Abstract
The emergence of new SARS-CoV-2 variants continues to pose an enormous public health concern. The SARS-CoV-2 infection disrupted host immune response accounting for cytokine storm has been linked to multiorgan failure and mortality in a significant portion of positive cases. Abruptly activated macrophages have been identified as the key pathogenic determinant of cytokine storm in COVID-19. Besides, reactive microglia have been known to discharge a surplus amount of proinflammatory factors leading to neuropathogenic events in the brains of SARS-CoV-2 infected individuals. Considering the fact, depletion of activated macrophages and microglia could be proposed to eradicate the life-threatening cytokine storm in COVID-19. Clodronate, a non-nitrogenous bisphosphonate drug has been identified as a potent macrophage and microglial depleting agent. While recent advancement in the field of liposome encapsulation technology offers the most promising biological tool for drug delivery, liposome encapsulated clodronate has been reported to effectively target and induce prominent phagocytic cell death in activated macrophages and microglia compared to free clodronate molecules. Thus, in this review article, we emphasize that depletion of activated macrophages and microglial cells by administration of liposome encapsulated clodronate can be a potential therapeutic strategy to diminish the pathogenic cytokine storm and alleviate multiorgan failure in COVID-19. Moreover, recently developed COVID-19 vaccines appear to render the chronic activation of macrophages accounting for immunological dysregulation in some cases. Therefore, the use of liposome encapsulated clodronate can also be extended to the clinical management of unforeseen immunogenic reactions resulting from activated macrophages associated adverse effects of COVID-19 vaccines.
Keywords: COVID-19, Cytokine storm, Inflammation, Macrophage, Microglia, Liposomal clodronate
1. Introduction
The ongoing outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) instigating the comorbid coronavirus disease 2019 (COVID-19) has become one of the leading causes of death worldwide within a short span of time [1]. Though different types of vaccines have been rapidly developed to attenuate the existing forms of SARS-CoV-2, the emergence of new viral variants represents a huge challenge to the health care system worldwide [2], [3], [4], [5]. Thus, the establishment of innovative therapeutic strategies and repurposing of available pharmacological agents against the innumerable pathogenic consequences of SARS-CoV-2 remains crucial [6], [7]. Notably, severe COVID-19 cases have been characterized by an overwhelming surge of cytokine levels in the circulation and in highly susceptible organs including the lungs and brain. Of note, the secretome of activated macrophages, mast cells, endothelial, epithelial cells and microglia appears to be the underlying basis of cytokine storm in COVID-19 [6], [7], [8], [9], [10]. Among various immunogenic cells, abruptly activated macrophages have been identified as a key pathogenic determinant of destructive pathogenic events in various organs including the lungs in COVID-19 [11], [12]. Besides, SARS-CoV-2 mediated activation of microglia leads to neuroinflammation and destructive neuropathological events in the brain [13]. As a result, a significant portion of COVID-19 patients exhibit comorbid clinical complications like macrophage activation syndrome (MAS), acute respiratory distress syndrome (ARDS), destructive neurological deficits, neuroregenerative failure and dementia [8], [7], [14]. Considering the aforementioned facts, elimination of the cellular basis of cytokine storm could be a promising therapeutic strategy to combat COVID-19 [15], [16], [17]. Depletion of hyper-activated macrophages and microglia by a highly specific pharmacologic agent might provide a prompt and effective medical relief against COVID-19. Clodronate, a non-nitrogen-containing bisphosphonate has been recognized for its role in the depletion of phagocytic macrophages in cancer metastasis and bone diseases [18], [19], [20]. Besides, clodronate has also been known to diminish pathogenically activated microglia in the brain [21]. However, the use of free clodronate molecules in specifically targeting abruptly activated immune cells appears to be a highly challenging approach against COVID-19. The recent scientific advancement in the liposome encapsulation technology offers the most promising scientific tool for drug delivery. Notably, liposome encapsulated clodronate has been reported to effectively target and induce prominent phagocytic cell death in activated macrophages and microglial cells compared to free clodronate molecules. Thus, this review article describes the deleterious effects of activated macrophages and microglia mediated hyperinflammation in COVID-19 and describes conclusive experimental evidence to underpin the beneficial effects of liposome encapsulated clodronate in some human diseases. Further, this article strongly emphasizes the repurposing of liposome encapsulated clodronate and its therapeutic perspective in effectively mitigating cytokine storm through inducing phagocytosis in pathogenic macrophages and microglia in COVID-19.
2. An overview of the detrimental roles of activated macrophages and cytokine storm in COVID-19
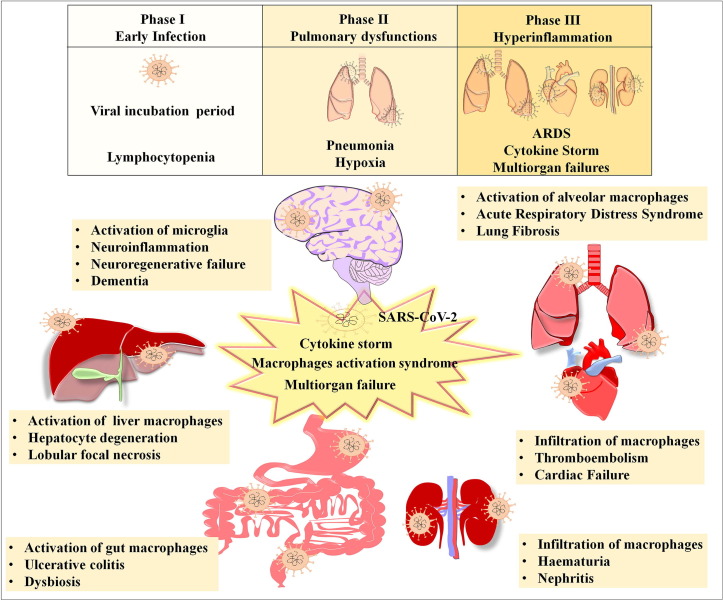
Upon infection, the progression of SARS-CoV-2 mediated clinical manifestation followed by the host immune responses over the period has been categorized into three pathological phases namely, 1) early infection phase, 2) pulmonary phase, and 3) severe hyperinflammation phase [22]. The early stage of COVID-19 has been characterized by the recruitment and spread of SARS-CoV-2 in the epithelial and blood cells via the cognate and non-cognate receptors accounting for pathogenic changes in haematological parameters including lymphocytopenia and haemostatic dysfunctions [23]. The second stage has been characterized by replication of SARS-CoV-2 and localized inflammation in the lungs, leading to pneumonia and hypoxia [22]. As the viral load increases in the hyperinflammatory phase, the cellular components of the immune-system are drastically activated and emit a surplus amount of proinflammatory molecules accounting for cytokine storm [24]. The hyperinflammatory phase has been characterized by fatal ARDS, and multi organ injury and failure. The lungs, liver, gastrointestinal system, heart, kidney and the brain are known to be highly vulnerable to SARS-CoV-2 mediated cytokine storm (Fig. 1 ).
Fig. 1.
Graphical representation of the key pathological hallmarks associated with activation of respective macrophages in various organs. The figure highlights SARS-CoV-2 mediated activation of macrophages, cytokine storm and clinical complications in lungs, liver, gastrointestinal tract, kidney and heart. Besides, the figure also indicates the SARS-CoV-2 mediated activation of microglial cells leading to neuroinflammation, regenerative failure in the brain and dementia.
While SARS-CoV-2 can directly infect the organs through receptors present on the host cells, multiorgan defects take place as a result of the activation of macrophage and uncontrollable immune response. Among various organs, the lungs are the primary direct target of SARS-CoV-2 mediated pathogenesis. The activated alveolar macrophages-mediated hyperinflammation in the lungs has been associated with pulmonary edema and fibrosis in critically ill COVID-19 patients [25]. Next, a considerable portion of COVID-19 positive cases show altered levels of liver enzymes, indicating the dysfunction of the hepatic system. It has been demonstrated that activation of the hepatic resident macrophage population and the invasion of pathogenic circulating macrophages leads to severe liver damage in COVID-19. Moreover, histopathological observation of liver autopsies from COVID‐19 victims indicated hepatic steatosis and prominent degeneration of hepatocytes [26]. Though COVID‐19 has primarily been characterized by respiratory syndrome, convincing clinical data have clearly established the association between COVID-19 and heart injury. As pre-existing cardiovascular diseases have been considered as the potential risk factor of COVID-19 related death, it has become increasingly evident that SARS-CoV-2 infection leads to arrhythmia, coronary defects, venous thromboembolism and myocardial failure in the subjects regardless of any history of heart diseases [27]. Thus, cardiac dysfunction has been recognized as a life-threatening clinical feature of COVID-19. Besides, COVID-19 appears to affect the gastrointestinal tract as a significant portion of subjects with COVID-19 display dysbiosis and ulcerative colitis [28]. As the severity of the disease progresses, COVID-19 escalates kidney injury with obvious signs of hematuria and nephritis due to inflammatory reactions in the glomerular compartments of the kidneys [29]. Further, neuroimaging and post-mortem brain studies have clearly indicated that SARS-CoV-2 infection leads to various neurological deficits and mental illnesses due to abrupt onset of neuroinflammation [8], [14]. As the unregulated activation of macrophages has been identified as a key cellular basis of hyperinflammation, the pathogenic events resulting from SARS-CoV-2 infection in various organs appears to be propagated via abnormally activated circulating or tissue resident macrophages. Recent reports revealed that activated microglia can interfere with neuroregenerative properties of the brain and induce neurocognitive impairments in COVID-19 [8], [7], [14] (Fig. 1).
Macrophages are the subset of myeloid cell lineage that differentiates from the bone marrow-derived monocytes in the blood. Macrophages are responsible for a wide range of immunological functions including antigen presentation, tissue remodeling and phagocytosis [30]. Macrophages are normally found in an inactivated state throughout the body as various cellular entities such as histiocytes in lymph nodes, Kupffer cells in the liver, Hofbauer cells in the placenta, alveolar macrophages in pulmonary alveoli, osteoclast in the bone, and microglia in the brain [31] (Fig. 1). The molecular stimulants released during the interaction between any sort of antigen and the host cells can provoke the activation of macrophages. The inflammasomes induced activation of the natural killer (NK) cells, T-cell mediated cytotoxicity and pyroptosis have been recognized as the major pathogenic events associated with activated macrophages [32]. Notably, the macrophages undergoing pyroptotic cell death specifically release large amounts of IL-1α, IL-1β, IL-18 and high mobility group box protein (HMGB)-1 [33]. Certain inflammatory cytokines that originated from macrophages, such as TNF-α and IL-1, can lead to thrombosis [34]. The macrophages that receive inflammatory signals recruit T-cells into the site of inflammation and feedback inflammatory signals from T-cells further activate macrophages, thereby leading to cytokine storm [35].
The SARS-CoV-2 infection causes activation of immune cells and elicits inflammatory responses in the host [36], [37]. The abnormally activated immune cells discharge an exceedingly large amount of immunoregulatory factors accounting for cytokine storm in COVID-19 patients [10], [38], [39], [40], [41]. Notably, cytokine release syndrome (CRS) in COVID-19 has been associated with abnormal levels of interferon (IFN)-γ, interferon γ-induced protein 10 kDa (IP-10), macrophage inflammatory protein (MIP)-1a, transforming growth factor (TGF)-β, tumor necrosis factor (TNF)-α, granulocyte colony-stimulating factor (G-CSF), and interleukins such as IL-2, IL-6, IL-7 and IL-10 [37], [42]. Among them, elevated levels of IL-6 have been known to exacerbate the pathogenic severity of COVID-19 through TNF-α mediated activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signalling [6], [43], [44]. Besides, an abnormal level of TGF-β has been identified to be involved in pulmonary fibrosis in COVID-19 [45], [25]. Moreover, prominent elevation of C-reactive protein (CRP), an inflammatory indicator associated with disease severity and fatality has also been reported in the serum samples of COVID-19 patients [46], [47]. In addition, the SARS-CoV-2 infection leads to the overproduction of inflammatory chemokines such as monocyte chemoattractant protein-1 (MCP-1) and the chemokine (C-X-C motif) ligand (CXCL)-1, 5, 10 in the circulation of COVID-19 patients [48]. The activated forms of myeloid cells appear to be the potential source of proinflammatory chemokines and cytokines in COVID-19 [37]. Among various immunogenic cells, the activated macrophages have been regarded as critical pathogenic determinants in COVID-19 as it induces tissue lesion through the abundant cytokine release [49]. The high level of inflammatory mediators like ILs and TNF, originating from the activated macrophages have been known to provoke NF-kB and mitogen-activated protein kinase (MAPK) signalling cascades in COVID-19 [50].
Besides, microglia, the resident macrophages of the brain, are known to be associated with the neuroimmune responses upon infection, injury and neurological illnesses. A “two-hit hypothesis” claims that COVID-19 patients exhibit neuroinflammation due to pre-activated microglia that were rendered by adverse experiences during their early life [51]. A cohort study by Schurink et al. reported the prominent signs for the activation of microglia with the formation of nodules in the post-mortem COVID-19 brains [52]. Thus, the establishment of treatment methods against the root cause of the hyperinflammatory condition has become highly mandatory for COVID-19.
3. Recapitulation of the key pharmacological agents proposed or attempted against COVID-19
Recently, many pharmacological agents have been examined and proposed to neutralize cytokine storm in COVID-19. Initially, convalescent plasma therapy (CPT) has been considered the most common therapy for COVID-19 [53]. However, CPT has been identified to have some risks of inducing allergic reactions, alveolar damage, and possible transmission of the human immunodeficiency virus (HIV) and hepatitis viruses (HBVs). Next, inhibitors of ACE-2 have also been considered as a preventive measure against COVID-19 as it blocks the SARS-CoV-2 entry into the host cells [54]. However, the use of ACE-2 inhibitors has been found to be associated with many adverse effects, interfering with key physiological functions [55]. As most of the inflammatory cytokines and their downstream signalling mediators have been linked to the activation of pathogenic NF-kB signalling, blockade of the NF-kB pathway using inhibitor of nuclear factor kappa-B kinase subunit (IKK)-β antagonists has been proposed to be effective against inflammation in COVID-19 [6]. IL-6-STAT3 blockers have also been proposed to ameliorate cytokine storm in COVID-19 [39]. High-dose methylprednisolone along with calcineurin inhibitors like cyclosporine, a T-cell blocking agent has been considered to neutralize the cytokine storm in COVID-19 [56], [57]. A few reports suggest the use of non-steroidal anti-inflammatory drugs (NSAIDs) to block pro-inflammatory responses mediated by cyclo-oxygenase (COX) and prostaglandins in COVID-19 [58]. The use of biosurfactants for their anti-inflammatory roles, cationic and antioxidant properties have been suggested for treatment against COVID-19 [59]. Previous studies revealed that glycyrrhizic derivatives possess antiviral effects against the SARS-CoV-2 and modify the inflammatory signatures in COVID-19 [60], [61]. The use of nutraceuticals for modulating the miRNAs responsible for cytokine storm has also been considered to manage COVID-19 [62]. Though there exists a number of proposed therapeutic strategies for COVID-19, further exprimental validation and clinical trials remain to be established. Though the above-mentioned drugs are somewhat prescribed to manage COVID-19, their specificity and efficacy are not concurrent and they are known to be associated with some adverse effects (Table 1 ). Therefore, identifying the highly specific drug that targets the ultimate cellular source of cytokine storm might be a valuable strategy to tackle COVID-19. Considering the facts, implementation of a drug that has the ability to specifically deplete the activated macrophages could be of potential therapeutic aid in COVID-19.
Table 1.
List of widely proposed or used drugs against COVID-19 with their advantages and disadvantages.
| No | Drugs | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| 1 | Cyclosporine | Immunosuppressive drug, decreases the disease severity in the majority of patients with systemic juvenile idiopathic arthritis (sJIA) associated MAS |
Neurotoxic and nephrotoxic effects, Kidney failure and hypertension |
[63] |
| 2 | Emapalumab | Human monoclonal antibody raised against interferon-γ, used in hemophagocytic lymphohistiocytosis and MAS |
Cause pyrexia, constipation and hypertension | [64] |
| 3 | Anakinra | An IL-1 receptor antagonist, commonly used against arthritis, used in combination with TCZ and corticosteroids to suppress the pro-inflammatory factors and MAS | Induces edema at the injection site and transaminase activity | [65] |
| 4 | Baricitinib along with remdesivir | A JAK-1/2 inhibitor, reduces serum levels of TNF-α, IL-1β, and IL-6 and plays a significant role in quick recovery from COVID-19 | Associated with upper respiratory tract infections, increased LDL, cholesterol, nausea and thrombocytosis |
[66] |
| 5 | Chloroquine and derivatives | Significantly decreases the production of pro-inflammatory factors and reduces disease severity in COVID-19 patients | Gastrointestinal discomfort, Ocular toxicity and cardiotoxicity |
[67] |
| 6 | Colchicine | Proposed to inhibit inflammasome signalling in COVID-19 | Gastrointestinal defects and Pulmonary embolism |
[68] |
| 7 | Etoposide | Potential candidate drug against MAS as it induces apoptosis in activated T cells and malignant tumor cells | Alopecia and gastrointestinal toxicity | [69] |
| 8 | Eculizumab | Immunosuppressant and improves survival and reduces hypoxia in randomized clinical trials | Meningococcal infection | [70] |
| 9 | Glycyrrhizic derivatives | Inhibit replication of SARS resulting in reduced pulmonary inflammation and microvascular permeability | Cardiac dysfunction edema, and hypertension |
[71] |
| 10 | Plasma exchange therapy | Improve survival rate and beneficial in treating cytokine storm associated secondary MAS |
Allergic reactions, alveolar damage and possible transmission of HIV and HBV | [72] |
| 11 | Rituximab | Anti-CD20 rituximab therapy has been proposed to attenuate MAS in COVID-19 | Induces hyper immune reactions | [73] |
| 12 | Tocilizumab | Proposed for treatment against COVID-19 as it reduces the circulating levels of CRP | Headache, hypertension and abnormal liver function | [74] |
4. Therapeutic significance of liposomal encapsulated clodronate in effective depletion of activated macrophages in various experimental conditions and diseases
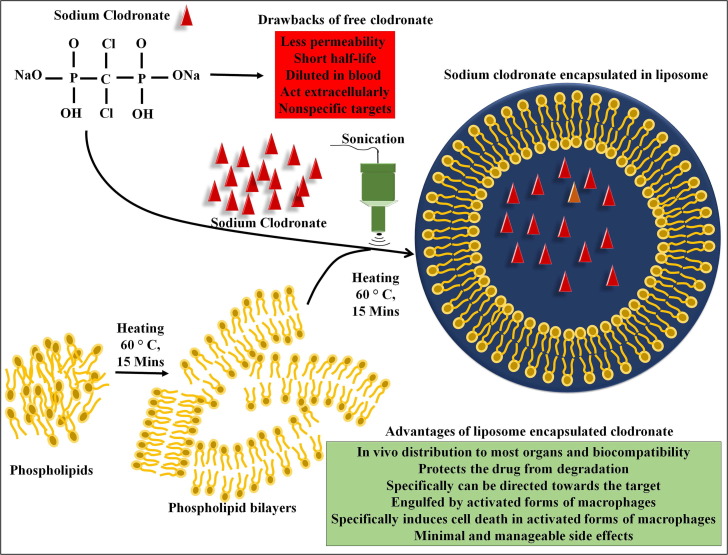
Clodronate has been proven to be a highly specific macrophage and microglia depleting drug [75], [76], [77]. Clodronate acts on the mineral surfaces of the bone and suppresses the osteoclast-mediated bone resorption [77]. Clodronate can enter into the macrophages through endocytosis and disrupt the mitochondrial production of adenosine triphosphate (ATP) leading to their apoptotic cell death [78], [79], [80]. Mönkkönen et al. demonstrated that upon the intake of clodronate by cells, intracellular accumulation of adenosine 5′ (b,g-dichloromethylene) triphosphate (AppCCl2p) inhibits adenine nucleotide translocator (ANT) and alters mitochondrial membrane potential, thereby initiating apoptotic signalling cascade [78], [81]. Clodronate has widely been used in the treatment of osteoporosis, osteoarthritis, myeloma and hypercalcaemia [75], [82]. In addition to its anti-bone resorptive capability, clodronate also exhibits anti-inflammatory and analgesic properties [83], [84]. Studies have shown that clodronate reduces the release of inflammatory cytokines such as TNF-α, IL-1b and IL-6 [85]. Clodronate-mediated depletion of macrophages appear to be effective in reducing the levels of proinflammatory cytokines [86], [87]. However, use of free clodronate molecules have some practical disadvantages due to their less permeability and short half-life. Alternatively, liposome-encapsulated clodronate has been identified to yield better results in targeting phagocytic macrophages. Initially, the conventional preparation of liposomes involve dissolving the phospholipid molecules into an appropriate organic solvent such as chloroform and methanol followed by evaporation of the organic solvent at 60 °C for 15 min to obtain thin lipid bilayer films. Further, incubation of a salt form of clodronate containing aqueous solution with lipid bilayer films at 60 °C for 15 min and simultaneous sonication can yield the encapsulation of clodronate in the liposomes. The non-encapsulated clodronate can be removed by centrifugation or filtration. In various in vitro and in vivo experiments, the effect of liposomal encapsulated clodronate has been tested against macrophages [18], [88] (Fig. 2 ). It has been well established that liposomes act as an efficient biological cargo of drugs and bioactive molecules. The liposome encapsulated drugs have several advantages including biocompatibility, self-assembly, and the ability to carry ample molecules. Given the wide range of physicochemical and biophysical properties of liposomes, they can be modified to acquire the desired characteristics [89]. Liposomal encapsulation protects the drug from degradation by the gastric acid, ensures the bioavailability of the effectors in the bloodstream and transportation to various organs, whereas ingestion of unbound small molecules can easily be destroyed by gastric acid and their half-life appears to be very minimal in the circulation [89]. Moreover, the systemic circulation of the free molecules may represent some nonspecific and undesirable effects. However, liposome encapsulation of drugs has many practical advantages as it can minimize the dilution of drugs in the circulation with longer half-life. Eventually, liposomal encapsulated drugs can specifically be directed towards or attracted by designated pathogenic cells and organs [90] (Fig. 2).
Fig. 2.
Schematic representation of steps involved in the preparation of liposomal clodronate. The figure provides an overview of experimental method for the generation of liposomal clodronate that includes the preparation of lipid bilayer films from phospholipids followed by incorporation of sodium chlodronates in liposomes with help of sonication treatment.
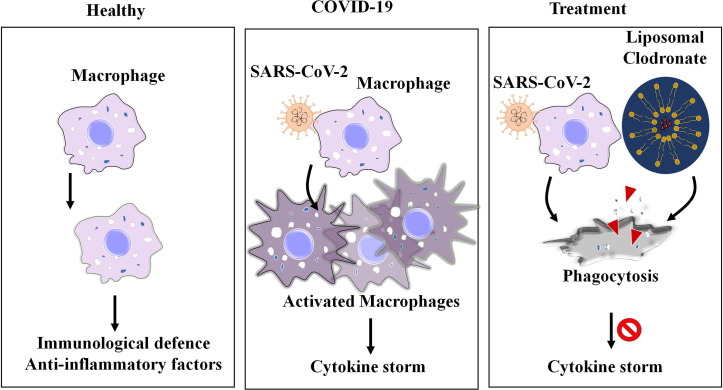
Earlier, Mönkkönen and Heath have reported that liposome-encapsulated clodronate is about 350-fold more effective in deactivating the macrophages than free clodronate molecules. While free clodronate appears to act on extracellular level, macrophages engulfs liposome-encapsulated clodronate thereby, committing to prominent phagocytic depletion [91] (Fig. 3 ). Moreover, Van Rooijen and Sanders described the macrophage ‘suicide’ technique, from which it has become apparent that liposome-encapsulated clodronate treatment specifically eleminates the phagocytic macrophages, while non-phagocytic cells are not vulnerable to liposome-encapsulated clodronate [92]. It has been reported that intravenous administration of 20 mg of clodronate encapsulated with unilamellar liposomes significantly reduced macrophages and diminished inflammation in an experimental rat model of arthritis, while treatment of free clodronate had no effect in targeting macrophages [93], [94]. The radioactive labelling-based animal experiments by Buiting have already demonstrated the in vivo distribution of clodronate in various organs including the liver and spleen [95]. Besides, the whole animal in vivo experiments described that the use of clodronate-liposome solution leads to efficient depletion of macrophages in the bone marrow, spleen, liver, lungs, brain, gut, peritoneal cavity, lymph nodes and in circulation thereby, suggesting liposome-encapsulated clodronate can spread to various organs in the body [96] (Fig. 2, Fig. 3, Fig. 4 ).
Fig. 3.
Graphical illustration for the clodronate liposomes mediated depletion of activated macrophages in COVID-19. The figure depicts the role of macrophages in healthy condition and SARS-CoV-2 mediated macrophage activation leading to cytokine storm, and depletion of activated macrophage by clodronate liposomes as a proposed treatment option to mitigate cytokine storm in COVID-19.
Fig. 4.
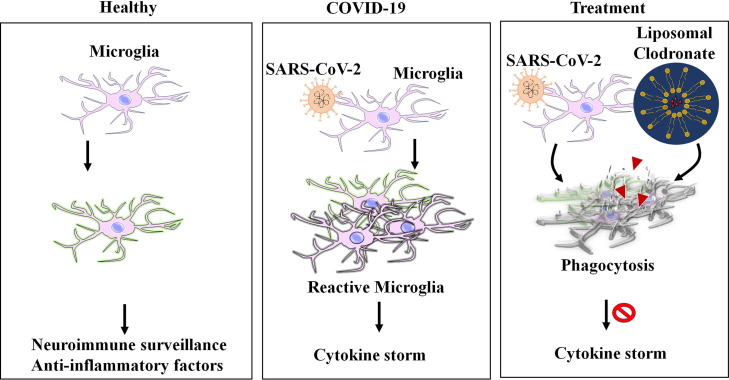
Graphical illustration for the clodronate liposomes mediated depletion of activated microglia in COVID-19. The figure depicts the function of the brain resident microglia in healthy condition, SARS-CoV-2 mediated activation of macrophages responsible for cytokine storm, and depletion of activated microglia by clodronate liposomes as a possible treatment option to eradicate cytokine storm in the brain of subjects with COVID-19.
Notably, pulmonary surfactant is a mixture of phospholipids and proteins secreted by the alveolar cells in the lungs. The pulmonary surfactants have high biocompatibility with liposomes that are used for encapsulation of drugs. Thus, formulated liposomes can efficiently be attracted and observed by the lungs via pulmonary surfactants. Ample reports indicated that liposomal inhalants represent emerging carriers in pulmonary drug delivery strategy and play an important role in treating various lung disorders including acute respiratory distress syndrome (ARDS). Elder et al. reported that intratracheal inhalation of liposomes containing clodronate efficiently depletes alveolar macrophages in experimental rats [97]. Recent data suggests the therapeutic effectiveness of liposome-encapsulated clodronate through intratracheal instillation against pulmonary fibrosis by inhibiting the macrophages in the lungs of LyzM-Cre/ Methyl-CpG–binding domain 2 lox/flox (Mbd2-CKO) mice [98].
Clodronate liposome treatment in a mouse model of colorectal cancer revealed a reduction in the expression of macrophage cell markers and cytokines such as IL-13, TGF-β, IL-10 and CCL-17 in the colon leading to arrest in tumor progression [99]. Besides, an experimental murine model of myeloma treated with clodronate liposomes has also showed a proper depletion of macrophages and considerable redcution of the tumor mass [20]. Satyanarayanan et al. reported that clodronate treatment-induced drastic reduction in peritoneal macrophages in a mouse model of peritonitis [100]. Besides, Kameka et al. demonstrated that a single dose of clodronate liposomes can be sufficient for the depletion of macrophages in the spleen and lungs of experimental chickens [101]. Moreover, the depletion of macrophages by clodronate-encapsulated liposomes have been reported to be highly efficient in infectious models such as Taenia crassiceps cysticercosis, influenza virus and dengue [102], [103], [104]. Roscic-Mrkic et al. demonstrated clodronate liposome-mediated depletion of monocyte/macrophages in a genetically modified mouse model with measles virus infection [105]. Several studies highlighted the depletion of macrophages in experimental mice by administration of clodronate-liposomes at weekly intervals [106], [107]. In the influenza pig model, Kim et al. reported the depletion of alveolar macrophages by liposome-encapsulated clodronate [81]. In addition, Mert et al. demonstrated the anti-inflammatory effect of liposome-encapsulated clodronate against activated macrophages and neutrophils in the carrageenan-induced inflammation model [84]. Histological and immunohistochemical studies in a mouse model of rheumatoid arthritis (RA) indicated that macrophage depletion by clodronate liposomes is beneficial against inflammation [108]. In an immunofluorescence based study by Wang et al. to analyse the effect of clodronate liposome against neuropathic pain in a rat model indicated a transient depletion of microglia in the spinal cord [109]. Moreover, animal experimental studies revealed that treatment of liposamal clodronate leads to depletion of microglia in the brain and retina [110], [111]. Besides, liposamal clodronate treatment has been reported to attenuate the zymosan-induced activation of NF-kB, Fos expression and hypothermia [112]. Han et al. demonstrated that the direct injection of liposome-encapsulated clodronate to the striatum of experimental animals result in depletion of microglia [113]. Moreover, liposome-encapsulated clodronate has also been considered as an antiosteolytic therapeutic agent in bone pain coupled with skeletal metastases in patients with breast cancer and multiple myeloma [114], [115]. In addition, clodronate has proven to be effective against bone-related diseases like osteoporosis, osteopenia, osteolytic lesions, and hypercalcemia [116]. A clinical trial by Frediani et al. indicated that intramuscular injection of clodronate provides therapeutic relief against osteoarthritis and bone marrow edema [117]. Besides, clodronate treatment appears to be effective against radiation induced bone necrosis in head and neck cancer patients [118]. Taken together, the use of clodronate has been prevalent in the treatment of breast cancer, bone metastasis, multiple myeloma, osteolytic lesions, and osteoporosis. Moreover, clodronate has been suggested to reduce inflammatory cytokines and also act against nitric oxide secretion from macrophages [119], [120].
5. Prospective for the use of liposome encapsulated clodronate for the treatment regime against COVID-19 and to manage COVID-19 vaccinations related adverse effects
It has become clearly evident that elevated levels of proinflammatory cytokines have been associated with various pathogenic events, irreversible multiorgan failure and mortality in a significant portion of severely affected COVID-19 patients. Thus, the pathogenic severity of COVID-19 has been directly linked to cytokine storm. Despite the accumulation of reports advocating the use of many anti-inflammatory drugs, COVID-19 has been refractory to the tailored treatment options. Though vaccinations have been implemented to prevent the spread of SARS-CoV-2 infection, improper vaccination drives and the emergence of new viral variants pose a huge challenge to the health care system in many countries. Therefore, establishment of better alternate therapeutics targeting the activated immune cells needs to be considered. Chronically activated macrophages have been very well established potential cellular sources of cytokine storm in COVID-19. Besides, neuropathogenic events and neuroinflammation resulting from SARS-CoV-2 infection have been reported to be associated with activated microglial cells in the brain. Therefore, it can be hypothesized that depletion of activated macrophages and microglial cells by a specific drug could be highly beneficial in eliminating the life-threatening cytokine storm in COVID-19. A plethora of preclinical studies and clinical trials in patients with cancer and bone disease has clearly demonstrated that liposome encapsulated clodronate has the ability to deplete macrophages responsible for cytokine storm. Considering a well-established specificity in depleting activated forms of macrophages, biocompatibility, widespread in vivo distribution, it can be proposed that liposome encapsulated clodronate might be an ideal therapeutic candidate to harness the activated macrophages and microglia in COVID-19. The liposome encapsulated clodronate can be delivered via non-invasive or minimal invasive routes in the forms of oral suspensions, intravenous injections and nasal spray. The use of liposome encapsulated clodronate can be expected to result in ablation of the abnormal cytokine storm and thereby preventing the multiorgan failure in COVID-19 (Fig. 3, Fig. 4).
While COVID-19 vaccines are safer and more effective in providing boosting immunity against SARS-CoV-2 infection with relatively very rare side effects, a set of recent clinical evidence indicates the activation of inflammatory cascade upon COVID-19 vaccination in a significant number of receivers. However, the degree of side effects varies among different formulations of vaccines generated against SARS-CoV-2. Notably, COVID-19 vaccinations have been known to be associated with thrombotic thrombocytopenia in some cases [121]. Ample reports indicate that COVID-19 vaccinated individuals have considerably increased risk of reinfection to SARS-CoV-2 [122], [123]. Notably, in some individuals, COVID-19 vaccination developed destructive macrophage activation and multisystem inflammatory disease similar to the pathogenesis seen in COVID-19 [124], [125]. In some cases, the immoral immune responses of COVID-19 vaccinations appear to be associated with chronic activation of various immune cells including macrophages resulting in cytokine storm and unforeseen adverse effects including MAS and ARDS [126]. Considering the facts, liposome encapsulated clodronate might also be a beneficial in depleting activated macrophages in individuals who exhibit hyperinflammation upon COVID-19 vaccination.
Though the use of clodronate can be effective in depleting the pathogenic macrophages, it might be associated with depletion of the physiological macrophages. The unpresented depletion of physiological macrophages might be related with immunological dysfunction in healthy situations. However, recent reports suggest that physiological macrophages can be repopulated over the time or upon the withdrawal of liposome-encapsulated clodronate. Moreover, ample reports suggested the depletion of macrophages by clodronate have resulted in the amelioration of various disease conditions with no side effects or drawbacks of the treatment. To note, oral administration of clodronate appears to be associated with gastrointestinal toxicities, esophagitis and diarrhoea. Chronic administration of liposome-encapsulated clodronate might be associated with hypercalcaemia. The incidence of adverse effects appears to be very low and are not life‐threatening as they can be managed by lowering the doses of clodronate. However, future experimental evaluations and pharmacodynamics of liposome encapsulated clodronate in preclinical models of COVID-19 needs to be considered.
6. Conclusion
The macrophage depleting drug, liposome encapsulated clodronate has gained a greater clinical advantage and use in the treatment of cancer-associated bone diseases and osteoporosis. The macrophages and microglia depleting effect of liposomal clodronate has already been well-established in vitro and in vivo preclinical models of malignant disorders and bone diseases. In addition, the reports on preclinical studies suggest the use of clodronate as an efficient therapeutic strategy to mitigate various human diseases that are associated with activated immune cells and severe inflammation. Therefore, we propose the transient use of liposome encapsulated clodronate as a potential therapeutic strategy to eliminate the pathogenic macrophages and microglia to defuse cytokine storm in COVID-19 (Fig. 3, Fig. 4). The proposed treatment regime of liposome encapsulated clodronate could be higly effective and beneficial in diminishing the cytokine storm responsible for life-threatening pathogenic events in COVID-19. Though the available reports on the use of clodronate for various disease conditions revealed no considerable adverse effects even when utilising a high dose and for a prolonged period of time, predictable and unknown side-effects associated with clodronate treatment may not be completely ignored.
Funding Source
There is no funding source for this article.
Author contributions
MK conceived the idea and generated the graphical illustrations. SR and NM performed the literature search and made the initial draft. SR, NM and MK contributed to the revision of article. All authors discussed the content and contributed to the final manuscript.
Ethical approval and informed consent
Not applicable.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
M.K. has been supported by, University Grants Commission, Faculty Recharge Programme (UGC-FRP), New Delhi, India. M.K. acknowledges RUSA2.0, Biological Sciences, Bharathidasan University, for a financial support and UGC-SAP and DST-FIST for the infrastructure of the Department of Animal Science, Bharathidasan University. Authors would like to thank Risna and Mercy Priyadharshini for proofreading the manuscript and helpful suggestions.
References
- 1.Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20:773. doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahimi F., Talebi Bezmin Abadi A. Implications of the emergence of a new variant of SARS-CoV-2, VUI-202012/01. Arch Med Res. 2021 doi: 10.1016/j.arcmed.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conti P., Caraffa A., Gallenga C.E., Kritas S.K., Frydas I., Younes A., et al. The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem. J Biol Regul Homeost Agents. 2021;35:1–4. [PubMed] [Google Scholar]
- 5.Moore J.P., Offit P.A. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325:821–822. doi: 10.1001/jama.2021.1114. [DOI] [PubMed] [Google Scholar]
- 6.Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:561–567. doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandasamy M. Perspectives for the use of therapeutic Botulinum toxin as a multifaceted candidate drug to attenuate COVID-19. Med Drug Discov. 2020;6:100042. doi: 10.1016/j.medidd.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rethinavel H.S., Ravichandran S., Radhakrishnan R.K., Kandasamy M. COVID-19 and Parkinson’s disease: Defects in neurogenesis as the potential cause of olfactory system impairments and anosmia. J Chem Neuroanat. 2021;115:101965. doi: 10.1016/j.jchemneu.2021.101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022:1–1. https://doi.org/10.1038/s41586-022-04802-1. [DOI] [PMC free article] [PubMed]
- 12.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020:1–8. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong GU, Lyu J, Kim K-D, Chung YC, Yoon GY, Lee S, et al. SARS-CoV-2 infection of microglia elicits proinflammatory activation and apoptotic cell death. Microbiol Spectr 2022:e0109122. https://doi.org/10.1128/spectrum.01091-22. [DOI] [PMC free article] [PubMed]
- 14.Radhakrishnan R.K., Kandasamy M. SARS-CoV-2-mediated neuropathogenesis, deterioration of hippocampal neurogenesis and dementia. Am J Alzheimers Dis Other Demen. 2022;37 doi: 10.1177/15333175221078418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peter AE, Sandeep BV, Rao BG, Kalpana VL. Calming the storm: natural immunosuppressants as adjuvants to target the cytokine storm in COVID-19. Front Pharmacol 2021;11. https://doi.org/10.3389/fphar.2020.583777. [DOI] [PMC free article] [PubMed]
- 16.Meidaninikjeh S., Sabouni N., Marzouni H.Z., Bengar S., Khalili A., Jafari R. Monocytes and macrophages in COVID-19: Friends and foes. Life Sci. 2021;269:119010. doi: 10.1016/j.lfs.2020.119010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeisberger S.M., Odermatt B., Marty C., Zehnder-Fjällman A.H.M., Ballmer-Hofer K., Schwendener R.A. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summan M., Warren G.L., Mercer R.R., Chapman R., Hulderman T., Van Rooijen N., et al. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 20.Opperman K.S., Vandyke K., Clark K.C., Coulter E.A., Hewett D.R., Mrozik K.M., et al. Clodronate-liposome mediated macrophage depletion abrogates multiple myeloma tumor establishment in vivo. Neoplasia N Y N. 2019;21:777–787. doi: 10.1016/j.neo.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohl A., Dehghani F., Korf H.-W., Hailer N.P. The bisphosphonate clodronate depletes microglial cells in excitotoxically injured organotypic hippocampal slice cultures. Exp Neurol. 2003;181:1–11. doi: 10.1016/s0014-4886(02)00049-3. [DOI] [PubMed] [Google Scholar]
- 22.Romagnoli S., Peris A., De Gaudio A.R., Geppetti P. SARS-CoV-2 and COVID-19: from the bench to the bedside. Physiol Rev. 2020;100:1455–1466. doi: 10.1152/physrev.00020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID‐19. Am J Hematol 2020:10.1002/ajh.25829. https://doi.org/10.1002/ajh.25829. [DOI] [PMC free article] [PubMed]
- 24.Mason M.D., Sydes M.R., Glaholm J., Langley R.E., Huddart R.A., Sokal M., et al. Oral sodium clodronate for nonmetastatic prostate cancer–results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873) J Natl Cancer Inst. 2007;99:765–776. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 25.P KM, Sivashanmugam K, Kandasamy M, Subbiah R, Ravikumar V. Repurposing of histone deacetylase inhibitors: A promising strategy to combat pulmonary fibrosis promoted by TGF-β signalling in COVID-19 survivors. Life Sci 2021;266:118883. https://doi.org/10.1016/j.lfs.2020.118883. [DOI] [PMC free article] [PubMed]
- 26.Nardo AD, Schneeweiss‐Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID‐19. Liver Int 2020:10.1111/liv.14730. https://doi.org/10.1111/liv.14730. [DOI] [PMC free article] [PubMed]
- 27.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57–69. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma P., Uppal N.N., Wanchoo R., Shah H.H., Yang Y., Parikh R., et al. COVID-19-associated kidney injury: A case series of kidney biopsy findings. J Am Soc Nephrol JASN. 2020;31:1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikitina E., Larionova I., Choinzonov E., Kzhyshkowska J. Monocytes and macrophages as viral targets and reservoirs. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verschoor C.P., Puchta A., Bowdish D.M.E. The macrophage. Methods Mol Biol Clifton NJ. 2012;844:139–156. doi: 10.1007/978-1-61779-527-5_10. [DOI] [PubMed] [Google Scholar]
- 32.Hyperinflammation A.U. On the pathogenesis and treatment of macrophage activation syndrome. Acta Paediatr Oslo Nor 1992. 2021 doi: 10.1111/apa.15900. [DOI] [PubMed] [Google Scholar]
- 33.Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otsuka R., Seino K.-I. Macrophage activation syndrome and COVID-19. Inflamm Regen. 2020;40:19. doi: 10.1186/s41232-020-00131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mokhtari T., Hassani F., Ghaffari N., Ebrahimi B., Yarahmadi A., Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol. 2020:1–16. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45:100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020:1–11. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santa Cruz A., Mendes-Frias A., Oliveira A.I., Dias L., Matos A.R., Carvalho A., et al. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keddie S., Ziff O., Chou M.K.L., Taylor R.L., Heslegrave A., Garr E., et al. Laboratory biomarkers associated with COVID-19 severity and management. Clin Immunol Orlando Fla. 2020;221:108614. doi: 10.1016/j.clim.2020.108614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang M, Chen X, Xu Y. A Retrospective Study of the C-Reactive Protein to Lymphocyte Ratio and Disease Severity in 108 Patients with Early COVID-19 Pneumonia from January to March 2020 in Wuhan, China. Med Sci Monit Int Med J Exp Clin Res 2020;26:e926393-1-e926393-8. https://doi.org/10.12659/MSM.926393. [DOI] [PMC free article] [PubMed]
- 47.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Upadhyay J., Tiwari N., Ansari M.N. Role of inflammatory markers in corona virus disease (COVID-19) patients: A review. Exp Biol Med Maywood NJ. 2020;245:1368–1375. doi: 10.1177/1535370220939477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouayed J., Bohn T. The link between microglia and the severity of COVID-19: The “two-hit” hypothesis. J Med Virol. 2021;93:4111–4113. doi: 10.1002/jmv.26984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schurink B., Roos E., Radonic T., Barbe E., Bouman C.S.C., de Boer H.H., et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID‐19: Systematic review. J Med Virol 2020:10.1002/jmv.25961. https://doi.org/10.1002/jmv.25961. [DOI] [PMC free article] [PubMed]
- 54.Behl T., Kaur I., Bungau S., Kumar A., Uddin M.S., Kumar C., et al. The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020;257:118075. doi: 10.1016/j.lfs.2020.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herman L.L., Padala S.A., Ahmed I., Bashir K. StatPearls Publishing; StatPearls, Treasure Island (FL): 2022. Angiotensin converting enzyme inhibitors (ACEI) [PubMed] [Google Scholar]
- 56.Ravelli A., De Benedetti F., Viola S., Martini A. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis successfully treated with cyclosporine. J Pediatr. 1996;128:275–278. doi: 10.1016/s0022-3476(96)70408-0. [DOI] [PubMed] [Google Scholar]
- 57.Mouy R., Stephan J.L., Pillet P., Haddad E., Hubert P., Prieur A.M. Efficacy of cyclosporine A in the treatment of macrophage activation syndrome in juvenile arthritis: report of five cases. J Pediatr. 1996;129:750–754. doi: 10.1016/s0022-3476(96)70160-9. [DOI] [PubMed] [Google Scholar]
- 58.Kragholm K., Torp-Pedersen C., Fosbol E. Non-steroidal anti-inflammatory drug use in COVID-19. Lancet Rheumatol. 2021 doi: 10.1016/S2665-9913(21)00144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subramaniam M.D., Venkatesan D., Iyer M., Subbarayan S., Govindasami V., Roy A., et al. Biosurfactants and anti-inflammatory activity: A potential new approach towards COVID-19. Curr Opin Environ Sci Health. 2020;17:72–81. doi: 10.1016/j.coesh.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng W, Huang X, Lai Y, Liu X, Jiang Y, Zhan S. Glycyrrhizic acid for COVID-19: findings of targeting pivotal inflammatory pathways triggered by SARS-CoV-2. Front Pharmacol 2021;12. [DOI] [PMC free article] [PubMed]
- 61.Zhao Z., Xiao Y., Xu L., Liu Y., Jiang G., Wang W., et al. Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment. ACS Appl Mater Interfaces. 2021;13:20995–21006. doi: 10.1021/acsami.1c02755. [DOI] [PubMed] [Google Scholar]
- 62.Desjarlais M., Wirth M., Lahaie I., Ruknudin P., Hardy P., Rivard A., et al. Nutraceutical targeting of inflammation-modulating micrornas in severe forms of COVID-19: A novel approach to prevent the cytokine storm. Front Pharmacol. 2020;11:602999. doi: 10.3389/fphar.2020.602999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poulsen N.N., von Brunn A., Hornum M., Blomberg J.M. Cyclosporine and COVID-19: Risk or favorable? Am J Transplant. 2020;20:2975–2982. doi: 10.1111/ajt.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cure E., Kucuk A., Cure M.C. Can emapalumab be life saving for refractory, recurrent, and progressive cytokine storm caused by COVID-19, which is resistant to anakinra, tocilizumab, and Janus kinase inhibitors. Indian J Pharmacol. 2021;53:226–228. doi: 10.4103/ijp.IJP_615_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kyriazopoulou E., Huet T., Cavalli G., Gori A., Kyprianou M., Pickkers P., et al. Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021;3:e690–e697. doi: 10.1016/S2665-9913(21)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopes M.I., Bonjorno L.P., Giannini M.C., Amaral N.B., Menezes P.I., Dib S.M., et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7:e001455. doi: 10.1136/rmdopen-2020-001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel M., Dominguez E., Sacher D., Desai P., Chandar A., Bromberg M., et al. Etoposide as salvage therapy for cytokine storm due to coronavirus disease 2019. Chest. 2021;159:e7–e11. doi: 10.1016/j.chest.2020.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diurno F., Numis F.G., Porta G., Cirillo F., Maddaluno S., Ragozzino A., et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 71.Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., et al. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 72.Khamis F., Al-Zakwani I., Al Hashmi S., Al Dowaiki S., Al Bahrani M., Pandak N., et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis. 2020;99:214–218. doi: 10.1016/j.ijid.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boekel L., Wolbink G.J. Rituximab during the COVID-19 pandemic: time to discuss treatment options with patients. Lancet Rheumatol. 2022;4:e154–e155. doi: 10.1016/S2665-9913(21)00418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossini M., Braga V., Gatti D., Gerardi D., Zamberlan N., Adami S. Intramuscular clodronate therapy in postmenopausal osteoporosis. Bone. 1999;24:125–129. doi: 10.1016/s8756-3282(98)00154-9. [DOI] [PubMed] [Google Scholar]
- 76.Rovetta G., Monteforte P., Balestra V. Intravenous clodronate for acute pain induced by osteoporotic vertebral fracture. Drugs Exp Clin Res. 2000;26:25–30. [PubMed] [Google Scholar]
- 77.Muratore M., Quarta E., Grimaldi A., Calcagnile F., Quarta L. Clinical utility of clodronate in the prevention and management of osteoporosis in patients intolerant of oral bisphosphonates. Drug Des Devel Ther. 2011;5:445–454. doi: 10.2147/DDDT.S12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lehenkari P.P., Kellinsalmi M., Näpänkangas J.P., Ylitalo K.V., Mönkkönen J., Rogers M.J., et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002;61:1255–1262. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- 79.Thompson K., Rogers M.J., Coxon F.P., Crockett J.C. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol Pharmacol. 2006;69:1624–1632. doi: 10.1124/mol.105.020776. [DOI] [PubMed] [Google Scholar]
- 80.Rosa R.G., Collavino K., Lakhani A., Delve E., Weber J.F., Rosenthal A.K., et al. Clodronate exerts an anabolic effect on articular chondrocytes mediated through the purinergic receptor pathway. Osteoarthritis Cartilage. 2014;22:1327–1336. doi: 10.1016/j.joca.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim H.M., Lee Y.-W., Lee K.-J., Kim H.S., Cho S.W., van Rooijen N., et al. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J Virol. 2008;82:4265–4274. doi: 10.1128/JVI.02602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adami S., Bolzicco G.P., Rizzo A., Salvagno G., Bertoldo F., Rossini M., et al. The use of dichloromethylene bisphosphonate and aminobutane bisphosphonate in hypercalcemia of malignancy. Bone Miner. 1987;2:395–404. [PubMed] [Google Scholar]
- 83.Bonabello A., Galmozzi M.R., Canaparo R., Serpe L., Zara G.P. Long-term analgesic effect of clodronate in rodents. Bone. 2003;33:567–574. doi: 10.1016/s8756-3282(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 84.Mert T., Sahin M., Sahin E., Yaman S. Anti-inflammatory properties of Liposome-encapsulated clodronate or Anti-Ly6G can be modulated by peripheral or central inflammatory markers in carrageenan-induced inflammation model. Inflammopharmacology. 2019;27:603–612. doi: 10.1007/s10787-019-00563-y. [DOI] [PubMed] [Google Scholar]
- 85.Dehghani F., Conrad A., Kohl A., Korf H.-W., Hailer N.P. Clodronate inhibits the secretion of proinflammatory cytokines and NO by isolated microglial cells and reduces the number of proliferating glial cells in excitotoxically injured organotypic hippocampal slice cultures. Exp Neurol. 2004;189:241–251. doi: 10.1016/j.expneurol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 86.van Rooijen N., Sanders A., van den Berg T.K. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 87.Kawanishi N., Mizokami T., Niihara H., Yada K., Suzuki K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem Biophys Rep. 2015;5:146–151. doi: 10.1016/j.bbrep.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weisser S.B., van Rooijen N., Sly L.M. Depletion and reconstitution of macrophages in mice. J Vis Exp JoVE. 2012;4105 doi: 10.3791/4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Samad A., Sultana Y., Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4:297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- 91.Mönkkönen J., Heath T.D. The effects of liposome-encapsulated and free clodronate on the growth of macrophage-like cells in vitro: the role of calcium and iron. Calcif Tissue Int. 1993;53:139–146. doi: 10.1007/BF01321893. [DOI] [PubMed] [Google Scholar]
- 92.Van Rooijen N., Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 93.Camilleri J.P., Williams A.S., Amos N., Douglas-Jones A.G., Love W.G., Williams B.D. Methods for assessing splenic macrophage depletion by liposome encapsulated clodronate. Inflamm Res. 1995;44:152–157. doi: 10.1007/BF01782812. [DOI] [PubMed] [Google Scholar]
- 94.Richards P.J., Williams A.S., Goodfellow R.M., Williams B.D. Liposomal clodronate eliminates synovial macrophages, reduces inflammation and ameliorates joint destruction in antigen-induced arthritis. Rheumatol Oxf Engl. 1999;38:818–825. doi: 10.1093/rheumatology/38.9.818. [DOI] [PubMed] [Google Scholar]
- 95.Buiting A.M., Zhou F., Bakker J.A., van Rooijen N., Huang L. Biodistribution of clodronate and liposomes used in the liposome mediated macrophage “suicide” approach. J Immunol Methods. 1996;192:55–62. doi: 10.1016/0022-1759(96)00034-8. [DOI] [PubMed] [Google Scholar]
- 96.Moreno S.G. Depleting macrophages in vivo with clodronate-liposomes. Methods Mol Biol Clifton NJ. 2018;1784:259–262. doi: 10.1007/978-1-4939-7837-3_23. [DOI] [PubMed] [Google Scholar]
- 97.Elder A.C.P., Gelein R., Oberdörster G., Finkelstein J., Notter R., Wang Z. Efficient depletion of alveolar macrophages using intratracheally inhaled aerosols of liposome-encapsulated clodronate. Exp Lung Res. 2004;30:105–120. doi: 10.1080/01902140490266510. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y., Zhang L., Wu G.-R., Zhou Q., Yue H., Rao L.-Z., et al. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci Adv. 2021;7:eabb6075. doi: 10.1126/sciadv.abb6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bader J.E., Enos R.T., Velázquez K.T., Carson M.S., Nagarkatti M., Nagarkatti P.S., et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am J Physiol Gastrointest Liver Physiol. 2018;314:G22–G31. doi: 10.1152/ajpgi.00229.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumaran Satyanarayanan S., El Kebir D., Soboh S., Butenko S., Sekheri M., Saadi J., et al. IFN-β is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nat Commun. 2019;10 doi: 10.1038/s41467-019-10903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kameka A.M., Haddadi S., Jamaldeen F.J., Moinul P., He X.T., Nawazdeen F.H.P., et al. Clodronate treatment significantly depletes macrophages in chickens. Can J Vet Res Rev Can Rech Veterinaire. 2014;78:274–282. [PMC free article] [PubMed] [Google Scholar]
- 102.Reyes J.L., Terrazas C.A., Alonso-Trujillo J., van Rooijen N., Satoskar A.R., Terrazas L.I. Early removal of alternatively activated macrophages leads to Taenia crassiceps cysticercosis clearance in vivo. Int J Parasitol. 2010;40:731–742. doi: 10.1016/j.ijpara.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 103.Tate M.D., Pickett D.L., van Rooijen N., Brooks A.G., Reading P.C. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84:7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fink K., Ng C., Nkenfou C., Vasudevan S.G., van Rooijen N., Schul W. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur J Immunol. 2009;39:2809–2821. doi: 10.1002/eji.200939389. [DOI] [PubMed] [Google Scholar]
- 105.Roscic-Mrkic B., Schwendener R.A., Odermatt B., Zuniga A., Pavlovic J., Billeter M.A., et al. Roles of macrophages in measles virus infection of genetically modified mice. J Virol. 2001;75:3343–3351. doi: 10.1128/JVI.75.7.3343-3351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lang P.A., Recher M., Honke N., Scheu S., Borkens S., Gailus N., et al. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatol Baltim Md. 2010;52:25–32. doi: 10.1002/hep.23640. [DOI] [PubMed] [Google Scholar]
- 107.Abdul-Careem M.F., Firoz Mian M., Gillgrass A.E., Chenoweth M.J., Barra N.G., Chan T., et al. FimH, a TLR4 ligand, induces innate antiviral responses in the lung leading to protection against lethal influenza infection in mice. Antiviral Res. 2011;92:346–355. doi: 10.1016/j.antiviral.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Q., Yuan R., Li C., Wei W., Shen W., Cui Y., et al. Macrophage depletion with clodronate-containing liposomes affects the incidence and development of rheumatoid arthritis. Z Rheumatol. 2019;78:996–1003. doi: 10.1007/s00393-018-0563-x. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y.-R., Mao X.-F., Wu H.-Y., Wang Y.-X. Liposome-encapsulated clodronate specifically depletes spinal microglia and reduces initial neuropathic pain. Biochem Biophys Res Commun. 2018;499:499–505. doi: 10.1016/j.bbrc.2018.03.177. [DOI] [PubMed] [Google Scholar]
- 110.Todd L., Palazzo I., Suarez L., Liu X., Volkov L., Hoang T.V., et al. Reactive microglia and IL1β/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J Neuroinflammation. 2019;16:118. doi: 10.1186/s12974-019-1505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Conedera F.M., Pousa A.M.Q., Mercader N., Tschopp M., Enzmann V. Retinal microglia signaling affects Müller cell behavior in the zebrafish following laser injury induction. Glia. 2019;67:1150–1166. doi: 10.1002/glia.23601. [DOI] [PubMed] [Google Scholar]
- 112.Takagi S., Murayama S., Torii K., Takemura-Morita S., Kurganov E., Nagaoka S., et al. Depletion of microglia and macrophages with clodronate liposomes attenuates zymosan-induced Fos expression and hypothermia in the adult mouse. J Neuroimmunol. 2020;344:577244. doi: 10.1016/j.jneuroim.2020.577244. [DOI] [PubMed] [Google Scholar]
- 113.Han X., Li Q., Lan X., El-Mufti L., Ren H., Wang J. Microglial depletion with clodronate liposomes increases proinflammatory cytokine levels, induces astrocyte activation, and damages blood vessel integrity. Mol Neurobiol. 2019;56:6184–6196. doi: 10.1007/s12035-019-1502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paterson A.H., Powles T.J., Kanis J.A., McCloskey E., Hanson J., Ashley S. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol. 1993;11:59–65. doi: 10.1200/JCO.1993.11.1.59. [DOI] [PubMed] [Google Scholar]
- 115.Powles T., Paterson S., Kanis J.A., McCloskey E., Ashley S., Tidy A., et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;20:3219–3224. doi: 10.1200/JCO.2002.11.080. [DOI] [PubMed] [Google Scholar]
- 116.Frediani B., Giusti A., Bianchi G., Dalle Carbonare L., Malavolta N., Cantarini L., et al. Clodronate in the management of different musculoskeletal conditions. Minerva Med. 2018;109:300–325. doi: 10.23736/S0026-4806.18.05688-4. [DOI] [PubMed] [Google Scholar]
- 117.Frediani B., Toscano C., Falsetti P., Nicosia A., Pierguidi S., Migliore A., et al. Intramuscular clodronate in long-term treatment of symptomatic knee osteoarthritis: a randomized controlled study. Drugs RD. 2020;20:39–45. doi: 10.1007/s40268-020-00294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel S., Patel N., Sassoon I., Patel V. The use of pentoxifylline, tocopherol and clodronate in the management of osteoradionecrosis of the jaws. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2021;156:209–216. doi: 10.1016/j.radonc.2020.12.027. [DOI] [PubMed] [Google Scholar]
- 119.Mönkkönen J., Makkonen N., Rogers M.J., Frith J.C., Auriola S. Effects of bisphosphonates on the inflammatory processes of activated macrophages. Phosphorus Sulfur Silicon Relat Elem. 1999;144:321–324. doi: 10.1080/10426509908546246. [DOI] [Google Scholar]
- 120.Mahajan S., Decker C.E., Yang Z., Veis D., Mellins E.D., Faccio R. Plcγ2/Tmem178 dependent pathway in myeloid cells modulates the pathogenesis of cytokine storm syndrome. J Autoimmun. 2019;100:62–74. doi: 10.1016/j.jaut.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bilotta C., Perrone G., Adelfio V., Spatola G.F., Uzzo M.L., Argo A., et al. COVID-19 vaccine-related thrombosis: a systematic review and exploratory analysis. Front Immunol. 2021;12:729251. doi: 10.3389/fimmu.2021.729251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Menni C., May A., Polidori L., Louca P., Wolf J., Capdevila J., et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ashizawa N., Takazono T., Umeda M., Yamamoto K., Kawakami A., Mukae H. Macrophage activation syndrome after BNT162b2 mRNA vaccination successfully treated with corticosteroids. Clin Exp Rheumatol. 2022;40:1060. doi: 10.55563/clinexprheumatol/a9hrmo. [DOI] [PubMed] [Google Scholar]
- 125.Yousaf A.R., Cortese M.M., Taylor A.W., Broder K.R., Oster M.E., Wong J.M., et al. Reported cases of multisystem inflammatory syndrome in children aged 12–20 years in the USA who received a COVID-19 vaccine, December 2020, through August, 2021: a surveillance investigation. Lancet Child Adolesc Health. 2022;6:303–312. doi: 10.1016/S2352-4642(22)00028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khan W.H., Hashmi Z., Goel A., Ahmad R., Gupta K., Khan N., et al. COVID-19 pandemic and vaccines update on challenges and resolutions. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.690621. [DOI] [PMC free article] [PubMed] [Google Scholar]