Abstract
Introduction
COVID-19 is currently a global pandemic, and initial reports of identified COVID-19 lockdown and limitations can adversely affect childhood obesity and metabolic health. Studies conducted in recent years have shown that the rate of obesity in childhood increases with the changing lifestyle with the pandemic. However, there is insufficient data on how the situation changes and how metabolism is affected in those, who are already obese. The aim of this paper was to determine how the pandemic affects the current status, severity, and metabolic parameters of obese children. We also attempted to show potential effects of metformin therapy.
Methods
The study was conducted with the participation of 101 patients with obesity (The mean age was 13.6 ± 2.2). The patients were evaluated using pre- and post-lockdown data with an interval of 6 months. The new classification system was used to determine the severity of obesity. All anthropometrics, metabolic parameters (Blood glucose, insulin, HbA1C, lipid profile), lifestyle, and comorbidities were evaluated by dividing the participants into various subgroups according to their obesity and metformin usage status.
Results
Our data shows that weight, height, BMI, BMI-SD, and BMI percentiles all increased significantly, after the pandemic started. The severity of obesity increased statistically (overweight decreases and class 2 obesity increases, p = 0.001). No change was observed in metabolic parameters. Surprisingly, a significant increase was observed in insulin and HOMA-IR values in the group with-metformin.
Discussion
Most studies about childhood obesity have only focused on obesity increases and pandemic relation. Our study showed that although there was no significant change in metabolic status at the end of a lockdown period, there was a serious increase in the severity of obesity. Metformin use had no effect on either obesity or metabolic parameters, and even an increase in insulin resistance indicators was observed.
Keywords: Covid-19 pandemic, Childhood obesity, Severity, Metabolic parameters, Metformin, Lipid profile, Disease Severity, Severity of obesity, Insulin, HbA1c Glycated hemoglobin A1c
Abbreviations: COVID-19, Coronavirus disease; FDA, Food and Drug Administration; BMI, kg/m2, Body mass index; SD, Standard deviation; TG, Triglyceride; TC, Total cholesterol; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; FBG, Fasting blood glucose; HbA1c, Glycated hemoglobin A1c; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance
1. Introduction
Coronavirus disease (COVID-19) is currently a global pandemic and spread extremely quickly. This disease, which is transmitted from person to person and causes serious deaths, naturally brought social isolation and restriction rules, such as lockdowns (Loades et al., 2020; Pietrobelli et al., 2020). After the outbreak of COVID-19, many countries across the world, including Turkey, imposed a general lockdown and social-behavioral limitations. Since 2020, the pandemic has caused many potential consequences, including long-term school closures, stay-at-home policies, and social limitations, which all exacerbate the spread of childhood obesity (Loades et al., 2020; Chung and Rhie, 2021; Rundle et al., 2020a). Initial reports from pandemic hot spots, identified higher rates of depression, social isolation, anxiety disorders, inactivity, eating disorders, and obesity rates in people, who imposed pandemic limitations (Loades et al., 2020; Fern á ndez -Aranda et al., 2020; Salari et al., 2020).
Childhood obesity, even without pandemic, causes serious co-morbidities such as glucose intolerance, insulin resistance, lipid elevation, type 2 diabetes mellitus, and there is no treatment other than lifestyle changes. Many recent studies have reported that severe obesity in childhood is usually much more associated with weight-related comorbidities, adverse metabolic profiles, and related conditions, such as depression (three times higher) and anxiety (five times higher) in adolescents with severe obesity (Chung and Rhie, 2021; Fox et al., 2016). Furthermore, recent studies emphasize that the number of children and adolescents with obesity has increased substantially during the pandemic and the severity of pediatric obesity continues to increase consistently (Fox et al., 2016; Skinner et al., 2018; Nam et al., 2017; Chang et al., 2021; Jenssen et al., 2021). With the considerable amount of unavoidable health consequences and pandemic related circumstances, recent studies have showed severe pediatric obesity gaining momentum and that it should be treated comprehensively (Fox et al., 2016; Chang et al., 2021; Jenssen et al., 2021; Nicodemo et al., 2021; Jia et al., 2020).
In normal stances, lifestyle interventions are a cornerstone treatment for children with obesity, but interventions, especially with the pandemic, are mostly ineffective and do not result in the amount of weight loss needed to reduce the risk for other chronic diseases in children with severe obesity (Chang et al., 2021; Nathan et al., 2016; Danielsson et al., 2012). Therefore, in recent years, the alarming increase in obesity severity has created a demand for potential treatment options to combat this disease (Nathan et al., 2016). However, adjuvant medical options for severe pediatric obesity remain limited. While metformin has only been approved by the Food and Drug Administration (FDA) for children above 10 years old, with type 2 diabetes mellitus, it is commonly used off-label to treat metabolic disorders in obese children as a second line therapy (McDonagh et al., 2014; Raman and Foster, 2021). In some studies, it has been suggested that in addition to lifestyle modification, metformin use reduces body mass index, insulin resistance, glucose intolerance, and the development of metabolic syndrome (Orchard et al., 2005; U.K.-Prospective-Diabetes-Study-Group, 1998; Nathan et al., 2009; Kelly et al., 2016; Knowler et al., 2002). Numerous studies and clinical trials have demonstrated that metformin adjuvant therapy with lifestyle changes may be effective in treating obesity (Chung and Rhie, 2021; Orchard et al., 2005; Kelly et al., 2013).
Several previous studies have shown that pandemic lockdown and quarantine can adversely affect childhood obesity with potential consequences (Pietrobelli et al., 2020; Nicodemo et al., 2021; An, 2020; Clemmensen et al., 2020). Indeed, according to recent reports, changing dietary and physical activity behavior due to the global pandemic is aggravating both obesity and metabolic problems in children (Chung and Rhie, 2021). Metabolic changes including the co-presence of obesity, abnormal glucose homeostasis, insulin changes, and dyslipidemia is an important determinants of cardiovascular risks (Atay and Bereket, 2016). Obese children have a higher risk of cardiovascular and renal complications, orthopedic problems, and hyperlipidemic comorbidities which increases with the severity of obesity (Atay and Bereket, 2016). Eventually, obesity-related predisposition directly causes cardiometabolic changes related to potential consequences all life-long (Albataineh et al., 2019).
The most critical long-term consequences of increased severity of childhood obesity are its persistence into adulthood, thus, in this article, we focus on the triangle relationship between pandemic lockdowns, severity of childhood obesity, and effect of metformin treatment as an adjuvant therapy to prevent and control obesity (Albataineh et al., 2019). The primary aim of this study was to investigate how the COVID-19 pandemic lockdown affects the current status, severity, and metabolic parameters of already obese children. The second aim was to evaluate the effect of metformin treatment on the severity of obesity and metabolic parameters among obese children during the pandemic.
2. Methods
2.1. Participants
In Turkey, full or partial lockdown policies were implemented periodically. People under the age of 20 were especially strictly prohibited from going out under certain conditions, while varying conditions prevailed for adults. During these prohibition periods, only emergency patients were accepted in the hospital. Schools were closed periodically and switched to online education between 2020 and 2021.
Our obesity outpatient clinic service is provided on certain days of the week within the Pediatric Endocrine Department. Here, patients are routinely evaluated in terms of anthropometric measurements and metabolic parameters. A special diet program for each obese child is planned by the pediatric dietician. It is recommended to exercise at least 1 h a day, 3 days a week. Diet and exercise compliance is questioned at each visit and recorded as “yes” or “no”. In our clinical practice, some children, who cannot lose weight with lifestyle changes and additionally, who have impaired fasting glucose, glucose intolerance, or insulin resistance, are started on metformin treatment (850 mg twice per day) along with standard lifestyle recommendations, with the family's consent.
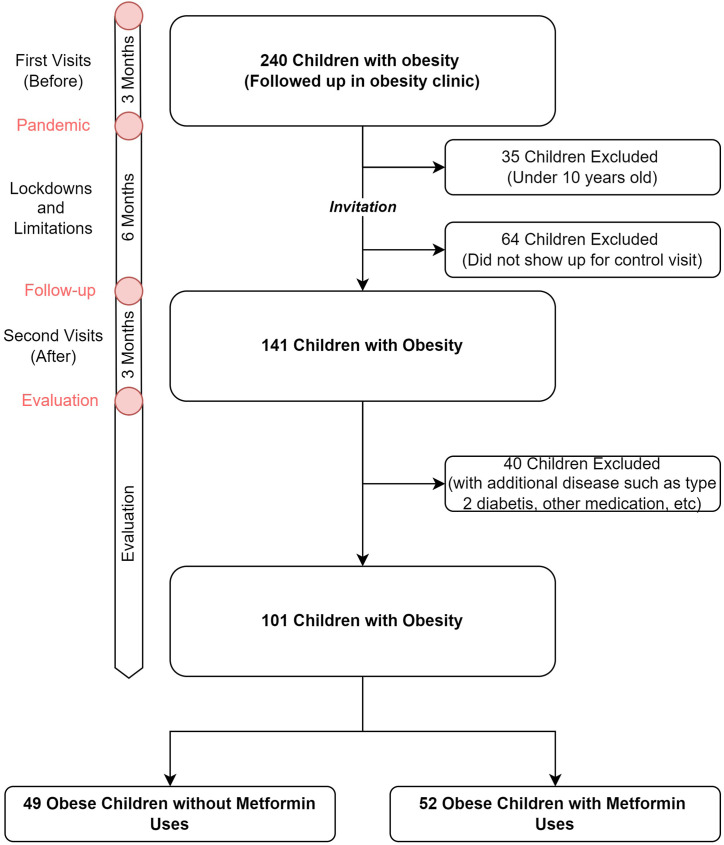
This present study was conducted in our obesity outpatient clinic, city of Kayseri, Erciyes University. The study was approved by the ethical committee of Erciyes University with approval number 202128. Two hundred-forty obese children were included in the study, who were already being followed up in our clinic and evaluated for routine control before the pandemic and lockdown period. These cases were invited to follow-up examinations after the lockdown periods. Complete or almost-complete distinct closure periods continued for approximately 6 months (Fig. 1 ). Children younger than 10 years of age, with missing file information in terms of anthropometric and metabolic parameters, and those, who did not agree to participate in the study were excluded. One hundred forty-one children accepted the invitation and were examined in the 6th month follow-up visits. The mean age was 13.6 (SD ± 2.2, between 10 and 18 years).
Fig. 1.
Timeline and flowchart of the study.
To determine the effect of metformin on the change in anthropometric and metabolic parameters during the lockdown, the patients were divided into two groups as without-metformin and with-metformin. The two main groups were as follows; 52 patients (51.5%) were taking metformin and 49 (48.5%) were non taking metformin (Before the pandemic, Fig. 1). The group with-metformin included children, who had been taking metformin for at least 6 months before the lockdown and those, who continued to use metformin during the lockdown. Forty children having an additional disease that could cause obesity, having type 2 diabetes, using medication other than metformin, and using metformin for less than 6 months and more than one year were excluded from the study. The average duration of metformin use was between 6 and 12 months. As a result, a total of 101 children were evaluated in the study.
2.2. Anthropometric and metabolic parameters
The calendar ages of the patients were calculated in terms of decimal age. In our obesity clinic, all measurements are made by the same trained nurse. Height and weight measurements were taken without shoes, with underwear, and they were measured and recorded with a digital weight and height meter belonging to Densi Industrial Weighing Systems, with a deviation of 0.1 kg per kilogram and 0.5 cm per centimeter, respectively. Each patient was given a detailed physical examination by the pediatric endocrine fellows.
Body mass index (BMI) was calculated by dividing the weight in kilograms by the height in square meters and BMI-standard deviation (SD) values were calculated for all ages and genders according to the standard for Turkey (Ozturk et al., 2008).
Since all children participating in the study were already overweight and obese, the study aimed to show the effect of the pandemic lockdowns more clearly and a new classification system was used to determine the severity of obesity (Kelly et al., 2013; Barlow, 2007; Wang, 2001). The participants were separated further into two main subgroups as mild obesity (includes overweight and class 1 obesity) and serious obesity (includes class 2 and class 3 obesity) based on the literature definitions (Chung and Rhie, 2021).
Classification for BMI:
-
-
Normal weight: BMI between 5th-85th percentile
-
-
Overweight: BMI between 85th- 95th percentile
-
-
Class 1 Obesity: BMI >95th percentile
-
-
Class 2 Obesity: BMI ≥120% of the 95th percentile
-
-
Class 3 Obesity: BMI ≥140% of the 95th percentile
Blood samples were drawn after an 8-h fast for determination of lipid profile [Triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL)], fasting blood glucose (FBG), insulin, and glycated hemoglobin A1c (HbA1c). Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the equation: HOMA-IR = Fasting insulin (μU/mL) x Fasting glucose (mg/dL)/405 (Keskin et al., 2005). Biochemical parameters were determined using Roche Cobas 8000 kits.
2.3. Statistical analysis
All data were anonymized before analysis. Continuous variables were described as the mean with standard deviation and range (minimum-maximum). Categorical variables were reported as frequency and percentages. The Kołmogorov–Smirnov test served to check normality. Age, weight, height, HbA1C, cholesterol, HDL, and LDL continuous variables were normal distribution and others showed abnormal distribution. According to normality, the paired t-test and Wilcoxon test were used to compare the difference of parameters between pre and post lockdown period. Chi square and McNemar-Bowker tests were used to determine the difference between dependent and independent groups of categorical variables, respectively. In addition, binary and hierarchical logistic regressions were performed for selected subgroups. Statistical analysis was performed in SPSS for Windows, version 21. A p-value < 0.05 was considered significant.
3. Results
Of all 101 children and adolescents included in the study, (n: 58) 57.4% were boys. The age range of the patients ranged from 10 to 18 years (Mean ± SD: 13.6 ± 2.2). The median interval between pre-lockdown (pre-pandemic) and post-lockdown pandemic interval visits was 5.3 months. Patients were divided into two main groups according to metformin usage status. Fifty-two patients (51.5%) were taking metformin treatment before the pandemic.
Table .1 shows all children's anthropometric and metabolic parameters and the comparisons of all parameters of children according to taking metformin treatment or not. Initially, 16% of all children were overweight and 30.7% were severely obese. Mean BMI and BMI-SD values were 30.3 ± 5.2 and 2.4 ± 0.7, respectively. When the anthropometric and metabolic parameters of the children, who received and did not receive metformin were compared, except for weight, height, and BMI (p = 0.007, p = 0.012, and p = 0.01, respectively), the severity of obesity and all the other parameters were found to be statistically similar between the two groups (Table .1).
Table 1.
Anthropometric and metabolic characteristics of children according to their metformin intake status.
| Without-Metformin, n = 49 | With-Metformin, n = 52 | p | ||
|---|---|---|---|---|
| Age (year) | 13.6 ± 2.2 (10–18.0) | 13.2 ± 2.4 (10.0–18.0) | 14.0 ± 2.4 (10.0–18.0) | 0.098 |
| Gender | ||||
| Girls | 43 (42.6%) | 22 (44.9%) | 21 (40.4%) | 0.399 |
| Boys | 58 (57.4%) | 27 (55.1%) | 31 (59.6%) | |
| Obesity classification | ||||
| Overweight | 16 (15.8%) | 10 (20.4%) | 6 (11.5%) | 0.205 |
| Class 1 | 54 (53.5%) | 26 (53.1%) | 28 (53.8%) | |
| Class 2 | 23 (22.8%) | 10 (20.4%) | 13 (25.0%) | |
| Class 3 | 8 (7.9%) | 3 (6.1%) | 5 (9.6%) | |
| Metabolic and anthropometric measurements | ||||
| Weight (kg) | 77.3 ± 20.5 (32.4–136.6) | 71.7 ± 20.8 (32.4–136.6) | 82.5 ± 18.9 (43.7–131.0) | 0.007 |
| Height (cm) | 159.1 ± 12.7 (121–188) | 155.9 ± 14.2 (121–188) | 162.1 ± 10.3 (139–186) | 0.012 |
| BMI (kg/m2) | 30.3 ± 5.2 (21.4–48.1) | 28.9 ± 4.7 (22.2–44.7) | 31.1 ± 5.4 (21.4–48.1) | 0.013 |
| BMI-SD | 2.4 ± 0.7 (1.0–4.6) | 2.3 ± 0.7 (1.0–4.3) | 2.5 ± 0.8 (1.1–4.7) | 0.383 |
| BMI-percent | 113.9 ± 20.1 (85.0–184.2) | 110.8 ± 17.7 (85–168) | 116.8 ± 22.0 (85–184.2) | 0.103 |
| BG (mg/dL) | 89.3 ± 9.4 (73–100) | 90.1 ± 8.7 (79–120) | 88.6 ± 10.1 (73–119) | 0.204 |
| HBA1C (%) | 5.5 ± 0.5 (4.1–6.5) | 5.5 ± 0.4 (4.7–6.5) | 5.6 ± 0.5 (4.1–6.5) | 0.743 |
| Insulin (μ IU/mL) | 18.9 ± 14.9 (1.1–68.4) | 20.2 ± 15.7 (2.5–68.4) | 17.6 ± 14.0 (1.1–64.8) | 0.128 |
|
Total Cholesterol (mg/dL) |
156.6 ± 32.6 (94–242) | 156.3 ± 31.2 (99–232) | 156.7 ± 34.0 (94–242) | 0.961 |
| TG (mg/dL) | 125.0 ± 66.7 (45–404) | 111.6 ± 47.5 (45–193) | 136.5 ± 78.4 (53–404) | 0.255 |
| HDL (mg/dL) | 44.6 ± 9.2 (25.0–63.2) | 44.9 ± 10.0 (30.8–63.2) | 44.2 ± 8.5 (25.0–60.0) | 0.769 |
| LDL (mg/dL) | 85.8 ± 24.8 (29.2–155.9) | 87.5 ± 26.8 (34.8–155.9) | 84.4 ± 23.2 (29.2–140.6) | 0.609 |
| HOMA-IR | 4.3 ± 3.5 (0.2–16.7) | 4.6 ± 3.7 (0.5–16.7) | 4.0 ± 3.3 (0.2–14.0) | 0.337 |
BMI: Body mass index. BMI-SD: Body mass index- standard deviation. BMI-percent: Body mass index-percent. BG: blood glucose. TG: triglyceride. HDL: high density lipoprotein. LDL: low density lipoprotein. HOMA-IR: homeostasis model assessment-insulin resistance.
Changes in anthropometric and metabolic parameters before and after the pandemic lockdowns are shown in Table .2 . While weight, height, BMI, BMI-SD, and BMI percent increased significantly after pandemic lockdown in all children, no change was observed in metabolic parameters (for BMI, p = 0.014; for the others p < 0.001). Similarly, the evaluation of pandemic related changes (pre and post lockdown) revealed that there were significant increases in anthropometric parameters in both the with- and without-metformin groups (Table .2). Furthermore, changes in terms of metabolic parameters were detected besides insulin and HOMA-IR with the pandemic lockdowns. Surprisingly, a significant increase was observed in the insulin and HOMA-IR values in the with-metformin group. In this group, pre-lockdown insulin level was 17.6 ± 14.0 and HOMA-IR was 4.0 ± 3.3, after-pandemic lockdowns these values increased to 23.7 ± 16.6 and 5.4 ± 3.8, respectively (p = 0.013, p = 0.012, respectively) (Table 2).
Table 2.
Change of metabolic and anthropometric parameters pre- and post-lockdown.
| All children, n: 101 |
Without-Metformin, n = 49 |
With-Metformin, n = 52 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-lockdown | After-lockdown | p | Pre-lockdown | After-lockdown | p | Pre-lockdown | After-lockdown | p | |
| Age (year) | 13.6 ± 2.2 (10.0–18.00); | 13.2 ± 2.4 (10.0–18.00); | 14.0 ± 2.0 (10.0–18.00); | ||||||
| Weight (kg) | 77.3 ± 20.5 (32.4–136.6) | 80.1 ± 21.1 (35.3–163.3) | <0.001 | 71.7 ± 20.8 (32.4–136.6) | 74.8 ± 20.5 (35.3–144.0) | <0.001 | 82.5 ± 18.9 (43.7–131.0) | 85.2 ± 20.5 (47.5–163.3) | 0.015 |
| Height (cm) | 159.1 ± 12.7 (121–188) | 161. ±12.3 (123–187) | <0.001 | 155.9 ± 14.2 (121–188) | 157.9 ± 13.5 (123.0–187.0) | <0.001 | 162.1 ± 10.3 (139–186) | 164.1 ± 10.4 (142–186) | <0.001 |
| BMI (kg/m2) | 30.3 ± 5.2 (21.4–48.1) | 30.4 ± 5.1 (20.6–49.8) | 0.014 | 28.9 ± 4.7 (22.2–44.7) | 29.4 ± 4.6 (20.6–45.4) | 0.012 | 31.1 ± 5.4 (21.4–48.1) | 31.3 ± 5.4 (22.4–49.8) | 0.347 |
| BMI-SD | 2.4 ± 0.7 (1.0–4.6) | 2.48 ± 0.9 (0.9–6.8) | <0.001 | 2.3 ± 0.7 (1.0–4.3) | 2.3 ± 0.7 (0.9–4.1) | <0.001 | 2.5 ± 0.8 (1.1–4.6) | 2.6 ± 1.1 (1.2–6.8) | <0.001 |
| BMI-percent | 113.9 ± 20.1 (85.0–184.2) | 117.3 ± 18.5 (88.6–190.8) | <0.001 | 110.8 ± 17.7 (85.0–168.0) | 115.0 ± 15.7 (88.6–161.0) | <0.001 | 116.8 ± 22.0 (85.0–184.0) | 119.5 ± 20.7 (94–190.8) | 0.046 |
| BG (mg/dL) | 89.3 ± 9.4 (73–100) | 92.3 ± 15.1 (76–180) | 0.061 | 90.1 ± 8.7 (79–120) | 91.1 ± 10.0 (80–147) | 0.442 | 88.6 ± 10.1 (73–119) | 93.5 ± 18.8 (76–180) | 0.064 |
| HBA1C (%) | 5.5 ± 0.5 (4.1–6.5) | 5.4 ± 0.3 (4.9–6.0) | 0.096 | 5.5 ± 0.4 (4.7–6.5) | 5.4 ± 0.3 (5.0–6.0) | 0.277 | 5.6 ± 0.5 (4.1–6.5) | 5.4 ± 0.3 (4.9–6.0) | 0.216 |
| Insulin (μIU/mL) | 18.9 ± 14.9 (1.1–68.4) | 21.2 ± 14.5 (4.0–87.0) | 0.116 | 20.2 ± 15.7 (2.5–68.4) | 18.7 ± 11.9 (4.0–45.9) | 0.589 | 17.6 ± 14.0 (1.1–64.8) | 23.7 ± 16.6 (5.7–87.0) | 0.013 |
|
Total Cholesterol (mg/dL) |
156.6 ± 32.6 (94–242) | 155.3 ± 36.3 (77–274) | 0.738 | 156.3 ± 31.2 (99–232) | 156.4 ± 32.9 (94–242) | 0.975 | 156.7 ± 34.0 (94–242) | 154.2 ± 39.4 (77–274) | 0.714 |
| TG (mg/dL) | 125.0 ± 66.7 (45–404) | 127.5 ± 57.4 (45–265) | 0.786 | 111.6 ± 47.5 (45–193) | 127.8 ± 67.4 (45–265) | 0.340 | 136.5 ± 78.4 (53–404) | 127.3 ± 48.1 (55–255) | 0.437 |
| HDL (mg/dL) | 44.6 ± 9.2 (25.0–63.2) | 44.0 ± 8.8 (24.0–63.8) | 0.393 | 44.9 ± 10.0 (30.8–63.2) | 43.3 ± 9.1 (24.0–57.0) | 0.127 | 44.2 ± 8.5 (25.0–60.0) | 44.6 ± 8.6 (31.1–63.8) | 0.731 |
| LDL (mg/dL) | 85.8 ± 24.8 (29.2–155.9) | 86.4 ± 29.0 (35.8–194.0) | 0.786 | 87.5 ± 26.8 (34.8–155.9) | 85.6 ± 28.7 (35.8–179.9) | 0.455 | 84.4 ± 23.2 (29.2–140.6) | 87.1 ± 29.7 (46.9–194.0) | 0.402 |
| HOMA-IR | 4.3 ± 3.5 (0.2–16.7) | 4.9 ± 3.5 (0.8–18.5) | 0.118 | 4.6 ± 3.7 (0.5–16.7) | 4.3 ± 3.2 (0.8–16.7) | 0.481 | 4.0 ± 3.3 (0.2–14.0) | 5.4 ± 3.8 (1.3–18.5) | 0.012 |
BMI: Body mass index. BMI-SD: Body mass index- standard deviation. BMI-percent: Body mass index-percent. BG: blood glucose. TG: triglyceride. HDL: high density lipoprotein. LDL: low density lipoprotein. HOMA-IR: homeostasis model assessment-insulin resistance.
When the severity of obesity was classified before the pandemic, 15.8% were overweight, 53.5% class 1 obese, 22.8% class 2 obese, and 7.9% class 3 obese (Table .3 ). In the overall pre-lockdown (pre-pandemic) group, 70 (69.3%) of the patients had mild obesity and 31 (30.7%) had serious obesity. After-pandemic lockdowns, the proportions changed as follows: 5.9% overweight, 55.4% class 1 obese, 29.7% class 2 obese, and 8.9% class 3 obese. After 6 months of pandemic lockdown intervals, the proportion of obesity changed as 62 (61.4%) of patients had mild obesity and 32 (38.6%) had serious obesity. After-pandemic, the severity of obesity increased statistically significantly (p = 0.001). It was similar in-patient groups taking metformin and not. The number of overweight children decreased after pandemic lockdowns in both groups, but the rate of class 2 obesity significantly increased. The ratio of class 2 obese children increased from 20.4% to 26.5% in the group without-metformin, and this rate increased from 25% to 32.7% in the group with-metformin (p = 0.012 and p = 0.046, respectively), (Table .3).
Table 3.
Pre-lockdown and post-lockdown obesity severity change of diet and exercise compliance.
| All children, n = 101 |
Without-Metformin, n = 49 |
With-Metformin, n = 52 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-lockdown | After-lockdown | p | Pre-lockdown | After-lockdown | p | Pre-lockdown | After-lockdown | p | ||
| Obesity classification | Overweight | 16 (15.8) | 6 (5.9) | 0.001 | 10 (20.4%) | 4 (8.2%) | 0.012 | 6 (11.5%) | 2 (3.8%) | 0.046 |
| Class 1 | 54 (53.5) | 56 (55.4) | 26 (53.1%) | 28 (57.1%) | 28 (53.8%) | 28 (53.8%) | ||||
| Class 2 | 23 (22.8) | 30 (29.7) | 10 (20.4%) | 13 (26.5%) | 13 (25.0%) | 17 (32.7%) | ||||
| Class 3 | 8 (7.9) | 9 (8.9) | 3 (6.1%) | 4 (8.2%) | 5 (9.6%) | 5 (9.6%) | ||||
| Dietary compliance | Yes | 60 (59.4) | 41 (40.6) | 0.003 | 27 (55.1%) | 18 (36.7%) | 0.050 | 33 (63.5%) | 23 (44.2%) | 0.025 |
| No | 41 (40.6) | 60 (59.4) | 22 (44.9%) | 31 (63.3%) | 19 (36.5%) | 29 (55.8%) | ||||
| Exercise | Yes | 57 (56.4) | 26 (25.7) | <0.001 | 27 (55.1%) | 12 (24.5%) | 0.003 | 30 (57.7%) | 14 (26.9%) | 0.001 |
| No | 44 (43.6) | 75 (74.3) | 22 (44.9%) | 37 (75.5%) | 22 (42.3%) | 38 (73.1%) | ||||
The dietary compliance and exercise status of children before and during the pandemic lockdown was roughly evaluated. A subgroup analysis of mild and serious obesity revealed that there were no significant changes between any groups besides exercise and diet compliance. During the pandemic lockdown period, 59.4% of the children reported that compliance with the diet deteriorated, while 74.3% reported that they did not exercise. These rates were reported as 40.6% and 43.6% in the pre-lockdown (pre-pandemic) visit. A significant change was observed in diet compliance and exercise rates compared with the pre-lockdown (pre-pandemic) period (p = 0.003 and p < 0.001, respectively) (Table 3). The result was similar between the subgroups of with-metformin and without-metformin.
Incremental change (delta value) in each variable at the two-time points of pre and post pandemic lockdown according to using metformin were also compared. The delta values were calculated by subtracting the value of the pre-lockdown from the value of the after-lockdown period. Comparison of the delta values between children without- or with-metformin revealed significant difference in only insulin levels at the two time points. The increase was more pronounced in the metformin group (1.7 vs. 7.0, p = 0.039). There were no other significant differences between the two-time points among the groups (Table 4 ).
Table 4.
Comparison of the difference in each anthropometric and metabolic parameters variable at pre- and post-lockdown according to metformin taking status.
| Delta values | Without-Metformin, n = 49 | With-Metformin, n = 52 | p |
|---|---|---|---|
| Weight (kg) | 3.3 ± 5.6 (−9.8-18.8) | 2.7 ± 7.6 (−16.3-32.2) | 0.636 |
| Height (cm) | 0.02 ± 0.02 (0.01–0.1) | 0.02 ± 0.03 (0.01–0.16) | 0.735 |
| BMI (kg/m2) | 0.6 ± 1.8 (−4.3-4.6) | 0.2 ± 2.2 (−5.2-7.1) | 0.338 |
| BMI-SD | 0.02 ± 0.4 (−0.9-1.0) | 0.1 ± 0.8 (−5.2-7.1) | 0.341 |
| BMI-percent | 4.2 ± 7.3 (−16.0-23.6) | 2.6 ± 9.2 (−18.1-26.8) | 0.361 |
| BG (mg/dl) | 1.1 ± 13.1 (−31.3-64.0) | 4.9 ± 19.7 (−31.0-89.0) | 0.250 |
| HBA1C (%) | 0.1 ± 0.5 (−1.0-1.0) | −0.1 ± 0.6 (−1.1-1.3) | 0.868 |
| Insulin (μ IU/mL) | −1.7 ± 17.6 (−50.7-36.5) | 7.0 ± 16.9 (−34.1-54.3) | 0.039 |
|
Total Cholesterol (mg/dL) |
0.1 ± 16.8 (−36.0-35.0) | −2.5 ± 41.4 (−96.0-150.0) | 0.740 |
| TG (mg/dL) | 16.1 ± 59.3 (−110.0-182.0) | −9.2 ± 61.5 (−206.0-100.0) | 0.087 |
| HDL (mg/dL) | −1.6 ± 5.9 (−14.8-8.7) | 0.3 ± 5.5 (−11.9-10.8) | 0.160 |
| LDL (mg/dL) | −1.9 ± 14.4 (−26.9-42.5) | 2.7 ± 19.6 (−47.4-53.7) | 0.272 |
| HOMA-IR | 0.3 ± 4.5 (−14.7-10.7) | 1.7 ± 4.0 (−12.7-7.9) | 0.063 |
BMI: Body mass index. BMI-SD: Body mass index- standard deviation. BMI-percent: Body mass index-percent. BG: blood glucose. TG: triglyceride. HDL: high density lipoprotein. LDL: low density lipoprotein. HOMA-IR: homeostasis model assessment-insulin resistance.
4. Discussion
The novel coronavirus has infected millions of people and caused a global pandemic, which is generating health, economic, and social emergency (Clemmensen et al., 2020; Chakraborty and Maity, 2020). Lifestyle limitations specifically covered for those under the age of 20, naturally included the childhood group as possibly most affected. Moreover, several studies have shown that children under these conditions are at significant risk of a sedentary lifestyle, negative changes in eating habits, and an increased stress burden (Chang et al., 2021). Healthy lifestyle became more difficult for obese children under pandemic lockdowns (Nicodemo et al., 2021; L ó pez -Bueno et al., 2020).
A microsimulation model to project the impact of COVID-19 on childhood obesity was published with results of possible childhood obesity rates increasing by 2.4% (An, 2020). Their data showed that pandemics might expose children to an increased risk of childhood obesity (An, 2020). Several studies about the impact of the COVID-19 pandemic in children have assessed the effects of staying home and school closure on increasing obesity, mostly based on personal reports (Almandoz et al., 2020; Zachary et al., 2020; Rundle et al., 2020b; He et al., 2020; Workman, 2020).
Reports to quantify the effect of lockdown on healthy children have revealed gains in the body weight of children (2.3 kg in younger and 1.7 kg in older) during lockdown (Chang et al., 2021; Jia et al., 2020; Al Hourani et al., 2021). The overall prevalence of children and adolescents with obesity increased substantially by 13.7%–15.4% between the pre-lockdown (pre-pandemic) and post-lockdown (Jenssen et al., 2021). Publications of children with pre-existing obesity showed that pandemics aggravate the severity of pediatric obesity during the COVID-19 pandemic era (Chang et al., 2021). The relevance of pandemic related weight, height, BMI, BMI-SD, and BMI percent increase is clearly supported by our findings. In addition to the increase in obesity severity, metabolic parameters were also evaluated in this study, and it was observed that there was no difference between before and after the lockdown period.
Metformin, an oral hypoglycemic agent, has only been approved for use in the diagnosis of type 2 diabetes in children. Although there are studies showing that metformin slightly reduces BMI and increases insulin sensitivity, there are also studies claiming that it has no long-term effect (Chung and Rhie, 2021). However, metformin could be recommended in certain obese children. We use metformin therapy in addition to diet and exercise recommendations in obese children with impaired fasting glucose IFG and impaired glucose tolerance IGT, or who cannot benefit from lifestyle changes in our clinical practice. In this present study, half of the obese children were taking metformin (6–12 months). Our data demonstrated that the children taking metformin had no significant changes in weight, BMI, or metabolic parameters compared to those not taking metformin during lockdown. There were similar changes observed in obesity proportion in both groups.
In numerous meta-analyses, benefits and risks of metformin in childhood obesity have been evaluated (McDonagh et al., 2014; Mead et al., 2016). McDonagh et al. considered that metformin significantly reduced weight and BMI. Their paper identified various highlights: patients with >35 baseline BMI showed greater reduction in BMI (−1.23; 95% CI, −1.66 to −0.79), the highest reduction showed in weight loss was observed at 6 months in the metformin use group, and a smaller change was observed in studies with more girls and higher age (>12 years) (McDonagh et al., 2014). Our mean BMI (31.1 ± 5.4) and mean age (13.6 ± 2.2) were possible determinants. Additionally, our group using metformin was using it for 6–12 months and had limited chances to implement lifestyle interventions due to pandemic lockdowns. Another subgroup analysis also showed a group using metformin for 6 months having a greater difference in BMI reduction (Mean −1.40, between −1.98 to −0.81) with 51% inconsistency (I2) compared to <6 or > 6-month groups (McDonagh et al., 2014; Kelly et al., 2016). Mead et al.'s meta-analysis related with metformin revealed a small reduction of BMI (He et al., 2020). Studies have attempted to explain metformin effects as an adjuvant therapy to children, who have tried and failed diet & exercise programs (Wiegand et al., 2010; Rezvanian et al., 2010). A randomized, triple masked, placebo-controlled study reported that beneficial effects of metformin are possibly much smaller in those, who failed in lifestyle changes (McDonagh et al., 2014; Rezvanian et al., 2010). Another metformin study with a yearlong use demonstrated no significant difference (Lavine et al., 2011). Metformin and other therapies might further contribute to weight loss and obesity control in childhood. Our results suggest that it was not effective in our sample with lifestyle restrictions due to lockdown. However, current data are limited.
This paper attempts to show an evaluation of the triangle relation of childhood obesity, metformin, and metabolic parameters, such as HbA1c, insulin/HOMA-IR, cholesterol, TG, HDL, and LDL. Half of the obese children included in the study consisted of those, who were started on metformin treatment because they did not benefit from lifestyle changes. A clear benefit of metformin on the control of BMI or weight could not be identified during the lockdown period. As a result, it was observed that metformin had no significant effect in preventing an increase in the severity of obesity or in regulating metabolic control.
Unexpectedly, while at least insulin resistance was expected to decrease in metformin users, a significantly increase was observed in insulin and HOMA-IR levels at the end of the lockdown period in this group. The incremental change of insulin was 7.0 ± 16.9 (range −34.1 to 54.3) and HOMA-IR was 1.7 ± 4.0 (range −12.7 to 7.9). The children enrolled in the study were similar in the pre-lockdown period in terms of obesity severity and metabolic parameters. The increase in insulin level in those who used only metformin after-pandemic lockdown may be due to these children being more prone to insulin resistance. Therefore, the lifestyle change during the stay-at-home lockdown period seems to have affected these children more. Although the rates of compliance with diet and exercising decreased in both groups, those using metformin may have behaved more comfortably by relying on the medicine they took. Further experimental investigations are needed to estimate this paradigm.
The literature shows that obesity over a pandemic time may affect children's lives worldwide. Our data supports this by showing a potential increase in the severity of obesity. A new obesity epidemic with this momentum may lead to, not only health problems, but also extreme economic consequences on a scale much larger than the COVID-19 infection (Jia et al., 2020; Nicola et al., 2020; Finkelstein et al., 2009). The most obvious finding to emerge from this study is that the severity of obesity in children rapidly deteriorated during the pandemic lockdown period. Controlling severe pediatric obesity is important to prevent for a next pandemic. Identifying when children are experiencing victimization and providing the needed support or resources in school and at home is an essential part of any obesity management plan. Lifestyle modification therapy is the main therapy with or without metformin, but the effects of metformin appear inefficient in children with severe obesity (Chung and Rhie, 2021). We want to emphasize that this momentum should be prevented immediately with various action plans, especially targeting lifestyle interventions.
This present study emphasizes that severity of obesity is gaining momentum among children with the pandemic and lockdowns. Rates of overweight and obesity are possibly continuing to grow in children and aggressive increases in obesity in almost all countries (Jia et al., 2020; An, 2020; Chakraborty and Maity, 2020; Rundle et al., 2020b; Di Renzo et al., 2020; Mattioli et al., 2020). Children, who were separated from their peers, cannot go to school, and adopted their lifestyle to watching television, bad sleep habits, an unhealthy diet, and a sedentary & restricted life (Nicodemo et al., 2021; L ó pez -Bueno et al., 2020). In our study, the deterioration in diet and exercise compliance was clearly observed. Based on the literature information and the data of this study, it can be said that the Covid 19 pandemic will shift the already epidemic childhood obesity to severe obesity, which is more difficult to manage. (Pietrobelli et al., 2020; Chakraborty and Maity, 2020; Rundle et al., 2020b). Governments and health care providers should make all possible efforts to minimize the occurrence of childhood obesity and severity.
4.1. Strengths & limitations of the study
This study provides more detailed information on childhood obesity during the pandemic. Our data not only evaluated anthropometric evaluations, but also the metabolic parameters of obese children. To our knowledge, this is one of the first studies evaluating the effects of metformin with metabolic and anthropometric data during the pandemic. Sample proportion with mild and severe obesity rates, and metformin use rates were homogeneous. All measurements were taken with the same standard nurse/personnel and laboratory evaluations were performed in the same central laboratory. The change in the severity of obesity was shown by the obesity classification based on new guidelines. Although pre-lockdown information was taken from file records, the study was conducted on children, who came after the pandemic started. In our study, together with the change in anthropometric and metabolic parameters, the change of compliance to diet and exercise during the lockdown period was roughly evaluated. These are the strengths of our study.
The present study had some limitations. First, data were collected at a single center and with a relatively small sample size with the limitations in terms of the generalization of these study results globally. Second, we proposed a potential impact of the metformin uses on obesity and severity; however, it is challenging to disentangle the contributions of various factors. Fatty liver and disproportionate body fat measurements, which are other decisive parameters in obesity assessment, could not be (before-after) evaluated because of the pandemic conditions. We believe that long-term studies with more prolonged time intervals are required to evaluate the relationship between the impact of metformin and the longitudinal effects of other severe obesity comorbidities such as hepatocellular injury, sleeping problems, cardiovascular changes, and psychometric changes. Policies such as closing schools and social isolation, which were implemented after the lockdown, undoubtedly affected obesity negatively. In the study, one of the critical factors of obesity indicators such as compliance with diet and exercise was evaluated only with the patient's or parent's “yes” and “no” answer, and detailed scales could not be used for evaluation.
5. Conclusion
Pandemic limitations and stay-at-home policies further exacerbate the situation in obese children by reducing diet and exercise compliance. These findings have supported those children, who already have all the risks of obesity in extreme situations, such as pandemics, have an increased risk of proportional changes to more severe obesity and metabolic disorders. For this reason, obese children should be monitored more closely and carefully by both parents and doctors during extraordinary conditions. Public health interventions need to be directed towards practices that promote a combination of healthy eating and physical activity at home to reduce the negative impact of COVID-19 on childhood obesity. Moreover, metformin, which can be used to prevent the metabolic disorder of obesity, does not appear to be effective on obesity control or severity of obesity. Systematic studies needed to identify how metformin interacts with other treatments that are believed to be linked to obesity control.
Data sharing statement
Deidentified individual participant data (including data dictionaries) will be made available, in addition to study protocols, the statistical analysis plan, and the informed consent form. The data will be made available upon publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to Nihal HATIPOGLU, nihalhatipoglu@yahoo.com or Bahadir M. Samur, mbahadirsamur@yahoo.com.
Article summary
Present study reveals how the pandemic affects obesity & severity of obesity, and the impact of metformin uses.
Disclosure
No financial disclosure exists to this study or for any of the authors.
Ethics & permissions
The study was approved by the ethical committee of Erciyes University (2021) (Ethical committee approval number: 202,128–January 06, 2021).
CRediT authorship contribution statement
Bahadir M. Samur: The study was conceived and designed by Bahadır M. Samur, MD and Nihal Hatipoglu, Prof. The Erciyes University Endocrinolgoy Clinic was used to evaluate patients, Conceptualization, Supervision, Data curation, Writing – review & editing, conceptualized coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Tugba G. Samur: Data curation, Formal analysis, Writing – review & editing, designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript, All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Ulku Gul-Sir: Data curation, Formal analysis, Writing – review & editing, designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript, All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Nihal Hatipoglu: The study was conceived and designed by Bahadır M. Samur, MD and Nihal Hatipoglu, Prof. The Erciyes University Endocrinolgoy Clinic was used to evaluate patients, Conceptualization, Supervision, Data curation, Writing – review & editing, conceptualized coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of competing interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any conflict interest in the subject matter or materials discussed in this manuscript. No funding was secured for this study.
Acknowledgements
The author(s) received no specific funding for this work.
References
- Al Hourani H., Alkhatib B., Abdullah M. Impact of COVID-19 lockdown on body weight, eating habits, and physical activity of Jordanian children and adolescents. Disaster Med. Public Health Prep. 2021:1–9. doi: 10.1017/dmp.2021.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albataineh S.R., Badran E.F., Tayyem R.F. Overweight and obesity in childhood: dietary, biochemical, inflammatory and lifestyle risk factors. Obes. Med. 2019;15 [Google Scholar]
- Almandoz J.P., Xie L., Schellinger J.N., Mathew M.S., Gazda C., Ofori A., et al. Impact of COVID-19 stay-at-home orders on weight-related behaviours among patients with obesity. Clin. Obes. 2020;10(5) doi: 10.1111/cob.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An R. Projecting the impact of the coronavirus disease-2019 pandemic on childhood obesity in the United States: a microsimulation model. J. Sport Health Sci. 2020;9(4):302–312. doi: 10.1016/j.jshs.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atay Z., Bereket A. Current status on obesity in childhood and adolescence: prevalence, etiology, co-morbidities and management. Obes. Med. 2016;3:1–9. [Google Scholar]
- Barlow S.E. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl. 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Chakraborty I., Maity P. Science of the Total Environment; 2020. COVID-19 Outbreak: Migration, Effects on Society, Global Environment and Prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.H., Chen Y.C., Chen W.Y., Chen C.Y., Hsu W.Y., Chou Y., et al. Weight gain associated with COVID-19 lockdown in children and adolescents: a systematic review and meta-analysis. Nutrients. 2021;13(10) doi: 10.3390/nu13103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.L., Rhie Y.-J. Severe obesity in children and adolescents: metabolic effects, assessment, and treatment. J. Obes. Metab. Synd. 2021;30(4):326. doi: 10.7570/jomes21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen C., Petersen M.B., Sørensen T.I.A. Will the COVID-19 pandemic worsen the obesity epidemic? Nat. Rev. Endocrinol. 2020;16:469–470. doi: 10.1038/s41574-020-0387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson P., Kowalski J., Ekblom Ö., Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch. Pediatr. Adolesc. Med. 2012;166(12):1103–1108. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- Di Renzo L., Gualtieri P., Pivari F., Soldati L., Attinà A., Cinelli G., et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J. Transl. Med. 2020;18(1):229. doi: 10.1186/s12967-020-02399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Aranda F., Casas M., Claes L., Bryan D.C., Favaro A., Granero R., et al. COVID-19 and implications for eating disorders. Eur. Eat Disord. Rev. 2020;28(3):239–245. doi: 10.1002/erv.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E.A., Trogdon J.G., Cohen J.W., Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff. 2009;28(5):w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- Fox C.K., Gross A.C., Rudser K.D., Foy A.M., Kelly A.S. Depression, anxiety, and severity of obesity in adolescents: is emotional eating the link? Clin. Pediatr. 2016;55(12):1120–1125. doi: 10.1177/0009922815615825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Xian Y., Lv X., He J., Ren Y. Changes in body weight, physical activity, and lifestyle during the semi-lockdown period after the outbreak of COVID-19 in China: an online survey. Disaster Med. Public Health Prep. 2020:1–6. doi: 10.1017/dmp.2020.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen B.P., Kelly M.K., Powell M., Bouchelle Z., Mayne S.L., Fiks A.G. COVID-19 and changes in child obesity. Pediatrics. 2021;147(5) doi: 10.1542/peds.2021-050123. [DOI] [PubMed] [Google Scholar]
- Jia P., Zhang L., Yu W., Yu B., Liu M., Zhang D., et al. Impact of COVID-19 lockdown on activity patterns and weight status among youths in China: the COVID-19 Impact on Lifestyle Change Survey (COINLICS) Int. J. Obes. 2020 doi: 10.1038/s41366-020-00710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.S., Barlow S.E., Rao G., Inge T.H., Hayman L.L., Steinberger J., et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- Kelly A.S., Fox C.K., Rudser K.D., Gross A.C., Ryder J.R. Pediatric obesity pharmacotherapy: current state of the field, review of the literature and clinical trial considerations. Int. J. Obes. 2016;40(7):1043–1050. doi: 10.1038/ijo.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin M., Kurtoglu S., Kendirci M., Atabek M.E., Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500–e503. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bueno R., López-Sánchez G.F., Casajús J.A., Calatayud J., Gil-Salmerón A., Grabovac I., et al. Health-related behaviors among school-aged children and adolescents during the Spanish covid-19 confinement. Front. Pediatr. 2020;8:573. doi: 10.3389/fped.2020.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine J.E., Schwimmer J.B., Van Natta M.L., Molleston J.P., Murray K.F., Rosenthal P., et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305(16):1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loades M.E., Chatburn E., Higson-Sweeney N., Reynolds S., Shafran R., Brigden A., et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59 doi: 10.1016/j.jaac.2020.05.009. 1218-39.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli A.V., Pinti M., Farinetti A., Nasi M. Obesity risk during collective quarantine for the COVID-19 epidemic. Obes. Med. 2020;20 doi: 10.1016/j.obmed.2020.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh M.S., Selph S., Ozpinar A., Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168(2):178–184. doi: 10.1001/jamapediatrics.2013.4200. [DOI] [PubMed] [Google Scholar]
- Mead E., Atkinson G., Richter B., Metzendorf M.I., Baur L., Finer N., et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst. Rev. 2016;11(11) doi: 10.1002/14651858.CD012436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H.K., Kim H.R., Rhie Y.J., Lee K.H. Trends in the prevalence of extreme obesity among Korean children and adolescents from 2001 to 2014. J. Pediatr. Endocrinol. Metab. 2017;30(5):517–523. doi: 10.1515/jpem-2016-0456. [DOI] [PubMed] [Google Scholar]
- Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E., Holman R.R., Sherwin R., et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan B.M., Rudser K.D., Abuzzahab M.J., Fox C.K., Coombes B.J., Bomberg E.M., et al. Predictors of weight-loss response with glucagon-like peptide-1 receptor agonist treatment among adolescents with severe obesity. Clin. Obes. 2016;6(1):73–78. doi: 10.1111/cob.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemo M., Spreghini M.R., Manco M., Wietrzykowska Sforza R., Morino G. Childhood obesity and COVID-19 lockdown: remarks on eating habits of patients enrolled in a food-education program. Nutrients. 2021;13(2) doi: 10.3390/nu13020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard T.J., Temprosa M., Goldberg R., Haffner S., Ratner R., Marcovina S., et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann. Intern. Med. 2005;142(8):611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk A., Mazicioglu M.M., Hatipoglu N., Budak N., Keskin G., Yazlak Z., et al. Reference body mass index curves for Turkish children 6 to 18 years of age. J. Pediatr. Endocrinol. Metab.: JPEM (J. Pediatr. Endocrinol. Metab.) 2008;21(9):827–836. doi: 10.1515/jpem.2008.21.9.827. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A., Pecoraro L., Ferruzzi A., Heo M., Faith M., Zoller T., et al. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in verona, Italy: a longitudinal study. Obesity. 2020;28(8):1382–1385. doi: 10.1002/oby.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman V., Foster C.M. Metformin treatment of pediatric obesity. Pediatrics. 2021;147(3) doi: 10.1542/peds.2020-044982. [DOI] [PubMed] [Google Scholar]
- Rezvanian H., Hashemipour M., Kelishadi R., Tavakoli N., Poursafa P. A randomized, triple masked, placebo-controlled clinical trial for controlling childhood obesity. World J. Pediatr. 2010;6(4):317–322. doi: 10.1007/s12519-010-0232-x. [DOI] [PubMed] [Google Scholar]
- Rundle A.G., Park Y., Herbstman J.B., Kinsey E.W., Wang Y.C. COVID-19-Related school closings and risk of weight gain among children. Obesity. 2020;28(6):1008–1009. doi: 10.1002/oby.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle A.G., Park Y., Herbstman J.B., Kinsey E.W., Wang Y.C. COVID-19–Related school closings and risk of weight gain among children. Obesity. 2020;28:1008–1009. doi: 10.1002/oby.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari N., Hosseinian-Far A., Jalali R., Vaisi-Raygani A., Rasoulpoor S., Mohammadi M., et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob. Health. 2020;16(1):57. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner A.C., Ravanbakht S.N., Skelton J.A., Perrin E.M., Armstrong S.C. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics. 2018;141(3) doi: 10.1542/peds.2017-3459. Pediatrics. 2018;142(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.K.-Prospective-Diabetes-Study-Group UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. U.K. Prospective Diabetes Study Group. Diabetes Care. 1998;21(1):87–92. doi: 10.2337/diacare.21.1.87. [DOI] [PubMed] [Google Scholar]
- Wang Y. Cross-national comparison of childhood obesity: the epidemic and the relationship between obesity and socioeconomic status. Int. J. Epidemiol. 2001;30(5):1129–1136. doi: 10.1093/ije/30.5.1129. [DOI] [PubMed] [Google Scholar]
- Wiegand S., l'Allemand D., Hübel H., Krude H., Bürmann M., Martus P., et al. Metformin and placebo therapy both improve weight management and fasting insulin in obese insulin-resistant adolescents: a prospective, placebo-controlled, randomized study. Eur. J. Endocrinol. 2010;163(4):585–592. doi: 10.1530/EJE-10-0570. [DOI] [PubMed] [Google Scholar]
- Workman J. How much may COVID-19 school closures increase childhood obesity? Obesity. 2020;28(10):1787. doi: 10.1002/oby.22960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary Z., Brianna F., Brianna L., Garrett P., Jade W., Alyssa D., et al. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes. Res. Clin. Pract. 2020;14(3):210–216. doi: 10.1016/j.orcp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]