Abstract
Bariatric surgery is currently the most effective treatment for obesity and associated comorbidities, including rapid resolution of type 2 diabetes mellitus (T2DM). Although the weight loss itself has substantial impact, bariatric surgery also has weight loss–independent effects on T2DM. Several variations of bariatric surgery exist, including the widely studied Roux-en-Y gastric bypass and vertical sleeve gastrectomy. The success of both of these bariatric surgeries was originally attributed to restrictive and malabsorptive modes of action; however, mounting evidence from both human and animal studies implicates mechanisms beyond surgery-induced mechanical changes to the gastrointestinal (GI) system. In fact, with bariatric surgery comes a spectrum of physiological responses, including postprandial enhancement of gut peptide and bile acids levels, restructuring of microbial composition, and changes in GI function and morphology. Although many of these processes are also essential for glucoregulation, the independent role of each in the success of surgery is still an open question. In this review, we explore whether these changes are necessary for the improvements in body mass and glucose homeostasis or whether they are simply markers of the physiological effect of surgery.
Keywords: type 2 diabetes, glucose metabolism, bariatric surgery
Introduction
Obesity and type 2 diabetes mellitus (T2DM) are epidemic in Western societies and are among the most costly and urgent health crises worldwide.1 Despite the fact that these two diseases go hand in hand, they are clinically treated as separate diseases. Therapeutic options for obesity are limited in both number and efficacy. At best, lifestyle intervention achieves only ~5% weight loss, and this can increase to 10% if combined with one of the few pharmacotherapy options. The opposite is true for treatment of T2DM, where there is an ever-expanding repertoire of pharmacotherapies. However, like obesity, T2DM is a progressive disease requiring continual adjustment of medications in order to achieve adequate glycemic control. Furthermore, most T2DM therapies counterproductively promote weight gain. Although large-scale treatment implementation is limited owing to the invasiveness and infrastructure needed to perform surgery, bariatric surgery is the most effective treatment that targets both obesity and T2DM simultaneously. In fact, weight loss after bariatric surgery is three times greater than that seen with behavioral modification or pharmaceutical therapy and is sustained over a 10-year period.2 Despite the inherent risk of surgery itself, bariatric procedures reduce overall mortality2,3 through the reduction of obesity comorbidities, such as heart disease,4 cancer,5 and T2DM,5 and this has been attributed to the ability of surgery to induce long-term metabolic benefits. Although T2DM is generally viewed as a chronic disease, these surgeries improve T2DM through mechanisms that are at least partly independent of weight loss, as remission in T2DM is often seen before patients are released from the hospital.6 The rapid and unprecedented resolution of T2DM has led to the increasing use of bariatric surgeries to specifically treat T2DM in less-obese patients.7 One focus of this review is to explore the specific weight loss–independent and –dependent changes in glucose homeostasis that drive the T2DM resolution.
The precise mechanisms by which bariatric surgery causes sustained weight loss and resolves T2DM remain elusive. The original hypothesis for the success of bariatric surgeries was focused on the anatomical changes induced by the respective surgeries. If a surgery reduced stomach size, then the surgery was believed to cause weight loss by restriction of the stomach and, thus, meal size, consequently limiting the number of calories that could be consumed. If surgery included rearrangement of the intestinal anatomy, then the surgery was thought to be malabsorptive owing to a loss of calories in the feces. However, it is becoming more accepted that these operations have mechanisms that reach beyond the changes in anatomy. In fact, the substantial metabolic improvements after bariatric surgery that surpass the effects of weight loss alone have led to these operations to often be referred to as “metabolic surgeries.”8–10 In addition, there are widespread physiological effects of surgery, including the 10-fold increases in postprandial gut peptide levels,11,12 increases in circulating bile acids,13,14 changes in the microbiome composition,9 and a change in intestinal morphology.15 A second major purpose of this review is to address a critical question as to whether these responses are simply a marker of the response of the gastrointestinal (GI) tract to the change in anatomy or whether they are necessary underlying mechanisms that drive the metabolic success of surgery.
The anatomy of VSG and RYGB
Several variations of bariatric surgery are currently performed; some alter both stomach and intestinal anatomy, while others only alter stomach anatomy. Perhaps the best-studied surgery is Roux-en-Y gastric bypass (RYGB). In this surgery, a small stomach pouch is surgically created from the proximal stomach and sutured to the mid-jejunum, while the remaining 95% of the stomach and the proximal intestine remain in the peritoneal cavity but are bypassed from nutritional access. In the mini-gastric bypass, instead of a pouch, a long gastric tube is formed and sutured to the mid-jejunum so that nutrient flow will bypass most of the stomach and the upper intestine. This surgery was designed to be a simpler and safer surgery with fewer major complications as compared with RYGB. Despite the greater simplicity, the degree of weight loss and improvements in obesity-related comorbidities over a 10-year period are similar to RYGB.16,17 In another surgery, the biliopancreatic diversion with duodenal switch, 70% of the stomach is removed along the greater curvature and the intestine is rerouted similar to RYGB; however, the intestine is by passed to a greater extent. This surgery results in robust weight loss and a greater remission of T2DM compared with RYGB yet causes significantly greater macro-and micronutrient malabsorption such that malnutrition is a frequent complication of the surgery.18
Notable bariatric surgeries that do not involve intestinal rearrangement include laparoscopic adjustable gastric banding and vertical sleeve gastrectomy (VSG). In gastric banding, a saline-filled band is placed around the superior portion of the stomach and is made adjustable by varying the amount of saline within the band. In VSG, ~80% of the stomach along the greater curvature is removed, and intestinal structure is unaltered. While both of these operations alter stomach size, they differ widely in efficacy of weight loss and reduction of obesity-associated comorbidities. A benefit of adjustable gastric banding is that it is minimally invasive with low rates of mortality and complications. However, a comprehensive meta-analysis of the literature concluded that adjustable gastric banding results in significantly less weight loss compared with other bariatric surgeries.19 In contrast, both human and rodent data suggest that VSG is nearly as effective as RYBG for resolving T2DM and inducing sustained weight loss.20,21 In this review, we primarily focus on the effects of RYGB and VSG, as these are two of the most commonly performed and studied procedures, and they provide an interesting comparison given their similar efficacy but drastically different anatomical rearrangements.
Bariatric surgery, glucose homeostasis, and T2DM resolution
The degree of T2DM remission reported after bariatric surgery ranges from 38% to 77%20–23 and depends on the type of surgery, the duration of disease, and the criteria used to define remission. In general, the reported rate of remission is greatest with biliopancreatic diversion, then RYGB, and finally VSG,20,21 and is more frequent in patients with greater weight loss and a shorter duration of disease.20 Furthermore, whether remission is defined by a fall in glycosylated hemoglobin to below 6.5% or 6% can differentiate the degree of remission reported between studies.20,21 A fall in glycosylated hemoglobin to below 6.0% is more conservative, and, when this criterion is used, the degree of remission falls to ~40%.20
That bariatric surgery causes significant improvements in glucose homeostasis and that the weight loss itself has a profound effect on improving glucose homeostasis is not in dispute. However, what is in dispute is the degree of the additional contribution of weight loss–independent effects on this improvement in glucose homeostasis. Early proponents of the weight loss–independent effects of surgery pointed to the rapid resolution of T2DM, which occurs within days postoperatively and before significant weight loss.6 In other words, we know that weight loss alone leads to significant improvements in T2DM, but the question remains if bariatric surgery adds additional non-weight-loss mechanisms to the resolution of T2DM. In the following subsection of this review, we intertwine discussion regarding weight loss–independent effects of bariatric surgery with discussion of the exact processes of glucoregulation that are altered by surgery.
Targeted glucoregulatory processes of bariatric surgery
Regulation of glucose homeostasis is a multiorgan integrative process for which bariatric surgery seems to act on several levels (Fig. 1). Clinical and preclinical studies have used many different end points to try to understand the impact of surgery on glucoregulation. These are summarized and defined in Table 1 and include glycosylated hemoglobin, fasting plasma glucose and insulin levels, basal endogenous glucose production (EGP), insulin-induced suppression of EGP, postprandial glucose and insulin levels, gut-independent nutrient-induced insulin secretion, and insulin-independent glucose disposal. Although dependent on the postoperative timing, bariatric surgery affects many of these end points and thus affects many aspects of glucoregulation. This multisystem effect likely contributes to its sustainable impact on T2DM resolution.
Figure 1.
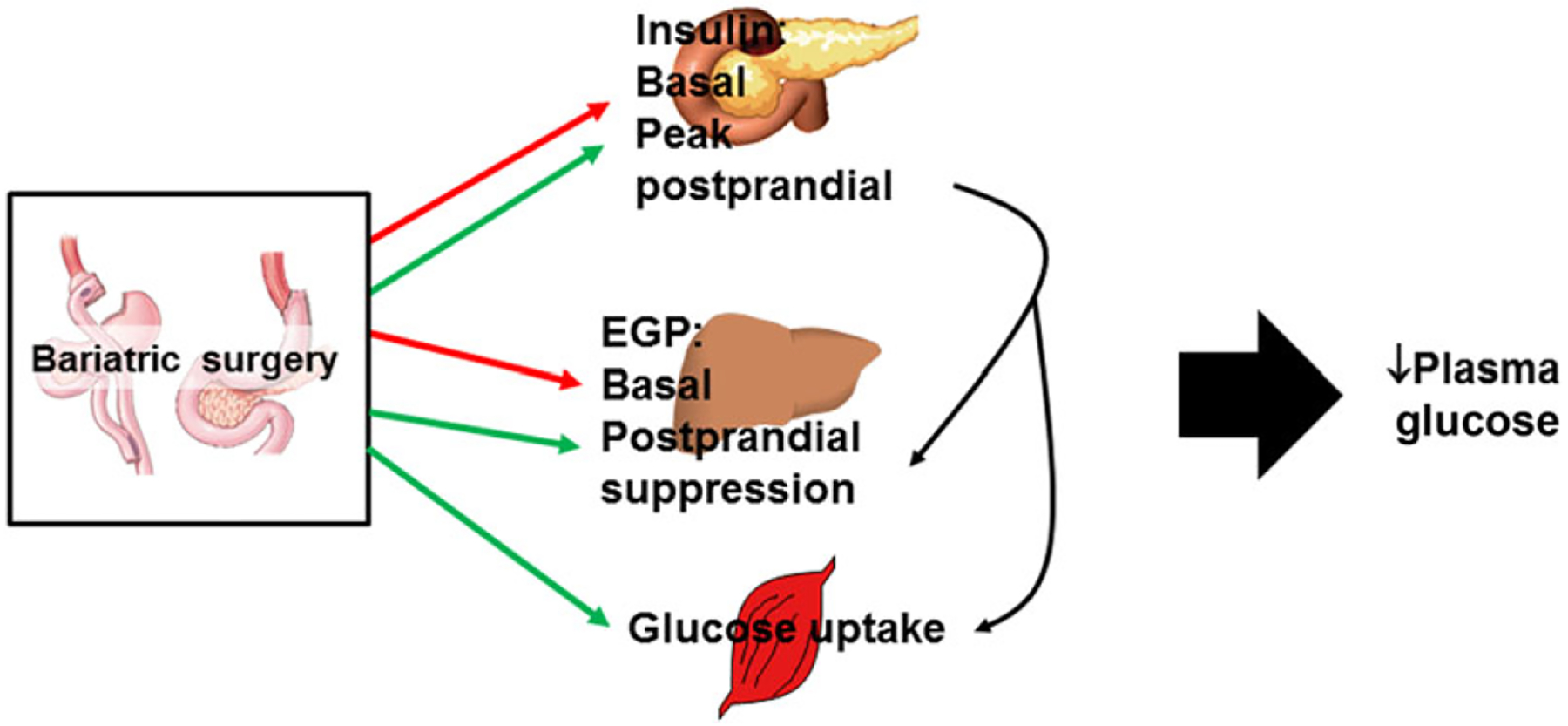
Bariatric surgery, including both RYGB and VSG, has widespread effects on glucose homeostasis. Early responses to surgery include a reduction in basal glucose and insulin levels (and consequently HOMA-IR) and a reduction in basal endogenous glucose production, as indicated in both human and rodent studies. Although unexplored in humans, rodents also demonstrate an early postoperative improvement in hepatic insulin sensitivity. Peak insulin levels in response to a meal are greater after surgery but then rapidly return to baseline. Last, and only after significant weight loss, peripheral insulin sensitivity and thus glucose uptake are increased. Red arrows, end points that are reduced; green arrows, end points that are increased.
Table 1.
The various end points examined when looking for changes in glucose homeostasis, what physiology they represent or are regulated by, and the impact of surgery
| End point | Physiology | Impact of surgery |
|---|---|---|
| Glycosylated hemoglobin | The amount of glucose-bound hemoglobin; represents long-term glucose control | Improved |
| Fasting glucose and insulin | Basal endogenous glucose production dictates fasting glucose | Early postoperative improvement |
| HOMA-IR | Equation based on fasting glucose and insulin and used as an index of insulin sensitivity | Early postoperative improvement |
| Basal endogenous glucose production | Dictated by glycogenolysis and gluconeogenesis, which is controlled by the ratio of insulin to glucagon | Early postoperative improvement |
| Postprandial endogenous glucose production | Suppressed by insulin | Early postoperative improvement in rats |
| Peripheral glucose uptake | Stimulated by insulin | Late postoperative improvement |
| Postprandial glucose | Regulated by gastric emptying rate, intestinal absorption, suppression of EGP, and insulin-mediated glucose disposal | Increased peak glucose but more rapid return to baseline |
| Postprandial insulin | Increased insulin suppresses EGP and stimulates glucose disposal; regulated by gut-dependent and gut-independent mechanisms | Increased peak insulin but more rapid return to baseline |
| Insulin response to an IV glucose load | Marker of nutrient sensing at the β cell and is gut independent | Increased |
| Incretin effect | The glucose-induced increase in gut peptides (GLP-1, GIP) increases insulin to a greater extent after an oral versus an IV glucose load | Increased secretion of GLP-1 |
Early after surgery (days to ~2 weeks) the most robust change in glucose homeostasis in patients with frank diabetes or with impaired glucose tolerance is the reduction in fasting plasma glucose and/or insulin levels and consequently homeostatic model assessment for assessing β-cell function and insulin resistance (HOMA-IR),24–29 an index of insulin sensitivity calculated using fasting glucose and insulin levels. Some of this rapid improvement could certainly occur through removal of glucotoxicity, which affects insulin secretion and insulin-mediated glucose disposal (i.e., the impact of chronically high glucose levels itself impairs insulin secretion and glucose disposal).30 Fasting glucose levels are also regulated by basal endogenous (primarily the liver but also the kidney) glucose production. However, clinically basal glucose production is a difficult end point to obtain, as it requires the use of a hyperinsulinemic euglycemic clamp, and is optimal when this technique is paired with glucose tracers to separate out changes in hepatic versus peripheral insulin sensitivity. Despite this difficulty, a few studies have been able to complete these experiments within days to weeks postoperatively and did, in fact, find that basal EGP was reduced.31,32 Whether insulin-induced suppression of EGP is enhanced in patients is less clear, as these studies are done under conditions where EGP is maximally suppressed. Thus, one study demonstrated improvements in insulin-induced suppression of EGP33 while another did not.31 This is in contrast to rodents, where both RYGB and VSG improved hepatic insulin resistance within 2 weeks postoperatively, and this effect was found to be independent of weight loss, as a weight-matched group did not demonstrate the same improvement.11 Postprandial suppression of EGP has been found to be enhanced once weight loss approached 20% in both RYGB and VSG patients, but whether this is an early or weight loss–independent effect is unknown.34
The source of glucose driving EGP is via breakdown of hepatic glycogen and gluconeogenesis. It has been found that energy restriction reduces EGP owing to decreases in glycogenolysis rather than reduction in gluconeogenesis, and this occurs very acutely after the onset of caloric restriction.35 While it is unknown whether bariatric surgery drives an early and specific decrease in gluconeogenesis versus glycogenolysis, in a rat model of T2DM induced by a combination of high-fat diet and low-dose streptozotocin, RYGB and VSG both decreased hepatic gluconeogenic gene expression 8 weeks postoperatively.36
Plasma glucose levels in response to an oral glucose load are an excellent physiological indicator of overall ability to handle nutrients. Gastric emptying is very rapid after both RYGB and VSG, and thus peak glucose levels, in both patients and rodents, are not typically reduced after surgery.37,38 However, there is a more rapid return to baseline compared with obese controls.37 Despite the rapid gastric emptying rate, RYGB still improved oral glucose tolerance to a greater extent than that observed in patients who had achieved a similar weight through dietary intervention.39 These authors also performed metabolomics profiling and found that branched-chain amino acids, thought to have an independent effect impairing glucose tolerance, were decreased more after RYGB compared with dietary intervention patients.40 Together, these data support a weight loss–independent effect on the glucose tolerance and metabolomics profile. Importantly, the degree of weight loss–independent effects may depend on T2DM status. Plum et al.41 found that patients with T2DM, but not those without T2DM, had greater improvements in insulin sensitivity and glucose disposition after RYGB compared with patients who lost an equivalent amount of weight with dietary intervention.
The amount of insulin secreted in response to a nutrient load is essential to suppress EGP and stimulate glucose clearance. Postprandial plasma insulin levels are often reported to be elevated after both RYGB and VSG.11,37,39 To distinguish between the impact of changes in insulin sensitivity and insulin secretion on glucose homeostasis, investigators often use a frequently sampled intravenous (IV) glucose tolerance test where plasma glucose and insulin levels are repeatedly measured after an IV glucose load. When this was done in patients 3 years postoperatively, RYGB lowered the insulin response to an IV glucose load42 but increased the response to oral glucose. Together, these results suggested that gut factors, such as the surgery-induced increases in glucagon-like peptide-1 (GLP-1), are critical for maintaining normal insulin secretion and, consequently, postprandial glucose homeostasis. These findings highlight a critical difference between the impact on bariatric surgery and weight loss on the insulin response to IV versus oral glucose loads.
In contrast to early hepatic effects, in both humans and rodents, it is very clear that improvements in peripheral insulin sensitivity and glucose disposal as assessed by hyperinsulinemic euglycemic clamps11,27,29,32,43 do not occur until after significant weight loss. In fact, long-term weight loss–adjusted results suggest that weight loss–independent metabolic effects are important early after surgery but that the sustained weight loss is more of a factor for the long-term reductions in basal glucose and insulin and consequent reductions in HOMAIR.44 Regardless, it is very clear that multiple processes of glucoregulation are altered by bariatric surgery. However, because of the potent degree of weight loss caused by bariatric surgery, dissociating the weight loss–independent from the–dependent effects of surgery will be difficult in clinical studies.
T2DM: cure or postponing the disease
Even while the majority of evidence points to metabolic surgery producing long-term weight loss,22 ~20% of patients either fail to lose weight or regain weight after bariatric surgery.45 If weight is regained, then it stands to reason that recidivism in T2DM will also occur. In one study, while 72% of RYGB patients had T2DM remission the first 2 years postoperatively, this was down to 30% of RYGB patients 15 years postoperatively (remission defined by a fasting glucose <110 mg/dL and no T2DM medications).6 These data suggest that bariatric surgery postpones rather than cures T2DM. Future studies are needed with detailed analysis of the factors that define recidivism in both obesity and T2DM after bariatric surgery, as identification would help determine if there is a specific population of patients who should not have surgery in the first place.
Gut peptides
Although there are conflicting data suggesting that the striking metabolic impact of bariatric surgery is due to weight loss alone, one factor not in dispute is the weight loss–independent effects on postprandial gut peptide secretion. These gut peptides have a variety of functions, including playing key roles in regulating glucose and energy homeostasis. Despite the clear changes in gut peptides, none have been shown to be independently necessary for the weight loss or improvements in glucose homeostasis in response to bariatric surgery. This section will review some of the more widely studied gut peptides and their independent effect, or lack thereof, on surgical outcome.
Ghrelin
Ghrelin is secreted by enteroendocrine cells within the stomach, duodenum, and pancreas,46 and plasma levels are highest during fasting.47 Ghrelin acts on receptors within the central nervous system (CNS), such as the hypothalamus and nucleus accumbens, to regulate food reward and long-term energy balance46,48 and is the only GI peptide that, when given exogenously, increases food intake in humans49 and rodents.47 Exogenous ghrelin administration has also been found to inhibit glucose-stimulated insulin release50–52 and reduce insulin sensitivity in peripheral tissues.53
Bariatric surgeries like RYGB and VSG remove nutrient access to a large portion of the stomach and proximal gut in the case of RYGB, and this led to the hypothesis that removal of ghrelin is an underlying mechanism driving the success of bariatric surgery. Generally, ghrelin levels are reported to be reduced after VSG,54,55 but, as reviewed by Tymitz et al.,56 this is less clear after RYGB, with some reports suggesting that postprandial and diurnal fluctuations of ghrelin were absent,57,58 increased,59 or not changed after RYGB.54 In humans and rodents, plasma ghrelin levels have been found to be substantially reduced after VSG but not after RYGB.60,61 Thus, to determine whether removal of ghrelin played a role in the outcome of VSG, we performed VSG in ghrelin-deficient and wild-type mice. We found that VSG was equally effective at reducing body mass and improving glucose tolerance in both groups of animals.60 Although genetic manipulation of ghrelin could have led to developmental compensations that potentially distort the role of ghrelin in surgical outcome, these data indicate that reduced ghrelin signaling is not necessary for the weight loss and improved glucose regulation that result from VSG.
GLP-1
GLP-1 is secreted from enteroendocrine L cells in a nutrient-dependent manner.62 GLP-1 has been shown to increase insulin and decrease glucagon secretion, a hormonal change that has a potent effect on inhibiting EGP and restraining postprandial glucose homeostasis. GLP-1 also delays gastric emptying and intestinal transit and reduces meal size through a G protein–coupled receptor (reviewed in Ref. 62). Because of these effects, the GLP-1 system remains a major target in the pharmaceutical pipeline of drugs to treat T2DM.63
Nutrient-driven increases in plasma GLP-1 are dramatic after both RYGB and VSG, increasing 10-fold over controls in a manner that is both conserved across species11,37,64–67 and independent of weight loss.11,40 Pharmacologic blockade of the GLP-1 receptor after RYGB or VSG inhibits prandial insulin release in humans64,65,68–70 and rodents.11 GLP-1 has therefore been implicated as a mechanism underlying weight loss and improvements in glucose homeostasis after bariatric surgery.
GLP-1 receptors are located on β cells but are also widely expressed in the CNS, and both populations of receptors have been found to play roles in glucose homeostasis.71,72 However, CNS administration of a GLP-1 receptor antagonist in rats had no impact on changes in body mass or food intake in response to RYGB.73 Moreover, whole-body GLP-1 receptor knockout (KO) mice also respond normally to VSG74 and RYGB,75 both in terms of weight loss and improvements in glucose regulation. Such an outcome indicates that increases in GLP-1 are not necessary for the major metabolic effects of either VSG or RYGB. However, there is a small population of patients, primarily after RYGB, that over time has increasing incidence of postprandial hypoglycemia or dumping syndrome, with both autonomic (sweating and heart palpitations) and neuroglycopenic (confusion) symptoms. Data are accumulating that suggest that enhanced GLP-1 receptor signaling causes patients to be more susceptible to this condition and that administration of a GLP-1 antagonist prevents postprandial hypoglycemia in these patients.68 This is an important finding, as dumping syndrome is an extremely limiting complication of RYGB that sometimes leads to surgery revision.
Thus, although mouse studies suggest that GLP-1 is not necessary for the success of bariatric surgery, acute manipulation of postprandial GLP-1 signaling blunts postprandial insulin excursions, suggesting an integrated rather than independent role in surgery success. However, the role of GLP-1 receptor signaling in susceptibility to dumping syndrome merits future research.
Other GI peptides
Other GI peptides that have been found to be altered after bariatric surgery include cholecystokinin (CCK),76,77 glucose inhibitory peptide,78,79 GLP-2,77,78 and peptide YY.67,80–82 Like ghrelin, there is some variability in the changes in gut peptides in VSG versus RYGB (Fig. 2). In general, peptides secreted from the upper GI tract, such as ghrelin (discussed above), CCK, and gastric inhibitory poly-peptide (GIP), have variable changes after RYGB but are increased by VSG.76,77 In contrast, peptides secreted from the distal gut, such as GLP-1, GLP-2, and PYY, are generally increased by both operations.76,83 These data highlight how a change in the anatomy of the GI tract results in changes in GI peptide responses to that surgery. Given the similarity of effects of these operations and the retained efficacy of surgery in genetic KO mouse models, it is not likely that any one of these factors alone contributes to the success of bariatric surgery. If these peptides are important, a more likely scenario is that some or all of these gut peptides act in concert to mediate some of the effects of these procedures. This idea has already been adopted in the pharmaceutical pipeline for T2DM and obesity in the form of dual and triagonists.84
Figure 2.
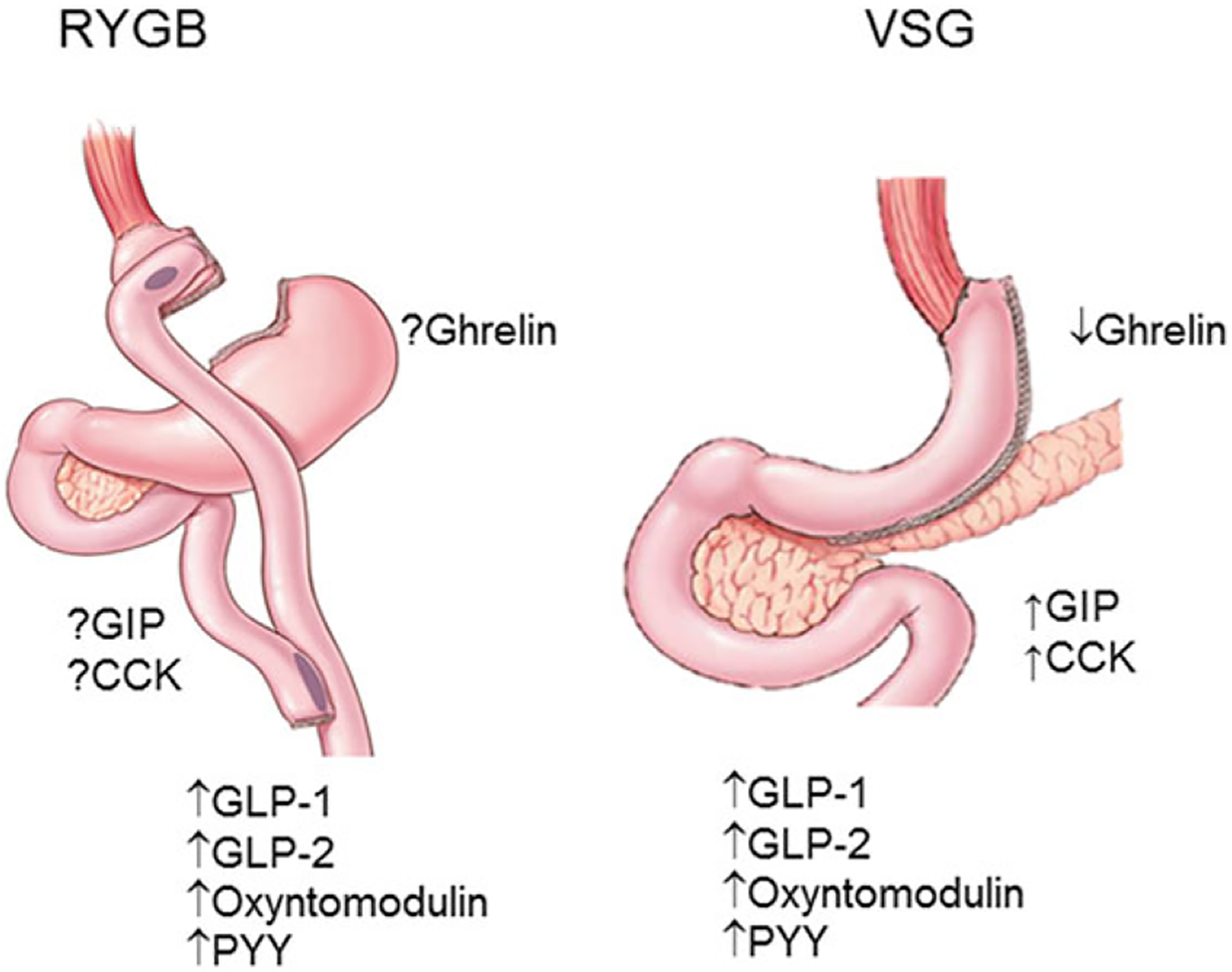
The variability in the changes in postprandial gut peptide levels after RYGB versus VSG. In general, peptides secreted from the upper GI tract, such as ghrelin, CCK, and GIP, have variable reported changes after RYGB but are increased by VSG. In contrast, peptides secreted from the distal gut, such as GLP-1, GLP-2, oxyntomodulin, and PYY, are increased by both operations.
Bile acids
Bile acids are derivatives of cholesterol synthesized in the liver and secreted into the duodenum that function to emulsify lipids, enabling digestion and absorption. After lipid absorption, bile acids remain in the intestinal lumen until they reach the terminal ileum, where they are reabsorbed into the bloodstream and recycled within the liver. Cholesterol-derived primary bile acids are secreted by the liver, while secondary bile acids are a product of bile acid hydroxylation by intestinal microbiota (Fig. 3). All bile acids can be conjugated to glycine or taurine within the liver to form bile salts.85 Thus, the term “bile acids” represents a pool of different molecules with potentially diverse biological activity. Although the traditionally known function of bile acids is lipid emulsification, they are also known to act as signaling molecules.
Figure 3.
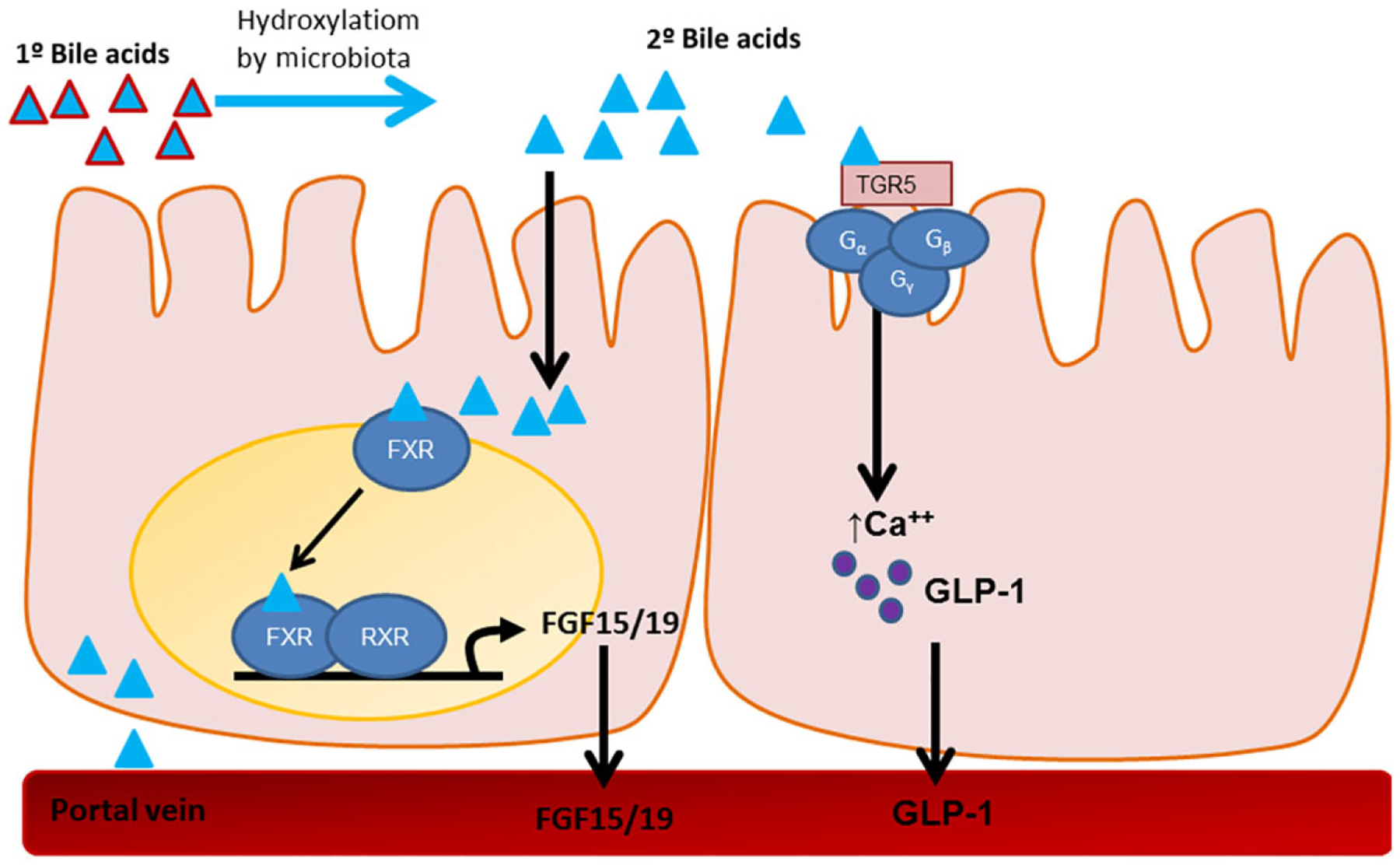
Primary bile acids secreted by the liver are hydroxylated by the microbiome in the intestinal tract to yield secondary bile acids. In addition to emulsification of lipids, bile acids act as hormones by activating two different receptors. One is a nuclear transcription factor called FXR. Once activated, FXR produces FGF19/15 (human/rodent analog), which is secreted into the circulation. FGF19/15 then acts on downstream metabolic pathways to regulate glucose and lipid homeostasis. The other receptor activated by bile acids is TGR5. TGR5 is a G protein–coupled receptor that, within the intestine, is known to regulate GLP-1 secretion.
Bile acid signaling influences several physiological processes associated with improvements in T2DM, including regulation of glucose and lipid metabolism,86 release of gut peptides,87 insulin sensitivity,88 and regulation of energy expenditure.89 In fact, the positive impact of bile acid sequestrants on T2DM underscores the important role of these molecules in regulation of glucose homeostasis.90 It has also been reported that obese patients have increased bile acid synthesis, preferential 12α-hydroxylation (a bile acid modification associated with insulin resistance), and impaired serum bile acid fluctuations.91 Therefore, when it was reported that bile acids were increased after bariatric surgery,92 attention turned to their role as a key mechanism leading to the metabolic improvements after bariatric surgery.
Bariatric surgery has been shown to alter multiple aspects of bile acid flux, including the amount, the composition, and the circulation of bile acids. RYGB increased the amount of total and specifically conjugated bile acids within the plasma in a weight loss–independent manner.93–95 In addition, the peaks in plasma bile acids were higher and occurred earlier after a meal compared to weight-matched control patients.96 Although bile acids are associated with improvements in glucose homeostasis,91 postprandial bile acids are reduced 1 month but increased 2 years after RYGB in humans.97 An interesting possibility is that it is not necessarily true that bariatric surgery increases bile acids, but rather that obesity reduces them and surgery returns the levels to normal. In support of this, morbidly obese patients have been found to have reduced postprandial increases in bile acids, and levels were returned to that of lean control levels in patients with RYGB.76,98
The impact of bariatric surgery on bile acids is, at least qualitatively, similar across species. A recent study found that total circulating and primary bile acids were increased after RYGB in humans, pigs, and rats, but there were some differences in the degree of increases in certain species of secondary and conjugated bile acids.99 Specifically, while there generally were increases in glycine-conjugated bile acids, the degree of the increase and the specific bile acid that changed varied between the species, while no species demonstrated changes in taurine-conjugated bile acids except very early after surgery. In humans 1 month postoperatively, there were significant increases in ursodeoxycholic acid and its glycine and taurine conjugates, suggesting that early alterations were via bacterial processing.100 In contrast, later postoperative increases were due to primary unconjugated bile acids as well as deoxycholic acid and its glycine conjugate. Similar to RYGB, ileal interposition in high fat–fed rodents, a surgery where a distal section of the ileum (where the majority of bile acid resorption occurs) is resected and repositioned within the proximal jejunum, resulted in increased total plasma bile acids and decreased bile acid excretion compared with sham-operated controls.101–103 This surgery also resulted in some weight loss (although not to the extent seen with RYGB or VSG) and improvements in glucose tolerance, hepatic triglycerides, and cholesterol levels, despite the fact that patients were maintained on a high-fat diet after surgery.103 Notably, reorganization of the intestine is not necessary for surgically induced changes in bile acids. VSG also increased circulating primary and taurine-conjugated bile acids in rodents13 and postprandial104 but not fasting105 total bile acids in humans, and, in humans and rodents, simply surgically diverting bile to the distal intestine is sufficient to cause an increase in circulating bile acids, weight loss, and improvements in glucose and lipid homeostasis.106,107
As signaling molecules, bile acids activate two different types of receptors. One is a nuclear transcription factor called farnesoid X receptor (FXR), which is highly expressed in the intestine, liver, adipose tissue, pancreas, and adrenal gland.90,108 Once activated by bile acids, FXR drives secretion of fibroblast growth factor 19 (FGF19) in humans (or FGF15 in rodents)109 (Fig. 3). FGF19/15, in turn, enters the circulation and provides negative feedback to inhibit bile acid synthesis in the liver, but also functions to regulate carbohydrate, lipid, and energy metabolism.110 For example, exogenous administration of FGF19 to dietary-induced obese or leptin-deficient mice causes weight loss, at least in part by increasing energy expenditure, and also improves glucose homeostasis.111
FGF19 is significantly increased after RYGB and VSG in rodents and human patients.107,112,113 In addition, FXR signaling is necessary for surgical outcome. For example, while VSG induces an initial reduction in food intake and body weight, Fxr KO mice regained all of the lost weight and body fat compared to sham-operated Fxr KO mice.112 Additionally, Fxr KO mice did not exhibit the known VSG-induced metabolic improvements in fasting blood glucose levels and glucose tolerance. Further research is required to distinguish among the impacts of the various populations of FXR receptors on surgical outcome.
Bile acids also activate a cell-surface G protein–coupled receptor called TGR5 (also GPBAR1 (Fig. 3)). These receptors are mainly expressed in the gall bladder, ileum, colon, brown and white adipose tissue, and to a lesser extent in skeletal muscle, liver, and immune cells.114 Bile acids act on TGR5 receptors to increase plasma GLP-1 and also to regulate glucose and lipid metabolism.115 Activation of TGR5 by the agonist oleanolic acid attenuated dietary-induced obesity and improved insulin resistance in mice.116 While FXR is necessary for both weight loss and improvements in glucose homeostasis after bariatric surgery, TGR5 plays only a partial role. Tgr5 KO mice lost a similar amount of weight after VSG to their wild-type counterparts, indicating that this receptor is not necessary for weight loss after VSG.117 However, after VSG, the Tgr5 KO mice had slightly reduced improvements in glucose tolerance and fasting blood glucose compared with the degree of improvement seen in the wild-type mice.
Bile acids show clear increases after both RYGB and VSG, a similar finding across species. While we know that bile acids activate both FXR and TGR5, it seems that activation of FXR is a more important overall signaling mechanism underlying the success of bariatric surgery. Thus, targeting FXR signaling is an important avenue for drug development to treat both obesity and T2DM.
Changes in the microbiome
Recent work has explored the importance of the intestinal microbiome in metabolism. The microbiome houses approximately three trillion bacteria and has been shown to regulate host metabolism.118 The microbiome has been shown to alter susceptibility to both obesity and T2DM,119–121 but obesity itself has also been found to alter the microbiome by reducing microbial diversity and bacterial gene richness.118,122
Bariatric surgeries manipulate the intestinal environment and also cause weight loss, and therefore have the potential to either directly or indirectly (through weight loss) influence the balance of bacterial composition and diversity within the gut.123 One of the first human studies to support this idea demonstrated that fecal microbiota (an index of the intestinal population) was distinct among normal-weight and obese patients and after RYGB.124 These authors identified a phylum-level compositional shift in the microbiome, with a relative decrease in the abundance of Firmicutes and an increase in the abundance of Gammaproteobacteria in RYGB versus both normal-weight and obese individuals. Additionally, obese and normal-weight individuals had distinct microbiomes. Unfortunately, the interpretation of the direct or indirect impact of RYGB on the microbiome may be skewed by the cross-sectional nature of the study. Subsequent studies have found a temporal shift in the microbiome with an increase in the phylum Proteobacteria by 3 months, and this was maintained at 6 months postsurgery compared with pre-RYGB.125,126 A similar shift happened 3 months after RYGB in patients who had T2DM at the time of surgery.127 The importance of these microbial shifts is highlighted in a study where a fecal transplant from humans after RYGB or vertical-banded gastroplasty to mice was found to restrain adiposity.128 While all of these data indicate that bariatric surgery is conducive to changes in the gut microbiome, they do not clarify whether this is due to a direct effect of the operation or whether it is indirect via surgery-induced weight loss, the reduced caloric intake, or even changes in macronutrient content of the diet, all factors shown to induce changes in the microbiome.129,130
All of these factors are easier to control for in preclinical studies where animals are matched for body mass or fat before surgery and experimental groups include a pair-fed or weight-matched sham-surgery control group. Still, these data are consistent with human studies in that rodents also demonstrate a shift in the microbiome after RYGB.131–133 In addition, fecal transplants from RYGB-treated mice to germ-free mice induced weight loss, while lean chow–fed mice administered fecal transplants from sham-surgery obese mice gained weight.131 Another study found that the change in the microbiome was conserved across species (humans, rats, mice), with an increase in the Gammaproteobacteria and verrucomicrobia, an effect that was independent of weight loss.131 As these animals were matched for body weight before surgery and were all maintained on the same diet, these data are an important indication that the impact of surgery on the microbiome is independent of weight loss. While most human studies have focused on changes in the microbiome after RYGB, we have also observed a shift in the microbiome after VSG.112
One way the microbiome could alter metabolism is through generation of metabolic by-products. Short-chain fatty acids, such as butyrate, are fermented by gut bacteria, and recent evidence suggests an association between butyrate-producing bacteria and the beneficial effects on metabolism in both mice and humans.134,135 In mice, oral administration of butyrate has been shown to improve insulin sensitivity and increase energy expenditure.134 Furthermore, diabetic mice colonized with feces from RYGB mice demonstrated improvements in glucose and lipid metabolism, and this was associated with increases in butyrate-producing bacteria.131
Several aspects of bariatric surgery, independent of weight loss, could induce a shift in microbial composition. For example, changes in the pH (as the number of acid-secreting cells in the stomach is reduced), changes in bile acid composition, alterations in nutrient handling and sensing, and changes in GI motility are all factors that could influence the microbiome. Because the intestinal microbiota plays an integral role in generating secondary bile acids, which are increased with surgery (discussed above), bile acid FXR signaling is an intriguing molecular link between the microbiome and host metabolism.136 While FXR deficiency blocks the VSG-induced reductions in body weight and improvements in glucose tolerance, it also blunts the ability of VSG to reduce Bacteroides and increase Roseburia.112 Altogether, these data suggest a microbiome–bile acid–FXR axis that is essential to the success of bariatric surgery.
Intestinal morphology
The intestinal mucosa is a highly plastic system where the turnover of human epithelial cells occurs every 3–5 days.137 The rapid cellular turnover is regulated by signaling pathways that dictate the rates of proliferation and atrophy. Changes in the balance of proliferation to atrophy lead to changes in overall mucosal mass138 and can involve changes in villus height, crypt depth, mucosal surface area, and/or intestinal weight.139
In rodents who receive operations where intestinal resection is performed, proliferation of the remaining tissue is very evident and likely occurs to create more absorptive cells for macro- and micronutrients.138 Resection of the majority of the proximal bowel in rats increases glucose uptake in the ileum and is associated with an increase in villus height and intestinal length rather than increased gene expression of glucose transporters.140 Thus, functional removal of one portion of the GI tract causes a compensatory response in the remaining tissue. Intestinal proliferation has been found in multiple studies after RYGB, where increases in bowel width, villus height, crypt depth, and overall cell proliferation141,142 in the alimentary and common intestinal limbs and an increase in bowel width within the biliopancreatic limb142 are observed in rats and mice. Other bariatric surgeries in rodents involving intestinal manipulation, such as duodenal jejunal bypass, placement of an duodenal endoluminal sleeve, and ileal interposition, also demonstrate intestinal hyperplasia.103,143,144 Of note, the degree of changes in intestinal proliferation in humans after bariatric surgery remains unknown.
Surgically induced cell proliferation requires surgical manipulation of the intestine, as VSG does not affect intestinal morphology in rats and mice.145,146 Given that RYGB and VSG often drive similar metabolic improvements, these data could indicate that changes in intestinal morphology are not necessary for surgical outcome. Recent data suggest that, although general intestinal cell proliferation does not occur, there are specific increases in GLP-1–secreting cells after VSG in mice.145 It is also possible that there is an increase in the nutrient-sensing machinery that drives GLP-1 secretion.
Recent data demonstrate that intestinal proliferation with diet-induced obesity is due to increased stem cell differentiation in mice.147 Thus, if both obesity and bariatric surgery drive intestinal proliferation, it is intriguing to consider that each circumstance drives a differential stem cell niche, one for which there are negative consequences (obesity-induced cancer) and one for which there are positive consequences (weight loss, improved metabolism).
Conclusions
We believe that weight loss–independent and weight loss–dependent mechanisms are both crucial to cause sustained remission in T2DM after surgery. Understanding the weight loss–independent mechanisms is important for a basic understanding of the pathology of the disease. Ultimately, the goal is to find simpler strategies, either surgical or pharmacological, that could be more widely implemented to treat the enormity of the health crisis caused by obesity and T2DM.
While the increase in gut peptides, especially GLP-1, has been implicated as a mechanism for the success of surgery, other widespread physiological effects, including changes in bile acids and in the microbiome, seem to be more robust when pooling insights obtained from both clinical and preclinical data. Regardless, a question in need of continual pursuit is whether these widespread changes simply represent the physiological adaptation of the GI tract in response to anatomical rearrangement or whether these changes act in concert or independently to drive the postoperative weight loss and/or improvements in glucose homeostasis.
Acknowledgments
The work of the laboratory is supported in part by National Institutes of Health Award DK082480 (D.A.S.) and by research funding from Ethicon Endo-Surgery, Inc. and Novo Nordisk A/S.
Conflicts of interest
Darleen Sandoval received research funding from Ethicon Endo-Surgery, Inc. and Novo Nordisk A/S. and Sanofi.
References
- 1.Makary MA et al. 2010. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery. Arch. Surg 145: 726–731. [DOI] [PubMed] [Google Scholar]
- 2.Sjöström L et al. 2007. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med 357: 741–752. [DOI] [PubMed] [Google Scholar]
- 3.Adams TD et al. 2007. Long-term mortality after gastric bypass surgery. N. Engl. J. Med 357: 753–761. [DOI] [PubMed] [Google Scholar]
- 4.Pontiroli AE & Morabito A. 2011. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann. Surg 253: 484–487. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafian H et al. 2011. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer 117: 1788–1799. [DOI] [PubMed] [Google Scholar]
- 6.Sjöström L et al. 2014. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 311: 2297–2304. [DOI] [PubMed] [Google Scholar]
- 7.Rubino F, Schauer PR, Kaplan LM & Cummings DE. 2010. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu. Rev. Med 61: 393–411. [DOI] [PubMed] [Google Scholar]
- 8.Stefater MA, Wilson-Pérez HE, Chambers AP, et al. 2012. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr. Rev 33: 595–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeley RJ, Chambers AP & Sandoval DA. 2015. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab. 21: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidal J, Jiménez A, de Hollanda A, et al. 2015. Metabolic surgery in type 2 diabetes: Roux-en-Y gastric bypass or sleeve gastrectomy as procedure of choice? Curr. Atheroscler. Rep 17: 58. [DOI] [PubMed] [Google Scholar]
- 11.Chambers AP et al. 2011. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calanna S et al. 2013. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia 56: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myronovych A et al. 2013. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 22: 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pournaras DJ et al. 2012. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 153: 3613–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mumphrey MB, Patterson LM, Zheng H & Berthoud H-R. 2013. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol. Motil 25: e70–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutledge R 2001. The mini-gastric bypass: experience with the first 1,274 cases. Obes. Surg 11: 276–280. [DOI] [PubMed] [Google Scholar]
- 17.Lee DY et al. 2012. Outcomes of laparoscopic Roux-en-Y gastric bypass versus laparoscopic adjustable gastric banding in adolescents. Obes. Surg 22: 1859–1864. [DOI] [PubMed] [Google Scholar]
- 18.Rubino F, Schauer PR, Kaplan LM & Cummings DE. 2010. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu. Rev. Med 61: 393–411. [DOI] [PubMed] [Google Scholar]
- 19.Chang S-H et al. 2014. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 149: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schauer PR et al. 2014. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N. Engl. J. Med 370: 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mingrone G et al. 2012. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med 366: 1577–1585. [DOI] [PubMed] [Google Scholar]
- 22.Buchwald H et al. 2004. Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724–1737. [DOI] [PubMed] [Google Scholar]
- 23.Schauer PR et al. 2012. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med 366: 1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camastra S et al. 2011. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 54: 2093–2102. [DOI] [PubMed] [Google Scholar]
- 25.Kashyap SR et al. 2010. Acute effects of gastric bypass versus gastric restrictive surgery on β-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int. J. Obes. (Lond.) 34: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickremesekera K, Miller G, Naotunne TD, et al. 2005. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes. Surg 15: 474–481. [DOI] [PubMed] [Google Scholar]
- 27.Albers PH et al. 2015. Enhanced insulin signaling in human skeletal muscle and adipose tissue following gastric bypass surgery. Am. J. Physiol. Regul. Integr. Comp. Physiol 309: R510–R524. [DOI] [PubMed] [Google Scholar]
- 28.Isbell JM et al. 2010. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 33: 1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos GM et al. 2010. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J. Gastrointest. Surg 14: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrannini E 2010. The stunned beta cell: a brief history. Cell Metab. 11: 349–352. [DOI] [PubMed] [Google Scholar]
- 31.de Weijer BA et al. 2013. Hepatic and peripheral insulin sensitivity do not improve 2 weeks after bariatric surgery. Obesity (Silver Spring) 21: 1143–1147. [DOI] [PubMed] [Google Scholar]
- 32.Dunn JP et al. 2012. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care 35: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bojsen-Møller KN et al. 2014. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 63: 1725–1737. [DOI] [PubMed] [Google Scholar]
- 34.Bradley D et al. 2014. Matched weight loss induced by sleeve gastrectomy or gastric bypass similarly improves metabolic function in obese subjects. Obesity (Silver Spring) 22: 2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christiansen MP, Linfoot PA, Neese RA & Hellerstein MK. 2000. Effect of dietary energy restriction on glucose production and substrate utilization in type 2 diabetes. Diabetes 49: 1691–1699. [DOI] [PubMed] [Google Scholar]
- 36.Yan Y et al. 2016. Roux-en-Y gastric bypass surgery suppresses hepatic gluconeogenesis and increases intestinal gluconeogenesis in a T2DM rat model. Obes. Surg 1–8. [DOI] [PubMed] [Google Scholar]
- 37.Chambers AP et al. 2014. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am. J. Physiol. Endocrinol. Metab 306: E424–E432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melissas J et al. 2007. Sleeve gastrectomy—a restrictive procedure? Obes. Surg 17: 57. [DOI] [PubMed] [Google Scholar]
- 39.Laferrere B et al. 2008. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab 93: 2479–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laferrère B et al. 2011. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl. Med 3: 80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plum L et al. 2011. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring) 19: 2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutia R et al. 2014. Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes 63: 1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregor MF et al. 2009. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sjöhölm K, Sjöström E, Carlsson LMS & Peltonen M. 2015. Weight change-adjusted effects of gastric bypass surgery on glucose metabolism: two- and 10-year results from the Swedish Obese Subjects (SOS) study. Diabetes Care 39: 625–631. [DOI] [PubMed] [Google Scholar]
- 45.Sheppard CE et al. 2013. The economic impact of weight regain. Gastroenterol. Res. Pract 2013: 379564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kojima M et al. 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660. [DOI] [PubMed] [Google Scholar]
- 47.Tschöp M, Smiley DL & Heiman ML. 2000. Ghrelin induces adiposity in rodents. Nature 407: 908–913. [DOI] [PubMed] [Google Scholar]
- 48.Cone JJ, McCutcheon JE & Roitman MF. 2014. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J. Neurosci 34: 4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wren AM et al. 2001. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab 86: 5992. [DOI] [PubMed] [Google Scholar]
- 50.Heppner KM et al. 2014. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes 63: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reimer MK, Pacini G & Ahrén B. 2003. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 144: 916–921. [DOI] [PubMed] [Google Scholar]
- 52.Tong J et al. 2010. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59: 2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vestergaard ET et al. 2008. Acute effects of ghrelin administration on glucose and lipid metabolism. J. Clin. Endocrinol. Metab 93: 438–444. [DOI] [PubMed] [Google Scholar]
- 54.Karamanakos SN, Vagenas K, Kalfarentzos F & Alexandrides TK. 2008. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann. Surg 247: 401–407. [DOI] [PubMed] [Google Scholar]
- 55.Bohdjalian A et al. 2010. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes. Surg 20: 535–540. [DOI] [PubMed] [Google Scholar]
- 56.Tymitz K, Engel A, McDonough S, et al. 2011. Changes in ghrelin levels following bariatric surgery: review of the literature. Obes. Surg 21: 125–130. [DOI] [PubMed] [Google Scholar]
- 57.Cummings DE et al. 2002. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med 346: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 58.Korner J et al. 2005. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J. Clin. Endocrinol. Metab 90: 359–365. [DOI] [PubMed] [Google Scholar]
- 59.Faraj M et al. 2003. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J. Clin. Endocrinol. Metab 88: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 60.Chambers AP et al. 2013. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 144: 50–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nosso G et al. 2016. Comparative effects of Roux-en-Ygastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm. Metab. Res 48: 312–317. [DOI] [PubMed] [Google Scholar]
- 62.Sandoval DA & D’Alessio DA. 2015. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol. Rev 95: 513–548. [DOI] [PubMed] [Google Scholar]
- 63.Vilsbøll T, Christensen M, Junker AE, et al. 2012. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344: d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jimenéz A et al. 2014. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes 63: 3372–3377. [DOI] [PubMed] [Google Scholar]
- 65.Jimenéz A, Casamitjana R, Viaplana-Masclans J, et al. 2013. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care 36: 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Umeda LM et al. 2011. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes. Surg 21: 896–901. [DOI] [PubMed] [Google Scholar]
- 67.Peterli R et al. 2009. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann. Surg 250: 234–241. [DOI] [PubMed] [Google Scholar]
- 68.Salehi M, Gastaldelli A & D’Alessio DA. 2014. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 146: 669–680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shah M et al. 2014. Contributionofendogenousglucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes 63: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salehi M, Prigeon RL & D’Alessio DA. 2011. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 60: 2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sandoval DA, Bagnol D, Woods SC, et al. 2008. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57: 2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith EP et al. 2014. The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab. 19: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye J et al. 2014. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am. J. Physiol. Regul. Integr. Comp. Physiol 306: R352–R362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson-Pérez HE et al. 2013. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide-1 receptor deficiency. Diabetes 62: 2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mokadem M, Zechner JF, Margolskee RF, et al. 2014. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol. Metab 3: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peterli R et al. 2012. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes. Surg 22: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobsen SH et al. 2012. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes. Surg 22: 1084–1096. [DOI] [PubMed] [Google Scholar]
- 78.Romero F et al. 2012. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg. Endosc 26: 2231–2239. [DOI] [PubMed] [Google Scholar]
- 79.Lee CJ et al. 2013. Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia. J. Clin. Endocrinol. Metab 98: E1208–E1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dimitriadis E et al. 2013. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann. Surg 257: 647–654. [DOI] [PubMed] [Google Scholar]
- 81.le Roux CW et al. 2007. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg 246: 780–785. [DOI] [PubMed] [Google Scholar]
- 82.Korner J et al. 2009. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int. J. Obes 33: 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romero F et al. 2012. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg. Endosc 26: 2231–2239. [DOI] [PubMed] [Google Scholar]
- 84.Rodgers RJ, Tschöp MH & Wilding JPH. 2012. Anti-obesity drugs: past, present and future. Dis. Model. Mech 5: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alphonse PAS & Jones PJH. 2016. Revisiting human cholesterol synthesis and absorption: the reciprocity paradigm and its key regulators. Lipids 51: 519–536. [DOI] [PubMed] [Google Scholar]
- 86.Porez G, Prawitt J, Gross B & Staels B. 2012. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J. Lipid Res 53: 1723–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Katsuma S, Hirasawa A & Tsujimoto G. 2005. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun 329: 386–390. [DOI] [PubMed] [Google Scholar]
- 88.Cariou B et al. 2006. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J. Biol. Chem 281: 11039–11049. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe M et al. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489. [DOI] [PubMed] [Google Scholar]
- 90.Lefebvre P, Cariou B, Lien F, et al. 2009. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev 89: 147–191. [DOI] [PubMed] [Google Scholar]
- 91.Haeusler RA, Astiarraga B, Camastra S, et al. 2013. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes 62: 4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nyhlin H, Brydon G, Danielsson A & Eriksson F. 1990. Bile acid malabsorption after intestinal bypass surgery for obesity. A comparison between jejunoileal shunt and biliointestinal bypass. Int. J. Obes 14: 47–55. [PubMed] [Google Scholar]
- 93.Patti ME et al. 2009. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 17: 1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kohli R et al. 2013. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J. Clin. Endocrinol. Metab 98: E708–E712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakatani H et al. 2009. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 58: 1400–1407. [DOI] [PubMed] [Google Scholar]
- 96.De Giorgi S et al. 2015. Long-term effects of Roux-en-Y gastric bypass on postprandial plasma lipid and bile acids kinetics in female non diabetic subjects: a cross-sectional pilot study. Clin. Nutr 34: 911–917. [DOI] [PubMed] [Google Scholar]
- 97.Dutia R et al. 2015. Temporalchangesinbileacidlevelsand 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int. J. Obes. (Lond.) 39: 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahmad NN, Pfalzer A & Kaplan LM. 2013. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int. J. Obes. (Lond.) 37: 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spinelli V et al. 2016. Influence of Roux-en-Y gastric bypass on plasma bile acid profiles: a comparative study between rats, pigs and humans. Int. J. Obes. (Lond.) 40: 1260–1267. [DOI] [PubMed] [Google Scholar]
- 100.Albaugh VL et al. 2015. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J. Clin. Endocrinol. Metab 100: E1225–E1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Strader AD, Clausen TR, Goodin SZ & Wendt D. 2009. Ileal interposition improves glucose tolerance in low dose streptozotocin-treated diabetic and euglycemic rats. Obes. Surg 19: 96–104. [DOI] [PubMed] [Google Scholar]
- 102.Strader AD et al. 2005. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am. J. Physiol. Endocrinol. Metab 288: E447–E453. [DOI] [PubMed] [Google Scholar]
- 103.Kohli R et al. 2010. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am. J. Physiol. Gastrointest. Liver Physiol 299: G652–G660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steinert RE et al. 2013. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring) 21: E660–E668. [DOI] [PubMed] [Google Scholar]
- 105.Haluźıková D et al. 2013. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity (Silver Spring) 21: 1335–1342. [DOI] [PubMed] [Google Scholar]
- 106.Flynn CR et al. 2015. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat. Commun 6: 7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferrannini E et al. 2015. Increased bile acid synthesis and deconjugation after biliopancreatic diversion. Diabetes 64: 3377–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mi L-Z et al. 2003. Structural basis for bile acid binding and activation of the nuclear receptor FXR. Mol. Cell 11: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 109.Cicione C, Degirolamo C & Moschetta A. 2012. Emerging role of fibroblast growth factors 15/19 and 21 as metabolic integrators in the liver. Hepatology 56: 2404–2411. [DOI] [PubMed] [Google Scholar]
- 110.Beenken A & Mohammadi M. 2009. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov 8: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fu L et al. 2004. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145: 2594–2603. [DOI] [PubMed] [Google Scholar]
- 112.Ryan KK et al. 2014. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Escalona A et al. 2016. Bile acid synthesis decreases after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis 12: 763–769. [DOI] [PubMed] [Google Scholar]
- 114.Thomas C, Auwerx J & Schoonjans K. 2008. Bile acids and the membrane bile acid receptor TGR5–connecting nutrition and metabolism. Thyroid 18: 167–174. [DOI] [PubMed] [Google Scholar]
- 115.Thomas C et al. 2009. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sato H et al. 2007. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun 362: 793–798. [DOI] [PubMed] [Google Scholar]
- 117.McGavigan AK et al. 2015. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. doi: 10.1136/gutjnl-2015-309871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ley RE et al. 2005. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U.S.A 102: 11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tremaroli V & Bäckhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249. [DOI] [PubMed] [Google Scholar]
- 120.Karlsson F, Tremaroli V, Nielsen J & Bäckhed F. 2013. Assessing the human gut microbiota in metabolic diseases. Diabetes 62: 3341–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sommer F & Bäckhed F. 2013. The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol 11: 227–238. [DOI] [PubMed] [Google Scholar]
- 122.Turnbaugh PJ et al. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 123.Aron-Wisnewsky J & Clement K. 2014. The effects of gastrointestinal surgery on gut microbiota: potential contribution to improved insulin sensitivity. Curr. Atheroscler. Rep 16: 454. [DOI] [PubMed] [Google Scholar]
- 124.Zhang H et al. 2009. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. U.S.A 106: 2365–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kong L-C et al. 2013. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr 98: 16–24. [DOI] [PubMed] [Google Scholar]
- 126.Furet J-P et al. 2010. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59: 3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Graessler J et al. 2013. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 13: 514–522. [DOI] [PubMed] [Google Scholar]
- 128.Tremaroli V et al. 2015. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 22: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ley RE, Turnbaugh PJ, Klein S & Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 130.Schwiertz A et al. 2010. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18: 190–195. [DOI] [PubMed] [Google Scholar]
- 131.Liou AP et al. 2013. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med 5: 178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li JV et al. 2011. Metabolic surgery profoundly influences gut microbial–host metabolic cross-talk. Gut 60: 1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li JV et al. 2011. Experimental bariatric surgery in rats generates a cytotoxic chemical environment in the gut contents. Front. Microbiol 2: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gao Z et al. 2009. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vrieze A et al. 2012. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143: 913–916.e7. [DOI] [PubMed] [Google Scholar]
- 136.Sayin SI et al. 2013. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17: 225–235. [DOI] [PubMed] [Google Scholar]
- 137.Groos S, Hünefeld G & Luciano L. 2001. Epithelial cell turnover–extracellular matrix relationship in the small intestine of human adults. Ital. J. Anat. Embryol 106: 353–361. [PubMed] [Google Scholar]
- 138.Shaw D, Gohil K & Basson MD. 2012. Intestinal mucosal atrophy and adaptation. World J. Gastroenterol 18: 6357–6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Drozdowski LA, Clandinin MT & Thomson ABR. 2009. Morphological, kinetic, membrane biochemical and genetic aspects of intestinal enteroplasticity. World J. Gastroenterol 15: 774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iqbal CW, Qandeel HG, Zheng Y, et al. 2008. Mechanisms of ileal adaptation for glucose absorption after proximal-based small bowel resection. J. Gastrointest. Surg 12: 1854–1864; discussion 1864–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.le Roux CW et al. 2010. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann. Surg 252: 50–56. [DOI] [PubMed] [Google Scholar]
- 142.Taqi E et al. 2010. The influence of nutrients, biliary–pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J. Pediatr. Surg 45: 987–995. [DOI] [PubMed] [Google Scholar]
- 143.Li B, Lu Y, Srikant CB, et al. 2013. Intestinal adaptation and Reg gene expression induced by antidiabetic duodenal–jejunal bypass surgery in Zucker fatty rats. Am. J. Physiol. Gastrointest. Liver Physiol 304: G635–G645. [DOI] [PubMed] [Google Scholar]
- 144.Habegger KM et al. 2013. Duodenal nutrient exclusion improves metabolic syndrome and stimulates villus hyperplasia. Gut 63: 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cavin J-B et al. 2015. Differences in alimentary glucose absorption and intestinal disposal of blood glucose following Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology 150: 454–464.e9. [DOI] [PubMed] [Google Scholar]
- 146.Mumphrey MB, Hao Z, Townsend RL, et al. 2015. Sleeve gastrectomy does not cause hypertrophy and reprogramming of intestinal glucose metabolism in rats. Obes. Surg 25: 1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beyaz S et al. 2016. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


