Abstract
Introduction:
Progressive agrammatic aphasia (PAA) can be associated with abnormal behaviors, however it is unknown whether behaviors occur and/or are different in patients with primary progressive apraxia of speech (PPAOS). We aimed to compare baseline and longitudinal behavioral symptomatology between PPAOS, patients with PAA and patients with both apraxia of speech and agrammatic aphasia (AOS-PAA).
Methods:
We recruited 89 for this study, 40 with PPAOS, 11 with PAA and 38 with AOS-PAA. Behavioral disturbances were evaluated using the frontal behavior inventory (FBI) which was also split into negative behaviors and disinhibition, and the 20-item behavioral assessment (20-BAS). Data analysis was performed using liner regression and linear mixed models.
Results:
Of the 89 patients in the study, 54% were women and the mean age at onset was 68 years. All patients, regardless of diagnosis, endorsed at least one symptom on FBI at baseline, most frequently verbal apraxia (100%), logopenia (95.6%), irritability (55.9%) and apathy (42.6%). On the 20-BAS, 47.6% of the patients endorsed at least one symptom, most commonly `crying more easily` (19.5%) and personality change (18.3%). PPAOS was the least behaviorally affected group, with differences between PPAOS and AOS-PAA mainly driven by negative behaviors. The four behavioral metrics showed average sensitivity and specificity to distinguish between groups. Behavioral disturbances worsened over time although rate of change across groups was similar.
Conclusion:
Behavioral disturbances, particularly negative behaviors, are more common and severe in patients with progressive agrammatic aphasia compared to patients with isolated apraxia of speech.
Keywords: Behavioral dysfunction, AOS, PPA, longitudinal, 20-BAS
Introduction
Apraxia of speech (AOS) is a disorder of motor programming/planning of speech, distinguishable from aphasia and dysarthria[1, 2]. AOS can occur as the sole presenting feature of a neurodegenerative disorder[2], in the absence of aphasia, referred to as primary progressive apraxia of speech (PPAOS)[3, 4]. The estimated prevalence of PPAOS is approximately 4.4 per 100,000[5]. Two subtypes of AOS have been described: phonetic and prosodic AOS[6, 7]. The phonetic subtype is characterized by a predominance of distorted sounds and distorted sound substitutions and additions, whereas the prosodic subtype is dominated by slow, prosodically segmented speech. Although some non-speech behavioral complaints were initially noted in a few patients[3], there was no shared pattern of behavioral abnormalities at initial presentation and behaviors did not cause problems in the household nor affected the patients` activities of daily living. However, to date, no study has specifically evaluated behavioral disturbances in PPAOS.
Patients with a neurodegenerative AOS can commonly also present with an agrammatic aphasia (AOS-PAA)[6], while less commonly patients can present with a progressive agrammatic aphasia (PAA)[8] whereby AOS is absent. Although some researchers consider all patients with PPAOS, AOS-PAA, and PAA to meet consensus criteria for the non-fluent/agrammatic variant of primary progressive aphasia (agPPA)[9], there is now a wealth of data demonstrating differences among these groups to support their separation, including differences on neuroimaging, progression and survival [6, 10, 8, 11]. The extent, if any, of behavioral disturbances in PPAOS is unknown. It is also unknown how behaviors in PPAOS compare to behaviors in AOS-PAA or PAA, and/or whether behaviors differ across AOS subtypes.
In this study we assessed a variety of behavioral features in a large cohort of PPAOS patients. We also compared behaviors in PPAOS to behaviors in AOS-PAA and PAA and investigated the influence of AOS subtype.
Materials and Methods
Participants
This study included all patients recruited by the Neurodegenerative Research Group (NRG) from the Department of Neurology, Mayo Clinic, Rochester MN, and enrolled into NIH-funded studies that included neurodegenerative AOS and/or agrammatic aphasia between July 6th, 2010 and November 10th, 2020. From a total of 89 patients, at their initial visit, 40 (45%) presented with PPAOS, 38 (43%) presented with AOS-PAA and 11 (12%) presented with PAA. Of 78 patients presenting with AOS (i.e., PPAOS + AOS-PAA), 40 (51%) had the phonetic subtype and 38 (49%) the prosodic subtype. We excluded all patients with any other neurological condition that could account for their presentation, including neurodegenerative disorders, such as behavioral variant frontotemporal dementia[12], and neurodevelopmental disorders. Patients were evaluated thoroughly by one of five speech-language pathologists (J.R.D., H.M.C., R.L.U., J.A.S, E.A.S), one of two behavioral neurologist (K.A.J, H.B.) and a neuropsychologist testing were overseen by a neuropsychologist (M.M.M). A final clinical diagnoses was based on consensus by at least 2 speech-language pathologists, as detailed previously[3]. Fifty-seven patients (64%) had more than one visits allowing for longitudinal analyses. The median time between visits was 1.1 years [range: 1.0–1.2]. In total, 238 patient visits were available for analysis, with an average of 2.37 visits per patient (range: 1-7). That is, an average of 2.37 years of follow-up (range: 1-7 years of follow-up).
Clinical evaluations
We utilized the Western Aphasia Battery (WAB)[13] to assess aphasia severity through the domains of lexical content, fluency, repetition, naming, and language comprehension, all of which, in sum, yielded an Aphasia Quotient (WAB-AQ). Two speech-language pathologists also reached consensus for an independent aphasia severity score which ranked between 0 (none) to 4 (severe). We used the Token Test Part V[14] to assess auditory comprehension; the Boston Naming Test (BNT)[15] to assess confrontation naming and the Northwestern Anagram Test (NAT)[16] to assess syntactic performance. Additional evidence of agrammatism was determined via spoken and written tasks (for details, see[8]). The Apraxia of Speech Rating Scale (ASRS) was used to assess the presence and prominence of a number of clinical features associated with AOS[3, 17] and the Motor Speech Disorders (MSD) scale[18] was used to assess severity of any motor speech disorder on speech intelligibility. The presence and severity of dysarthria was determined based on performance during all speech tasks. We also included Non-Verbal Oral Apraxia rating (NVOA) as an assessment of praxis for non-speech movements of speech muscles that can occur with or without AOS[19]. We used the Montreal Cognitive Assessment battery (MoCA)[20] to assess general cognitive function.
Behavioral Assessment
For behavioral assessment, we used two independent caregiver-report inventories. First, the frontal behavior inventory (FBI)[21], a tool developed to detect behavioral disturbances associated with frontotemporal dementia. It consists of 24 items, all of which are scored on a 0 to 3 scale (0 = absent behavior; 1 = occasional; 2 = moderate, 3 = severe or frequent). A behavior was considered present with a score of ≥1. The sum of all items yields the total FBI score (max=72). From the FBI, the 12 items of apathy, spontaneity, indifference, inflexibility, concreteness, personal neglect, disorganization, inattention, loss of insight, logopenia, verbal apraxia, and alien hand sum to form the FBI negative (type A or deficient) behavior subscore (max=36). The remaining 12 items, namely, preservation, irritability, excessive jocularity, poor judgment, inappropriateness, impulsivity, restlessness, aggression, hyperorality, hypersexuality, utilization behavior, and incontinence sum to form the FBI disinhibition (type B or positive) behavior subscore (max=36).
The second behavioral inventory was a 20-item behavioral assessment (20-BAS) that we specifically designed to capture a wide range of behavioral disturbances, particularly behaviors beyond those captured in the FBI. It is a qualitative measure that judges the behaviors as present/absent based on the carer’s response to the question. To calculate the 20-BAS, we assigned a score of 1 to mean the behavior is present and 0 if the behavior is absent; hence, the maximum score on 20-BAS is 20.
Statistical Analysis
All analyses were done using SPSS 27.0. Normally distributed variables were compared using one-way ANOVA across 3 groups, whereas non-normally distributed variables were compared with the Kruskal-Wallis test. Categorical variables were compared using the chi-square test. Baseline comparisons were performed adjusting for disease duration using linear regression models. We log-transformed the dependent variable to reduce skewness in these regression models. Similarly, as AOS subtype distribution differed between PPAOS and AOS-PAA, linear regression models adjusting for AOS subtype were utilized. For individual item comparisons between any 2 groups on the FBI and 20-BAS, the Mann Whitney U test was used, adjusting for multiple comparisons via the Benjamini-Hochberg correction. To assess for group-wise discrimination we performed an AUC analysis for each of the four main behavior metrics.
For longitudinal analysis, within group comparisons were made using the patients’ change scores calculated over visits one and two with Wilcoxon Signed Ranks Test. Additionally, we fit linear mixed models to patients’ log-transformed scores to model change overtime by group. The models included data from all individuals with two or more time-points and time was calculated as years from the first visit. The models included random participant-specific intercepts and slopes. Group-wise differences in rates of change were assessed by testing the group-by-time interaction using likelihood ratio tests.
Results
Demographic and clinical results
The PPAOS, AOS-PAA and PAA groups did not differ on demographic variables, except for PAA that had a shorter time from onset to first evaluation compared to the other groups (Table 1). AOS subtype differed across groups, with PPAOS consisting predominantly of prosodic AOS while AOS-PAA consisted predominantly of phonetic AOS. PPAOS patients performed better on the MoCA than AOS-PAA and PAA patients. As expected, PPAOS outperformed the other groups on all language measures and PPAOS and AOS-PAA performed worse than PAA on measures of motor speech.
Table 1.
Demographic and clinical variables across groups
| Variable | PPAOS (n=40) | AOS-PAA (n=38) | PAA (n=11) | p-Value |
|---|---|---|---|---|
| Gender (% male) | 17 (42.5) | 20 (52.6) | 4 (36.4) | 0.527 |
| Handedness (% right) | 34 (85.0) | 36 (94.7) | 10 (90.9) | 0.633 |
| Education (years) | 16.00 (13.25, 18.00) | 16.00 (12.00, 16.25) | 14.00 (12.00, 16.00) | 0.556 |
| Age at onset (years) | 70.09 (61.81, 74.12) | 66.73 (57.79, 70.25) | 68.26 (60.97, 75.16) | 0.309 |
| Age at first visit (years) | 72.31 (64.36, 79.02) | 70.21 (61.11, 73.60) | 69.45 (64.59, 76.31) | 0.270 |
| Onset to baseline (years) | 3.29 (2.29, 4.69) | 3.04 (2.06, 5.15) | 1.58 (1.17, 2.33) | 0.008a |
| Phonetic subtype (%) | 12 (30.0) | 28 (73.7) | NA | <0.001b |
| MoCA | 28.00 (26.00, 29.00) | 24.00 (20.00, 25.00) | 23.00 (21.00, 25.00) | <0.001a,b |
| WAB-AQ | 97.60 (96.08, 99.15) | 85.50 (81.38, 93.33) | 89.20 (79.30, 92.70) | <0.001a,b |
| WAB-fluency | 10.00 (9.00, 10.00) | 6.00 (5.00, 9.00) | 6.00 (6.00, 9.00) | <0.001a,b |
| WAB-repetition | 9.70 (9.40, 10.00) | 9.00 (7.86, 9.60) | 9.40 (9.00, 9.60) | <0.001b |
| WAB-spont. speech | 20.00 (19.00, 20.00) | 15.50 (14.00, 19.00) | 16.00 (14.00, 19.00) | <0.001a,b |
| WAB-info. content | 10.00 (10.00, 10.00) | 9.00 (9.00, 10.00) | 10.00 (8.00, 10.00) | <0.001a,b |
| Aphasia severity | 0.00 (0.00, 0.00) | 1.00 (1.00, 1.63) | 1.50 (1.00, 2.00) | <0.001a,b |
| Token Test | 20.00 (19.25, 21.00) | 16.50 (12.00, 19.00) | 16.00 (9.50, 18.50) | <0.001a,b |
| Boston Naming Test | 14.50 (13.00, 15.00) | 13.00 (12.00, 14.00) | 13.00 (9.75, 14.25) | 0.003b |
| NAT | 10.00 (9.00, 10.00) | 6.00 (5.00, 8.50) | 5.00 (2.50, 8.00) | <0.001a,b |
| Dysarthria severity | 0.00 (0.00, 1.50) | 0.00 (0.00, 0.63) | 0.00 (0.00, 0.00) | 0.044a |
| ASRS | 16.00 (12.00, 21.75) | 15.50 (11.75, 23.25) | 2.00 (1.00, 5.00) | <0.001a,c |
| NVOA | 29.00 (22.50, 32.00) | 26.50 (18.50, 30.00) | 29.00 (25.00, 30.00) | 0.125 |
| Letter fluency (FAS) | 24.00 (16.50, 33.00) | 18.00 (10.00, 21.00) | 12.00 (9.00, 22.00) | 0.001a,b |
| Action fluency | 12.00 (10.00, 16.00) | 8.00 (6.00, 11.75) | 7.00 (2.00, 14.00) | <0.001a,b |
| MSD | 7.00 (6.00, 8.00) | 6.00 (5.00, 7.25) | 10.00 (10.00, 10.00) | <0.001a,c |
Values are shown as median (q1, q3). PPAOS = primary progressive apraxia of speech; AOS-PAA = apraxia of speech- progressive agrammatic aphasia; PAA = progressive agrammatic aphasia; NA = not applicable, n.s. = not significant; MoCA = Montreal Cognitive Assessment. ASRS = Apraxia of Speech Rating Scale; MSD = Motor speech disorder rating; NAT = Northwestern Anagram Test; NVOA = Non-Verbal Oral Apraxia rating; WAB = Western Aphasia Battery; WAB-AQ = Western Aphasia Battery-Aphasia Quotient
PPAOS differs from PAA
PPAOS differs from AOS-PAA
PAA differs from AOS-PAA
Baseline behavioral analysis
All patients, regardless of diagnosis, endorsed at least one symptom from the FBI at baseline, most frequently verbal apraxia (100%) followed by logopenia (95.6%), consistent with having a speech-language disorder (Table 2). Ignoring those two items, 85.3% exhibited at least one behavioral disturbance. Irritability (55.9%), apathy (42.6%), inflexibility (39.7%), inattention (39.7%), preservation (39.7%), indifference (38.2%) and concreteness (36.8%) were commonly present. Similarly, on the 20-BAS (Table 3), 47.6% of carers endorsed at least one symptom, most frequently `crying more easily` (19.5%) followed by `personality change` (18.3%).
Table 2.
Individual FBI parameters at the baseline. All parameters are scored between 0-3. The number of patients for whom the symptom was endorsed is shown (percentage in parentheses).
| Frontal Behavior Inventory | PPAOS (n=30) | AOS-PAA (n=29) | PAA (n=9) | Total (n=68)* |
|---|---|---|---|---|
| Any item | 30 (100.0) | 29 (100.0) | 9 (100.0) | 68 (100.0) |
| Any non-speech related item** | 21 (70.0) | 28 (96.6) | 9 (100.0) | 58 (85.3) |
| Any negative behavior | 30 (100.0) | 29 (100.0) | 9 (100.0) | 68 (100.0) |
| Any disinhibition behavior | 20 (66.7) | 26 (89.7) | 8 (88.9) | 54 (79.4) |
| Apathy† | 14 (46.7) | 11 (37.9) | 4 (44.4) | 29 (42.6) |
| Spontaneity† | 5 (16.7) | 11 (37.9) | 3 (33.3) | 19 (27.9) |
| Indifference† | 11 (36.7) | 12 (41.4) | 3 (33.3) | 26 (38.2) |
| Inflexibility† | 6 (20.0) | 18 (62.1) | 3 (33.3) | 27 (39.7) |
| Concreteness† | 6 (20.0) | 15 (51.7) | 4 (44.4) | 25 (36.8) |
| Personal Neglect† | 5 (16.7) | 3 (10.3) | 1 (11.1) | 9 (13.2) |
| Disorganization† | 3 (10.0) | 11 (37.9) | 2 (22.2) | 16 (23.5) |
| Inattention† | 6 (20.0) | 15 (51.7) | 6 (66.7) | 27 (39.7) |
| Loss of Insight† | 3 (10.0) | 8 (27.6) | 3 (33.3) | 14 (20.6) |
| Logopenia† | 28 (93.3) | 28 (96.6) | 9 (100.0) | 65 (95.6) |
| Verbal Apraxia† | 30 (100.0) | 29 (100.0) | 9 (100.0) | 68 (100.0) |
| Perseveration | 7 (23.3) | 14 (48.3) | 6 (66.7) | 27 (39.7) |
| Irritability | 18 (60.0) | 15 (51.7) | 5 (55.6) | 38 (55.9) |
| Excessive Jocularity | 3 (10.0) | 2 (6.9) | 0 (0.0) | 5 (7.4) |
| Poor Judgment | 2 (6.7) | 7 (24.1) | 3 (33.3) | 12 (17.6) |
| Inappropriateness | 3 (10.0) | 4 (13.8) | 2 (22.2) | 9 (13.2) |
| Impulsivity | 2 (6.7) | 8 (27.6) | 1 (11.1) | 11 (16.2) |
| Restlessness | 6 (20.0) | 9 (31.0) | 3 (33.3) | 18 (26.5) |
| Aggression | 5 (16.7) | 5 (17.2) | 2 (22.2) | 12 (17.6) |
| Hyperorality | 0 (0.0) | 3 (10.3) | 1 (11.1) | 4 (5.9) |
| Hypersexuality | 0 (0.0) | 1 (3.4) | 1 (11.1) | 2 (2.9) |
| Utilization Behavior | 1 (3.3) | 0 (0.0) | 1 (11.1) | 2 (2.9) |
| Incontinence | 5 (16.7) | 2 (6.9) | 2 (22.2) | 9 (13.2) |
| Alien Hand† | 2 (6.7) | 1 (3.4) | 0 (0.0) | 3 (4.4) |
PPAOS = primary progressive apraxia of speech; AOS-PAA = apraxia of speech- progressive agrammatic aphasia; PAA = progressive agrammatic aphasia; FBI = frontal behavior inventory.
21 patients out of 89 in total did not have FBI data.
Items other than verbal apraxia and logopenia.
Negative behaviors
Table 3.
Individual 20-item behavioral assessment score parameters at the baseline. All parameters are scored as being present or absent. The number of patients who endorse the symptom is shown (in brackets is the percentage).
| 20-BAS | PPAOS (n=38) | AOS-PAA (n=34) |
PAA (n=10) | Total (n=82)* |
|---|---|---|---|---|
| Any item | 11 (28.9) | 20 (58.8) | 8 (80.0) | 39 (47.6) |
| Personality Change | 3 (7.9) | 9 (26.5) | 3 (30.0) | 15 (18.3) |
| Selfish Behavior | 1 (2.6) | 4 (11.8) | 2 (20.0) | 7 (8.5) |
| Paranoid Behavior | 0 (0.0) | 3 (8.8) | 1 (10.0) | 4 (4.9) |
| Crying More Easily | 6 (15.8) | 7 (20.6) | 3 (30.0) | 16 (19.5) |
| Laughing Excessively and/or Inappropriately | 1 (2.6) | 2 (5.9) | 0 (0.0) | 3 (3.7) |
| Craving Sweet Foods | 0 (0.0) | 6 (17.6) | 1 (10.0) | 7 (8.5) |
| Wandering or Pacing | 0 (0.0) | 3 (8.8) | 0 (0.0) | 3 (3.7) |
| Hoarding of Objects | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (1.2) |
| Touching and Aligning Objects | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (1.2) |
| Clockwatching | 0 (0.0) | 2 (5.9) | 0 (0.0) | 2 (2.4) |
| Superstitious Rituals | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Excessive Humming or Singing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Excessive Worrying | 1 (2.6) | 3 (8.8) | 1 (10.0) | 5 (6.1) |
| Other Obsessive/Compulsive Like Behaviors | 2 (5.3) | 3 (8.8) | 1 (10.0) | 6 (7.3) |
| Repetitive Motor Behavior (e.g., Leg Tapping or Rubbing) | 0 (0.0) | 3 (8.8) | 0 (0.0) | 3 (3.7) |
| Loss of Sex Drive (Libido) | 4 (10.5) | 3 (8.8) | 3 (30.0) | 10 (12.2) |
| Recent Divorce or Job Change | 1 (2.6) | 1 (2.9) | 0 (0.0) | 2 (2.4) |
| New Habits (e.g., Smoking) | 1 (2.6) | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Hyperreligiosity | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Very Fidgety | 1 (2.6) | 5 (14.7) | 1 (10.0) | 7 (8.5) |
PPAOS = primary progressive apraxia of speech; AOS-PAA = apraxia of speech- progressive agrammatic aphasia; PAA = progressive agrammatic aphasia; 20-BAS = 20-item behavioral assessment score.
17 patients out of 89 in total did not have 20-BAS data.
After controlling for disease duration, PPAOS patients were less impaired than both AOS-PAA and PAA patients on the FBI total score and 20-BAS (Table 4). PPAOS were also less impaired on the FBI negative behavior score than AOS-PAA and less impaired on the FBI disinhibition score than PAA. No differences were identified between AOS-PAA and PAA. The differences between PPAOS and AOS-PAA remained significant after controlling for AOS subtype (Table 5); of note, we observed no differences in the main behavioral measures across AOS subtypes (Table 5). In our comparison of individual items from the FBI and 20-BAS, the only differences that survived correction for multiple comparisons, where between PPAOS and AOS-PAA for inflexibility (corrected p=0.004), disorganization (corrected p=0.010), inattention (corrected p=0.008), logopenia (corrected p=0.002) and verbal apraxia (corrected p=0.006). Of note, all these behaviors are considered as negative symptoms.
Table 4.
Linear regression results with behavioral parameters when adjusted for disease duration. The reference group is shown in brackets. Significant results are presented in bold.
| Unstandardized Coefficients |
Standardized Coefficient |
||||
|---|---|---|---|---|---|
| Variable | B | Std. Error | Beta | T | P value |
| FBI Total Score | |||||
| Disease duration (years) | .047 | .042 | .136 | 1.130 | .263 |
| AOS-PAA (PPAOS) | .574 | .177 | .393 | 3.237 | .002 |
| PAA (PPAOS) | .585 | .270 | .274 | 2.167 | .034 |
| AOS-PAA (PAA) | −.011 | .272 | −.008 | −.041 | .968 |
| FBI Negative Behavior Score | |||||
| Disease duration (years) | .027 | .037 | .087 | .727 | .470 |
| AOS-PAA (PPAOS) | .546 | .157 | .421 | 3.482 | .001 |
| PAA (PPAOS) | .429 | .239 | .227 | 1.796 | .077 |
| AOS-PAA (PAA) | .117 | .240 | .090 | .487 | .628 |
| FBI Disinhibition Score | |||||
| Disease duration (years) | .070 | .046 | .190 | 1.527 | .132 |
| AOS-PAA (PPAOS) | .366 | .195 | .237 | 1.883 | .064 |
| PAA (PPAOS) | .633 | .296 | .280 | 2.136 | .037 |
| AOS-PAA (PAA) | −.266 | .298 | −.172 | −.892 | .375 |
| 20-BAS | |||||
| Disease duration (years) | −.015 | .035 | −.049 | −.440 | .661 |
| AOS-PAA (PPAOS) | .399 | .145 | .309 | 2.750 | .007 |
| PAA (PPAOS) | .454 | .228 | .234 | 1.989 | .050 |
| AOS-PAA (PAA) | −.055 | .231 | −.042 | −.237 | .813 |
PPAOS = primary progressive apraxia of speech; AOS-PAA = apraxia of speech- progressive agrammatic aphasia; PAA = progressive agrammatic aphasia; FBI = frontal behavior inventory; 20-BAS = 20-item behavioral assessment score.
Table 5.
Linear regression with diagnosis and AOS subtype. The reference group is shown in brackets. Significant results are presented in bold.
| Unstandardized Coefficients |
Standardized Coefficient |
||||
|---|---|---|---|---|---|
| Variable | B | Std. Error | Beta | T | Significance |
| FBI Total Score | |||||
| AOS-PAA (PPAOS) | .488 | .206 | .333 | 2.374 | .021 |
| Phonetic AOS (Prosodic) | .182 | .206 | .124 | .886 | .380 |
| FBI Negative Behavior Score | |||||
| AOS-PAA (PPAOS) | .441 | .180 | .337 | 2.453 | .017 |
| Phonetic AOS (Prosodic) | .218 | .180 | .166 | 1.208 | .232 |
| FBI Disinhibition Score* model p=0.153 | |||||
| AOS-PAA (PPAOS) | .313 | .225 | .206 | 1.390 | .170 |
| Phonetic AOS (Prosodic) | .120 | .226 | .079 | .533 | .596 |
| 20- BAS | |||||
| AOS-PAA (PPAOS) | .323 | .159 | .253 | 2.028 | .046 |
| Phonetic AOS (Prosodic) | .181 | .159 | .142 | 1.135 | .260 |
PPAOS = primary progressive apraxia of speech; AOS-PAA = apraxia of speech- progressive agrammatic aphasia; AOS = apraxia of speech; FBI = frontal behavior inventory; 20-BAS: 20-item behavioral assessment score.
Sensitivity and specificity values for each of the four main behavioral metrics to differentiate between PPAOS, AOS-PAA and PAA is shown in Table 6. None of the four behavioral metrics provided excellent cut-points to distinguish between the different diagnostic category. The 20-BAS was best to differentiate between PPAOS and PAA although sensitivity and specificity were good but not excellent.
Table 6.
Sensitivity and specificity of each of the four main behavioral metrics
| PPAOS vs AOS-PAA | PPAOS vs PAA | AOS-PAA vs PAA | |
|---|---|---|---|
| FBI total score | |||
| AUC (95% CI) | 0.72 (0.57, 0.83) | 0.68 (0.46, 0.83) | 0.58 (0.37, 0.76) |
| P-value | 0.004 | 0.12 | 0.49 |
| Cut-point | 9 | 9 | — |
| Decision rule | AOS-PAA if >9 | PAA if >9 | — |
| Sensitivity | 69% | 56% | — |
| Specificity | 67% | 67% | — |
| FBI negative behaviors | |||
| AUC (95% CI) | 0.74 (0.59, 0.84) | 0.66 (0.44, 0.82) | 0.60 (0.39, 0.77) |
| P-value | 0.002 | 0.16 | 0.39 |
| Youden cut-point | 7 | 6 | — |
| Decision | AOS-PAA if >7 | PAA if >6 | — |
| Sensitivity | 69% | 56% | — |
| Specificity | 67% | 57% | — |
| FBI disinhibition behaviors | |||
| AUC (95% CI) | 0.64 (0.49, 0.76) | 0.69 (0.47, 0.84) | 0.54 (0.33, 0.73) |
| P-value | 0.07 | 0.09 | 0.76 |
| Youden cut-point | 1 | 3 | — |
| Decision | AOS-PAA if >3 | PAA if >3 | — |
| Sensitivity | 52% | 56% | — |
| Specificity | 70% | 70% | — |
| BAS 20 | |||
| AUC (95% CI) | 0.66 (0.53, 0.77) | 0.74 (0.54, 0.87) | 0.56 (0.36, 0.73) |
| P-value | 0.01 | 0.01 | 0.59 |
| Youden cut-point | 1 | 1 | — |
| Decision | AOS-PAA if >1 | PAA if > 1 | — |
| Sensitivity | 59% | 80% | — |
| Specificity | 71% | 71% | — |
Longitudinal behavioral analysis
Figure 1 shows the frequency of behavioral symptoms over the first two consecutive visits (average interval=1.8yrs) across the cohort, demonstrating an increase in the frequency of many behaviors. Across the cohort, the FBI total score, FBI negative behavior subscore, FBI disinhibition subscore and 20-BAS score increased at the second visit compared to the first visit (Table 7). At the group level, PPAOS worsened significantly over time in the FBI negative behavior subscore and 20-BAS; AOS-PAA worsened in the FBI total score and FBI disinhibition subscore; and PAA did not change over time (Table 7). We did not observe any differences across groups in the annualized rate of change for the main behavioral scores (Table 8).
Figure 1:
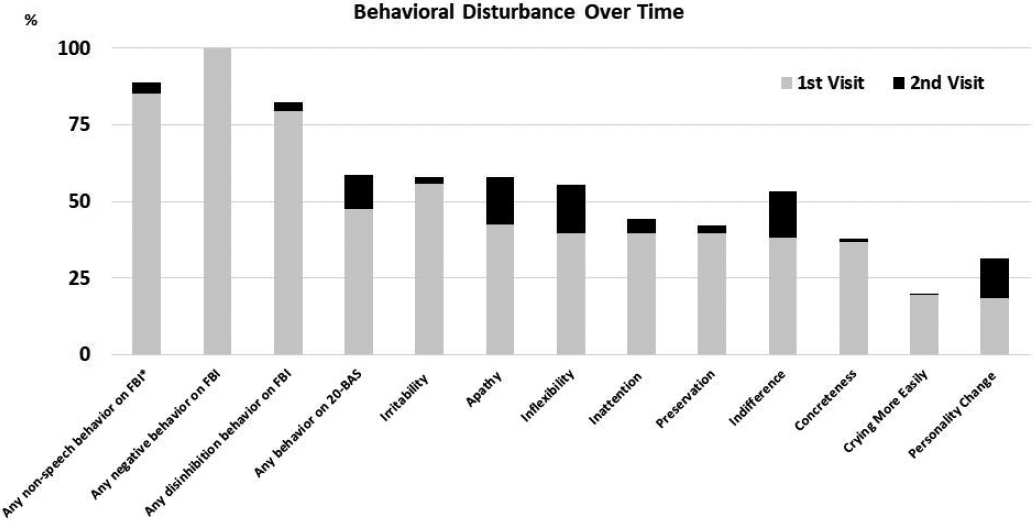
Frequency of each behaavior at baseline and follow-up visit across all patients.* items other than verbal apraxia and logopenia.
Table 7.
A comparison of behavioral scores over time.
| PPAOS (n=20) | AOS-PAA (n=11) | PAA (n=7) | Total (n=38) | |
|---|---|---|---|---|
| FBI Total Score | ||||
| 1st Visit | 7.00 (3.00, 12.00) | 13.00 (7.50, 18.00) | 9.00 (7.50, 14.00) | 9.00 (5.00, 15.00) |
| 2nd visit | 10.50 (6.00, 16.75) | 14.00 (10.00, 30.50) | 24.50 (7.25, 27.75) | 12.00 (6.00, 24.50) |
| p value | 0.167 | 0.041 | 0.611 | 0.017 |
| FBI Negative Behavior Score | ||||
| 1st Visit | 5.00 (3.00, 9.25) | 8.00 (5.50, 14.50) | 7.00 (4.50, 11.00) | 7.00 (4.00, 11.00) |
| 2nd Visit | 8.50 (5.25, 14.00) | 10.00 (6.00, 18.50) | 14.50 (5.25, 18.75) | 9.00 (5.50, 16.50) |
| p value | 0.038 | 0.166 | 0.345 | 0.011 |
| FBI Disinhibition Score | ||||
| 1st Visit | 2.00 (0.00, 3.00) | 3.00 (1.00, 5.50) | 3.00 (2.00, 4.00) | 2.00 (1.00, 4.00) |
| 2nd Visit | 1.50 (0.00, 4.00) | 4.00 (2.00, 13.00) | 7.00 (1.50, 12.50) | 3.00 (1.00, 9.00) |
| p value | 0.850 | 0.012 | 0.671 | 0.042 |
| 20-BAS Score | ||||
| 20-BAS | PPAOS (n=26) | AOS-PAA (n=13) | PAA (n=6) | Total (n=45) |
| 1st Visit | 0.00 (0.00, 1.00) | 1.00 (0.00, 2.00) | 1.00 (0.75, 2.00) | 0.00 (0.00, 1.25) |
| 2nd Visit | 1.00 (0.00, 2.00) | 1.00 (0.00, 4.00) | 4.00 (0.00, 7.00) | 1.00 (0.00, 3.00) |
| p value | 0.006 | 0.205 | 0.465 | 0.004 |
Data is shown median (q1,q4). Significant comparisons are in bold.
PPAOS = primary progressive apraxia of speech; AOS-PAA = apraxia of speech- progressive agrammatic aphasia; PAA = progressive agrammatic aphasia; FBI = frontal behavior inventory; 20-BAS: 20-item behavioral assessment
Table 8.
Annualized rate of change for our main behavioral outcomes calculated for the patients with more than one visit. Estimates are from a linear mixed effects model with log-transformed responses. Data are shown as estimated annual rate of increase in log-transformed response (95% CI).
| Annualized increase | PPAOS (n=23) | AOS-PAA (n=12) | PAA (n=7) | p-Value |
|---|---|---|---|---|
| FBI | 0.22 (0.15, 0.30) | 0.24 (0.12, 0.36) | 0.34 (0.06, 0.61) | 0.74 |
| FBI Negative behavior | 0.22 (0.15, 0.29) | 0.21 (0.10, 0.32) | 0.27 (0.01, 0.54) | 0.84 |
| FBI Disinhibition Behavior | 0.14 (0.07, 0.21) | 0.27 (0.14, 0.39) | 0.43 (0.14, 0.72) | 0.14 |
| PPAOS (n=28) | AOS-PAA (n=15) | PAA (n=6) | ||
| 20-BAS | 0.15 (0.09, 0.21) | 0.08 (−0.02, 0.18) | −0.07 (−0.35, 0.21) | 0.30 |
PPAOS = primary progressive apraxia of speech; AOS-PAA = apraxia of speech- progressive agrammatic aphasia; PAA = progressive agrammatic aphasia; FBI = frontal behavior inventory; 20-BAS: 20-item behavioral assessment score.
Discussion
Our findings provide important insights into behavioral phenomenology in progressive apraxia of speech and agrammatic aphasia. Behavioral disturbances were frequently observed in our cohort, albeit mild, and worsened over time. Behavioral differences were more common in the patients with agrammatic aphasia (i.e., AOS-PAA and PAA) compared to PPAOS. The difference between PPAOS and AOS-PAA stemmed mainly from greater negative behaviors in AOS-PAA, whereas greater disinhibition in PAA distinguishes it from PPAOS.
In our relatively large cohort of 89 patients, a significant majority of caregivers endorsed at least one behavioral symptom even at baseline. The most common abnormal behaviors were irritability and apathy observed in 56% and 43% respectively, both of which were exhibited across diagnoses. A previous study of 14 agPPA patients, which may most closely align with our PAA group, reported about 30% frequency of irritability at baseline[22]. Also, despite their small sample sizes, previous studies reported apathy frequently in agPPA, ranging from 33% to 64%[22-24]. It should be stressed, however, that the median total FBI score in our cohort was 9 which represents relatively mild severity of behavioral abnormalities.
We found that PPAOS was the least behaviorally affected group. This result is, perhaps, not unexpected given the comparatively focal nature of neurodegeneration in PPAOS involving superior premotor cortex[3]. Involvement of the ventral frontotemporal network and degeneration of the left uncinate fasciculus were reported to be associated with behavioral symptoms in a PPA cohort[25]. Specifically, FBI total score, negative behavior, and disinhibition subscores were shown to be negatively correlated with left orbitofrontal cortex and anterior temporal cortex thickness[25]. The presence of irritability and apathy have been suggested to be correlated with reduced grey matter intensity in right lateral orbitofrontal cortex; and irritability correlated with reduced grey matter intensity in right anterior cingulate in PPA[22]. Interestingly, despite typically lacking involvement in any of these areas, PPAOS patients in this study had frequent irritability and apathy. This finding may indicate general disruption of the salience network rather than disruption of specific regions within the network[26]. Future network level studies may help clarify this hypothesis.
In contrast, AOS-PAA patients typically have more widespread neurodegeneration extending into inferior and middle frontal lobes, insula, and the body of corpus callosum[6, 8], and our AOS-PAA group exhibited more behavioral symptoms than PPAOS, especially in negative behaviors. Differences were observed in the domains of inattention, inflexibility, and disorganization. Attention, flexibility, and organization are all executive functions and are supported mainly by frontal lobe, disruption of which is consistent with the neuroanatomical correlates of AOS-PAA. Similarly, earlier work demonstrated that PAA was associated with degeneration in prefrontal and anterior temporal lobes[8], and in line with that, we found that PAA endorsed more behavioral disturbances than PPAOS. Interestingly, what distinguished PPAOS from PAA was the disinhibition component of FBI. We did not identify any specific items that might explain this difference, likely due to relatively small sample size of PAA group. On the other hand, PAA scored higher in the disinhibition component than PPAOS and AOS-PAA, the latter not reaching statistical significance. This result is concordant with the fact that PAA shows greater neurodegeneration in the orbitofrontal cortex and anterior temporal lobe than AOS-PAA[8].
Behavioral disturbances in speech-language disorders are increasingly studied and the deterioration has been shown to be multifaceted. In this study, we found the FBI to be a useful screening tool that investigates a range of behaviors. However, there are still aspects not captured by the FBI. We developed the 20-BAS as a complementary tool to the FBI to assess those features. It is a quick bedside test with 20 items, scored as present/absent, that can be reported by caregivers of any educational level. On top of its simplicity and practicality, in this first report of the 20-BAS, we show that it is useful. It detected behavioral disturbance in 47.6% of patients, with PPAOS being behaviorally less effected than AOS-PAA and PAA, mirroring the FBI findings. The most endorsed items were `crying more easily` (19.5%) and `personality change` (18.3%), frequency of the latter approached one third in PAA and AOS-PAA with PPAOS being relatively spared (8%). This result is not surprising; personality is a function of the frontal lobe which is involved in AOS-PAA and PAA, but not in PPAOS. Modifying 20-BAS into a quantitative measure would likely increase its sensitivity at the cost of time and complexity.
The results from both inventories also showed that behavioral symptoms worsened over time, although no differences were observed across diagnoses. Behavioral disturbances can result in significant caregiver burden[27, 24] and therefore it is important to assess their nature to address the needs of the patient and the caregiver. The presence of agrammatic aphasia is a clear risk fact for behavioral dyscontrol in this speech-language cohort and our results support the separation of PPAOS from patients with agrammatic aphasia.
Acknowledgements:
We thank Ms. Boland from Mayo Clinic for her role in the neuropsychological testing.
Funding sources:
The study was funded by NIH grants R01-DC12519, R01-DC14942 and R01-DC010367.
Footnotes
Conflict of interests: The authors have no conflicts of interest to declare
Statement of ethics: This study protocol was reviewed and approved by the Mayo Clinic Institutional Review Board, approval numbers [09-008772, 12-008988, 17-010087, 17-002468]. All patients provided written informed consented to participate in the study.
Data Availability:
The raw data used for this study is available upon request to the corresponding author.
References
- 1.McNeil MR, Doyle PJ, Wambaugh JL. Apraxia of Speech: a treatable disorder of motor planning and programming. In: Nadeau SE, Gonzalez Rothi LJ, editors. Aphasia and language: theory to practice. New York: Guilford Press; 2000. p. 221–66. [Google Scholar]
- 2.Duffy J. Apraxia of Speech in degenerative neurologic disease. Aphasiology. 2006;20(6):511–27. [Google Scholar]
- 3.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012. May;135(Pt 5):1522–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, et al. The evolution of primary progressive apraxia of speech. Brain. 2014. Oct;137(Pt 10):2783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitwell JL, Duffy JR, Strand EA, Machulda MM, Tosakulwong N, Weigand SD, et al. Sample size calculations for clinical trials targeting tauopathies: a new potential disease target. J Neurol. 2015. Sep;262(9):2064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013. Jul 23;81(4):337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utianski RL, Duffy JR, Clark HM, Strand EA, Botha H, Schwarz CG, et al. Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang. 2018. Sep;184:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tetzloff KA, Duffy JR, Clark HM, Utianski RL, Strand EA, Machulda MM, et al. Progressive agrammatic aphasia without apraxia of speech as a distinct syndrome. Brain. 2019. Aug 1;142(8):2466–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa Se, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011:WNL. 0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tetzloff KA, Duffy JR, Clark HM, Strand EA, Machulda MM, Schwarz CG, et al. Longitudinal structural and molecular neuroimaging in agrammatic primary progressive aphasia. Brain. 2018. Jan 1;141(1):302–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitwell JL, Martin P, Duffy JR, Clark HM, Utianski RL, Botha H, et al. Survival Analysis in Primary Progressive Apraxia of Speech and Agrammatic Aphasia. Neurol Clin Pract. 2021. Jun;11(3):249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain : a journal of neurology. 2011. Sep;134(Pt 9):2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kertesz A. Western Aphasia Battery (Revised). San Antonio, Tx: PsychCorp; 2007. [Google Scholar]
- 14.De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain : a journal of neurology. 1962. Dec;85:665–78. [DOI] [PubMed] [Google Scholar]
- 15.Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Arch Clin Neuropsychol. 1999. Aug;14(6):481–7. [PubMed] [Google Scholar]
- 16.Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. American journal of Alzheimer's disease and other dementias. 2009. Oct-Nov;24(5):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strand EA, Duffy JR, Clark HM, Josephs K. The Apraxia of Speech Rating Scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord. 2014. Sep-Oct;51:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yorkson K, Strand EA, Miller R, Hillel A, Smith K. Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. J Medical Speech-Language Pathology. 1993;1(1):35–46. [Google Scholar]
- 19.Botha H, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology. 2014. May 13;82(19):1729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005. Apr;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 21.Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. The Canadian journal of neurological sciences. 1997. Feb;24(1):29–36. [DOI] [PubMed] [Google Scholar]
- 22.Rohrer JD, Warren JD. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci. 2010. Jun 15;293(1-2):35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Langenhove T, Leyton CE, Piguet O, Hodges JR. Comparing Longitudinal Behavior Changes in the Primary Progressive Aphasias. J Alzheimers Dis. 2016. Jun 18;53(3):1033–42. [DOI] [PubMed] [Google Scholar]
- 24.Wong S, Irish M, Husain M, Hodges JR, Piguet O, Kumfor F. Apathy and its impact on carer burden and psychological wellbeing in primary progressive aphasia. J Neurol Sci. 2020. Sep 15;416:117007. [DOI] [PubMed] [Google Scholar]
- 25.D'Anna L, Mesulam MM, Thiebaut de Schotten M, Dell'Acqua F, Murphy D, Wieneke C, et al. Frontotemporal networks and behavioral symptoms in primary progressive aphasia. Neurology. 2016. Apr 12;86(15):1393–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Botha H, Utianski RL, Whitwell JL, Duffy JR, Clark HM, Strand EA, et al. Disrupted functional connectivity in primary progressive apraxia of speech. Neuroimage Clin. 2018;18:617–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diehl-Schmid J, Schmidt EM, Nunnemann S, Riedl L, Kurz A, Forstl H, et al. Caregiver burden and needs in frontotemporal dementia. J Geriatr Psychiatry Neurol. 2013. Dec;26(4):221–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used for this study is available upon request to the corresponding author.


