body mass index; SD = standard deviation; 3D-CRT = 3D-conformal radiotherapy; IMRT = intensity-modulated radiotherapy. Relevant comorbidities were defined as morbidities that may impair treatment conduct (e.g., unstable cardiac disease).
| Mean (SD) | Median | Range | ||
|---|---|---|---|---|
| Age (years) | 61.68 (9.98) | 61.5 | 33–85 | |
| BMI (kg/m2) | 27.29 (4.78) | 26.7 | 17.1–43.9 | |
| ECOG performance status | 0 | 0–2 | ||
| Tumor size (mm) | 15.46 (8.86) | 13 | 2–55 | |
| N (%) | ||||
| Comorbidity | Relevant | Not relevant | None | Unknown |
| Heart | 0 (0) | 36 (13.1) | 209 (76.3) | 29 (10.6) |
| Lung | 0 (0) | 14 (5.1) | 231 (84.3) | 29 (10.6) |
| Kidney | 0 (0) | 7 (2.5) | 238 (86.9) | 29 (10.6) |
| Axillary surgery | ||||
| Sentinel lymph node biopsy | 236 (86.1) | |||
| Axillary dissection | 37 (13.5) | |||
| No axillary staging | 1 (0.4) | |||
| (y)pT category | ||||
| T0 (after neoadjuvant treatment) | 6 (2.2) | |||
| T1a | 12 (4.4) | |||
| T1b | 73 (26.6) | |||
| T1c | 130 (47.4) | |||
| T2 | 52 (19.0) | |||
| T3 | 1 (0.4) | |||
| T4 | 0 (0) | |||
| pN category | ||||
| N0 | 255 (93.0) | |||
| N1mic | 4 (1.5) | |||
| N1 | 15 (5.5) | |||
| cM stage | ||||
| M0 | 266 (97.1) | |||
| M1 | 0 (0) | |||
| Unknown | 8 (2.9) | |||
| Laterality | ||||
| Right | 132 (48.2) | |||
| Left | 142 (51.8) | |||
| Histological type | ||||
| Invasive-ductal | 213 (77.8) | |||
| Invasive-lobular | 45 (16.4) | |||
| Medullary | 2 (0.7) | |||
| Other | 11 (4.0) | |||
| Unknown | 3 (1.1) | |||
| Estrogen receptor | ||||
| Negative | 37 (13.5) | |||
| Positive | 222 (81.0) | |||
| Unknown | 15 (5.5) | |||
| Progesterone receptor | ||||
| Negative | 50 (18.3) | |||
| Positive | 207 (75.5) | |||
| Unknown | 17 (6.2) | |||
| HER2-status | ||||
| 0 | 122 (44.5) | |||
| 1+ | 90 (32.9) | |||
| 2+ | 33 (12.0) | |||
| 3+ | 14 (5.1) | |||
| Unknown | 15 (5.5) | |||
| Chemotherapy-status | ||||
| None | 165 (60.2) | |||
| Neoadjuvant | 16 (5.8) | |||
| Adjuvant | 85 (31.1) | |||
| Unknown | 8 (2.9) | |||
| Endocrine therapy | ||||
| None | 35 (12.8) | |||
| Started at time of radiotherapy | 82 (29.9) | |||
| Planned | 137 (50) | |||
| Unknown | 20 (7.3) | |||
| Type of endocrine therapy | ||||
| Tamoxifen | 117 (42.7) | |||
| Aromatase inhibitors | 65 (23.7) | |||
| Unknown | 57 (20.8) | |||
| Not applicable | 35 (12.8) | |||
| Radiotherapy technique | ||||
| 3D-CRT | 120 (43.8) | |||
| IMRT | 150 (54.7) | |||
| Unknown | 4 (1.5) | |||
| Boost | ||||
| 6 MeV photons only | 137 (50) | |||
| 6-MeV photons and other photon energies | 128 (46.7) | |||
| Photons and electrons | 1 (0.4) | |||
| Others | 2 (0.7) | |||
| Unknown | 6 (2.2) | |||
3.2. Efficacy
In summary, three local recurrences in the breast were observed (36, 72 and 75 months after radiotherapy) and one regional nodal recurrence in the axilla (17 months after radiotherapy). Two of the three local recurrences and the single nodal recurrence occurred in ER-negative patients. Five patients (1.82%) were diagnosed with distant metastases (range 7–84 months). A second malignancy after breast cancer therapy occurred in 11 patients (4.0%; range 4–74 months). Three of these patients had contralateral breast cancer, occurring 4, 11, and 52 months after radiation therapy. Furthermore, two patients developed lung cancer. Ten patients died during follow-up (3.65%). Two of these deaths were potentially related to breast cancer. The others did not show any relation to breast cancer. The observed cancer-related events are listed in Table 2.
Table 2.
Patterns of recurrence.
| Patterns of recurrence | |
|---|---|
| Event | n (%) |
| In-breast recurrence | 3 (1.1) |
| Regional nodal recurrence | 1 (0.36) |
| Distant metastases | 5 (1.82) |
| Contralateral breast cancer | 3 (1.1) |
| Other second malignancies | 8 (2.92) |
| Cancer-related death | 2 (0.73) |
| Death of other/unknown cause | 8 (2.92) |
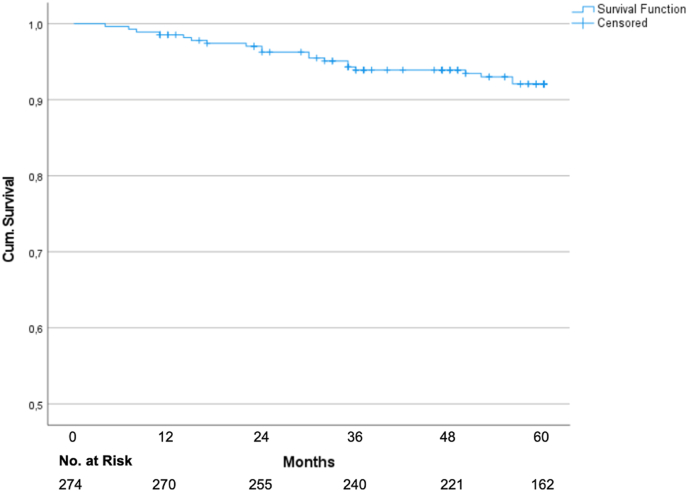
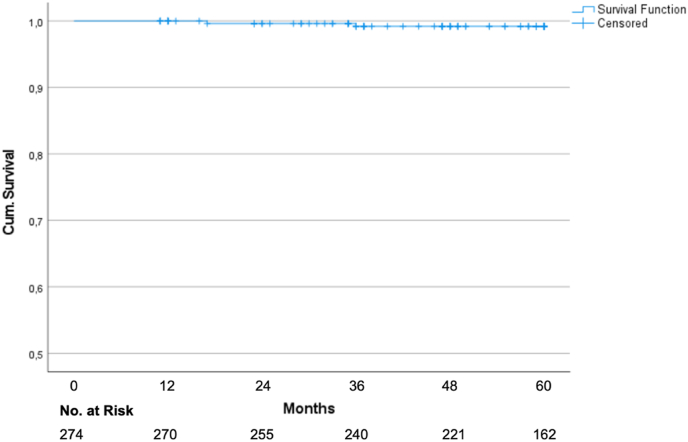
The Kaplan-Meier analysis yielded a five-year disease-free survival of 92.1% (95% CI 89.0–95.2%; Fig. 1). The local control rate and locoregional control rate were 99.6% (95% CI: 98.81–100%) and 99.2% (95% CI: 98.03–100%; Fig. 2) after 5 years. The overall survival was 97.2% after 5 years.
Fig. 1.
Kaplan Meier graph for disease-free survival.
Fig. 2.
Kaplan Meier graph for locoregional control.
4. Toxicity
High-grade toxicity was very rare. Only two women (0.7%) suffered a long-term toxicity of grade 3 (telangiectasia and lymphedema of the breast). In most patients the highest registered radiation side-effects were of grade 1. The cumulative rate of grade 2 toxicity was 7.3%. The highest proportions of grade 1 toxicities were fibrosis (13.1%), hyperpigmentation (11.7%) and lymphedema of the breast (7.3%). Fortunately, no long-term side-effects were reported for 63.5% of patients (Table 3).
Table 3.
Worst toxicity grades during follow-up.
| Toxicities |
Grade |
||||
|---|---|---|---|---|---|
| n (%) | 0 | 1 | 2 | 3 | 4 |
| Fibrosis | 233 (85.1) | 36 (13.1) | 5 (1.8) | 0 (0) | 0 (0) |
| Telangiectasia | 257 (93.8) | 16 (5.8) | 0 (0) | 1 (0.4) | 0 (0) |
| Hyperpigmentation | 241 (87.9) | 32 (11.7) | 1 (0.4) | 0 (0) | 0 (0) |
| Brachial plexopathy | 268 (97.8) | 3 (1.1) | 3 (1.1) | 0 (0) | 0 (0) |
| Lung fibrosis | 268 (97.8) | 4 (1.5) | 2 (0.7) | 0 (0) | 0 (0) |
| Lymphedema breast | 246 (89.8) | 20 (7.3) | 7 (2.5) | 1 (0.4) | 0 (0) |
| Lymphedema arm | 265 (96.7) | 5 (1.8) | 4 (1.5) | 0 (0) | 0 (0) |
| Highest grade reached by patients | 174 (63.5) | 78 (28.5) | 20 (7.3) | 2 (0.7) | 0 (0) |
Since 3D-CRT and IMRT were used relatively similar across the study centers, the long-term side-effects of this analysis were compared between both techniques. Baseline characteristics between patients receiving 3D-CRT and IMRT were similar (Supplementary table 1). Chi-squared-tests showed that most toxicities did not differ significantly between 3D-CRT and IMRT. Only fibrosis and lymphedema of the arm showed significantly lower grades in favor of IMRT (Table 4, Table 5).
Table 4.
Distribution of the different grades of fibrosis according to the use of IMRT and 3D-CRT (p = 0.009 for the comparison of IMRT vs. 3D-CRT). 3D-CRT = 3D-conformal radiotherapy; IMRT = intensity-modulated radiotherapy.Table 4: Distribution of the different grades of fibrosis according to the use of IMRT and 3D-CRT (p = 0.009 for the comparison of IMRT vs. 3D-CRT). 3D-CRT = 3D-conformal radiotherapy; IMRT = intensity-modulated radiotherapy.
| 3D-CRT |
IMRT |
Total |
||
|---|---|---|---|---|
| n (%) | ||||
| Fibrosis | Grade 0 | 93 (77.5) | 136 (90.7) | 229 (84.8) |
| Grade 1 | 23 (19.2) | 13 (8.7) | 36 (13.3) | |
| Grade 2 | 4 (3.3) | 1 (0.6) | 5 (1.9) | |
| Total | 120 (100) | 150 (100) | 270 (100) | |
| p = 0.009 | ||||
Table 5.
Distribution of the different grades of lymphedema according to the use of IMRT and 3D-CRT (p = 0.044 for the comparison of IMRT vs. 3D-CRT). 3D-CRT = 3D-conformal radiotherapy; IMRT = intensity-modulated radiotherapy.
| 3D-CRT |
IMRT |
Total |
||
|---|---|---|---|---|
| n (%) | ||||
| Lymphedema arm | Grade 0 | 115 (95.8) | 146 (97.3) | 261 (96.7) |
| Grade 1 | 1 (0.9) | 4 (2.7) | 5 (1.8) | |
| Grade 2 | 4 (3.3) | 0 (0) | 4 (1.5) | |
| Total | 120 (100) | 150 (100) | 270 (100) | |
| p = 0.044 | ||||
5. Discussion
This analysis shows promising results regarding long-term toxicities and tumor control using HF with SIB in 16 fractions.
By now, several trials have explored HF with SIB for breast cancer patients. The largest patient cohort was treated at Humanitas Research Hospital and Cancer Center in Milan, Italy. Several publications from this group have analyzed the outcome of patients treated with 40.5 Gy to the whole breast and 48 Gy to the tumor bed in 15 fractions within a prospective trial. Due to the varying patient numbers and follow up-periods of these publications, we decided to limit our discussion to the most recent report on 450 patients with a median follow-up of 6 years [19]. Local control was excellent at 98.9% with grade 2 skin toxicity in only 1.4% of patients. Cosmesis was excellent or good in 98.9% of patients. Osa et al. studied the same regimen within a prospective trial with 404 patients [25]. At 5 years, local control was 99.2% and grade 3 toxicity occurred in 1% of patients, 82% if patients had a favorable cosmetic outcome. Two trials from Italy studied a 20-fraction regimen with 45 Gy to the breast and a SIB of 50 Gy to the tumor bed [18,20]. With a median of 117 months, the retrospective analysis of Cante et al. provides one of the longest follow-up periods for hypofractionation with SIB. At 10 years, local control was excellent at 97.3% and grade ≥2 was rare with 7% of patients experiencing fibrosis and 5% telangiectasia. Cosmetic outcome was excellent or good in 87.8% of patients. Freedman et al. published long-term data on a 20 fraction-regimen with 45 Gy to the breast and 56 Gy to the boost volume with a median follow-up of 69 months [21]. Again, local control was excellent with 97.3% at 5 years. Patient-reported outcomes were collected using the Breast Cancer Treatment Outcome Scale (BCTOS) and showed no significant changes over the follow-up period.
Only limited data from randomized controlled trials on HF with SIB have been published so far. Two of those trials compared HF with SIB to conventional fractionation while two trials compared it to HF with a sequential boost.
Researchers from Brussels conducted a trial of conventionally fractionated radiotherapy compared to tomotherapy with 42 Gy to the whole breast and 51 Gy to the boost volume in 15 fractions [11]. There was a trend towards decreased skin toxicity at 2 years with SIB. Furthermore, there was a significantly higher proportion of patients with a decline in pulmonary function at 2 years with conventional fractionation. The other randomized controlled trial from Thailand enrolled 73 patients and randomized them to conventional fractionation or 43.2 Gy to the whole breast and 52.8 Gy to the boost volume in 16 fractions [23]. With a median follow-up of more than 10 years, there were no significant differences in terms of local control, overall survival or late toxicity between the two arms.
Van Hulle et al. recently published two-year results on 150 evaluable patients from their randomized controlled trial comparing hypofractionated whole-breast radiotherapy with a sequential boost to a 15-fraction regimen of 40.05 Gy to the whole breast and a SIB of 46.8 Gy (negative margin) or 49.95 Gy (positive margin) to the tumor bed [22]. There was no grade 3 toxicity. No significant differences were observed between the two treatment arms in terms of late toxicity and cosmetic outcome.
Recently, 5-year data from the IMPORT HIGH-trial were presented [26]. This is a 3-arm randomized controlled trial with 2617 patients. HF-WBRT with 40.05 Gy in 15 fractions followed by a sequential boost of 8 × 2 Gy was compared to two different 15-fraction SIB regimens. Both experimental arms used a reduced dose of 36 Gy to the uninvolved breast and delivered 40 Gy only to a partial breast volume surrounding the tumor bed. The SIB doses were 48 Gy and 53 Gy, respectively. Non-inferiority regarding local tumor recurrence at 5 years was established for the 48 Gy-arm, but not for the 53 Gy-arm. Late toxicity was higher in the 53 Gy-arm. Overall, marked and moderate adverse events occurred in less than 5% and 12–14% of patients, respectively.
Our results compare favorably to the above-mentioned studies. The patient population includes a high proportion of low-risk patients; 93% of the patients were node-negative and 81% ER-positive. In all studies addressing HF with SIB, the local control rate was well above 95%, even in the two reports with ten-year follow-up [18,23].
Nevertheless, further follow-up is necessary, especially in patients with luminal tumors, that have risk of recurrence well beyond 10 years [27]. More research is needed to analyze the role of boost irradiation in the context of biological subtypes and contemporary systemic therapy [28].
Furthermore, toxicity rates were favorable in our analysis and in line with previously published findings. Dosimetric studies show improved dose homogeneity with SIB which may impact the risk of late normal tissue effects, especially outside of the boost area [29]. Despite the increased single dose in the tumor bed with SIB, there are no signs of an increased risk of local fibrosis. Our results suggest that advanced radiotherapy techniques may decrease the risk of fibrosis. Due to the retrospective nature of this analysis, it was not possible to discern between fibrosis within and beyond the boost area. In fact, several of the above-mentioned studies preferentially employed IMRT/VMAT for treatment planning and delivery for SIB [19,25]. These techniques may further improve dose homogeneity in the breast; however, the benefit may differ according to the size of the boost volume and the tumor location. Furthermore, we found a lower risk of lymphedema in patients treated with IMRT. As regional nodal irradiation was not permitted and most patients received sentinel lymph node biopsy for axillary staging, this difference may be explained by differences in the incidental exposure to the axilla [30,31]. Our findings related to the choice of radiotherapy technique should be regarded as hypothesis-generating and need to be analyzed in future studies.
The strengths of this analysis include the sample size, the length of follow-up and the prospective design of the original trials with a nearly identical treatment protocol. Participation was broad with university hospitals, teaching hospitals as well as private practices, most of which went on to enroll patients in the follow-up phase III HYPOSIB trial (NCT02474641). Due to the retrospective collection of follow-up data and the use of telephone interviews and mailed questionnaires in a subgroup of patients, toxicity may have been underreported. Multivariate analysis was not feasible because of the small number of recurrences and toxicity events. No data on cosmetic outcome were collected. Extrapolation to patients requiring regional nodal irradiation is not possible, several prospective trials are ongoing regarding this patient group. Constraints for heart and lung doses are outdated from the current perspective.
Based on both ARO-trials, the randomized HYPOSIB-trial with the same radiation regimen was started in 2015 and completed enrollment in early 2019. Patient characteristics and patterns of fractionation in the standard arm have been published [32]. Acute toxicity-data were presented at the ASTRO annual meeting and demonstrated a favorable safety profile for moderate HF with SIB [33]. Long-term results of HYPOSIB and RTOG 1005 (NCT01349322) trials will allow definitive conclusions on the efficacy and safety of HF with SIB.
6. Conclusion
In summary, our study demonstrates excellent locoregional control and tolerability of HF with SIB. Further data from prospective randomized trials are necessary to determine whether HF with SIB may replace HF with sequential boost administration as the standard of care.
Funding
No funding was available for these trials. Regarding this publication, we acknowledge financial support by Land Schleswig-Holstein within the funding programme Open Access Publikationsfonds.
Regulatory aspects
The ARO-2010-01 and the ARO-2013-04 trials were approved by the institutional review board at the Universität zu Lübeck (09–194 and 13–071) as well as the local institutional review boards. This analysis was approved by the institutional review board at Christian-Albrechts-Universität Kiel (D434/20). All patients provided their informed consent before inclusion in the trials.
Declaration of competing interest
D. Krug received honoraria from MSD Sharp & Dome and research funding from Merck KGaA. The other authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.05.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Meattini I., Becherini C., Boersma L., Kaidar-Person O., Marta G.N., Montero A., et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022;23:e21–31. doi: 10.1016/s1470-2045(21)00539-8. [DOI] [PubMed] [Google Scholar]
- 2.Haviland J.S., Owen J.R., Dewar J.A., Agrawal R.K., Barrett J., Barrett-Lee P.J., et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/s1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 3.Hickey B.E., James M.L., Lehman M., Hider P.N., Jeffery M., Francis D.P., et al. Fraction size in radiation therapy for breast conservation in early breast cancer. Cochrane Database Syst Rev. 2016;7:CD003860. doi: 10.1002/14651858.cd003860.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Offersen B.V., Alsner J., Nielsen H.M., Jakobsen E.H., Nielsen M.H., Krause M., et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III trial: the DBCG HYPO trial. J Clin Oncol. 2020;38:3615–3625. doi: 10.1200/jco.20.01363. [DOI] [PubMed] [Google Scholar]
- 5.Owen J.R., Ashton A., Bliss J.M., Homewood J., Harper C., Hanson J., et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467–471. doi: 10.1016/s1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 6.Shaitelman S.F., Lei X., Thompson A., Schlembach P., Bloom E.S., Arzu I.Y., et al. Three-year outcomes with hypofractionated versus conventionally fractionated whole-breast irradiation: results of a randomized, noninferiority clinical trial. J Clin Oncol : Official Journal of the American Society of Clinical Oncology. 2018:JCO1800317. doi: 10.1200/jco.18.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelan T.J., Pignol J.-P., Levine M.N., Julian J.A., MacKenzie R., Parpia S., et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/nejmoa0906260. [DOI] [PubMed] [Google Scholar]
- 8.Andrade T.R.M., Fonseca M.C.M., Segreto H.R.C., Segreto R.A., Martella E., Nazário A.C.P. Meta-analysis of long-term efficacy and safety of hypofractionated radiotherapy in the treatment of early breast cancer. Breast. 2019;48:24–31. doi: 10.1016/j.breast.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Aly M.M.O.M., Abo-Madyan Y., Jahnke L., Wenz F., Glatting G. Comparison of breast sequential and simultaneous integrated boost using the biologically effective dose volume histogram (BEDVH) Radiat Oncol. 2016;11:16. doi: 10.1186/s13014-016-0590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parijs H.V., Reynders T., Heuninckx K., Verellen D., Storme G., Ridder M.D. Breast conserving treatment for breast cancer: dosimetric comparison of different non-invasive techniques for additional boost delivery. Radiat Oncol. 2014;9:36–37. doi: 10.1186/1748-717x-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parijs H.V., Miedema G., Vinh-Hung V., Verbanck S., Adriaenssens N., Kerkhove D., et al. Short course radiotherapy with simultaneous integrated boost for stage I-II breast cancer, early toxicities of a randomized clinical trial. Radiat Oncol. 2012;7 doi: 10.1186/1748-717x-7-80. 80–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krug D., Köder C., Häfner M.F., Arians N., Harrabi S.B., Koerber S.A., et al. Acute toxicity of normofractionated intensity modulated radiotherapy with simultaneous integrated boost compared to three-dimensional conformal radiotherapy with sequential boost in the adjuvant treatment of breast cancer. Radiat Oncol. 2020;15:235. doi: 10.1186/s13014-020-01652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hörner-Rieber J., Forster T., Hommertgen A., Haefner M.F., Arians N., König L., et al. Intensity modulated radiation therapy (IMRT) with simultaneously integrated boost shortens treatment time and is noninferior to conventional radiation therapy followed by sequential boost in adjuvant breast cancer treatment: results of a large randomized phase III trial (IMRT-MC2 trial) Int J Radiat Oncol Biol Phys. 2021;109:1311–1324. doi: 10.1016/j.ijrobp.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Forster T., Hommertgen A., Häfner M.F., Arians N., König L., Harrabi S.B., et al. Quality of life after simultaneously integrated boost with intensity-modulated versus conventional radiotherapy with sequential boost for adjuvant treatment of breast cancer: 2-year results of the multicenter randomized IMRT-MC2 trial. Radiother Oncol. 2021;163:165–176. doi: 10.1016/j.radonc.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Choi K.H., Ahn S.J., Jeong J.U., Yu M., Kim J.H., Jeong B.K., et al. Postoperative radiotherapy with intensity-modulated radiation therapy versus 3-dimensional conformal radiotherapy in early breast cancer: a randomized clinical trial of KROG 15-03. Radiother Oncol. 2020;154:179–186. doi: 10.1016/j.radonc.2020.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Dellas K., Vonthein R., Zimmer J., Dinges S., Boicev A.D., Andreas P., et al. Hypofractionation with simultaneous integrated boost for early breast cancer: results of the German multicenter phase II trial (ARO-2010-01) Strahlenther Onkol. 2014;190:646–653. doi: 10.1007/s00066-014-0658-5. [DOI] [PubMed] [Google Scholar]
- 17.Krug D., Baumann R., Krockenberger K., Vonthein R., Schreiber A., Boicev A., et al. Adjuvant hypofractionated radiotherapy with simultaneous integrated boost after breast-conserving surgery: results of a prospective trial. Strahlenther Onkol. 2020;197:48–55. doi: 10.1007/s00066-020-01689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cante D., Petrucci E., Sciacero P., Piva C., Ferrario S., Bagnera S., et al. Ten-year results of accelerated hypofractionated adjuvant whole-breast radiation with concomitant boost to the lumpectomy cavity after conserving surgery for early breast cancer. Med Oncol. 2017;34:152. doi: 10.1007/s12032-017-1020-4. [DOI] [PubMed] [Google Scholar]
- 19.Franceschini D., Fogliata A., Spoto R., Dominici L., Faro L.L., Franzese C., et al. Long term results of a phase II trial of hypofractionated adjuvant radiotherapy for early-stage breast cancer with volumetric modulated arc therapy and simultaneous integrated boost. Radiother Oncol. 2021;164:50–56. doi: 10.1016/j.radonc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Franco P., Zeverino M., Migliaccio F., Cante D., Sciacero P., Borca V.C., et al. Intensity-modulated and hypofractionated simultaneous integrated boost adjuvant breast radiation employing statics ports of tomotherapy (TomoDirect): a prospective phase II trial. J Cancer Res Clin. 2014;140:167–177. doi: 10.1007/s00432-013-1560-8. [DOI] [PubMed] [Google Scholar]
- 21.Freedman G.M., Anderson P.R., Bleicher R.J., Litwin S., Li T., Swaby R.F., et al. Five-year local control in a phase II study of hypofractionated intensity modulated radiation therapy with an incorporated boost for early stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;84:888–893. doi: 10.1016/j.ijrobp.2012.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulle H.V., Desaunois E., Vakaet V., Paelinck L., Schoepen M., Post G., et al. Two-year toxicity of simultaneous integrated boost in hypofractionated prone breast cancer irradiation: comparison with sequential boost in a randomized trial. Radiother Oncol. 2021;158:62–66. doi: 10.1016/j.radonc.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Saksornchai K., Jaruthien T., Nantavithya C., Shotelersuk K., Rojpornpradit P. Long-term results of hypofractionation with concomitant boost in patients with early breast cancer: a prospective study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0258186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the radiation therapy Oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-c. [DOI] [PubMed] [Google Scholar]
- 25.Osa E.-O.O., DeWyngaert K., Roses D., Speyer J., Guth A., Axelrod D., et al. Prone breast intensity modulated radiation therapy: 5-year results. Int J Radiat Oncol Biol Phys. 2014;89:899–906. doi: 10.1016/j.ijrobp.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coles C., Haviland J.S., Kirby A.M., Bhattacharya I., Brunt A.M., Chan C., et al. OC-0291 IMPORT HIGH trial: dose escalated simultaneous integrated boost radiotherapy in early breast cancer. Radiother Oncol. 2021;161:S197–S199. doi: 10.1016/s0167-8140(21)06840-7. [DOI] [Google Scholar]
- 27.Pedersen R.N., Esen B.Ö., Mellemkjær L., Christiansen P., Ejlertsen B., Lash T.L., et al. Jnci J National Cancer Inst; 2021. The incidence of breast cancer recurrence 10-32 Years after primary Diagnosis. djab202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fodor A., Brombin C., Mangili P., Borroni F., Pasetti M., Tummineri R., et al. Impact of molecular subtype on 1325 early-stage breast cancer patients homogeneously treated with hypofractionated radiotherapy without boost: should the indications for radiotherapy be more personalized? Breast. 2021;55:45–54. doi: 10.1016/j.breast.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwicker F., Hoefel S., Kirchner C., Huber P.E., Debus J., Schempp M. Hypofractionated radiotherapy with simultaneous-integrated boost after breast-conserving surgery compared to standard boost-applications using helical tomotherapy with TomoEdge. Anticancer Res. 2021;41 doi: 10.21873/anticanres.14957. 1909–20. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt M., Pin Y., Pflumio C., Mathelin C., Pivot X., Noel G. Incidental axillary dose delivery to axillary lymph node levels I–III by different techniques of whole-breast irradiation: a systematic literature review. Strahlenther Onkol. 2021;197:820–828. doi: 10.1007/s00066-021-01808-y. [DOI] [PubMed] [Google Scholar]
- 31.Lee J., Kim S.-W., Son S.H. Dosimetric evaluation of incidental irradiation to the axilla during whole breast radiotherapy for patients with left-sided early breast cancer in the IMRT era. Medicine. 2016;95 doi: 10.1097/md.0000000000004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krug D., Vonthein R., Schreiber A., Boicev A.D., Zimmer J., Laubach R., et al. Impact of guideline changes on adoption of hypofractionation and breast cancer patient characteristics in the randomized controlled HYPOSIB trial. Strahlenther Onkol. 2020;197:802–811. doi: 10.1007/s00066-020-01730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunst J., Krug D., Schreiber A., Boicev A.D., Zimmer J., Laubach R., et al. Patient reported experience with treatment modalities and safety of adjuvant breast radiotherapy - first results of the randomized HYPOSIB – study. Int J Radiat Oncol Biol Phys. 2020;108:S13. doi: 10.1016/j.ijrobp.2020.07.2091. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.