Abstract
Background
The burden of carbapenem resistance is not well studied in the Middle East. We aimed to describe the molecular epidemiology and outcome of carbapenem-resistant Enterobacterales (CRE) infections from several Saudi Arabian Centers.
Methods
This is a multicenter prospective cohort study conducted over a 28-month period. Patients older than 14 years of age with a positive CRE Escherichia coli or Klebsiella pneumoniae culture and a clinically established infection were included in this study. Univariate and multivariable logistic models were constructed to assess the relationship between the outcome of 30-day all-cause mortality and possible continuous and categorical predictor variables.
Results
A total of 189 patients were included. The median patient age was 62.8 years and 54.0% were male. The most common CRE infections were nosocomial pneumonia (23.8%) and complicated urinary tract infection (23.8%) and 77 patients (40.7%) had CRE bacteremia. OXA-48 was the most prevalent gene (69.3%). While 100 patients (52.9%) had a clinical cure, 57 patients (30.2%) had died within 30 days and 23 patients (12.2%) relapsed. Univariate analysis to predict 30-day mortality revealed that the following variables are associated with mortality: older age, high Charlson comorbidity index, increased Pitt bacteremia score, nosocomial pneumonia, CRE bacteremia and diabetes mellitus. In multivariable analysis, CRE bacteremia remained as an independent predictor of 30 day all-cause mortality [AOR and 95% CI = 2.81(1.26–6.24), p = 0.01].
Conclusions
These data highlight the molecular epidemiology and outcomes of CRE infection in Saudi Arabia and will inform future studies to address preventive and management interventions.
Keywords: Carbapenem-resistant Enterobacterales (CRE), Saudi Arabia, OXA-48, Multidrug resistant gram negative bacteria
Introduction
The emergence of carbapenem-resistant Enterobacterales (CRE) infections represents a significant public health threat [1, 2]. The World Health Organization (WHO) considers CRE infection a priority that needs to be addressed globally [3]. Carbapenems are considered the treatment of choice for extended-spectrum β-lactamase bacteria and the last line of defense against Enterobacterales. CRE infections are associated with poor outcomes due to limited treatment options and inappropriate initial therapy [4–6]. Evidence also suggests that infections with CRE are associated with worse outcomes when compared with susceptible Enterobacterales [4, 5, 7, 8]. Furthermore, CRE infection is associated with longer hospital stays and higher healthcare costs [5, 7–9].
Knoweldge regarding the molecular epidemiology of CRE in Gulf countries and in the Middle East in general is limited. In Saudi Arabia, studies have shown that the most prevalent Carbapenemase strain is OXA-48 followed by NDM [10, 11]. Most of these studies are limited by small sample sizes and retrospective designs. Saudi’s wide geographic area and the continuous influx of international and domestic pilgrims are likely factors affecting CRE strain distribution. The exact molecular epidemiology and impact of CRE on outcomes are still not clear. Treatment options for CRE infection remain inadequate. Available options are limited by pharmacokinetics challenges, toxicity, and availability. Understanding the molecular epidemiology of CRE in the region and the impact it has on health care is needed to inform local guidelines.
Our aim in this study is to describe the molecular epidemiology, clinical features, and outcomes of patients infected with CRE from several hospitals in Saudi Arabia. We also aimed to identify predictors of mortality in this population.
Methods
Patients in this multicenter prospective cohort study were recruited over a 28-month period from August 2018 through November 2020 in eight hospital systems in the Kingdom of Saudi Arabia. Hospitals with a median of 462.5 beds in the cities of Jeddah, Makkah, Dammam, and Riyadh participated in enrolling patients. Patients were excluded from participating in the study if they were 14 years of age or less. The final study population consisted of individuals above the age of 14 years with a positive CRE Escherichia coli or Klebsiella pneumoniae culture and a clinically established infection.
Susceptibility testing was performed using the Vitek 2 system (bioMérieux, Marcy L’étoile, France) and N-291 card. Phenotypic confirmation of CRE was performed using the Clinical Laboratory Standards Institute (CLSI) methodology, which includes the ertapenem, imipenem, and meropenem E-test and modified Hodge test (MHT). All confirmed isolates of CRE from the culture were then tested using the Xpert Carba-R Kit following the manufacturer’s recommendation for rapid detection and differentiation of the blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP gene sequences linked to carbapenem resistance in gram-negative bacteria. The Xpert Carba-R Assay will generate a negative or non-Detected result when testing samples resistance to Carbapenem but containing other than blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP gene sequences. The interpretation of the minimum inhibitory concentration (MIC) for carbapenems is based on CLSI guidelines; resistance to ertapenem was considered if MIC was ≥ 2 μg/ml and resistance to meropenem and imipenem was considered if MICs were ≥ 4 μg/ml.
The primary outcome in this study was 30-day all-cause mortality. Length of hospital stay, attributable mortality, relapse within 30 days, and acute kidney injury were recorded. Demographic and clinical characteristics including age, gender, comorbidities, Charlson Comorbidity Index, Apache II score, Pitt bacteremia score, type of CRE infection, organism isolates, location in the hospital at the time of CRE culture, molecular typing, and type of treatment patients received were considered as possible exposures and predictors of 30 day all-cause mortality. Susceptibility to and minimum inhibitory concentrations (MICs) for meropenem, imipenem, ertapenem, and ceftazidime-avibactam (CAZ-AVI) treatments were obtained for the CRE bacteria isolates. Normality of the continuous variables was assessed using Shapiro–Wilk and Kolmogorov–Smirnov tests and by comparing median and mean values.
The associations between 30 day all-cause mortality and continuous and categorical predictors were described using the Kruskal–Wallis and Chi-Square tests. Univariate logistic models were also constructed to assess the relationship between the outcome of 30 day all-cause mortality and possible continuous and categorical predictor variables. All predictor variables with a p-value < 0.1 in univariate analysis models were included in a multivariable logistic model to predict 30 day all-cause mortality. A similar analysis was conducted for the group of patients who were bacteremic (n = 77). Analyses were performed using SAS software, version 9.4, and statistical significance was determined at an α = 0.05 level. The study was approved by the institutional review boards at all participating hospitals.
Results
The study population included 189 patients greater than 14 years of age with a positive CRE culture and a clinically established infection. The median patient age was 62.8 years and 54.0% were male (Table 1). Just over one-fifth (20.1%) of the participants had heart disease and 42.3% had diabetes mellitus. Almost one-third (32.8%) of the patients had a non-hematological malignancy and 19.6% had a cerebral vascular accident comorbidity. The median Charlson comorbidity index was six at baseline. The APACHE II median score for patients who required ICU admission was 19 (n = 112) while the Pitt bacteremia median score for patients with bacteremia was two (n = 110) (Table 1).
Table 1.
Demographic and baseline clinical characteristics of patients with CRE infection (n = 189)
| Variable | n (%) | Median (IQR) |
|---|---|---|
| Age (years) | 62.8 (44.4–73.5) | |
| Gender | ||
| Male | 102 (54.0) | |
| Female | 87 (46.0) | |
| Baseline Comorbidities | ||
| Heart Disease | 38 (20.1) | |
| Peripheral vascular disease | 9 (4.8) | |
| Cerebral vascular accident | 37 (19.6) | |
| Chronic kidney disease | 28 (14.8) | |
| End stage renal disease | 10 (5.3) | |
| HIV | 1 (0.5) | |
| Dementia | 19 (10.1) | |
| COPD | 6 (3.2) | |
| Connective tissue disease | 5 (2.7) | |
| Peptic ulcer disease | 7 (3.7) | |
| Mild liver disease | 7 (3.7) | |
| Moderate and severe liver disease | 22 (11.6) | |
| Diabetes Mellitus | 80 (42.3) | |
| Non-hematological malignancy | 62 (32.8) | |
| Hematological malignancy | 11 (5.8) | |
| Solid organ transplant | 8 (4.2) | |
| Bone marrow transplant | 4 (2.1) | |
| Immunosuppressive medication | 41 (21.7) | |
| Charlson comorbiditiy index | 6 (3–8) | |
| APACHE II score1 | 19 (9.0–31.5) | |
| Pitt bacteremia score2 | 2 (1–6) | |
| Type of CRE infection3 | ||
| CLABSI | 28 (14.8) | |
| HAP/VAP | 45 (23.8) | |
| Complicated UTI | 45 (23.8) | |
| Complicated intra-abdominal infection | 32 (16.9) | |
| Skin and soft tissue infection | 20 (10.6) | |
| Joint/bone infection | 6 (3.2) | |
| Primary bacteremia (source unidentified) | 15 (7.9) | |
| Other | 19 (10.1) | |
| Organism isolates4 | ||
| Escherichia coli | 21 (11.1) | |
| Klebsiella pneumoniae | 165 (87.3) | |
| Both E. coli and K. pneumoniae | 2 (1.1) | |
| CRE bacteremia | 77 (40.7) | |
| Location at time of CRE culture | ||
| Emergency Room | 20 (10.6) | |
| Medical Floor | 52 (27.5) | |
| Surgical Floor | 21 (11.1) | |
| Hematology/Oncology Floor | 29 (15.3) | |
| OB/GYN Floor | 1 (0.5) | |
| ICU | 57 (30.2) | |
| Other | 9 (4.8) | |
| Molecular typing5 | ||
| KPC | 1 (0.5) | |
| NDM | 32 (16.9) | |
| IMP | 1 (0.5) | |
| OXA_48 | 131 (69.3) | |
| None identified | 25 (13.2) | |
IQR interquartile range, CLABSI central line-associated blood stream infection, HAP hospital-acquired pneumonia, CRE Carbapenem-resistant Enterobacteriaceae
1For n = 112 patients, 2For n = 110 patients, 3More than one CRE infection type possible
per patient, 4The isolates were identified at the species level following conventional schemes, percentages may total < 100 due to missing values, 5More than one molecular typing possible per patient
Almost half of the patients had either hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) (23.8%) or a complicated urinary tract infection (UTI) (23.8%) caused by CRE (Table 1). The majority of organisms isolated were Klebsiella pneumoniae (87.3%). Seventy-seven patients (40.7%) had CRE bacteremia and approximately one-third (30.2%) of patients were in the ICU at the time they received a positive CRE culture (Table 1).
For the isolates tested, the median MIC was greatest for meropenem with a value of 16 followed by imipenem with a value of 8. Of the 143 isolates tested for CAZ-AVI, 103 (72.0%) were susceptible, 35 (24.5%) were resistant, and 5 (3.5%) were intermediate at baseline. For the isolates resistant to CAZ-AVI, the molecular typing was KPC (n = 1), NDM (n = 24), and OXA-48 (n = 15) with some overlap between KPC and NDM being found in 1 patient and NDM and OXA-48 being found in 8 patients. The median MIC was smallest for CAZ-AVI with a value of 2 for the isolates tested against this antibiotic. Molecular typing revealed over two-thirds (69.3%) of patients were infected with OXA-48 CRE (Table 1).
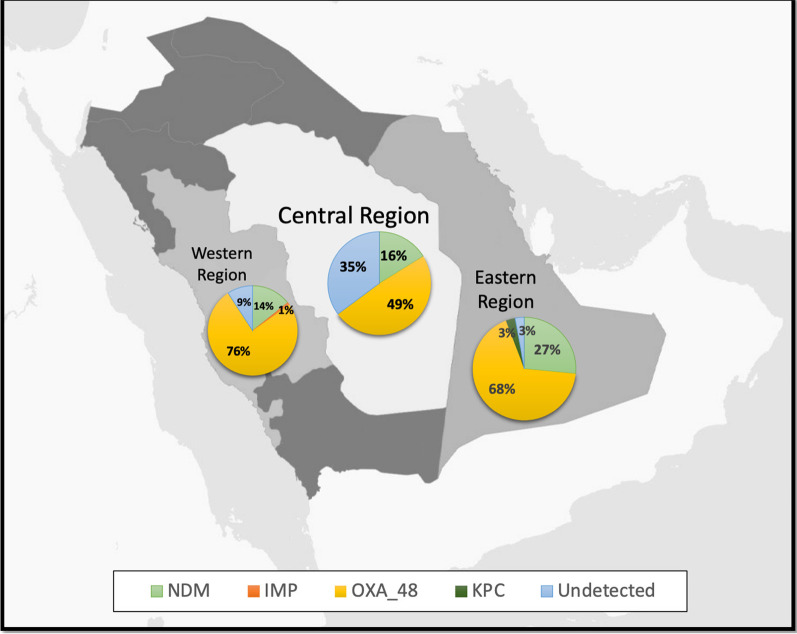
Overall, the study included 114 patients from western region hospitals, 41 patients from the central region of the Kingdom, and 34 patients from the Eastern province (Fig. 1). While 78.9% of patients in the western region had OXA_48 molecular typing identified in their isolates, 43.9% of patients in the central region and 67.7% of patients in the eastern region had OXA_48 identified in their isolates. A similar percentage of the patient isolates were identified as NDM in the western (14.9%) and central (14.6%) regions compared to 26.5% of patient isolates being identified as NDM in the eastern region. Almost one-third of isolates (31.7%) from patients in the central region had no molecular typing detected (Fig. 1).
Fig. 1.
Percentages of CRE molecular typing identified in different regions of Saudi Arabia
While 69.3% of patients took a carbapenem as part of their treatment regimen, 47.1% of patients received CAZ-AVI (Table 2). Approximately one-fifth (20.1%) of patients received an aminoglycoside at some point during their treatment regimen (Table 2). There were 100 patients (52.9%) with a clinical cure and 57 patients (30.2%) had died within 30 days (Table 3). Over one-third (36.5%) of patients had acute kidney injury and 23 patients (12.2%) relapsed within 30 days (Table 3).
Table 2.
Treatment regimen for patients with CRE infection
| Variable | n (%) |
|---|---|
| Any carbapenem (meropenem, imipenem, ertapenem) | 131 (69.3) |
| Aminoglycoside (amikacin, gentamicin) | 38 (20.1) |
| Aztreonam | 11 (5.8) |
| CAZ-AVI | 89 (47.1) |
| Colistin | 78 (41.3) |
| Tigecycline | 62 (32.8) |
CAZ-AVI ceftazidime-avibactam
Table 3.
Outcomes of patients with CRE infections (n = 189)
| Variable | n (%) |
|---|---|
| 30 day all-cause mortality | 57 (30.2) |
| Attributable mortality | 44 (23.4) |
| Clinical cure | 100 (52.9) |
| Relapse within 30 days | 23 (12.2) |
| Acute kidney injury | 69 (36.5) |
The median age of patients who died within 30 days at 64.7 years was significantly different than the median age, 60.3 years, in the group of patients who did not die within 30 days (Kruskal–Wallis Test = 4.8, df = 1, p = 0.03). Similarly, the association of Charlson comorbidity index with 30 day mortality was significant with a median of 6.0 in the group of patients who died within 30 days compared to a median of 5.5 in the group of patients who did not die within 30 days (Kruskal–Wallis Test = 9.1, df = 1, p = 0.003). The median values of Pitt bacteremia were also significantly different between the two groups with a value of 6.0 in the group of patients who died compared to a median value of 1.0 in the group of patients who did not die within 30 days (Kruskal–Wallis Test = 15.3, df = 1, p < 0.0001).
There was a statistically significant difference between the patients who had a HAP/VAP CRE infection with regards to 30 day all-cause mortality (Chi-Square = 5.7, df = 1, p = 0.02). While 35.1% of the patients who died within 30 days had a HAP/VAP infection, 18.9% of patients who did not die within 30 days had a HAP/VAP infection. The association between having CRE bacteremia and 30 day all-cause mortality was also statistically significant with 57.9% of patients who died also having CRE bacteremia compared to 33.3% of patients who did not die having CRE bacteremia (Chi-Square = 9.9, df = 1, p = 0.002). Presenting with the comorbid condition of Diabetes Mellitus was also significantly associated with 30 day all-cause mortality with 54.4% of patients who died having this condition compared to 37.1% of patients who did not die within 30 days having this condition (Chi-Square = 4.9, df = 1, p = 0.03).
There was no significant association identified between CRE treatment with CAZ-AVI and 30 day all-cause mortality (Chi-Square = 0.8, df = 1, p = 0.37). Twenty four (27.0%) of the patients who took CAZ-AVI ended up dying within 30 days of all-cause mortality. Approximately one third (n = 33, 33.0%) of patients who did not take CAZ-AVI (n = 97) ended up dying within 30 days of all-cause mortality.
In further looking at the association of CAZ-AVI treatment with 30 day all-cause mortality, among the patients with isolates that tested sensitive to CAZ-AVI and who took CAZ-AVI as part of their treatment regimen compared to the rest of the patients, there was no significant relationship. The association between those who tested sensitive compared to those with resistant or intermediate sensitivity results to CAZ-AVI for the patients who took CAZ-AVI was also not significant with regards to 30 day all-cause mortality. In addition, taking CAZ-AVI treatment did not significantly predict whether or not patients had a clinical cure [OR and 95% CI = 1.52 (0.86–2.71), p = 0.15]. The outcome of acute kidney injury was not significantly associated with CAZ-AVI treatment [OR and 95% CI 1.15 (0.63–2.08), p = 0.65].
Patients with CRE bacteremia (n = 77) had a median age of 63.6 years, a median Charlson comorbidity index of 6.0, and a median Pitt bacteremia score of 3.0. Among the bacteremic patients, the association between having CAZ-AVI as part of the treatment regimen and 30 day all-cause mortality was significant [odds ratio (OR) and 95% CI = 0.32 (0.12–0.81), p = 0.02] showing a protective effect of the treatment in univariate analysis. After controlling for age, Charlson comorbidity index, and Pitt bacteremia score the association between treatment with CAZ-AVI and 30 day all-cause mortality was no longer significant [AOR and 95% CI = 0.33 (0.10–1.06), p = 0.06]. After adjusting for these predictors, a one-unit increase in Pitt bacteremia score was associated with an increase of 29.0% in the odds of 30 day all-cause mortality [AOR and 95% CI = 1.29 (1.09–1.52), p = 0.004].
Univariate logistic models were constructed to predict the outcome of 30 day all-cause mortality based on demographic and clinical patient characteristics (Table 4). Univariate analysis revealed that a one-unit increase in the Charlson comorbidity index was associated with a 20.0% increase in the odds of dying within 30 days from all causes [OR and 95% CI = 1.20 (1.07–1.33), p = 0.001] and patients with a HAP/VAP infection had an increased odds by a factor of 2.3 of dying within 30 days from all causes [OR and 95%CI = 2.31 (1.15–4.64), p = 0.02]. For patients with a diagnosis of diabetes mellitus at baseline, the odds of dying within 30 days from all-causes increased by a factor of 2.2 times compared to patients without this diagnosis [OR and 95% CI = 2.02 (1.08–3.79), p = 0.03]. All predictor variables with a p < 0.1 were included in the multivariable logistic model looking at the association of clinical characteristics and 30 day all-cause mortality (Tables 4, 5). After adjusting for all predictors with a p < 0.1 from univariate analyses, patients with CRE bacteremia had an increased odds by 281% of dying within 30 days from all causes [AOR and 95% CI = 2.81 (1.26–6.24), p = 0.01]. (Table 5).
Table 4.
Univariate analysis of patient characteristics associated with 30 day all-cause mortality
| Parameter | OR 95% CI | p-value |
|---|---|---|
| Age (years) | 1.02 (1.01–1.04) | 0.01 |
| Charlson comorbidity index | 1.20 (1.07–1.33) | 0.001 |
| Pitt bacteremia score | 1.32 (1.15–1.50) | < 0.0001 |
| APACHE II score | 0.99 (0.95–1.02) | 0.43 |
| CLABSI | 2.74 (1.21–6.22) | 0.02 |
| HAP/VAP | 2.31 (1.15–4.64) | 0.02 |
| Complicated UTI | 0.50 (0.22–1.12) | 0.09 |
| Complicated intra-abdominal infection | 0.37 (0.14–1.03) | 0.06 |
| Skin and soft tissue infection | 0.75 (0.26–2.17) | 0.60 |
| Joint/bone infection | < 0.001 (< 0.001–> 999.99) | 0.98 |
| Primary bacteremia (source unidentified) | 2.17 (0.75–6.30) | 0.15 |
| CRE bacteremia | 2.75 (1.45–5.21) | 0.002 |
| NDM | 1.49 (0.67–3.31) | 0.32 |
| OXA48 | 1.35 (0.68–2.71) | 0.39 |
| Received CAZ-AVI | 0.75 (0.40–1.40) | 0.37 |
| Cardiac disease | 1.69 (0.81–3.55) | 0.16 |
| Diabetes Mellitus | 2.02 (1.08–3.79) | 0.03 |
| Non-hematological malignancy | 1.30 (0.22–3.37) | 0.44 |
| Hematological malignancy | 0.86 (0.67–2.49) | 0.83 |
| Immunosuppressive medication | 1.10 (0.52–2.32) | 0.81 |
OR odds ratio, CI confidence interval
Table 5.
Multivariable association of characteristics with 30 day all-cause mortality (n = 189)
| Parameter1 | OR 95% CI | p-value |
|---|---|---|
| Age (years) | 1.01 (0.99–1.04) | 0.29 |
| Charlson comorbidity index | 1.08 (0.94–1.24) | 0.28 |
| HAP/VAP | 1.90 (0.78–4.65) | 0.16 |
| Diabetes Mellitus | 1.59 (0.77–3.32) | 0.21 |
| CRE bacteremia | 2.81 (1.26–6.24) | 0.01 |
| CLABSI | 1.50 (0.49–4.58) | 0.48 |
| Complicated intra-abdominal infection | 0.45 (0.14–1.51) | 0.20 |
| Complicated UTI | 0.69 (0.26–1.84) | 0.46 |
OR odds ratio, CI confidence interval
1Categories for characteristics with missing values not included in the table
Discussion
Our study shows that OXA-48 carbapenemase is the most common in Saudi Arabia followed by NDM carbapenemase. On the other hand, the prevalence of Klebsiella pneumoniae carbapenemases (KPC) in the country is quite rare. Mortality in patients with CRE infection is very high which is consistent with other studies published globally [4, 12, 13]. The majority of the CRE in our cohort was Klebsiella pneumonia.
About two-thirds of our patients received carbapenem as part of the treatment regimen during their hospital stay. tThis likely occurred because some of the centers utilized combination therapy for the treatment of CRE infections or patients may have received carbapenem before identification of carbapenem resistance. Around 50% of the cohort received CAZ-AVI for the treatment of CRE infection. CAZ-AVI is a new β-lactam/β-lactamase inhibitor combination with in vitro activity against KPCs and OXA-48 producing Enterobacterales. Several studies showed that CAZ-AVI is effective for the treatment of CRE infection [14–17]. In our study, patients who received CAZ-AVI for CRE were less likely to die compared to those who did not receive it; however, this difference was not statistically significant. There are several reasons for this finding. Approximately one quarter of the isolates tested for susceptibility to CAZ-AVI were resistant to it. In addition, almost 30% of patients in the study cohort were either carrying the NDM gene or an unidentified gene. CAZ-AVI does not have activity against NDM carbapenemases and the efficacy of its combination with aztreonam is less certain when compared to its efficacy as a monotherapy for OXA-48 and KPC [18]. The sample size in this cohort was also not powered to detect the survival benefit of antibiotics intervention. Further examination of patients with CRE infection and bacteremia showed that CAZ-AVI has a protective effect in univariate analysis. Despite being expensive, the use of CAZ-AVI slightly exceeded colistin use which is very nephrotoxic and less effective than CAZ-AVI [15, 16, 19]. Although tigecycline has a black box warning of increased mortality, it is one of the few options available to treat CRE [20], and it was used in about a third of our cohort. Other CRE-active agents were not used because they are not yet available in Saudi Arabia such as eravacycline, plazomicin, meropenem-vaborbactam, and imipenem-cilastatin-relebactam. The latter two are less attractive in our region given their lack of activity against OXA-48, the predominant type of carbapenemase. KPC is not common in the region at this time and can be treated with CAZ-AVI [21].
We identified several predictors for 30-day mortality in the univariate analysis. These included older age, higher comorbidity index, higher pitt bacteremia score, and having diabetes as a comorbidity. These predictors were all consistent with what has been shown in previous studies [22, 23]. HAP/VAP and CRE bacteremia both were associated with a more than two fold increase in mortality. CRE bacteremia has been shown to be associated with a substantial increase in mortality in several studies. After adjusting for potential confounders, CRE bacteremia remained an independent risk factor for mortality.
Our study has several strengths including the prospective design and recruiting patients from several tertiary care hospitals from different geographic locations in Saudi Arabia. Furthermore, we collected information on baseline clinical characteristics and several time-varying covariates. We acknowledge several limitations in our study including the sample size in our cohort precluding identification of strong predictors of mortality and an inability to compare the efficacy of different antibiotic treatments for CRE infection. This was due to the heterogeneity of treatment between centers and to the lack of specific protocols for CRE therapy.
In conclusion, this study improved our knowledge regarding the burden of CRE infection in the region. It also highlights the molecular epidemiology and current therapeutic approach for CRE treatment in Saudi Arabia. This information will inform local and global preventive and therapeutic intervention protocols for CRE infection.
Acknowledgements
Not applicable.
Author contributions
BA conceptualized and designed the study and drafted the manuscript. BA and EH carried out the statistical analysis. EH contributed to the study design. YJ, WA, KJ, HT, AS, RM, MA and AT provided patients, contributed to the interpretation of data and critically revised the manuscript. MQ performed molecular analysis and contributed to the interpretation of data. KO, RO, WH, DA, RS, MS, MH and LH enrolled patients and carried out data collection and curation. All authors reviewed and approved the final manuscript. All authors read and approved the final manuscript.
Funding
No funding of any kind has been received; this data has been generated as part of the routine work of the hospitals.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the institutional review board of King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia (IRB #2018-57). We confirm that all methods were carried out in accordance with relevant guidelines and regulations of Helsinki declaration. The informed consent was waived by the Research Ethics Committee of King Faisal Specialist Hospital and Research Center.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017;8(4):460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75(2):115–120. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO: 2017.
- 4.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20(7):1170–1175. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 6.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villegas MV, Pallares CJ, Escandón-Vargas K, Hernández-Gómez C, Correa A, Álvarez C, Rosso F, Matta L, Luna C, Zurita J, et al. Characterization and clinical impact of bloodstream infection caused by carbapenemase-producing Enterobacteriaceae in seven Latin American Countries. PLoS ONE. 2016;11(4):e0154092. doi: 10.1371/journal.pone.0154092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewardson AJ, Marimuthu K, Sengupta S, Allignol A, El-Bouseary M, Carvalho MJ, Hassan B, Delgado-Ramirez MA, Arora A, Bagga R, et al. Effect of carbapenem resistance on outcomes of bloodstream infection caused by Enterobacteriaceae in low-income and middle-income countries (PANORAMA): a multinational prospective cohort study. Lancet Infect Dis. 2019;19(6):601–610. doi: 10.1016/S1473-3099(18)30792-8. [DOI] [PubMed] [Google Scholar]
- 9.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18(1):54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 10.Alotaibi F. Carbapenem-Resistant Enterobacteriaceae: an update narrative review from Saudi Arabia. J Infect Public Health. 2019;12(4):465–471. doi: 10.1016/j.jiph.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Moghnieh RA, Kanafani ZA, Tabaja HZ, Sharara SL, Awad LS, Kanj SS. Epidemiology of common resistant bacterial pathogens in the countries of the Arab League. Lancet Infect Dis. 2018;18(12):e379–e394. doi: 10.1016/S1473-3099(18)30414-6. [DOI] [PubMed] [Google Scholar]
- 12.Daikos GL, Petrikkos P, Psichogiou M, Kosmidis C, Vryonis E, Skoutelis A, Georgousi K, Tzouvelekis LS, Tassios PT, Bamia C, et al. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2009;53(5):1868–1873. doi: 10.1128/AAC.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17(7):726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 14.Alraddadi BM, Saeedi M, Qutub M, Alshukairi A, Hassanien A, Wali G. Efficacy of ceftazidime-avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae. BMC Infect Dis. 2019;19(1):772. doi: 10.1186/s12879-019-4409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakeam HA, Alsahli H, Albabtain L, Alassaf S, Al Duhailib Z, Althawadi S. Effectiveness of ceftazidime-avibactam versus colistin in treating carbapenem-resistant Enterobacteriaceae bacteremia. Int J Infect Dis. 2021;109:1–7. doi: 10.1016/j.ijid.2021.05.079. [DOI] [PubMed] [Google Scholar]
- 16.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin infect Dis. 2018;66(2):163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temkin E, Torre-Cisneros J, Beovic B, Benito N, Giannella M, Gilarranz R, Jeremiah C, Loeches B, Machuca I, Jiménez-Martín MJ et al. Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother. 2017; 61(2). [DOI] [PMC free article] [PubMed]
- 18.Tamma PD, Hsu AJ. Defining the role of novel β-lactam agents that target carbapenem-resistant gram-negative organisms. J Pediatric Infect Dis Soc. 2019;8(3):251–260. doi: 10.1093/jpids/piz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eljaaly K, Bidell MR, Gandhi RG, Alshehri S, Enani MA, Al-Jedai A, Lee TC. Colistin nephrotoxicity: meta-analysis of randomized controlled trials. Open Forum Infect Dis. 2021;8(2):ofab026. doi: 10.1093/ofid/ofab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-increased-risk-death-iv-antibacterial-tygacil-tigecycline.
- 21.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, Lagacé-Wiens PRS, Walkty A, Denisuik A, Golden A, et al. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs. 2018;78(1):65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Han X, Li Y, Li M. Assessment of mortality-related risk factors and effective antimicrobial regimens for treatment of bloodstream infections caused by carbapenem-resistant Enterobacterales. Antimicrob Agents Chemother. 2021;65(9):e0069821. doi: 10.1128/AAC.00698-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17(12):1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.